Introduction
Microglia, differentiated from the active phagocytic
amoeboid monocytes (1,2), are brain resident immunocompetent
cells originating from bone marrow-derived monocytes that invade
the brain during embryogenesis. Activated microglia produce
factors, such as reactive nitrogen species (RNS), inflammatory
factors, reactive oxygen species (ROS) and any other neurovirulent
factors, which injure neurons and result in neurodegenerative
diseases, such as Parkinson’s disease (PD) or Alzheimer’s disease
(AD) (3,4). The considerable contribution of
microglial activation as a risk factor to the pathogenesis of PD
has previously been proposed (5–7).
Several factors have been identified and are known to provoke
microglial activition. Lipopolysaccharide (LPS), the major portion
of the outer membrane of Gram-negative bacteria, is regarded as the
main risk factor responsible for microglial activation (8–10).
Among the LPS-induced reactions, the overproduction of nitric oxide
(NO) generated by NO synthase (NOS) has received much attention in
activated microglia. Inducible NOS (iNOS) is the most important NOS
involved in microglial activation. In a physiological
microenvironment, NO causes vasoconstriction, acts as a
neuroendocrine mediator in the central nervous system (CNS), and
has protective functions in anti-inflammatory pathways. Elevated NO
levels may also be beneficial in response to immunological stimuli
as a defense mechanism against microbial or viral insults.
Additionally, NO can also be produced in response to factors
resulting from chronic inflammatory conditions, such as PD and AD
(11).
Geniposide, one of the major iridoid glycosides in
fruits (12), has been used in
folk medicine in certain countries due to its antitumor (13,14) and anti-oxidative activities
(15). Currently, geniposide is
known for its neuritogenic and neuroprotective actions (16) and is suggested for the treatment
of neurodegenerative disorders. It has been demonstrated that
geniposide may reduce neuroinflammation and repress brain
microglial activation (17).
However, the mechanism involved remains unclear.
In the present study, we explored the effects of
geniposide on microglial activation. Our data demonstrate that
geniposide attenuates the production of ROS, NO and iNOS by
blocking the phosphorylation of p38, ERK1/2 and nuclear factor-κB
(NF-κB) induced by LPS in N9 cells. Thus, the present study
delivers important new insights into the molecular pathways that
may contribute to the proposed beneficial effects of geniposide in
the treatment of neurodegenerative disorders.
Materials and methods
Chemicals and reagents
Geniposide was obtained from Chengdu Push
Biotechnology Co., Ltd. (Chengdu, China). LPS,
2′,7′-dichlorofluorescein diacetate (DCFH-DA),
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT),
and β-mercaptoethanol were purchased from Sigma (St. Louis, MO,
USA). The ERK1/2 inhibitor (PD98059) and p38 inhibitor (SB203580)
were obtained from Invitrogen (Carlsbad, CA, USA). Reagent kits
used for the measurement of NOS and extracellular NO were obtained
from the Nanjing Jiancheng Bioengineering Institute (Nanjing,
China). 3-Amino,4-aminomethyl-2′,7′-difluorescein
diacetate (DAF-FM DA) and hematoxylin and eosin (H&E) were
acquired from the Beyotime Institute of Biotechnology (Jiangsu,
China). Iscove’s modified Dulbecco’s medium (IMDM), fetal bovine
serum (FBS) and antibiotics (penicillin/streptomycin) were
purchased from Gibco-BRL (Rockville, MD, USA). TRIzol was obtained
from Sangon Biological Engineering Technology and Services
(Shanghai, China). Anti-NF-κB p65 polyclonal antibody was purchased
from the Beyotime Institute of Biotechnology. Anti-inhibitory
factor-κB-α (IκB-α), anti-p38, anti-phospho-p38 (p-p38),
anti-ERK1/2, anti-phospho-ERK1/2 (p-ERK1/2) and anti-β-actin
polyclonal antibodies were purchased from Santa Cruz Biotechnology,
Inc. (Santa Cruz, CA, USA). Anti-iNOS antibody was supplied from
Cell Signaling Technology, Inc. (Boston, MA, USA).
Cell culture and treatment
The N9 murine microglial cell line was a gift from
Dr Yun Bai (Third Military Medical University, Chongqing, China).
The cells were cultured in IMDM supplemented with 10% FBS, 50 μM
β-mercaptoethanol, penicillin (100 U/ml) and streptomycin (100
μg/ml) and maintained at 37°C with 5% CO2 in a
humidified incubator. Pancreatin (0.25%) was used to digest and
passage the culture. The N9 microglial cells were plated into
6-well plates for most of the experiments. When the cells reached
subconfluence they were pre-treated for 24 h with IMDM culture
medium containing various concentrations of geniposide. The cells
were then exposed to LPS (1 μg/ml) diluted in culture medium for 4
h at 37°C after washing twice with phosphate-buffered saline (PBS,
pH 7.4).
Cell viability assay
The MTT conversion test was used to assess the cell
viability (18). N9 microglial
cells were seeded at a density of 7.5×103 cells/well in
96-well plates containing 150 μl of IMDM medium with 10% FBS and
grown to subconfluence. The cells then received 150 μl of IMDM
medium with 10% FBS plus various concentrations of geniposide (1,
10, 100 and 200 μg/ml) or LPS (10, 100 and 1,000 ng/ml) and were
incubated for 24 h, respectively. The cells were then washed with
PBS (pH 7.4) and incubated with MTT (5 mg/ml) in a culture medium
for 3 h at 37°C. The medium was discarded and the formazan blue,
which formed in the cells, was dissolved in 100 μl of DMSO. The
optical density at 490 nm was determined with a Sunrise Remote
Microplate Reader (Grodig, Austria). Cell viability in each well
was presented as percentage of the control level (treated with the
vehicle).
Preparations of RNA extraction and
reverse transcription-polymerase chain reaction (RT-PCR)
Total-RNA was extracted using TRIzol reagent
(Takara, Inc., Dalian, China) according to the manufacturer’s
instructions and RNA concentration was determined using a DNA/RNA
GeneQuant Calculator (from Amersham Biosciences, Piscataway, NJ,
USA). Reverse transcription was carried out in 10 μl of the
reaction mixture containing 1 μg of total RNA, 25 pmol of oligo(dT)
primer, 10 nmol of dNTP mixture, 20 units of RNase inhibitor and
2.5 units of AMV reverse transcriptase (Bioer Technology Co.,
Hangzhou, China) at 42°C for 1 h. PCR amplification was performed
in 20 μl PCR reaction mixture containing 1 μl of cDNA reaction
mixture, 10 nmol of dNTP mixture, 10 pmol of upstream and
downstream primers and 2 units of BioReady rTaq polymerase (Bioer
Technology Co.). PCR amplification to detect differences among the
samples was set as follows: 4 min at 94°C for initial denaturation,
30 cycles x 30 sec at 94°C, 45 sec at 53°C, and 45 sec at 72°C for
iNOS; 30 cycles × 30 sec at 94°C, 30 sec at 54°C, and 30 sec at
72°C for β-actin. Primers used were: β-actin (386 bp) upstream,
5′-GATGGT GGGAATGGGTCAGA-3′ and downstream,
5′-GGAGAGCA TAGCCCTCGTAGAT-3′; iNOS (650 bp)
upstream, 5′-TGGA GCGAGTTGTGGATTGTC-3′ and
downstream, 5′-CCCTTT GTGCTGGGAGTCAT-3′. The PCR
product was electro-phoresed on a 1.5% agarose gel containing 0.1
μg/ml dye (GoldView; SBS Genetech Co., Beiing, China). Gels were
visualized and photographed by a Gel Doc 2000 image analyzer
(Bio-Rad, Hercules, CA, USA). β-actin was used for normalization.
The ratios of the emissions incorporated into the PCR products of
the examined gene to the β-actin products were calculated to
evaluate relative changes in the mRNA expression levels of the
examined genes.
Western blot analysis
For isolation of total cell extracts, treated N9
cells were washed twice in ice-cold PBS and lysed with RIPA lysis
buffer (50 mM Tris with pH 7.4, 150 mM NaCl, 1% Triton X-100, 1%
sodium deoxycholate, 0.1% SDS and 0.05 mM EDTA). Samples were
centrifuged at 12,000 x g for 15 min at 4°C and the supernatant was
collected as total cell lysate. Nuclear and cytoplasmic protein
were extracted with the Nuclear and Cytoplasmic Protein Extraction
kit according to the manufacturer’s instructions (Beyotime
Institute of Biotechnology). Protein concentrations were determined
using the Bicinchoninic Acid Protein Assay kit (Beyotime Institute
of Biotechnology). The cell lysates with 5X loading buffer (125 mM
Tris-HCl, pH 6.8, 10% SDS, 8% dithiothreitol, 50% glycerol
and 0.5% bromochlorophenol blue) were boiled for 10 min, and 50 μg
of protein were loaded per lane on 8–12% SDS-polyacrylamide
gels. Proteins were separated and transferred to 0.45 μm
polyvinylidene fluoride membranes. Membranes were blocked with 5%
skim milk in PBS with 0.1% Tween-20 (PBST) for 1 h. Primary
antibodies against iNOS, NF-κB, IκB-α, p38, p-p38, ERK1/2, p-ERK1/2
and β-actin in PBST were incubated with membranes overnight at 4°C.
The membranes were then washed in PBST 3 times and incubated with
the bound horseradish peroxidase-conjugated secondary antibody for
1 h. After the final wash, protein bands were developed using
enhanced chemiluminescence reagents and densitometric analysis was
performed with the ChemDoc System (Bio-Rad).
Measurement of intracellular ROS
formation
Intracellular ROS formation was assessed based on
the ROS-mediated conversion of non-fluorescent DCFH-DA into DCFH,
as the fluorescence intensity of DCFH reflects enhanced oxidative
stress. Briefly, cells seeded in black 6-well plates were washed
twice with PBS (pH 7.4) and were pre-incubated with various
concentrations of geniposide (20, 40, 80 and 160 μg/ml) for 24 h.
Thereafter, cells were washed twice with (PBS, pH 7.4) and then
exposed to 1 μg/ml of LPS for 4 h and incubated in PBS containing
20 μM of DCFH-DA at 37°C for 2 h in the dark. Subsequently, the
fluorescence of cells in each well was measured at an excitation
wavelength of 485 nm and an emission wavelength of 530 nm using a
FLx800 fluorescence microplate reader (Bio-Tek Instruments, Inc.,
Winooski, VT, USA). Fluorescence values were calculated after
subtracting background fluorescence levels, as measured under
identical conditions but without DCFH-DA.
Flow cytometry for the measurement of
intracellular NO formation
The production of intracellular NO in N9 microglial
cells was evaluated using the NO-specific fluorescent dye, DAF-FM
DA, as described previously (19). Cells seeded in 6-well plates were
detached by 0.125% EDTA-free trypsin and suspended in PBS (pH 7.4)
containing 5 μM of DAF-FM DA at 37°C for 20 min. The cells were
then washed 3 times and re-suspended with PBS (pH 7.4). The
fluorescence in the cells was quantitatively analyzed at an
emission wavelength of 515 nm and an excitation wavelength of 495
nm using a Vantage SE flow cytometer system (Becton-Dickinson, San
Jose, CA, USA).
Analysis of NOS activity
For the assay activity of NOS, cells were seeded in
6-well plates (2×105 cells/well) and treated as
previously described. Cells were washed and immediately mixed with
ice-cold RIPA lysis buffer. The homogenate was centrifuged at
12,000 x g at 4°C for 15 min. The supernatant was harvested and
stored at −80°C. The protein content was detected by the
bicinchoninic acid (BCA) method, using bovine serum albumin as a
reference standard. NOS activities were all determined using
commercially available kits. All procedures completely complied
with the manufacturer’s instructions. Briefly, NOS activities was
determined at 530 nm by the reaction of NO with nucleophiles to
form a chromophoric production.
Measurement of extracellular NO
release
The concentration of nitrites
(NO2−) and nitrates
(NO3−), stable end products of NO, were
determined by the Griess reaction as previously described (20). NO production in the culture medium
was determined by measuring the optical density at 550 nm and
expressed as units/milligram protein.
H&E staining
Cells were seeded in 6-well plates (2×105
cells/well) and placed in a little glass to form a cell climbing
slice. The cells were fixed in 4% formaldehyde for 20 min, and then
rinsed in PBS for 1 min. The cells were incubated for 10 min in
hematoxylin solution. Subsequently, the cells were incubated in
HCl-ethanol (1%) for 10 sec. Subsequent rinsing was followed by
incubation in eosin for 1.5 min. The specimens were covered with
coverslips and were visualized and photographed under a
microscope.
Statistical analysis
Each experiment was performed at least 3 times. The
data are expressed as the means ± standard deviation, analyzed
using the SPSS 11.0 software. The differences between the 2 groups
were analyzed using the Student’s t-test. Differences in the data
between 2 or more groups were analyzed by the analysis of variance
method. P<0.05 indicated that the differences were statistically
significant.
Results
Scavenging effect of geniposide on the
extracellular and intracellular NO production induced by LPS in N9
microglial cells
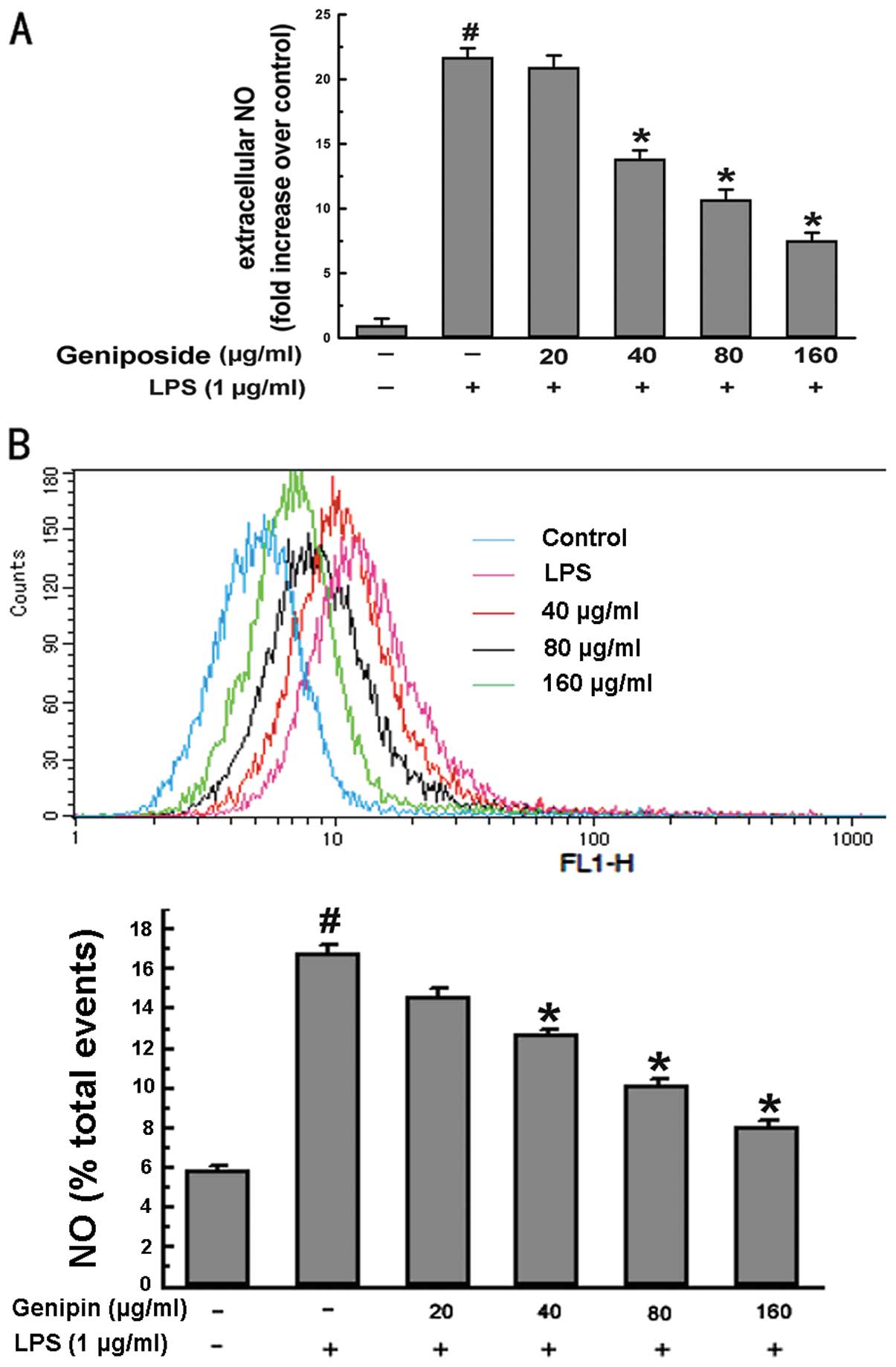
To investigate the effect of geniposide on
LPS-induced extracellular NO production in N9 microglial cells, the
cell culture medium was harvested and the concentration of
accumulated nitrite, the oxidative product of NO, was determined by
the Griess method. The cells were pre-treated with various
concentrations of geniposide (20, 40, 80 and 160 μg/ml) for 20 h
and then co-treated with LPS (1 μg/ml) for 4 h. The results
indicated that extracellular NO induced by LPS in N9 cells
increased by 22-fold compared to the vehicle-treated control group
(P<0.05) (Fig. 1A). By
contrast, pre-treatment with geniposide for 4 h before exposure to
LPS suppressed the production of NO in a dose-dependent manner.
In addition, we assessed intracellular NO by
monitoring the changes in fluorescence intensity. Intracellular NO
in N9 cells increased by 3-fold in response to LPS as compared to
the controls. The cells that were pre-treated with geniposide
before exposure to LPS also showed suppressed intracellular NO
levels in a dose-dependent manner (Fig. 1B). These data illustrate that
geniposide attenuates increasing NO levels induced by LPS in
cells.
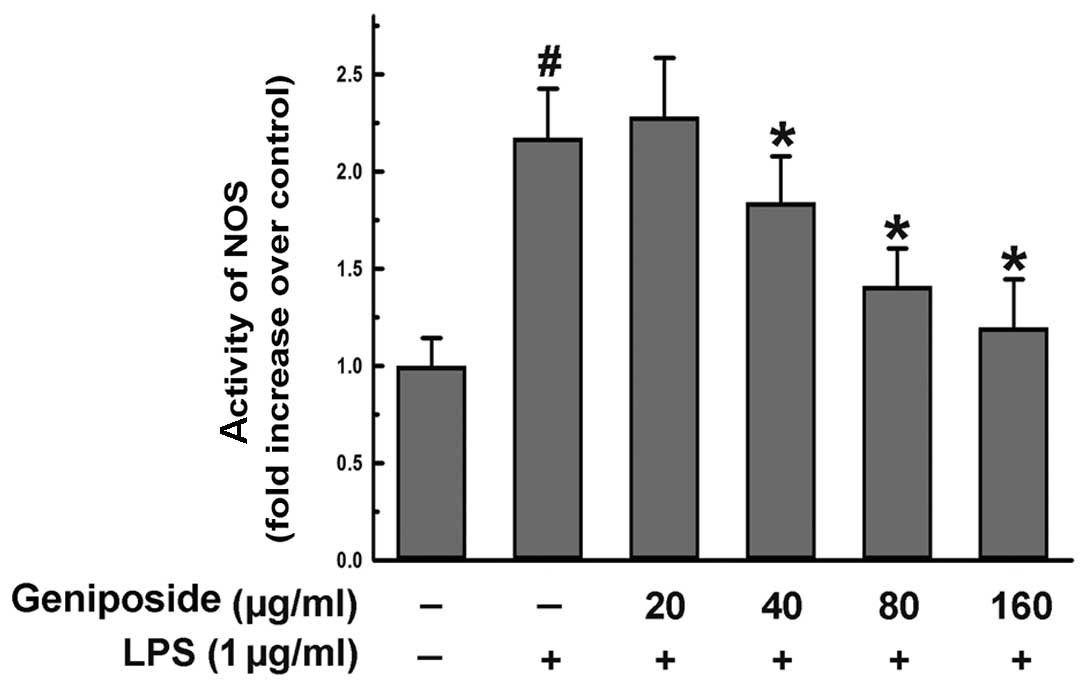
Effect of geniposide on NOS activity
induced by LPS in N9 microglial cells
NO was generated by NOS. To investigate the activity
of NOS, we used commercially available kits as described in
Materials and methods. The cells were pre-treated with geniposide
(20, 40, 80 and 160 μg/ml) for 20 h and then exposed to LPS (1
μg/ml) for 4 h. The activity of NOS induced by LPS was reduced by
geniposide in a dose-dependent manner in the N9 cells (Fig. 2). These results demonstrate that
geniposide suppresses the production of NO by inhibiting NOS
activity.
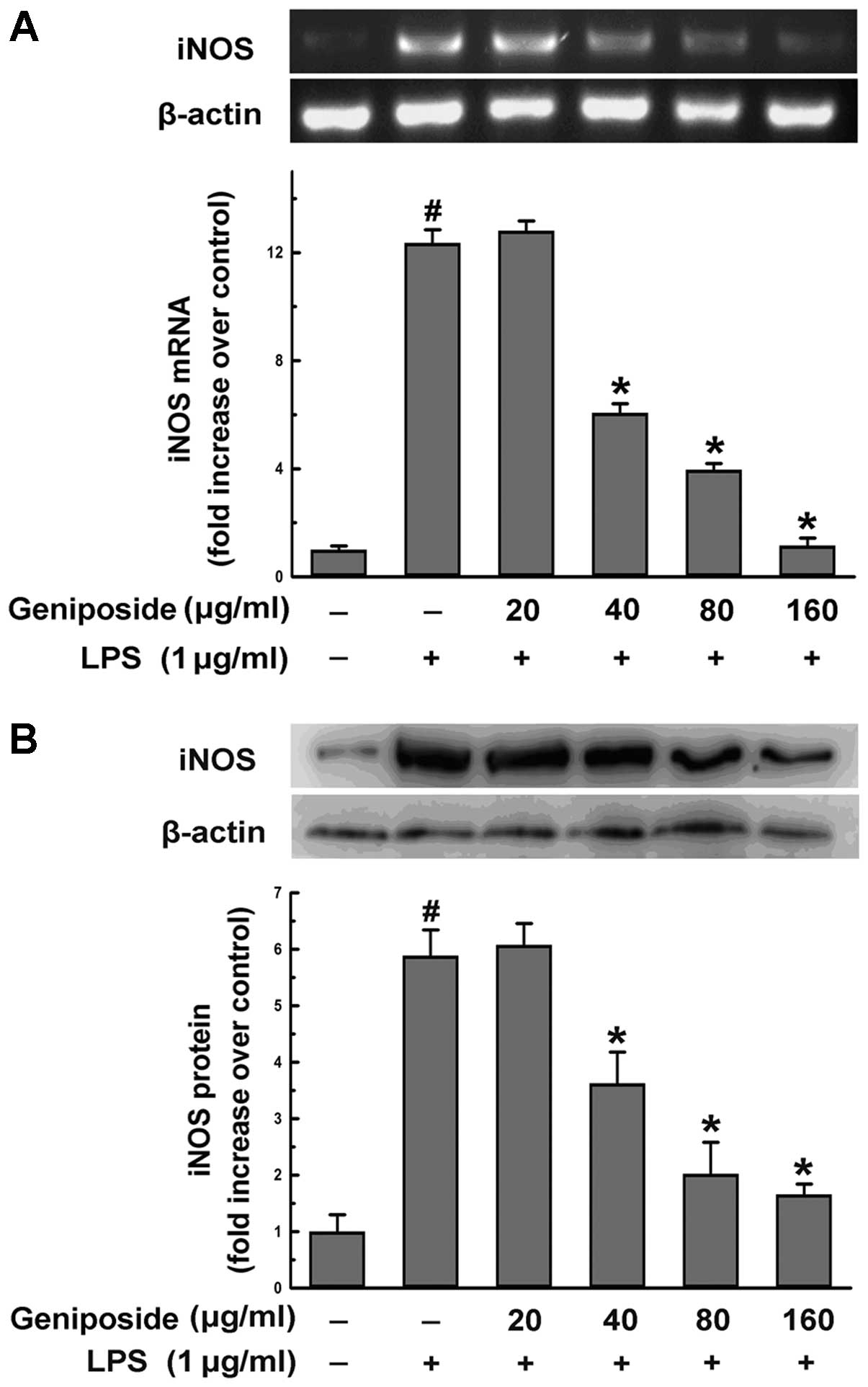
Effect of geniposide on iNOS production
induced by LPS in N9 microglial cells
iNOS plays an important role in generating the
production of NO in cells. The expression of iNOS is usually
increased in neuron cells induced by inflammatory factors, such as
LPS. To explore whether geniposide suppressing the production of NO
is associated with the expression of iNOS, we examined the mRNA
levels of iNOS by RT-PCR and the protein level of iNOS using
western blot analysis. The cells were pre-treated with geniposide
(20, 40, 80 and 160 μg/ml) for 20 h and then exposed to LPS (1
μg/ml) for 4 h. The expression of iNOS mRNA induced by LPS was
significantly reduced (35.0, 50.8 and 91.7%; P<0.05) when
geniposide was administered at a concentration of over 40 μg/ml
(Fig. 3A). The increased levels
of the iNOS protein induced by LPS were reduced by geniposide
pre-treatment in a dose-dependent manner (Fig. 3B). Our results demonstrated that
the increasing activity of NOS in N9 cells induced by LPS was
relative to the expression of iNOS and that geniposide inhibited
the upregulated gene expression of iNOS.
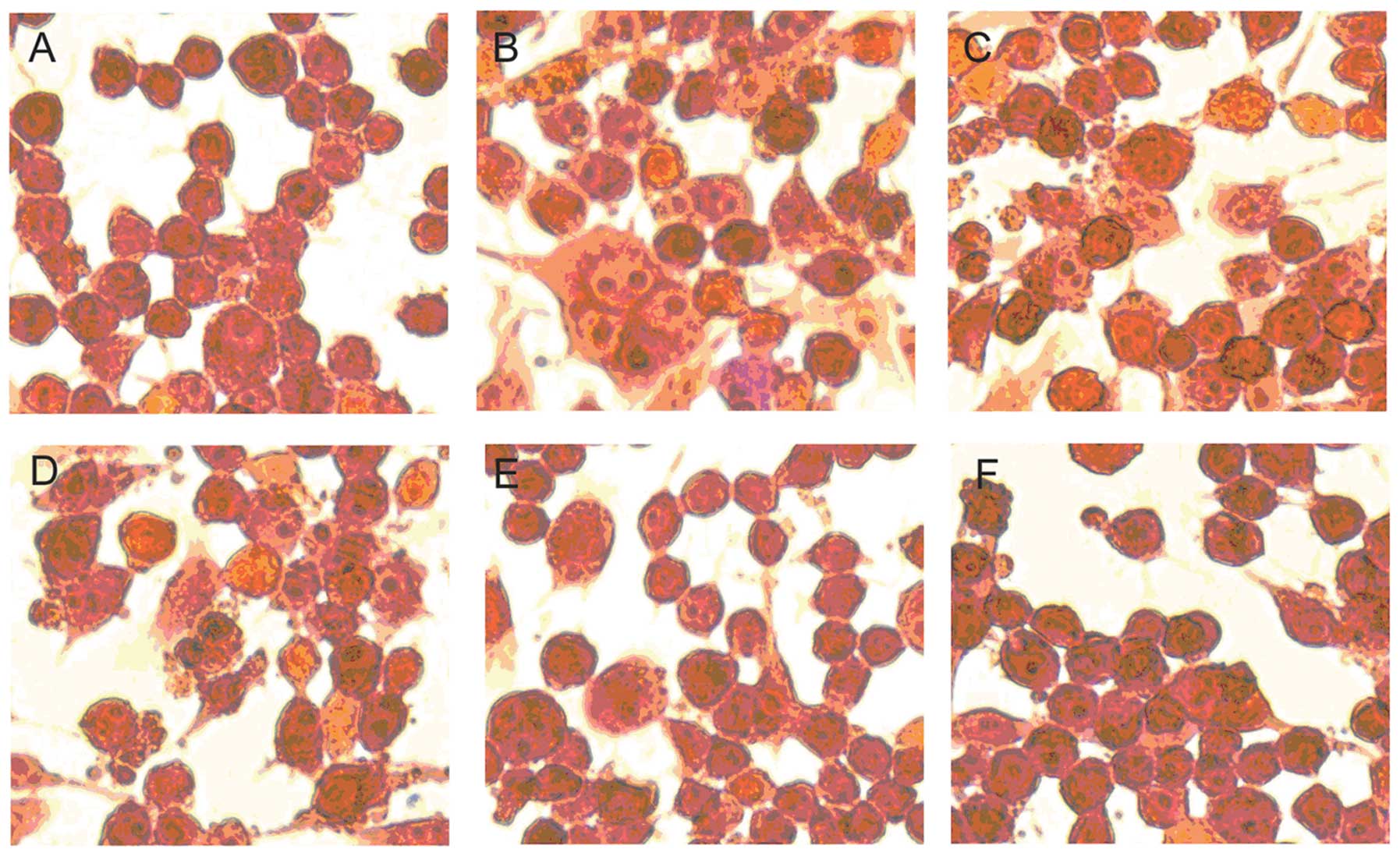
Suppressive effect of geniposide on
microglial activation induced by LPS
To clarify the effect of geniposide on microglial
activation induced by LPS, we observed the change of morphology of
the N9 cells by H&E staining after treatment with different
metarials. The cells were pre-treated with various concentrations
of geniposide (20, 40, 80 and 160 μg/ml) for 20 h and co-treated
with LPS (1 μg/ml) for 4 h. The relatively less microglial
activation in the vehicle-treated cells was observed (Fig. 4A). By contrast, after being
exposed to LPS (1 μg/ml) for 4 h, the cell morphology showed
variant cells with an amoeba-like shape (Fig. 4B). It revealed that LPS
stimulation induced a marked microglial activation. On the other
hand, the microglial activation induced by LPS was suppressed by
pre-treatment with geniposide for 24 h (Fig. 4C–F). These results suggest that
geniposide suppresses microglial activation induced by LPS in a
dose-dependent manner.
Inhibitory effect of geniposide on
intracellular ROS formation in N9 cells
The production of ROS is a risk factor in microglial
activation induced by inflammatory factors. Therefore, we assessed
intracellular ROS induced by LPS in N9 cells by monitoring the
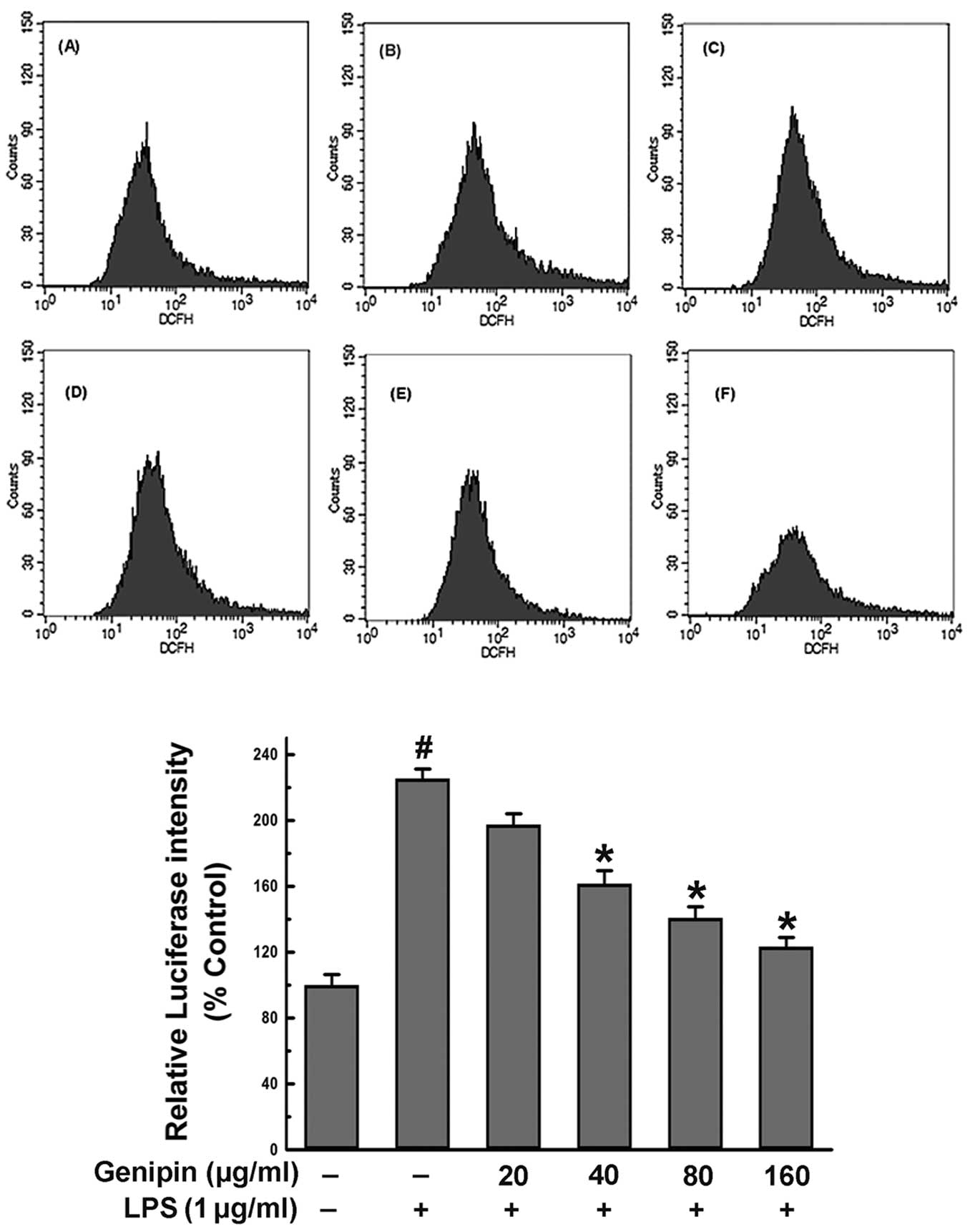
changes in fluorescence intensity of the probe, DCFH-DA (Fig. 5). The results indicated that the
amount of cells with fluorescence induced by 1 μg/ml LPS for 4 h
significantly increased by 2.26-fold compared to the
vehicle-treated control group (P<0.05). By contrast,
pre-treatment with geniposide for 20 h before exposure to LPS
suppressed the production of DCFH fluorescence in a dose-dependent
manner. The suppressive effect of geniposide (160 μg/ml) was
considered to be significant (54.6%) compared to the group treated
with LPS alone (P<0.05). These results suggest that one of the
reasons that geniposide suppresses microglial activation is the
decrease in the production of ROS.
Geniposide and LPS treatment display no
inhibitory effect on N9 cell viability
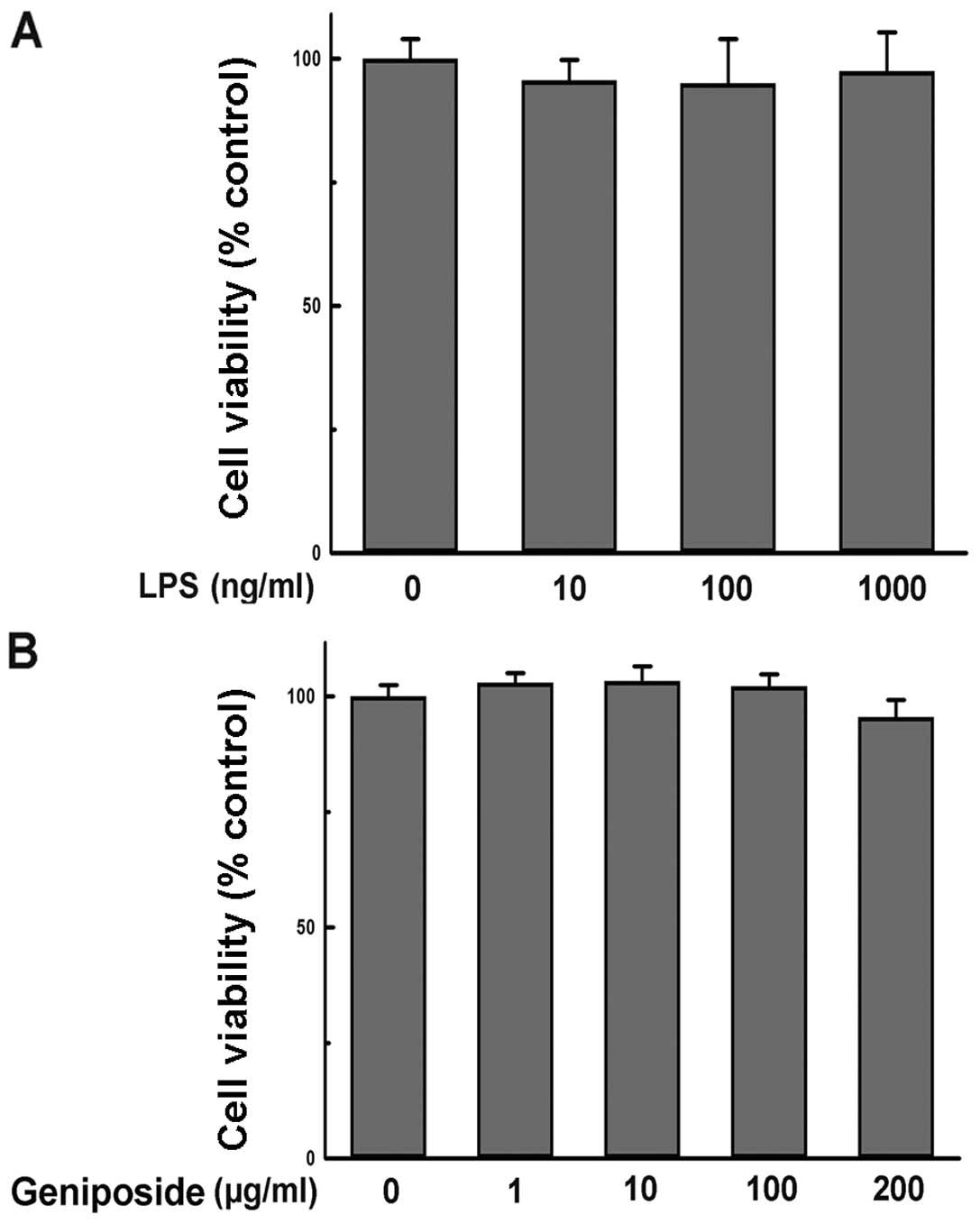
To evaluate whether the administered concentration
of geniposide or LPS influenced N9 cell viability, the effect of
geniposide and LPS on N9 cell viability was determined by MTT
assay. The results showed that incubation with LPS (10–1,000 ng/ml)
alone for 24 h had no effect on the viability of N9 cells (Fig. 6A). Additionally, treatment with
geniposide (1–200 μg/ml) for 24 h showed no inhibitory effect on N9
cell viability in comparison with the vehicle-treated group
(Fig. 6B). Thus, the effect of
geniposide in preventing microglial activation was not caused by
the cytotoxicity of reagents in previous experiments.
Effect of geniposide on the
phosphorylation of ERK and p38 mitogen-activated protein kinase
(MAPK) induced by LPS in N9 cells
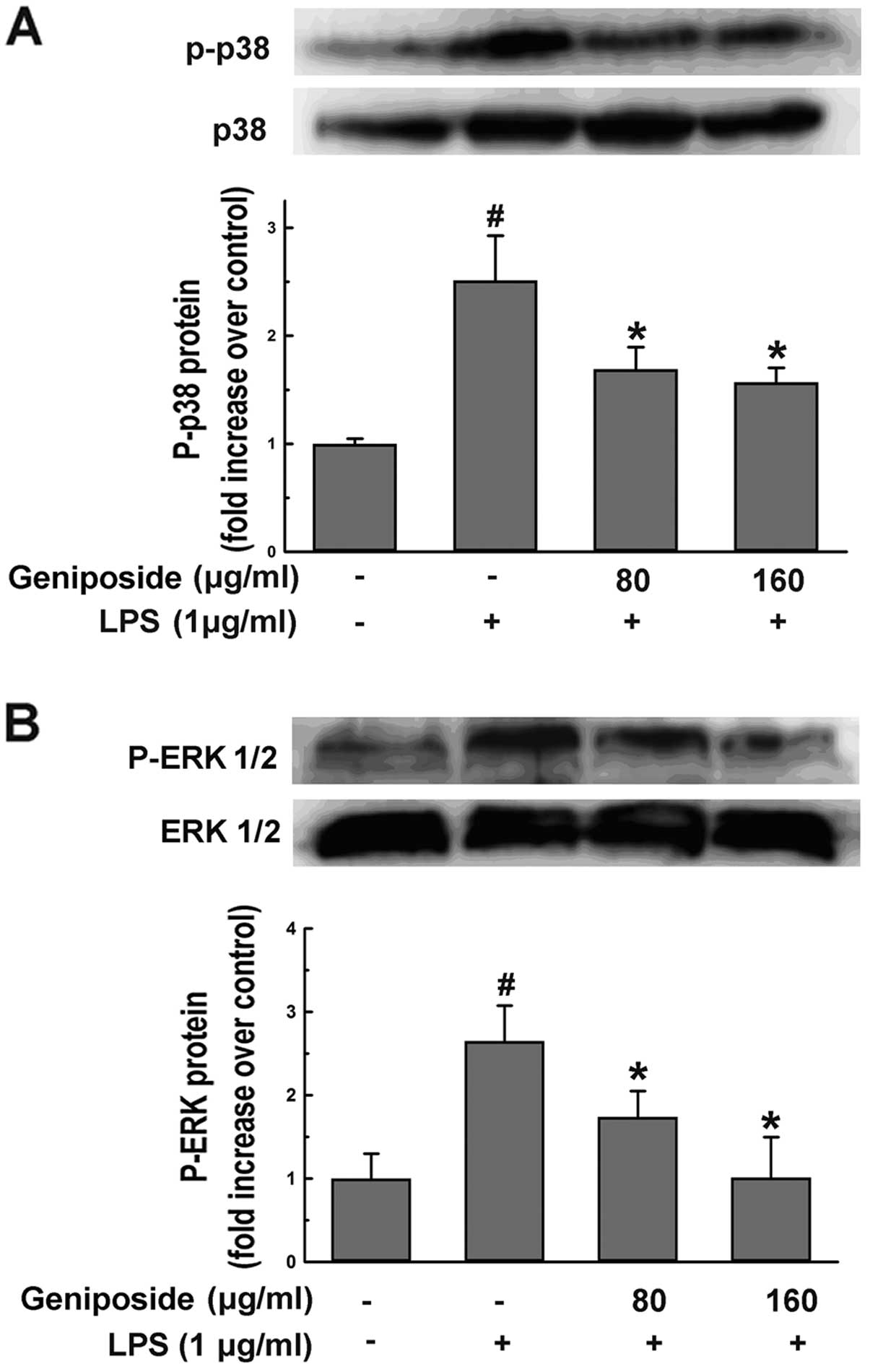
To investigate whether geniposide influences
underlying pathways followed by the upregulation of ROS, NO and
iNOS levels induced by LPS in microglial cells, we examined the
effect of geniposide on LPS-induced p38 and ERK1/2 activation in N9
cells. The cells were pre-treated with geniposide (80 and 160
μg/ml) for 20 h and then co-treated with LPS (1 μg/ml) for 30 min.
The LPS-induced phosphorylation of p38 (Fig. 7A) and ERK1/2 (Fig. 7B) was inhibited by geniposide.
These findings suggested that the phosphorylation of p38 and ERK1/2
involved in the production of ROS, NO and iNOS induced by LPS was
downregulated by geniposide.
Effect of p38 and ERK1/2 inhibitors on
iNOS protein expression induced by LPS in N9 cells
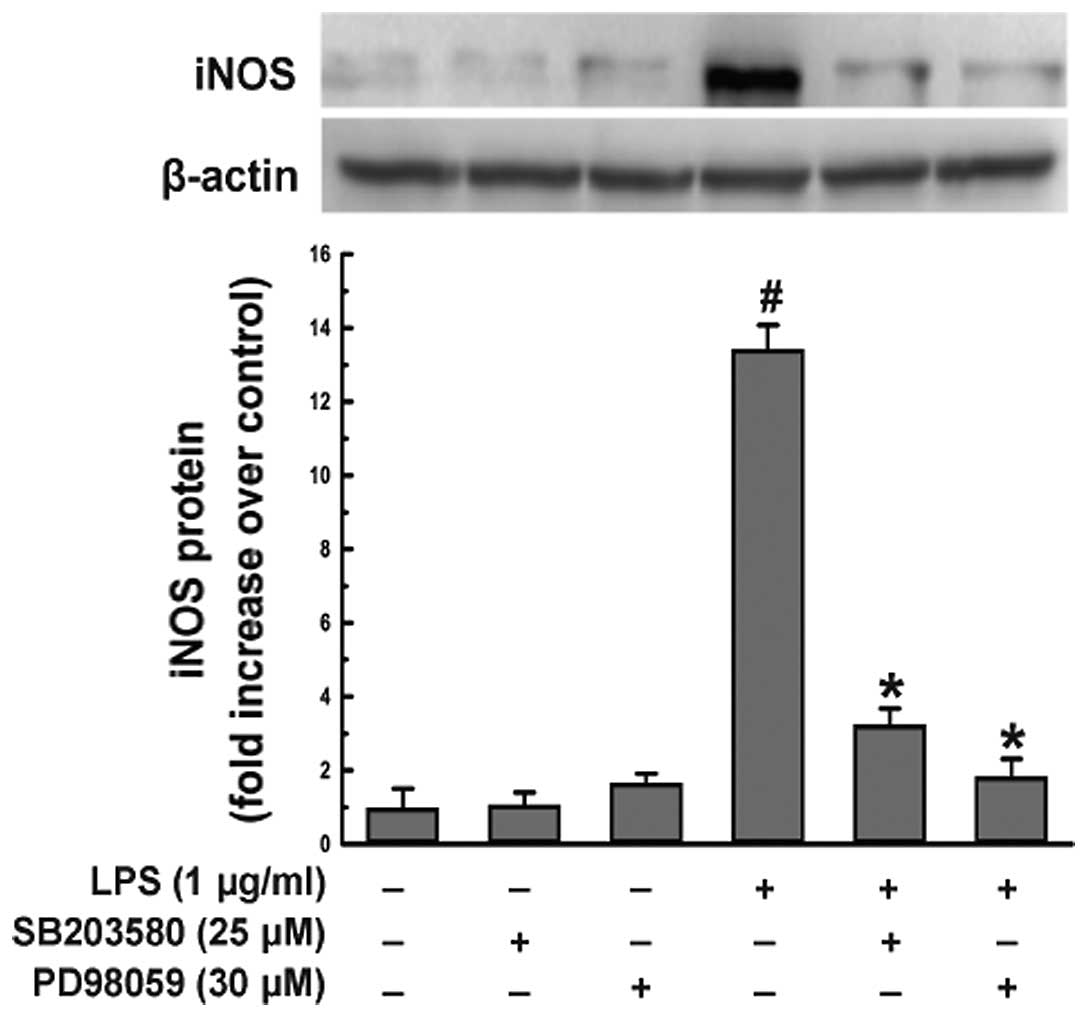
To confirm that geniposide suppresses the expression
of iNOS induced by LPS in microglial cells via the p38 and ERK1/2
MAPK signaling pathway, N9 cells were pre-treated with SB203580 (25
μM), p38 MAPK inhibitor, or PD98059 (30 μM), ERK1/2 inhibitor, for
1 h and exposed to LPS (1 μg/ml) for 20 h. Treatment with LPS for
20 h induced a remarkable upregulation of the iNOS protein level in
N9 cells (Fig. 8). By contrast,
the iNOS protein level decreased significantly following
pre-treatment with SB203580 or PD98059 (P<0.05) compared to the
LPS-treated group. The iNOS protein level was not decreased when N9
cells were treated with SB203580 or PD98059 alone compared to the
vehicle-treated group (Fig.
8).
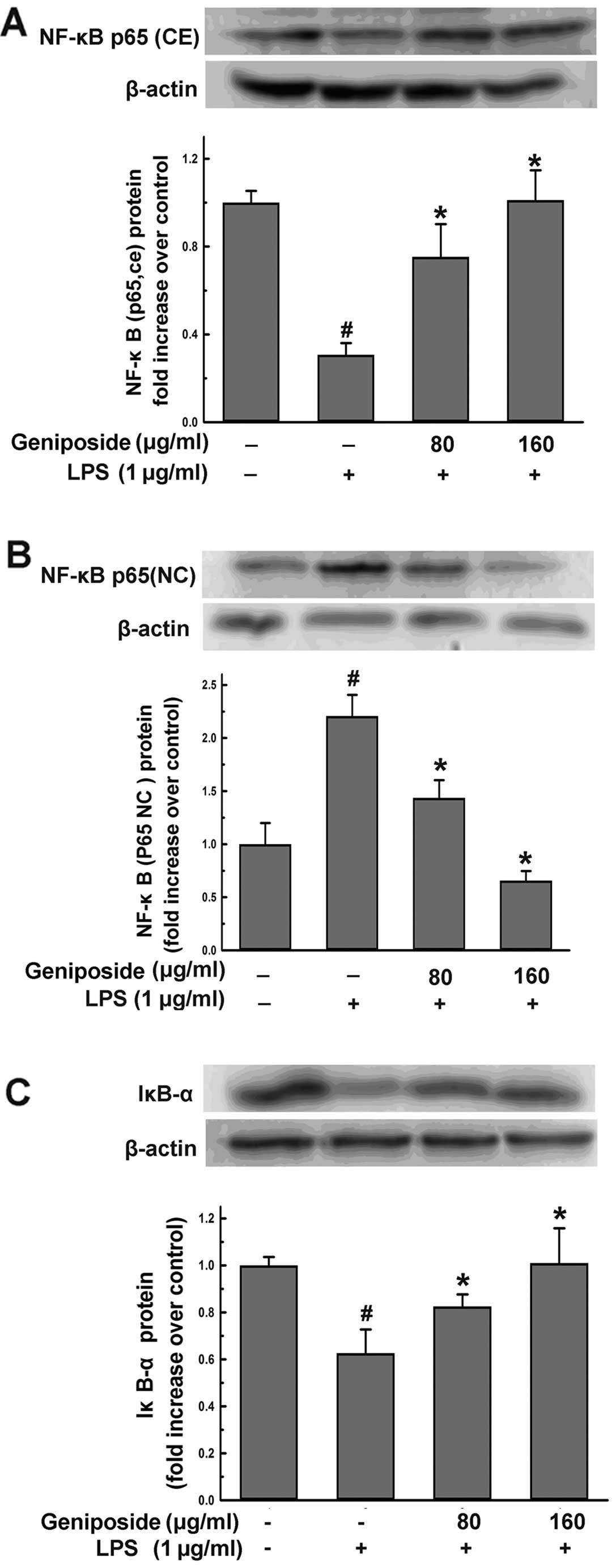
Geniposide inhibits liberation of IκB
from NF-κB complex and suppresses NF-κB translocation into nucleus
induced by LPS in N9 cells
To determine whether the downregulation of ROS, NO
and iNOS levels in microglial cells is regulated by the NF-κB
signaling pathway, we examined the NF-κB levels in the nucleus and
endochylema in N9 cells, respectively, and also examined the IκB-α
levels in the endochylema by western blot analysis. N9 cells were
pre-treated with geniposide (80 and 160 μg/ml) for 18 h and then
co-treated with LPS (1 μg/ml) for 6 h. The treatment with LPS for 6
h induced a remarkable downregulation of NF-κB in the nucleus and
an upregulation of NF-κB in the endochylema (Fig. 9). By contrast, the intra-nuclear
NF-κB protein increased significantly by pre-treatment with
geniposide (80 and 160 μg/ml) (P<0.05) compared to the
LPS-treated groups. Inversely, the levels of NF-κB in the
endochylema decreased markedly (P<0.05) compared to those of the
LPS-treated groups (Fig. 9A and
B). During the activation of NF-κB, its inhibitory protein,
IκB-α, degraded rapidly in the LPS-treated N9 cells (Fig. 9C). However, IκB-α levels increased
significantly by pre-treatment with geniposide (80 and 160 μg/ml)
(P<0.05). These results indicate that geniposide inhibits NF-κB
activation and the degradation of IκB-α induced by LPS in N9
cells.
Discussion
As a type of cell of the macrophage lineage in the
CNS, microglial cells are quiescent in the normal brain. However,
these cells may be activated by LPS during bacterial infection
(21,22). It is well known that the activated
microglia produce NO and pro-inflammatory cytokines, such as TNF-α,
which alter their cellular functions and initiate an inflammatory
cascade associated with several neurodegenerative diseases
(21,23,24). To the best of our knowledge, our
results demonstrate for the first time that geniposide blocks the
underlying pathways of p38, ERK1/2 and NF-κB followed by the
decrease in ROS, NO and iNOS levels in LPS-treated microglial
cells. Our data suggest that geniposide may be beneficial for the
treatment of neurodegenerative diseases.
NO is accepted as an active messenger molecule in
the cardiovascular and nervous systems. NO is formed endogenously
by the conversion of L-arginine to L-citrulline by NOS (25). It has been shown that activated
microglial cells kill neurons via NO from iNOS by inhibiting
neuronal respiration (26). We
found that pre-treatment with geniposide not only inhibited NO
secretion, but also suppressed iNOS at both the protein and mRNA
levels in LPS-treated N9 cells. This is one of the proposed
mechanisms by which geniposide prevents microglial activation
induced by LPS.
Quiescent microglia cells are round and small, but
LPS-induced microglial cells are disfigured and increase in size,
becoming disfigured. The cells pre-treated with various
concentrations of geniposide (20, 40, 80 and 160 μg/ml) did not
become disfigured by LPS treatment (Fig. 4). ROS are normal metabolites of
oxidation-reduction reaction in cells, whereas overproduction of
ROS induces a series of physiopathological events (27). Based on this fact, the suppression
of ROS may be an effective way to protect cells from inflammatory
damage. Therefore, we examined the intracellular ROS formation in
N9 cells. Our findings clearly showed that geniposide suppressed
the production of ROS in a dose-dependent manner. There was no
significant cytotoxic effect on cell viability when N9 cells were
incubated with geniposide (1–200 μg/ml) for 24 h, implying that the
downregulation of NO, iNOS and ROS levels by geniposide was not due
to a decrease in cell numbers.
Of note, the effects of geniposide on the
downregulation of ROS, NO and iNOS levels in LPS-treated microglial
cells occurred through the activation of multiple signaling
pathways. We showed that geniposide inhibited the signaling
pathways which LPS activated (p38 and ERK1/2 MAPK) by acting on
NF-κB and IκB-α. It has been documented that NF-κB is a modulator
of iNOS expression in microglial cells (28). We found that geniposide remarkably
downregulated the increase in phosphorylated levels of p38 MAPK and
ERK1/2 in LPS-treated N9 cells. The N9 cells were pre-treated with
specific MAPK inhibitors, SB203580 against p38 MAPK and PD98059
against ERK1/2, and then the expression of iNOS was determined. It
was shown that the inhibitors significantly suppressed the
overexpression of the iNOS protein in the LPS-treated N9 cells.
These results suggested that the phosphorylation of p38 MAPK and
ERK1/2 was attenuated in the suppression of NO, ROS and iNOS by
geniposide in LPS-treated N9 cells.
The NF-κB translocation into the nucleus is preceded
by the phosphorylation of IκB-α, a protein that normally sequesters
the NF-κB complex in the cytosol in an inactive form. Once
phosphorylated, IκB-α undergoes ubiquitination and proteolytic
degradation (29). Furthermore,
the LPS-induced NF-κB activation and subsequent NO and iNOS
production, a pivotal role for p38 MAPK and ERK1/2, in underlying
pathways of microglial cells was examined. Thus, on the basis of
our results, it was suggested that geniposide inhibited IκB-α
proteolytic degradation and subsequent translocation of NF-κB into
the nucleus.
AD is an age-dependent neurodegenerative disorder of
the cortex and hippocampus, eventually leading to cognitive
impairment of the brain. Only a limited number of therapeutic
options are currently available to treat this disease. In the
present study, we targeted the MAPK-NF-κB signaling pathway as a
novel approach to the management of AD. Geniposide inhibited
microglial activation, attenuated ROS production or the production
of NO and the expression of iNOS by blocking p38, ERK1/2 and IκB-α
activation induced by LPS in N9 cells. These effects of geniposide
are significant for suppressing the injury of neurons and
preventing and treating several neurodegenerative diseases, such as
PD or AD. In this study, we investigated various other signaling
pathways and their involvement in the effects induced by geniposide
in order to discover additional treatment options for
neurodegenerative diseases.
Abbreviations:
|
NO
|
nitric oxide;
|
|
iNOS
|
inducible nitric oxide synthase;
|
|
ROS
|
reactive oxygen species;
|
|
LPS
|
lipopolysaccharide;
|
|
MAPK
|
mitogen-activated protein kinase;
|
|
NF-κB
|
nuclear factor-κB
|
Acknowledgements
We are grateful for financial support
received from the National Nature Science Foundation of China
(project no. 81070222) and the Major National Science and
Technology Projects of China (project no. 2010ZX09401-306-1-1) and
the science and technology innovation ability of construction
projects of Chongqing (project no. CSTC, 2010AA5058).
References
|
1.
|
WF HickeyH KimuraPerivascular microglial
cells of the CNS are bone marrow derived and present antigen in
vivoScience239290292198810.1126/science.32760043276004
|
|
2.
|
RB RockG GekkerS HuRole of microglia in
central nervous system infectionsClin Microbiol
Rev17942964200410.1128/CMR.17.4.942-964.200415489356
|
|
3.
|
H WilmsL ZeccaP RosenstielInflammation in
Parkinson’s diseases and other neurodegenerative diseases: cause
and therapeutic implicationsCurr Pharm Des13192519282007
|
|
4.
|
J PalaceInflammation versus
neurodegeneration: consequences for treatmentJ Neurol
Sci2594649200710.1016/j.jns.2006.05.07217418237
|
|
5.
|
YS KimTH JohMicroglia, major player in the
brain inflammation: their roles in the pathogenesis of Parkinson’s
diseaseExp Mol Med38333347200616953112
|
|
6.
|
J RogersD MastroeniB
LeonardNeuroinflammation in Alzheimer’s disease and Parkinson’s
disease: are microglia pathogenic in either disorder?Int Rev
Neurobiol822352462007
|
|
7.
|
PL McGeerEG McGeerGlial reactions in
Parkinson’s diseaseMov Disord234744832008
|
|
8.
|
L QinY LiuT WangNADPH oxidase mediates
lipopolysaccharide-induced neurotoxicity and proinflammatory gene
expression in activated microgliaJ Biol
Chem27914151421200410.1074/jbc.M30765720014578353
|
|
9.
|
XL BiJY YangYX DongResveratrol inhibits
nitric oxide and TNF-alpha production by
lipopolysaccharide-activated microgliaInt
Immunopharmacol585193200515589480
|
|
10.
|
YM ZhuNS AzahriD YuPJ WollEffects of COX-2
inhibition on expression of vascular endothelial growth factor and
interleukin-8 in lung cancer cellsBMC
Cancer8218200810.1186/1471-2407-8-21818671849
|
|
11.
|
RN SahaK PahanRegulation of inducible
nitric oxide synthase gene in glial cellsAntioxid Redox
Signal8929947200610.1089/ars.2006.8.92916771683
|
|
12.
|
T AkaoK KobayashiM AburadaEnzymic studies
on the animal and intestinal bacterial metabolism of geniposideBiol
Pharm Bull1715731576199410.1248/bpb.17.15737735197
|
|
13.
|
SW WangCY LaiCJ WangInhibitory effect of
geniposide on aflatoxin B1-induced DNA repair synthesis in primary
cultured rat hepatocytesCancer
Lett65133137199210.1016/0304-3835(92)90157-Q1511417
|
|
14.
|
CH PengCN HuangSP HsuCJ WangPenta-acetyl
geniposide-induced apoptosis involving transcription of NGF/p75 via
MAPK-mediated AP-1 activation in C6 glioma
cellsToxicology238130139200710.1016/j.tox.2007.05.02917651887
|
|
15.
|
JH LiuF YinLX GuoXH DengYH
HuNeuroprotection of geniposide against hydrogen peroxide induced
PC12 cells injury: involvement of PI3 kinase signal pathwayActa
Pharmacol Sin30159165200910.1038/aps.2008.2519151742
|
|
16.
|
M YamazakiK ChibaNeurotrophic effects of
genipin on Neuro2a cellsJ Health
Sci51687692200510.1248/jhs.51.687
|
|
17.
|
KN NamYS ChoiHJ JungGenipin inhibits the
inflammatory response of rat brain microglial cellsInt
Immunopharmacol10493499201010.1016/j.intimp.2010.01.01120123040
|
|
18.
|
T MosmannRapid colorimetric assay for
cellular growth and survival: application to proliferation and
cytotoxicity assaysJ Immunol
Methods655563198310.1016/0022-1759(83)90303-46606682
|
|
19.
|
M LantornoH ChenJA KimGhrelin has novel
vascular actions that mimic PI 3-kinase-dependent actions of
insulin to stimulate production of NO from endothelial cellsAm J
Physiol Endocrinol
Metab292756764200710.1152/ajpendo.00570.200617106060
|
|
20.
|
L MarcocciJJ MaguireMT Droy-LefaixL
PackerThe nitric oxide-scavenging properties of Ginkgo biloba
extract EGb 761Biochem Biophys Res
Commun201748755199410.1006/bbrc.1994.17648003011
|
|
21.
|
F Gonzalez-ScaranoG BaltuchMicroglia as
mediators of inflammatory and degenerative diseasesAnnu Rev
Neurosci22219240199910.1146/annurev.neuro.22.1.21910202538
|
|
22.
|
G StollS JanderThe role of microglia and
macrophages in the pathophysiology of the CNSProg
Neurobiol58233247199910.1016/S0301-0082(98)00083-510341362
|
|
23.
|
GW KreutzbergMicroglia: a sensor for
pathological events in the CNSTrends
Neurosci19312318199610.1016/0166-2236(96)10049-78843599
|
|
24.
|
L MedaMA CassatellaGI SzendreiActivation
of microglial cells by β-amyloid protein and
IFN-γNature3746476501995
|
|
25.
|
B MayerK SchmidtP HumbertE
BöhmeBiosynthesis of endothelium-derived relaxing factor: a
cytosolic enzyme in porcine aortic endothelial cells
Ca2+-dependently converts L-arginine into an activator
of soluble guanylyl cyclaseBiochem Biophys Res
Commun164678685198910.1016/0006-291X(89)91513-12573351
|
|
26.
|
A Bal-PriceGC BrownInflammatory
neurodegeneration mediated by nitric oxide from activated
glia-inhibiting neuronal respiration, causing glutamate release and
excitotoxicityJ Neurosci21648064912001
|
|
27.
|
G ZalbaA FortunoG San JoseOxidative
stress, endothelial dysfunction and cerebrovascular
diseaseCerebrovasc Dis24Suppl 1S24S29200710.1159/000107376
|
|
28.
|
HW JungCH YoonKM ParkHexane fraction of
Zingiberis Rhizoma Crudus extract inhibits the production of
nitric oxide and proinflammatory cytokines in LPS-stimulated BV2
microglial cells via the NF-kappaB pathwayFood Chem
Toxicol47119011972009
|
|
29.
|
HL PahlActivators and target genes of
Rel/NF-κB transcription factorsOncogene18685368661999
|