Introduction
It is well known that ischemic preconditioning (IPC)
has adaptive cardioprotective effect (1). To date, this concept has been
extended to preconditioning induced by non-ischemic stress, such as
temperature (2), hypoxia
(3,4), anesthetic (5,6)
and reactive oxygen species (ROS) (7–9).
Recently, we have demonstrated that hydrogen
peroxide (H2O2) preconditioning protects PC12
cells against apoptosis induced by oxidative stress (10–13). This cytoprotection by
H2O2 preconditioning is associated with
blockade of the decrease in the expression of Bcl-2 and generation
of ROS (10), as well as
overexpression of inducible nitric oxide synhase (iNOS) and
cycloxygenase-2 (COX-2) (11),
activation of the Janus tyrosine kinases (JAK)-signal transducer
activator of transcription (STAT) pathway (12) and the transcription factor,
nuclear factor-κB (NF-κB) (13).
These findings suggest that the molecular mechanisms responsible
for H2O2 preconditioning-elicited adaptive
cytoprotection may be complex and related to multiple genes and
signaling pathways.
Inducible heme oxygenase-1 (HO-1), also known as
HSP32 (heat shock protein of 32 kDa), is a stress response protein,
which is response to multiple oxidative insults, such as heme, UV
light, heavy metal, glutathione depletion and
H2O2. This enzyme catalyzes the stepwise
degradation of heme to release free iron and equimolar
concentrations of carbon monoxide (CO) and the linear tetrapyrrol
biliverdin, which is converted to bilirubin by the enzyme
biliverdin reductase (14).
Increasing evidence has demonstrated the potent antioxidant
activity of the heme-derived metabolites produced by HO-1 catalysis
(biliverdin and bilirubin) and the cytoprotective effects of CO on
vascular endothelium and neuronal cells (14–17). In addition, the HO-1-deficient
mice exhibit a serious damage of iron metabolism, resulting in
liver and kidney oxidative insult and inflammation (18). Cells from mice with a target
deletion of HO-1 are much more sensitive to apoptosis induced by
serum deprivation, an effect that is significantly attenuated by
overexpression of HO-1 (19).
HO-1 induction in the brain also reduces stroke-related ischemic
injury and might contribute to the main neuroprotective effect of
statins (20). A recent study has
demonstrated that induction of HO-1 is involved in the
neuroprotection of chondroitin sulfate against oxidative stress
(21). Therefore, it is now
widely accepted that induction of HO-1 expression represents an
adaptive response that enhances cell resistance to noxious stimuli,
including oxidative stress. Interestingly, the previous studies
have shown that hyperbaric oxygen (HBO; i.e. exposure to pure
oxygen under high ambient pressure) pretreatment confers an
adaptive protection against H2O2-induced DNA
damage in blood cells (22). This
protection is associated with HO-1 induction (23). However, whether HO-1 is implicated
in the adaptive cytoprotective effect of H2O2
preconditioning in neuronal cells is unclear.
Recently, the role of phosphatidylinositol
3-kinase/Akt (PI3K/Akt) pathway in transcriptional regulation has
gained attention. PI3Ks and their downstream target Akt (also known
as protein kinase B) are a conserved family of signal transduction
enzymes which play important roles in suppressing apoptosis and in
promoting cell growth and proliferation (21,24–26). Salinas et al (27) reported that the PI3K/Akt pathway
participates in nerve growth factor (NGF)-elicited attenuation of
the intracellular ROS by regulating the expression of HO-1. In
addition, in human neuroblastoma SH-SY5Y cells subjected to
oxidative stress, such as H2O2,
PI3K/Akt-mediated induction of HO-1 contributes to the
neuroprotective effect of chondroitin sulfate, an endogenous
perineuronal net glycosamino glycan (21). The participation of the survival
pathway PI3K/Akt in the regulation of HO-1 has also described in
other cellular context, including the response to endotoxin
(28), arsenite (29) and carnosol (30).
In the present study, we analyzed the following
questions: i) effects of H2O2 preconditioning
on the expression of HO-1 and Akt; ii) roles of HO-1 and PI3K/Akt
pathway in the protective effects of H2O2
preconditioning against oxidative stress injury; iii) regulatory
effect of PI3k/Akt on the induction of HO-1 by
H2O2 preconditioning. The findings of this
study provide new evidence that H2O2
preconditioning protects PC12 cells against oxidative stress injury
by inducing HO-1 via the PI3K/Akt signaling pathway.
Materials and methods
Materials
3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyltetrazolium bromide
(MTT), propidium iodide (PI), RNase,
2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA), rhodamine 123
(Rh123) and zinc protoporphyrin IX (ZnPP) were purchased from
Sigma-Aldrich (St. Louis, MO, USA). RPMI-1640 medium, horse serum
and fetal bovine serum (FBS) were supplied by Gibco-BRL (Calsbad,
CA, USA). HO-1 antibody was purchased from StressGen Biotech
(Victoria, BC, Canada). Total (t)-Akt and phosphorylated (p)-Akt
antibodies were from Cell Signaling Technology (Danvers, MA, USA).
Ly294002 was supplied by Calbiochem (Schwalbach, Germany).
Caspase-Glo 3/7 kit was purchased from Promega (Madison, WI,
USA).
Cell culture and preconditioning
protocols
The rat pheochromocytoma cell line, PC12 cell, was
obtained from the Sun Yat-sen University Experimental Animal Center
(Guangzhou, China). PC12 cells were grown in RPMI-1640 medium
supplemented with 5% heat-inactivated horse serum and 10% FBS at
37°C under an atmosphere of 5% CO2 and 95% air.
PC12 cells were preconditioned with 100 μM
H2O2 for 90 min, followed by 24 h recovery
and subsequent exposure to 300 μM H2O2
for 12 h. HO-1 inhibitor (ZnPP) at 15 μM or PI3K inhibitor
(Ly294002) at 25 μM was administered 20 min before
preconditioning with 100 μM H2O2.
Determination of cell viability
Cell viability was determined by the conventional
MTT reduction assay. The PC12 cells were plated at a density of
5×104 cells/well in 96-well plates. After the indicated
treatments, cells were co-incubated with MTT solution (a final
concentration of 0.5 mg/ml) for 4 h. The medium was removed and 150
μl dimethyl sulphoxide (DMSO) was added to each well. The
formazan dye crystal was solubilized for 15 min and absorbance was
measured at 570 nm with a microplate reader (Molecular Devices,
Sunnyvale, CA, USA). The mean optical density (OD) in the indicated
groups was used to calculate percentage of cell viability according
to the formula below: percentage of cell viability = OD treatment
group/OD control group x 100%. Experiments were preformed in
triplicate.
Flow cytometry analysis of apoptosis
After different treatments, PC12 cells were
harvested and washed twice with phosphate buffer solution (PBS) and
fixed with 70% ice-cold ethanol. After centrifugation, PC12 cells
were adjusted to a concentration of 1×106 cells/ml and
then 0.5 ml RNase (1 mg/ml in PBS) was added to a 0.5 ml cell
sample. After gentle mixing with 50 mg/l PI, mixed cells were
filtered and incubated in the dark at 4°C for 30 min before flow
cytometric analysis. The PI fluorescence of individual nuclei was
measured by a flow cytometer (Beckman-Coulter, Los Angeles, CA,
USA). In the DNA histogram, the amplitude of the sub-G1 DNA peak,
which is lower than the G1 DNA peak, represents the number of
apoptotic cells.
Assay for caspase-3/-7 activity
PC12 cells were plated in 96-well plates at a
density of 1×104 cells/well. After the indicated
treatments, caspases-3 and -7 activation were measured by
caspase-Glo 3/7 assay (Promega) according to the manufacture’s
instructions. The assay provides a proluminescent caspase-3/-7
substrate which can be cleaved to aminoluciferin. The released
aminoluciferin is a substrate which is consumed by luciferase,
generating a luminescent signal. The signal is proportional to
caspase-3/-7 activity. The experiment was performed at least three
times with similar outcomes.
Measurement of intracellular ROS
generation
Intracellular ROS levels were determined by
fluorescent DCF derived from cell-permeable DCFH-DA. After
treatment with indicated conditioned mediums, PC12 cells were
incubated with 10 μM DCFH-DA solution at 37°C for 30 min in
the dark. DCF fluorescence was measured over the entire field of
vision with a fluorescent microscope connected to an imaging system
(BX50-FLA; Olympus, Tokyo, Japan). Mean fluorescence intensity
(MFI) of DCF from 3 random fields was analyzed with ImageJ 1.41o
software (National Institutes of Health (NIH), Bethesda, MD,
USA).
Measurement of mitochondrial membrane
potential (ΔΨm)
ΔΨm was monitored by a fluorescent dye Rh123, a
cell-permeable cationic dye that preferentially enters into
mitochondria based on the highly negative ΔΨm. Depolarization of
ΔΨm results in the loss of Rh123 from the mitochondria and a
decrease in intracellular fluorescence. In the present study, Rh123
(100 mg/l) was added to cell cultures for 45 min at 37°C and
fluorescence was measured over the entire field of vision by using
a fluorescence microscope connected to an imaging system (BX50-FLA;
Olympus). MFI of Rh123 from 3 random fields was analyzed with
ImageJ 1.41o software and the MFI was taken as an index of the
level of ΔΨm.
Western blotting assay
At the end of the treatments, PC12 cells were
harvested and re-suspended in ice-cold cell lysis solution and the
homogenate was centrifuged at 10,000 × g for 15 min at 4°C. After
quantitated with the BCA protein assay kit (Kangchen Biotech,
Shanghai, China), proteins were separated by 12% SDS-PAGE. The
proteins in the gel were transferred into polyvinylidene difluoride
(PVDF) membrane. After blocking with 5% fat-free dry milk in TBS-T
for 1 h at room temperature, the membrane was incubated with the
primary antibodies specific to HO-1 (1:1,000 dilution), t-Akt
(1:1,000 dilution), p-Akt (1:1,000 dilution), or horseradish
peroxidase (HRP)-conjugated β-actin (1:5,000 dilution) with gentle
agitation at 37°C overnight followed by further incubation with
HRP-conjugated secondary antibodies (1:5,000 dilution; Wuhan Boster
Biological Technology, Ltd., Wuhan, China) for 1.5 h at room
temperature. The immunoreactive signals were visualized using an
enhanced chemiluminescence (ECL) detection system (Applygen
Technologies, Inc., Beijing, China). For quantifying the protein
expression, the X-ray films were scanned and analyzed with ImageJ
1.41o software.
Data analysis and statistics
All data were presented as the mean ± SD.
Differences between groups were analyzed by one-way analyses of
variance (ANOVA) with SPSS 13.0 (SPSS, Inc.). P<0.05 was
considered to indicate statistical significance.
Results
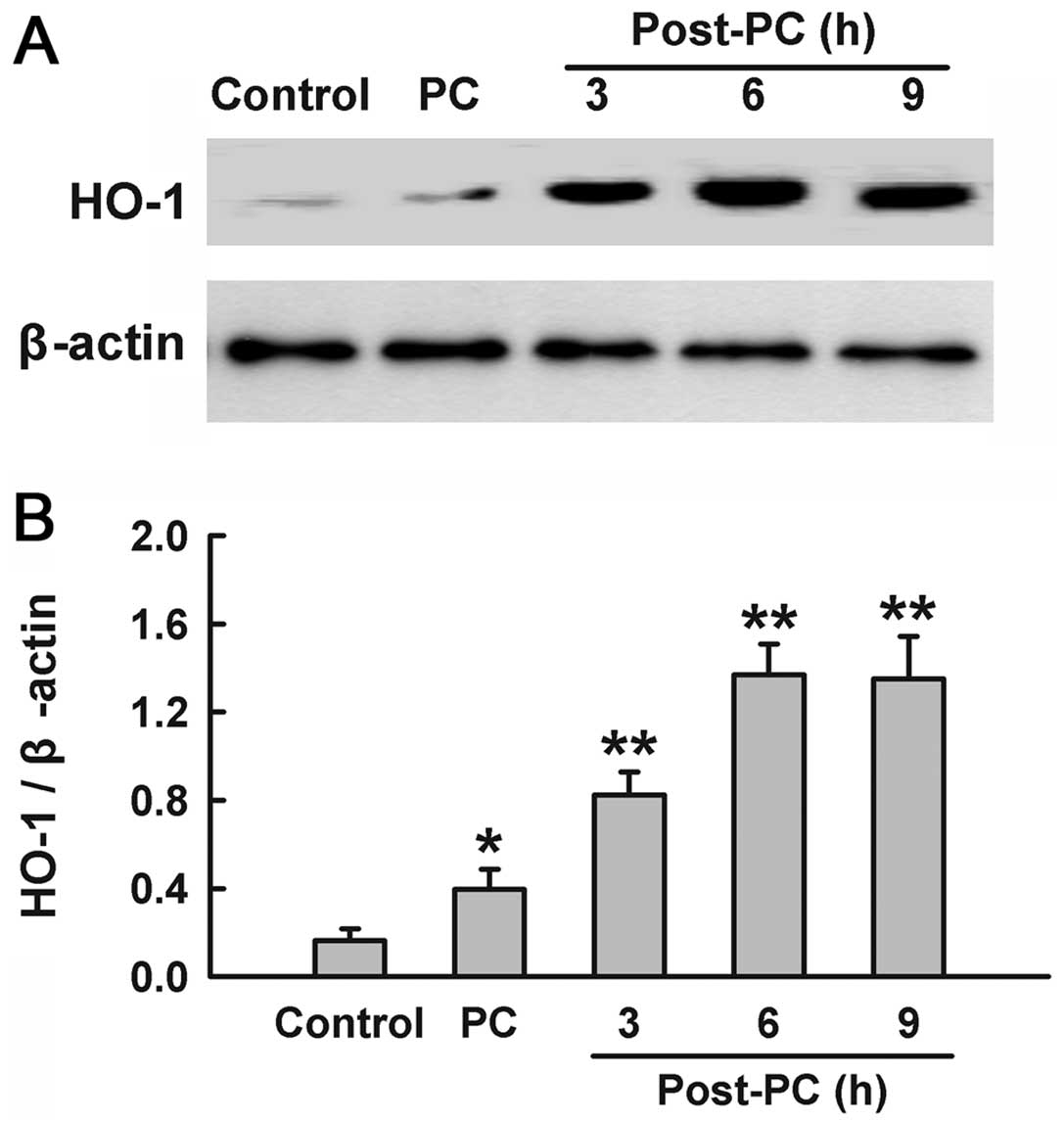
Preconditioning with
H2O2 upregulates expression of HO-1
To identify whether H2O2
preconditioning induces the expression of HO-1, PC12 cells were
treated with 100 μM H2O2 for 90 min,
and the samples were harvested at the indicated times (3, 6 and 9
h) after H2O2 preconditioning. The results of
western blotting analysis (Fig.
1) showed that treatment with H2O2
induced a significant increase in HO-1 expression compared with the
control group. Within 3–9 h after H2O2
preconditioning, there was a consistent increase in the expression
of HO-1, which peaked at 6 h.
HO-1 contributes to the cytoprotection of
H2O2 preconditioning against oxidative
stress-induced injury
To confirm whether HO-1 is involved in the adaptive
cytoprotection of H2O2 preconditioning, we
first examined the role of HO-1 in the protective effect of
H2O2 preconditioning against cytotoxicity
induced by H2O2. As shown in Fig. 2A, exposure of PC12 cells to
H2O2 at 300 μM for 12 h obviously
attenuated cell viability (P<0.01). Preconditioning with 100
μM H2O2 inhibited the 300 μM
H2O2-induced decrease in cell viability.
Preconditioning with 100 μM H2O2 for
90 min alone did not markedly alter the viability. Importantly,
this anti-cytotoxic effect of H2O2
preconditioning was blocked by treatment with 15 μM ZnPP for
20 min prior to preconditioning with H2O2,
indicating that HO-1 mediates the adaptive cytoprotection of
H2O2 preconditioning against cytotoxicity
induced by oxidative stress.
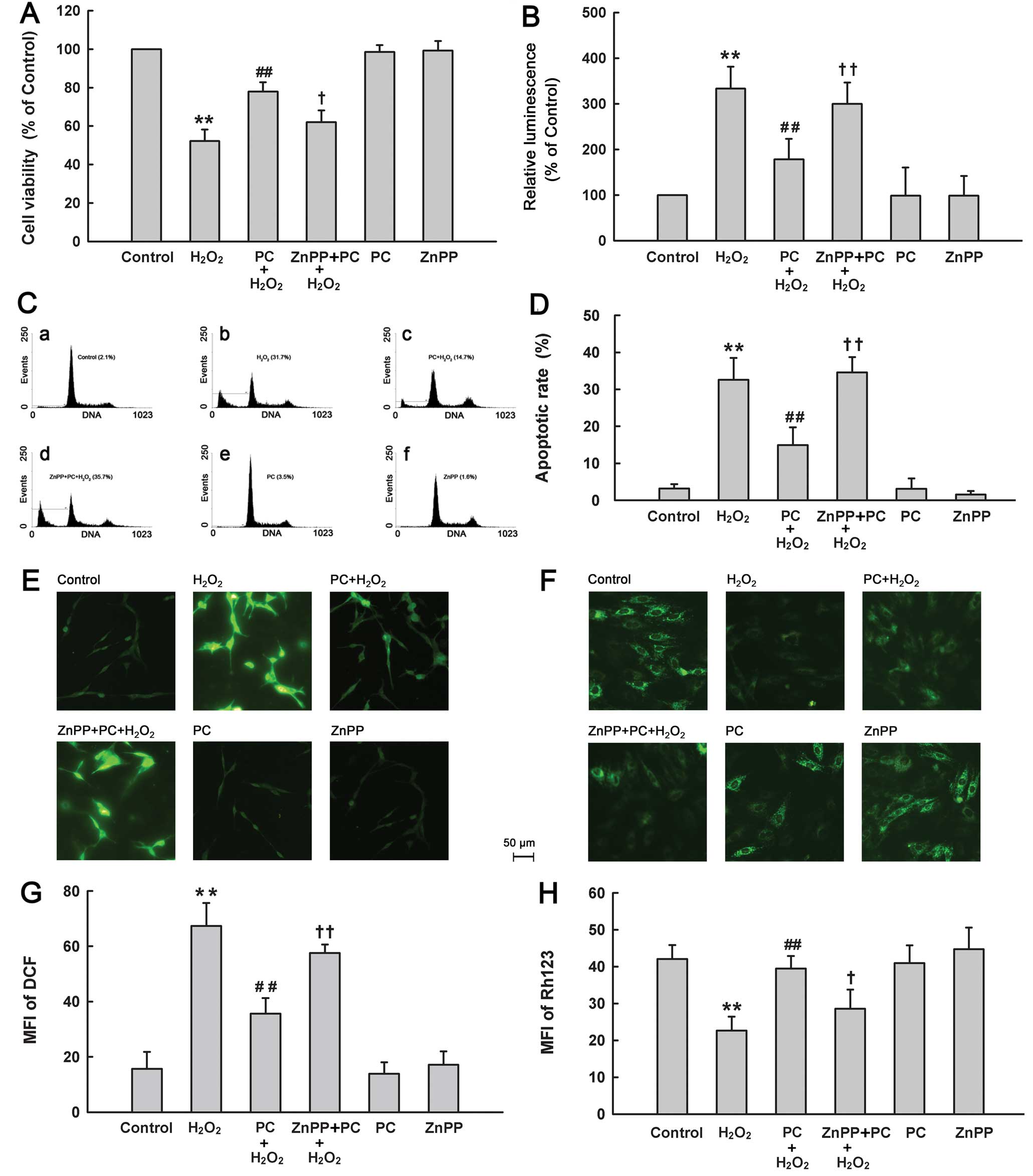 | Figure 2Effects of different treatments on
cell injury of PC12 cells. After the indicated treatments, (A) cell
viability, (B) activities of caspases-3/-7, (C and D) apoptosis, (E
and G) ROS generation and (F and H) ΔΨm were evaluated. Control
group, untreated PC12 cells. H2O2 group,
cells were treated with 300 μM H2O2
for 12 h. PC+H2O2 group, cells were
preconditioned with 100 μM H2O2 for 90
min before exposure to 300 μM H2O2 for
12 h. ZnPP+PC+H2O2 group, cells were treated
with ZnPP (15 μM) for 20 min before
H2O2 preconditioning, followed by exposure to
300 μM H2O2 for 12 h. PC group, PC12
cells were treated with 100 μM H2O2
for 90 min followed by a further 12 h culture. ZnPP group, PC12
cells were treated with 15 μM ZnPP for 20 min followed by a
further 12 h culture. Data were presented as mean ± SD, n=3.
**P<0.01 vs. control group; #P<0.05 vs.
H2O2 group; +P<0.05,
++P<0.01 vs. PC+H2O2 group. |
Secondarily, we detected the role of HO-1 in the
cytoprotection of H2O2 preconditioning from
H2O2-elicited apoptosis. Exposure to 300
μM H2O2 obviously elevated the
caspases-3/-7 activation in PC12 cells (Fig. 2B). The increased activities of
caspases-3 and -7 induced by H2O2 were
inhibited by 100 μM H2O2
preconditioning. However, ZnPP at 15 μM blocked the
protective effect of H2O2 preconditioning
against the H2O2-induced caspases-3/-7
activation. In addition, the results of flow cytometric analysis
(Fig. 2C and D) showed that
exposure of cells to 300 μM H2O2 for
12 h obviously enhanced the percentage of apoptotic cells
(P<0.01), which was reduced by preconditioning with
H2O2. Preconditioning with 100 μM
H2O2 alone had no significant effect on
apoptosis. Notably, treatment with 15 μM ZnPP for 20 min
before H2O2 preconditioning obviously
abrogated the anti-apoptotic effect of H2O2
preconditioning. These results suggest that HO-1 is implicated in
the anti-apoptotic effect of preconditioning with
H2O2.
Next, we also found involvement of HO-1 in
H2O2 preconditioning-induced antioxidative
stress and mitochondrial protection. As shown in Fig. 2E–H, preconditioning with 100
μM H2O2 considerably attenuated ROS
generation (Fig. 2E and G) and a
loss of ΔΨm (Fig. 2F and H)
induced by 300 μM H2O2. However, these
protective effects of H2O2 preconditioning
were reversed by treatment with 15 μM ZnPP prior to
H2O2 preconditioning. Alone, ZnPP did not
affect ROS generation or ΔΨm.
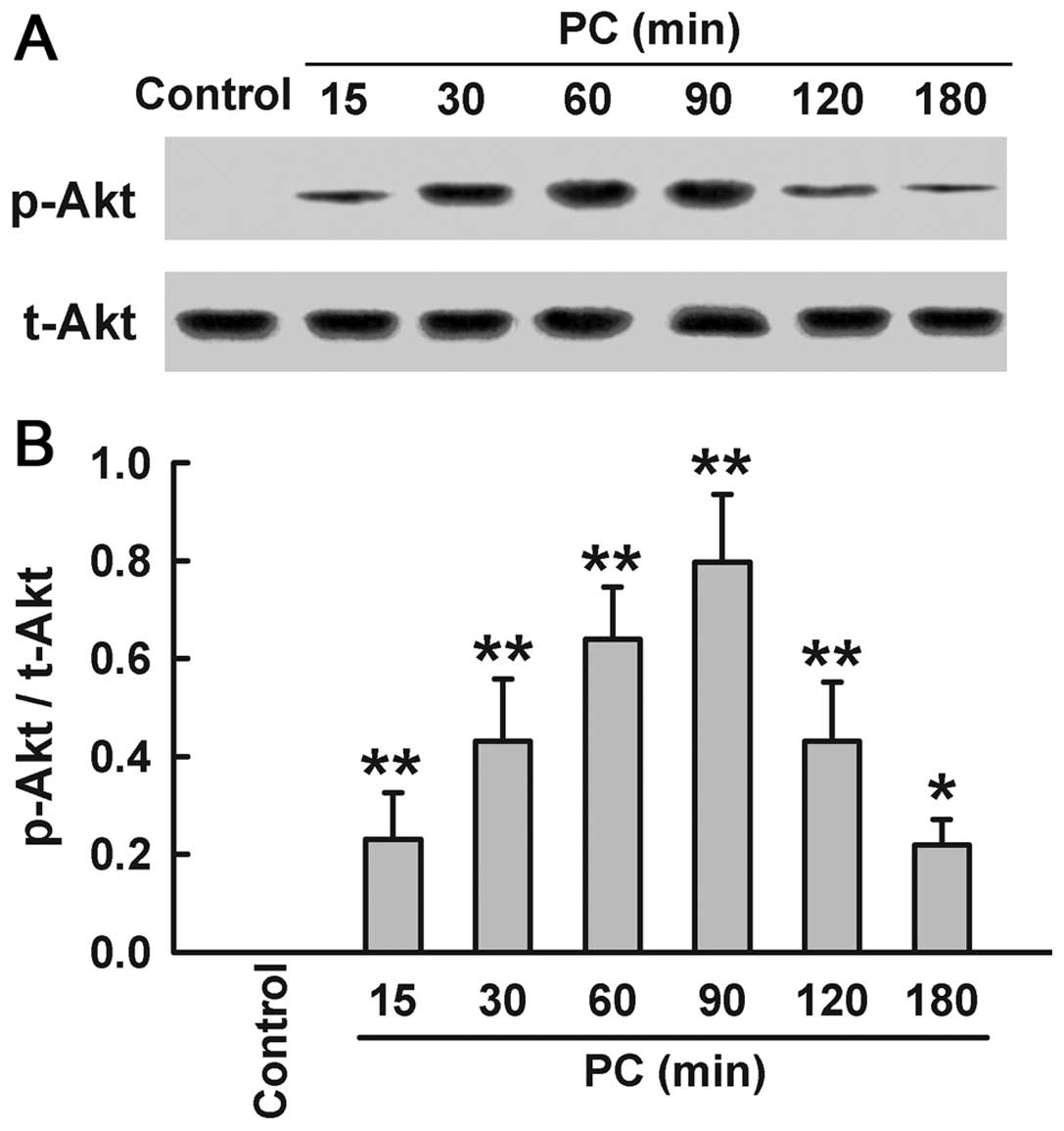
Preconditioning with
H2O2 enhances phosphorylation of Akt
Since Akt activation induces HO-1 expression, we
explored the effect of H2O2 preconditioning
on activation of Akt. Preconditioning with 100 μM
H2O2 upregulated the expression of p-Akt at
specific times (15, 30, 60, 90, 120 and 180 min after
H2O2 preconditioning), compared with the
control group (Fig. 3). Within
15–90 min after H2O2 preconditioning, the
expression of p-Akt increased in a time-dependent manner, peaking
at 90 min, and then gradually decreased at 120 and 180 min.
However, H2O2 preconditioning had no effect
on t-Akt expression.
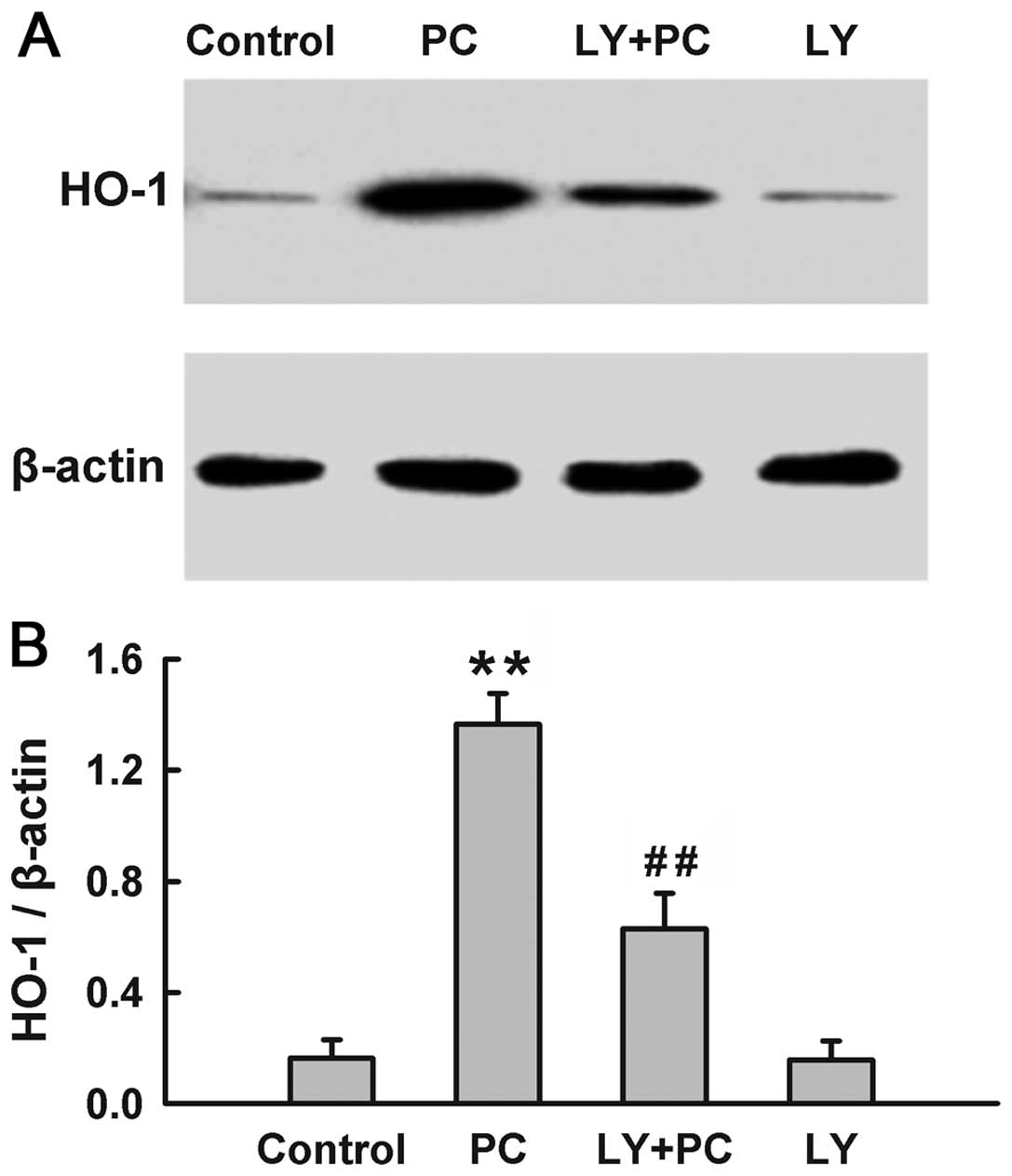
The PI3K/Akt pathway modulates the
induction of HO-1 induced by H2O2
preconditioning
Since both HO-1 and Akt were activated by
H2O2 preconditioning, we explored the
influence of PI3K/Akt pathway on the induction of HO-1 by
preconditioning with H2O2. The expression of
HO-1 was significantly upregulated by H2O2
preconditioning (Fig. 4). The
H2O2 preconditioning-induced overexpression
of HO-1 was blocked by treatment with Ly294002 (25 μM), a
selective inhibitor of PI3K/Akt, which was administered for 20 min
before H2O2 preconditioning. Alone, Ly294002
did not alter the basal expression of HO-1. These findings suggest
that the H2O2 preconditioning-induced
overexpression of HO-1 is dependent on the activation of the
PI3K/Akt pathway.
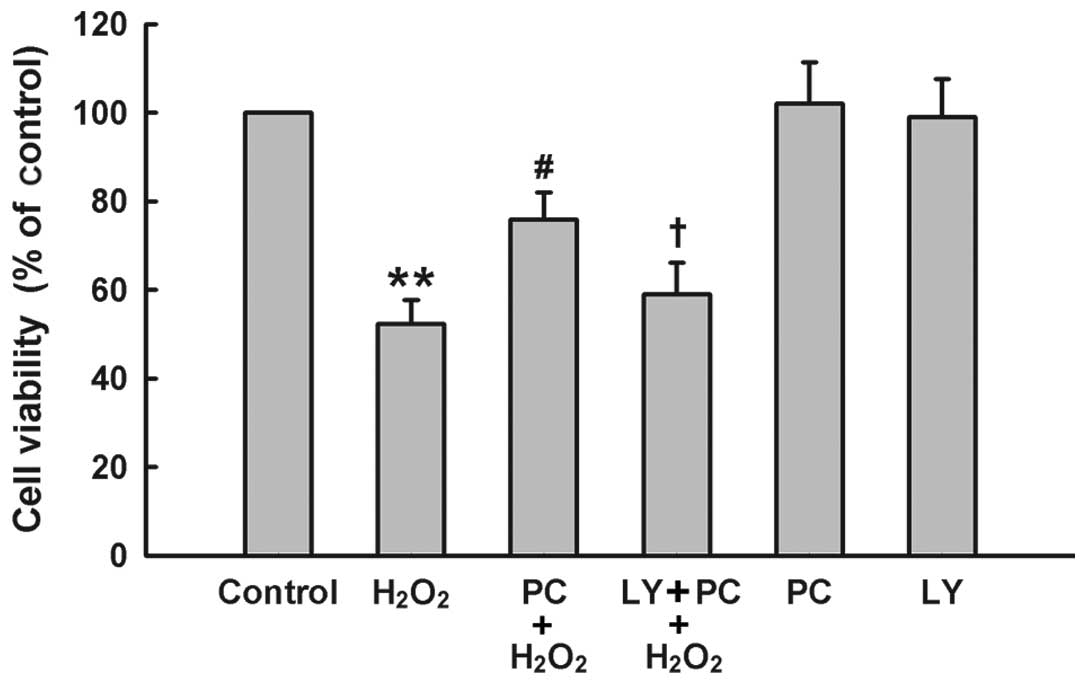
The PI3K/Akt pathway mediates the
cytoprotective effect of H2O2 preconditioning
against oxidative stress-induced cytotoxicity
To further demonstrate the role of PI3K/Akt pathway
in the cytoprotection of H2O2 preconditioning
against oxidative stress, PC12 cells were treated with Ly294002 (25
μM) for 20 min prior to H2O2
preconditioning. The results of Fig.
5 showed that H2O2 preconditioning
protected PC12 cells against H2O2-induced
cytotoxicity, evidenced by an increase in cell viability. Treatment
of cells with Ly294002 at 25 μM significantly blocked the
anti-cytotoxic effect of H2O2
preconditioning. Ly294002 alone had no effect on cell viability in
PC12 cells. These findings indicate that the PI3K/Akt pathway
participates in the protection of H2O2
preconditioning against H2O2-induced
cytotoxicity in PC12 cells.
Discussion
Based on our previous studies (10–13), this study further demonstrates
that PC12 cells have intrinsic mechanisms that respond to a brief
exposure to oxidative stress by enhancing cellular resistance to
the induction of oxidative injuries by subsequent sustained
oxidative exposure. Here, we provide new evidence for a key
mechanism that the PI3K/Akt-HO-1 pathway plays a critical role in
the adaptive cytoprotective effect of oxidative
(H2O2) preconditioning against oxidative
stress injuries in PC12 cells. This is strongly supported by the
findings that i) H2O2 preconditioning
enhanced the expression of HO-1; ii) inhibition of HO-1 by ZnPP
blocked the cytoprotection of H2O2
preconditioning against oxidative injuries, evidenced by the
decreases in cell viability and ΔΨm, and increases in apoptotic
cells, ROS generation as well as caspases-3 and -7 activities; iii)
the expression of p-Akt was upregulated by
H2O2 preconditioning; iv) Ly294002, a
selective inhibitor of PI3K, attenuated H2O2
preconditioning-induced overexpression of HO-1, indicating the
regulatory effect of the PI3K/Akt pathway on the expression of
HO-1; v) Ly294002 blocked the protective effect of
H2O2 preconditioning against oxidative
stress-elicited cytotoxicity, suggesting the involvement of the
PI3K/Akt pathway in the adaptive cytoprotection of preconditioning
with H2O2.
HO is the rate-limiting enzyme of microsomal heme
degradation. Three isoforms of HO, HO-1, HO-2 and HO-3, have been
characterized. It has been shown that both HO-2 and HO-3 are
constitutively expressed whereas HO-1 is an inducible isoform with
low basal expression (14). HO-2
functions as a physiologic regulator of cellular function and HO-3
appears to have only low enzyme activity, whereas HO-1 plays a
critical role in modulating tissue responses to injury in
pathophysiologic states (21,27,31). HO-1 is induced by a variety of
cell- and species-dependent stress factors including oxidative
stress (27,31,32). Increasing evidence reveals that
HO-1 has antioxidant (14,21,27,33),
anti-apoptotic (19,32), and cyto-protective effects,
including neuroprotection (14,20,21,33). Therefore, the role of HO-1 in
adaptive cytoprotection has been investigated.
In human proximal tubular (HK-2) cells, HO-1 is
involved in the protective effect of oxidant preconditioning
against lethal oxidant injury (8). In human lymphocytes, HO-1 mediates
the adaptive cytoprotection of HBO preconditioning (22). In addition, cardiac ischemic
preconditioning fails to occur in HO-1 knockout mice, suggesting an
important role of HO-1 in mediating tissue protection by ischemic
preconditioning. HO-1 also contributes to the cardioprotection of
H2O2 preconditioning from oxidative stress in
rat neonatal cardiomyocytes (9).
However, whether HO-1 is implicated in the neuroprotective effect
of H2O2 preconditioning against oxidative
stress injury remains unknown. In the present study, we found that
preconditioning with H2O2 upregulated the
expression of HO-1 in PC12 cells. Inhibition of HO-1 by ZnPP
significantly blocked the adaptive cytoprotection of
H2O2 preconditioning against oxidative stress
injuries, characterized by increases in cytotoxicity, apoptotic
cells, activities of caspases-3/-7, ROS generation and a loss of
MMP, suggesting that HO-1 contributes to the anti-cytotoxic,
anti-apoptotic and antioxidative effects as well as mitochondrial
improvement induced by H2O2 preconditioning.
Our findings are comparable with those previous studies (8,9,22).
This study and others (8,22) reveal that HO-1 may be an important
intrinsic mediator involved in preconditioning-induced adaptive
cytoprotection, in particular, oxidative preconditioning.
Accumulating evidence indicates that HO-1 is highly
inducible by agents causing oxidative stress, such as
H2O2 (14,22,32). HO-1 induction is often connected
with increased resistance to oxidant-mediated cell injury. Multiple
mechanisms are involved in the protection of HO-1 from
pathophysiological conditions. One of the key mechanisms may be
associated with its antioxidant effect. For example, bilirubin, one
of the main byproducts of the catabolism of heme by HO-1, acts as a
radical scavenger (32);
nanomolar amounts of bilirubin can reduce micromolar amounts of
H2O2 (34).
The increased formation of this anti-oxidant could therefore
explain the observed roles of HO-1 in the adaptive protection of
H2O2 preconditioning. Besides an increased
bililrubin production, both CO and ferritin (another product of
HO-1 enzyme activity) have also been shown to have an antioxidant
effect (32,35,36), which might also contribute to the
cytoprotection of H2O2 preconditioning.
Moreover, other antioxidant enzymes may be regulated by byproducts
of HO-1 activity, thus contributing to ROS detoxification. For
example, HO-1 activates the expression of mitochondrial superoxide
dismutase in neonatal rat astroglia challenged with dopamine
(37). Furthermore, it has been
demonstrated that upregulation of HO-1 improves mitochondrial
function and prevents ATP depletion after oxidative stress
(38). Noteworthily, some reports
have suggested a duality of effects of HO-1 overexpression in
oxidative stress (39,40). The release of ferric iron from the
porphyrin ring of heme may result in detrimental effects, because
this form of iron is known to catalyze oxidative stress (41).
Akt is a central node in cell signaling downstream
of growth factors, cytokines, and other cellular stimuli. Akt can
promote cell survival and protect against apoptosis initiated by
the mitochondrial pathway through phosphorylation and inhibition of
the mitochondrial pro-apototic proteins Bad, Bax and caspase-9
(42). Since HO-1 is induced by
H2O2 preconditioning, and has been identified
as a new substrate of Akt (43),
we explored the effect of preconditioning with
H2O2 on the activation of Akt. The results of
this study showed that preconditioning markedly enhanced the
expression of p-Akt, indicating that Akt is activated by
preconditioning with H2O2. These results are
consistent with previous evidence that Akt is rapidly activated in
response to strong oxidants, such as H2O2
(44,45) and that oxidative preconditioning
increases Akt activation in L-cells (7). In agreement with findings of
previous studies (9,43), we found that Ly294002, a selective
inhibitor of PI3K, blocked the induction of HO-1 by
H2O2 preconditioning, suggesting that the
PI3K/Akt pathway mediates the expression of HO-1. Similarly, recent
studies have shown the transcriptional regulation of HO-1 by the
PI3K/Akt pathway in response to nerve growth factor and to the
antioxidant polyphenol, carnosol (27,30). Importantly, our data showed that
treatment with Ly294002 also blocked the protective effects of
H2O2 preconditioning against cytotoxicity
induced by H2O2, which is comparable with the
findings reported by Han et al (7) and Angeloni et al (9). These results suggest that the
PI3K/Akt pathway is involved in the adaptive effect of
H2O2 preconditioning.
In conclusion, we have provided new evidence to
elucidate an important mechanism responsible for the adaptive
cytoprotective effect of H2O2 preconditioning
against oxidative stress-induced injuries, including cytotoxicity,
apoptosis and mitochondrial dysfunction in PC12 cells. We have
observed that activation of PI3K/Akt-HO-1 pathway is involved in
the protective effects of oxidative preconditioning. A better
understanding of the role of PI3K/Akt-HO-1 pathway in the adaptive
cytoprotection against oxidative stress may provide new therapeutic
approaches for oxidative stress-related diseases. The findings of
this study also support the notion that the lower levels of ROS
generated by physiological metabolism may continually precondition
cells and defend them against oxidative stress-induced insults
under both physiological and pathophysiological conditions.
Acknowledgements
This study was supported by the
Science and Technology Planning Project of the Guangdong province
in China (no. 2010B080701035).
References
|
1.
|
CE MurryRB JenningsKA
ReimerPreconditioning with ischemia: a delay of lethal cell injury
in ischemic
myocardiumCirculation7411241136198610.1161/01.CIR.74.5.1124
|
|
2.
|
I KhaliulinAP HalestrapMS
SuleimanTemperature preconditioning is optimal at 26°C and confers
additional protection to hypothermic cardioplegic ischemic
arrestExp Biol Med (Maywood)2367367452011
|
|
3.
|
HJ LinCT WangKC NiuHypobaric hypoxia
preconditioning attenuates acute lung injury during high-altitude
exposure in rats via up-regulating heat-shock protein 70Clin
Sci121223231201110.1042/CS2010059621599636
|
|
4.
|
D ShuklaS SaxenaP JayamurthyHypoxic
preconditioning with cobalt attenuates hypobaric hypoxia-induced
oxidative damage in rat lungsHigh Alt Med
Biol105769200910.1089/ham.2008.102819278353
|
|
5.
|
MJ de KlaverL ManningLA PalmerIsoflurane
pretreatment inhibits cytokine-induced cell death in cultured rat
smooth muscle cells and human endothelial
cellsAnesthesiology972432200212131100
|
|
6.
|
M ZauggE LucchinettiC GarciaAnaesthetics
and cardiac preconditioning. Part II. Clinical implicationsBr J
Anaesth91566576200310.1093/bja/aeg20614504160
|
|
7.
|
H HanH WangH LongOxidative preconditioning
and apoptosis in L-cells. Roles of protein kinase B and
mitogen-activated protein kinasesJ Biol
Chem2762635726364200110.1074/jbc.M01113620011331278
|
|
8.
|
HT LeeH XuA Ota-SetlikOxidant
preconditioning protects human proximal tubular cells against
lethal oxidant injury via p38 MAPK and heme oxygenase-1Am J
Nephrol23324333200310.1159/00007291412915776
|
|
9.
|
C AngeloniE MotoriD
FabbriH2O2 preconditioning modulates phase II
enzymes through p38 MAPK and PI3K/Akt activationAm J Physiol Heart
Circ Physiol300H2196H2205201121478407
|
|
10.
|
XQ TangJQ FengJ ChenProtection of
oxidative preconditioning against apoptosis induced by
H2O2 in PC12 cells: mechanisms via MMP, ROS,
and Bcl-2Brain
Res10575764200510.1016/j.brainres.2005.07.07216129420
|
|
11.
|
XQ TangHM YuJL ZhiInducible nitric oxide
synthase and cyclooxgenase-2 mediate protection of hydrogen
peroxide preconditioning against apoptosis induced by oxidative
stress in PC12 cellsLife
Sci79870876200610.1016/j.lfs.2006.03.010
|
|
12.
|
HM YuJL ZhiY CuiRole of the JAK-STAT
pathway in protection of hydrogen peroxide preconditioning against
apoptosis induced by oxidative stress in PC12
cellsApoptosis11931941200610.1007/s10495-006-6578-916547593
|
|
13.
|
M ZhangRX GuoLQ MoNuclear factor-kappaB
mediates cytoprotection of hydrogen peroxide preconditioning
against apoptosis induced by oxidative stress in PC12 cellsClin Exp
Pharmacol Physiol36304311200910.1111/j.1440-1681.2008.05066.x
|
|
14.
|
K ChenK GunterMD MainesNeurons
overexpressing heme oxygenase-1 resist oxidative stress-mediated
cell deathJ
Neurochem75304313200010.1046/j.1471-4159.2000.0750304.x10854275
|
|
15.
|
MD MainesBile pigments: newcomers to the
cell signaling arenaToxicol
Sci71910200310.1093/toxsci/71.1.912520070
|
|
16.
|
F MazzaA GoodmanG LombardoHeme oxygenase-1
gene expression attenuates angiotensin II-mediated DNA damage in
endothelial cellsExp Biol Med (Maywood)228576583200312709590
|
|
17.
|
J ChenY TuC MoonHeme oxygenase-1 and heme
oxygenase-2 have distinct roles in the proliferation and survival
of olfactory receptor neurons mediated by cGMP and bilirubin,
respectivelyJ
Neurochem8512471261200310.1046/j.1471-4159.2003.01776.x
|
|
18.
|
KD PossS TonegawaReduced stress defense in
heme oxygenase 1-deficient cellsProc Natl Acad Sci
USA941092510930199710.1073/pnas.94.20.109259380736
|
|
19.
|
CD FerrisSR JaffreyA SawaHaem oxygenase-1
prevents cell death by regulating cellular ironNat Cell
Biol1152157199910559901
|
|
20.
|
A KretzC SchmeerS TauschSimvastatin
promotes heat shock protein 27 expression and Akt activation in the
rat retina and protects axotomized retinal ganglion cells in
vivoNeurobiol Dis21421430200610.1016/j.nbd.2005.08.00316168661
|
|
21.
|
N CanasT ValeroM VillarroyaChondroitin
sulfate protects SH-SY5Y cells from oxidative stress by inducing
heme oxygenase-1 via phosphatidylinositol 3-kinase/AktJ Pharmacol
Exp Ther323946953200710.1124/jpet.107.12350517885094
|
|
22.
|
G SpeitC DennogU EichhornInduction of heme
oxygenase-1 and adaptive protection against the induction of DNA
damage after hyperbaric oxygen
treatmentCarcinogenesis2117951799200010.1093/carcin/21.10.179511023535
|
|
23.
|
A RothfussP RadermacherG SpeitInvolvement
of heme oxygenase-1 (HO-1) in the adaptive protection of human
lymphocytes after hyperbaric oxygen (HBO)
treatmentCarcinogenesis2219791985200110.1093/carcin/22.12.197911751428
|
|
24.
|
T HaY HuL LiuTLR2 ligands induce
cardioprotection against ischaemia/reperfusion injury through a
PI3K/Akt-dependent mechanismCardiovasc
Res87694703201010.1093/cvr/cvq11620421349
|
|
25.
|
T HaF HuaX LiuLipopolysaccharide-induced
myocardial protection against ischaemia/reperfusion injury is
mediated through a PI3K/Akt-dependent mechanismCardiovasc
Res78546553200810.1093/cvr/cvn037
|
|
26.
|
MA ArrudaAG RossiMS de FreitasHeme
inhibits human neutrophil apoptosis: involvement of
phosphoinositide 3-kinase, MAPK, and NF-kappaBJ
Immunol17320232030200410.4049/jimmunol.173.3.202315265937
|
|
27.
|
M SalinasR DiazNG AbrahamNerve growth
factor protects against 6-hydroxydopamine-induced oxidative stress
by increasing expression of heme oxygenase-1 in a
phosphatidylinositol 3-kinase-dependent mannerJ Biol
Chem2781389813904200310.1074/jbc.M209164200
|
|
28.
|
SW ChungYH ChenMA PerrellaRole of Ets-2 in
the regulation of heme oxygenase-1 by endotoxinJ Biol
Chem28045784584200510.1074/jbc.M40912520015590657
|
|
29.
|
VN IvanovTK HeiCombined treatment with
EGFR inhibitors and arsenite upregulated apoptosis in human
EGFR-positive melanomas: a role of suppression of the PI3K-Akt
pathwayOncogene24616626200510.1038/sj.onc.120812515580309
|
|
30.
|
D MartinAI RojoM SalinasRegulation of heme
oxygenase-1 expression through the phosphatidylinositol
3-kinase/Akt pathway and the Nrf2 transcription factor in response
to the antioxidant phytochemical carnosolJ Biol
Chem27989198929200410.1074/jbc.M30966020014688281
|
|
31.
|
G KikuchiT YoshidaM NoguchiHeme oxygenase
and heme degradationBiochem Biophys Res
Commun338558567200510.1016/j.bbrc.2005.08.02016115609
|
|
32.
|
H ParfenovaS BasuroyS
BhattacharyaGlutamate induces oxidative stress and apoptosis in
cerebral vascular endothelial cells: contributions of HO-1 and HO-2
to cytoprotectionAm J Physiol Cell
Physiol290C1399C1410200610.1152/ajpcell.00386.200516371440
|
|
33.
|
OG RosslerI BauerHY ChungGlutamate-induced
cell death of immortalized murine hippocampal neurons:
neuroprotective activity of heme oxygenase-1, heat shock protein
70, and sodium seleniteNeurosci
Lett362253257200410.1016/j.neulet.2004.03.033
|
|
34.
|
S DoreM TakahashiCD FerrisBilirubin,
formed by activation of heme oxygenase-2, protects neurons against
oxidative stress injuryProc Natl Acad Sci
USA9624452450199910.1073/pnas.96.5.244510051662
|
|
35.
|
GF VileS Basu-ModakC WaltnerHeme oxygenase
1 mediates an adaptive response to oxidative stress in human skin
fibroblastsProc Natl Acad Sci
USA9126072610199410.1073/pnas.91.7.26078146161
|
|
36.
|
MP SoaresA UshevaS BrouardModulation of
endothelial cell apoptosis by heme oxygenase-1-derived carbon
monoxideAntioxid Redox
Signal4321329200210.1089/15230860275366637012006183
|
|
37.
|
D FrankelK MehindateHM SchipperRole of
heme oxygenase-1 in the regulation of manganese superoxide
dismutase gene expression in oxidatively-challenged astrogliaJ Cell
Physiol1858086200010.1002/1097-4652(200010)185:1%3C80::AID-JCP7%3E3.0.CO;2-W10942521
|
|
38.
|
L BornmanCM SteinmannGS GerickeIn vivo
heat shock protects rat myocardial mitochondriaBiochem Biophys Res
Commun246836840199810.1006/bbrc.1998.87179618299
|
|
39.
|
DM SuttnerPA DenneryReversal of HO-1
related cytoprotection with increased expression is due to reactive
ironFASEB J1318001809199910506583
|
|
40.
|
DM SuttnerK SridharCS LeeProtective
effects of transient HO-1 overexpression on susceptibility to
oxygen toxicity in lung cellsAm J
Physiol276L443L451199910070108
|
|
41.
|
JM GutteridgeDA RowleyB
HalliwellSuperoxide-dependent formation of hydroxyl radicals and
lipid peroxidation in the presence of iron salts. Detection of
‘catalytic’ iron and anti-oxidant activity in extracellular
fluidsBiochem J20660560919826293469
|
|
42.
|
BD ManningLC CantleyAkt/PKB signaling:
navigating
downstreamCell12912611274200710.1016/j.cell.2007.06.00917604717
|
|
43.
|
M SalinasJ WangM Rosa de SagarraProtein
kinase Akt/PKB phosphorylates heme oxygenase-1 in vitro and in
vivoFEBS Lett5789094200410.1016/j.febslet.2004.10.07715581622
|
|
44.
|
D MartinM SalinasN FujitaCeramide and
reactive oxygen species generated by H2O2
induce caspase-3-independent degradation of Akt/protein kinase BJ
Biol Chem2774294342952200210.1074/jbc.M20107020012213802
|
|
45.
|
X WangKD McCulloughTF FrankeEpidermal
growth factor receptor-dependent Akt activation by oxidative stress
enhances cell survivalJ Biol
Chem2751462414631200010.1074/jbc.275.19.1462410799549
|