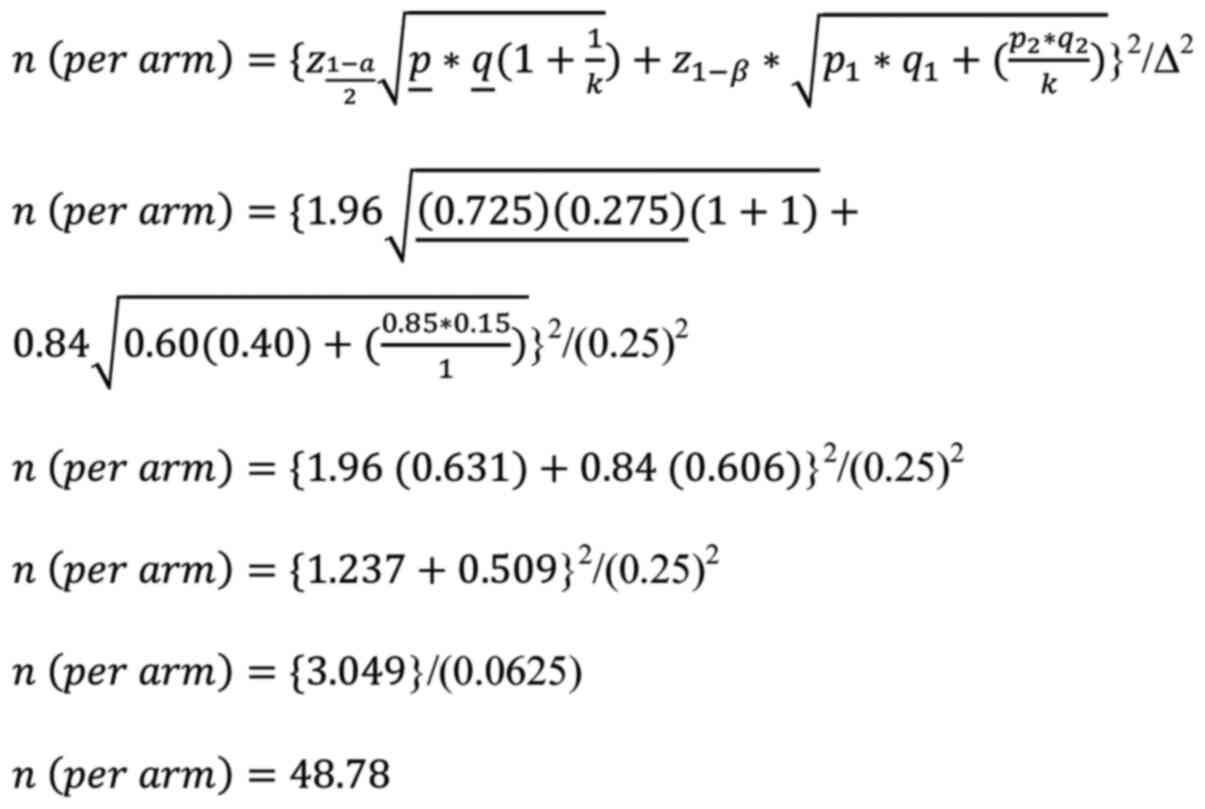Introduction
Since the epidemic of human immunodeficiency virus
(HIV), >84 million individuals have been infected with the virus
worldwide, with >40 million related deaths (1). Patients who have HIV not only
experience the deterioration of their immune system, but also the
worsening of the gastrointestinal system, with deficient nutrient
absorption, as well as nutrient metabolism (2). This critical condition affects their
overall quality of life (QoL) and intensifies the progression of
the disease. One of the metabolic disorders associated with the
prognosis of HIV infection is the malabsorption of vitamin D
(Vit-D), resulting in Vit-D deficiency (VDD). Previous studies have
demonstrated that the majority of HIV-infected individuals have
deficient levels (<20 ng/ml) or insufficient levels (<30
ng/ml) of Vit-D (3,4). Although the development of
antiretroviral therapy (ART), such as efavirenz has been a
revolutionary innovation for HIV-infected individuals, no
improvements have been documented regarding Vit-D absorption and
metabolism (5).
Among HIV-positive individuals, VDD is linked to
high levels of inflammation caused by the upregulation of
inflammatory markers, including stimulated monocyte phenotypes
(CCR2+ and CX3CR1), tumor necrosis factor-α (TNF-α) and
interleukin (IL)-6(6). This
condition further leads to the development of comorbidities, tissue
dysfunction, the progression of autoimmune deficiency syndrome
(AIDS), and ultimately, in premature death in HIV-infected
individuals (7).
Vit-D is a pro-hormone that exerts anti-inflammatory
effects (8). Optimal levels of
Vit-D are not only required for the proper absorption of
phosphorous and calcium (9), but
also they play a critical role in other critical physiological,
muscle, aging, skeletal and non-skeletal processes (10), including the prevention of tumor
growth, and the control of infections, allergies and autoimmune
diseases, the production of antibodies, the improvement of
flexibility and strength in muscle, and the normal regulation of
blood clotting and thyroid function (11-15).
It is well-established that cells displaying the cluster of
differentiation 4 (CD4) are greatly influenced by HIV infection,
presenting as a high viral load (VL), count and other blood profile
parameters. Previous studies have demonstrated an association
between VDD and suboptimal levels of CD4 cell counts, impaired
T-cell function, and increased levels of inflammatory markers among
HIV-infected individuals undergoing ART (16,17).
Even with ART, HIV-infected individuals present
suboptimal levels of Vit-D. This condition has been shown to be
associated with the progression of comorbidities, including
tuberculosis (TB), diabetes mellitus, cardiovascular diseases and
osteoporosis (14,18). HIV infection itself is a risk
factor for the premature development of numerous diseases, whereas
VDD can be a catalyst for these pathological processes. Given the
role of Vit-D in maintaining the adaptive, as well as innate immune
response, the present study aimed to examine the hypothesis that
Vit-D supplementation plays a crucial role in improving the
CD4-cell count, blood count (CBC) and levels of liver markers among
HIV-infected individuals undergoing ART.
Patients and methods
Study design and setting
A multicenter, double-masked, parallel-group,
individually randomized placebo-controlled clinical trial for
HIV-diagnosed cases treated with ART was conducted in Lahore
(Pakistan), according to the ‘Consolidated Standards of Reporting
Trials’ (CONSORT) (19) for
parallel-group randomized trials. In the present study,
HIV-positive patients prescribed with ART in the HIV treatment
centers of three major public sector hospitals in Lahore, Pakistan,
were recruited with a 1:1 allocation ratio. The study was conducted
in those hospitals that host HIV/AIDS treatment centers operated by
the Punjab AIDS Control Program. These hospitals include Mayo
Hospital, Jinnah Hospital and Government-Said Mitha Teaching
Hospital. Predefined inclusion and exclusion criteria were used to
recruit the participants in the present study.
Inclusion and exclusion criteria
The selection of participants was based on the
following inclusion criteria: i) HIV-positive patients undergoing
ART who were ≥18 years of age; and ii) Vit-D levels <50 ng/ml.
The following exclusion criteria were defined for the study: i) A
history of Vit-D supplementation at a dose >100,000 IU over the
past 3 months; and ii) pregnant and lactating women.
Sample size
Considering the role of olive oil (the placebo used
in the present study) in improving the lipid profile of
HIV-infected individuals (20), it
was assumed that 60% of patients in the control group and 85% of
patients in the treatment group (25% absolute increase) would
attain >30% of their baseline Vit-D status at 6 months. This
allowed the authors to determine a sample of 98 participants (49
per group) with a 5% significance level, 95% confidence interval
(CI) and 80% power using the following formula:
where, is the critical value for α at 95% CI
(1.96), z1-β is the critical
value for β at 80% power of the study (0.84), k is the allocation
ratio (1), p1 is
the incidence proportion of the control group (0.60),
q1 is 1-p1 (0.40),
p2 is the incidence proportion of the treatment
group (0.85), q2 is 1-p2
(0.15), p̲ is (0.725), q̲ is 1-p̲
(0.275) and Δ is the absolute difference between the proportions of
both groups (0.25).
The calculated sample size of 98 participants (49
per group) was then inflated 16% to account for the loss to
follow-up cases, making a total sample size of 114 participants (57
per group).
Baseline (pretest) assessment
A baseline assessment was conducted on the
participants who fulfilled the inclusion criteria. A structured
questionnaire was administered to collect the demographic details
of the participants, including their occupation, sex, marital
status, education, monthly family income, exposure to sunlight and
HIV screening status. Recently registered patients with HIV were
recruited in the study, and a 3 ml blood sample was obtained to
assess their baseline values of CD4-cell count and CBC.
Intervention
All the participants recruited in the present study
were enrolled for 24 weeks. Participants allocated to the control
group were provided with a placebo (1 ml commercially available
extra virgin olive oil). The placebo was administered orally in the
form of oil. The participants in the experimental group received a
100,000 IU vitamin D3 ampule administered orally by trained staff
in different multicentric setups. Olive oil was used as a placebo
as it has a similar appearance and texture as vitamin D3 ampules,
and its consumption is harmless. The commercial product name of the
study medication was ED-3, manufactured by GT Pharma (Pvt.) Ltd.
(https://khasmart.pk/product/ed3-injection-200000-an-oral-i-m/?srsltid=AfmBOooc9CTGGTvIkF7UR8vr7NhezQELH_l13NNxw3r66VTX-ezE4afx).
Each ampule had 200,000 IU vitamin D3 with a thick and transparent
liquid texture, whereas the product name of the placebo medication
was olive oil procured commercially. Both products, the placebo and
Vit-D supplement, were stored in a well-closed container at room
temperature (25-30˚C).
Outcomes
The primary outcome was to determine whether the
proposed oral high dose of vitamin D3 is sufficient for achieving
the physiological concentration (30-50 ng/ml) in HIV-positive
patients under ART after 24 weeks. The secondary outcome was to
determine whether this intervention improves CD4-cell count, VL
count, liver markers, such as serum glutamate pyruvate transaminase
(SGPT), serum glutamic-oxaloacetic transaminase (SGOT), alkaline
phosphatase (AP) and total serum bilirubin (SB) in the study
participants. The efficacy outcome and the tertiary outcome were to
determine the effects of Vit-D supplementation on the CBC [i.e.,
total leukocyte count (TLC) and hematocrit (HCT)], total neutrophil
count, monocyte count, platelet count, hemoglobin (Hb) count and
lymphocyte count] in HIV-infected patients.
Safety endpoints
The following safety endpoints were included:
Mortality, pregnancy, the incidence of hyperkalemia (high levels of
potassium in blood), and any adverse effects due to study Vit-D
supplementation (Vit-D toxicity signs and symptoms including
persistent nausea, vomiting, increased thirst, dehydration and
muscle weakness).
Randomization
Once written consent was obtained, eligible
participants were assigned to a screening number by the recruitment
hospital. The statistician, unrelated to the study, prepared and
maintained the hospital randomization list prior to the start of
recruitment. Of note, one hospital randomization list and three
separate participant randomization lists (one for each hospital)
were prepared. The subject identification code was then designated
using the screening number during the study. Equal numbers were
assigned to the participants of the supplemented and placebo
groups. There were no restrictions (for example, stratification or
block size). A screening number was given in the format of
‘XXXS###’ (for example, 101S001), which indicated the following:
‘XXX’: Site number, ‘S’: Screening; ‘###’: Order of subjects to
participate at each site.
Implementation
A random allocation sequence was then generated
using an Excel sheet by a researcher unrelated to the study. Equal
numbers were assigned to the supplemented and placebo groups. The
study pharmacy used this sequence to label pairs of ampules that
contained placebo and Vit-D supplement bearing the numbers assigned
to both groups. The staff assigned consecutive ID numbers to the
participants based on the order in which they were enrolled, and
the hospital pharmacy then supplied ampules of placebo and Vit-D
supplement bearing this ID number.
Blinding
All study participants, doctors and staff nurses who
enrolled participants and performed study assessments were blinded
to the allocation. The Vit-D supplement and placebo with a label of
ID number were presented in identical 1 ml ampules and had the same
appearance and texture.
Laboratory methods
The quantification of 25(OH)D in human serum and
plasma was used to diagnose Vit-D deficiency. Serum 25(OH)D
concentrations were measured using chemiluminescent microparticle
immunoassay (CMIA) (sensitivity 99-100%; measurement range, 0.02 to
30.5 nmol/l) technology in Chughtai Laboratories in Lahore,
Pakistan. CMIA is a delayed one-step immunoassay for quantitatively
determining 25(OH)D in human serum and plasma. All other
biochemical testing was performed in the same laboratory following
standard procedures and techniques.
Follow-up assessment
Participants in the study were allocated an HIV
enrollment card. Patients were advised to return to the treatment
center after the initial dose of supplementation on the 5th, 12th
and 24th weeks for follow-up visits. The doses were administered
orally in the 1st, 5th and 12th weeks of the study, whereas a 3-ml
blood sample for outcome measurements was obtained in the 24th
week. The patients were monitored at follow-up visits at the
centers and were also assessed for any medical or nutritional
complications and adverse events. Local community health workers
tracked down participants who did not attend follow-up visits.
Those who missed three consecutive visits were withdrawn from the
study.
Timeline of the study
Participant enrollment began on November 22, 2019.
However, due to the COVID-19 pandemic, enrollment remained very
low. Enrollment ended on July 31, 2021, while the study was
concluded on January 31, 2022.
Ethical considerations
The study was approved by the Punjab AIDS Control
Program (D. No. PACP/Admin/26285), as well as by the Institutional
Review Board (IRB), University of Punjab (D. No. 292/IIM). Written
informed consent was obtained from all the participants recruited
in the study. In addition, the present study is registered at
clinical trial.gov (NCT05306704).
Statistical analysis
Data were analyzed using SPSS version 25 software
(IBM Corp.). Frequencies and percentages were calculated for the
descriptive statistics of qualitative variables, while the mean and
standard deviation were calculated for quantitative variables. As
all the pairs of variables were skewed distributed (the results of
the normality test are presented in Table SI), to evaluate the difference in
outcome for the treatment group before and after the intervention,
the Wilcoxon signed-rank test was used, while assessing the
difference in outcome between the treatment and placebo groups,
analysis of covariance (ANCOVA) was applied. Both tests were
applied at a 95% CI. A value of P<0.05 was considered to
indicate a statistically significant difference.
Results
Study participants
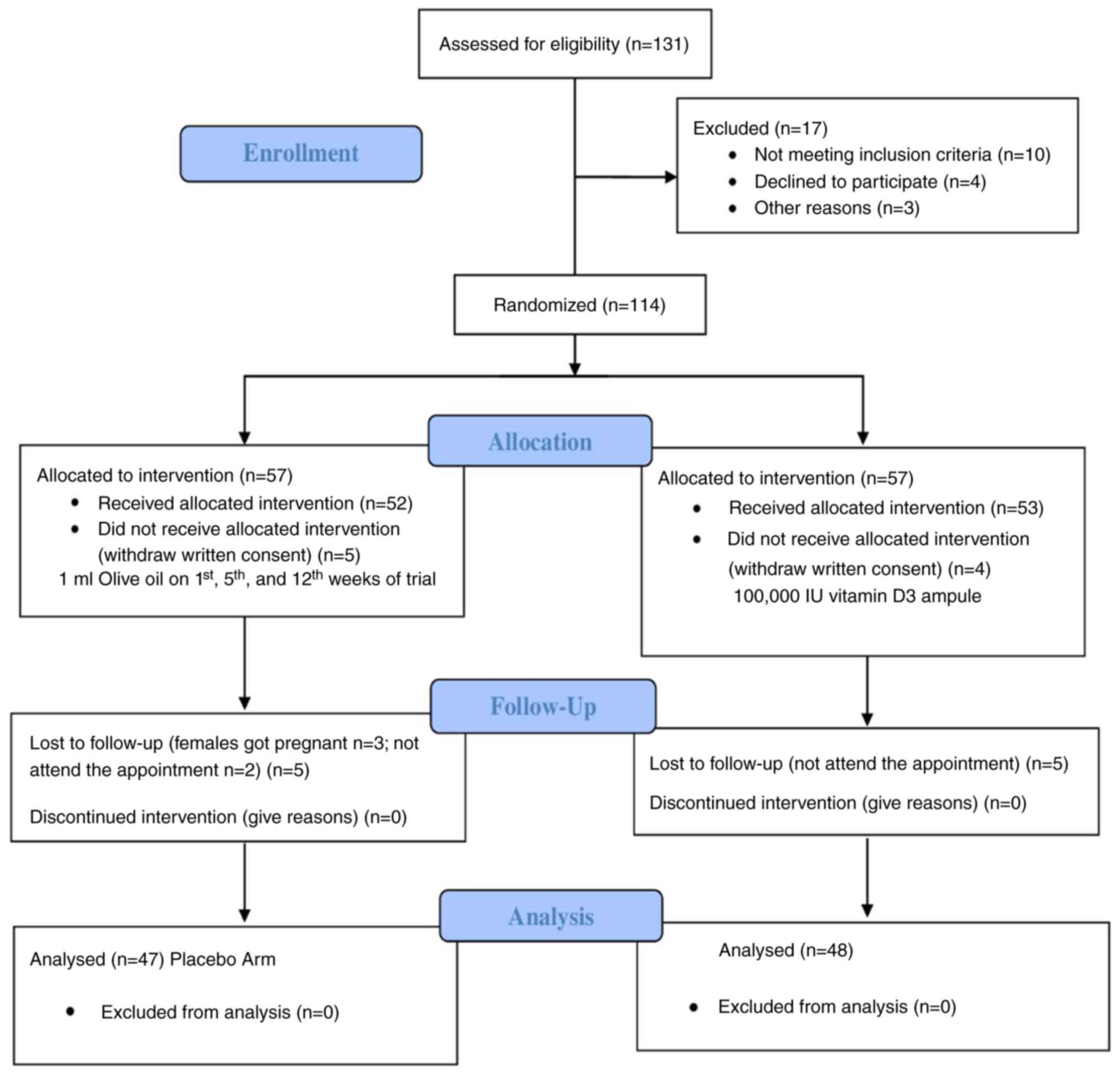
To enroll 114 participants in the present study, a
total of 131 patients were screened. All the enrolled participants
were then randomized, and 57 participants were recruited in each of
the two groups, i.e., the placebo and Vit-D-supplemented groups.
Nevertheless, a total of 9 participants withdrew written consent, 8
of whom were lost during the follow-up period, while 2 female
participants became pregnant during the trial. Consequently, a
sample of 47 participants in the placebo group and 48 participants
in the Vit-D supplementation group were included in the final data
analysis (Fig. 1).
Socio-demographic characteristics of
the participants
The majority of the participants was male (84.2%),
literate (60.0%), process/factory workers (37.9%), and married
(65.3%), with only a small number of participants being exposed to
regular sunlight (12.6%), with a mean age of 33.68±7.78 years
(Table I). Other characteristics
of the participants are also presented in Table I.
 | Table ISocio-demographic characteristics of
the study participants. |
Table I
Socio-demographic characteristics of
the study participants.
| | Placebo (n=47) | 25(OH)-D
(n=48) | Total |
|---|
| Socio-demographic
characteristics | Min | Max | Mean ± SD | Min | Max | Mean ± SD | Min | Max | Mean ± SD |
|---|
| Age (years) | 19 | 53 | 33.1±7.4 | 19 | 55 | 34.1±8.1 | 19 | 55 | 33.6±7.7 |
| Basal circulating
25(OH)- | 21.0 | 42.9 | 25.0±4.8 | 21.0 | 44.4 | 27.8±5.3 | 20.0 | 44.4 | 26.9±5.1 |
| D (nmol/l) | | | | | | | | | |
| | No. of
participants | | % | No. of
participants | | % | No. of
participants | | % |
| Sex | | | | | | | | | |
|
Male | 41 | | 87.2 | 39 | | 81.3 | 80 | | 84.2 |
|
Female | 3 | | 6.4 | 6 | | 12.5 | 9 | | 9.5 |
|
Transgender | 3 | | 6.4 | 3 | | 6.3 | 6 | | 6.3 |
| Literacy | | | | | | | | | |
|
Literate | 25 | | 53.2 | 32 | | 66.7 | 57 | | 60.0 |
|
Illiterate | 22 | | 46.8 | 16 | | 33.3 | 38 | | 40.0 |
| Occupation | | | | | | | | | |
|
Skilled
trade worker | 12 | | 25.5 | 12 | | 25.0 | 24 | | 25.3 |
|
Process/factory
worker | 16 | | 34.1 | 20 | | 41.7 | 36 | | 37.9 |
|
Elementary
worker | 15 | | 31.9 | 11 | | 22.9 | 26 | | 27.4 |
|
Sex
worker | 4 | | 8.5 | 5 | | 10.4 | 09 | | 9.5 |
| Marital status | | | | | | | | | |
|
Married | 30 | | 63.8 | 32 | | 66.7 | 62 | | 65.3 |
|
Unmarried | 17 | | 36.2 | 16 | | 33.3 | 33 | | 34.7 |
| Sunlight
exposure | | | | | | | | | |
|
Yes | 7 | | 14.9 | 5 | | 10.4 | 12 | | 12.6 |
|
No | 40 | | 85.1 | 43 | | 89.6 | 83 | | 87.4 |
Pre and post-intervention comparisons
of 25(OH)-D levels, CD4 cell count, viral load, hepatic markers and
CBC for the treatment group
The analysis revealed a significant (P<0.05)
difference for all parameters before and after the 25(OH)-D
supplementation (Table II).
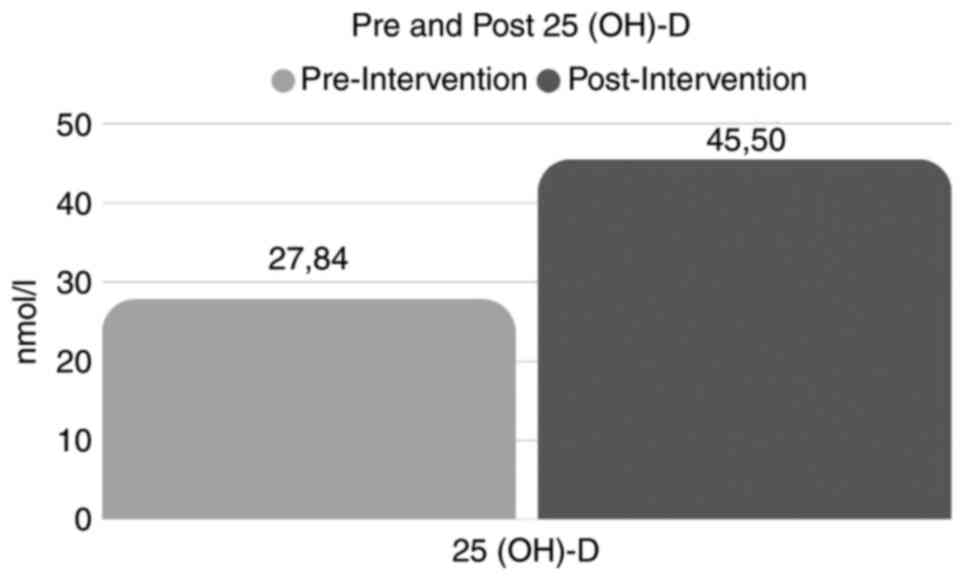
Significant increases (P<0.05) were observed in the
concentration of 25(OH)-D levels, CD4 cell count, SGPT, SGOT, SB,
AP, Hb, TLC, HCT, % neutrophils, % lymphocytes, % monocytes,
platelets and eosinophils. However, a significant (P<0.05)
decrease was observed in the VL count (Table II and Fig. 2).
 | Table IIComparison of 25(OH)-D circulating
levels, CD4-positive cells, viral load, liver markers, and complete
blood count for the Vit-D-treated group. |
Table II
Comparison of 25(OH)-D circulating
levels, CD4-positive cells, viral load, liver markers, and complete
blood count for the Vit-D-treated group.
| Pair nos. | Pairs | V1a Mean ± SD | V4b Mean ± SD | Z Score | P-value |
|---|
| Pair 1 | Pre 25 (OH)-D -
post 25 (OH)-D (nmol/l) | 27.84±5.32 | 45.50±8.74 | -6.208 | <0.001 |
| Pair 2 | Pre CD4 - post CD4
(cells/mm3) | 436.96±140.75 | 642.85±264.76 | -5.694 | <0.001 |
| Pair 3 | Pre VL - post VL
(viral particles/ml) |
10942.85±17646.20 | 740.13±945.60 | -4.038 | <0.001 |
| Pair 4 | Pre SGPT - post
SGPT (U/l) | 34.10±17.87 | 37.84±14.12 | -3.112 | 0.002 |
| Pair 5 | Pre SGOT - post
SGOT (U/l) | 35.67±19.11 | 41.72±22.31 | -4.108 | <0.001 |
| Pair 6 | Pre serum bilirubin
- post serum bilirubin (mg/dl) | 0.60±0.22 | 1.02±1.07 | -4.147 | <0.001 |
| Pair 7 | Pre-AP - post-AP
(U/l) | 205.18±115.46 | 329.44±252.92 | -4.657 | <0.001 |
| Pair 8 | Pre Hb - post Hb
(g/dl) | 13.14±1.87 | 14.96±1.59 | -4.978 | <0.001 |
| Pair 9 | Pre TLC - post TLC
(cells/l) | 5.87±2.20 | 8.57±2.57 | -5.498 | <0.001 |
| Pair 10 | Pre HCT - post HCT
(%) | 37.22±9.72 | 46.08±5.54 | -5.126 | <0.001 |
| Pair 11 | Pre neutrophil -
post neutrophil (%) | 45.54±12.67 | 57.45±10.61 | -5.549 | <0.001 |
| Pair 12 | Pre lymphocytes -
post lymphocytes (%) | 30.83±10.41 | 38.24±7.36 | -4.298 | <0.001 |
| Pair 13 | Pre monocytes -
post monocytes (%) | 5.42±3.25 | 7.46±3.03 | -4.657 | <0.001 |
| Pair 14 | Pre platelet - post
platelet (platelets/nl) | 230.10±94.03 | 321.81±98.32 | -4.684 | <0.001 |
| Pair 15 | Pre eosinophil -
post eosinophil (%) | 2.17±1.49 | 3.91±1.73 | -4.764 | <0.001 |
Differences in circulating parameters
comparing the Vit-D-supplemented group to the placebo group
In a following step, the values of some of the
circulating parameters analyzed in Table II, were compared between the
Vit-D-treated group and the placebo group (Table III). The analysis revealed
significantly (P<0.05) higher values in the treatment group for
the 25(OH)-D concentration, CD4 cell count, Hb, TLC, HCT, %
neutrophils, % lymphocytes and platelets. The comparison was
performed in the patients treated with the placebo compared to the
patients supplemented with Vit-D, when their mean values were
adjusted for their pre-intervention mean values. However, no
significant differences (P>0.05) were observed for the VL count,
SGPT, SGOT, SB, AP, % monocytes and % eosinophils between the
Vit-D-supplemented patients and the patients administered the
placebo post-intervention. These results highlight that there was a
significant (P<0.05) difference when patients undergoing ART are
supplemented with Vit-D, suggesting that Vit-D supplementation is
more beneficial than ART alone.
 | Table IIIComparison of outcomes for treatment
and control group post-intervention. |
Table III
Comparison of outcomes for treatment
and control group post-intervention.
| | Mean | |
|---|
| Pairs | Treatment
group | Control group | Mean
difference | F-value | P-value |
|---|
| 25 (OH)-D
(nmol/l) | 41.41 | 36.24 | 5.16 | 12.18 | <0.001 |
| CD4-cells
(cells/mm3) | 789.32 | 635.0 | 154.32 | 16.32 | <0.001 |
| VL (viral
particles/ml) | 742.10 | 668.48 | 73.62 | 0.118 | 0.732 |
| SGPT (U/l) | 37.89 | 34.42 | 3.47 | 1.29 | 0.259 |
| SGOT (U/l) | 38.28 | 33.94 | 4.33 | 2.03 | 0.158 |
| SB | 1.02 | 0.70 | 0.32 | 3.13 | 0.08 |
| AP (U/l) | 321.58 | 255.53 | 66.05 | 2.01 | 0.160 |
| Hb (g/dl) | 14.87 | 13.73 | 1.14 | 12.85 | <0.001 |
| TLC (cells/l) | 8.68 | 6.68 | 2.00 | 24.54 | <0.001 |
| HCT (%) | 46.16 | 40.84 | 5.32 | 16.75 | <0.001 |
| Neutrophils
(%) | 57.49 | 50.04 | 7.45 | 11.26 | <0.001 |
| Lymphocytes
(%) | 37.80 | 32.49 | 5.31 | 7.85 | <0.01 |
| Monocytes (%) | 7.380 | 6.457 | 0.922 | 0.95 | 0.089 |
| Platelets
(platelets/nl) | 314.84 | 255.79 | 59.05 | 9.981 | <0.01 |
| Eosinophils
(%) | 3.93 | 3.41 | 0.513 | 1.55 | 0.217 |
Adverse events
No adverse events were reported during the duration
of the trial.
Discussion
Similar to previous studies (21-23),
the present study also confirmed insufficient levels of Vit-D among
HIV-infected individuals undergoing ART (28.08±5.40 nmol/l). The
primary source of Vit-D is exposure to sunlight. Although Pakistan
is a country with high levels of sunlight exposure (Lahore has
>70% high-intensity sunny daylight hours per year). However, the
majority of the participants recruited in the present study
reported no sunlight exposure (85.1%). This reduced exposure could
be due to weak health conditions and concomitant infections caused
by HIV (24).
Previous research has also exhibited the stable
persistence of CD4-positive cells as a significant obstacle to the
treatment of HIV with ART (25).
This condition occurs together with suboptimal levels of other
circulating parameters. Taken together, this is a challenging issue
among patients with HIV undergoing ART. The present study also
revealed suboptimal levels of CD4 cell count, VL count, SGOT, SGPT,
SB, AP, Hb, TLC, HCT, neutrophils, lymphocytes, monocytes and
platelet counts in HIV-infected individuals on ART. However, after
administering the corresponding dose of Vit-D, there was a
significant (P<0.05) improvement in circulating Vit-D levels,
CD4 cell count, Hb, TLC, HCT, neutrophils, lymphocytes and platelet
counts.
Previous similar studies (22,26,27)
have also demonstrated the critical role of Vit-D supplementation
in improving physiological concentrations among HIV-infected
individuals. The study conducted by Piloya et al (22) found that daily Vit-D
supplementation for 16 weeks was well-tolerated and effectively
improved Vit-D levels among HIV-infected individuals. However, no
significant (P>0.05) changes were observed in the CD4 cell
count. By contrast, some studies claim the role of Vit-D
supplementation in reducing the CD4-cell count among HIV-infected
individuals under ART (26,27).
However, the present study found a significant (P<0.05) role of
Vit-D supplementation in achieving optimal levels for CD4-positive
cells. Moreover, the present study suggests an association between
Vit-D supplementation and improved enzyme/immune levels, including
Hb, TLC, HCT, neutrophil percentage, lymphocyte percentage and
platelets.
Further, the Vit-D-treated arm exhibited a
significant within-group reduction in VL; however, the
between-group comparison did not yield significant results. This
pattern is consistent with prior HIV randomized controlled trials
where Vit-D supplementation corrected deficiency and modestly
improved immune indices without producing a clear between-group VL
benefit once baseline values and ART effects were accounted for.
For example, a previous randomized trial found vitamin D3 did not
lower the risk of unsuppressed VL after 6 months of ART (RR 1.10;
95% CI, 0.87-1.41), despite biochemical repletion, suggesting any
observed within-group VL decline (28). This likely reflects ART
optimization, regression-to-the-mean, or adherence effects rather
than a direct antiviral action of Vit-D alone (26,28,29).
In the present study, mild elevations in SGPT and
SGOT were observed in the Vit-D-supplemented group. Such changes in
the levels of transaminases are commonly reported among individuals
living with HIV undergoing ART and are frequently attributed to
drug-induced hepatocellular stress or underlying infection, rather
than overt liver injury. For instance, studies in African settings
have demonstrated a low incidence of clinically significant SGPT
and SGOT elevations during ART, suggesting that mild enzyme
elevations may often reflect subclinical effects or systemic
inflammation rather than true hepatic damage (30,31).
Nevertheless, given that liver enzyme elevations were observed,
even if mild, this finding highlights the need for the ongoing
monitoring of hepatic function when high-dose Vit-D is administered
in ART-treated populations, to ensure early identification and
management of potential hepatotoxicity.
Similarly, the post-intervention AP level exceeded
the conventional adult reference range. AP is a composite enzyme
with hepatic and bone isoforms; increased bone-specific AP is
common during vitamin D repletion due to enhanced osteoblastic
activity and bone remodeling (24). Efavirenz and tenofovir exposure has
also been associated with elevated AP levels in HIV-positive
individuals (5), possibly through
effects on bone metabolism. While no adverse events were reported
herein, future studies should consider AP isoenzyme fractionation
and concurrent bone turnover markers to differentiate hepatic from
skeletal sources.
Nutritional deficiencies cause the onset and
progression of various diseases, including AIDS, by dysregulating
the immune system. Similarly, Vit-D has a marked effect on the
regulation of adaptive, as well as innate immune responses.
Although ART is considered to be a revolutionary treatment for
patients with HIV as it prolongs their life and improves their QoL,
it cannot be neglected that the initiation of some ARTs has been
associated with VDD in previous studies (24). Therefore, the present study
strongly suggests that the setup of certain ARTs would be
implemented with Vit-D supplementation, particularly in
Vit-D-deficient patients. Optimal levels of Vit-D have
antimicrobial, anti-inflammatory and immunomodulatory properties
that reduce the risk of acquiring a number of infections (16,17).
In summary, in the present study, high-dose vitamin
D3 supplementation in Vit-D-deficient adults living with HIV and
undergoing ART resulted in significant improvements in CD4 cell
counts and hematological indices, without significant between-group
differences in VL or hepatic markers. The absence of calcium
measurements is a limitation however, as high-dose Vit-D may, in
rare cases, cause hypercalcemia; future studies are thus required
to incorporate calcium and renal function monitoring. The selected
100,000 IU bolus dose was based on previous randomized controlled
trials and aligns with the Endocrine Society's recommendations for
deficiency correction, remaining within the safe upper intake level
equivalent (<10,000 IU/day) (24,28,29).
The findings of the present study therefore suggest a supportive,
although not curative, role of Vit-D in enhancing selected immune
and hematologic outcomes in this context. Optimal levels of Vit-D
in the human body control the production of Th17 cells,
Th-1-associated cytokines and T-lymphocytes, and encourage the
proliferation of Th-2-associated cytokines. Notably, Vit-D supports
antiviral and antibacterial immunity. It also attenuates disease
progression among HIV-infected individuals and should, therefore,
be made a part of the regular treatment of HIV.
The present study has some limitations which should
be mentioned. First, the present study was not a dose-response
study. Therefore, it is unclear whether a lower dose than the one
used would help improve the studied parameters. Second, although no
adverse events were encountered during the study, biochemical
safety monitoring and calcium status were not performed with the
high-dose of Vit-D supplementation. The increase observed in the
levels of transaminases was likely due to the ART, increasing with
Vit-D supplementation. Strategies to preserve liver function need
to be implemented (further research is required in this context).
Furthermore, the present study experienced an attrition rate of
17%, the initial sample size was prospectively increased by ~16% to
account for anticipated loss to follow-up, based on previous HIV
trial experiences in similar settings. Attrition was balanced
between intervention and control groups, and baseline
characteristics did not differ significantly between completers and
non-completers. Therefore, the risk of attrition bias is considered
minimal, and a per-protocol analysis was retained to reflect the
physiological effect of high-dose Vit-D in participants who
completed the regimen. While this approach may limit
generalizability to all individuals initiating supplementation, it
provides a robust estimate of efficacy under ideal adherence
conditions. Finally, the present study did not examine the
long-term effects of high-dose Vit-D supplementation and the
sustainability of improved parameters, as the study period was
relatively short. Therefore, further trials need to be conducted
with long follow-ups and different dosages in diverse settings.
In conclusion, Vit-D supplementation in deficient
HIV-infected individuals undergoing ART significantly improved CD4
cell counts and selected hematological parameters, without
producing significant changes in VL or hepatic markers. Nutritional
deficiencies can cause dysregulation in the immune system, while
nutritional supplementation can help protect individuals from
infections by regulating the immune system. In this context, Vit-D
supplements play an instrumental role.
While these results support the potential of Vit-D
as a safe, low-cost adjunct to optimize immune-related hematologic
outcomes, further research with longer follow-up periods, calcium
monitoring and varied dosing regimens is warranted to confirm and
extend these findings.
Supplementary Material
Results of the normality test.
Acknowledgements
The authors acknowledge Mayo Hospital, Jinnah
Hospital, and Government Said Mitha Teaching Hospital (Lahore,
Pakistan), for their support and cooperation. The authors would
like to thank the Neurobiology Research Group, Faculty of Medicine,
University of Valladolid (Soria, Spain) for their support and
involvement in the study. CIBEROBN is an initiative of Instituto de
Salud Carlos III, Spain.
Funding
Funding: Funding was provided by the Ministry of Science and
Innovation, the Government of Spain, and the European Union
(project PID2022-141358OBI00).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
FM, JS, RS, HWA, MY and JF carried out the
investigation and methodology. ER performed data curation and was
involved in the writing of the manuscript. AMCSM was involved in
the formal analysis and data validation. DFL was involved in study
supervision, in the conceptualization of the study, in project
administration and in the writing of the original draft of the
manuscript. Authors FM and JS confirm the authenticity of all the
raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Punjab AIDS
Control Program (D. No. PACP/Admin/26285) as well as by the
Institutional Review Board (IRB), University of Punjab (D. No.
292/IIM). Written informed consent was obtained from all the
participants recruited in the study, and they were informed that
they could withdraw their participation from the study at any point
in time. The present study is also registered at clinicaltrial.gov. The NCT number of this study on
clinical trial.gov is 05306704. All subjects provided
written informed consent in accordance with the Declaration of
Helsinki and the 2013 Fortaleza revision..
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
United Nations Program on HIV/AIDS
(UNAIDS): Global HIV & AIDS statistics. UNAIDS, Geneva, 2024.
https://www.unaids.org/en/resources/fact-sheet#:~:text=Global%20HIV%20statistics,AIDS%2Drelated%20illnesses%20in%202023.
Accessed August 2, 2024.
|
|
2
|
Perpetue FFD, Charles KT and Anatole PC:
Nutritional Assessment of HIV/AIDS patients in Centre Region of
Cameroon: A pilot study. J Food Sci Nutr Res. 4:77–93. 2021.
|
|
3
|
Kanwal W and Rehman A: High prevalence of
vitamin D deficiency in HIV-infected individuals in comparison with
the general population across Punjab province, Pakistan. Saudi J
Biol Sci. 30(103484)2023.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wardani IS, Hatta M, Mubin RH, Bukhari A,
Mulyanto Massi MN, Djaharuddin I, Bahar B, Aminuddin and
Wahyuni S: Serum vitamin D receptor and High Mobility Group Box-1
(HMGB1) levels in HIV-infected patients with different
immunodeficiency status: A cross-sectional study. Ann Med Sur
(Lond). 63(102174)2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Welz T, Childs K, Ibrahim F, Poulton M,
Taylor CB, Moniz CF and Post FA: Efavirenz is associated with
severe vitamin D deficiency and increased alkaline phosphatase.
AIDS. 24:1923–1928. 2010.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lucas RM, Gorman S, Geldenhuys S and Hart
PH: Vitamin D and immunity. F1000Prime Rep. 6(118)2014.PubMed/NCBI View
Article : Google Scholar
|
|
7
|
Jiménez-Sousa MÁ, Martínez I, Medrano LM,
Fernández-Rodríguez A and Resino S: Vitamin D in human
immunodeficiency virus infection: Influence on immunity and
disease. Front Immunol. 9(458)2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Nazarabadi PN, Etemad Z, Hoseini R and
Moradi F: Anti-Inflammatory effects of a period of aerobic training
and vitamin D supplementation in postmenopausal women with
metabolic syndrome. Int J Prev Med. 13(60)2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Akimbekov NS, Digel I, Sherelkhan DK and
Razzaque MS: Vitamin D and phosphate interactions in health and
disease. Adv Exp Med Biol. 1362:37–46. 2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Fernández-Lázaro D, Hernández JLG,
Lumbreras E, Mielgo-Ayuso J and Seco-Calvo J: 25-Hydroxyvitamin D
serum levels linked to single nucleotide polymorphisms (SNPs)
(rs2228570, rs2282679, rs10741657) in Skeletal muscle aging in
institutionalized elderly men not supplemented with vitamin D. Int
J Mol Sci. 23(11846)2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Grant WB, Boucher BJ, Al Anouti F and Pilz
S: Comparing the evidence from observational studies and randomized
controlled trials for non-skeletal health effects of vitamin D.
Nutrients. 14(3811)2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Joseph P, Pais P, Gao P, Teo K, Xavier D,
Lopez-Jaramillo P, Yusoff K, Santoso A, Gamra H, Talukder SH, et
al: Vitamin D supplementation and adverse skeletal and non-skeletal
outcomes in individuals at increased cardiovascular risk: Results
from the International Polycap Study (TIPS)-3 randomized controlled
trial. Nutr Metab Cardiovasc Dis. 33:434–440. 2023.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Al-Dahy LB, Al-Sammarraie MRH and Al
Chaabawi AJ: Effect study's the role of serum vitamin D3 level in
Hashimoto's thyroiditis disease. J Pharm Negative Results.
13:1088–1090. 2022.
|
|
14
|
Sudfeld CR, Giovannucci EL, Isanaka S,
Aboud S, Mugusi FM, Wang M, Chalamilla G and Fawzi WW: Vitamin D
status and incidence of pulmonary tuberculosis, opportunistic
infections, and wasting among HIV-infected Tanzanian adults
initiating antiretroviral therapy. J Infect Dis. 207:378–385.
2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Musselwhite LW, Andrade BB, Ellenberg SS,
Tierney A, Belaunzaran-Zamudio PF, Rupert A, Lederman MM, Sanne I,
Sierra-Madero JG and Sereti I: Vitamin D, D-dimer, interferon γ,
and sCD14 levels are independently associated with immune
reconstitution inflammatory syndrome: A prospective, international
study. EBioMedicine. 4:115–123. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Legeai C, Vigouroux C, Souberbielle JC,
Bouchaud O, Boufassa F, Bastard JP, Carlier R, Capeau J, Goujard C,
Meyer L, et al: Associations between 25-hydroxyvitamin D and
immunologic, metabolic, inflammatory markers in treatment-naive
HIV-infected persons: The ANRS CO9 «COPANA» cohort study. PLoS One.
18(e74868)2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Von Essen MR, Kongsbak M, Schjerling P,
Olgaard K, Ødum N and Geisler C: Vitamin D controls T cell antigen
receptor signaling and activation of human T cells. Nat Immunol.
11:344–349. 2010.PubMed/NCBI View
Article : Google Scholar
|
|
18
|
Pinzone MR, Di Rosa M, Malaguarnera M,
Madeddu G, Focà E, Ceccarelli G, d'Ettorre G, Vullo V, Fisichella
R, Cacopardo B and Nunnari G: Vitamin D deficiency in HIV
infection: An underestimated and undertreated epidemic. Eur Rev Med
Pharmacol Sci. 17:1218–1232. 2013.PubMed/NCBI
|
|
19
|
Moher D, Hopewell S, Schulz KF, Montori V,
Gøtzsche PC, Devereaux PJ, Elbourne D, Egger M and Altman DG:
CONSORT. CONSORT 2010 explanation and elaboration: updated
guidelines for reporting parallel group randomized trials. Int J
Surg. 10:28–55. 2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Olalla J, de Lomas JM, Chueca N,
Pérez-Stachowski X, De Salazar A, Del Arco A, Plaza-Díaz J, De la
Torre J, Prada JL, García-Alegría J, Fernández-Sánchez F and García
F: Effect of daily consumption of extra virgin olive oil on the
lipid profile and microbiota of HIV-infected patients over 50 years
of age. Medicine (Baltimore). 98(e17528)2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Eckard AR, Tangpricha V, Seydafkan S,
O'Riordan MA, Storer N, Labbato D and McComsey GA: The relationship
between vitamin D status and HIV-related complications in
HIV-infected children and young adults. Pediatr Infect Dis J.
32:1224–1229. 2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Piloya TW, Bakeera-Kitaka S, Kisitu GP,
Idro R and Cusick SE: Vitamin D status and associated factors among
HIV-infected children and adolescents on antiretroviral therapy in
Kampala, Uganda. PLoS One. 16(e0253689)2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Rutstein R, Downes A, Zemel B, Schall J
and Stallings V: Vitamin D status in children and young adults with
perinatally acquired HIV infection. Clin Nutr. 30:624–628.
2011.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Holick MF, Binkley NC, Bischoff-Ferrari
HA, Gordon CM, Hanley DA, Heaney RP, Murad MH and Weaver CM:
Endocrine Society. Evaluation, treatment, and prevention of vitamin
D deficiency: An Endocrine Society clinical practice guideline. J
Clin Endocrinol Metab. 96:1911–1930. 2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Siliciano JD, Kajdas J, Finzi D, Quinn TC,
Chadwick K, Margolick JB, Kovacs C, Gange SJ and Siliciano RF:
Long-term follow-up studies confirm the stability of the latent
reservoir for HIV-1 in resting CD4+ T cells. Nat Med. 9:727–728.
2003.PubMed/NCBI View
Article : Google Scholar
|
|
26
|
Alvarez N, Aguilar-Jimenez W and Rugeles
MT: The potential protective role of vitamin D supplementation on
HIV-1 infection. Front Immunol. 10(2291)2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Verma P, Shrivastava A, Siddiqui SA, Yadav
RK, Singh MV, Tripathi A, Maurya M and Mishra N: Effect of vitamin
D supplementation on CD4 count in HIV-infected children and
adolescents in north India: A non-randomized comparative study. J
Trop Pediatr. 68(fmac066)2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Muhihi A, Fawzi WW, Aboud S, Nagu TJ,
Ulenga N, Wang M, Mugusi F and Sudfeld CR: Cholecalciferol
supplementation does not affect the risk of HIV progression, viral
suppression, comorbidities, weight loss, and depression among
Tanzanian adults initiating antiretroviral therapy: Secondary
outcomes of a randomized trial. J Nutr. 152:1983–1990.
2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Sudfeld CR, Mugusi F, Muhihi A, Aboud S,
Nagu TJ, Ulenga N, Hong B, Wang M and Fawzi WW: Efficacy of vitamin
D3 supplementation for the prevention of pulmonary tuberculosis and
mortality in HIV: A randomised, double-blind, placebo-controlled
trial. Lancet HIV. 7:e463–e471. 2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Dusingize JC, Hoover DR, Shi Q, Mutimura
E, Rudakemwa E, Ndacyayisenga V, Gakindi L, Mulvihill M, Sinayobye
JD, Musabeyezu E and Anastos K: Association of abnormal liver
function parameters with HIV serostatus and CD4 count in
antiretroviral-naive Rwandan women. AIDS Res Hum Retroviruses.
31:723–730. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ocama P, Castelnuovo B, Kamya MR, Kirk GD,
Reynolds SJ, Kiragga A, Colebunders R and Thomas DL: Low frequency
of liver enzyme elevation in HIV-infected patients attending a
large urban treatment centre in Uganda. Int J STD AIDS. 21:553–557.
2010.PubMed/NCBI View Article : Google Scholar
|