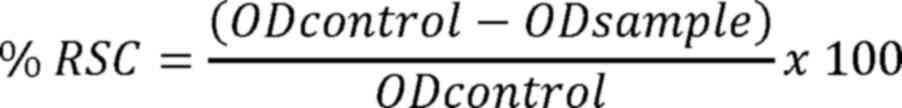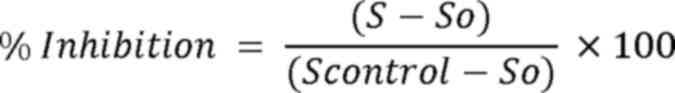Introduction
Viticulture and vinification are agricultural
activities with a significant socio-economic impact on a global
scale (1). The European Union (EU)
is the world-leading wine producer and consumer (2). According to the European Commission,
the total average wine production in the EU for the financial year
2021-2022 was 152,932,000 hectoliters (3). Despite its alcohol content, a
low-to-moderate wine consumption has been associated with
beneficial health effects, due to its high abundance in bioactive
phytochemicals (4,5). More specifically, previous studies
have demonstrated that wine is a rich source of polyphenols, plant
secondary metabolites that possess potent antioxidant (6), anti-inflammatory (7) and cardioprotective (8,9)
properties.
The wine phenolic composition and content is
dependent on several parameters and has a substantial impact on its
organoleptic characteristics and health-promoting effects (10). The grapevine variety is a key
determinant of the phenolic content, with red varieties containing
higher levels of phenol substances than white varieties. In
addition to the cultivar, the pedoclimatic conditions, such as soil
composition, temperature, relative humidity, exposure to sunlight,
rainfall and wind, contribute significantly to the grape phenolic
composition (11). Furthermore,
the vinification process constitutes a decisive factor in the wine
phenolic content. During the red wine vinification process, the
grape juice is fermented with all grape parts, including the skin
and seeds, resulting in a higher polyphenol extraction in the final
product (12). On the contrary,
during the white wine vinification process, the grape skin is
removed prior to grape juice fermentation, hence decreasing the
phenolic concentration (13).
Finally, the process of wine aging, particularly in oak barrels,
has a considerable impact on the phenolic composition of red wines
(12). To be more specific,
phenolic compounds released from oak barrels enhance the phenolic
content of the aged wine and contribute to color stability, thus
protecting against oxidation (14,15).
Additionally, during the red wine maturation and aging process,
co-pigmentation reactions occur, favored by the presence of oxygen,
leading to polymerization or formation of new, more complex and
stable pigments (16,17).
Greece is one of the oldest wine-producing regions
worldwide, with the first evidence of vinification dating back to
the 3rd millennium B.C., during the Minoan civilization. The
nutritional value and the health benefits of wine were widely
recognized in Ancient Greece. Therefore, wine was an integral part
of the daily regimen, also related to economic, social, religious
and cultural aspects. Currently, Greece is one the first
wine-producing territories in the EU, with 3.2% of the EU area
occupied by vines (18). Of
particular interest is that the Greek vineyard hosts ~200
indigenous grape cultivars of Vitis vinifera L., 60 of which
are extensively used in the winemaking process for the production
of fine red and white wines (19).
The dominant Greek red wine varieties are
Agiorgitiko and Xinomavro. Agiorgitiko is traditionally cultivated
in the Nemea region, in the northeastern part of the Peloponnese,
and contributes to the production of the protected designation of
origin (PDO) Nemea (20).
Xinomavro is cultivated in Northern Greece and contributes to the
production of the PDO Naoussa (21). As regards the Greek white wine
varieties, Assyrtiko and Malagouzia are definitely the most
recognizable. Assyrtiko, a white wine variety considered one of the
finest in the Mediterranean basin, originates from the volcanic
Aegean island of Santorini and contributes to the production of PDO
Santorini (22). Finally,
Malagouzia, a white wine variety saved from near extinction in the
recent past, is mainly cultivated in Central Greece and Macedonia
(23).
Based on the aforementioned information, the aim of
the present study was to determine the phenolic content and
antioxidant capacity of four indigenous Greek wines, namely the red
wine varieties Agiorgitiko and Xinomavro, as well as the white wine
varieties, Assyrtiko and Malagouzia. Towards this purpose, a
complete set of in vitro cell-free screening techniques was
applied for assessing the antiradical, reducing and antigenotoxic
properties of the wine varieties (24). The present study aims to improve
the current knowledge as regards the bioactivity of these
indigenous Greek grape varieties by assessing their bioactive
compound content and antioxidant potency, thus enhancing their
competitiveness and recognition abroad.
Materials and methods
Sample information and
preparation
A total of 32 commercial wines, eight of each wine
variety, produced from various regions across Greece and bottled in
750 ml wine bottles, were randomly selected and acquired from a
local wine store in Larissa, Greece. The alcohol content of
Agiorgitiko wines ranged from 13.5 to 15% v/v with an average of
14.1% v/v, that of Xinomavro wines from 12.5 to 14.5% v/v with an
average of 13% v/v, that of Assyrtiko wines from 13.5 to 14.8% v/v
with an average of 14.2% v/v, and that of Malagouzia wines from
12.5 to 13.8% v/v with an average of 13% v/v. Each bottle was
opened and the wine was divided into aliquots and stored at 4˚C,
until further analysis.
Determination of wine total phenolic
content (TPC). Folin-Ciocalteu assay
The TPC was evaluated using the Folin-Ciocalteu
phenol reagent (FCR; Merck KGaA), as previously described by
Singleton et al (25). In
detail, 20 µl of each wine sample (dilution 1:2 in deionized water
(dH2O) for red wines, no dilution for white wines) was
added to test tubes containing 1 ml dH2O. Subsequently,
100 µl FCR were added and the mixture was incubated in the dark at
room temperature (RT) for 3 min. Following incubation, 280 µl of
25% w/v sodium carbonate anhydrous (Na2CO3)
solution (Honeywell Research Chemicals) and 600 µl dH2O
were sequentially added and the mixture was incubated for 1 h in
the dark at RT. Following incubation, the optical density (OD) was
monitored at 765 nm using a UV/Visible spectrophotometer (U-1500,
Hitachi, Ltd.). For the determination of TPC, a standard curve was
prepared using various concentrations (50-1,500 µg/ml) of gallic
acid. The results were expressed as mg of gallic acid equivalents
(GAE)/ml of wine sample.
Determination of wine antioxidant
properties. 2,2-Diphenyl-1-picrylhydrazyl radical
(DPPH•) scavenging assay
DPPH• scavenging capacity was evaluated
on the basis of the method described in the study by Brand-Williams
et al (26). More
specifically, 50 µl of each wine sample (0.25-8 µl/ml for red wines
and 2.5-80 µl/ml for white wines) serially diluted in
dH2O was mixed with 900 µl of methanol (MeOH) and 50 µl
of DPPH• solution (2 mM; Alfa Aesar) in MeOH. In each
experiment, a blank containing 1 ml MeOH and a negative control
containing 950 µl MeOH and 50 µl DPPH• solution in MeOH
were prepared. Furthermore, vitamin C (Merck KGaA) was used as a
positive control. The samples were vortexed vigorously and
incubated in the dark at RT for 20 min. The OD was then measured at
517 nm using a UV/Visible spectrophotometer (U-1500, Hitachi,
Ltd.). The radical scavenging capacity percentage (% RSC) was
calculated using the following equation:
The half-maximal inhibitory concentration (IC50) was
calculated from the linear regression curve by plotting the % RSC
against the corresponding concentrations. The IC50 value represents
the concentration of the wine sample required to neutralize the 50%
of the corresponding free radicals. All analyses were carried out
in triplicate and at least in two separate occasions.
2,2'-Azinobis-(3-ethylbenzothiazoline-6-sulfonic
acid) radical (ABTS•+) scavenging assay.
ABTS•+ scavenging capacity was evaluated on the basis of
the method described in the study by Cano et al (27). More elaborately, 400 µl
dH2O, 500 µl ABTS solution (1 mM; Alfa Aesar) in
dH2O, 50 µl hydrogen peroxide
(H2O2; 30 µM; Merck KGaA), and 50 µl
horseradish peroxidase (HRP; 6 µM; SERVA Electrophoresis GmbH) were
sequentially added to test tubes. A blank containing 450 µl
dH2O, 500 µl ABTS solution and 50 µl
H2O2, as well as a negative control
comprising 400 µl dH2O, 500 µl ABTS solution, 50 µl
H2O2 and 50 µl HRP were also prepared.
Furthermore, vitamin C was used as a positive control. The samples
were vortexed vigorously and incubated in the dark at RT for 45
min. Subsequently, 50 µl of each wine sample (0.3125-20 µl/ml for
red and white wines) serially diluted in dH2O was added,
the samples were vortexed, and the OD was monitored at 730 nm using
a UV/Visible spectrophotometer (U-1500, Hitachi, Ltds). The % RSC
was calculated using the aforementioned equation. The IC50 value
was calculated from the linear regression curve by plotting the %
RSC against the corresponding concentrations. All analyses were
carried out in triplicate and at least in two separate
occasions.
Superoxide radical
(O2•−) scavenging assay. The
O2•− scavenging capacity was evaluated based
on the method described in the study by Gülçin et al
(28). More specifically, 50 µl of
each wine sample (0.25-8 µl/ml for red wines and 0.625-20 µl/ml for
white wines) diluted in dH2O was added to test tubes and
mixed with 625 µl Tris-HCl buffer (16 mM, pH 8.0), 125 µl nitro
blue tetrazolium (NBT; 300 µM; SERVA Electrophoresis GmbH), 125 µl
nicotinamide adenine dinucleotide (NADH; 468 µM; SERVA
Electrophoresis GmbH) and 125 µl phenazine methosulfate (PMS; 60
µM; Merck KGaA). A blank containing 800 µl Tris-HCl buffer, 125 µl
NBT and 125 µl NADH, as well as a negative control comprising 675
µl Tris-HCl buffer, 125 µl NBT, 125 µl NADH and 125 µl PMS were
prepared. Moreover, ellagic acid (Merck KGaA) was used as a
positive control. The samples were vortexed vigorously and
incubated in the dark at RT for 5 min. The OD was then measured at
560 nm using a UV/Visible spectrophotometer (U-1500, Hitachi.
Ltd.). The % RSC was calculated using the aforementioned equation.
The IC50 value was calculated from the linear regression curve by
plotting the % RSC against the corresponding concentrations. All
analyses were carried out in triplicate and at least in two
separate occasions.
Reducing power assay. The reducing power was
evaluated on the basis of the method described in the study by Yen
and Duh (29). In detail, 50 µl of
each wine sample (0.25-8 µl/ml for red wines and 1.25-40 µl/ml for
white wines) diluted in phosphate buffer (0.2 M, pH 6.6) was added
to test tubes and mixed with 200 µl phosphate buffer and 250 µl of
1% w/v potassium ferricyanide {K3[Fe(CN)6]}
(PanReac AppliChem, ITW Reagents) in dH2O. In each
experiment, a blank containing 500 µl phosphate buffer and a
negative control containing 250 µl phosphate buffer and 250 µl of
1% w/v potassium ferricyanide in dH2O were prepared.
Additionally, vitamin C was used as a positive control. The samples
were vortexed vigorously and incubated at 50˚C for 20 min.
Subsequently, 250 µl of 10% trichloroacetic acid (TCA; Merck KGaA)
were added to the mixture and the samples were centrifuged (875 x
g, 10 min, 25˚C). Following centrifugation, 700 µl of the
supernatant was transferred to new test tubes and 250 µl
dH2O and 50 µl of 0.1% iron (III) chloride (Merck KGaA)
in dH2O were added. The samples were vortexed and
incubated in the dark at RT for 10 min. Finally, the OD was
measured at 700 nm using a UV/Visible spectrophotometer (U-1500,
Hitachi, Ltd.). An absorbance unit 0.5 (AU0.5) value was calculated
from the linear regression curve by plotting the OD at 700 nm
against the corresponding concentrations. The AU0.5 value
represents the concentration of the wine sample required to achieve
an OD of 0.5 at 700 nm. All analyses were carried out in triplicate
and at least in two separate occasions.
Cupric ion reducing antioxidant capacity (CUPRAC)
assay. The reducing ability against copper ions was evaluated
on the basis of the method described in the study by Apak et
al (30). More specifically,
250 µl copper (II) chloride dihydrate (CuCl2) solution
(0.01 M; (Merck KGaA), 250 µl neocuproine (Nc) ethanolic solution
(0.0075 M; Merck KGaA), 250 µl ammonium acetate
(NH4CH3CO2) solution (1 M, pH 7.0;
Honeywell Research Chemicals), 225 µl dH2O, and 50 µl of
each wine sample (0.25-8 µl/ml for red wines and 1.25-40 µl/ml for
white wines) diluted in dH2O were mixed in test tubes.
In each experiment, a blank comprising 250 µl CuCl2
solution, 250 µl NH4CH3CO2
solution and 525 µl dH2O, and a negative control
containing 250 µl CuCl2 solution, 250 µl Nc solution,
250 µl NH4CH3CO2 solution and 275
µl dH2O were prepared. Furthermore, vitamin C was used
as a positive control. The mixture was vortexed vigorously and
incubated in the dark at RT for 30 min. The optical density was
then measured at 450 nm using a UV/Visible spectrophotometer
(U-1500, Hitachi, Ltd.). An AU0.5 value was calculated from the
linear regression curve by plotting the OD at 450 nm against the
corresponding concentrations. All analyses were carried out in
triplicate and at least in two separate occasions.
Plasmid DNA relaxation assay. The protective
ability against oxidative DNA damage was evaluated on the basis of
the method previously described by Paul et al (31). More specifically, 3 µl of each wine
sample (0.5-8 µl/ml for red wines and 2.5-40 µl/ml for white wines)
diluted in sterilized dH2O was mixed with 2 µl plasmid
DNA pBluescript II SK (+) (3.2 µg) (Stratagene, Agilent
Technologies), 1 µl of sterilized phosphate-buffered saline (PBS;
0.01 M, pH 7.4) (Gibco, Thermo Fischer Scientific, Inc.) and 4 µl
of 2,2'-azobis(2-amidinopropane) dihydrochloride (AAPH; 95 mM;
Merck KGaA) in sterilized PBS. A negative control comprising 8 µl
sterilized PBS and 2 µl plasmid DNA, and a positive control
comprising 4 µl sterilized PBS, 2 µl plasmid DNA and 4 µl of AAPH
were also prepared. Vitamin C was used as a standard. Furthermore,
the plasmid DNA was treated with the highest concentration of each
wine sample in order to assess its effects on supercoiled
conformation. The samples were vortexed and incubated for 45 min at
37˚C for the thermal decomposition of AAPH and the generation of
peroxyl radicals (ROO•). Following incubation, 3 µl of
loading buffer were added and the samples were loaded on a 0.8%
agarose gel (SERVA Electrophoresis GmbH), stained with ethidium
bromide (10 µg/ml) for 30 min at RT and electrophoresed at 70 V for
60 min. The gel was then exposed to UV using the MultiImage Light
Cabinet (ProteinSimple). The image was captured and analyzed using
a quantification software (AlphaView software, AlphaInnotech). The
protective ability of the wine samples was calculated through the
following equation:
where ‘S’ stands for the percentage of supercoiled
plasmid DNA in samples, ‘So’ stands for the percentage of
supercoiled plasmid DNA in the positive control, and ‘Scontrol’
stands for the percentage of supercoiled plasmid DNA in the
negative control.
The IC50 value was calculated from the linear
regression curve by plotting the % inhibition against the
corresponding concentrations. All analyses were conducted at least
in two separate occasions.
Statistical analysis
One-way analysis of variance (ANOVA), followed by
the Holm-Sidak post hoc test, were performed for multiple pairwise
comparisons between the mean IC50 or AU0.5 values of the different
wine varieties. An unpaired t-test was performed to compare the
mean IC50 or AU0.5 values between the red and white wine varieties.
Pearson's correlation coefficient (r) was calculated to determine
the correlation between the TPC and antioxidant capacity of the red
and white wine varieties. All data are presented as the mean ±
standard error of the mean (SEM). A value of P<0.05 was
considered to indicate a statistically significant difference. All
statistical analyses were performed using GraphPad Prism version
8.0.1 for Windows, GraphPad Software, Inc.
Results
TPC and antioxidant capacity of the
different wine varieties
According to the results obtained, all wine
varieties exhibited potent antioxidant activities (Fig. 1). Among them, the red wine variety
Xinomavro demonstrated the most potent antioxidant capacity, an
assertion supported by the lowest IC50 or AU0.5 values in all
cell-free assays tested. By contrast, the white wine variety
Malagouzia exhibited the weakest antioxidant properties, as denoted
by the highest IC50 or AU0.5 values, in all cell-free assays
examined.
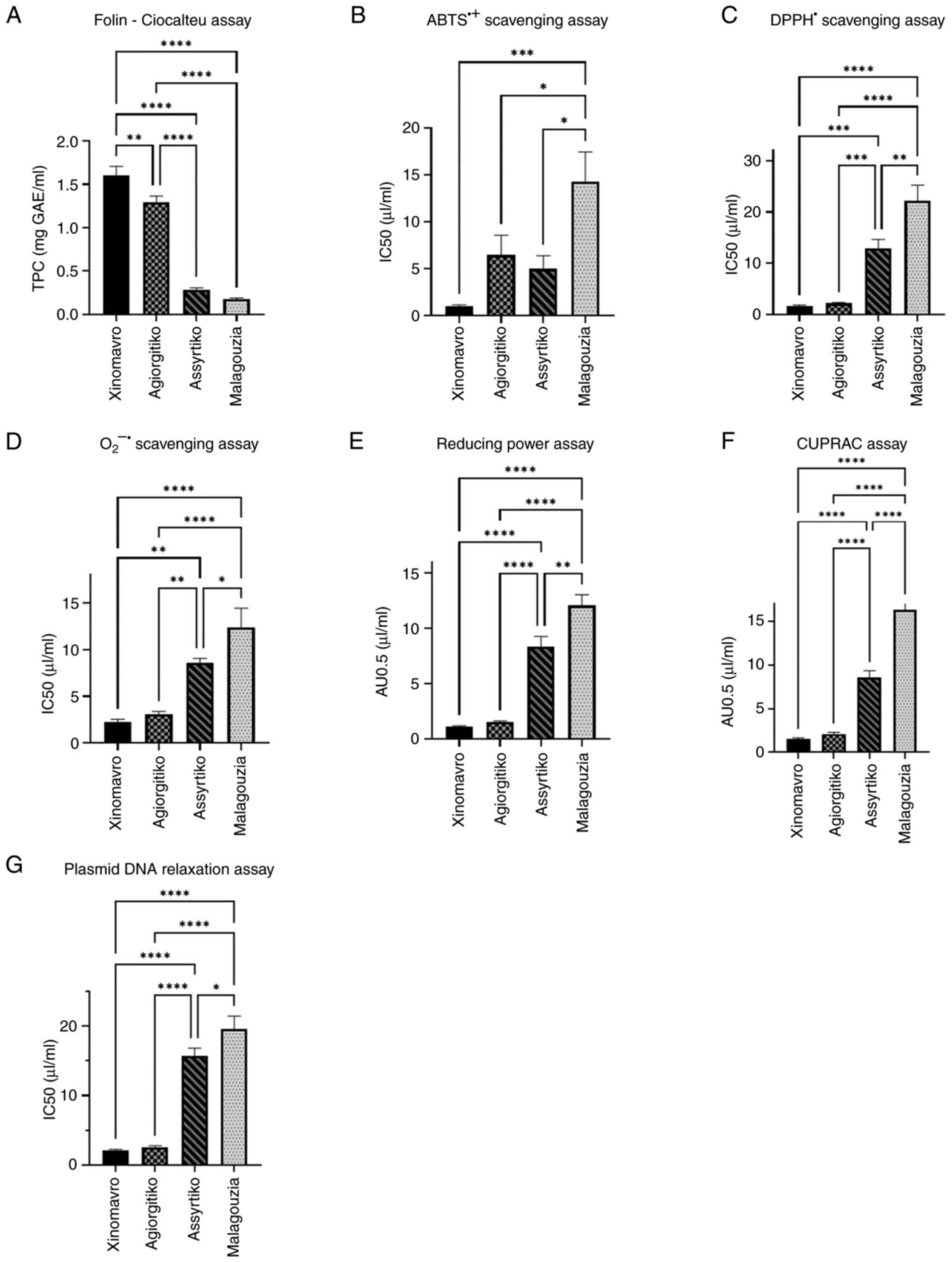 | Figure 1TPC and the antioxidant activities of
Xinomavro, Agiorgitiko, Assyrtiko and Malagouzia wine varieties
determined using (A) Folin-Ciocalteu assay, (B) ABTS•+
scavenging assay, (C) DPPH• scavenging assay, (D)
O2•- scavenging assay, (E) reducing power
assay, (F) CUPRAC assay, and (G) plasmid DNA relaxation assay.
*P<0.05, **P<0.01,
***P<0.001 and ****P<0.0001. TPC, total
phenolic content; ABTS•+,
2,2'-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) radical;
DPPH•, 2,2-diphenyl-1-picrylhydrazyl radical;
O2•-, superoxide radical; CUPRAC, cupric ion
reducing antioxidant capacity IC50, half-maximal inhibitory
concentration; AU0.5, absorbance unit 0.5. |
To begin with TPC, measured using the
Folin-Ciocalteu method and presented in Fig. 1A, Xinomavro exhibited the highest
TPC, which was calculated at 1.602±0.106 mg GAE/ml, whereas
Malagouzia exhibited the lowest, which was calculated at
0.178±0.013 mg GAE/ml. The TPC of Agiorgitiko was calculated at
1.291±0.072 mg GAE/ml and that of Assyrtiko was calculated at
0.284±0.072 mg GAE/ml. The statistical analysis revealed
significant differences between the red and white wine varieties,
as well as a significant difference between Xinomavro and
Agiorgitiko wine varieties.
As regards ABTS•+ radical scavenging
assay, illustrated in Fig. 1B,
Xinomavro exhibited the highest capacity to neutralize the
corresponding free radicals, whereas Malagouzia demonstrated the
lowest. More specifically, the IC50 value calculated for Xinomavro
was 0.985±0.153 µl/ml, that for Agiorgitiko was 6.441±2.114 µl/ml,
that for Assyrtiko was 5.004±1.385 µl/ml and that for Malagouzia
was 14.29±3.145 µl/ml. A finding of particular interest was that
the IC50 value of Assyrtiko was lower than that of Agiorgitiko. The
statistical analysis revealed a significant difference between all
wine varieties and Malagouzia. Moreover, the IC50 value calculated
for vitamin C was 2.298±0.044 µg/ml (data not shown).
As regards the DPPH• radical scavenging
assay depicted in Fig. 1C,
Xinomavro exhibited the highest efficacy to scavenge the
corresponding free radicals, whereas Malagouzia exhibited the
lowest. In particular, the IC50 value calculated for Xinomavro was
1.591±0.260 µl/ml, that for Agiorgitiko was 2.210±0.176 µl/ml, that
for Assyrtiko was 12.870±1.808 µl/ml and that for Malagouzia was
22.200±3.046 µl/ml. The statistical analysis revealed significant
differences between tje red and white wine varieties, as well as a
significant difference between the Assyrtiko and Malagouzia wine
varieties. In addition, the IC50 value calculated for vitamin C was
4.565±0.183 µg/ml (data not shown).
As regards the O2•- radical
scavenging assay, illustrated in Fig.
1D, Xinomavro demonstrated the most potent ability to scavenge
corresponding free radicals, while by contrast, Malagouzia
exhibited the weakest. In particular, the IC50 value for Xinomavro
was calculated as 2.277±0.260 µl/ml, that for Agiorgitiko was
calculated as 3.076±0.300 µl/ml, that for Assyrtiko was calculated
as 8.550±0.503 µl/ml and that for Malagouzia was calculated as
12.34±2.08 µl/ml. The statistical analysis revealed significant
differences between the red and white wine varieties, as well as a
significant difference between the Assyrtiko and Malagouzia wine
varieties. Additionally, the IC50 value calculated for ellagic acid
was 255.430±8.500 µg/ml (data not shown).
In terms of the reducing power assay, presented in
Fig. 1E, Xinomavro exhibited the
most prominent reducing properties, whereas Malagouzia exhibited
the weakest. In particular, the AU0.5 value calculated for
Xinomavro was 1.091±0.100 µl/ml, that for Agiorgitiko was
1.548±0.077 µl/ml, that for Assyrtiko was 8.361±0.909 µl/ml and
that for Malagouzia was 12.08±0.974 µl/ml. Statistically
significant differences were observed between the red and white
wine varieties. Additionally, a statistically significant
difference was observed between the Assyrtiko and Malagouzia wine
varieties. Moreover, the AU0.5 value calculated for vitamin C was
1.700±0.062 µg/ml (data not shown).
As regards the CUPRAC assay, presented in Fig. 1F, Xinomavro exhibited the highest
reducing properties, whereas Malagouzia exhibited the lowest. To be
more specific, the AU0.5 value calculated for Xinomavro was
1.531±0.140 µl/ml, that for Agiorgitiko was 2.105±0.188 µl/ml, that
for Assyrtiko was 8.552±0.798 µl/ml and that for Malagouzia was
16.24±1.292 µl/ml. The statistical analysis revealed significant
differences between the red and white wine varieties, as well as a
significant difference between the Assyrtiko and Malagouzia wine
varieties. Moreover, the AU0.5 value calculated for vitamin C was
6.551±0.050 µg/ml (data not shown).
Finally, concerning the plasmid DNA relaxation
assay, depicted in Fig. 1G,
Xinomavro exhibited the highest efficacy to inhibit the formation
of the corresponding free radicals, whereas Malagouzia demonstrated
the lowest. More elaborately, the IC50 value calculated for
Xinomavro was 2.078±0.163 µl/ml, that for Agiorgitiko was
2.547±0.218 µl/ml, that for Assyrtiko was 15.70±1.125 µl/ml and
that for Malagouzia was 19.62±1.830 µl/ml. The statistical analysis
revealed significant differences between the red and white wine
varieties, as well as a significant difference between the
Assyrtiko and Malagouzia wine varieties. In addition, the IC50
value calculated for vitamin C was 300.302±21.852 µg/ml (data not
shown).
TPC and antioxidant capacity of the
red and white wine varieties
In order to compare the TPC and the antioxidant
properties between the red and white wine varieties, the IC50 or
AU0.5 values of the wine samples of the red or white wine varieties
were pooled together and the mean IC50 or AU0.5 values were
calculated. According to the results obtained, substantial
differences were observed between the red and white wine varieties.
To be more specific, the red wine varieties demonstrated a higher
phenolic content and more potent antioxidant activities than the
white wine varieties, and all differences were statistically
significant (Fig. 2).
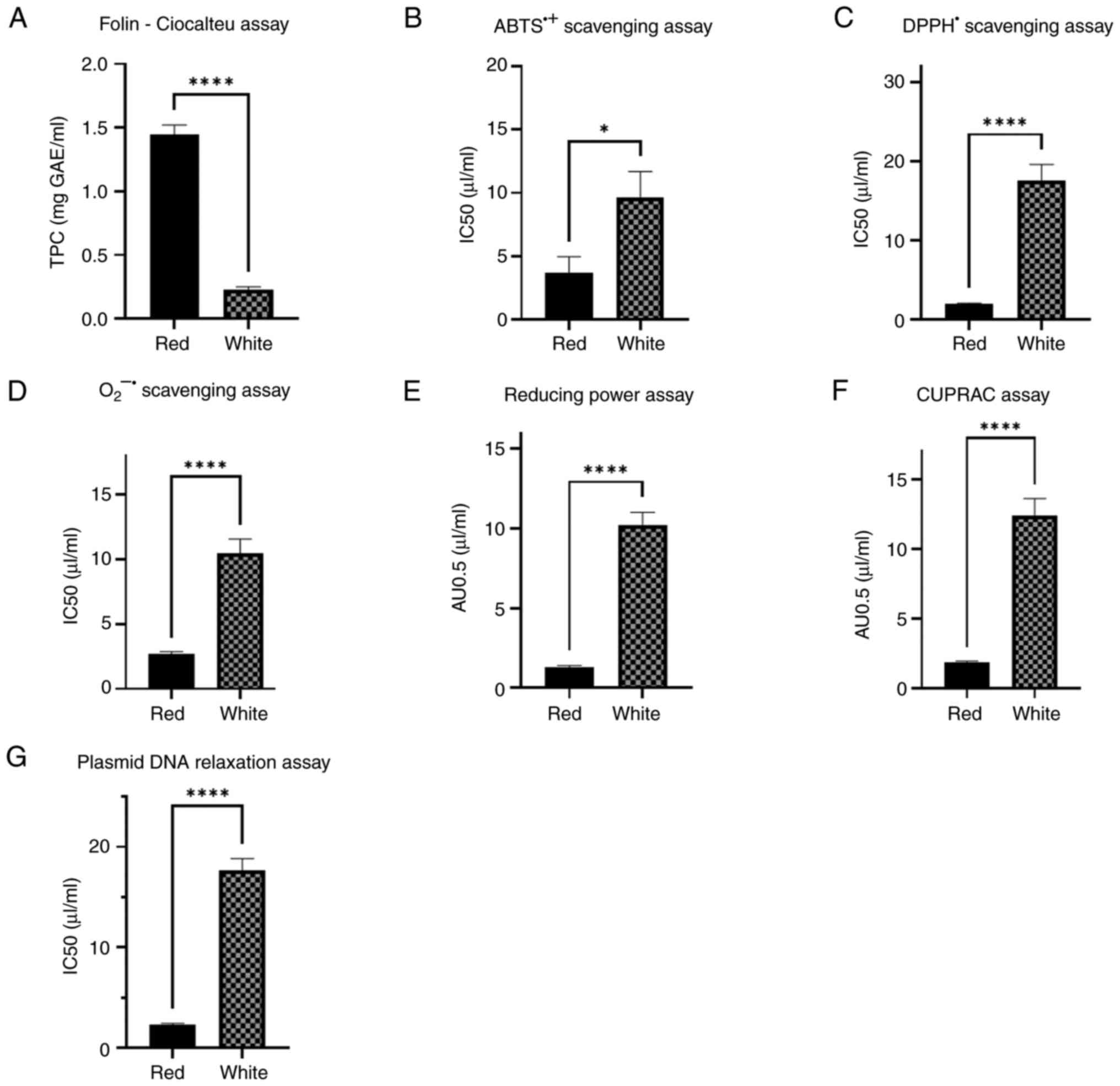 | Figure 2TPC and the antioxidant activities of
the red and white wine varieties determined using the (A)
Folin-Ciocalteu assay, (B) ABTS•+ scavenging assay, (C)
DPPH• scavenging assay, (D) O2•-
scavenging assay, (E) reducing power assay, (F) CUPRAC assay, and
(G) plasmid DNA relaxation assay. *P<0.05 and
****P<0.0001. TPC, total phenolic content;
ABTS•+, 2,2'-azinobis-(3-ethylbenzothiazoline-6-sulfonic
acid) radical; DPPH•, 2,2-diphenyl-1-picrylhydrazyl
radical; O2•-, superoxide radical; CUPRAC,
cupric ion reducing antioxidant capacity IC50, half-maximal
inhibitory concentration; AU0.5, absorbance unit 0.5. |
As regards the Folin-Ciocalteu assay, illustrated in
Fig. 2A, the TPC for the red wine
varieties was calculated 1.446±0.074 mg GAE/ml and that for the
white wine varieties was estimated at 0.231±0.019 mg GAE/ml. As
regards the ABTS•+ radical scavenging assay, presented
in Fig. 2B, the IC50 value for the
red wine varieties was estimated at 3.713±1.243 µl/ml and that for
the white wine varieties was estimated at 9.648±2.048 µl/ml.
Concerning the DPPH• radical scavenging assay (Fig. 2C), the IC50 value for the red wine
varieties was calculated at 1.900±0.171 µl/ml and that for the
white wine varieties was calculated at 17.530±2.093 µl/ml. In terms
of the O2•- radical scavenging assay,
presented in Fig. 2D, the IC50
value for the red wine varieties was calculated at 2.676±0.218
µl/ml, while the IC50 value for the white wine varieties was
calculated at 10.45±1.144 µl/ml.
With respect to the reducing power assay,
illustrated in Fig. 2E, the AU0.5
value for the red wine varieties was calculated at 1.319±0.085
µl/ml and that for the white wine varieties was calculated at
10.22±0.803 µl/ml. Concerning the CUPRAC assay (Fig. 2F), the AU0.5 value for the red wine
varieties was estimated at 1.818±0.135 µl/ml and that for the white
wine varieties was estimated at 12.39±1.234 µl/ml.
Finally, as regards the antigenotoxic properties,
evaluated using plasmid DNA relaxation assay and presented in
Fig. 2G, the IC50 value for the
red wine varieties was calculated at 2.312±0.145 µl/ml and that for
the white wine varieties was calculated at 17.66±1.155 µl/ml.
Correlation between the TPC and
antioxidant capacity of the red and white wine varieties
Pearson's correlation coefficient (r) was calculated
to determine the correlation between the TPC and antioxidant
properties of the red and white wine varieties. The correlation
analysis demonstrated significant negative correlations in almost
all cases, indicating that the higher the TPC, the lower the IC50
or AU0.5 value, and as a result, the more potent the antioxidant
capacity.
As regards the red wine varieties (Fig. 3), significant negative correlations
were observed between the TPC and ABTS•+ radical
scavenging assay (r=-0.5580l; Fig.
3A), the O2•- radical scavenging assay
(r=-0.5881; Fig. 3C), the reducing
power assay (r=-0.5009; Fig. 3D),
the CUPRAC assay (r=-0.5965; Fig.
3E) and the plasmid DNA relaxation assay (r=-0.5381; Fig. 3F). The negative correlation
detected between TPC and DPPH• radical scavenging assay
(r=-0.3093; Fig. 3B) was not
significant.
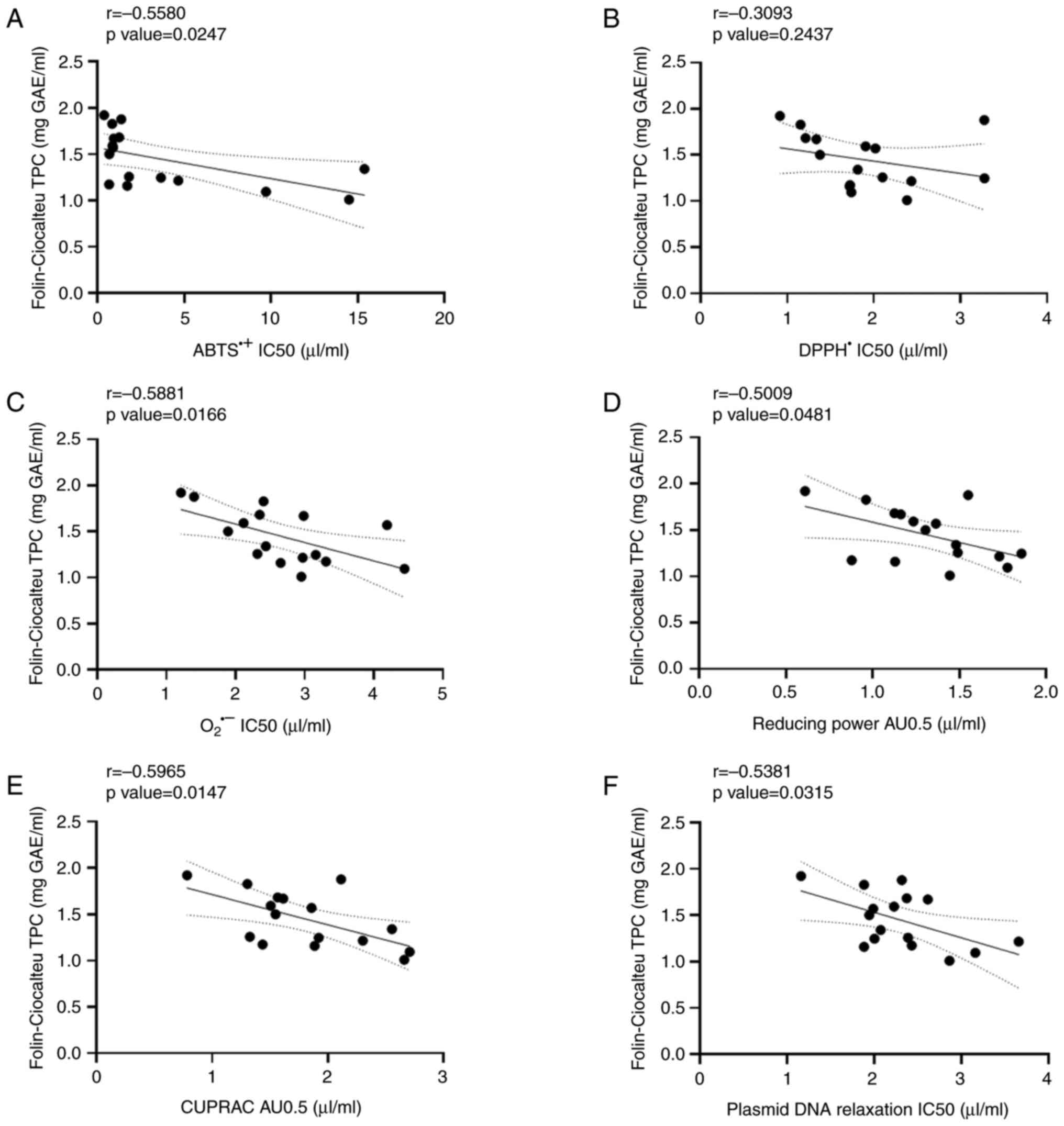 | Figure 3Correlation analysis between TPC and
(A) ABTS•+ scavenging assay, (B) DPPH•
scavenging assay, (C) O2•- scavenging assay,
(D) reducing power assay, (E) CUPRAC assay, and (F) plasmid DNA
relaxation assay in the red wine varieties. TPC, total phenolic
content; ABTS•+,
2,2'-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) radical;
DPPH•, 2,2-diphenyl-1-picrylhydrazyl radical;
O2•-, superoxide radical; CUPRAC, cupric ion
reducing antioxidant capacity IC50, half-maximal inhibitory
concentration; AU0.5, absorbance unit 0.5. |
Concerning the white wine varieties (Fig. 4), significant negative correlations
were observed between TPC and ABTS•+ radical scavenging
assay (r=-0.6584; Fig. 4A), the
DPPH• radical scavenging assay (r=-0.6119; Fig. 4B), the O2•-
radical scavenging assay (r=-0.6740; Fig. 4C), the reducing power assay
(r=-0.8329; Fig. 4D), the CUPRAC
assay (r=-0.8539; the Fig. 4E) and
the plasmid DNA relaxation assay (r=-0.5693; Fig. 4F).
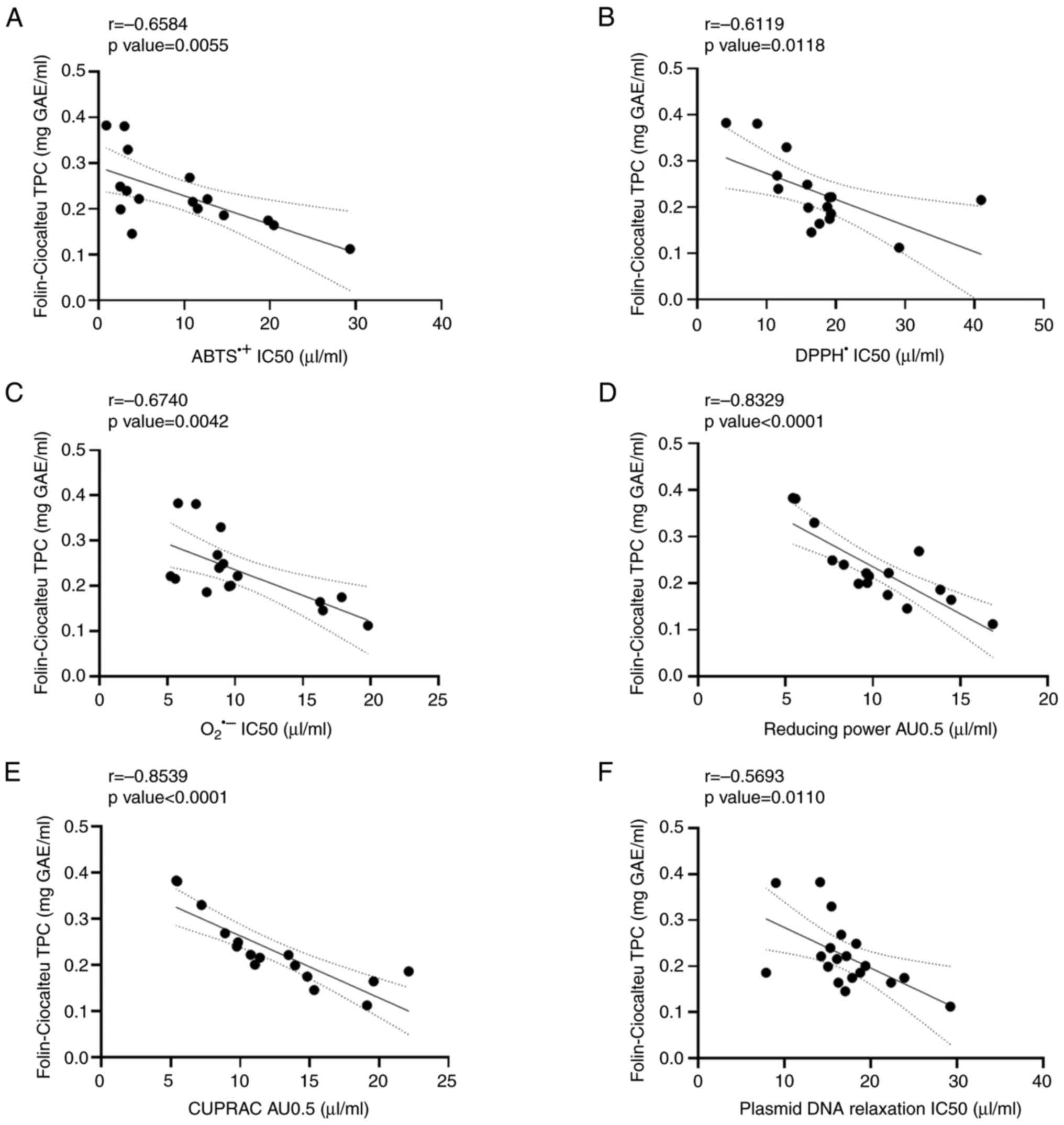 | Figure 4Correlation analysis between TPC and
(A) ABTS•+ scavenging assay, (B) DPPH•
scavenging assay, (C) O2•- scavenging assay,
(D) reducing power assay, (E) CUPRAC assay, and (F) plasmid DNA
relaxation assay in the white wine varieties. TPC, total phenolic
content; ABTS•+,
2,2'-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) radical;
DPPH•, 2,2-diphenyl-1-picrylhydrazyl radical;
O2•-, superoxide radical; CUPRAC, cupric ion
reducing antioxidant capacity IC50, half-maximal inhibitory
concentration; AU0.5, absorbance unit 0.5. |
Discussion
In the present study, the phenolic content and the
antioxidant potency of four native Greek wine varieties, and more
specifically, the red wine varieties, Xinomavro and Agiorgitiko,
and the white wine varieties, Assyrtiko and Malagouzia, were
thoroughly evaluated using a methodology previously proposed by the
authors (24). The utilization of
a panel of reliable and valid in vitro cell-free assays
represents the first line of screening towards the investigation of
the antioxidant activities of natural products that are
particularly rich in polyphenolic compounds. It has been
well-established that antioxidants exert their protective effects
by acting as free radical scavengers, as reducing agents, as metal
chelators and as enhancers of antioxidant gene expression (32,33).
For this reason, in the present study, the antioxidant activities
of Greek wine varieties were assessed multifacetedly on the basis
of their antiradical, reducing, and antigenotoxic properties.
Polyphenolic compounds are the main bioactive
phytochemicals of wine that exhibit antioxidant potency (34). More specifically, polyphenols
protect wine from oxidation, thus extending its shelf life, and
exert health-promoting effects in biological systems (35-38).
However, the polyphenolic content and profile differs significantly
between the red and white wine varieties and, in general, red wine
varieties exhibit higher concentrations of phenolic compounds than
the white wine varieties (12,39).
In particular, the concentration of phenolic compounds in red wines
is ~6-fold higher than that in white wines, due to the fact that
the red juice is fermented with the grape skins and seeds, wherein
the phenolic compounds are mainly concentrated (40). As regards the polyphenolic profile,
tannins and anthocyanins are the most abundant polyphenols in red
wines, whereas white wines are particularly rich in phenolic acids
(41).
The first objective of the present study was to
determine the polyphenolic content of the four native Greek wine
varieties using the Folin-Ciocalteu method. According to the
results obtained, the TPC of red wine varieties was significantly
higher than that of the white wine varieties, a finding supported
by scientific literature (42-47).
As mentioned above, the main reason for the higher levels of
polyphenolic compounds in red wines is that the fermentation
process of red juice includes all grape parts, resulting in greater
polyphenol extraction in the final product (48). Furthermore, in the present study,
as regards the TPC of different wine varieties, Xinomavro
demonstrated the highest concentration of polyphenolic compounds,
whereas Malagouzia exhibited the lowest. A significant difference
was also detected between the TPC of the Xinomavro and Agiorgitiko
red wine varieties, with Xinomavro exhibiting higher levels of
polyphenols. A previous study by the authors investigated the
phenolic content and the antioxidant and antimutagenic properties
of wine extracts derived from Xinomavro, Agiorgitiko, Assyrtiko and
Malagouzia wine varieties, using a battery of in vitro
cell-free assays (49). That study
reported that the TPC of the red wine extracts was higher than that
of the white wine extracts; however, there were no significant
differences between the TPC of Xinomavro and Agiorgitiko wine
extracts (49).
The quantitative and the qualitative polyphenolic
profile determines the biological actions of wine (34,50).
For the purpose of evaluating the bioactive load of polyphenolic
compounds, the antioxidant activities of the four native Greek wine
varieties were assessed on the basis of their free radical
scavenging capacity and their reducing properties. According to the
results obtained, the red wine varieties demonstrated more potent
antioxidant activities than the white wine varieties in all
cell-free assays examined. This finding may be attributed to the
higher polyphenolic content of the red wine varieties, which is in
accordance with findings obtained from previous studies (51-55).
Moreover, in the present study, concerning the antioxidant
activities of the different wine varieties, Xinomavro exhibited the
strongest antioxidant efficacy, whereas Malagouzia demonstrated the
weakest in all cell-free assays tested. The obtained results are in
line with the findings obtained using the Folin-Ciocalteu method,
with Xinomavro wine variety exhibiting the highest TPC and
Malagouzia wine variety the slightest.
Finally, the present study investigated the
correlation between the TPC and antioxidant activities of the red
and white wine varieties. The Pearson's correlation coefficient (r)
revealed significant negative correlations between the TPC and
antioxidant assays in almost all cases, suggesting that the
antioxidant properties of red and white wine varieties are
significantly affected by polyphenolic content. In particular, the
higher the TPC, the greater the antioxidant activities. As regards
the red wine varieties, similar levels of significant strong
negative correlations were observed between the TPC and all
antioxidant assays, apart from the DPPH• scavenging
assay, wherein the negative correlation was not significant.
Concerning the white wine varieties, significant strong negative
correlations were observed between the TPC and all antioxidant
assays. A finding of particular interest was that the highest
degree of correlation was observed between the TPC, and the
reducing power and CUPRAC assays. To be more specific, these in
vitro test tube assays examine the ability of a sample to
reduce ferric (Fe3+) to ferrous (Fe2+) ions
and cupric (Cu2+) to cuprous (Cu1+) ions,
respectively. Reducing agents are strong electron donors, that can
reduce oxidized intermediates of lipid peroxidation process in
biological systems (56).
Prior to summarizing the conclusions of the
particular research, it should be underlined that the findings need
to be viewed in light of some limitations. As previously stated,
the present study represents the first-line test for evaluating the
phenolic content and antioxidant capacity of commercial wines of
Agiorgitiko, Xinomavro, Assyrtiko and Malagouzia. Hence, it
provides some early, yet valuable, indications of the bioactivity
of these indigenous Greek varieties. It has to be mentioned that at
this phase of the research project, we did not perform liquid
chromatography-high-resolution mass spectrometry to identify and
characterize the main phenolics of the selected wines in order to
correlate them with their antioxidant properties. Furthermore, the
sugar content and flavor are both variables that could have been
considered in the determination of the antioxidant potency of
wines. To the best of our knowledge, high performance liquid
chromatography (HPLC), Fehling's method for reducing sugars and
various enzymatic analyses are widely used for the determination of
the sugar content; however, no such investigations have been
conducted in the present study. Finally, the volatile composition
of wine is closely related to its characteristic aroma and flavor
(57). Among the other volatile
flavor compounds, esters, formed by reactions occurring between
alcohols and acids, are responsible for the primary fruit and
floral aromas and flavors in wines (58,59).
However, the present study did not perform any analysis to
determine the volatile composition of the selected wines and,
therefore, it is not safe to draw any conclusions as regards the
effect of wine flavor on the parameters measured.
Conclusively, the present study reported that the
indigenous Greek wine varieties, Xinomavro, Agiorgitiko, Assyrtiko
and Malagouzia, are rich sources of phytochemical constituents and
exert strong biological activities, attributed to their
polyphenolic content. The red wine varieties demonstrate a higher
TPC and exhibit more prominent antioxidant activities than the
white wine varieties. In addition, among the different wine
varieties, Xinomavro exhibited the highest concentration of
phenolic compounds and the most robust antioxidant activities.
Overall, the findings of the present study dictate that these
native Greek wines are highly bioactive, particularly Xinomavro,
and their moderate consumption may be related to beneficial health
effects, due to their potent antioxidant properties.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FT and DK conceptualized the study. FT and MG were
involved in the study methodology. FT, PV and ZS were involved in
the formal analysis and in data curation. FT, MG and PV were
involved in the writing and preparation of the original draft. PV
was involved in the writing, reviewing and editing of the
manuscript. FT was involved in visualization (creation of the
figures for the article). DK supervised the study and was involved
in project administration. FT and DK confirm the authenticity of
all the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
DK is an Editor of the journal, but had no personal
involvement in the reviewing process, or any influence in terms of
adjudicating on the final decision, for this article. The other
authors declare that they have no competing interests.
References
|
1
|
Buja LM: The history, science, and art of
wine and the case for health benefits: Perspectives of an
oenophilic cardiovascular pathologist. Cardiovasc Pathol.
60(107446)2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Corsinovi P and Gaeta D: The European wine
policies: Regulations and strategies. Palgrave Handb Wine Ind Econ.
265–290. 2019.
|
|
3
|
European Commission:sDirectorate-General
for Agriculture and Rural Development: Wine production and opening
stocks. Available from: https://agridata.ec.europa.eu/extensions/DashboardWine/WineProduction.html.
Accessed October 3, 2023].
|
|
4
|
Castaldo L, Narváez A, Izzo L, Graziani G,
Gaspari A, Minno GD and Ritieni A: Red wine consumption and
cardiovascular health. Molecules. 24(3626)2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Markoski MM, Garavaglia J, Oliveira A,
Olivaes J and Marcadenti A: Molecular properties of red wine
compounds and cardiometabolic benefits. Nutr Metab Insights.
9:51–57. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Giovinazzo G, Carluccio MA and Grieco F:
Wine polyphenols and health. In: Bioactive Molecules in Food:
Reference Series in Phytochemistry. Mérillon JM and Ramawat K
(eds). Springer, Cham. pp1135-1155, 2019.
|
|
7
|
Di Renzo L, Marsella LT, Carraro A,
Valente R, Gualtieri P, Gratteri S, Tomasi D, Gaiotti F and De
Lorenzo A: Changes in LDL oxidative status and oxidative and
inflammatory gene expression after red wine intake in healthy
people: A randomized trial. Mediators Inflamm.
2015(317348)2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Liberale L, Bonaventura A, Montecucco F,
Dallegri F and Carbone F: Impact of red wine consumption on
cardiovascular health. Curr Med Chem. 26:3542–3566. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Poli A, Marangoni F, Avogaro A, Barba G,
Bellentani S, Bucci M, Cambieri R, Catapano AL, Costanzo S,
Cricelli C, et al: Moderate alcohol use and health: A consensus
document. Nutr Metab Cardiovasc Dis. 23:487–504. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Merkyte V, Longo E, Windisch G and Boselli
E: Phenolic compounds as markers of wine quality and authenticity.
Foods. 9(1785)2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Shah MH, Rafique R, Rafique T, Naseer M,
Khalil U, Rafique R, Shah MH, Rafique R, Rafique T, Naseer M, et
al: Effect of climate change on polyphenols accumulation in
grapevine. 2021.
|
|
12
|
Gutiérrez-Escobar R, Aliaño-González MJ
and Cantos-Villar E: Wine polyphenol content and its influence on
wine quality and properties: A review. Molecules.
26(718)2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Haseeb S, Alexander B, Santi RL, Liprandi
AS and Baranchuk A: What's in wine? A clinician's perspective.
Trends Cardiovasc Med. 29:97–106. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhang B, Cai J, Duan CQ, Reeves MJ and He
F: A review of polyphenolics in oak woods. Int J Mol Sci.
16:6978–7014. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Rasines-Perea Z, Jacquet R, Jourdes M,
Quideau S and Teissedre PL: Ellagitannins and
flavano-ellagitannins: Red wines tendency in different areas,
barrel origin and ageing time in barrel and bottle. Biomolecules.
9(316)2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Martínez-Gil A, Del Alamo-Sanza M and
Nevares I: Evolution of red wine in oak barrels with different
oxygen transmission rates. Phenolic compounds and colour. LWT.
158(113133)2022.
|
|
17
|
Pfahl L, Catarino S, Fontes N, Graça A and
Ricardo-Da-Silva J: Effect of barrel-to-barrel variation on color
and phenolic composition of a red wine. Foods.
10(1669)2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Vineyards in the EU - statistics -
Statistics Explained, 2022. Available from: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Vineyards_in_the_EU_-_statistics.
Accessed July 3, 2023].
|
|
19
|
Biniari K, Xenaki M, Daskalakis I, Rusjan
D, Bouza D and Stavrakaki M: Polyphenolic compounds and
antioxidants of skin and berry grapes of Greek Vitis vinifera
cultivars in relation to climate conditions. Food Chem.
307(125518)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kazou M, Pagiati L, Dotsika E, Proxenia N,
Kotseridis Y and Tsakalidou E: The microbial terroir of the nemea
zone agiorgitiko cv.: A first metataxonomic approach. Aust J Grape
Wine Res. 2023:1–18. 2023.
|
|
21
|
Koufos GC, Mavromatis T, Koundouras S,
Fyllas NM, Theocharis S and Jones GV: Greek wine quality assessment
and relationships with climate: Trends, future projections and
uncertainties. Water (Switzerland). 14(573)2022.
|
|
22
|
Frankel C: Volcanoes and Wine: From
Pompeii to Napa. Chicago, The University of Chicago Press,
2019.
|
|
23
|
Skiada FG, Grigoriadou K and Eleftheriou
EP: Micropropagation of Vitis vinifera L. cv. ‘Malagouzia’ and
‘Xinomavro’. Cent Eur J Biol. 5:839–852. 2010.
|
|
24
|
Veskoukis A, Kerasioti E, Priftis A, Kouka
P, Spanidis Y, Makri S and Kouretas D: A battery of translational
biomarkers for the assessment of the in vitro and in vivo
antioxidant action of plant polyphenolic compounds: The biomarker
issue. Curr Opin Toxicol. 13:99–109. 2019.
|
|
25
|
Singleton LV, Orthofer R, Lamuela-Raventós
MR, Singleton VL, Orthofer R and Lamuela-Raventós RM: [14] Analysis
of total phenols and other oxidation substrates and antioxidants by
means of folin-ciocalteu reagent. Methods in Enzymology.
299:152–178. 1999.
|
|
26
|
Brand-Williams W, Cuvelier ME and Berset
C: Use of a free radical method to evaluate antioxidant activity.
LWT Food Sci Technol. 28:25–30. 1995.
|
|
27
|
Cano A, Hernández-Ruíz J, García-Cánovas
F, Acosta M and Arnao MB: An end-point method for estimation of the
total antioxidant activity in plant material. Phytochem Anal.
9:196–202. 1998.
|
|
28
|
Gülçin I, Küfrevioǧlu ÖI, Oktay M and
Büyükokuroǧlu ME: Antioxidant, antimicrobial, antiulcer and
analgesic activities of nettle (Urtica dioica L.). J
Ethnopharmacol. 90:205–215. 2004.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yen GC and Duh PD: Antioxidative
properties of methanolic extracts from peanut hulls. J Am Oil Chem
Soc. 70:383–386. 1993.
|
|
30
|
Apak R, Güçlü K, Özyürek M and Karademir
SE: Novel total antioxidant capacity index for dietary polyphenols
and vitamins C and E, using their cupric ion reducing capability in
the presence of neocuproine: CUPRAC method. J Agric Food Chem.
52:7970–7981. 2004.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Paul T, Young MJ, Hill IE and Ingold KU:
Strand cleavage of supercoiled DNA by water-soluble peroxyl
radicals. The overlooked importance of peroxyl radical charge.
Biochemistry. 39:4129–4135. 2000.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Aguilar TAF, Navarro CBH and Pérez JAM:
Endogenous antioxidants: A review of their role in oxidative
stress. A master regul oxidative stress-transcr factor Nrf2,
2016.
|
|
33
|
Nimse SB and Pal D: Free radicals, natural
antioxidants, and their reaction mechanisms. RSC Adv.
5:27986–28006. 2015.
|
|
34
|
Buljeta I, Pichler A, Šimunović J and
Kopjar M: Beneficial effects of red wine polyphenols on human
health: Comprehensive review. Curr Issues Mol Biol. 45:782–798.
2023.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Ditano-Vázquez P, Torres-Peña JD,
Galeano-Valle F, Pérez-Caballero AI, Demelo-Rodríguez P,
Lopez-Miranda J, Katsiki N, Delgado-Lista J and
Alvarez-Sala-Walther LA: The fluid aspect of the mediterranean diet
in the prevention and management of cardiovascular disease and
diabetes: The role of polyphenol content in moderate consumption of
wine and olive oil. Nutrients. 11(2833)2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Barbalho SM, Ottoboni AMM, Fiorini AMR,
Guiguer ÉL, Nicolau CCT, de Alvares Goulart R and Flato UAP: Grape
juice or wine: Which is the best option? Crit Rev Food Sci Nutr.
60:3876–3889. 2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Li L and Sun B: Grape and wine polymeric
polyphenols: Their importance in enology. Crit Rev Food Sci Nutr.
59:563–579. 2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Tekos F, Skaperda Z, Vardakas P, Kyriazi
D, Maravelis GC, Poulas K, Taitzoglou IA, Nepka C and Kouretas D:
Redox biomarkers assessment after oral administration of wine
extract and grape stem extract in rats and mice. Molecues.
28(1574)2023.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Myrtsi ED, Koulocheri SD, Iliopoulos V and
Haroutounian SA: High-throughput quantification of 32 bioactive
antioxidant phenolic compounds in grapes, wines and vinification
byproducts by LC-MS/MS. Antioxidants (Basel).
10(1174)2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Hornedo-Ortega R, González-Centeno MR,
Chira K, Jourdes M and Teissedre PL, Hornedo-Ortega R,
González-Centeno MR, Chira K, Jourdes M and Teissedre PL: Phenolic
compounds of grapes and wines: Key compounds and implications in
sensory perception. Chem Biochem Winemak Wine Stab Aging, 2020.
|
|
41
|
Onache PA, Florea A, Geana EI, Ciucure CT,
Ionete RE, Sumedrea DI and Tița O: Assessment of bioactive phenolic
compounds in musts and the corresponding wines of white and red
grape varieties. Appl Sci. 13(5722)2023.
|
|
42
|
Pandeya A, Rayamajhi S, Pokhrel P and Giri
B: Evaluation of secondary metabolites, antioxidant activity, and
color parameters of Nepali wines. Food Sci Nutr. 6:2252–2263.
2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Paixão N, Perestrelo R, Marques JC and
Câmara JS: Relationship between antioxidant capacity and total
phenolic content of red, rosé and white wines. Food Chem.
105:204–214. 2007.
|
|
44
|
Preserova J, Ranc V, Milde D, Kubistova V
and Stavek J: Study of phenolic profile and antioxidant activity in
selected Moravian wines during winemaking process by FT-IR
spectroscopy. J Food Sci Technol. 52:6405–6414. 2015.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Fermo P, Comite V, Sredojević M, Ćirić I,
Gašić U, Mutić J, Baošić R and Tešić Ž: Elemental analysis and
phenolic profiles of selected italian wines. Foods.
10(158)2021.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Zhu L, Zhang Y, Deng J, Li H and Lu J:
Phenolic concentrations and antioxidant properties of wines made
from North American grapes grown in China. Molecules. 17:3304–3323.
2012.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Radeka S, Rossi S, Bestulić E, Budić-Leto
I, Ganić KK, Horvat I, Lukić I, Orbanić F, Jurjević TZ and Dvornik
Š: Bioactive compounds and antioxidant activity of red and white
wines produced from autochthonous croatian varieties: Effect of
moderate consumption on human health. Foods.
11(1804)2022.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Nemzer B, Kalita D, Yashin AY and Yashin
YI: Chemical composition and polyphenolic compounds of red wines:
Their antioxidant activities and effects on human health-a review.
Beverages. 8(1)2021.
|
|
49
|
Tekos F, Makri S, Skaperda ZV, Patouna A,
Terizi K, Kyriazis ID, Kotseridis Y, Mikropoulou EV, Papaefstathiou
G, Halabalaki M and Demetrios K: Assessment of antioxidant and
antimutagenic properties of red and white wine extracts in vitro.
Metab. 11(436)2021.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Lucarini M, Durazzo A, Lombardi-Boccia G,
Souto EB, Cecchini F and Santini A: Wine polyphenols and health:
Quantitative research literature analysis. Appl Sci.
11(4762)2021.
|
|
51
|
Fernández-Pachón MS, Villaño D,
García-Parrilla MC and Troncoso AM: Antioxidant activity of wines
and relation with their polyphenolic composition. Anal Chim Acta.
513:113–118. 2004.
|
|
52
|
Tarko T, Duda-Chodak A, Sroka P, Satora P
and Jurasz E: Polish wines: Characteristics of cool-climate wines.
J Food Compos Anal. 23:463–468. 2010.
|
|
53
|
Li H, Wang X, Li Y, Li P and Wang H:
Polyphenolic compounds and antioxidant properties of selected China
wines. Food Chem. 112:454–460. 2009.
|
|
54
|
Tauchen J, Marsik P, Kvasnicova M,
Maghradze D, Kokoska L, Vanek T and Landa P: In vitro antioxidant
activity and phenolic composition of Georgian, Central and West
European wines. J Food Compos Anal. 41:113–121. 2015.
|
|
55
|
Staško A, Brezová V, Mazúr M, Čertík M,
Kaliňák M and Gescheidt G: A comparative study on the antioxidant
properties of Slovakian and Austrian wines. LWT Food Sci Technol.
41:2126–2135. 2008.
|
|
56
|
Gaschler MM and Stockwell BR: Lipid
peroxidation in cell death. Biochem Biophys Res Commun.
482:419–425. 2017.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Zhu F, Du B and Li J, Zhu F, Du B and Li
J: Aroma compounds in wine. Grape Wine Biotechnol, 2016.
|
|
58
|
Styger G, Prior B and Bauer FF: Wine
flavor and aroma. J Ind Microbiol Biotechnol. 38:1145.
2011.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Liu S, Lou Y, Li Y, Zhao Y, Laaksonen O,
Li P, Zhang J, Battino M, Yang B and Gu Q: Aroma characteristics of
volatile compounds brought by variations in microbes in winemaking.
Food Chem. 420(136075)2023.PubMed/NCBI View Article : Google Scholar
|