Introduction
Cancer remains one of the leading causes of
mortality worldwide, with the number of new cases rising each year.
Chemotherapy, although a mainstay in cancer treatment, is
associated with high toxicity and significant side-effects. This
approach targets not only tumor cells, but also healthy cells,
resulting in severe adverse outcomes such as nausea, fatigue, organ
damage and immune suppression. These effects often necessitate
treatment interruption, thereby compromising therapeutic efficacy
(1). Furthermore, the development
of resistance to chemotherapeutic agents remains a major challenge
in cancer treatment (2). These
limitations underscore the need for alternative adjuvant therapies
capable of exerting antitumor effects without inducing toxicity,
thereby allowing for reduced dosage and/or duration of conventional
chemotherapy without compromising efficacy. Natural compound-based
therapies have shown promise as complementary strategies that
improve the quality of life of patients and support uninterrupted
treatment.
Brazil harbors a vast biodiversity of plants
containing natural bioactive compounds with a high biotechnological
potential for the pharmaceutical industry. Among these, a wide
variety of plant-derived polysaccharides have attracted
considerable attention for their immunomodulatory and antitumor
properties. Pectins, a family of covalently linked D-galacturonic
acid-rich polysaccharides abundant in the primary cell walls of
fruits, exhibit diverse biological activities, including
anti-inflammatory, antioxidant, immunoregulatory and antitumor
effects, and may also serve as carriers for targeted drug delivery
(3). In numerous fruits, pectic
polysaccharide structures undergo chemical and enzymatic
modifications during ripening, leading to substantial
intramolecular changes in the pectic chain (4). Pectins derived from diverse
biological sources are known to exhibit considerable structural
variability, and such differences in chemical composition have been
associated with distinct biological activities. Notably,
accumulating evidence indicates that pectins may exert antitumor
effects through the modulation of tumor cell proliferation,
adhesion and apoptosis (3). Pectin
derived from papaya has been shown to reduce the viability and
induce the necroptosis of colon and prostate cancer cell lines
(5). Previous studies have
demonstrated that pectic polysaccharides extracted from potatoes
(6) and sugar beet (7) significantly inhibited the
proliferation of HT-29 cells in vitro. Furthermore, apple
pectin has been reported to promote the apoptosis and reduce the
adhesion of 4T1 breast cancer cells (8). Notably, pectins display low toxicity
and minimal side-effects on normal cells compared to conventional
chemotherapeutic agents (9). These
properties highlight pectins as molecules with potential to serve
as adjuvants in cancer therapy, exerting antitumor effects and
potentially enabling a reduction in the use of chemotherapeutic
agents associated with severe side effects.
Prunes are the dried fruits of Prunus
domestica L. (European plum; P. domestica), a tree
cultivated on all continents, with its fruits widely consumed
worldwide (10). Pectins extracted
from P. domestica fruits have been reported to exhibit
antioxidant (11),
gastroprotective (12,13) and anti-inflammatory activities
(14). Recently, Vaz da Luz et
al (15) demonstrated that
isolated side chains of pectins (type I arabinogalactans) with
different molar masses, obtained from prune tea infusions,
displayed varying antitumor effects. However, unlike the study by
Vaz da Luz et al (15), the
present study aimed to evaluate the antitumor activity of pectins
present in the prune pectic fraction obtained with hot water (PWH),
which consists of a mixture of rhamnogalacturonans with type I
arabinogalactan side chains and low-methyl-esterified
homogalacturonan (13). It is
well-established that a mixture of pectic polysaccharides in
solution can elicit different biological effects compared to those
produced by individual polysaccharide chains (4). To the best of our knowledge, the
antitumor activity of pectins in the PWH fraction has not been
previously investigated, which justifies the present
investigation.
In light of the above, the present study aimed to
evaluate the antitumor activity of the PWH fraction obtained from
prunes in B16F10 murine melanoma cells. The present study assessed
its cytotoxicity on both tumor and normal cells, as well as its
effects on tumor cell migration, colony formation and
morphology.
Materials and methods
Purification, characterization and
solubilization of polysaccharide fraction
The prune pectin fraction (PWH) was characterized
and kindly provided by the Department of Biochemistry at the
Federal University of Paraná (Paraná, Brazil). Pitted prunes (dried
fruits from P. domestica purchased at a local market in
Curitiba, Brazil; LA VIOLETERA®) were freeze-dried and
milled. The extraction of pectic polysaccharides was carried out
using hot water in order to obtain the molecules more tightly bound
to the cell wall. Moreover, hot-water extraction results in a
higher pectin yield compared to cold-water extraction. The water
extract was obtained by filtration, and the polysaccharides were
recovered by ethanol (3 vol.) precipitation and lyophilization,
originating the fractions. A homogeneous fraction was analyzed by
sugar composition, high-performance steric exclusion
chromatography, methylation, and nuclear magnetic resonance
spectroscopy analyses. The PWH comprises rhamnogalacturonans with
type I arabinogalactans as side chains, and low-methyl esterified
homogalacturonan (9). The
freeze-dried pectin fraction was solubilized in the cell growth
medium [RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.),
supplemented with 10% fetal bovine serum (FBS, Gibco; Thermo Fisher
Scientific, Inc.) and 1% antibiotic (penicillin 10,000 U and
streptomycin 10 mg/l), MilliporeSigma] and stored at -20˚C until
its use.
Cell groups were divided into a control group (CT)
and a group treated with prune pectins (PWH). The control group
received only the cell growth medium without PWH. For cytotoxicity
analysis, doxorubicin (DX, Pfizer®) at a concentration
of 2.5 µg/ml was used as a positive control. In this case, the same
control medium was used, supplemented with the chemotherapy
drug.
Cell lines and cell culture
The B16F10 murine melanoma cell line (BCRJ, 0046)
and BALB/c 3T3 normal cell line (clone A31, ATCC, CCL-163) were
kindly provided by the Laboratory of Inflammatory and Neoplastic
Cells, Department of Cell Biology, UFPR, Curitiba, Brazil, and were
initiated and maintained according to specific recommendations for
each line. The BALB/c 3T3 cell line, derived from fibroblasts of
BALB/c mouse embryos, is non-tumorigenic and widely employed as a
representative model of normal cells in contrast to tumor cell
lines. Given that fibroblasts are distributed throughout the body,
this lineage provides an appropriate model for assessing normal
cell function in studies of viability and cytotoxicity. Cells were
cultured in growth medium and maintained in an incubator (Sanyo
Scientific MCO-18AC) at 37˚C, 90% humidity, and 5% CO2
for 72 h. All experiments were performed in biological
triplicates.
Analysis of cell viability
To determine the concentrations to be used in the
present study, a cell viability and cytotoxicity test was carried
out to determine the concentrations that were not cytotoxic to
normal cells. For this, the colorimetric tests of reduction of
diphenyltetrazolium bromide (MTT) and neutral red (NR) were
performed by the protocols proposed by Mosmann (16) and Repetto et al (17), respectively. The B16F10 cells
(5x102 cells/well) and BALB/c 3T3 (2x103
cells/well) were exposed to 0, 2.5, 5, 10, 100 and 800 µg/ml PWH,
and 2.5 µg/ml DX for 72 h. Briefly, the MTT cell viability assay
was performed by first seeding the cells into 96-well plates and
treating them as described in the experimental design. Following
treatment, the wells were aspirated to remove the culture medium
(RPMI-1640, MilliporeSigma; containing fetal bovine serum 10%
Gibco; Thermo Fisher Scientific, Inc.), and 100 µl MTT solution
[3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide 5
mg/ml in PBS, MilliporeSigma, pH 7.4] were added to each well. The
plates were incubated for 3.5 h at 37˚C in a humidified atmosphere
with 5% CO2 to allow the formation of formazan crystals
by metabolically active cells. Following incubation, the MTT
solution was discarded, and 100 µl dimethyl sulfoxide (DMSO,
MilliporeSigma) were added to each well to solubilize the formazan
crystals. The plates were gently shaken to ensure complete
solubilization, and the absorbance was measured at 540 nm using a
microplate reader (TECAN® Infinite M200; Tecan Group,
Ltd.). The absorbance values obtained were directly proportional to
the number of viable cells.
The neutral red cell viability assay was performed
by first treating cells cultured in 96-well plates according to the
experimental protocol. Following treatment, the medium (RPMI-1640,
MilliporeSigma; containing fetal bovine serum 10% Gibco; Thermo
Fisher Scientific, Inc.) was removed, and 100 µl Neutral Red
solution (MilliporeSigma, 40 µg/ml in culture medium) were added to
each well. The cells were then incubated for 3 h at 37˚C in a 5%
CO2 atmosphere to allow dye uptake by viable cells.
Following incubation, the dye solution was removed, and the cells
were washed gently with 150 µl PBS (MilliporeSigma) to eliminate
excess dye. Subsequently, 100 µl destaining solution (50% ethanol,
49% distilled water, and 1% glacial acetic acid, MilliporeSigma)
was added to each well to extract the dye from the lysosomes. The
plate was shaken until the complete solubilization of the dye, and
the absorbance was measured at 540 nm using a microplate reader
(TECAN® Infinite M200). The absorbance values obtained
were directly proportional to the number of viable cells. The
results obtained from the MTT and neutral red assays (measured by
absorbance) were converted into percentages, using the control
group (without treatment with the PWH fraction) as having 100%
viability.
Cytotoxicity assay
The assay was performed through the crystal violet
method, as previously described by Bonnekoh et al (18). The B16F10 cells (2x103
cells/well) were plated in a new 96-well plate and exposed to 0, 10
and 100 µg/ml PWH. Cell viability was measured in 24, 48 and 72 h.
Following the treatment period, the medium (RPMI-1640
MilliporeSigma; containing fetal bovine serum 10% Gibco; Thermo
Fisher Scientific, Inc.) was removed, and the cells were gently
washed with 150 µl PBS to eliminate non-adherent cells and debris.
Subsequently, 100 µl of 4% paraformaldehyde solution
(MilliporeSigma) were added to each well to fix the cells for 20
min at room temperature. Following fixation, the wells were washed
again with PBS, and 100 µl 0.1% crystal violet solution (prepared
in 20% methanol, Merck KGaA) were added to stain the cells at room
temperature for 10 min. Excess dye was removed by rinsing the plate
under running distilled water until the background was clear. After
drying, the bound dye was solubilized by the addition of 100 µl 10%
acetic acid (MilliporeSigma) to each well. The plate was gently
shaken to ensure complete solubilization. The results were obtained
using a TECAN® Infinite M200 device at 590 nm. The data
are presented as a percentage relative to the CT group at 24 h.
Cell migration (wound healing)
assay
Following 72 h of exposure to 0, 10 and 100 µg/ml
PWH, the B16F10 surviving cells were plated again (2x104
cells/well) in a new 96-well plate with culture medium (RPMI-1640
MilliporeSigma; containing fetal bovine serum 10% Gibco; Thermo
Fisher Scientific, Inc.) to perform the cell migration assay
(‘scratch’ method) (19). Briefly,
after the cells reached full confluency, cell proliferation was
inhibited with the use of RPMI growth medium lacking in FBS (1%),
and a straight scratch was made in the monolayer using a sterile
200 µl pipette tip. The wells were then gently washed with PBS to
remove detached cells. Images of the scratch area were captured
immediately (0 h) and following 24 h of incubation at 37˚C in a 5%
CO2 atmosphere using an inverted microscope
(BIOVAL® XDS-1B). The wound closure area was analyzed
using ImageJ® software 1.52 (National Institutes of
Health), and the migration rate was expressed as the percentage of
area covered by cells after 24 h compared to time 0(20).
Colony formation assay
Following 72 h of exposure to 0, 10 and 100 µg/ml
PWH, B16F10 surviving cells were plated again at a reduced
concentration (1x102 cells/well) in a 24-well plate to
perform the colony formation assay analyses (21). This assay is expected to evaluate
the ability of a single cell to form new colonies. For this,
surviving cells were maintained only in the growth medium
(RPMI-1640 MilliporeSigma; containing fetal bovine serum 10% Gibco;
Thermo Fisher Scientific, Inc.) and incubated at 37˚C for 96 h. At
the end of the incubation period, the colonies were fixed with 4%
paraformaldehyde at room temperature for 15 min and then stained
with 0.1% crystal violet solution (prepared in 20% methanol) for 10
min at room temperature. Excess dye was washed off with distilled
water, and the plates were dried in air. The images were acquired
using an inverted microscope (BIOVAL® XDS-1B), and
colonies containing >50 cells were counted using
ImageJ® software 1.52 (National Institutes of
Health).
Analysis of cell morphology
This assay aimed to evaluate morphological changes
in B16F10 cells following exposure to the PWH fraction. The cells
(5x10³ cells/well) were seeded in 24-well plates and treated with
0, 10, or 100 µg/ml PWH. Cell morphology was assessed at the start
of treatment and again after 24 h. At each time point, the cells
were fixed with 4% paraformaldehyde at room temperature for 10 min
and stained with 0.25% crystal violet (prepared in methanol) at
room temperature for 10 min (22).
The area occupied by the cells in culture was quantified through
digital analysis of images captured using a light microscope
(BIOVAL® XDS-1B) at x400 magnification. Images were
processed using ImageJ software 1.52 (National Institutes of
Health), applying threshold-based segmentation to differentiate
cells from the background. The total cell area was subsequently
measured in pixels.
Reverse transcription-quantitative PCR
(RT-qPCR)
RNA isolation from the B16F10 cell samples following
72 h of PWH treatment was performed using TRIzol®
reagent (Bio-Rad Laboratories, Inc.) according to the
manufacturer's recommendations. The extracted RNA samples were then
converted to cDNA using the iScript Reverse Transcription Supermix
for RT-qPCR kit (cat. no. 1708841, Bio-Rad Laboratories, Inc.). The
reactions were taken to the thermocycler (Applied Biosystems;
Thermo Fisher Scientific, Inc.), and the samples were incubated in
three cycles: 1st cycle of 5 min at 25˚C, 2nd cycle of 20 min at
46˚C, and 3rd cycle of 1 min at 95˚C. The RT-qPCR reaction was
performed using the one-step RT-PCR SYBR®-Green kit
(cat. no. 1725270, Bio-Rad Laboratories, Inc.) and the appropriate
primers for each reaction, as follows: Focal adhesion kinase (FAK)
forward, 5'-TCTGTGGAATTGGCAATCGG-3' and reverse,
5'-TGGATGGTCTGCACTTGGTT-3'; beta actin (ACTB) forward,
5'-CTGTATTCCCCTCCATCGTG-3' and reverse,
5'-GGGTCAGGATACCTCTCTTGC-3'; hypoxanthine-guanine
phosphoribosyltransferase (HPRT1) forward,
5'-GTTGGGCTTACCTCACTGCT-3' and reverse, 5'-TAATCACGACGCTGGGACTG-3'.
The samples were analyzed in a QuantStudioTM 5 device (Applied
Biosystems; Thermo Fisher Scientific, Inc.). For each reaction, a
control RT (without conversion of RNA to cDNA) was performed. The
relative quantification was measured according to the pre-set
threshold fluorescence level of the target gene (FAK) compared to
the endogenous controls used (ACTB and HPRT1). ACTB and HPRT1 were
utilized for normalization. The quality and purity of total RNA
were evaluated using a NanoDrop spectrophotometer (Thermo Fisher
Scientific, Inc.). Samples exhibited A260/A280 ratios between 1.8
and 2.0 and A260/A230 ratios >1.8, indicating minimal
contamination by proteins or phenolic compounds and falling within
commonly accepted parameters for gene expression analysis. For each
RT-qPCR reaction, 4.5 µl cDNA, synthesized from a standardized RNA
concentration of 10 ng/µl, was used, corresponding to 45 ng total
RNA per reaction. The assay performance was monitored using two
endogenous reference genes (ACTB and HPRT1), selected for their
stability across the analyzed Mus musculus samples.
No-reverse transcriptase (RT-) controls were included to confirm
the absence of genomic DNA contamination. Each sample was tested in
triplicate, and the relative gene levels were normalized using the
2-ΔΔCq method (23).
Statistical analysis
The normal distribution of values was verified using
the Shapiro-Wilk test. One-way ANOVA followed by Tukey's test was
performed in all analyses, apart from cell proliferation, which was
analyzed using two-way ANOVA (mixed model) followed by the
Bonferroni post hoc test, both through GraphPad Prism8
Software® (Dotmatics). P<0.05 was considered to
indicate a statistically significant difference. Tukey's test was
used as a post hoc test. Data are presented as the mean ± SD of at
least three independent experiments.
Results
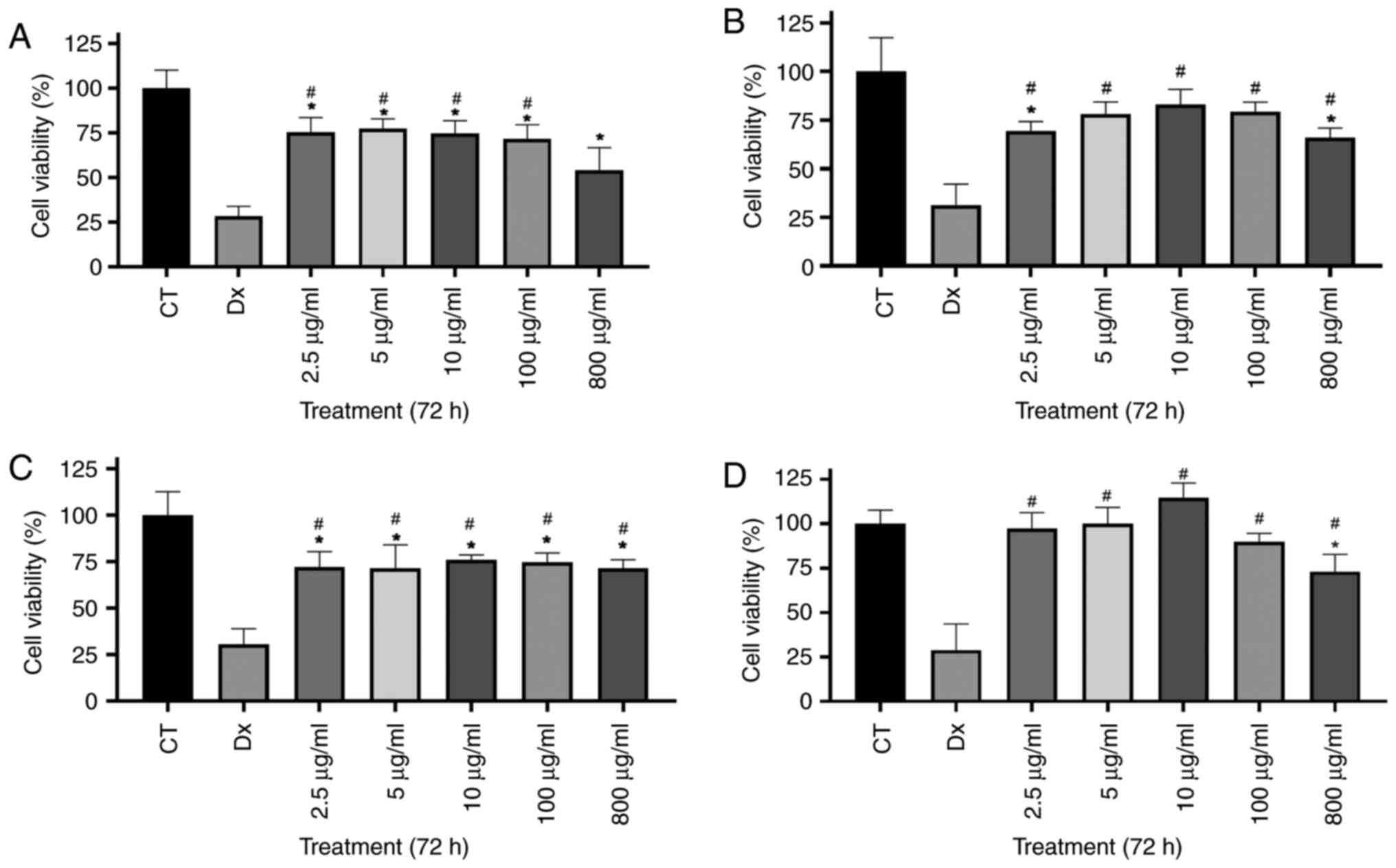
Cell viability
In the NR assay, all PWH concentrations
significantly reduced the viability of the B16F10 cells compared
with the CT group (Fig. 1A). In
the BALB/c 3T3 cells, a significant reduction was observed only
following treatment with 2.5 and 800 µg/ml PWH in relation to the
CT group (Fig. 1B). The MTT assay
revealed a similar viability profile for the B16F10 cells as in the
NR assay (Fig. 1C). In the BALB/c
3T3 cells, cytotoxicity was detected only following treatment with
800 µg/ml PWH, although it remained lower than that induced by DX
(Fig. 1D). Overall, PWH was less
cytotoxic than DX across all concentrations and assays (Fig. 1A-C), apart from the concentration
of 800 µg/ml in the NR assay (Fig.
1A). These assays suggest that the PWH fraction exhibits
greater cytotoxicity toward tumor cells than toward normal
cells.
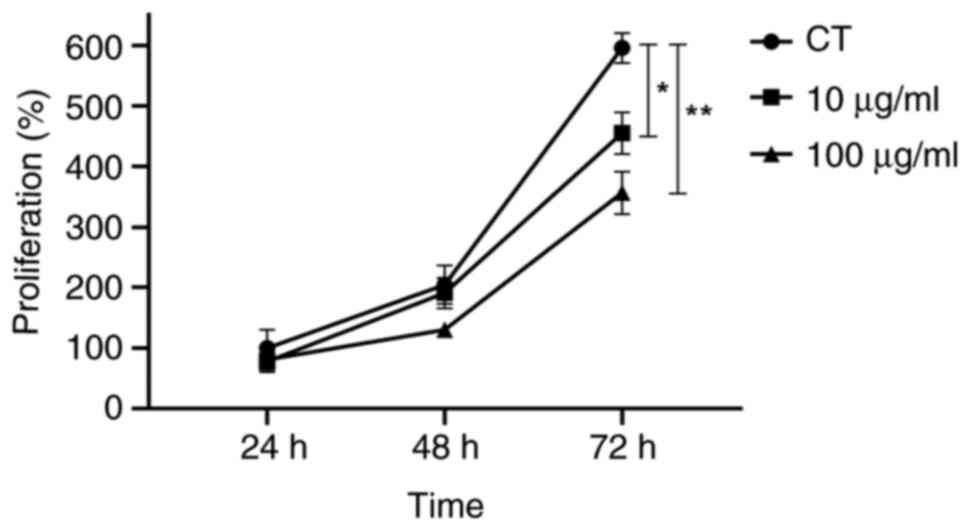
Cytotoxicity assay
Based on the cell viability data, concentrations
were selected to avoid cytotoxic effects on normal cells while
effectively reducing tumor cell viability. Subsequent experiments
were performed using 10 and 100 µg/ml PWH, with the control (CT)
group remaining polysaccharide-free (0 µg/ml). The results
demonstrated the cytotoxicity of the PWH fraction on tumor cells
over time. PWH did not significantly affect cell viability at 24 or
48 h compared to the CT group. However, after 72 h, viability was
markedly reduced at both concentrations, with decreases of 23.6 and
40.3%, respectively (Fig. 2).
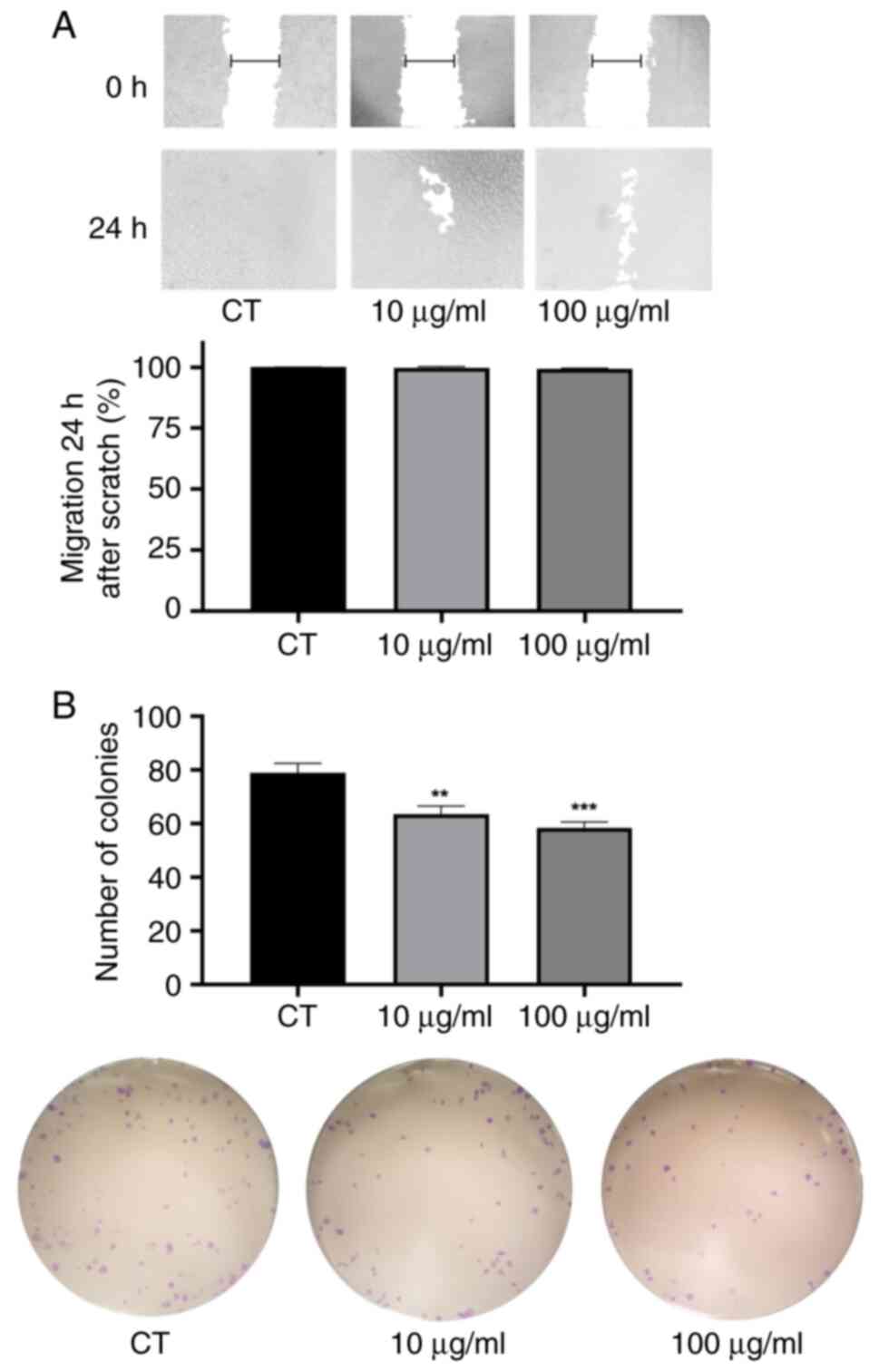
Cell migration and colony formation
capacity
In the migration assay, tumor cells treated with the
PWH fraction closed the scratch to a similar extent as untreated
cells, indicating that PWH did not significantly impair cell
migration at either concentration within 24 h (Fig. 3A).
By contrast, PWH effectively reduced colony
formation at both concentrations. Compared to the CT group, the
number of new colonies decreased by 19.6% in the cells treated with
10 µg/ml PWH and by 25.9% in those exposed to 100 µg/ml PWH
(Fig. 3B). These data indicated
that tumor cells exhibited a reduced proliferation and diminished
colony formation capacity following treatment with PWH.
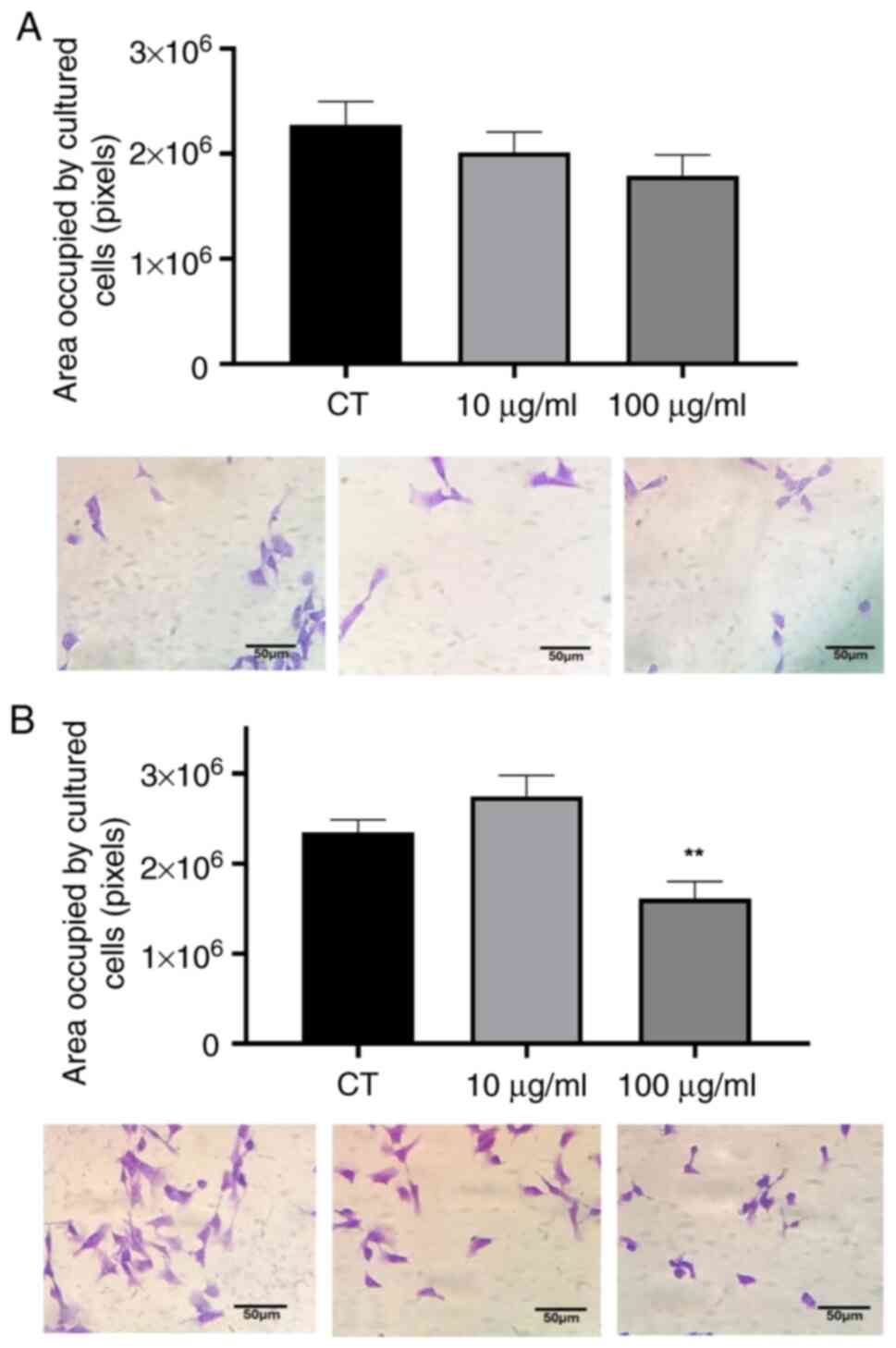
Area occupied by cultured cells
Morphological changes were observed in the cells
exposed to PWH under an inverted microscope. To confirm and further
investigate these changes, a detailed morphological analysis was
performed. Immediately after treatment (0 h), no noticeable
differences were detected between the treated and control cells
(Fig. 4A). However, following 24 h
of exposure, the cells treated with 100 µg/ml PWH exhibited a 30%
reduction in the area occupied by cultured cells compared to the
control group (Fig. 4B),
suggesting that contact with the polysaccharides in the PWH
fraction interfered with the cytoskeletal organization of tumor
cells.
RT-qPCR
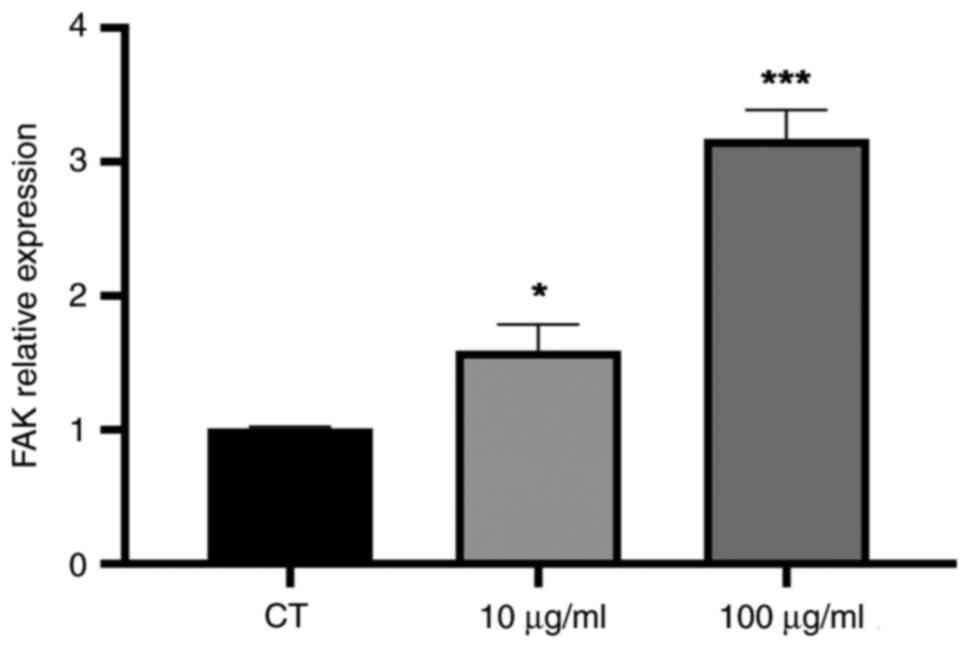
Based on the observed effects on cell proliferation,
colony formation and morphology, it was hypothesized that PWH may
influence the focal adhesion points of cells. To determine this,
FAK gene expression was quantified. FAK is a central regulator that
promotes focal adhesion to the extracellular matrix and
cytoskeletal remodeling. The results revealed the altered
expression of the FAK gene in the B16F10 cells treated with PWH,
with a 57% increase at the concentration of 10 µg/ml and a 3-fold
increase at the concentration of 100 µg/ml (Fig. 5).
Discussion
The results of the present study demonstrated that
PWH exerted specific effects on B16F10 cells, including reduced
viability, decreased colony formation, fewer cytoplasmic extensions
and an altered FAK gene expression. These changes are closely
linked to melanoma differentiation and invasiveness (24-26).
Even the lowest concentration of PWH (2.5 µg/ml) was sufficient to
reduce B16F10 cell viability following 72 h of treatment. From a
pharmacological perspective, the ability to decrease cancer cell
viability at low concentrations are highly desirable (27). It was also observed that the
reduction in cell viability was similar at both low and high
concentrations, a phenomenon previously reported in studies
involving pectins (28). Notably,
the effect of PWH on normal cell viability was substantially lower
than that of DX, consistent with other reports highlighting the low
toxicity of pectins (29,30).
Cell cytotoxicity assays over time are essential for
understanding the mechanisms of action of any proposed treatment.
In the present study, a concentration-dependent reduction in tumor
cell viability was observed after 72 h of exposure to PWH. Similar
effects of pectins on tumor cell cultures have been reported in
other studies (5,31,32).
Of note, despite the 10-fold difference between the lowest and
highest PWH concentrations (10 and 100 µg/ml), the effect on B16F10
cell viability was not proportionally greater at the higher
concentration. This suggests that the impact of the PWH fraction on
cell viability may reach a plateau beyond a certain concentration,
indicating that the cellular pathways involved are already fully
modulated, and increasing the concentration to 100 µg/ml does not
further enhance the response. Indeed, a similar non-proportional
reduction in viability with increasing concentrations of a
comparable polysaccharide fraction has been reported previously
(33).
Pectins from various sources have been reported to
reduce tumor cell migration (34,35),
including the migration of B16F10 cells (36). In contrast, in the present study,
treatment with PWH did not produce significant changes in the
migration rate of B16F10 cells. Nevertheless, the potential effect
of the polysaccharides on cell migration cannot be ruled out, as
the present study focused solely on migration at 0 and 24 h after
scratch creation, which may have overlooked any early delays in
movement immediately following the ‘wound’. Additionally, employing
alternative techniques to evaluate migratory capacity could provide
further insights.
The process of metastasis involves not only the
ability of cells to migrate, but also the capacity of a single cell
to proliferate and form a new colony in a tissue different from its
primary origin. Studies investigating the effects of
polysaccharides on tumor cell colony formation have reported
promising results (37,38). These findings are consistent with
those of the present study, in which PWH significantly reduced
colony formation at its highest concentration (100 µg/ml).
In the present study, PWH at 100 µg/ml reduced the
cytoplasmic area of B16F10 cells. Since cell-cell contact is a key
mechanism for proliferation, the reduction in cell area and
dendritic projections may lead to decreased release of critical
growth factors necessary for sustaining cell proliferation
(39). Furthermore, the data
presented herein indicated that exposure to PWH was associated with
a reduced cell area alongside increased FAK gene expression in
B16F10 murine melanoma cells. Cytoskeletal reorganization plays a
crucial role in the adaptation of a cell to specific exogenous
stimuli or inhibitors present in the surrounding microenvironment.
Consequently, the signaling proteins involved in this
reorganization are essential for maintaining cell morphology and
regulating biophysical dynamics (40). Focal adhesion sites are specialized
regions where the cytoskeleton connects with the extracellular
matrix (ECM). These sites rely on the coordinated activity of
integrins, the cytoskeleton and signaling proteins, such as FAK and
Src. They are essential for maintaining cellular architecture and
sensing mechanical cues from the environment. The FAK-Src pathway
can be activated by various signals involved in cell survival,
invasion and adhesion. FAK is a central regulator of tumor cell
motility and invasiveness. Upon activation, via integrin-ECM
interactions or growth factor signaling, FAK autophosphorylates at
Tyr397 and recruits Src kinases, promoting focal adhesion turnover
and cytoskeletal remodeling through targets such as paxillin,
p130Cas and Rho GTPases. FAK signaling also enhances MMP-2 and
MMP-9 expression, supporting extracellular matrix degradation and
invasion, and contributes to invadopodia formation and EMT-like
phenotypes in melanoma (25,41).
Notably, research has shown that an increased FAK expression is
directly associated with heightened cancer aggressiveness (42), which is in contrast to the findings
of the present study. However, it was hypothesized that the pectins
present in PWH may impair cell adhesion to the surrounding
environment. This could explain why, unable to adhere efficiently
to their microenvironment, the cells remodel their cytoskeleton and
display a reduced morphology compared to the control group.
Consequently, in the absence of optimal adhesion and spreading, a
compensatory mechanism may be triggered, leading cells to
upregulate FAK in an attempt to form additional focal adhesion
points for survival. Further detailed investigations of the
mechanisms involved in the FAK-Src signaling pathway are required
to clarify these findings.
The present study has certain limitations which
should be mentioned. The analysis of additional genes related to
cell proliferation and migration/invasion, as well as their
corresponding protein expression, was not performed here and should
be addressed in future work.
In conclusion, the pectins from prunes present in
the PWH fraction reduced the viability of B16F10 murine melanoma
cells, while exhibiting minimal toxicity toward normal BALB/c 3T3
cells, exhibiting lower cytotoxicity than the chemotherapeutic
agent DX. Additionally, PWH inhibited malignant colony formation
and appeared to affect focal adhesion in the B16F10 cell line.
These results provide preliminary evidence supporting PWH as a
potential compound for future cancer research.
Acknowledgements
The authors are grateful to Dr Edvaldo da Silva
Trindade and Dr Fernanda Fogagnoli Simas from the Laboratory of
Inflammatory and Neoplastic Cells - Federal University of Paraná,
Curitiba, Brazil, for kindly providing the cell lines.
Funding
Funding: The present study was funded by the Araucaria
Foundation (Official Agreement no. 051/2017), CNPq (grant nos.
404717/2026-0, 310731/2021-6 and 403295/2021-1), and fellowships
from CAPES (Funding code 001, PROEX - Grant no.
88881.924191/2023-01).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
APB, SCSB and RFA conducted the assays. GDOF and FFW
analyzed the photo-derived data. LMCC isolated and characterized
the polysaccharides. MHA contributed to the experimental design. KN
and LCF assisted in the analysis and discussion of the results. FI
was involved in the experimental design, and data analysis and
interpretation. APB and FI confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Use of artificial intelligence tools
During the preparation of this work, AI tools were
used to improve the readability and language of the manuscript or
to generate images, and subsequently, the authors revised and
edited the content produced by the AI tools as necessary, taking
full responsibility for the ultimate content of the present
manuscript.
References
|
1
|
Hossain MB and Haldar Neer AH:
Chemotherapy. Cancer Treat Res. 185:49–58. 2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Riganti C and Contino M: New strategies to
overcome resistance to chemotherapy and immune system in cancer.
Int J Mol Sci. 20(4783)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Sultana N: Biological properties and
biomedical applications of pectin and pectin-based composites: A
review. Molecules. 28(7974)2023.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Pedrosa LF, Raz A and Fabi JP: The complex
biological effects of pectin: Galectin-3 targeting as potential
human health improvement? Biomolecules. 12(289)2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Prado SBRD, Ferreira GF, Harazono Y, Shiga
TM, Raz A, Carpita NC and Fabi JP: Ripening-induced chemical
modifications of papaya pectin inhibit cancer cell proliferation.
Sci Rep. 7(16564)2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ogutu FO, Mu TH, Sun H and Zhang M:
Ultrasonic modified sweet potato pectin induces apoptosis like cell
death in colon cancer (HT-29) cell line. Nutr Cancer. 70:136–145.
2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Maxwell EG, Colquhoun IJ, Chau HK,
Hotchkiss AT, Waldron KW, Morris VJ and Belshaw NJ: Modified sugar
beet pectin induces apoptosis of colon cancer cells via an
interaction with the neutral sugar side-chains. Carbohydr Polym.
136:923–929. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Delphi L and Sepehri H: Apple pectin: A
natural source for cancer suppression in 4T1 breast cancer cells in
vitro and express p53 in mouse bearing 4T1 cancer tumors, in vivo.
Biomed Pharmacother. 84:637–644. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Li DQ, Li J, Dong HL, Li X, Zhang JQ,
Ramaswamy S and Xu F: Pectin in biomedical and drug delivery
applications: A review. Int J Biol Macromol. 185:49–65.
2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ortega-Vidal J, Ruiz-Martos L, Salido S
and Altarejos J: Proanthocyanidins in pruning wood extracts of four
european plum (Prunus domestica L.) cultivars and their hLDHA
inhibitory activity. Chem Biodivers. 20(e202200931)2023.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Konrade D, Gaidukovs S, Vilaplana F and
Sivan P: Pectin from fruit- and berry-juice production by-products:
Determination of physicochemical, antioxidant and rheological
properties. Foods. 12(1615)2023.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Markov PA, Paderin NM, Chelpanova TI,
Efimtseva EA, Nikitina IR and Popov SV: Gastroprotective and
antidepressant-like effect of plum pectin (Prunus domestica L.)
under water-immobilization stress in laboratory mice. Vopr Pitan.
92:16–25. 2023.PubMed/NCBI View Article : Google Scholar : (In Russian).
|
|
13
|
Cantu-Jungles TM, Maria-Ferreira D, da
Silva LM, Baggio CH, Werner MF, Iacomini M, Cipriani TR and
Cordeiro LM: Polysaccharides from prunes: Gastroprotective activity
and structural elucidation of bioactive pectins. Food Chem.
146:492–499. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Popov SV, Ovodova RG, Golovchenko VV,
Khramova DS, Markov PA, Smirnov VV, Shashkov AS and Ovodov YS:
Pectic polysaccharides of the fresh plum Prunus domestica L.
isolated with a simulated gastric fluid and their anti-inflammatory
and antioxidant activities. Food Chem. 143:106–113. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Vaz da Luz KT, Gonçalves JP, de Lima
Bellan D, Visnheski BRC, Schneider VS, Cortes Cordeiro LM, Vargas
JE, Puga R, da Silva Trindade E, de Oliveira CC and Simas FF:
Molecular weight-dependent antitumor effects of prunes-derived type
I arabinogalactan on human and murine triple wild-type melanomas.
Carbohydr Res. 535(108986)2024.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Mosmann T: Rapid colorimetric assay for
cellular growth and survival: Application to proliferation and
cytotoxicity assays. J Immunol Methods. 65:55–63. 1983.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Repetto G, del Peso A and Zurita JL:
Neutral red uptake assay for the estimation of cell
viability/cytotoxicity. Nat Protoc. 3:1125–1131. 2008.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Bonnekoh B, Wevers A, Jugert F, Merk H and
Mahrle G: Colorimetric growth assay for epidermal células cultures
by their crystal violet binding capacity. Arch Dermatol Res.
281:487–490. 1989.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Liang CC, Park AY and Guan JL: In vitro
scratch assay: A convenient and inexpensive method for analysis of
cell migration in vitro. Nat Protoc. 2:329–333. 2007.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Borges BE, Appel MH, Cofré AR, Prado ML,
Steclan CA, Esnard F, Zanata SM, Laurindo FR and Nakao LS: The
flavo-oxidase QSOX1 supports vascular smooth muscle cell migration
and proliferation: Evidence for a role in neointima growth. Biochim
Biophys Acta. 1852:1334–1346. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Boia-Ferreira M, Basílio AB, Hamasaki AE,
Matsubara FH, Appel MH, Da Costa CRV, Amson R, Telerman A, Chaim
OM, Veiga SS and Senff-Ribeiro A: TCTP as a therapeutic target in
melanoma treatment. Br J Cancer. 117:656–665. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Naliwaiko K, Luvizon AC, Donatti L,
Chammas R, Mercadante AF, Zanata SM and Nakao LS: Guanosine
promotes B16F10 melanoma cell differentiation through PKC-ERK 1/2
pathway. Chem Biol Interact. 173:122–128. 2008.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Maddodi N and Setaluri V: Prognostic
significance of melanoma differentiation and trans-differentiation.
Cancers (Basel). 2:989–999. 2010.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Qi X, Chen Y, Liu S, Liu L, Yu Z, Yin L,
Fu L, Deng M, Liang S and Lü M: Sanguinarine inhibits melanoma
invasion and migration by targeting the FAK/PI3K/AKT/mTOR
signalling pathway. Pharm Biol. 61:696–709. 2023.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Raineri A, Fasoli S, Campagnari R, Gotte G
and Menegazzi M: Onconase restores cytotoxicity in
dabrafenib-resistant A375 human melanoma cells and affects cell
migration, invasion and colony formation capability. Int J Mol Sci.
20(5980)2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Cobs-Rosas M, Concha-Olmos J,
Weinstein-Oppenheimer C and Zúñiga-Hansen ME: Assessment of
antiproliferative activity of pectic substances obtained by
different extraction methods from rapeseed cake on cancer cell
lines. Carbohydr Polym. 117:923–932. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Amaral SDC, Barbieri SF, Ruthes AC, Bark
JM, Brochado Winnischofer SM and Silveira JLM: Cytotoxic effect of
crude and purified pectins from Campomanesia xanthocarpa Berg on
human glioblastoma cells. Carbohydr Polym.
224(115140)2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Chen GT, Fu YX, Yang WJ, Hu QH and Zhao
LY: Effects of polysaccharides from the base of Flammulina
velutipes stipe on growth of murine RAW264.7, B16F10 and L929
cells. Int J Biol Micromol. 107:2150–2156. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Xiang T, Yang R, Li L, Lin H and Kai G:
Research progress and application of pectin: A review. J Food Sci.
89:6985–7007. 2024.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Prado SBRD, Santos GRC, Mourão PAS and
Fabi JP: Chelate-soluble pectin fraction from papaya pulp interacts
with galectin-3 and inhibits colon cancer cell proliferation. Int J
Biol Macromol. 126:170–178. 2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wu XQ, Fu JY, Mei RY, Dai XJ, Li JH, Zhao
XF and Liu MQ: Inhibition of liver cancer HepG2 cell proliferation
by enzymatically prepared low-molecular citrus pectin. Curr Pharm
Biotechnol. 23:861–872. 2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Angeli RF, Bialli AP, Baal SCS, dos Santos
EF, Filho PSL, Schneider VS, Fernandes LC, Naliwaiko K, Cordeiro
LMC and Iagher F: Antitumor activity of polysaccharides obtained
from guavira fruit industrial waste on murine melanoma cells.
Bioact Carbohydrates Diet Fibre. 33(100469)2025.
|
|
34
|
Fan Y, Sun L, Yang S, He C, Tai G and Zhou
Y: The roles and mechanisms of homogalacturonan and
rhamnogalacturonan I pectins on the inhibition of cell migration.
Int J Biol Macromol. 106:207–217. 2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
do Prado SBR, Shiga TM, Harazono Y, Hogan
VA, Raz A, Carpita NC and Fabi JP: Migration and proliferation of
cancer cells in culture are differentially affected by molecular
size of modified citrus pectin. Carbohydr Polym. 211:141–151.
2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Wikiera A, Kozioł A, Mika M and Stodolak
B: Structure and bioactivity of apple pectin isolated with
arabinanase and mannanase. Food Chem 15:.
388(133020)2022.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Cao XY, Liu JL, Yang W, Hou X and Li QJ:
Antitumor activity of polysaccharide extracted from Pleurotus
ostreatus mycelia against gastric cancer in vitro and in vivo. Mol
Med Rep. 12:2383–2389. 2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Adami ER, Corso CR, Turin-Oliveira NM,
Galindo CM, Milani L, Stipp MC, do Nascimento GE, Chequin A, da
Silva LM, de Andrade SF, et al: Antineoplastic effect of pectic
polysaccharides from green sweet pepper (Capsicum annuum) on
mammary tumor cells in vivo and in vitro. Carbohydr Polym.
201:280–292. 2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Vayssade M, Sengkhamparn N, Verhoef R,
Delaigue C, Goundiam O, Vigneron P, Voragen AG, Schols HA and Nagel
MD: Antiproliferative and proapoptotic actions of okra pectin on
B16F10 melanoma cells. Phytother Res. 24:982–989. 2010.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Nikkhah M, Strobl JS, De Vita R and Agah
M: The cytoskeletal organization of breast carcinoma and fibroblast
cells inside three dimensional (3-D) isotropic silicon
microstructures. Biomaterials. 31:4552–4561. 2010.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Xu B, Song G and Ju Y: Effect of focal
adhesion kinase on the regulation of realignment and tenogenic
differentiation of human mesenchymal stem cells by mechanical
stretch. Connect Tissue Res. 51:373–379. 2011.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Zhao J and Guon JL: Signal transduction by
focal adhesion kinase in cancer. Cancer Metastasis Rev. 28:35–40.
2009.PubMed/NCBI View Article : Google Scholar
|