Introduction
In human saliva, α-amylase is the most abundant
protein (1), accounting for
40–50% of salivary protein (2),
and has the important capacity to rapidly alter the physical
properties of starch in the oral cavity (3). Aging or radiation therapy for head
and neck cancer leads to severe salivary gland dysfunction and
consequential xerostomia (dry mouth syndrome), resulting in
hampered speech, dental problems, difficulties with swallowing and
food mastication, impaired taste, and nocturnal oral discomfort
(4–6).
Mesenchymal stem cells (MSCs) have been isolated
from various tissues, such as bone marrow (7), muscle (8), skin (9), and adipose tissue (10). Among them, adipose tissue contains
100- to 300-fold more MSCs than the bone marrow (11). Recent studies have identified
adipose-derived stem cells (ASCs) that can differentiate along
multiple pathways, including into osteogenic, adipogenic, myogenic,
and chondrogenic lineages, if an appropriate environment is
provided (12–17). Thus, ASCs have increasingly gained
importance due to their abundance in tissues and easy availability
for extraction (1).
Plant hormones are small organic molecules commonly
used to increase grain production (18,19). Among the hormones, gibberellic
acid (GA3), a plant growth regulator, is used worldwide
to increase the growth of fruits, such as strawberries, grapes, and
date palm (20) and of some
vegetables, such as tomatoes, cabbages, cauliflower, peppers, and
olives (21–23). Signal transduction pathways of
GA3 enable aleurone cells to modulate hydrolase
production, mainly α-amylase, in response to hormonal and
environmental stimuli. These enzymes digest the stored starch and
other nutrients in the endosperm to support the growth of young
seedlings.
Although GA3 is widely used in
agriculture, its effects on human health have not been well
explored. Thus, we focused on the potential effects of
GA3 and demonstrated a novel induction approach that
buccal fat pad (BFP)-derived ASCs differentiate into salivation
cells with GA3 treatment.
Materials and methods
Primary culture of human ASCs
BFPs were obtained from healthy donors at Chiba
University Hospital, Chiba, Japan. All donors provided written
informed consent for a protocol reviewed and approved by the
institutional review board of Chiba University. To isolate ASCs, we
performed the centrifuge methods described previously (12). Briefly, the adipose tissues were
harvested, washed extensively with PBS, minced for 10 min with fine
scissors, and enzymatically digested at 37°C for 40 min with 0.1%
collagenase (Wako, Osaka, Japan). An equal volume of control medium
(Dulbecco’s modified Eagle’s medium/F-12; Sigma-Aldrich Co., St.
Louis, MO) containing 10% fetal bovine serum (FBS; Sigma Aldrich
Co.) and 50 U/ml penicillin and streptomycin (Sigma Aldrich Co.)
was then added to neutralize the collagenase. The cell suspension
was centrifuged at 1,300 rpm (260 × g) for 5 min to obtain a
high-density ASC pellet, which was resuspended in control medium.
After being counted using trypan blue, the cells were plated at a
concentration of 5×105 cells/100-mm cell culture dishes
(BD Biosciences, Franklin Lakes, NJ) and kept in the control medium
at 37°C in 5% CO2.
Flow cytometric analysis of ASCs
Cultured ASCs were washed twice in cold PBS
supplemented with 2% FBS (Sigma-Aldrich Co.) and resuspended to a
concentration of about 1×106 cells/antibody test and
labeled with anti-human CD73-PE, CD90-FITC, CD105-PerCP, CD31-PE,
CD34-PerCP, and CD45-FITC antibodies for 20 min at room temperature
in the dark (BD Biosciences). The labeled cells were analyzed using
a fluorescence-activated cell sorter (FAC; BD Biosciences).
Negative control stains were performed using FITC-, PE- and
PerCP-conjugated mouse IgG1 κ isotypes (BD Biosciences). Data were
analyzed using FlowJo software (Tree Star, Inc., Ashland, OR).
Differentiation culture conditions
To induce osteogenic differentiation, ASCs were
cultured in an osteogenic differentiation basal medium containing
osteogenic supplement (Invitrogen, Carlsbad, CA). After 3 weeks,
osteogenic differentiation was evaluated with alkaline phosphatase
(ALP) staining (Primary Cell Co., Ltd., Hokkaido, Japan).
Adipogenic differentiation of ASCs was induced by adipocyte
differentiation basal medium containing an adipogenic supplement
(Chemicon International, Inc., Temecula, CA) for 4 weeks. After
induction, the cells were stained with Oil Red O (Sigma). To induce
neural differentiation, ASCs were grown in neural differentiation
medium (Thermo Fisher Scientific, Rockford, IL) for 3 days. The
induced cells were subjected to immunocytochemical analysis to
assess the expression of nestin (Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA), a neural marker.
GA3 cytotoxicity
ASCs were seeded at a density of 1×104
cells/60-mm cell culture dishes (BD Biosciences) in the control
medium with the indicated concentrations of GA3 for the
indicated time points. The effect of GA3 cytotoxicity on
the numbers of ASCs was determined using phase-contrast microscopy
and a trypan blue exclusion test.
Treatment of ASCs with
GA3
The ASCs at 80% confluence were incubated in the
control medium with the indicated concentrations of GA3.
ASCs were harvested for extraction of total-RNA and protein at 0,
7, 14, 21 and 28 days after 1 mM GA3 treatment.
Preparation of cDNA
Total-RNA was isolated using TRIzol Reagent
(Invitrogen), according to the manufacturer’s instructions. cDNA
was generated from 5 μg of total-RNA using Ready-To-Go You-Prime
First-Strand Beads (GE Healthcare, Buckinghamshire, UK) and
oligo(dt) primer (Sigma-Genosys, Ishikari, Japan), according to the
manufacturer’s instructions.
mRNA expression analysis
To evaluate the expression levels of α-amylase in
ASCs, real-time quantitative reverse transcriptase-polymerase chain
reaction (qRT-PCR) was performed. qRT-PCR was carried out with one
method using a LightCycler FastStart DNA Master SYBR-Green I kit
(Roche Diagnostics GmbH, Mannheim, Germany). The PCR reactions
using the LightCycler apparatus were performed in a final volume of
20 μl of a reaction mixture consisting of 2 μl of FirstStart DNA
Master SYBR-Green I mix, 3 mM MgCl2, and l μM primers,
according to the manufacturer’s instructions. The reaction mixture
was loaded into glass capillary tubes and subjected to an initial
denaturation at 95°C for 10 min, followed by 45 rounds of
amplification at 95°C (10 sec) for denaturation, 62°C (10 sec) for
annealing, and 72°C (10 sec) for extension, with a temperature
slope of 20°C/sec. Amplified products were analyzed by 3% agarose
gel electrophoresis to ascertain size and purity. The transcript
amounts for the target genes were estimated from the respective
standard curves and normalized to the glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) transcript amount determined in corresponding
samples. The following primers were used: α-amylase, forward,
5′-ATTTTCATGTCGCCCGTTGT-3′ and reverse,
5′-CCCATGTGATGGACCAATGTC-3′; GAPDH, forward,
5′-CATCTCTGCCCCCTCTGCTGA-3′ and reverse,
5′-GGATGACCTTGCCCACAGCCT-3′.
Protein extraction
The cells were washed twice with cold PBS and
centrifuged briefly. The cell pellets were incubated at 4°C for 30
min in a lysis buffer (7 M urea, 2 M thiourea, 4% w/v CHAPS, and 10
mM Tris pH 7.4) with a proteinase inhibitor cocktail (Roche
Diagnostics). The protein concentration was measured with the BCA
Protein Assay kit (Thermo Scientific).
Evaluation of α-amylase protein
expression by western blot analysis
Protein extracts were electrophoresed on 4–12%
Bis-Tris gels, transferred to nitrocellulose membranes
(Invitrogen), and blocked for 1 h at room temperature in Blocking
One (Nacalai Tesque, Kyoto, Japan). The membranes were washed three
times with 0.1% Tween-20 in Tris-buffered saline and incubated with
anti-human α-amylase (1:100 dilution) and β-actin (1:1,000
dilution) monoclonal antibodies (Santa Cruz Biotechnology, Inc.)
overnight at 4°C. The membranes were washed again and incubated for
1 h at room temperature with a 1:2,500 of goat anti-mouse IgG (H+L)
HRP conjugate (Promega, Madison, WI) as a secondary antibody.
Finally, the membranes were detected using SuperSignal West Pico
Chemiluminescent substrate (Thermo Fisher Scientific) and
immunoblotting was visualized by exposing the membranes to ATTO
Light-Capture II (ATTO, Tokyo, Japan). Signal intensities were
quantitated using the CS Analyzer version 3.0 software (ATTO).
Results
Isolation of ASCs from human BFPs
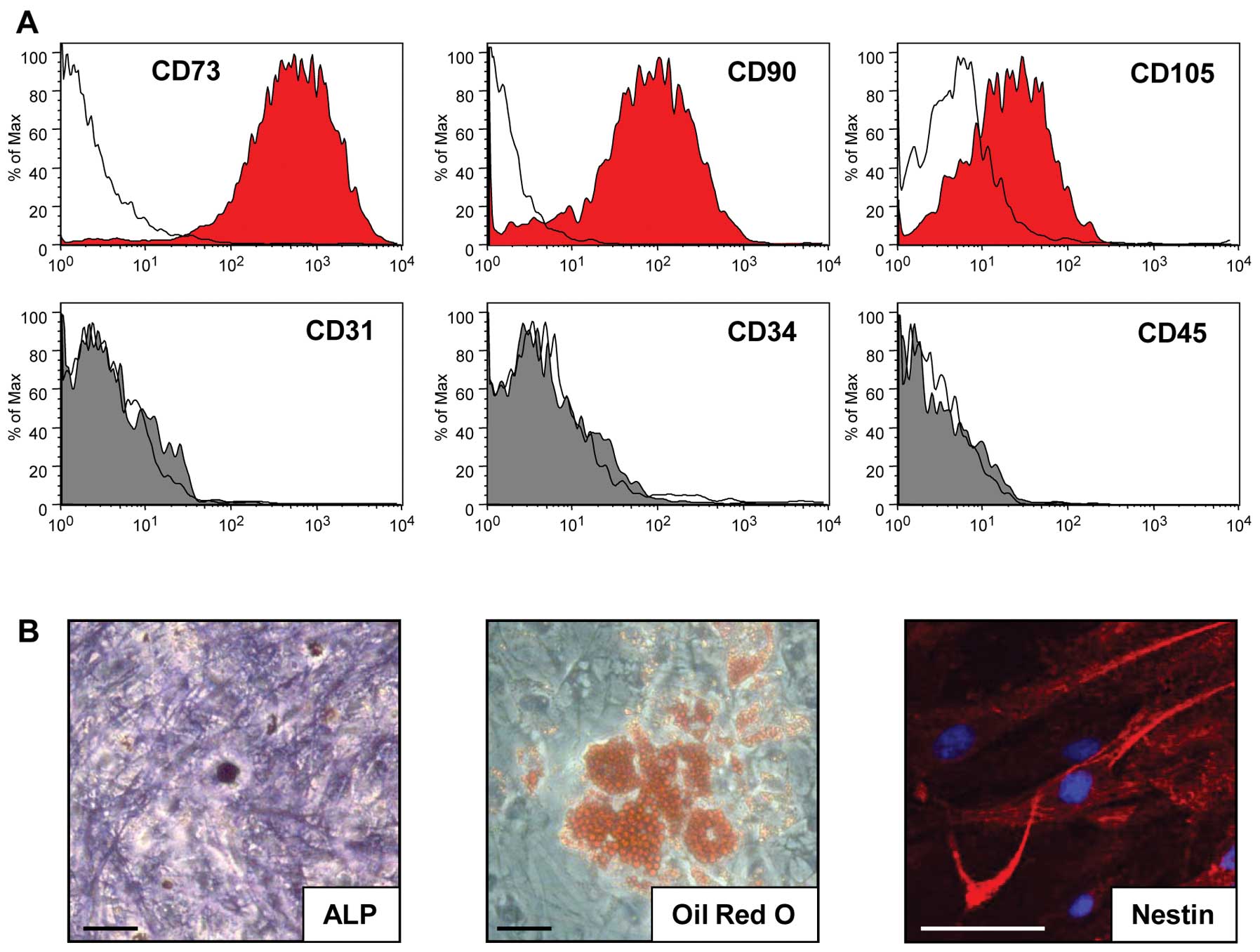
FACS analysis of BFP-derived ASCs at the fifth
passage showed that the cells expressed the cell surface markers,
CD73, CD90, and CD105 but not CD31, CD34 and CD45 (Fig. 1A). These results are consistent
with the definition that MSCs must express CD73, CD90 and CD105, as
suggested by Dominici et al (24). ASCs did not spontaneously
differentiate during culture expansion. To determine whether ASCs
from BFPs can differentiate into various cell types, such as
osteoblasts, adipocytes, and neural cells in vitro, ASCs
were cultured in specific selection media. After 3 weeks in the
osteogenic medium culture, the cells differentiated into
osteoblasts, which were confirmed with strong ALP staining
(Fig. 1B). After 4 weeks in the
adipogenic differentiation culture, the cells differentiated into
lipid-laden cells that were stained with Oil Red O (Fig. 1B). After 3 days of neural
differentiation culture, the ASCs differentiated into neural cells,
which were confirmed with immunocytochemistry for nestin (Fig. 1B). These results showed that ASCs
from BFPs can multidifferentiate.
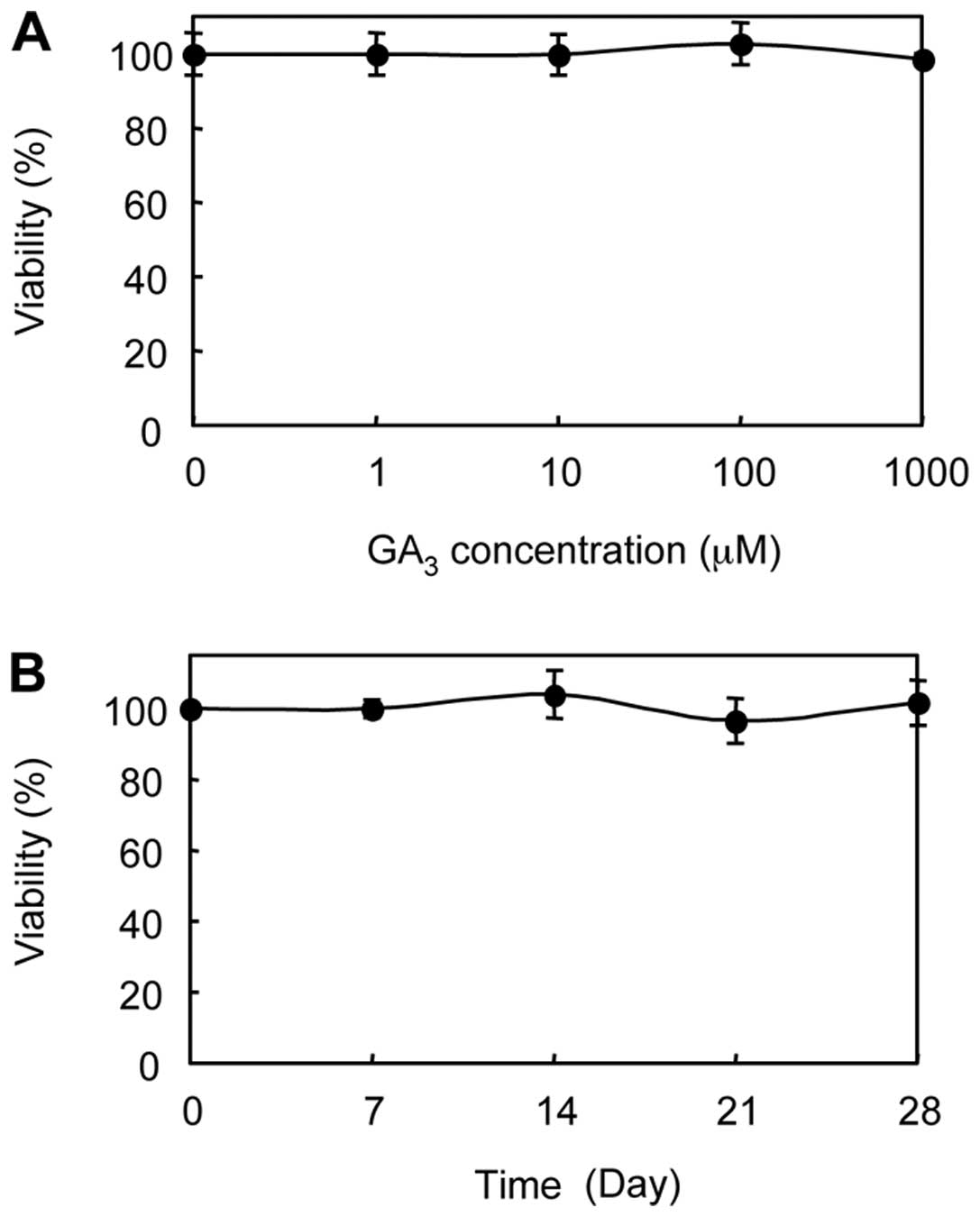
Cytotoxicity of GA3
Ishii et al (25) reported that plant hormones are
closely related to anticancer therapy. We treated the ASCs with
GA3 to determine the cytotoxic effect. GA3,
up to 1 mM, did not affect the cell viability of ASCs in a dose- or
time-dependent manner (Fig. 2).
In addition, there were no morphologic changes when we challenged
the ASCs with GA3 (data not shown).
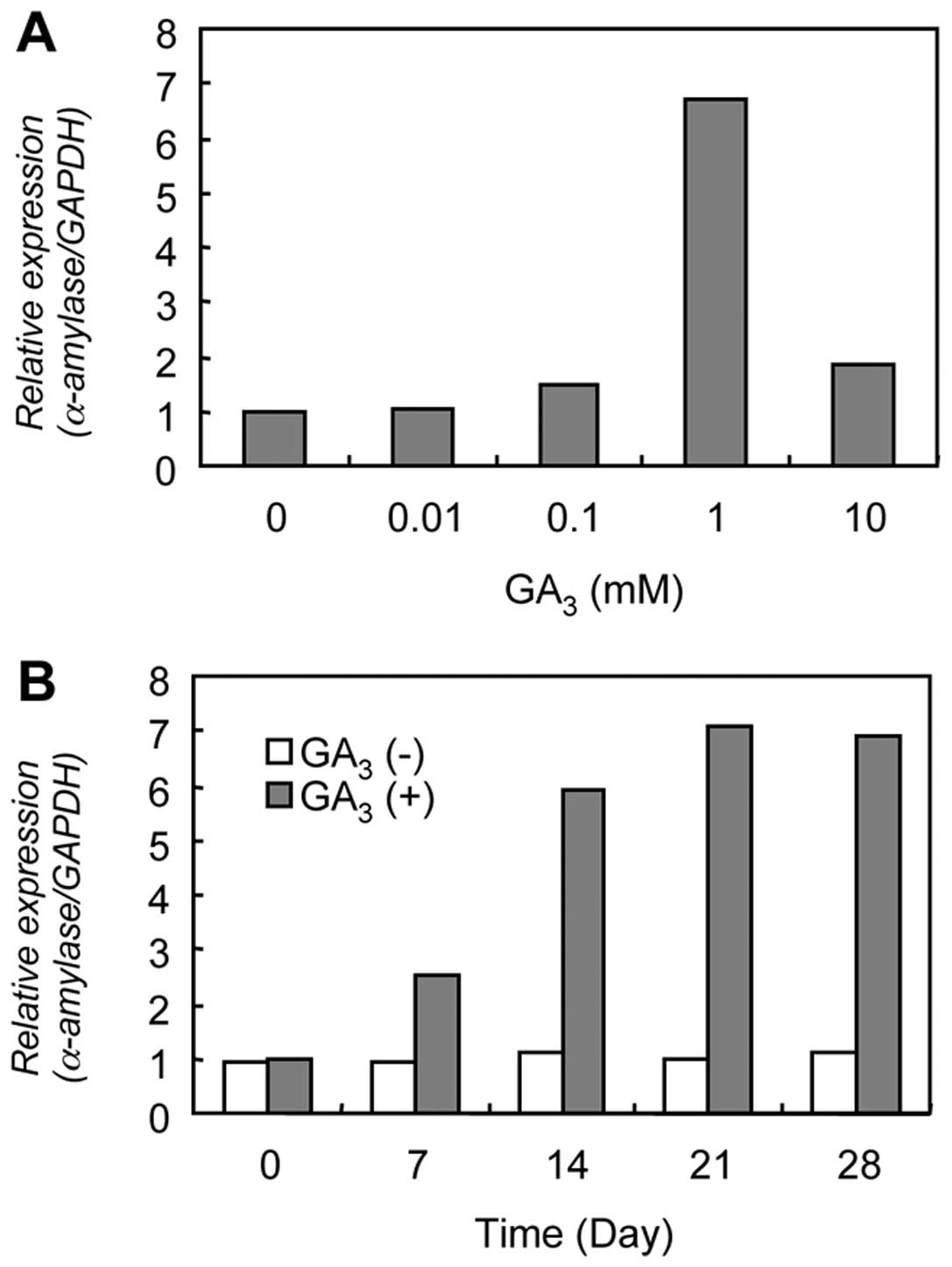
Evaluation of α-amylase mRNA
expression
The result of qRT-PCR analysis for α-amylase mRNA
expression is shown in Fig. 3.
Higher α-amylase mRNA expression was found after treatment with 1
mM GA3 for 14 days. α-amylase mRNA expression reached
its maximum on 21 days after 1 mM GA3 treatment, which
was 7-fold than that of resting conditions (0 day).
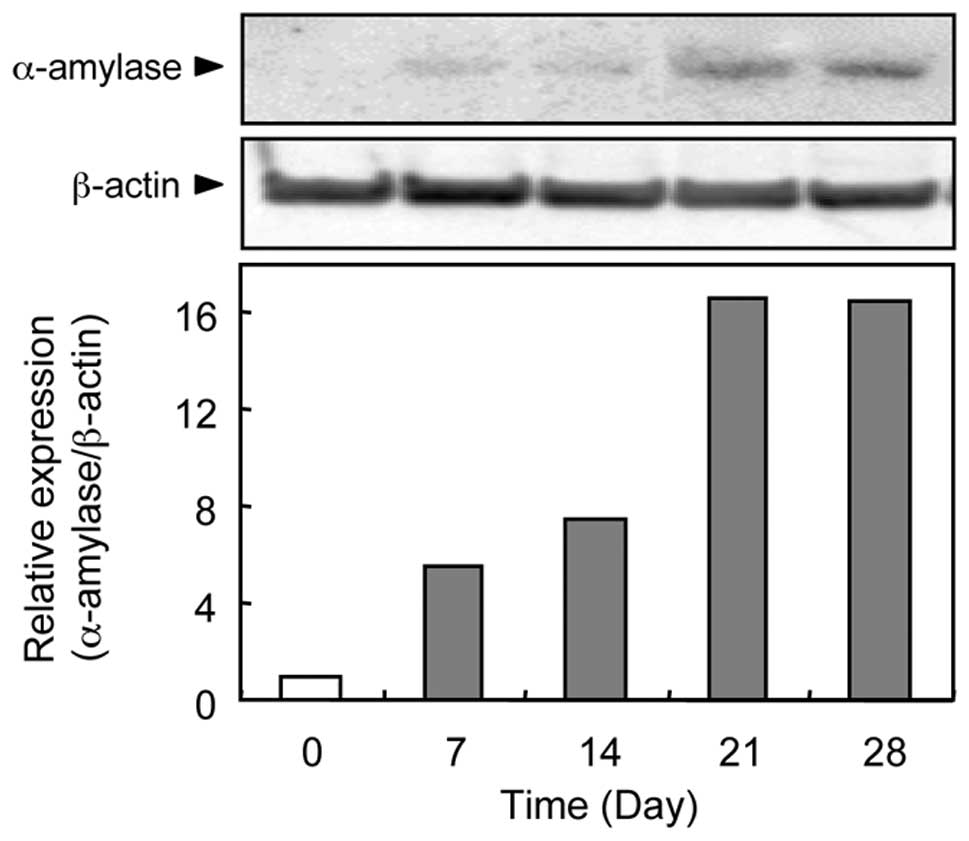
Evaluation of α-amylase protein
expression
We performed western blot analysis to determine the
α-amylase protein expression status in the GA3-treated
ASCs. Representative results of western blot analysis for α-amylase
protein expression are shown in Fig.
4. We did not detect any α-amylase protein bands under resting
conditions (0 day). α-amylase protein became evident 7 days after
treatment with GA3, reaching a maximal level on Day
21.
Discussion
The current study showed that GA3, a
plant growth regulator, plays an important role in regulating
α-amylase in BFP-derived ASCs and that the induction method could
be an emerging potential therapeutic approach for regenerating
salivary glands.
ASCs have been recognized as an efficient source of
adult stem cells because of their easy accessibility, minimal
morbidity upon harvesting, and abundance of stem cells compared
with bone marrow-derived MSCs (11). Moreover, ASCs can be propagated
more rapidly, and they retain their mesenchymal pluripotency after
multiple passages (15). We
isolated ASCs from BFPs, adipose-encapsulated masses in the oral
cavity, and revealed that BFP-derived ASCs showed positive MSC
markers and pluripotency. BFPs are an easy source for dentists and
oral surgeons who treat patients for dry mouth syndrome.
The digestion of dietary starch in humans is
initiated by salivary α-amylase, an endo-enzyme that hydrolyzes
starch into maltose, maltotriose, and larger oligosaccharides.
Salivary α-amylase accounts for 40 to 50% of protein in human
saliva and rapidly alters the physical properties of starch. This
amylolytic digestion begins during mastication in the oral cavity
and continues in the stomach (1–3).
Gibberellins were identified initially in the 1930s
as a product of a fungus, which caused excessive shoot elongation.
Further studies found that gibberellins are also involved in other
processes, e.g., promoting flowering and seed germination (18). One gibberellin, GA3,
accelerates and improves the yield of a wide variety of plants by
increasing cell division (18,26). Early in seed germination, the
embryo synthesizes GA3, which diffuses to the aleurone
cells in which GA3 acts as a signal to activate
synthesis and secretion of α-amylases and other hydrolases. While
GA3 is widely used in agriculture, only a few
experiments have examined the possible toxic effects in mammals. A
previous study reported that gibberellin derivatives had strong
anticancer activities by inhibiting topoisomerase I activity in
rodents (27). To determine the
effect of GA3 on cell viability in ASCs, we carried out
a cytotoxic assay of ASCs using several concentrations of
GA3 for a maximum of 28 days. GA3 never
affected cell viability or cell morphology up to 1 mM. However,
some groups reported that exposure of GA3 induced
oxidative stress and histopathological changes to rats (28,29). Therefore, further studies with
more in vivo samples are needed to address the status of
α-amylase expression after GA3 treatment in greater
detail.
The aleurone layer of cereal grains is the most
widely studied and best characterized system for studying the
activity of GA3. To date, at least one GA3
receptor is present in the plasma membrane (30) and there is evidence of a number of
other components of the pathways, including Ca2+
(31,32), lipases (33), cGMP (34), protein phosphatases (35), an endoplasmic reticulum-located
Ca2+-ATPase, inositol-1,4,5-triphosphates, and
Ca2+/calmodulin (36)
at the early stage of GA3 signal transduction. The
GA3-regulated myb gene, GAmyb, may be a component of the
GA3 response pathway and has been shown to transactivate
the α-amylase promoter (37). In
the present study, we found that GA3 regulated α-amylase
expression in human ASCs, suggesting that mammalian cells also may
have a GA3 response pathway. Since the mammalian signal
transduction pathways of GA3 are unknown, further
studies are required to reveal the pathway for α-amylase
expression.
The potential effects of GA3 on human
health have not been explored. This is the first report to show
that GA3 treatment can increase the expression of
cellular α-amylase and that our induction method might be a useful
therapeutic application for salivary gland regeneration.
Acknowledgements
We thank Dr Hiroshi Mizuno and Dr
Morikuni Tobita, Juntendo University, Japan, for helpful
discussions and critical review of the manuscript; Lynda C.
Charters for editing this manuscript; and Dr Hiroshi Nakajima and
Dr Hiroaki Takatori, Department of Molecular Genetics, Graduate
School of Medicine, Chiba University, for assistance with the FACS
experiments.
References
|
1.
|
FG OppenheimE SalihWL SiqueiraSalivary
proteome and its genetic polymorphismsAnn NY Acad
Sci10982250200710.1196/annals.1384.03017303824
|
|
2.
|
RE NobleSalivary alpha-amylase and
lysozyme levels: a non-invasive technique for measuring parotid vs
submandibular/sublingual gland activityJ Oral
Sci428386200010.2334/josnusd.42.8310989590
|
|
3.
|
C HoeblerA KarinthiMF DevauxPhysical and
chemical transformations of cereal food during oral digestion in
human subjectsBr J
Nutr80429436199810.1017/S00071145980014949924264
|
|
4.
|
TE DanielsPC FoxSalivary and oral
components of Sjögren’s syndromeRheum Dis Clin North
Am18571589199211292733
|
|
5.
|
A VissinkFR BurlageFK SpijkervetPrevention
and treatment of the consequences of head and neck radiotherapyCrit
Rev Oral Biol Med14213225200310.1177/15441113030140030612799324
|
|
6.
|
A VissinkJ JansmaFK SpijkervetOral
sequelae of head and neck radiotherapyCrit Rev Oral Biol
Med14199212200310.1177/154411130301400305
|
|
7.
|
MF PittengerAM MackaySC BeckMultilineage
potential of adult human mesenchymal stem
cellsScience284143147199610.1126/science.284.5411.143
|
|
8.
|
A AsakuraStem cells in adult skeletal
muscleTrends Cardiovasc
Med13123128200310.1016/S1050-1738(03)00024-012691677
|
|
9.
|
M BelicchiF PisatiR LopaHuman skin-derived
stem cells migrate throughout forebrain and differentiate into
astrocytes after injection into adult mouse brainJ Neurosci
Res77475486200410.1002/jnr.2015115264217
|
|
10.
|
PA ZukM ZhuH MizunoMultilineage cells from
human adipose tissue: implications for cell-based therapiesTissue
Eng7211228200110.1089/10763270130006285911304456
|
|
11.
|
K LinY MatsubaraY MasudaCharacterization
of adipose tissue-derived cells isolated with the Celution
systemCytotherapy10417426200810.1080/1465324080198297918574774
|
|
12.
|
PA ZukM ZhuP AshjianHuman adipose tissue
is a source of multipotent stem cellsMol Biol
Cell1342794295200212475952
|
|
13.
|
JK FraserR SchreiberB StremPlasticity of
human adipose stem cells toward endothelial cells and
cardiomyocytesNat Clin Pract Cardiovasc Med3Suppl
1S33S37200610.1038/ncpcardio044416501628
|
|
14.
|
TA MoseleyM ZhuMH HedrickAdipose-derived
stem and progenitor cells as fillers in plastic and reconstructive
surgeryPlast Reconstr Surg118Suppl
3121S128S200610.1097/01.prs.0000234609.74811.2e16936551
|
|
15.
|
H NakagamiR MorishitaK MaedaAdipose
tissue-derived stromal cells as a novel option for regenerative
cell therapyJ Atheroscler
Thromb137781200610.5551/jat.13.7716733294
|
|
16.
|
AM ParkerAJ KatzAdipose-derived stem cells
for the regeneration of damaged tissuesExp Opin Biol
Ther6567578200610.1517/14712598.6.6.56716706604
|
|
17.
|
X BaiK PinkernellYH SongGenetically
selected stem cells from human adipose tissue express cardiac
markersBiochem Biophys Res
Commun353665671200710.1016/j.bbrc.2006.12.10317196165
|
|
18.
|
AL SilverstoneT SunGibberellins and the
green revolutionTrends Plant
Sci512200010.1016/S1360-1385(99)01516-2
|
|
19.
|
M AshikariH SakakibaraS LinCytokinin
oxidase regulates rice grain
productionScience309741745200510.1126/science.111337315976269
|
|
20.
|
RJ WeavorGrowth of graps in relation to
gibberellinAdv Chem Ser2889108196110.1021/ba-1961-0028.ch010
|
|
21.
|
FG GustafsonInfluence of gibberellic acid
on setting and development of fruit in tomatoPlant
Physiol35521523196010.1104/pp.35.4.52116655381
|
|
22.
|
S ArousM BoussaidM MarrakchiPlant
regeneration from zygotic embryo hypocotyls of Tunisian chilli
(Capsicum annuum L.)J Appl Hort317222001
|
|
23.
|
A Chaari-RkhisM MaalejS Ouled MessaoudIn
vitro vegetative growth and flowering of olive tree in response to
GA3 treatmentAfr J Biotechnol5209723022006
|
|
24.
|
M DominiciK Le BlancI MuellerMinimal
criteria for defining multipotent mesenchymal stromal cells. The
International Society for Cellular Therapy position
statementCytotherapy8315317200610.1080/14653240600855905
|
|
25.
|
Y IshiiH KiyotaS SakaiInduction of
differentiation of human myeloid leukemia cells by jasmonates,
plant
hormonesLeukemia1814131419200410.1038/sj.leu.240342115229618
|
|
26.
|
M AsahinaH IwaiA KikuchiGibberellin
produced in the cotyledon is required for cell division during
tissue reunion in the cortex of cut cucumber and tomato
hypocotylsPlant Physiol129201210200210.1104/pp.01088612011351
|
|
27.
|
J ChenZ SunY ZhangSynthesis of gibberellin
derivatives with anti-tumor bioactivitiesBioorg Med Chem
Lett1954965499200910.1016/j.bmcl.2009.07.09019679470
|
|
28.
|
A TroudiIB AmaraN SoudaniOxidative stress
induced by gibberellic acid on kidney tissue of female rats and
their progeny: biochemical and histopathological studiesJ Physiol
Biochem67307316201110.1007/s13105-011-0076-421305369
|
|
29.
|
N ErinB AfacanY ErsoyGibberellic acid, a
plant growth regulator, increases mast cell recruitment and alters
Substance P
levelsToxicology2547581200810.1016/j.tox.2008.09.02018948165
|
|
30.
|
S GilroyRL JonesPerception of gibberellin
and abscisic acid at the external face of the plasma membrane of
barley (Hordeum vulgare L.) aleurone protoplastsPlant
Physiol10411851192199412232156
|
|
31.
|
M WangBV DuijnAW SchramAbscisic acid
induces a cytosolic calcium decrease in barley aleurone
protoplastsPlant Mol Biol24697419911825201
|
|
32.
|
DS BushEffects of gibberellic acid and
environmental factors on cytosolic calcium in wheat aleurone
cellsPlanta19988891996
|
|
33.
|
S GilroyA TrewavasSignal sensing and
signal transduction across the plasma membraneThe Plant Plasma
MembraneC LarssonIM MollerSpringer-VerlagBerlin2032321990
|
|
34.
|
A KuoS CappellutiM
Cervantes-CervantesOkadaic acid, a protein phosphatase inhibitor,
blocks calcium changes, gene expression, and cell death induced by
gibberellin in wheat aleurone cellsPlant
Cell8259269199610.1105/tpc.8.2.2598742711
|
|
35.
|
SP PensonRC SchuurinkA FathcGMP is
required for gibberellic acid-induced gene expression in barley
aleuronePlant Cell823252333199610.1105/tpc.8.12.232512239379
|
|
36.
|
X ChenM ChangB WangCloning of a
Ca(2+)-ATPase gene and the role of cytosolic Ca2+ in the
gibberellin dependent signaling pathway in aleurone cellsPlant
J113633711997
|
|
37.
|
F GublerR KallaJK
RobertsGibberellin-regulated expression of a myb gene in barley
aleurone cells: evidence for Myb transactivation of a high-pI
α-amylase gene promoterPlant Cell71879189119958535141
|