Introduction
Atherosclerosis is the major cause of cardiovascular
disease (CVD), which is the leading cause of death in developed
countries (1). During the last
decade, increasing amount of evidence supports chronic systemic
vascular inflammation as an essential requirement for the
progression of atherosclerosis and that the innate and adaptive
immune cells play an integral role (2). Activation of vascular endothelial
and smooth muscle cells by several endogenous or exogenous moieties
causes up regulation of inflammatory cytokines like interleukin
(IL)-1β and IL-18, augment proliferation, accumulation of oxidized
low density lipoprotein (oxLDL) and extracellular matrix (ECM)
components by the activation of intracellular pattern-recognition
receptors (PRRs) (3,4).
IL-1β, IL-1α and IL-1 receptor antagonist (Ra) are
members of the IL-1 family protein encoded by genes located on
chromosome 2 (5). IL-1Ra is known
to be a natural competitor for similar binding sites with IL-1β,
which is an important mediator of cellular processes such as
differentiation, proliferation and apoptosis (5). IL-1β has been implicated for the
recruitment of inflammatory cells to infection sites, while IL-18
is important for IFN-γ production and enhancement of natural killer
cell activity (5,6). IL-1β and IL-18 are produced from
their 31 and 24 kDa inactive forms to their 17 and 18 kDa
bio-active forms, respectively, through a proteolytic cleavage by
caspase-1 and this depends on a complex protein platform called the
inflammasome (7–9).
Previous studies have identified the NLRP1, NLRP3,
IPAF/NLRC4 and AIM2 inflammasomes to activate caspase-1 (10). These complex proteins consist of
three major components: a receptor [nucleotide oligomerisation
domain (NOD)-like receptor (NLR)], which acts as an intracellular
sensor and induces complex formation; in most cases an adaptor
protein [apoptosis speck-like protein containing (ASC) a caspase
recruitment domain (CARD) and caspase-1] (10). It has been hypothesized that upon
stimulation of the inflammasome receptors by exogenous and
endogenous stimuli like pathogens, cellular stress, low
K+ concentrations, crystals or uric acid, the receptors
undergo conformational changes forming inflammasome complexes by
either recruiting an ASC via their pyrin-pyrin interaction before
binding to caspase-1, or binding directly to pro-caspase-1 through
CARD-CARD interaction (9,11–13). This leads to activation of
caspase-1, which in turn converts inactive IL-1β to its active form
(14). Unlike caspase-1 and NLRs
that are expressed in resting cells, IL-1β mRNA is believed
to emanate from the activation of the NF-κB pathway; or microbial
induction via Toll-like receptor, C-type lectin receptor or
RIG-1-like receptor’s ligand binding; or through MyD88 activation
via IL-1 receptor binding (10).
CARD8 is a member of the CARD family and has been
implicated to be a co-regulator in both inflammatory and apoptotic
signaling pathways, although their exact role in these processes
remain elusive and thus needs to be further explored (15). The CARD8 gene is located on
chromosome 19q13 (16) and
polymorphisms in this gene have been associated with several
auto-inflammatory diseases, such as inflammatory bowel disease
(IBD) and rheumatoid arthritis (RA) (17,18). Five isoforms of CARD8 have been
identified but a contradictory functional role has been reported
between the 48- and 54-kDa isoforms (19). The 48-kDa isoform has been
implicated to interact directly with caspase-1 to induce apoptosis,
while the 54-kDa isoform suppresses caspase mediated apoptosis
(19,20). The isoforms have been reported to
show a different expression pattern in different tissues and tumor
cell lines (19).
Concurrently, studies have been carried out to
explore the functional role of NLRP3 and CARD8 mainly in
inflammatory (monocytes and macrophages) and cancer cells, but less
is known about their functional role in vascular cells. Due to the
expression of IL-1 family proteins in vascular cells and in
atherosclerotic plaque (21–23) together with detrimental effects
associated with excess IL-1β, it is therefore crucial to understand
how vascular cells sense infection and metabolic stress and
initiate vascular cell inflammation. The aim of this study is to
examine the actual role of NLRP3 inflammasome and CARD8 protein in
IL-1β expression and release from aortic smooth muscle cells
(AOSMCs).
Materials and methods
Materials
Human AOSMC, OPTI-MEM reduce serum medium,
Lipofectamine 2000, AOSMC Basal medium (M-231–500) and specific
primers for Tucan 47F, Tucan 48F, Tucan 54F and Tucan Ex-9-R
(19); and TaqDNA polymerase
(18038/042) were purchased from Invitrogen (Stockholm, Sweden),
Universal negative control and specific siRNA’s for NLRP3
and CARD8 were obtained from Sigma-Aldrich (Stockholm,
Sweden), E.Z.N.A. total-RNA kit I from Omega Biotech (Doraville,
GA, USA), 2X TaqMan Universal PCR Master Mix and probes for gene
expression analysis were from Applied Biosystems (Foster City, CA),
tumor necrosis factor-α (TNF-α) from PeproTech (Stockholm, Sweden),
IL-1β and IL-1Ra kit for ELISA was obtained from DuoSet®
Development System (R&D Systems, UK). Primary antibodies
against NLRP3 (MAB3724) and CARD8 (PAB0218) were obtained from
Abnova Corp. (Taipei City, Taiwan). 5X Green Go reaction buffer
(M719A) was purchased from Promega Biotech AB (Stockholm,
Sweden).
Cell culture
Human AOSMC cells were grown in smooth muscle cell
growth medium with medium change every second day. For experiments,
passages 5-10 cells were used and 2.5x105 cells/well
were seeded in 6-well plates and treated with or without TNF-α for
1, 6, 24 or 48 h. Cell culture supernatants were used for measuring
IL-1β and IL-1Ra expression.
Knockdown of NLRP3 and CARD8
Knockdown of NLRP3 and CARD8 were performed on
2x105 cells/well seeded in 6-well plates. The
transfection mixture was prepared by using 4 μl of Lipofectamine
2000 and 10 or 20 pmol of NLRP3 (Hs0200313821) or
CARD8 (Hs0034180) siRNA, respectively, to 500 μl of
Opti-MEM. The tubes were incubated for 20 min. The mixture was then
added to cells and incubated for 8 h followed by addition of
antibiotic free growth medium and incubated for 24 h. Media was
then replaced and cells stimulated with 50 ng/ml TNF-α and
incubated for further 24 h.
Quantitative real-time-polymerase chain
reaction (qRT-PCR)
In accordance with the manufacturer’s instruction,
total-RNA was extracted using the E.Z.N.A. total-RNA kit. Using
random polyhexamers, RNaseOUT and Superscript II, 0.75 mg of RNA
was reverse-transcribed into cDNA. For gene expression analysis,
IL-1β (Hs001740097), NLRP3 (Hs00366465),
IL-1RN (Hs00277299), CARD8 (Hs01088228) and
cyclophilin A (Hs04194521) were used, cDNA was amplified in
the 7900HT Fast Real-Time PCR system (Applied Biosystems) on fast
optical 96-well plates according to the manufacturer’s
instructions. Each sample was analyzed in duplicate and values
obtained were normalized with cyclophilin A.
Enzyme-linked immunosorbent assay
(ELISA)
We employed the ELISA technique for the detection of
IL-1β and IL-1Ra secreted in cell culture supernatant using the
DuoSet® Development System kits. In accordance with the
manufacturer’s instruction with slight modification, 0.05%
Tween-PBS was used as reagent diluent in place of 1% BSA. Samples
were diluted in equal volumes with 0.05% Tween-PBS for IL-1β
quantification, but not for IL-1Ra, and each sample was analyzed in
duplicate.
Reverse transcription-polymerase chain
reaction (RT-PCR)
cDNA obtained from reverse transcriptase as
mentioned above was amplified in a 50 μl PCR mixture.
Tucan-54F/Ex9-R, Tucan-48F/Ex9-R and Tucan-47F/Ex9-R were used as
forward and reverse primer pairs as previously described (19). The PCR mixture was amplified as
follows: 10 min at 94˚C followed by 40 cycles at 45 sec at 94˚C, 30
sec at 58˚C, and 90 sec at 72˚C. The cycling procedure was followed
by 10 min extension at 72˚C. The PCR mixture was viewed under UV
light post electrophoresis on an ethidium bromide stained 1%
agarose gel.
Western blot analysis
Protein lysates from transfected ASOMC were
subjected to electrophoresis on 8 or 12% SDS-polyacrylamide gels
and further transfered to PVDF membrane. Membranes were blocked
with 5% non-fat milk in 0.01% Tween-PBS with shaking for 4 h and
incubated overnight with primary antibodies (1:1,000) at 4˚C,
followed by 2 h incubation with 1:1,000 dilution of secondary
antibody for NLRP3 (anti-mouse IgG from GE Healthcare, Uppsala
Sweden) and CARD8 (anti-rabbit IgG from AH Diagnostics, Stockholm,
Sweden).
Statistical analysis
The independent two-tailed Student’s t-test and one
way ANOVA was used for data analysis and results are expressed as
mean ± SD. The P-value was considered to be statistically
significant at P≤0.05.
Results
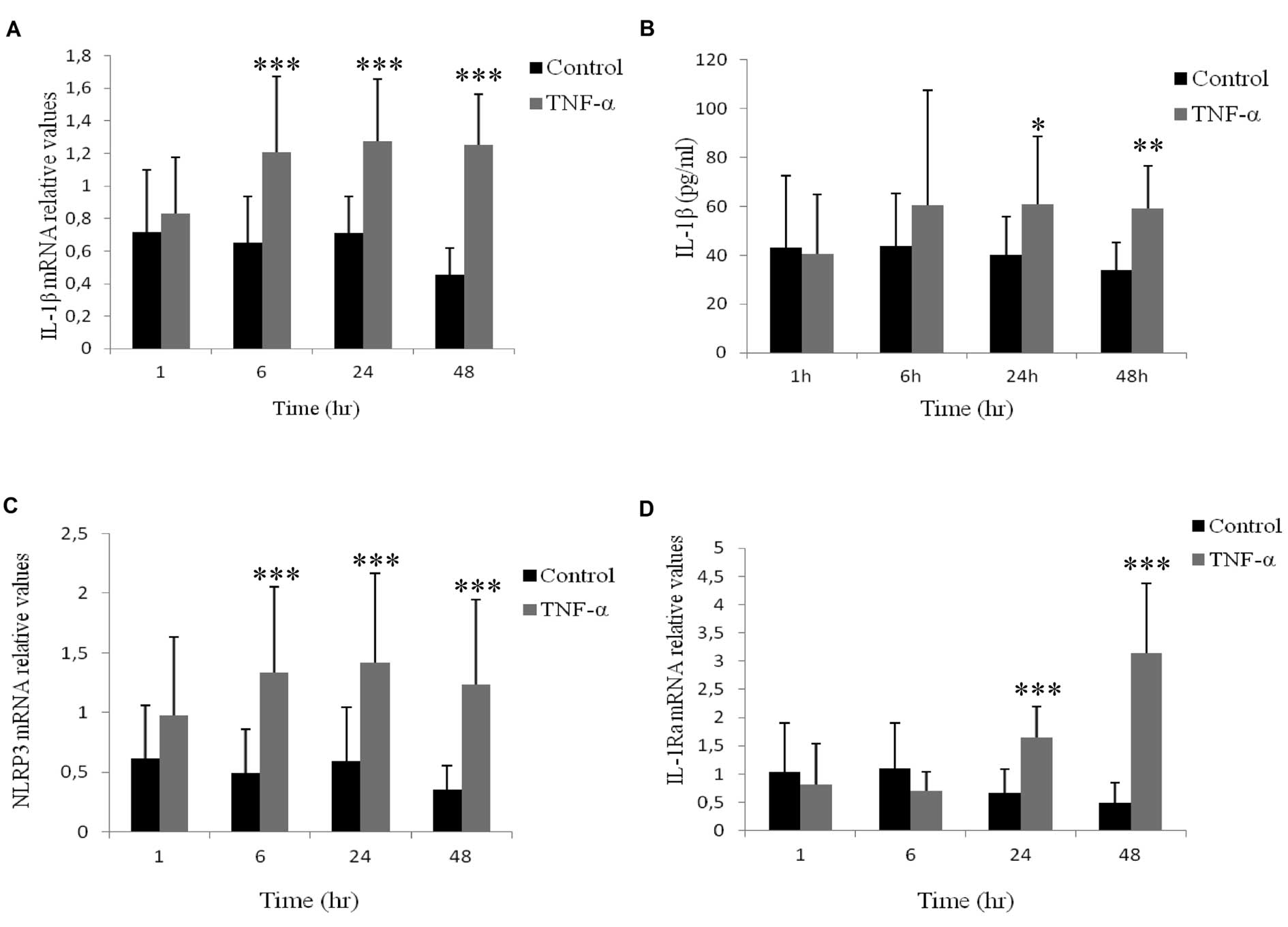
Effects of TNF-α on the expression and
release of IL-1β and its regulators in AOSMCs
qRT-PCR revealed that stimulation of AOSMC with 50
ng/ml of TNF-α for different time points (1–48 h), induces a
2–3-fold increment of IL-1β mRNA expression, compared to
their unstimulated controls (Fig.
1A). Furthermore, ELISA was used to quantify IL-1β protein
release to the media from AOSMC stimulated with 50 ng/ml TNF-α and
significant increases were observed after 24 and 48 h (Fig. 1B). We further analyzed the effects
of TNF-α on IL-1β regulators: IL-1Ra, which is a natural competitor
with IL-1β for the binding site to the IL-1 receptors; NLRP3, which
is essential for NLRP3 inflammasome assembly; and CARD8 a
co-inflammatory regulator. qRT-PCR revealed that stimulation of
AOSMC with 50 ng/ml of TNF-α for different time points (1–48 h),
induces a 1–3-fold increase in NLRP3 mRNA expression at time
points 6, 24 and 48 h and a 2.5–6-fold increment of IL-1Ra
mRNA expression at 24 and 48 h, compared to their respective
unstimulated controls (Fig. 1C and
D). CARD8 levels were not affected by TNF-α (data not
shown).
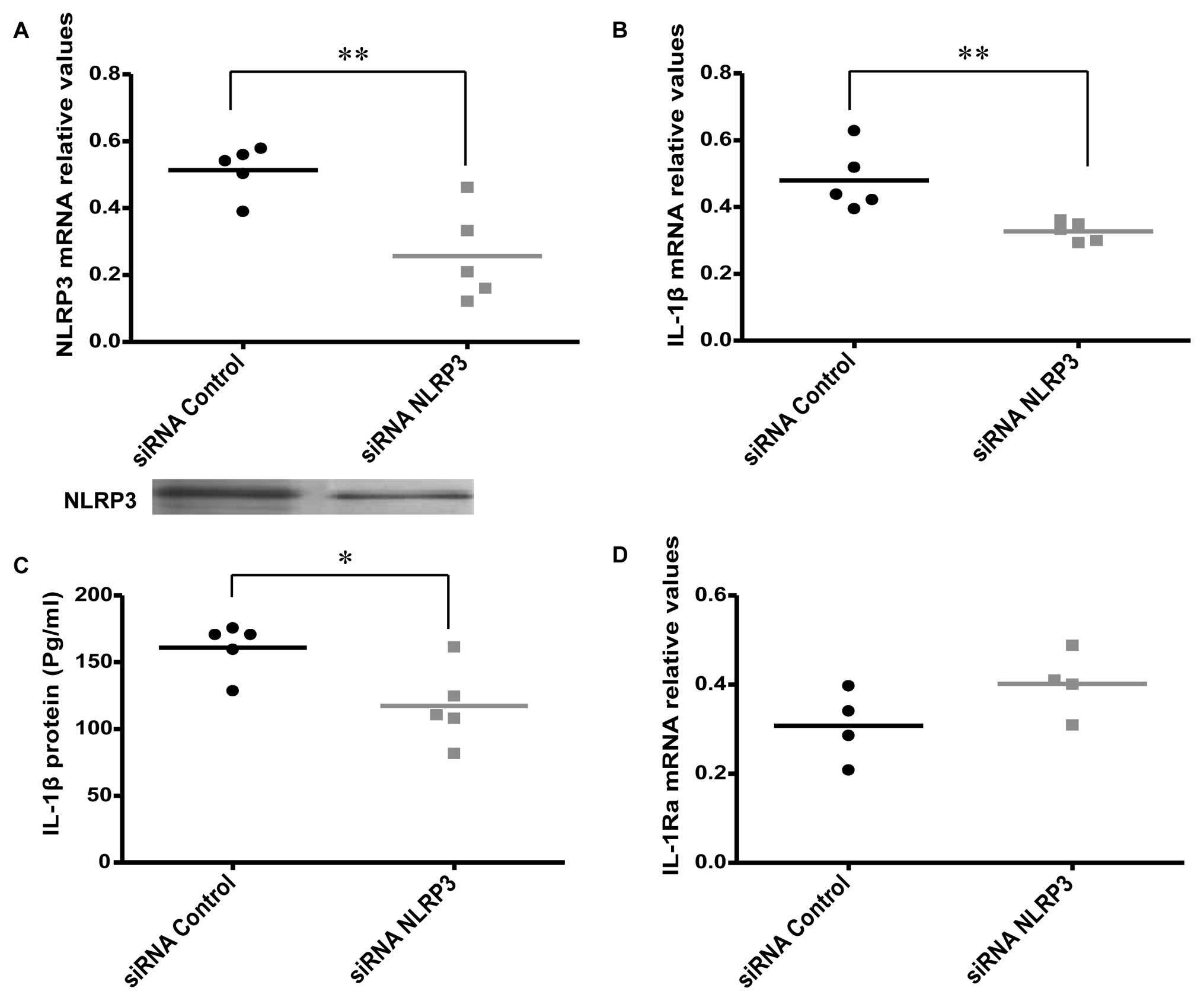
Effects of NLRP3 knockdown on IL-1β
expression and release
Specific siRNAs were used to knockdown the NLRP3
gene to investigate the effect on the expression and release of
IL-1β in AOSMC (Fig. 2A). The
knockdown of NLRP3 was associated with a significant decrease in
IL-1β expression and release (Fig. 2B
and C), while a non-significant, but borderline increment of
IL-1Ra mRNA expression was revealed (P=0.07; Fig. 2D). We also found that there was no
difference in IL-1Ra protein release 24 h after NLRP3
knockdown, compared to the control (data not shown).
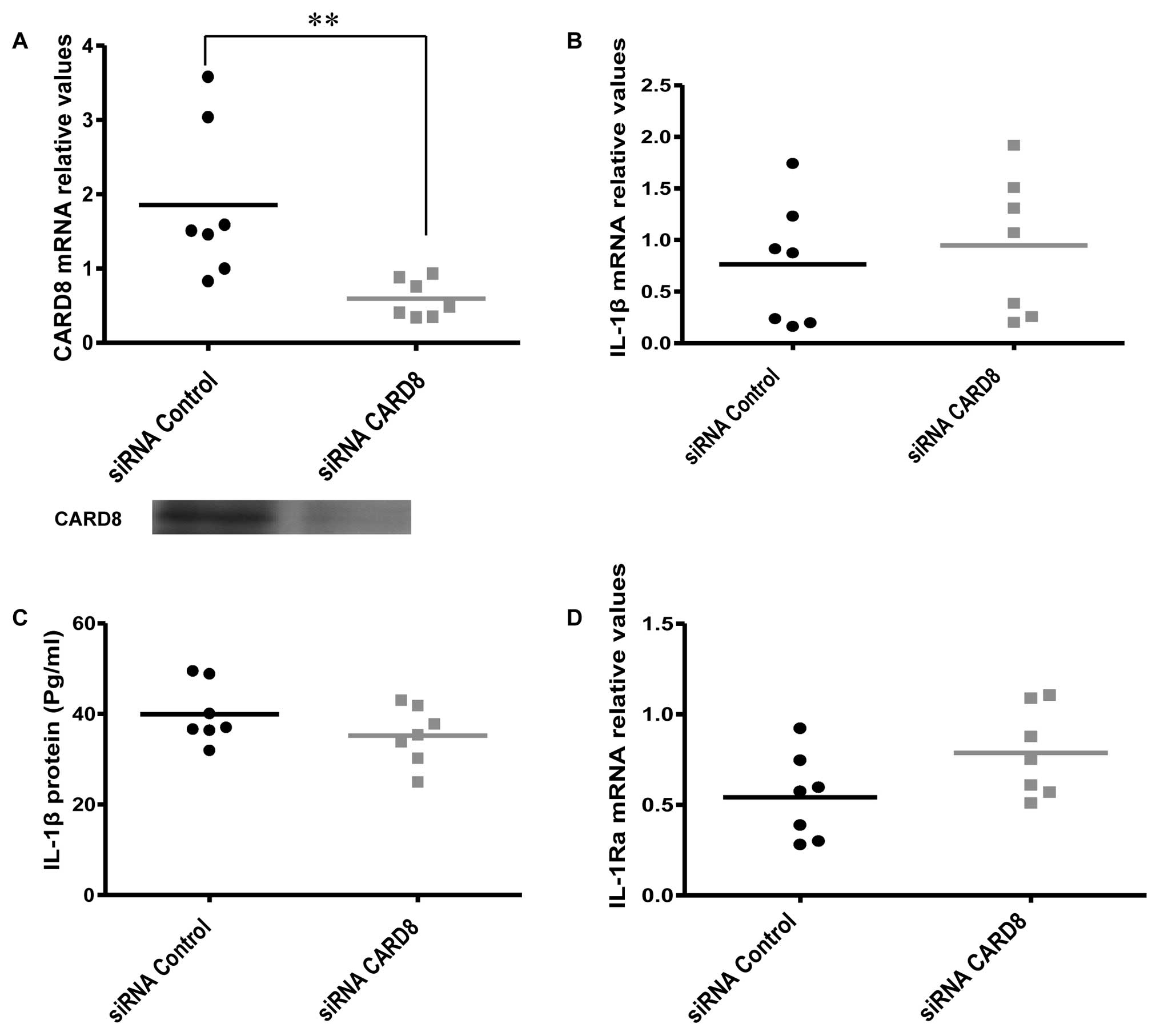
Effects of CARD8 knockdown on IL-1β
expression and release
CARD8 has been reported to inhibit caspase-1
activity and also negatively regulate NF-κB activation in monocytes
and other cell lines (15). We
therefore hypothesized that knockdown of CARD8 may increase the
expression and release of IL-1β. However, no significant change was
evident in IL-1β mRNA (Fig.
3B) and protein release (Fig.
3C) but the IL-1Ra mRNA expression showed a
non-significant, but borderline increment (P=0.08) (Fig. 3D) in AOSMC after knockdown of
CARD8.
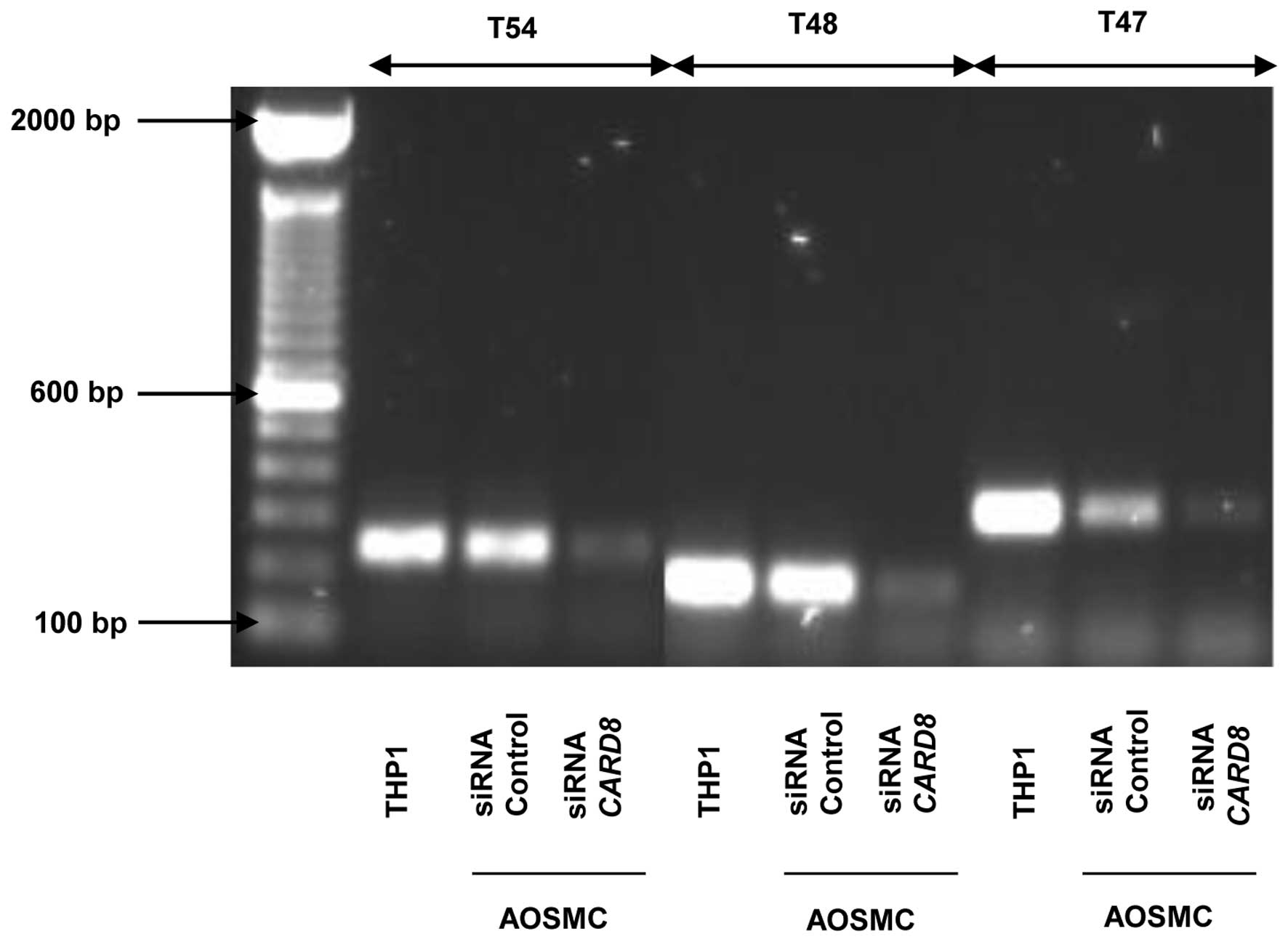
Expression of CARD8 isoforms in
AOSMCs
Considering the contradictory functional role
reported between the CARD8 (T54 and T48) isoforms (19), we quest to determine which
isoforms of CARD8 are expressed in AOSMC. We found that AOSMC
express the T47, T48 and T54 isoforms and they were all knockdown
by our siRNA. THP1 cells were used as positive control since it has
previously been shown to express several CARD8 isoforms (19). Our results show that T54 is 250
bp, T48 is 200 bp while T47 is 300 bp (Fig. 4).
Discussion
Existing evidence suggests that vascular cells
actively contribute to atherogenic inflammation as they express
several inflammatory moieties, which are overexpressed in
atherosclerotic plaques. It is now evident that NLRP3 forms an
integral part of the inflammasome that catalyzes the maturation of
inactive IL-1β and IL-18 (24).
CARD8 on the other hand has been reported to be an inhibitor of
caspase-1 and a negative regulator of NF-κB and conflicting
evidence has been shown regarding CARD8 and some inflammatory
diseases like IBD and RA (15,17,18,25).
Consistent with other studies, we also found that
TNF-α affects IL-1β expression and release (21,26). Expression of IL-1β and
NLRP3 mRNA increased proportionally with time, while
IL-1Ra significantly increased at 24 and 48 h after
treatment. This suggests that, during cellular stress or exposure
to a foreign ligand, elevated levels of IL-1β are required to
execute its protective role, but over time excess production of
IL-1β could become detrimental. Thus, the increase of IL-1Ra after
24 h could be to compensate for IL-1β activity and to maintain
cellular homeostasis. In addition, our data supports the hypothesis
that pathogens, cellular stress or cytokines (TNF-α) stimulate
NLR’s to form inflammasome complexes which leads to the activation
and secretion of IL-1β. To study the role of NLRP3 and CARD8 in
IL-1β expression and release from AOSMC, cells were
transfected with either siRNA against the NLRP3 or
CARD8 genes. We observed that knockdown of NLRP3 led
to a decrease of IL-1β mRNA and protein secretion. Our
results are similar to findings reported by Zhu et al
(27), who showed that silencing
of the NLRP3 gene using siRNA, decreased the IL-1β release
from Kupffer cells, hence antagonizing liver ischemic injury. The
reason for the decreased IL-1β mRNA after NLRP3
knockdown is unclear, but we suggest that, IL-1β mRNA may
undergo self regulation, due to the decrease in IL-1β protein
secreted from the cells. Our results are consistent with studies
carried out by Yamasaki et al (28), and Kankkunen et al
(29). On the other hand, from
the observation that CARD8 is an inhibitor of caspase-1 and a
negative regulator of NF-κB (15), we derived a hypothesis that
knockdown of the CARD8 gene may augment caspase-1 and NF-κB
activities and thus increase the IL-1β expression and
release. However, no significant change in IL-1β mRNA or
protein release was evident.
Contrary to NLRP3, the role of CARD8 in
IL-1β and IL-1Ra expression and release in AOSMCs may
therefore be limited. However, a contradictory functional role has
been demonstrated in the CARD8 isoforms as they show different
expression pattern in different cell types and tumor cell lines
with some cells expressing both (19). We further sought to determine
which isoforms of CARD8 are expressed in AOSMC and found an
expression of the 47, 48 and 54 kDa isoforms and that they were all
knockdown by siRNA. However, due to lack of studies regarding
CARD8, more studies are required to fully elucidate the functions
of CARD8 and to demonstrate its role in inflammasome formation and
activity.
In conclusion, our results show that TNF-α induces
IL-1β, IL-1Ra and NLRP3 but not CARD8; knockdown of NLRP3
gene significantly decreases 1L-1β expression and release and AOSMC
express the 47, 48 and 54 kDa isoforms of CARD8, but the
effect of CARD8 on IL-1β and IL-1Ra seems to be limited. Our
data therefore suggest that NLRP3 but not CARD8 play an important
role in IL-1β expression and release in AOSMC and could be a
therapeutic target or marker for atherosclerosis. Increased
understanding of the mechanisms of initiation and progression of
atherogenic vascular inflammation may lead to new approaches in the
development of novel therapeutics for atherosclerosis and other
inflammatory diseases.
Acknowledgements
This study was supported by grants
from the Swedish Research Council, the Swedish Heart-Lung
Foundation, the Swedish Fund for Research without Animal
Experiments, the Swedish Heart and Lung Association, the Foundation
of Olle Engkvist.
Abbreviations:
|
IL
|
interleukin;
|
|
NLRP
|
NLR family, containing pyrin
domain;
|
|
CARD
|
caspase recruitment domain;
|
|
AOSMCs
|
aortic smooth muscle cells;
|
|
siRNA
|
short interfering RNA;
|
|
TNF-α
|
tumor necrosis factor-α;
|
|
Ra
|
receptor antagonist;
|
|
CVD
|
cardiovascular disease;
|
|
oxLDL
|
oxidized low density lipoprotein;
|
|
ECM
|
extracellular matrix;
|
|
PRR
|
pattern-recognition receptor;
|
|
IFN-γ
|
interferon γ;
|
|
NLR
|
NOD-like receptor or NOD and LRR
containing;
|
|
NLRC
|
NLR family, containing CARD
domain;
|
|
AIM2
|
absent in melanoma 2;
|
|
NOD
|
nucleotide-binding oligomerization
domain;
|
|
ASC
|
apoptosis speck-like protein contaning
CARD;
|
|
NF-κB
|
nuclear factor-κ-light-chain-enhancer
of activated B cells;
|
|
IBD
|
inflammatory bowel disease;
|
|
RA
|
rheumatoid arthritis;
|
|
PBS
|
phosphate-buffered saline;
|
|
BSA
|
bovine serum albumin
|
References
|
1.
|
P LibbyInflammation in
atherosclerosisNature420868874200210.1038/nature0132312490960
|
|
2.
|
GK HanssonA HermanssonThe immune system in
atherosclerosisNat Immunol12204212201110.1038/ni.200121321594
|
|
3.
|
E WestphalM HerzbergI NeumannL BeibeiC
PilowskiC LiK WerdanH LoppnowNeutrophils process interleukin-1beta
and interleukin-18 precursors in a caspase-1-like fashion -
processing is inhibited by human vascular smooth muscle cellsEur
Cytokine Netw171928200616613759
|
|
4.
|
H LoppnowK WerdanW BuerkeVascular cells
contribute to atherosclerosis by cytokine- and
innate-immunity-related inflammatory mechanismsInnate
Immun146387200810.1177/175342590809124618713724
|
|
5.
|
C GabayC LamacchiaG PalmerIL-1 pathways in
inflammation and human diseasesNat Rev6232241201020177398
|
|
6.
|
M SahooI Ceballos-OlveraL del BarrioF
ReRole of the inflammasome, IL-1β, and IL-18 in bacterial
infectionsScientificWorldJournal11203720502011
|
|
7.
|
MG NeteaA SimonF van de VeerdonkBJ
KullbergJW van der MeerLA JoostenIL-1beta processing in host
defense: beyond the inflammasomesPLoS
Pathog6e1000661201010.1371/journal.ppat.100066120195505
|
|
8.
|
JA GracieSE RobertsonIB
McInnesInterleukin-18J Leukoc
Biol73213224200310.1189/jlb.0602313
|
|
9.
|
K SchroderJ TschoppThe
inflammasomesCell140821832201010.1016/j.cell.2010.01.04020303873
|
|
10.
|
F BauernfeindA AblasserE BartokS KimJ
Schmid-BurgkT CavlarV HornungInflammasomes: current understanding
and open questionsCell Mol Life
Sci68765783201110.1007/s00018-010-0567-421072676
|
|
11.
|
F MartinonV PétrilliA MayorA TardivelJ
TschoppGout-associated uric acid crystals activate the NALP3
inflammasomeNature440237241200610.1038/nature0451616407889
|
|
12.
|
V PétrilliS PapinC DostertA MayorF
MartinonJ TschoppActivation of the NALP3 inflammasome is triggered
by low intracellular potassium concentrationCell Death
Diff1415831589200717599094
|
|
13.
|
F MartinonL AgostiniE MeylanJ
TschoppIdentification of bacterial muramyl dipeptide as activator
of the NALP3/cryopyrin inflammasomeCurr
Biol1419291934200410.1016/j.cub.2004.10.02715530394
|
|
14.
|
L AgostiniF MartinonK BurnsMF McDermottPN
HawkinsJ TschoppNALP3 forms an IL-1β-processing inflammasome with
increased activity in Muckle-Wells autoinflammatory
disorderImmunity203193252004
|
|
15.
|
M RazmaraSM SrinivasulaL WangJL PoyetBJ
GeddesPS DiStefanoJ BertinES AlnemriCARD-8 protein, a new CARD
family member that regulates caspase-1 activation and apoptosisJ
Biol Chem2771395213958200210.1074/jbc.M10781120011821383
|
|
16.
|
H ZhangW FuNDPP1 is a novel CARD domain
containing protein which can inhibit apoptosis and suppress
NF-kappaB activationInt J Oncol2010351040200211956601
|
|
17.
|
A FontalbaV Martinez-TaboadaO GutierrezC
PipaonN BenitoA BalsaR BlancoJL Fernandez-LunaDeficiency of the
NF-kappaB inhibitor caspase activating and recruitment domain 8 in
patients with rheumatoid arthritis is associated with disease
severityJ
Immunol17948674873200710.4049/jimmunol.179.7.486717878386
|
|
18.
|
DPB McGovernH ButlerT AhmadM PaolucciDA
van HeelK NegoroP HysiJ RagoussisSPL TravisLR CardonDP JewellTUCAN
(CARD8) genetic variants and inflammatory bowel
diseaseGastroenterology13111901196200610.1053/j.gastro.2006.08.00817030188
|
|
19.
|
RD BagnallRG RobertsMM MirzaT TorigoeNJ
PrescottCG MathewNovel isoforms of the CARD8 (TUCAN) gene evade a
nonsense mutationEur J Hum
Genet16619625200810.1038/sj.ejhg.520199618212821
|
|
20.
|
M YamamotoT TorigoeK KamiguchiY HirohashiK
NakanishiC NabetaH AsanumaT TsuramaT SatoF HataA novel isoform of
TUCAN is overexpressed in human cancer tissues and suppresses both
caspase-8- and caspase-9-mediated apoptosisCancer
Res6587068714200510.1158/0008-5472.CAN-04-464916204039
|
|
21.
|
D WågsäterK JattaP OcayaJ DimbergA
SirsjöExpression of IL-1beta, IL-1 receptor type I and IL-1
receptor antagonist in human aortic smooth muscle cells: effects of
all-trans-retinoic acidJ Vasc Res43377382200616804330
|
|
22.
|
PS OlofssonY SheikineK JattaM GhaderiA
SamnegårdP ErikssonA SirsjöA functional interleukin-1 receptor
antagonist polymorphism influences atherosclerosis development. The
interleukin-1beta:interleukin-1 receptor antagonist balance in
atherosclerosisCirc J7315311536200910.1253/circj.CJ-08-1150
|
|
23.
|
J GaleaJ ArmstrongP GadsdonH HoldenSE
FrancisCM HoltInterleukin-1 beta in coronary arteries of patients
with ischemic heart diseaseAtheroscler Thromb Vasc
Biol1610001006199610.1161/01.ATV.16.8.10008696938
|
|
24.
|
WP ArendG PalmerC GabayIL-1, IL-18, and
IL-33 families of cytokinesImmunol
Rev2232038200810.1111/j.1600-065X.2008.00624.x18613828
|
|
25.
|
SF FisherMM MirzaCM OnnieD SoarsCM LewisNJ
PrescottCG MathewJ SandersonA ForbesC TodhunterCombined evidence
from three large british association studies rejects TUCAN/CARD8 as
an IBD susceptibility
geneGastroenterology13220782080200710.1053/j.gastro.2007.03.08617484911
|
|
26.
|
P LibbyJM OrdovasKR AugerAH RobbinsLK
BirinyiCA DinarelloEndotoxin and tumor necrosis factor induce
inter-leukin-1 gene expression in adult human vascular endothelial
cellsAm J Pathol12417918519863526909
|
|
27.
|
P ZhuL DuanJ ChenA XiongQ XuH ZhangF
ZhengZ TanF GongM FangGene silencing of NALP3 protects against
liver ischemia-reperfusion injury in miceHum Gene
Ther22853864201110.1089/hum.2010.14521128730
|
|
28.
|
K YamasakiJ MutoKR TaylorAL CogenD AudishJ
BertinEP GrantAJ CoyleA MisaghiHM HoffmanRL GalloNLRP3/cryopyrin is
necessary for interleukin-1beta (IL-1beta) release in response to
hyaluronan, an endogenous trigger of inflammation in response to
injuryJ Biol
Chem2841276212771200910.1074/jbc.M80608420019258328
|
|
29.
|
P KankkunenL TeirilaJ RintahakaH AleniusH
WolffS Matikainen(1,3)-beta-glucans activate both dectin-1 and
NLRP3 inflammasome in human macrophagesJ
Immunol18463356342201010.4049/jimmunol.090301920421639
|