Introduction
The incidence of incisional hernias after laparotomy
because of visceral surgical interventions is in the range of 5–20%
(1,2), frequently requiring surgical repair.
The National Health Statistics Center quotes approximately 200,000
incisional hernia repairs each year in the United States alone
(3).
A variety of mitogenic and/or angiogenic factors
such as VEGF, FGF or PDGF have been successfully tested for
experimental improvement of wound healing (4) and PDGF, -BB, bFGF and GM-CSF made it
into the clinics. Novel delivery systems aiming at a continuous
release of the factors or living cell therapy (4) as well as gene transfer strategies
may further improve the therapeutic gains (5).
Transforming growth factor-β (TGF-β) expression has
been demonstrated throughout wound healing and has been shown to
regulate many processes involved in tissue repair, including
production of ECM, proteases, protease inhibitors, migration,
chemotaxis and proliferation of macrophages, fibroblasts of the
granulation tissue, epithelial and capillary endothelial cells
(6). Copper plays a key role in
angiogenesis and in the synthesis and stabilization of
extracellular matrix skin proteins, which are critical processes of
skin formation (7).
However, there is also reasonable evidence that
easily available standard drugs or factors acting on or promoting
the collagen synthesis, such as ascorbic acid (AA) or stanozolol,
an anabolic steroid with fibrinolytic properties, are also
beneficial for wound healing. Antioxidants such as Emblica
officinalis were recently reported to improve wound healing
through upregulation of collagen and extracellular signal-related
kinases (8). Significant
reductions of plasma AA concentrations in the post-operative period
of patients are associated with an increase in post-operative
complications (9). Pre-treatment
with AA increased wound healing of excision wounds in mice exposed
to whole body γ irradiation (10).
This prompted us to compare the efficacy of
topically applied AA in comparison to stanozolol, TGF-β, copper
peptide and controls in midline laparotomy incisional wounds in a
diabetic mouse model. The laparotomy model was chosen because of
the high socio-economic impact of incisional hernias that
complicate 11% of all abdominal wall closures (2). Maximum tensile strength testing was
intended for measuring the functional outcome and histology and
morphometry to elucidate possible modes of action.
Materials and methods
Diabetes induction
Female BALB-C mice aged 4–5 months were injected
intraperitoneally (i.p.) on Days 0, 2, 4 and 7 with 150 mg/kg body
weight (bw) streptozotocin (STZ; Sigma-Aldrich Chemie GmbH,
Steinheim, Germany) solved in 0.9% NaCl. Additional injections were
administered if the blood glucose level was below 250 mg/dl.
Animals with a blood glucose level below 220 mg/dl received 150
mg/kg bw STZ, animals with blood glucose levels between 220–250
mg/dl received 120 mg/kg bw STZ until the desired blood glucose
level of 250 mg/dl was reached. All mice were kept on a light/dark
(12 h/12 h) cycle at 23°C and received food (standard lab chow) and
water ad libitum. The care of the animals was consistent
with the guidelines laid down by law. All experiments were
conducted with the approval of the local institutional review
board.
Laparotomy incision wound
Surgery was performed under aseptic conditions. Mice
were anesthetized intraperitoneally with 1 ml/100g body weight
Avertin (2,2,2-Tribromoethanol and Pentanol in PBS; Sigma-Aldrich,
München, Germany). The skin of the ventral abdominal wall was
shaved and wiped off with 70% isopropyl alcohol. After midline skin
incision over a length of 18 mm, the fascia was exposed and the
linea alba was incised over a length of 15 mm. The linea alba was
closed again with 3–4 single button sutures Prolene® 6-0
(Ethicon; Johnson & Johnson Medical GmbH, Norderstedt,
Germany). After preparation of a subcutaneous pouch for the drugs
solved in 0.2 ml hydrogel, the skin was closed with 5–6 single
button sutures.
Treatment groups and follow-up
Control animals (group 1) received hydrogel only,
consisting of 12.5% hydroxypropylcellulose in phosphate buffer (pH
7.4). Group 2 animals received hydrogel with 10 ng/ml TGF-β. Group
3 animals received hydrogel enriched with 100 μm copper
peptide/100 g, whereas group 4 animals received hydrogel added with
100 μm/100 g stanozolol. Group 5 animals received hydrogel
with 100 μm/100 ml AA. All gels were produced and portioned
in a standardized procedure (Itemp, Aachen, Germany). The groups
consisted of 12–14 animals each.
All animals survived the operation. Half of the
animals were sacrificed on Day 3 and the other half 14 days after
surgery. The skin sutures were removed after 6 days. After
euthanasia with an overdose of pentobarbital (Sigma-Aldrich Chemie
GmbH) the tissue was sampled for biomechanical and histologic
evaluation.
Tensile strength measurements
From all specimens obtained 14 days after surgery,
3-mm wide test strips were punched out from the central area of the
skin wound vertically to the craniocaudal axis. The test strips had
a defined hour barbell form with 3-mm width at the narrowest part
constituting a predetermined breaking point. The skin and subcutis
were dissected from the linea alba and tested separately. The
breaking strength test device consisted of 2 opposing gripping jaws
to fix the tissue strip (11).
The electric motor driven gripping jaws were moved apart with a
constant strain rate of 0.5 mm/s under displacement control. Time,
force and displacement were recorded during the stretching of the
tissue. Endpoint was the disrupture of the sample. A position
encoder (WA300) was used to register the stretching distance; a
force transducer (S2, maximum value 150 N) was used to quantify the
power impacting on the tissue strip. The resulting values were
processed by a multiple channel PC measuring device (Spider 8) and
plotted as way-power curve (software: Catman 4.5; all HBM Hottinger
Baldwin Messtechnik, Darmstadt, Germany). The maximum breaking
strength was determined from the stress-strain curve. Specimens
sampled on Day 3 after surgery were not considered for
biomechanical testing due to the early time point.
Histology and immunohistochemistry
Ten-micrometer thick slides of paraffin embedded
probes were stained with hematoxylin and eosin (H&E) according
to standard protocols. H&E sections were used to assess the
morphology of the scars and the thicknesses as measure for the
progress of remodeling. The thickness of the individual skin layers
were measured with the image analyzing software Diskus 4.80
(Hilgers Königswinter, Germany). The sections were quantified in a
blinded manner by an independent observer. Picrosirius red staining
was used to quantify the collagen content by means of polarization
microscopy. Collagen content was expressed as percentage of
red-stained pixels assessed with the image analyzing software KS300
(Kontron AG, Eching, Germany).
Immunohistochemical staining of endothelial cells
was performed using a monoclonal antibody against CD31 (BD
Biosciences Pharmingen, Heidelberg, Germany). Antibody binding was
visualized via a 3-step staining procedure using a biotinylated
polyclonal anti-rat Ig-G secondary antibody (DakoCytomation GmbH,
Hamburg, Germany) and the streptavidin horseradish peroxydase
reaction together with the DAB detection system. The vessel
densities were assessed using a Weibel grid and expressed as
percentual vessel surface area. The proliferation rate was assessed
using a Weibel grid to count anti-Ki67-positive cells in defined
areas.
Statistical analysis
The unpaired Student’s t-test for samples of unequal
variances was used to calculate statistical significance. The data
were expressed as the mean ± SD. The significance level for the
sample distribution was defined as p<0.05.
Results
Macroscopic appearance
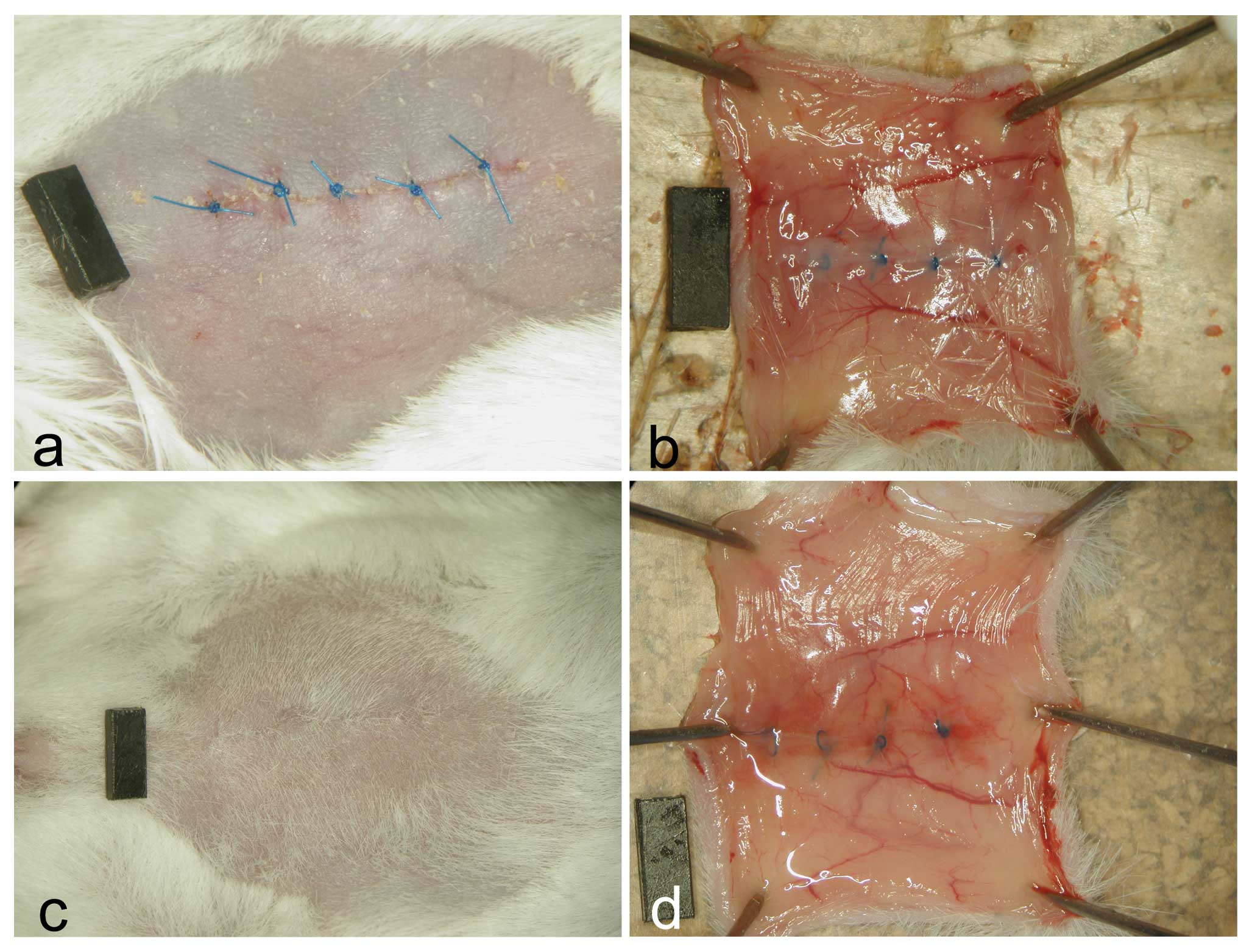
As shown in Fig.
1, the wounds of all animals were correctly adapted on Day 3
showing on the peritoneal side hyperemia and diffuse minor
hemorrhages that were evident also on Day 14. The main branches of
the inferior epigastric arteries appeared dilated. No group
differences were observed.
Tensile strength
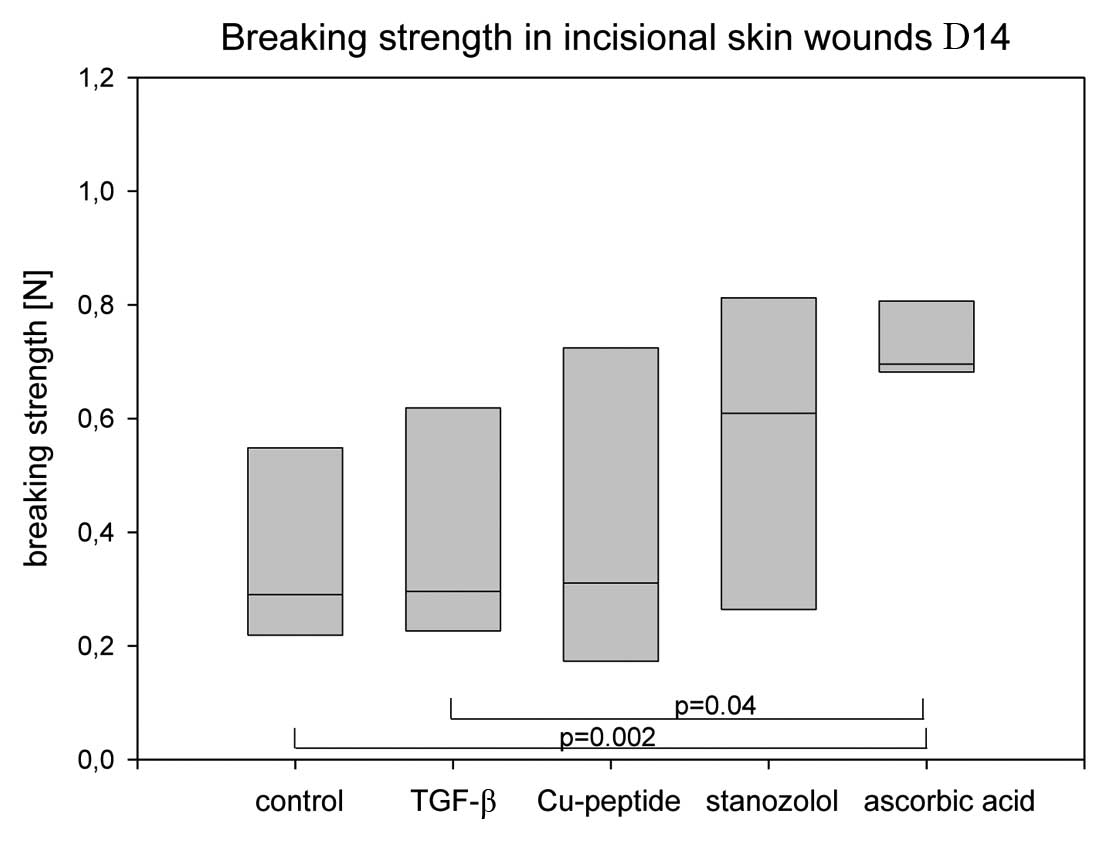
Fig. 2 shows the
lowest breaking strength in the controls with a mean of 0.38 N. The
highest maximum tensile strengths were found in the AA group with a
mean of 0.74 N. The stanozolol group showed a mean of 0.56 N. There
was a significant difference of the AA group in comparison to the
control group (p=0.002) and to the TGF-β group (p=0.04). The
variability of the values of the AA group was significantly less
than in all other groups. The other groups failed to reach the
level of significance as compared to the control group because of
the high dispersion coefficient.
The maximum tensile strengths of the linea alba
itself did not show significant differences between the individual
groups (data not shown). Histology, however, revealed that this
result was mainly caused by the small width of the linea alba
(150–300 μm) which resulted in test specimens that consisted
mainly of musculature. In all cases the breaking did not occur
within the scar, but paramedially in the musculature. Thus, a
reliable measurement of the impact of the agents on the healing of
the linea alba scar itself was not possible.
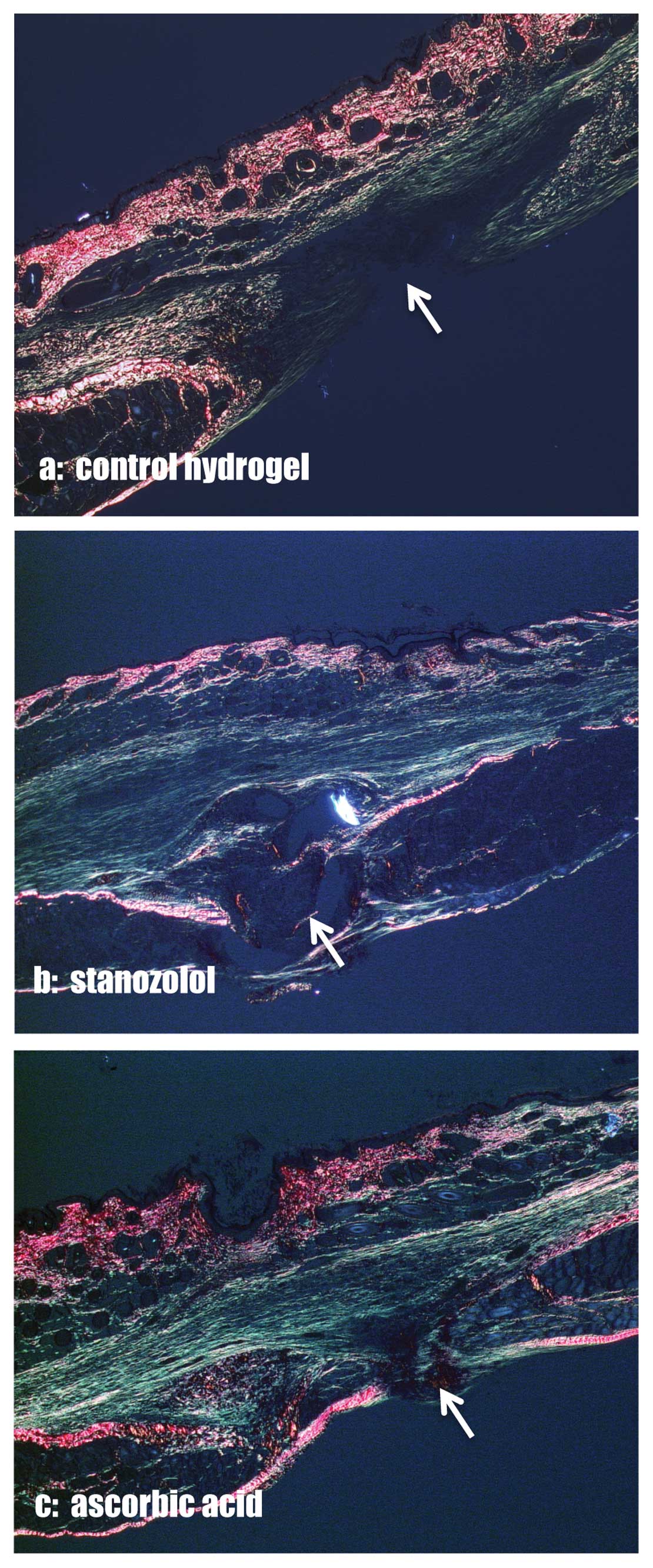
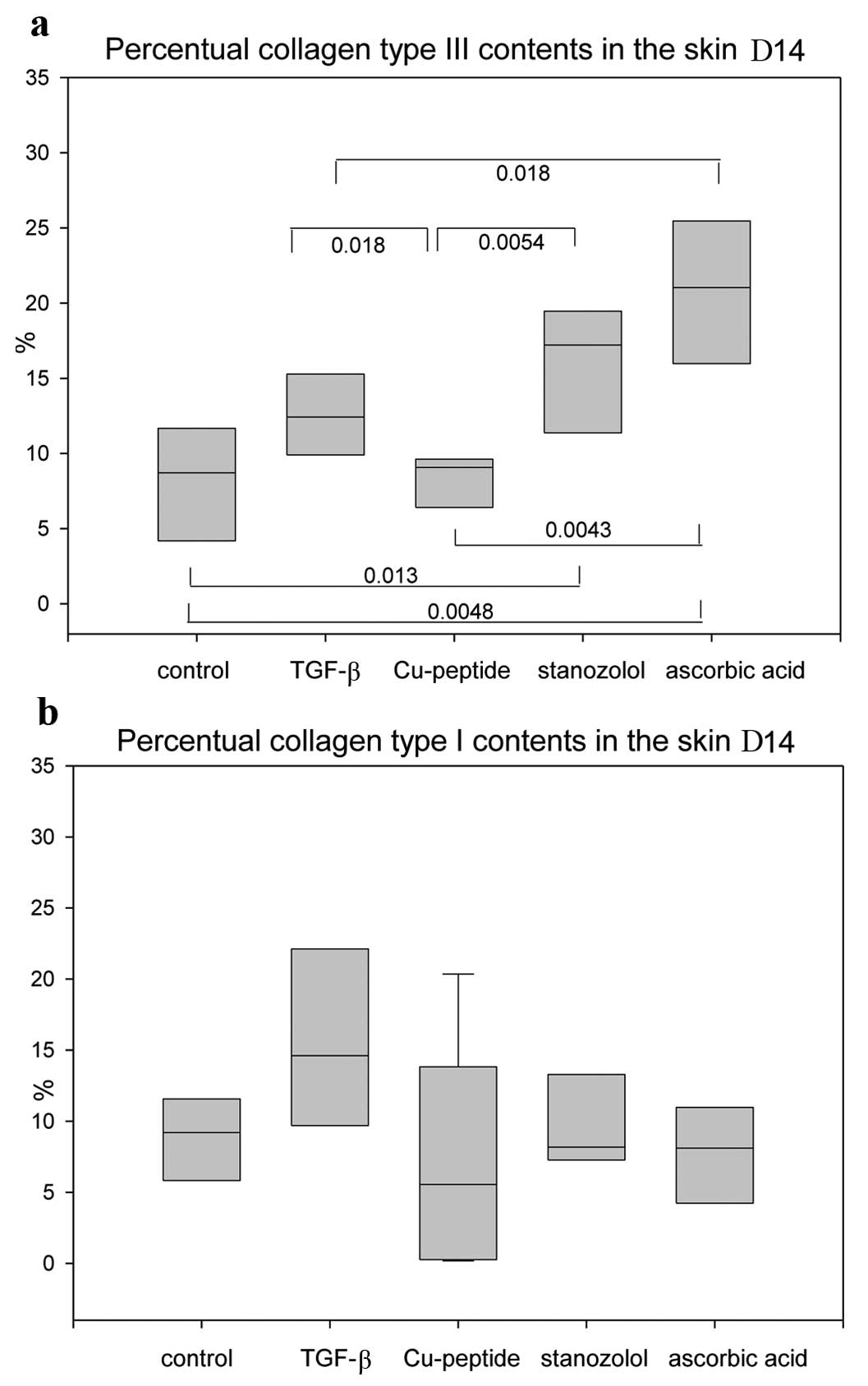
Collagen contents
The percentage of collagen type I and III in the
area of interest was assessed in picrosirius red stained specimens
(Fig. 3). Type III collagen was
significantly increased in the skin of the stanozolol and AA groups
compared to the control group on Day 14 (Fig. 4a). The collagen type I contents,
however, did not vary between the groups (Fig. 4b). In the linea alba itself no
differences in the collagen type I/III ratios were observed.
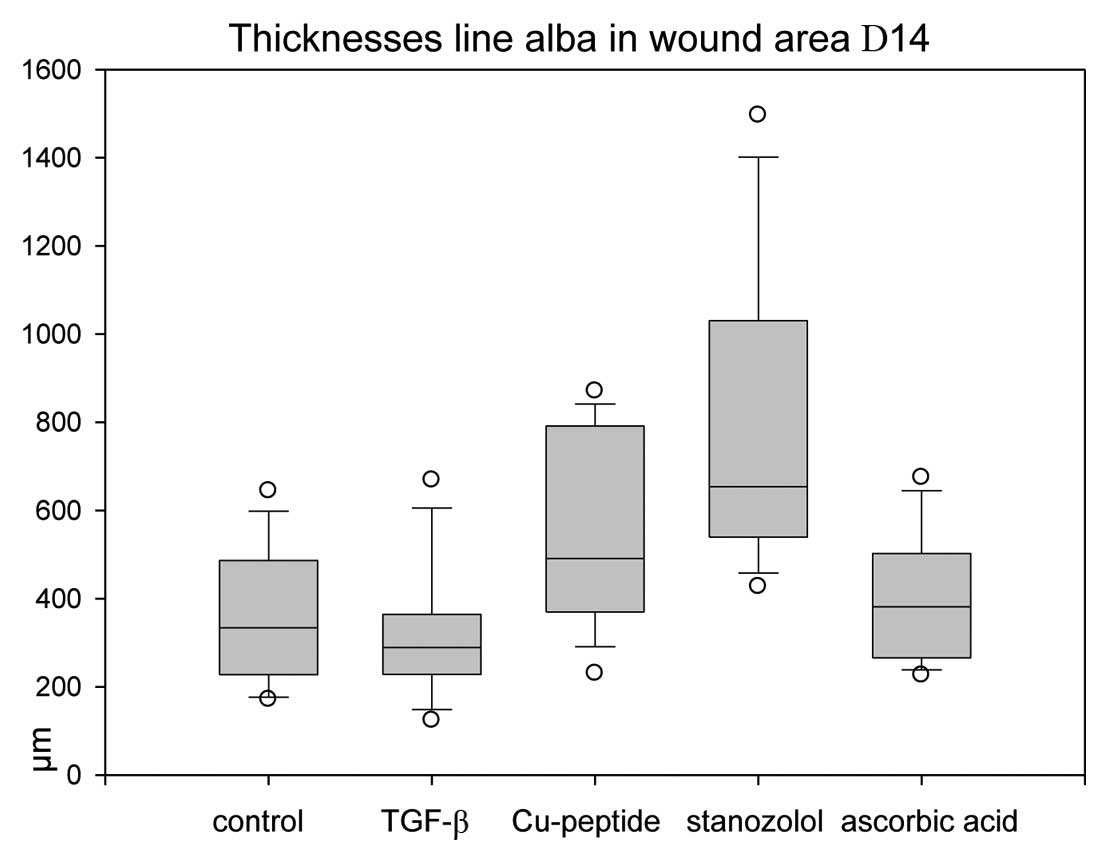
Thicknesses of the scar tissue
Measurements of the scar dimensions in the midline
show that significantly higher values were observed in copper
peptide and stanozolol animals than in the other groups (Fig. 5). Despite the higher values for
collagen type III, AA-treated wounds did not result in higher scar
thicknesses.
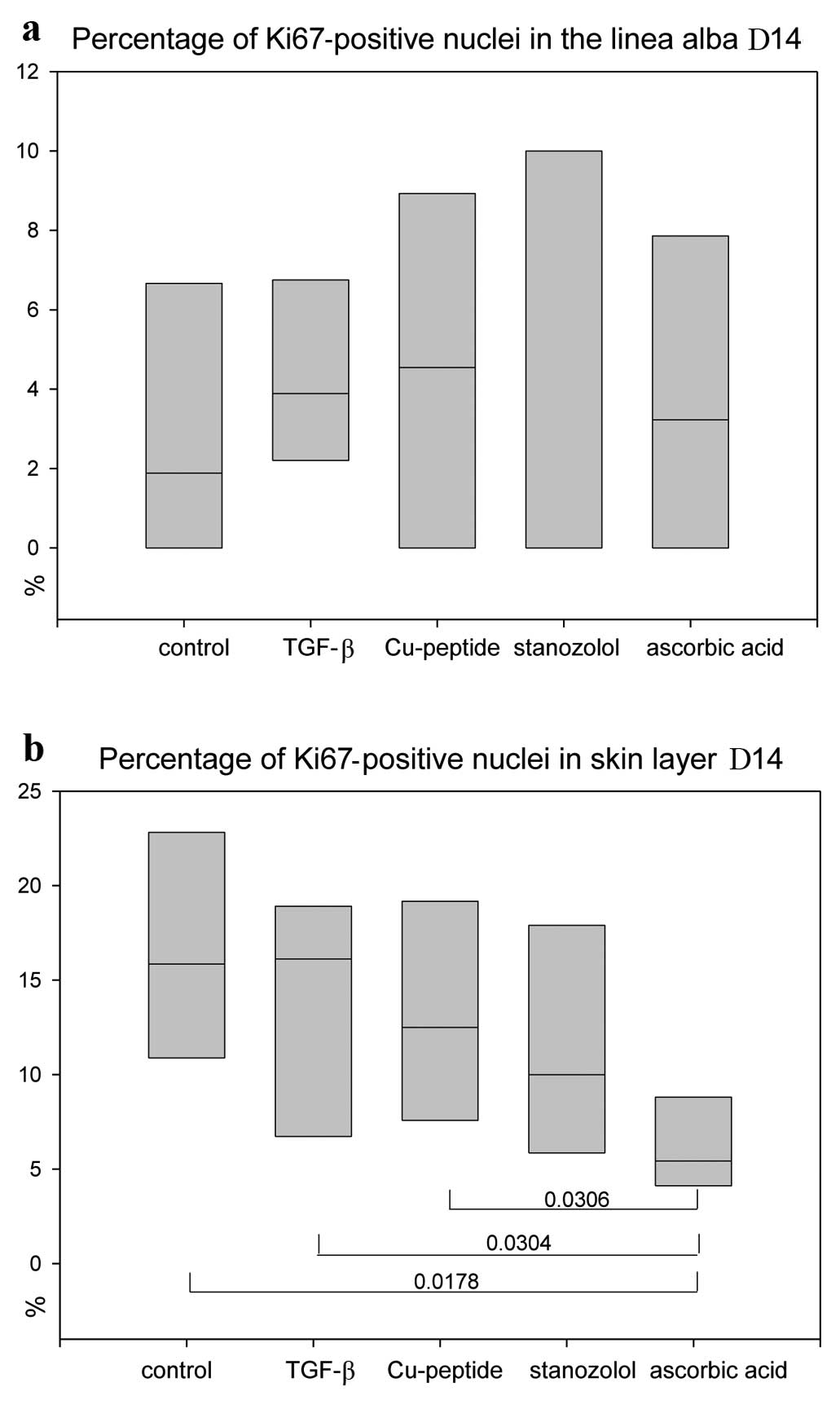
Proliferation rate
Anti-Ki67 stains revealed no significant differences
in the proliferation status of the linea alba scar (Fig. 6a), but significantly lower values
for AA-treated animals in the skin layer (Fig. 6b), indicating an earlier
remodelling process.
Discussion
Irrespective of the identification of numerous risk
factors, such as suboptimal closure techniques or wound infections
(12–16), the incidence of incisional hernias
after laparotomy remains high. Midline laparatomies may result in
incidences of 15–20% (1). This
calls for new approaches in optimised wound treatment, e.g., by
application of pro-angiogenic and pro-mitogenic substances or
agents. In a rat model of incisional hernias, significant increases
of fascial wound breaking strength were seen after treatment with
delayed release of bFGF (17).
The aim of our study was to elucidate possible effects of single
doses of AA and stanozolol in comparison to TGF-β and copper
peptide on laparotomy wound healing.
Wound healing is a complex process with numerous
cell types, signal transduction pathways and factors involved. This
complex process may be disturbed by diseases like diabetes, which
is known to be associated with a variety of alterations in
connective tissue metabolism, as a result of which diabetics face
the problem of poor wound healing (18). Diabetic macroangiopathy and
microangiopathy make tissues relatively ischemic, impairing wound
healing and increasing the risk of infection (19). Macrophages are dysfunctional due
to glycosylation and thus they do not adequately debride wounds or
combat infection (19,20). Angiogenesis may be severely
impaired, further delaying wound healing (19). This results, even in induced
murine diabetes, in more pronounced effects of proangiogenic and
promitogenic growth factors in diabetic than in normoglycemic
animals (21,22).
Stanozolol was developed in the 1950s in an attempt
to dissociate the anabolic and androgenic effects of testosterone.
The anabolic steroid stanozolol has been particularly helpful
because it has one of the largest anabolic/androgenic ratios. In
addition, stanozolol has substantial fibrinolytic properties.
Stanozolol is approved for use in the treatment of hereditary
angioedema. Stanozolol was also significantly capable of
stimulating the pro-collagenase production by skin fibroblasts
(23). It was also reported that
stanozolol stimulates the production of prostaglandin E2 (PGE2) and
the matrix metalloproteinase collagenase and stromelysin in human
skin fibroblasts (24). Falanga
et al (25) showed that
stanozolol enhances collagen synthesis in vitro in a
dose-dependent manner. Stanozolol also increases the mRNA levels of
alpha1 (I) and alpha1 (III) procollagen and, to a similar extent,
upregulated TGF-β1 mRNA and peptide levels.
AA contributes to several metabolic processes
including efficient hydroxylation of HP in elastin, collagen and
proteins with collagenous domains. AA is required for the
hydroxylation of proline residues in procollagen and HP stabilizes
the collagen triple helical structure. Consequently, ascorbate
stimulates procollagen secretion (26). Low levels of vitamin C may result
in higher incidence of complications and retarded wound healing
(4,27).
Copper introduced into wound dressings was
hypothesized to enhance wound repair (7). Application of wound dressings
containing copper oxide to wounds inflicted in genetically
engineered diabetic mice (C57BL/KsOlaHsd-Leprdb)
resulted in increased gene and in situ upregulation of
proangiogenic factors, increased blood vessel formation and
enhanced wound closure as compared to control dressings (7).
Among many molecules known to influence wound
healing, TGF-β1 has the broadest spectrum of actions, affecting all
cell types that are involved in all stages of wound healing
(26). Both positive and negative
effects of TGF-β1 on wound healing have been reported (28).
Our study showed that single doses of AA only had a
significant effect on the healing of incisional skin wounds in
terms of higher tensile strength. TGF-β and copper peptide had no
effect, whereas stanozolol treated wounds had a tendency towards
higher values. The content of Collagen type III was also markedly
increased in animals treated with AA or stanozolol. The
proliferation marker Ki67 showed the lowest values for AA-treated
incisional wounds indicating a faster healing and remodelling in
the skin layer, but not in the linea alba connective tissue.
The limited beneficial results of our experimental
setting indicate that single dose treatment is inferior to a
repetitive or delayed release of the factors. Stumpf et al
(29) developed a drug-eluting
platform device that enables continuous release of AA from a wound
dressing. It would be desirable to develop release systems that may
be implanted at time of surgery in order to optimize or accelerate
laparotomy wound healing.
Acknowledgements
The authors appreciate the technical
assistance of Kerstin Bahr, Institute of Functional and Clinical
Anatomy, University Medical Center of the Johannes Gutenberg
University Mainz.
References
|
1.
|
J HöerM AnurovS TitkovaU KlingeC TönsA
OttingerV SchumpelikInfluence of suture material and suture
technique on collagen fibril diameters in midline laparotomiesEur
Surg Res32359367200011182620
|
|
2.
|
TA SantoraJJ RoslynIncisional herniaSurg
Clin North Am735575701993
|
|
3.
|
EJ GravesBS GiullumDetailed diagnoses and
procedures, National hospital discharge survey, 1995Vital Health
Stat13114619979429338
|
|
4.
|
D LangemoJ AndersonD HansonS HunterP
ThompsonME PosthauerNutritional considerations in wound careAdv
Skin Wound
Care19297303200610.1097/00129334-200607000-0000716885642
|
|
5.
|
RE GiuntaT HolzbachC TaskovPS HolmMA
KonerdingD SchamsE BiemerB GänsbacherAdVEGF165 gene transfer
increases survival in overdimensioned skin flapsJ Gene
Med7297306200510.1002/jgm.67515515117
|
|
6.
|
M ValluruCA StatonMW ReedNJ
BrownTransforming growth factor beta and endoglin signaling
orchestrate wound healingFront
Physiol28999201110.3389/fphys.2011.0008922164144
|
|
7.
|
G BorkowJ GabbayR DardikAI EidelmanY
LavieY GrunfeldS IkherM HuszarRC ZatcoffM MarikovskyMolecular
mechanisms of enhanced wound healing by copper oxide-impregnated
dressingsWound Repair
Regen18266275201010.1111/j.1524-475X.2010.00573.x20409151
|
|
8.
|
M SumitraP ManikandanVS GayathriP
MahendranL SugunaEmblica officinalis exerts wound healing action
through upregulation of collagen and extracellular signal-regulated
kinases (ERK1/2)Wound Repair
Regen1799107200910.1111/j.1524-475X.2008.00446.x19152656
|
|
9.
|
A RümelinT HumbertO LuhkerA DrescherU
FauthMetabolic clearance of the antioxidant ascorbic acid in
surgical patientsJ Surg Res1294651200516085104
|
|
10.
|
GC JagetiaGK RajanikantKV Mallikarjun
RaoAscorbic acid increases healing of excision wounds of mice whole
body exposed to different doses of
gamma-radiationBurns33484494200710.1016/j.burns.2006.08.02517223272
|
|
11.
|
T WolloscheckA GaumannA TerzicA HeintzT
JungingerMA KonerdingInguinal hernia: measurement of the
biomechanics of the lower abdominal wall and the inguinal
canalHernia8233241200410.1007/s10029-004-0224-715098100
|
|
12.
|
LA IsraelssonT JonssonSuture length to
wound length ratio and healing of midline laparotomy incisionsBr J
Surg8012841286199310.1002/bjs.18008010208242299
|
|
13.
|
JJ HoerK JungeA SchachtruppU KlingeV
SchumpelickInfluence of laparotomy closure technique on collagen
synthesis in the incisional
regionHernia69398200210.1007/s10029-002-0070-412209295
|
|
14.
|
J WissingTJ van VroonhovenME
SchattenkerkHF VeenRJ PonsenJ JeekelFascia closure after midline
laparotomy: results of a randomized trialBr J
Surg74738741198710.1002/bjs.18007408313307992
|
|
15.
|
C Gutiérrez de la PenaC Medina AchiricaE
Dominguez-AdameJ Medina DiézPrimary closure of laparotomies with
high risk of incisional hernia using prosthetic material: analysis
of usefulnessHernia7134136200312687426
|
|
16.
|
TS de Vries ReilinghD van GeldereB
LangenhorstD de JongGJ van der WiltH van GoorRP BleichrodtRepair of
large midline incisional hernias with polypropylene mesh:
comparison of three operative techniquesHernia85699200414586775
|
|
17.
|
DA DubayX WangMA KuhnMC RobsonMG FranzThe
prevention of incisional hernia formation using a delayed-release
polymer of basic fibroblast growth factorAnn
Surg240179186200410.1097/01.sla.0000131576.12153.ab15213634
|
|
18.
|
JL Wilkinson-BerkaEL FletcherAngiotensin
and bradykinin: targets for the treatment of vascular and
neuroglial pathology in diabetic retinopathyCurr Pharm
Des1033133330200410.2174/138161204338317915544518
|
|
19.
|
V FalangaWound healing and its impairment
in the diabetic
footLancet36617361743200510.1016/S0140-6736(05)67700-816291068
|
|
20.
|
D AronsonEJ RayfieldHow hyperglycemia
promotes atherosclerosis: Molecular mechanismsCardiovasc
Diabetol1110200210.1186/1475-2840-1-112119059
|
|
21.
|
M AckermannT WolloscheckA WellmannVW LiWW
LiMA KonerdingPriming with a combination of proangiogenic growth
factors improves wound healing in normoglycemic miceInt J Mol
Med27647653201121373751
|
|
22.
|
M AckermannT WolloscheckA WellmannVW LiWW
LiMA KonerdingPriming with a combination of proangiogenic growth
factors enhances wound healing in streptozotocin-induced diabetes
in miceEur Surg Res478189201110.1159/00032814321720165
|
|
23.
|
JK WrightAJ SmithTE CawstonBL HazlemanThe
effect of the anabolic steroid, stanozolol, on the production of
procollagenase by human synovial and skin fibroblasts in
vitroAgents Actions28279282198910.1007/BF019674152556901
|
|
24.
|
AJ EllisTE CawstonEJ MackieThe
differential effects of stanozolol on human skin and synovial
fibroblasts in vitro: DNA synthesis and receptor bindingAgents
Actions413743199410.1007/BF019863918079819
|
|
25.
|
V FalangaAS GreenbergL ZhouSM OchoaAB
RobertsA FalabellaY YamaguchiStimulation of collagen synthesis by
the anabolic steroid stanozololJ Invest
Dermatol11111931197199810.1046/j.1523-1747.1998.00431.x9856839
|
|
26.
|
B KaplanB GonulS DincerFN Dincer KayaA
BabulRelationships between tensile strength, ascorbic acid,
hydroxyproline, and zinc levels of rabbit full-thickness incision
wound healingSurg Today2004747751200415338346
|
|
27.
|
KJ DesnevesBE TodorovicA CassarTC
CroweTreatment with supplementary arginine, ascorbic acid and zinc
in patients with pressure ulcers: a randomised controlled trialClin
Nutr24979987200510.1016/j.clnu.2005.06.01116297506
|
|
28.
|
XJ WangG HanP OwensY SiddiquiAG LiRole of
TGF beta-mediated inflammation in cutaneous wound healingJ Investig
Dermatol Symp
Proc11112117200610.1038/sj.jidsymp.565000417069018
|
|
29.
|
U StumpfM MichaelisD KlassertJ CinatlJ
AltrichterJ WindolfJ HergenrötherM ScholzSelection of proangiogenic
ascorbate derivatives and their exploitation in a novel
drug-releasing system for wound healingWound Repair
Regen19597607201110.1111/j.1524-475X.2011.00718.x22092798
|