Introduction
Coronary artery syndrome is the most common cause of
death among women in developed countries (1). The coronary artery syndrome is
initiated by atherosclerosis, which is complexed with dyslipidemia
and an inflammatory process.
Angina pectoris (AP) is paroxysmal thoracic pain
that is sometimes accompanied by a feeling of suffocation (2). AP is most often due to ischemia of
the myocardium and is precipitated by effort or excitement. Several
biomarkers have been developed to diagnose coronary artery disease,
including lipid and inflammatory markers (3), although the markers are not
prognostic. It is well-known that the apoB/apoA-I ratio is
important to predict the risk of coronary artery disease (CAD)
(4). There have been many
non-invasive biochemical measures used to predict cardiovascular
risk, such as lipid and lipoprotein metabolism, inflammation, and
oxidative stress (5–7). Recently, the apolipoprotein
composition in lipoprotein and high-density lipoprotein (HDL)
sub-fractions has been shown to change in the sera from patients
with acute coronary syndrome (ACS). Huang et al (8) reported that plasma ApoAV is
associated with ACS. Tashiro et al (9) reported that pre β1-HDL is elevated
in patients with unstable angina pectoris. Furthermore, we recently
reported an increase in apoC-III in HDL2 from a male
with a myocardial infarction (10). Similarly, Lee et al
(11) reported that low-density
lipoprotein (LDL)-containing apoC-III is an independent risk factor
for coronary events in diabetic patients. These findings
collectively raised the possibility of a relationship between
increased lipid and apoC-III, oxidative modification, and
inflammatory processes. In ACS, oxidative stress constitutes an
integral part of plaque rupture and platelet activation (12). The oxidative modification of LDL,
which is considered a strong risk factor for atherosclerosis and
ACS, occurs through the release of pro-inflammatory and oxidative
signals. The composition and functional correlations of HDL is also
associated with the incidence of metabolic syndrome as described in
our previous report (13).
Elevated triglycerides (TG) and low cholesterol (C) content in HDL
is a major characteristic of the metabolic syndrome (14) and of myocardial infarction (MI)
(10). A low HDL-C level is the
most common lipid abnormality observed in families with premature
coronary heart disease (CHD) (15).
There have been many studies attempting to establish
non-invasive biomarkers for the early detection of risk for CHD,
including AP and MI. In the current study, to detect unique
parameters in lipoprotein levels, lipid and apolipoprotein
compositions, and enzyme activities were analyzed between females
with AP and controls.
Materials and methods
Patients and controls
Female patients with stable AP (n=22) were selected
using the following criteria: the presence of chest or arm
discomfort that is rarely described as pain, but is reproducibly
associated with physical exertion or stress and relieved within
5–10 min of rest and/or administration of sublingual nitroglycerin.
The diagnosis was confirmed with a treadmill exercise test and
coronary angiography in all patients. Patients did not take any
medications, except for statins, prior to hospitalization. Age- and
gender-matched reference subjects (n=20) were recruited from
healthy volunteers who underwent regular health evaluations at the
Health Center of Yeungnam University Hospital (Daegu, Korea). They
had unremarkable medical records without a history of
endocrinological disorders. Heavy alcohol consumers (>30 g
EtOH/day) and those who had taken prescribed drugs to treat
hyperlipidemia, diabetes mellitus, or hypertension were excluded.
Informed consent was obtained from all patients and the control
group prior to enrollment in the study. The Institutional Review
Board at the Medical Center of Yeungnam University approved the
protocol.
Isolation of lipoproteins
After overnight fasting, blood was collected using a
vacutainer (BD Bio Sciences, Franklin Lakes, NJ, USA) containing
EDTA (final concentration, 1 mM). Plasma was isolated by low-speed
centrifugation and stored at −80˚C until analysis.
Very low-density lipoproteins (VLDL, d<1.019
g/ml), LDL (1.019<d<1.063), HDL2
(1.063<d<1.125) and HDL3 (1.125<d<1.225)
were isolated from individual patient and control sera via
sequential ultracentrifugation (16), with the density adjusted by the
addition of NaCl and NaBr in accordance with standard protocols.
Samples were centrifuged for 24 h at 10˚C at 100,000 × g using a
Himac CP 90α (Hitachi, Tokyo, Japan) at the Instrumental Analysis
Center of Yeungnam University.
For each of the lipoproteins which were individually
purified, total cholesterol (TC) and TG measurements were obtained
using commercially available kits (cholesterol, T-CHO, and TG,
Cleantech TS-S; Wako Pure Chemical, Osaka, Japan). The protein
concentrations of lipoproteins were determined via the Lowry
protein assay, as modified by Markwell et al (17) using the Bradford assay reagent
(Bio-Rad, Seoul, South Korea) with bovine serum albumin (BSA) as a
standard. To assess the degree of oxidation of individual LDL, the
concentration of oxidized species in LDL was determined by the
thiobarbituric acid reactive substances (TBARS) method using
malondialdehyde (MDA) as a standard (18).
Ferric reducing ability of plasma
assay
The ferric reducing ability of plasma (FRAP) was
determined using the method described by Benzie and Strain
(19) with a slight modification,
as described previously (20).
The antioxidant activities of the individual HDL fractions (20 μg
each) were then estimated by measuring the increase in absorbance
induced by the generated ferrous ions.
Cholesteryl ester conversion assay
Cholesteryl ester conversion was performed via
lecithin:cholesterol acyltransferase (LCAT) assays, as previously
described (21). An equal amount
of individual lipoproteins (in 50 μl) from each patient was
utilized as the enzyme source. ApoA-I-rHDL containing radiolabeled
cholesterol (1 μCi of [14C]-4-cholesterol/69 μg of
cholesterol/1 mg of apoA-I) was used as a substrate, and the apoA-I
was then expressed using an E. coli expression system, as
described previously (21).
Discoidal rHDL was prepared via the sodium cholate dialysis method
using initial molar ratios of palmitoyloleoyl phosphatidylcholine
(POPC)-cholesterol-apoA-I-sodium cholate at a ratio of 95:5:1:150
(wt/wt/wt/wt). The reaction was initiated by the addition of
individual serum, and the mixture was then incubated for 1 h at
37˚C. Next, the esterified cholesterol and free cholesterol were
separated via thin layer chromatography, and the activity was
expressed as the percentage conversion rate of cholesteryl ester
from free cholesterol.
Cholesteryl ester transfer assay
An rHDL-containing apoA-I and cholesteryl oleate was
synthesized in accordance with the method described by Cho
(20) using trace amounts of
[3H]-cholesteryl oleate (TRK886, 3.5 μCi/mg of apoA-I;
GE Healthcare) with a slight modification (22). The CE-transfer reaction was
allowed in 300 μl reaction mixtures that contained equal amounts of
the individual lipoproteins (20 μl, 10–20 μg of protein) as a
cholesteryl ester transfer protein (CETP) source and rHDL-agarose
(50 μl, 0.25 mg/ml) and human LDL (50 μl, 0.25 mg/ml) as a CE-donor
and CE-acceptor, respectively. After incubation at 37˚C, the
reaction was halted via brief centrifugation (10,000 × g) for 3 min
at 4˚C. The supernatant (150 μl) was then subjected to
scintillation counting, and the percentage transfer of
[3H]-CE from rHDL to LDL was calculated.
Paraoxonase assay
Paraoxonase-1 (PON-1) activity toward paraoxon was
determined by evaluating the hydrolysis of paraoxon into
p-nitrophenol and diethylphosphate, which was catalyzed by
the enzyme (23). PON-1 activity
was then determined by measuring the initial velocity of
p-nitrophenol production at 37˚C, as determined by measuring
the absorbance at 405 nm (microplate reader, Bio-Rad model 680;
Bio-Rad, Hercules, CA, USA), as described previously (13).
Platelet activating
factor-acetylhydrolase (PAF-AH) assay
The individual lipoprotein fractions (10 μl, 20 μg)
were used as an enzyme source for the PAF-AH reaction with an
Lp-PLA2 assay conducted according to the method
described by Boyd et al (24). Briefly, [3H]-platelet
activating factor (hexadecyl-2-acetyl
sn-glyceryl-3-phosphorylcholine, NET910, 0.1 mCi/ml; Perkin-Elmer
Life and Analytical Sciences, Boston, MA, USA) and
1-O-hexadecyl-2-acetyl-sn-glycero-3-phosphocholine were used as
substrates for the reaction. A substrate solution containing 10 μl
of [3H]-PAF (1 μCi, 50 μM) and 12 μM of cold PAF was
incubated using each HDL solution as a source for 30 min. The
reaction was then stopped by vortexing the solutions with 600 μl of
CHCl3:MeOH (2:1, v/v), after which the aqueous layer
(150 μl) was removed. The aqueous layer was then vortexed again
with CHCl3, after which it was centrifuged and the upper
phase was used for scintillation counting.
Electromobility of lipoproteins
In order to compare the electromobility of the
patient and control samples, the migration of each lipoprotein
(LDL, HDL2 and HDL3) was evaluated by agarose
electrophoresis. The gels were then dried and stained with 0.125%
Coomassie Brilliant Blue, after which the relative band intensities
were compared via band scanning using Gel Doc® XR
(Bio-Rad) with Quantity One software (version 4.5.2).
Western blot analysis
The apolipoprotein/lipoprotein compositions were
compared via sodium dodecyl sulfate-polyacylamide gel
electrophoresis (SDS-PAGE) with identical protein loading
quantities (6 μg of total protein per lane) from individual
HDL3, and the levels of expression of apolipoprotein
were analyzed via immunodetection. Anti-human apoC-III antibody
(AB821) was purchased from Chemicon (Temecula, CA, USA). Anti-human
CETP antibody (ab19012) and LCAT antibody (ab786) were purchased
from Abcam (Cambridge, UK). The relative band intensity (BI) was
compared via band scanning with Gel Doc® XR (Bio-Rad)
using the Quantity One software (version 4.5.2).
Data analysis
All data are expressed as the mean ± SD from at
least three independent experiments with duplicate samples. Data
comparisons were assessed by the Student’s t-test using the SPSS
program (version 14.0; SPSS, Inc., Chicago, IL, USA).
Results
Lipid and protein composition in
lipoprotein
The serum TC concentrations were similar between the
groups (204±57 and 200±32 mg/dl, respectively), which remained in
the normal range, as suggested by the guidelines of the National
Cholesterol Education Program (NCEP)-adult treatment panel
(ATP)-III. The LDL-C level was similar between the groups (105±38
and 108±33 mg/dl for the AP patients and controls, respectively).
However, the HDL-C level was slightly lower in the AP patients than
the controls. The ratio of HDL-C-to-TC was significantly lower in
the AP patients (34±2%) compared to the control group (40±4%). The
serum TG level was not significantly different between the groups
(136–175 mg/dl).
Properties of lipoprotein are good biomarkers which
reflect the progress of cardiovascular and renal disease. As shown
in Table I, the AP group had a
similar composition of lipid and protein in VLDL with the control
group. Although the TC and protein content in LDL was similar
between the groups, the TG content in LDL was significantly higher
in the AP group (38 mg of TG/mg of TP) compared to the control
group (30 mg of TG/mg of TP). In the HDL2 fraction, the
AP group had a much lower TC content and a higher TG content (44%
higher TC and 32% lower TG) than the control group. Immunodetection
revealed that the level of expression of apoC-III was elevated in
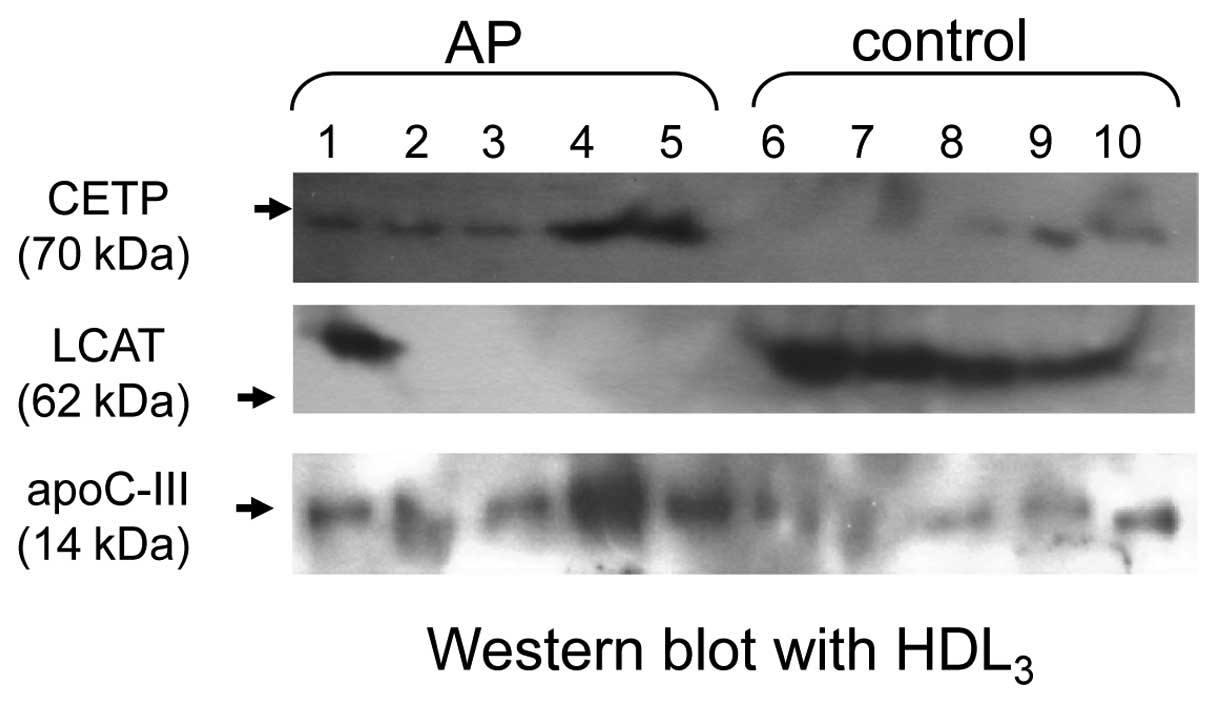
the HDL3 fraction of the AP group (Fig. 1).
 | Table ILipid and protein composition of
lipoproteins from patients. |
Table I
Lipid and protein composition of
lipoproteins from patients.
| Angina pectoris
(n=22) | Control (n=20) |
|---|
|
|
|
|---|
| TC (mg/dl) | TG (mg/dl) | TP (mg/ml) | TC (mg/dl) | TG (mg/dl) | TP (mg/dl) |
|---|
| VLDL | 120±64 | 203±140 | 2.8±0.1 | 130±31 | 243±46 | 2.8±0.1 |
| LDL | 1095±231 | 256±65a | 6.6±0.2 | 909±177 | 165±15 | 5.5±1.3 |
|
HDL2 | 46±14a | 82±24a | 2.0±0.1 | 75±14 | 52±28 | 1.8±0.1 |
|
HDL3 | 64±16 | 16±7 | 3.6±0.5 | 58±22 | 24±22 | 3.8±0.1 |
CETP and LCAT activity
As shown in Table
II, although the CE-transfer activity of the VLDL fraction was
similar between the groups (~2–3% CE-transfer), the LDL fraction of
the AP group had 2-fold increased CE-transfer activity. The CETP
activity of the HDL fraction also increased in the AP group (a 70
and 34% increase for HDL2 and HDL3,
respectively), compared to the control. Immunodetection revealed
that CETP was highly expressed in the HDL3 fraction of
the AP group (Fig. 1).
 | Table IILCAT and CETP activities in
lipoprotein fractions. |
Table II
LCAT and CETP activities in
lipoprotein fractions.
| Angina pectoris
(n=22) | Control (n=20) |
|---|
| CETP
activitya |
| VLDL | 2.6±0.6 | 3.1±0.1 |
| LDL | 5.5±0.3c | 2.0±1.0 |
|
HDL2 | 17±2.1c | 10±3.6 |
|
HDL3 | 35.4±6.6c | 26.8±2.1 |
| LCAT
activityb |
|
HDL2 | 1.2±0.7 | 2.0±1.5 |
|
HDL3 | 3.5±1.3c | 12.3±2.1 |
The LCAT activity was significantly lower in the
HDL3 fraction of the AP group, while no difference
existed in the HDL2 fraction between the groups. The
LCAT activity for CE-conversion from FC was lowered in the
HDL2 fraction in the AP and control groups, as shown in
Table II. The level of
expression of LCAT was nearly undetectable in the AP group (lane
1–5) except in one patient (Fig.
1).
Antioxidant activity of HDL2
and HDL3 was decreased in the AP group
The HDL3 from the AP group had weaker
antioxidant activity (172% increase from the initial level) than
the control group (198% increase) when the same amount of protein
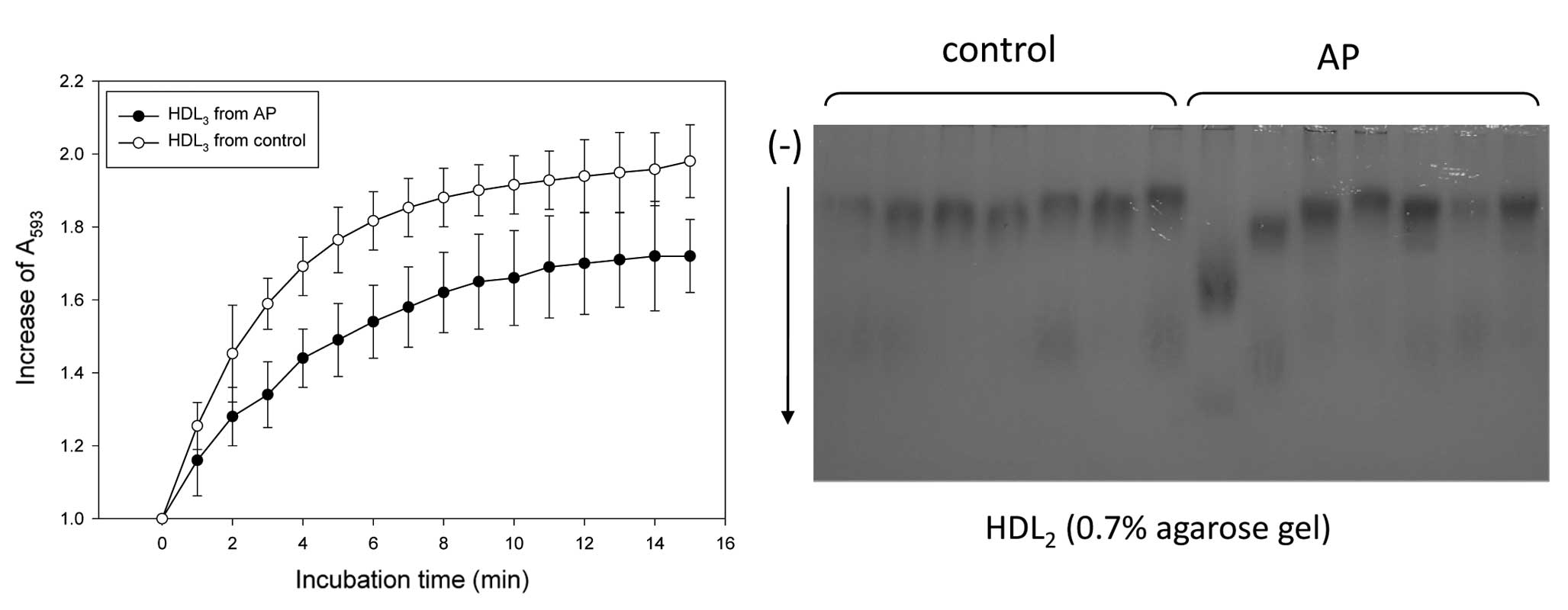
in HDL (1.5 mg/ml) was used as an antioxidant source (Fig. 2A). The extent of oxidation in the
native state was compared by relative electrophoretic mobility on
0.7% agarose gel electrophoresis. The HDL2 from the AP
group migrated faster than the control group without cupric ion
treatment, indicating that HDL2 of the AP group was more
oxidized in the native state (Fig.
2B). More highly oxidized HDL has a faster mobility due to a
smaller particle size and an increase in charge. In particular,
HDL2 from the AP group was 2-fold more susceptible to
cupric ion-mediated oxidation, as shown in Fig. 3, indicating that the antioxidant
potential was significantly decreased in the AP group.
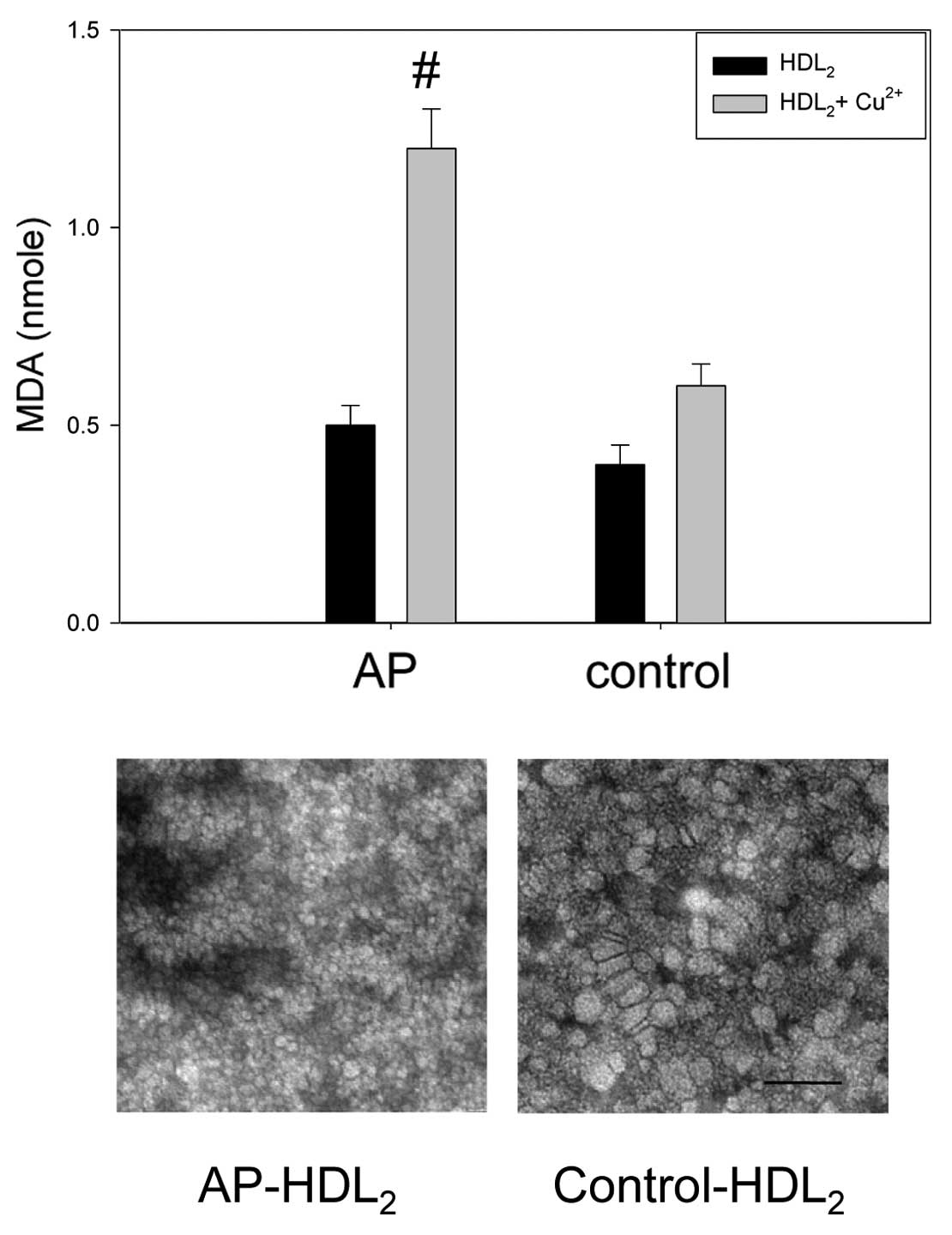
Specifically, electron microscopy revealed that HDL2
from the AP group had a smaller particle size than the control;
HDL2 from the AP group was 18–20 nm in width and length,
while HDL2 from the control group was 22–25 nm in width
and length. These results suggest that more highly oxidized HDL has
faster electromobility and reduced particle size.
HDL-associated paraoxonase and
PAF-AH
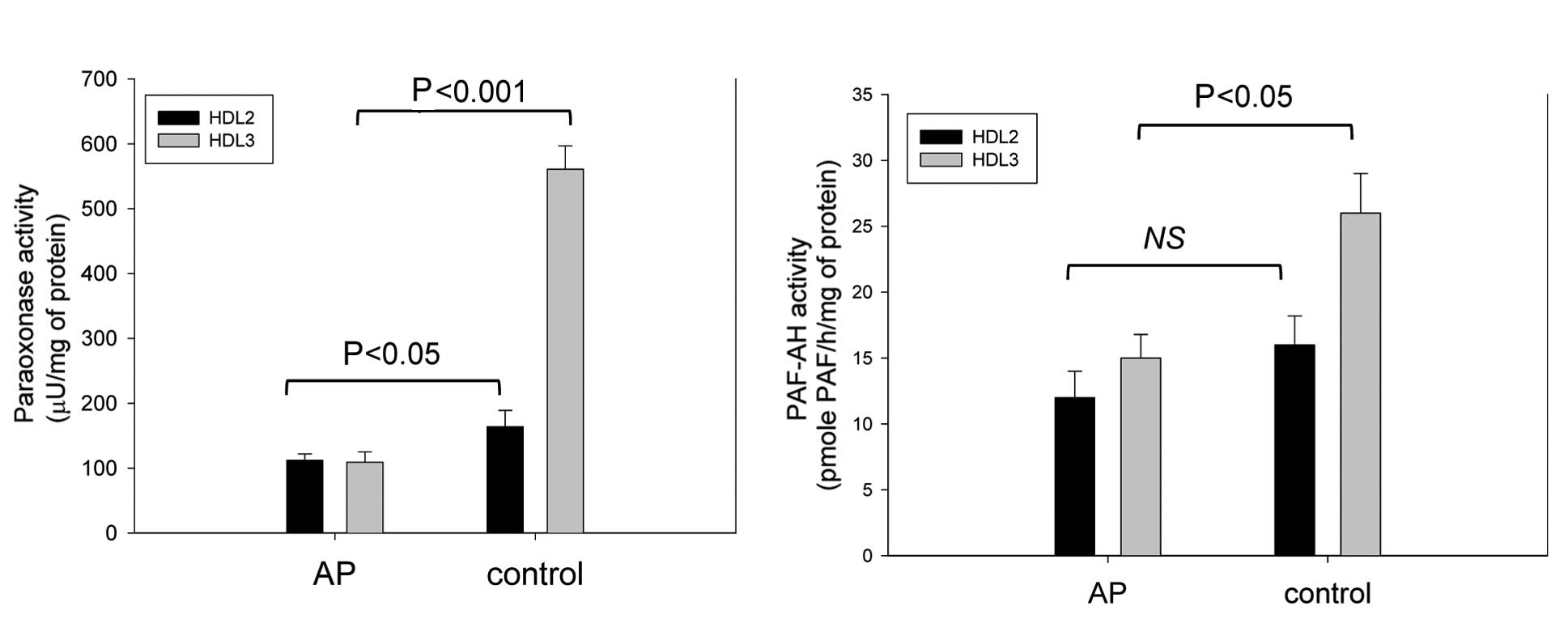
The HDL2-associated PON activity was
lower in the AP group than in the control group (112±10 vs. 164±25
μU/mg of protein) (Fig. 4A).
Moreover, the AP group had a 3-fold lower
HDL3-associated PON activity than the control group
(109±16 vs. 561±36 μU/mg of protein, respectively).
Although there was no significant difference in the
HDL2 fraction used as the PAF-AH source, the activity
was significantly lower in the AP group when the HDL3
fraction was used (Fig. 4).
HDL3 from the AP group showed 40% less activity than the
control group (15±2 and 26±3 pmole PAF/h/mg of protein for the AP
and control groups, respectively).
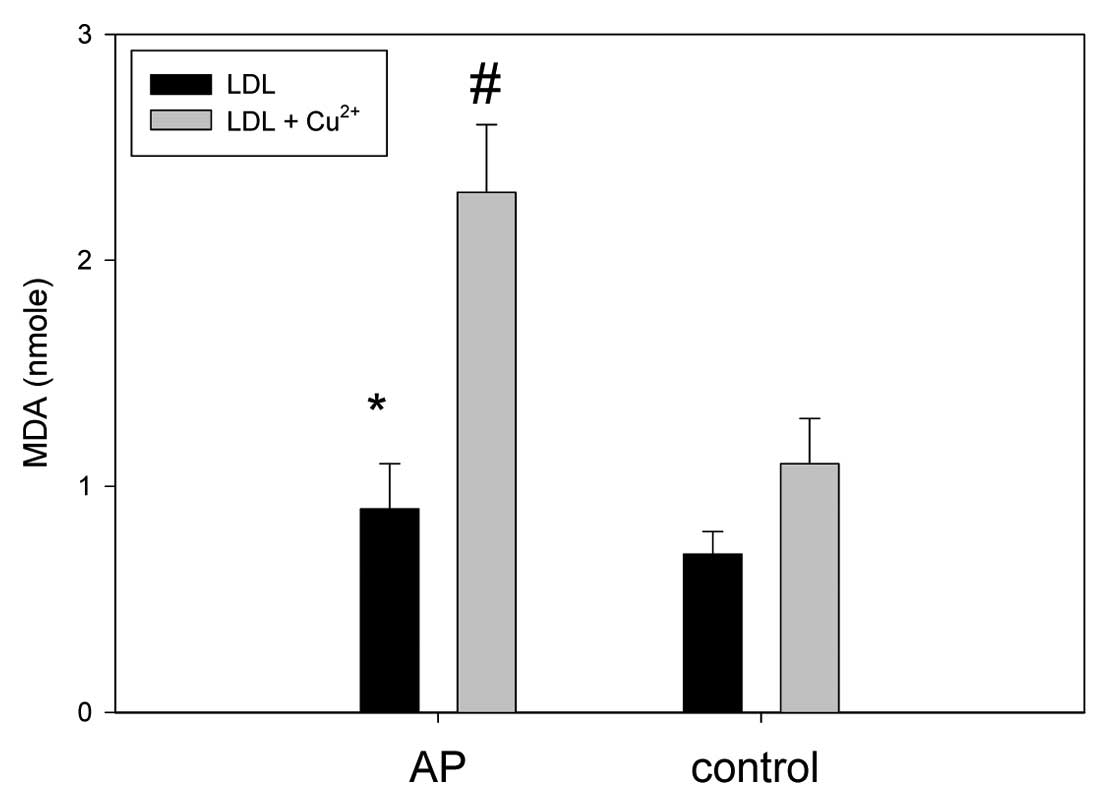
LDL from AP patient was more
oxidized
The LDL from the AP patient had ~1.8-fold higher
levels of MDA than the control without cupric ion treatment,
indicating a greater extent of oxidation of LDL in the AP group in
the native state (Fig. 5). Under
treatment with cupric ion (final 10 μM), the LDL from the AP and
control group showed 2.3 and 1.1 nmole of MDA, respectively,
suggesting that the AP group LDL was more sensitive to cupric-ion
mediated oxidation.
Discussion
In addition to a change in serum lipid parameters,
lipid and protein compositions in lipoproteins have emerged as a
parameter which is associated with the progress of metabolic
diseases, such as metabolic syndrome (13,14) and CHD (15). In fact, structural and functional
changes in HDL are more dramatic in the acute phase, such as viral
infections (25) and after
cardiac surgery (26).
Although the AP group had similar levels of TC and
LDL-C, the AP group had a lower ratio of HDL-C/TC. While the TC
content was lower in HDL2 from the AP group, the TG
content was significantly elevated. An increase in TG in the serum
is a potent inflammatory factor and is associated with the
incidence of CAD (27).
Accumulation of serum TG in HDL has been correlated with the
incidence of cardiovascular disease (4,10).
TG-enriched lipoprotein is more inflammatory in vascular events
(28). An elevated TG/HDL-C ratio
is associated with increased insulin resistance and cardiovascular
events (29). The current report
suggests that the serum TG was more highly accumulated in the LDL
and HDL2 fractions, rather than in the VLDL fraction,
which is similar to the results of a previous report involving a
male MI patient (10) that showed
a strong and consistent association of hypertriglyceridemia with
enriched LDL fraction. Recently, TG/HDL-C was shown to be a strong
independent predictor of mortality in women with an ischemia
syndrome (4). Several reports
have suggested that an increased TG level is associated with
elevation of apoC-III in lipoproteins; apoC-III in VLDL and LDL is
linked with CHD and senescence (11,30). In the current study, TG in
HDL2 and LDL, and CETP activity were elevated in AP
patients, suggesting that apoC-III in HDL is also a risk factor for
coronary events in female AP patients (Table I and Fig. 1).
It is known that serum CETP is an atherogenic
factor. CETP promotes the transfer of CE from HDL to VLDL and LDL
in exchange for TG, which moves in the opposite direction. The
exchange of CE and TG between lipoproteins is linked to elevated
levels of TG-enriched lipoprotein, which is pro-inflammatory and
pro-atherogenic (31). CETP is an
independent risk factor for CHD and metabolic syndrome (32). In addition, we recently reported
that the metabolic syndrome in male patients is characterized by a
38% higher serum cholesteryl ester transfer protein (CETP) activity
than the control group (10). The
increase in TG is also associated with elevated level of apoC-III
in the serum and lipoproteins in male MI patients (10). Furthermore, CETP activity is not
decreased when apoC-III-enriched HDL is used as a CETP source
(20). The current report showed
that the AP group had an elevated level of apoC-III in
HDL3.
With the alteration in the lipid content in HDL,
many reports have suggested that HDL particle size is associated
with cardiovascular events (9).
Zeller et al (33)
proposed that the smaller particle size of HDL is associated with
young age in patients with acute MI. In addition, Arsenault et
al (34) reported that a
decreased HDL particle size is associated with an adverse
cardiometabolic risk profile. They also proposed that a small HDL
particle size was associated with an increased CHD risk.
Interestingly, the HDL particle size was inversely related to CETP
activity, serum TG concentration, body mass index, and C-reactive
protein.
One of the beneficial virtues of HDL is exerting
antioxidant activity. The increase in oxidation susceptibility in
the AP group might be linked to alteration of lipid and protein
composition in HDL. In the AP group, HDL2-TC was ~40%
lower than the control, while HDL2-TG was elevated by
60%. Moreover, LCAT activity in HDL2 and HDL3
was 40 and 72% lower in the AP group, respectively, compared to the
control. Using immunodetection techniques, LCAT expression was
undetectable in the HDL3 fraction of the AP group with
the exception of one patient, while the LCAT band was detected in
the control (Fig. 1). The
decrease in LCAT activity and expression may contribute to the loss
of antioxidant activity and oxidation sensitivity.
In addition, human serum PON (EC 3.1.1.2) is an
HDL-associated calcium-dependent enzyme, and has strong antioxidant
activity. It catalyzes the hydrolysis of oxidized fatty acids from
phospholipids and prevents the accumulation of oxidized lipids in
lipoproteins, particularly LDL (23). PON activity and -SH levels have
been shown to be lower in CAD patients (35), which suggests that reduced PON
activity may contribute to the severity of CAD. PAF-AH (EC
3.1.1.47) is also involved in the antioxidant and anti-inflammatory
functions associated with the surfaces of HDL (36), and is a
Ca2+-independent enzyme belonging to group 7 of the
PLA2 family (37).
PAF-AH degrades oxidized phospholipids and platelet activating
factor, which is a pro-inflammatory factor. Thus, PAF-AH may
function as a profoundly anti-atherogenic enzyme. These three
enzymes were coincidentally lowered in the HDL fraction of the AP
group, which is in good agreement with decreased antioxidant
activity.
In conclusion, the current results strongly support
the interrelationship between CETP activity, the serum TG level and
its distribution, apoC-III expression, and that the change in HDL
particle size and antioxidant ability are intimately correlated,
especially in the onset of the female with AP.
Acknowledgements
This study was supported by the National Research
Foundation (NRF) through the Aging-associated Vascular Disease
Research Center at Yeungnam University [R13-2005-005-01003-0
(2010)]. The authors thank Jinwoo Hong, Wonil Choi, Jungwon Lee,
Jaemin Jeon, Youngseok Lee and Jinwook Bae at Chunma Honors School
of Yeungnam University for their helpful technical assistance.
References
|
1
|
W RosamondK FlegalK FurieA GoK GreenlundN
HaaseSM HailpernM HoV HowardB KisselaS KittnerD Lloyd-JonesM
McDermottJ MeigsC MoyG NicholC O’DonnellV RogerP SorlieJ
SteinbergerT ThomM WilsonY HongAmerican Heart Association
Statistics Committee and Stroke Statistics SubcommitteeHeart
disease and stroke statistics-2008 update: a report from the
American Heart Association Statistics Committee and Stroke
Statistics
SubcommitteeCirculation117e25e146200810.1161/CIRCULATIONAHA.107.18799818086926
|
|
2
|
C CannonE BraunwaldUnstable angina and
non-ST elevation myocardial infarction In: Harrison’s Principles of
Internal MedicineDL KasperE BraunwaldAS FauciSL HauserDL LongoJL
Jameson16th editionMcGraw-HillNew York144414482005
|
|
3
|
E JawadR AroraChronic stable angina
pectorisDis Mon54671689200810.1016/j.disamonth.2008.06.009
|
|
4
|
V BittnerBD JohnsonI ZinehWJ RogersD
VidoOC MarroquinCN Bairey-MerzG SopkoThe triglyceride/high-density
lipoprotein cholesterol ratio predicts all-cause mortality in women
with suspected myocardial ischemia: a report from the Women’s
Ischemia Syndrome Evaluation (WISE)Am Heart
J157548555200919249427
|
|
5
|
K ThygesenJS AlpertHD WhiteJoint
ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial
InfarctionAS JaffeFS AppleM GalvaniHA KatusLK NewbyJ RavkildeB
ChaitmanPM ClemmensenM DellborgH HodP PorelaR UnderwoodJJ BaxGA
BellerR BonowEE Van der WallJP BassandW WijnsTB FergusonPG StegBF
UretskyDO WilliamsPW ArmstrongEM AntmanKA FoxCW HammEM OhmanML
SimoonsPA Poole-WilsonEP GurfinkelJL Lopez-SendonP PaisS MendisJR
ZhuLC WallentinF Fernández-AvilésKM FoxAN ParkhomenkoSG PrioriM
TenderaLM Voipio-PulkkiA VahanianAJ CammR De CaterinaV DeanK
DicksteinG FilippatosC Funck-BrentanoI HellemansSD KristensenK
McGregorU SechtemS SilberM TenderaP WidimskyJL ZamoranoJ MoraisS
BrenerR HarringtonD MorrowM LimMA Martinez-RiosS SteinhublGN
LevineWB GiblerD GoffM TubaroD DudekN Al-AttarUniversal definition
of myocardial
infarctionCirculation11626342653200710.1161/CIRCULATIONAHA.107.18739717951284
|
|
6
|
G WalldiusI JungnerIs there a better
marker of cardiovascular risk than LDL cholesterol? Apolipoproteins
B and A-I-new risk factors and targets for therapyNutr Metab
Cardiovasc Dis17565571200710.1016/j.numecd.2007.02.01017631989
|
|
7
|
S TsimikasJT WillersonPM RidkerC-Reactive
protein and other emerging blood biomarkers to optimize risk
stratification of vulnerable patientsJ Am Coll
Cardiol47C19C31200610.1016/j.jacc.2005.10.06616631506
|
|
8
|
XS HuangSP ZhaoQ ZhangL BaiM HuElevated
plasma apolipoprotein AV in acute coronary syndrome is positively
correlated with triglyceride and C-reactive proteinChin Med J
(Engl)12214081412200919567162
|
|
9
|
J TashiroO MiyazakiY NakamuraA MiyazakiI
FukamachiH BujoY SaitoPlasma pre beta1-HDL level is elevated in
unstable angina
pectorisAtherosclerosis204595600200910.1016/j.atherosclerosis.2008.10.01519054517
|
|
10
|
KH ChoDG ShinSH BaekJR KimMyocardial
infarction patients showed altered lipoprotein properties and
functions when compared with stable angina pectoris patientsExp Mol
Med416776200910.3858/emm.2009.41.2.009
|
|
11
|
SJ LeeH CamposLA MoyeFM SacksLDL
containing apolipoprotein CIII is an independent risk factor for
coronary events in diabetic patientsArterioscler Thromb Vasc
Biol23853858200310.1161/01.ATV.0000066131.01313.EB12637336
|
|
12
|
M HartfordO WiklundL Mattsson HulténA
PerssonT KarlssonJ HerlitzK CaidahlC-reactive protein,
interleukin-6, secretory phospholipase A group IIA and
intercellular adhesion molecule-1 in the prediction of late outcome
events after acute coronary syndromesJ Intern
Med262526536200710.1111/j.1365-2796.2007.01862.x
|
|
13
|
KH ParkDG ShinJR KimKH ChoThe functional
and compositional properties of lipoproteins are altered in
patients with metabolic syndrome with increased cholesteryl ester
transfer protein activityInt J Mol Med251291362010
|
|
14
|
T McLaughlinF AbbasiK ChealJ ChuC
LamendolaG ReavenUse of metabolic markers to identify overweight
individuals who are insulin resistantAnn Intern
Med139802809200310.7326/0003-4819-139-10-200311180-0000714623617
|
|
15
|
JJ Genest JrSS Martin-MunleyJR McNamaraJM
OrdovasJ JennerRH MyersSR SilbermanPW WilsonDN SalemEJ
SchaeferFamilial lipoprotein disorders in patients with premature
coronary artery
diseaseCirculation8520252033199210.1161/01.CIR.85.6.20251534286
|
|
16
|
RJ HavelHA EderJH BragdonThe distribution
and chemical composition of ultracentrifugally separated
lipoproteins in human serumJ Clin
Invest3413451353195510.1172/JCI10318213252080
|
|
17
|
MA MarkwellSM HaasLL BieberNE TolbertA
modification of the Lowry procedure to simplify protein
determination in membrane and lipoprotein samplesAnal
Biochem87206210197810.1016/0003-2697(78)90586-998070
|
|
18
|
MS BloisAntioxidant determinations by the
use of a stable free
radicalNature18111991200195810.1038/1811199a0
|
|
19
|
IF BenzieJJ StrainThe ferric reducing
ability of plasma (FRAP) as a measure of antioxidant power: the
FRAP assayAnal Biochem2397076199610.1006/abio.1996.02928660627
|
|
20
|
KH ChoSynthesis of reconstituted
high-density lipoprotein (rHDL) containing apoA-I and apoC-III: the
functional role of apoC-III in rHDLMol
Cells27291297200910.1007/s10059-009-0037-819326075
|
|
21
|
JM HanTS JeongWS LeeI ChoiKH ChoStructural
and functional properties of V156K and A158E mutants of
apolipoprotein A-I in the lipid-free and lipid-bound statesJ Lipid
Res46589596200510.1194/jlr.M400468-JLR20015716588
|
|
22
|
KH ChoJY LeeMS ChoiJM ChoJS LimYB ParkA
peptide from hog plasma that inhibits human cholesteryl ester
transfer proteinBiochim Biophys
Acta1391133144199810.1016/S0005-2760(97)00197-59554982
|
|
23
|
HW EckersonCM WyteBN La DuThe human serum
paraoxonase/arylesterase polymorphismAm J Hum
Genet351126113819836316781
|
|
24
|
HF BoydSC FellST FlynnDM HickeyRJ IfeCA
LeachCH MacpheeKJ MillinerKE MooresIL PintoRA PorterDA RawlingsSA
SmithIG StansfieldDG TewCJ TheobaldCM WhittakerN-1 substituted
pyrimidin-4-ones: novel, orally active inhibitors of
lipoprotein-associated phospholipase A2Bioorg Med Chem
Lett1025572561200010.1016/S0960-894X(00)00510-211086729
|
|
25
|
KH ChoSH ParkJE ParkYO KimI ChoiJJ KimJR
KimThe function, composition, and particle size of high-density
lipoprotein were severely impaired in an oliguric phase of
hemorrhagic fever with renal syndromeClin
Biochem415664200810.1016/j.clinbiochem.2007.10.00717996200
|
|
26
|
A JahangiriMC de BeerV NoffsingerLR
TannockC RamaiahNR WebbDR van der WesthuyzenFC de BeerHDL
remodeling during the acute phase responseArterioscler Thromb Vasc
Biol29261267200910.1161/ATVBAHA.108.17868119008529
|
|
27
|
PE McBrideTriglycerides and risk for
coronary heart diseaseJ Am Med
Assoc298336338200710.1001/jama.298.3.33617635897
|
|
28
|
P LibbyFat fuels the flame
triglyceride-rich lipoproteins and arterial
inflammationCirculation100299301200710.1161/01.RES.0000259393.89870.5817307968
|
|
29
|
R OstfeldD MookherjeeM SpinelliD HoltzmanA
ShoyebM SchaeferS DoddamaniD SpevackY DuA triglyceride/high-density
lipoprotein ratio > or = 3.5 is associated with an increased
burden of coronary artery disease on cardiac catheterizationJ
Cardiometab Syndr113152006
|
|
30
|
KH ParkDG ShinJR KimKH
ChoSenescence-related truncation and multimerization of
apolipoprotein A-I in high-density lipoprotein with an elevated
level of advanced glycated end products and cholesteryl ester
transfer activityJ Gerontol A Biol Sci Med
Sci65600610201010.1093/gerona/glq034
|
|
31
|
MJ ChapmanWL GoffM GuerinA
KontushCholesteryl ester transfer protein: at the heart of the
action of lipid-modulating therapy with statins, fibrates, niacin,
and cholesteryl ester transfer protein inhibitorsEur Heart
J31149164201010.1093/eurheartj/ehp399
|
|
32
|
MJ ChapmanTherapeutic elevation of
HDL-cholesterol to prevent atherosclerosis and coronary heart
diseasePharmacol
Ther111893908200610.1016/j.pharmthera.2006.02.00316574234
|
|
33
|
M ZellerD MassonM FarnierL LorgisV
DeckertJP Pais de BarrosC DesrumauxP SicardJ GroberD BlacheP
GambertL RochetteY CottinL LagrostHigh serum cholesteryl ester
transfer rates and small high-density lipoproteins are associated
with young age in patients with acute myocardial infarctionJ Am
Coll Cardiol5019481955200710.1016/j.jacc.2007.06.05217996559
|
|
34
|
BJ ArsenaultI LemieuxJP DesprésP GagnonNJ
WarehamES StroesJJ KasteleinKT KhawSM BoekholdtHDL particle size
and the risk of coronary heart disease in apparently healthy men
and women: the EPIC-Norfolk prospective population
studyAtherosclerosis206276281200910.1016/j.atherosclerosis.2009.01.044
|
|
35
|
M GurM AslanA YildizR DemirbagR YilmazS
SelekO ErelI OzdogruParaoxonase and arylesterase activities in
coronary artery diseaseEur J Clin
Invest36779787200610.1111/j.1365-2362.2006.01727.x17032345
|
|
36
|
K KarasawaClinical aspects of plasma
platelet-activating factor-acetylhydrolaseBiochim Biophys
Acta176113591372200610.1016/j.bbalip.2006.06.01717049457
|
|
37
|
DA SixEA DennisThe expanding superfamily
of phospholipase A(2) enzymes: classification and
characterizationBiochim Biophys
Acta1488119200010.1016/S1388-1981(00)00105-011080672
|