Introduction
The IGF-1/PTEN/Akt/FoxO signaling pathway exerts
important physiological effects on many types of animal cells
(1–3). Transduction of signals through the
insulin-like growth factor 1 (IGF-1) receptor triggers a multiple
series of intra-cellular phosphorylation events as well as those
activating several signaling pathways which prevent cell death
(4). The phosphatidylinositol
3-kinase (PI3K)/Akt pathway predominantly activated by IGF-1 is a
strong cell survival cascade. Akt [also called protein kinase B
(PKB)] is a serine/threonine protein kinase downstream of PI3K
(5) and is an important regulator
of cell proliferation, cell growth and cell survival (6). To date, three members of the Akt
family have been isolated: Akt-1, Akt-2 and Akt-3. Although they
are products of different genes, they are closely related to one
another, with >80% amino acid sequence identity. The three genes
are expressed differentially, with a broader expression for Akt-1
and Akt-2 and a more restricted expression for Akt-3 (7). Phosphatase and tensin homologue
deleted on chromosome 10 (PTEN) is a tumor-suppressor gene which
encodes a dually specific phosphatase that recognizes both lipid
and peptide substrates, including phosphatidylinositol (3,4,5)-trisphosphate (PIP3), a product of
PI3K. Through its lipid phosphatase activity, PTEN controls Akt
signaling and its downstream targets responsible for cell size,
cell migration, cell cycle, cell death and focal adhesion formation
(8). A downstream target of
IGF-1/PTEN/Akt signaling is the O subfamily of forkhead box (FoxO)
proteins, which is phosphorylated and thereby inhibited by
activated Akt (9). Four members,
FoxOs, FoxO3a, FoxO1, FoxO4 and FoxO6, have been reported in
mammalian cells (10).
Phosphorylation of FoxO proteins by Akt results in cytoplasmic
retention and inactivation, and consequently inhibits the
expression of FoxO-regulated genes which control the cell cycle,
cell death and cell metabolism. The shuttling of FoxO between the
cytoplasm and nucleus is a key step of cell apoptosis (11).
The water-immersion-and-restraint-stress (WRS) rat
has consistently been used as an animal model of gastric mucosal
lesions (12–16). Previous studies have reported that
activated neutrophils are critically involved in the development
and healing of WRS-induced gastric mucosal injury (17). In addition, the gastrointestinal
tract has been identified as one of the most sensitive target
tissues for IGF-1, which is responsible for various important
biological functions, including promotion of the differentiation of
various cell types and potent anti-apoptotic activity (4). It has been reported that
reperfusion-induced hepatic apoptosis may be reduced by increasing
IGF-1 production (18). IGF-1 has
also been shown to reduce tissue injury through prevention of cell
death in animal models of renal ischemia/reperfusion (I/R)
(19). Among the various
activities of IGF-1, its anti-apoptotic activity has been shown to
play an important role in the reduction of I/R-induced tissue
injury by attenuating inflammatory responses (18,19). In particular, previous studies
demonstrated that gastric ulceration triggered an ∼3-fold increase
in IGF-1 expression in epithelial cells of the ulcer margin. The
upregulation of IGF-1 in the gastric ulcer margin accelerated
gastric ulcer healing by promoting cell re-epithelialization,
proliferation and COX-2 expression via the PI3K pathway (17,20,21). Furthermore, it was reported that
IGF-1 reduced WRS-induced gastric mucosal injury by inhibiting
gastric accumulation of neutrophils through inhibition of caspase-3
activation by PI3K/Akt signaling (22). Our previous studies demonstrated
the cell-specific and age-dependent expression patterns of FoxO4
and FoxO3a proteins in the duodenum, and some involvement in the
development and growth performance of the rat duodenum (23). We also found that FoxO4 is a
primary forkhead transcriptional factor localized in the
gastrointestinal tracts of the pig (24).
The aim of the present study was to determine
whether the IGF-1/PTEN/Akt/FoxO signaling pathway is involved in
the protection against gastric ulcers. In the rat gastric-ulcer
model, we analyzed the expression and localization of IGF-1, PTEN,
Akt and FoxO by immunohistochemistry and real-time polymerase chain
reaction (PCR), respectively. In addition, we detected cell
apoptosis through the TUNEL method and the measurement of caspase-3
activity.
Materials and methods
WRS-induced gastric mucosal lesion
formation in rats
All experiments were carried out on intact male
Sprague-Dawley rats (9–11 weeks old; Qinglongshan Experimental
Animal Breeding Farm, Nanjing, China), weighing 200–220 g. All
procedures were designed in accordance with accepted ethical
standards for animal experimentation and the guidelines established
by the Institutional Animal Care and Use Committee, Nanjing
Agricultural University. Uniform commercial diets used in the
experiment were also purchased from Qinglongshan Experimental
Animal Breeding Farm. Regular rat chow and tap water were allowed
ad libitum. Rats were housed individually at room
temperature (25°C) with a 12:12 h light/dark cycle and humidity of
65–70%. Before each experiment, animals were deprived of food but
not water for 24 h. The animals were then placed in a restraint
cage and immersed in a water bath (20±2°C) to the level of the
xiphoid process as described previously (12). Some animals were sacrificed after
3 and 7 h of WRS, and the rest were normally fed starting 1 h later
and sacrificed at various time points (4, 8 and 15 days) after 7 h
WRS. The animals were anesthetized by an intraperitoneal injection
of ether. Their stomachs were removed and filled with 2 ml of 1%
formalin and immersed in 1% formalin for 24 h. The stomachs were
then cut along the greater curvature and examined for mucosal
lesions. Since most gastric mucosal lesions were linear and almost
always <2 mm wide, the total length (mm) of each linear
hemorrhagic erosion was measured as the ulcer index (UI) (mm) by an
independent observer blinded to the previous treatment, as
previously described (25).
Assessment of apoptotic cell number
The sections were rehydrated as described above, and
the terminal deoxynucleotidyl transferase UTP nick-end labeling
(TUNEL) method was performed with the TUNEL apoptosis kit direct
(Beyotime Institute of Biotechnology) as described previously
(26). Briefly, gastric mucosal
cells were counterstained with
2-(4-amidinophenyl)-1H-indole-6-carboxamidine dihydrochloride
(DAPI) (Beyotime Institute of Biotechnology) to label the DNA of
all nuclei, and fragmented DNA was end-labeled with fluoroscein
isothiocyanate (FITC)-labeled dUTP using terminal transferase. The
gastric mucosa was then observed using a fluorescence microscope.
Sections that were pretreated with DNase I to nick all DNA served
as positive controls. For negative controls, dUTP was omitted,
resulting in uniformly negative staining. Ten optical fields,
∼500–1000 cells, were counted in each slide under high power (x400)
microscopy, and the number of positive cells per field was
expressed as the apoptotic index. These experiments were performed
in triplicate with 6 mice/group/experiment.
Measurement of caspase-3 activity
Activity of caspase-3 was detected using a
commercially available caspase-3 activity kit (Beyotime Institute
of Biotechnology) with Ac-DEVD-pNA as the colorimetrically specific
substrate. In brief, gastric mucosal samples (n=6 for each
treatment) were weighed and homogenized in lysis buffer containing
10 mM/l HEPES/KOH (pH 7.2), 2 mM/l EDTA, 0.1% CHAPS, 5 mM/l
dithiothreitol, 1 mM/l phenylmethyl-sulfonylfluoride, 10 μg/ml
aprotinin and 20 μg/ml leupeptin. The lysate was centrifuged at
20,000 x g for 10 min at 4°C, and supernatants were incubated for 7
h at 37°C with 10 μl caspase-3 substrate (Ac-DEVD-pNA, 2 mM/l).
Substrate cleavage was measured with a spectrofluorometer at 405 nm
and was corrected as enzyme activity according to standard curve
content in the lysate. The activity of caspase-3 was expressed as
values of enzyme activity compared with the control (27,28).
Immunohistochemical analysis
Antibodies for FoxO1/FKHR (no. 9462, lot 2),
FoxO3A/FKHRL1 (no. 9467, lot 4), FoxO4/AFX (no. 9472, lot 1) and
total PKB/Akt (no 9292, lot 1) were purchased from Cell Signaling
Technology (Beverly, MA, USA). Antibodies for PTEN (no. sc-9145,
lot C0707) were obtained from Santa Cruz Biotechnology, Inc. (Santa
Cruz, CA, USA). Antibodies for IGF-1 (BA0939) were obtained from
Boster Bio-engineering (Wuhan, China). ABC kits were obtained from
BioGenex Laboratories, Inc. (San Ramon, CA, USA) and
3,3′-diaminobenzidine tetrachloride (DAB) was purchased from Sigma
Chemical Co. (St. Louis, MO, USA). All other chemicals were
purchased commercially and were of reagent grade.
After transferral through a graded series of alcohol
and xylene, gastric mucosal samples were embedded in paraffin and
sectioned (7 μm). The sample sections were mounted on slides and
processed for immunohistochemical analysis, which was conducted
using a protocol similar to the method used in our previous reports
(29). Briefly, sections were
incubated overnight at room temperature with a polyclonal rabbit
immunoaffinity-purified antiserum directed against IGF-1 (1:200),
PTEN (1:400), total Akt (1:400); and FoxO1 (1:400), FoxO3a (1:400)
and FoxO4 proteins (1:500). The specific protein immunoreactivity
was visualized with an Elite ABC kit and 0.05% DAB in 10 mM
PBS-buffered saline containing 0.01% H2O2 for
5 min. Specificity of the antibody was examined using normal rabbit
serum (NRS) instead of the primary antibody. In order to identify
structural components and cell morphology, the sections were
counter-stained with hematoxylin and mounted with coverslips.
Relative levels of immunostaining between animals and cell types
were repeated at least four times and evaluated by three
independent observers.
Total-RNA isolation and reverse
transcription
Gastric mucosal samples were collected and stored in
liquid nitrogen until the time of RNA isolation. Total-RNA was
isolated after homogenizing gastric mucosa in TRIzol®
reagent (Invitrogen, USA) using the manufacturer’s protocol. RNA
quality was evaluated by examining a portion on a RNA gel. Bands of
18S and 28S were clear, and there was little smearing, indicating
that the quality was acceptable. However, the 18S band was not as
dark as expected, suggesting that some slight RNA degradation had
occurred.
Reverse transcription reactions (RT) were performed
using RT reagent kits with gDNA Eraser (Takara, China). The total
reaction volume of 20 μl contained 2 μl 5X gDNA Eraser buffer, 1 μl
gDNA Eraser, 1 μg total-RNA, 4 μl 5X RT buffer, 1 μl RT enzyme mix,
1 μl RT primer mix and sufficient nuclease-free H2O. The
RT reaction was carried out at 42°C for 2 min, 37°C for 15 min,
followed by a denaturation step at 85°C for 15 sec and cooling on
ice.
Real-time PCR analysis of gene
expression
Quantification of all transcripts was performed by
real-time quantitative PCR using the ABI 7300 PRISM system (Applied
Biosystems, USA). PCR products for 9 genes (IGF-1,
PTEN, Akt-1, Akt-2, Akt-3,
FoxO1, FoxO3a, FoxO4 and HPRT) were
detected by SYBR Green chemistry. The sequences and GenBank
accession nos. of the primer sets used for amplification of the
target genes are presented in Table
I. PCR reactions were run in triplicates in a total volume of
20 μl (consisting of SYBR Premix Ex Taq, ROX Reference Dye, 200 nM
each of the sequence-specific primers and 100 ng equivalent of
cDNA). The amplification conditions were as follows: DNA polymerase
activation at 95°C for 30 sec, followed by 40 amplification cycles
at 95°C for 5 sec, and 60°C for 31 sec. At the end of the
amplification cycles, a melting curve analysis was performed to
verify specific amplification.
 | Table I.Primers used for real-time PCR
analysis. |
Table I.
Primers used for real-time PCR
analysis.
| Gene and sequence
reference (GenBank no.) | Primer
sequence | Size of PCR product
(bp) | Annealing
temperature (°C) |
|---|
| HPRT (X62085) | F:
5′-AGTGATGATGAACCAGGTTA-3′ | 556 | 58.0 |
| R:
5′-ATTATAGTCAAGGGCATATC-3′ | | |
| IGF-1
(BC086374) | F:
5′-TGGTGGACGCTCTTCAGTTC-3′ | 168 | 58.0 |
| R:
5′-GCTTCAGCGGAGCACAGTAC-3 | | |
| PTEN
(NM031606) | F:
5′-AGCGTGCGGATAATGACAAG-3′ | 151 | 56.0 |
| R:
5′-GGATTTGATGGCTCCTCTACTG-3′ | | |
| Akt-1
(NM033230) | F:
5′-TAGGCATCCCTTCCTTACAG-3′ | 269 | 58.0 |
| R:
5′-GCCCGAAGTCCGTTATCT-3′ | | |
| Akt-2
(NM017093) | F:
5′-GAGCCGAGTCCTACAGAATACC-3′ | 263 | 58.0 |
| R:
5′-GGCCATCTTTGTCCAGCATA-3′ | | |
| Akt-3
(NM031575) | F:
5′-AACGACCAAAGCCAAATACA-3′ | 498 | 58.0 |
| R:
5′-CCCCATTAACATATTCCATCAC-3′ | | |
| FoxO1
(NM001191846) | F:
5′-CGTCCTCGAACCAGCTCAA-3′ | 292 | 57.4 |
| R:
5′-TTGGCGGTGCAAATGAATAG-3′ | | |
| FoxO3a
(NM001106395) | F:
5′-TTCGCAACGACCCAATGA-3′ | 331 | 57.4 |
| R:
5′-TCCAAGCTCCCATTGAACAT-3′ | | |
| Fox04
(NM001106943) | F:
5′-GGTGCCCTACTTCAAGGACAA-3′ | 148 | 58.0 |
| R:
5′-ATCGGGGTTCAGCATCCA-3′ | | |
The comparative CT method was used for relative
quantification of target gene expression levels (ABI PRISM Sequence
Detection System, Applied Biosystems). The quantity of each
measured cDNA sample was normalized to the endogenous gene
HPRT (a housekeeping gene), and all samples were measured in
triplicate. The mean values of the replicate wells run for each
sample were calculated and divided by HPRT to obtain a
normalized value for each transcript (30).
Statistical analysis
Statistical analyses were performed by using SPSS
17.0. Values are expressed as the means ± standard error of the
mean (SEM). The data were analyzed using a one-way analysis of
variance (ANOVA) and with Fisher’s protected least significant
difference (LSD) tests. P<0.05 was considered to indicate a
statistically significant result. All experiments were repeated at
least 3 times, and representative data are shown.
Results
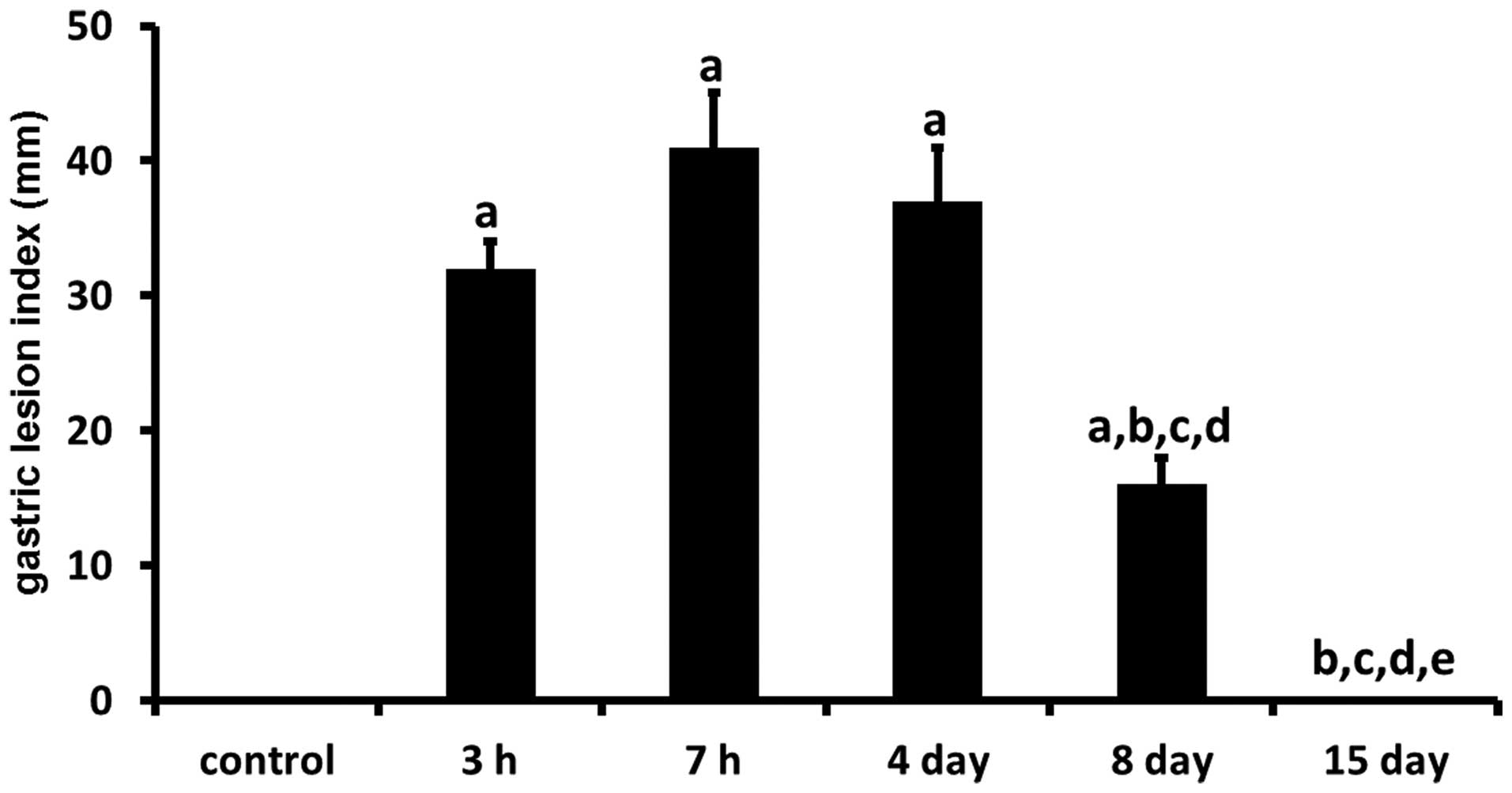
Effects of various time durations of WRS
and healing on the UI in rats
In the evaluation of WRS-induced gastric mucosal
injury in rats (n=6), the total length of lesions in the stomach
was expressed as the morphologic index of gastric ulcers (Fig. 1). In contrast to the normal
appearance of the gastric mucosa in control rats, numerous
hemorrhagic lesions were observed in the gastric mucosa of rats
subjected to WRS. The number of gastric mucosal lesions increased
time-dependently in the gastric mucosa of rats exposed to WRS for
various time durations (0, 3 and 7 h). Exposure to 3 h WRS resulted
in the formation of gastric mucosal lesions. When the WRS was
extended up to 7 h, the number of gastric mucosal lesions was
higher than that at 3 h (P>0.05). In the gastric mucosa, the
lengths of lesions were 32.2±2.7 mm after 3 h of WRS and 41.6±4.9
mm after 7 h of WRS.
In contrast, during the healing of gastric lesions,
the number of gastric lesions showed a progressive decrease at 4, 8
and 15 days following 7 h of WRS. At Day 8, the number of gastric
lesions was significantly less than that at Day 4 (P<0.05). In
the gastric mucosa, the lengths of lesions were 37.2±4.3 mm at Day
4 and 16.6±2.4 mm at Day 8. No gastric lesions were observed in the
gastric mucosa of rats at Day 15.
Effect of WRS on apoptosis in the gastric
mucosa
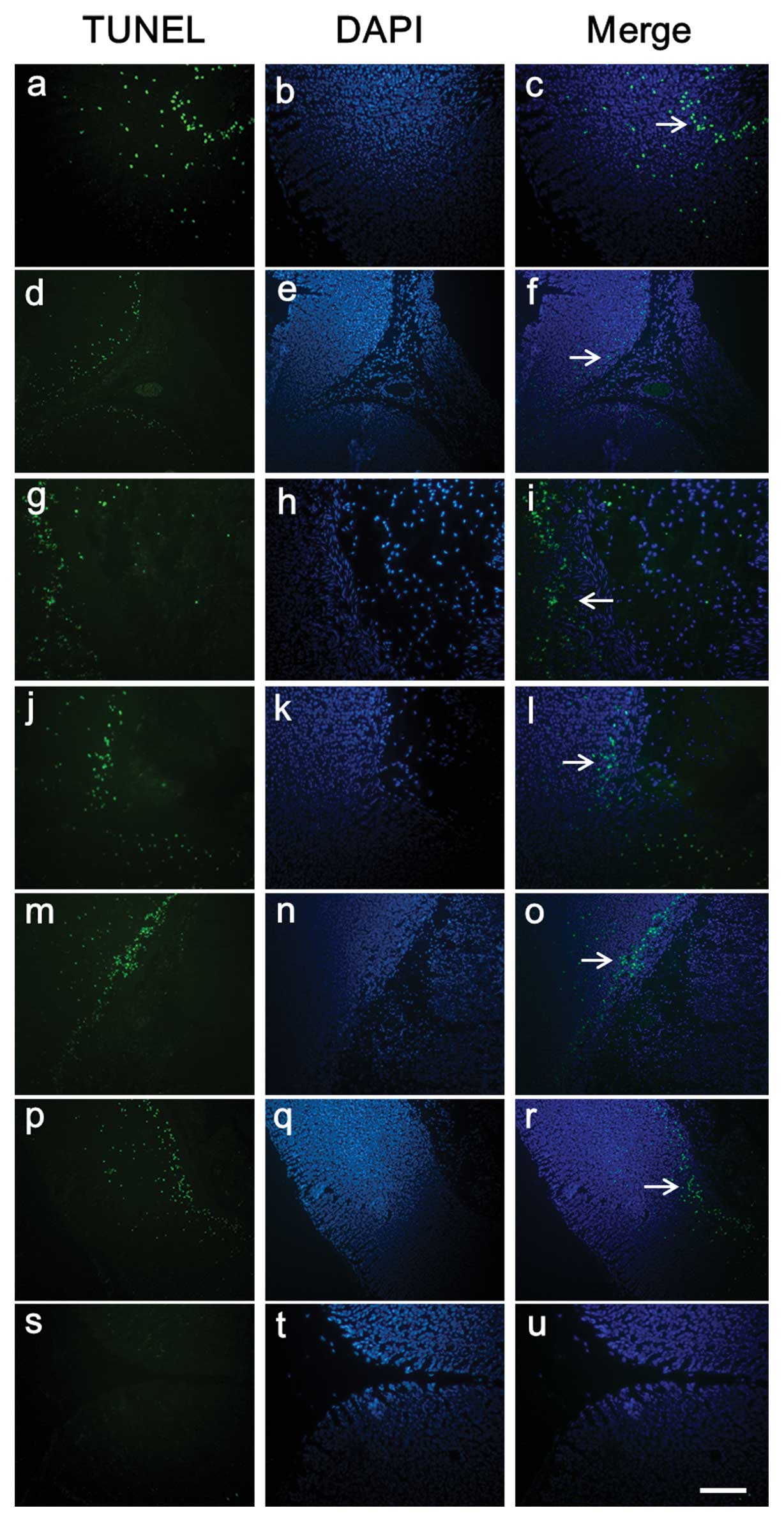
Gastric tissue sections were stained using the TUNEL
method to determine the quantity and distribution of apoptotic
cells as well as nuclear condensation and fragmentation (n=5).
Tissue sections exposed to DNase I, which causes DNA fragmentation,
showed intense staining of all nuclei and were used as positive
controls for the TUNEL method (data not shown). Sections stained
using the previous procedure but without the use of the TdT enzyme,
showed no staining and were used as negative controls (Fig. 2s–u). In the gastric mucosa of the
non-WRS rats, little labeling at the mucosal layer close to the
muscularis mucosa was found (Fig.
2a–c). In the gastric mucosa of rats subjected to WRS, many
apoptotic cells were observed throughout the entire thickness of
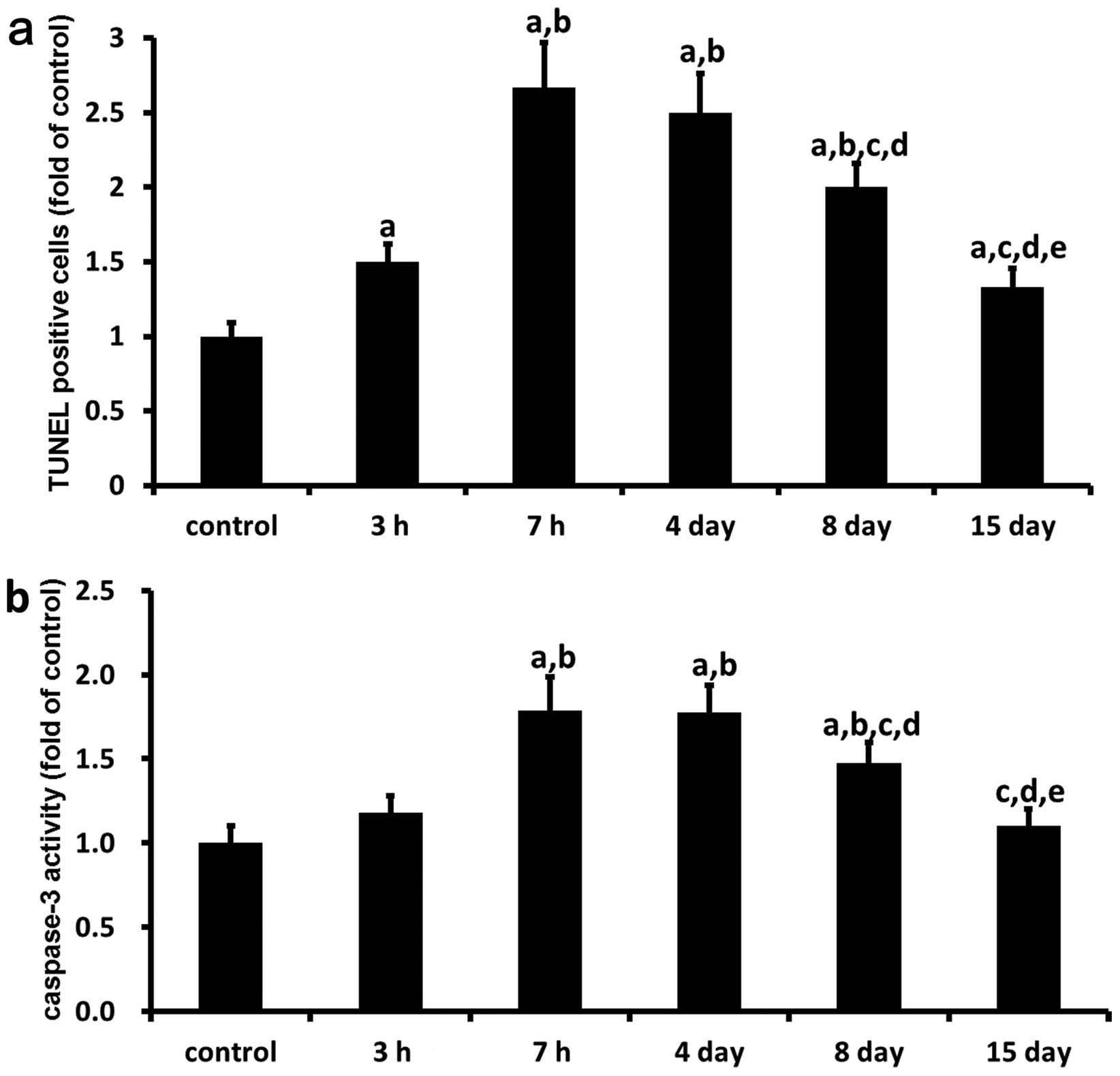
the mucosal layer and muscularis mucosa (Fig. 2d–r). Furthermore, the number of
apoptotic cells in the gastric mucosa of the rats subjected to 3
and 7 h of WRS were significantly increased by 50 (P<0.05) and
167% (P<0.05), respectively, compared with that in the control
group (Fig. 3a). In addition,
from Days 14 and 15 after 7 h of WRS, the number of apoptotic cells
in the gastric mucosa decreased gradually (Fig. 3a). By Day 8 following 7 h of WRS,
the number of apoptotic cells in the gastric mucosa were
significantly decreased by 37 (P<0.05) and 30% (P<0.05)
respectively, compared to the number in the group subjected to 7 h
of WRS or Day 4. Nevertheless, no significant difference between
the 15-day group and the control group was observed.
In order to reconfirm the cell apoptosis in gastric
lesion development and healing, caspase-3 activity in the gastric
ulcer margin of rats was detected by colorimetric analysis (n=6).
Caspase-3 activity in the gastric ulcer margin after 3 and 7 h of
WRS were also time-dependently enhanced by 18 and 79% (P<0.05),
compared with the control group (Fig.
3b). However, at Days 4, 8 and 15 after 7 h of WRS, the
caspase-3 activity decreased gradually with time, compared with the
7-h WRS group. At 8 days after 7 h of WRS, the caspase-3 activity
was significantly decreased by 31 (P<0.05) and 30% (P<0.05)
respectively, compared with the 7-h WRS group and the group at 4
days (P<0.05). There was no significant difference between the
15-day group and the control group.
Immunohistochemical localization of
IGF-1, PTEN, total Akt, FoxO1, FoxO3a and FoxO4 in the gastric
mucosa of rats after WRS
To assess the localization of IGF-1, PTEN, total
Akt, FoxO1, FoxO3a and FoxO4 in rat gastric ulcers (n=6), sections
from normal and ulcerated gastric mucosa were stained with specific
antibodies against these proteins. In normal rat gastric mucosa,
PTEN (Fig. 4e), total Akt
(Fig. 4h), and FoxO1 (Fig. 4k) were found mainly in the
cellular cytoplasm of the fundic glands in lamina propria close to
the muscularis mucosa. The results indicated that strong staining
of IGF-1 (Fig. 4b), FoxO3a
(Fig. 4n) and FoxO4 (Fig. 4q) in the gastric mucosa was
primarily concentrated in the cell cytoplasm of the fundic glands
in whole lamina propria. However, in rat gastric ulcers, IGF-1
(Fig. 4a–c), total Akt (Fig. 4g–i), FoxO3a (Fig. 4m–o) and FoxO4 (Fig. 4p–r) were localized proximal to the
base of the ulcer margin and were also present in the granulation
tissue of gastric ulcers. The expression patterns of PTEN (Fig. 4d–f) and FoxO1 (Fig. 4j–l) did not change in the rat
gastric ulcers, compared with the control rats. No marked staining
of PTEN and FoxO1 was found around the ulcer margin and granulation
tissue.
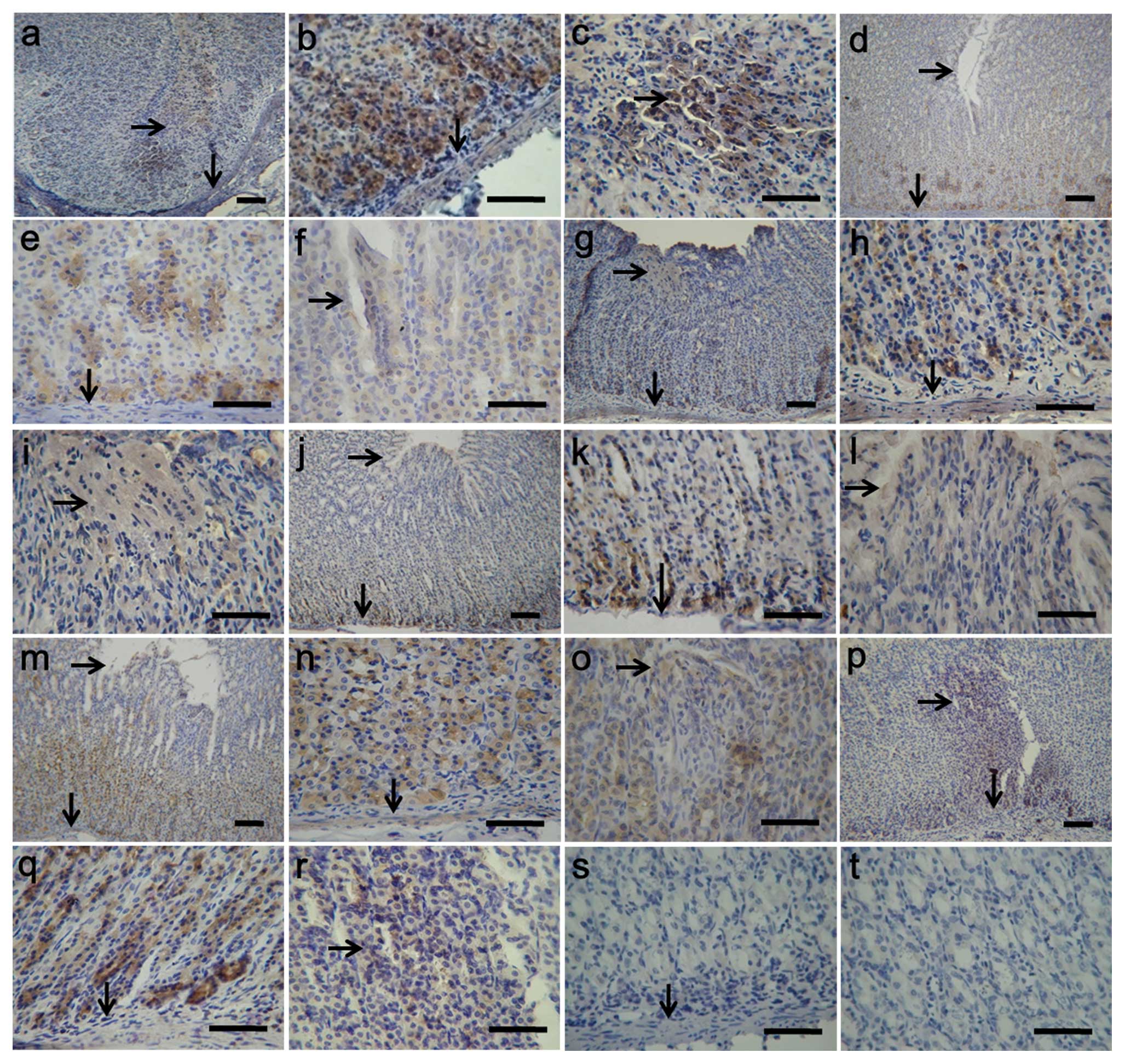 | Figure 4.Immunohistochemical localization of
IGF-1, PTEN, total Akt, FoxO1, FoxO3a and FoxO4 in the gastric
mucosa of rats after WRS. The immunohistochemical signals appear
brown and the counterstained background appears blue in color. The
figures indicate immunohistochemical localization of (a–c) IGF-1,
(d–f) PTEN, (g–i) total Akt, (j–l) FoxO1, (m–o) FoxO3a and (p–r)
FoxO4. In the control sections, normal albumin bovine was used
instead of the primary antibody (s and t). ↓, Muscularis
mucosa; →, gastric mucosal ulcer. Bar, 50 μm. |
Relative expression of IGF-1, PTEN,
Akt-1, Akt-2, Akt-3, FoxO1, FoxO3a and FoxO4 in the gastric mucosa
of rats after WRS
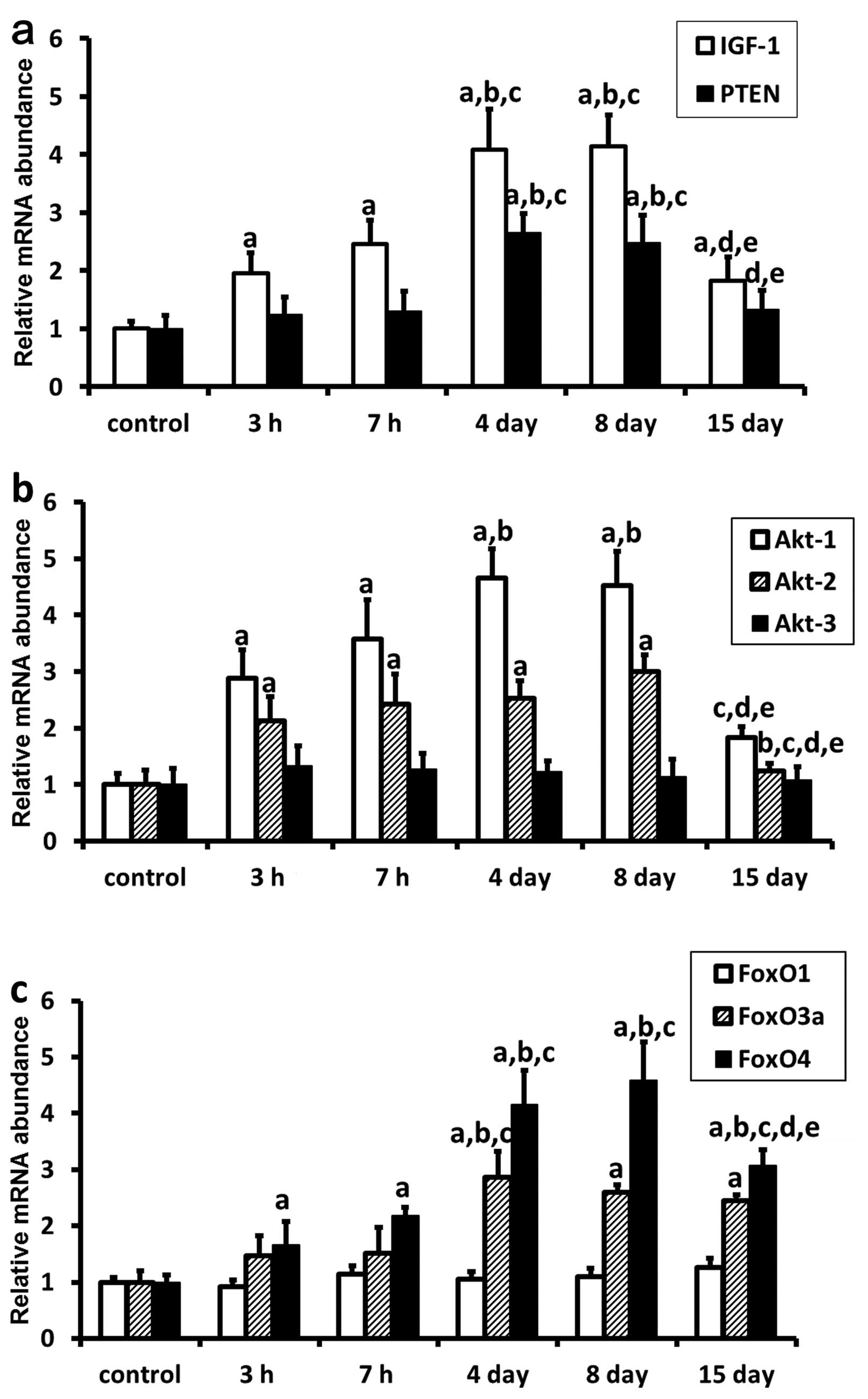
Expression levels of selected genes were analyzed
using real-time PCR (n=6). Amplification products were identified
by melting curve profile analysis and confirmed by gel
electrophoresis and sequencing. The data showed the relative
transcript of each target gene normalized to HPRT. The
real-time RT-PCR analysis of a 168 bp (transcript) of IGF-1,
a 151 bp of PTEN, a 269 bp of Akt-1, a 263 bp of
Akt-2, a 498 bp of Akt-3, a 292 bp of FoxO1, a
331 bp of FoxO3a, a 148 bp of FoxO4, and a 556 bp of
HPRT is shown in Fig. 5.
All selected genes were transcriptionally active. In the rat
gastric ulcers, mRNA transcript levels of IGF-1 (Fig. 5a), Akt-2 (Fig. 5b) and FoxO4 (Fig. 5c) were upregulated in the gastric
ulcer margin, with a peak 8 days after 7 h of WRS. The mRNA
transcript levels of Akt-2 returned to near baseline levels
at 15 days following 7 h of WRS. However, the mRNA transcript
levels of IGF-1 and FoxO4 in the WRS groups were
still significant higher than those in the non-WRS group
(P<0.05). Similarly, mRNA transcript levels of PTEN
(Fig. 5a), Akt-1 (Fig. 5b) and FoxO3a (Fig. 5c) were also upregulated in the
gastric ulcer margin, with a peak at 4 days following 7 h of WRS,
and returned to near baseline levels at 15 days following 7 h of
WRS. In addition, the results showed that Akt-3 (Fig. 5b) and FoxO1 (Fig. 5c) mRNA transcript levels in rats
subjected to WRS had no significant difference during the
development and healing of WRS-induced gastric ulcers.
Discussion
The WRS rat has long been used as a model animal of
gastric mucosal lesions (12). As
shown in the present study, in the evaluation of WRS-induced
gastric mucosal injury in rats, the gastric mucosa exposed to WRS
of various durations lasting 0, 3 and 7 h, time-dependently
increased the number of gastric mucosal lesions. Moreover, in the
healing of WRS-induced gastric ulcers, the number of gastric
lesions showed a progressive decrease at 4, 8 and 15 days after 7 h
of WRS. These results are consistent with previous studies
(12,26,31–34), suggesting that the rat gastric
ulcer model is well established.
Apoptosis is normally observed in the
gastrointestinal tract and plays an important role in the
maintenance of normal gastrointestinal homeostasis and mucosal
integrity (35). Recent studies
have demonstrated that apoptosis is critically involved in gastric
ulceration 24 h after ulcer induction (36,37). Caspases are causative enzymes that
induce apoptosis and are always present in intact cells, playing
important roles in the pathogenesis of tissue injury by activating
neutrophils. Nevertheless, all known stimuli that induce apoptosis
initiate events that culminate in caspase activation (38). Previous reports showed that IGF-1
and capsaicin administration to rats markedly reduced WRS-induced
gastric mucosal injury by inhibiting gastric accumulation of
neutrophils through inhibition of caspase-3 activation (22,39). In the present study, we
systematically investigated apoptosis in the development and
healing of WRS-induced gastric ulcers. We found that WRS induced
increases in both gastric caspase-3 activity and the number of
TUNEL-positive cells during the development of gastric ulcers.
Thereafter, during the healing of gastric ulcers, both gastric
caspase-3 activity and the number of TUNEL-positive cells decreased
slowly in 15 days. This suggests that apoptosis plays a role in the
development and healing of WRS-induced gastric ulcers.
The IGF-1/PTEN/Akt/FoxO signaling pathway plays
critical roles in the regulation of cell survival, growth,
differentiation and migration in many cell types and tissues
(3,40). To confirm whether the
IGF-1/PTEN/Akt/FoxO signaling pathway plays a critical role in the
development and healing of WRS-induced gastric ulcers, localization
and expression of IGF-1, PTEN, Akt and FoxO proteins were evaluated
in the present study. The results revealed that IGF-1 was localized
proximal to the base of the ulcer margin and was also present in
the granulation tissue of gastric ulcers. Meanwhile, mRNA
transcript levels of IGF-1 were upregulated in the gastric
ulcers, with a peak 8 days after 7 h of WRS. In the
gastrointestinal tract, IGF-1 is secreted by salivary and other
exocrine glands (41). It has
been reported that gastric ulceration triggered an ∼3-fold increase
in IGF-1 expression in epithelial cells of the ulcer margins
(17). Additionally, studies
using diabetic and arthritic rat models have demonstrated a delay
in gastric ulcer healing attributed to a decrease in IGF-1 mRNA in
the gastric mucosa (42,43). Subsequently, injection of
exogenous IGF-1 to these diabetic and arthritic rats was found to
accelerate ulcer healing. Moreover, direct injection of IGF-1 into
the ulcers was also shown to accelerate the healing of cryo-induced
rat gastric ulcers (44). Under
in vitro conditions, exogenous IGF-1 has been shown to
promote migration and proliferation in intrahepatic biliary
epithelial cells and in a wounded monolayer of rabbit gastric
epithelial cells (20,45). Related findings revealed that
IGF-1 is upregulated in injured skin, bone and brain (17). Accordingly, our results suggest
that IGF-1 plays a key role in combating ulcers through regulation
of its downstream genes as noted during the development and healing
of gastric ulcers.
In addition, Nguyen et al (17) found that upregulation of IGF-1 in
gastric ulcer margins enhanced gastric ulcer healing by promoting
cell re-epithelialization, proliferation and COX-2 expression via
the PI3K pathway. Furthermore, it was also reported that IGF-1
reduced WRS-induced gastric mucosal injury by inhibiting gastric
accumulation of neutrophils through inhibition of caspase-3
activation by PI3K/Akt signaling (22). In the present study, total Akt
protein was localized around gastric ulcer margins and was also
present in granulation tissue. In addition, gastric ulceration
triggered increases in Akt-1 and Akt-2 mRNA expression during the
development and healing of gastric ulcers. mRNA transcript levels
of Akt-3 did not change significantly during the development
and healing of WRS-induced gastric ulcers. As described above,
these results suggest that Akt-1 and Akt-2 participate in the
inhibition of caspase-3 activation in rat gastric ulcers.
The PTEN gene is a tumor suppressor that is
frequently deleted or mutated in human cancers, and which controls
Akt signaling. Its downstream targets are responsible for
regulating many physiologically and pathologically significant
processes, such as cellular proliferation, survival, growth and
motility (8). Results of the
present study found that the localization pattern of PTEN did not
change in rat gastric ulcers, compared with normal rats. It was
observed mainly in the cell cytoplasm of the fundic glands in the
lamina propria close to the muscularis mucosa. No marked staining
of PTEN was noted around the ulcer margins and granulation tissue.
mRNA transcript levels of PTEN were also upregulated in gastric
ulcer margins, achieving a peak 4 days following 7 h of WRS, and
returned to a near baseline level at Day 15 following 7 h of WRS.
Many researchers have investigated PTEN in gastric cancer (46–48). In particular, Wang et al
(47) revealed that caspase-3
activity was related to upregulation of PTEN in human gastric
cancer MGC-803 cells. In accordance with our results, PTEN may be
involved in the regulation of cell apoptosis by Akt signaling.
In control rats, strong staining of FoxO3a and FoxO4
in the gastric mucosa was primarily concentrated in the cellular
cytoplasm of the fundic glands in the whole lamina propria. In rat
gastric ulcers, FoxO3a and FoxO4 were localized proximal to the
base of the ulcer margins and were also present in the granulation
tissue of gastric ulcers. Furthermore, mRNA transcript levels of
FoxO3a and FoxO4 were upregulated in gastric ulcers,
reaching a peak between Day 4 and 8 following 7 h of WRS. FoxO1 was
localized in the cellular cytoplasm of the fundic glands close to
the muscularis mucosa in normal gastric mucosa. However, its
staining was not observed around the mucosal ulcer. mRNA transcript
levels of FoxO1 did not change siginificantly during the
development and healing of WRS-induced gastric ulcers.
FoxO1, FoxO3a and FoxO4 are all downstream effectors
of the IGF-1/PTEN/Akt pathway. These FoxO proteins participate in a
growing number of physiologic processes including cell
proliferation, apoptosis, stress resistance, differentiation and
metabolism (49). The present
results were consistent with our previous studies (23,24) and suggest that FoxO3a and FoxO4
are the primary forkhead transcriptional factors localized to the
gastrointestinal tracts. Liu et al (50) showed that FoxO1 mRNA was expressed
at lower levels in the duodenum subcutaneous adipose tissue and
pancreas than in other tissues of pigs. In addition,
phosphorylation of FoxO proteins by PKB results in cytoplasmic
retention and inactivation, which consequently inhibits the
expression of FoxO-regulated genes that control the cell cycle,
cell death and cell metabolism. The shuttling of FoxOs between the
cytoplasm and nucleus is a key step in apoptosis (11). FoxO3a and FoxO4 may thereby be
involved in the development and healing of WRS-induced gastric
ulcers, which, in part, are associated with the regulation of
apoptosis.
In conclusion, these observations raise the
possibility that the IGF-1/PTEN/Akt/FoxO signaling pathway plays
certain role(s) in protecting against ulcers through the regulation
of cellular apoptosis as observed during the development and
healing of rat gastric ulcers.
Acknowledgements
This study was supported by the
National Nature Science Foundation of China (no. 31172206) and a
Grant-in-Aid for the Innovative Training of Doctoral Students in
Jiangsu Province of China (CXLX11-0699).
References
|
1.
|
DH CastrillonL MiaoR KolliparaJW HornerRA
DePinhoSuppression of ovarian follicle activation in mice by the
transcription factor
Foxo3aScience301215218200310.1126/science.108633612855809
|
|
2.
|
A CarneroC Blanco-AparicioO RennerW LinkJF
LealThe PTEN/PI3K/AKT signalling pathway in cancer, therapeutic
implicationsCurr Cancer Drug
Targets8187198200810.2174/15680090878429365918473732
|
|
3.
|
P ReddyL LiuD AdhikariOocyte-specific
deletion of Pten causes premature activation of the primordial
follicle
poolScience319611613200810.1126/science.115225718239123
|
|
4.
|
PV CarrollTreatment with growth hormone
and insulin-like growth factor-I in critical illnessBest Pract Res
Clin Endocrinol Metab15435451200110.1053/beem.2001.016211800516
|
|
5.
|
G SongG OuyangS BaoThe activation of
Akt/PKB signaling pathway and cell survivalJ Cell Mol
Med95971200510.1111/j.1582-4934.2005.tb00337.x15784165
|
|
6.
|
M CullyH YouAJ LevineTW MakBeyond PTEN
mutations: the PI3K pathway as an integrator of multiple inputs
during tumorigenesisNat Rev
Cancer6184192200610.1038/nrc181916453012
|
|
7.
|
JA Fresno VaraE CasadoJ de CastroP CejasC
Belda-IniestaM Gonzalez-BaronPI3K/Akt signalling pathway and
cancerCancer Treat Rev30193204200415023437
|
|
8.
|
NR LeslieCP DownesPTEN function: how
normal cells control it and tumour cells lose itBiochem
J382111200410.1042/BJ2004082515193142
|
|
9.
|
DA CrossDR AlessiP CohenM AndjelkovichBA
HemmingsInhibition of glycogen synthase kinase-3 by insulin
mediated by protein kinase
BNature378785789199510.1038/378785a08524413
|
|
10.
|
A SenguptaJD MolkentinJH PaikRA DePinhoKE
YutzeyFoxO transcription factors promote cardiomyocyte survival
upon induction of oxidative stressJ Biol
Chem28674687478201110.1074/jbc.M110.17924221159781
|
|
11.
|
BM BurgeringGJ KopsCell cycle and death
control: long live ForkheadsTrends Biochem
Sci27352360200210.1016/S0968-0004(02)02113-812114024
|
|
12.
|
M AdachiG HoriuchiN
IkematsuIntragastrically administered lysophosphatidic acids
protect against gastric ulcer in rats under water-immersion
restraint stressDig Dis
Sci5622522261201110.1007/s10620-011-1595-0
|
|
13.
|
SX YiY PengXR ChangN PengJ YanYP LinEffect
of pre-moxibustion on apoptosis and proliferation of gastric mucosa
cellsWorld J
Gastroenterol1321742178200710.3748/wjg.v13.i15.217417465496
|
|
14.
|
SN NieXM QianXH WuRole of TFF in healing
of stress-induced gastric lesionsWorld J
Gastroenterol917721776200312918118
|
|
15.
|
YM LiGM LuXP ZouZS LiGY PengDC FangDynamic
functional and ultrastructural changes of gastric parietal cells
induced by water immersion-restraint stress in ratsWorld J
Gastroenterol12336833722006
|
|
16.
|
P JiangL ChangCS PanYF QiCS TangProtective
role of metallothionein in stress-induced gastric ulcer in
ratsWorld J
Gastroenterol1127392743200510.3748/wjg.v11.i18.273915884113
|
|
17.
|
T NguyenJ ChaiA LiT AkahoshiT TanigawaAS
TarnawskiNovel roles of local insulin-like growth factor-1
activation in gastric ulcer healing: promotes actin polymerization,
cell proliferation, re-epithelialization, and induces
cyclooxygenase-2 in a phosphatidylinositol 3-kinase-dependent
mannerAm J Pathol17012191228200710.2353/ajpath.2007.060745
|
|
18.
|
N HaradaK OkajimaH KuriharaN
NakagataStimulation of sensory neurons by capsaicin increases
tissue levels of IGF-I, thereby reducing reperfusion-induced
apoptosis in
miceNeuropharmacology5213031311200710.1016/j.neuropharm.2007.01.016
|
|
19.
|
MA DaemenC van ‘t VeerG DeneckerInhibition
of apoptosis induced by ischemia-reperfusion prevents inflammationJ
Clin Invest104541549199910.1172/JCI697410487768
|
|
20.
|
S WatanabeXE WangM HiroseInsulin-like
growth factor I plays a role in gastric wound healing: evidence
using a zinc derivative, polaprezinc, and an in vitro rabbit wound
repair modelAliment Pharmacol
Ther1211311138199810.1046/j.1365-2036.1998.00408.x
|
|
21.
|
SN NieHC SunXH WuXM QianCyclooxygenase 2,
pS2, inducible nitric oxide synthase and transforming growth factor
alpha in gastric adaptation to stressWorld J
Gastroenterol1035373541200415526382
|
|
22.
|
J ZhaoN HaradaK SobueH KatsuyaK
OkajimaInsulin-like growth factor-I reduces stress-induced gastric
mucosal injury by inhibiting neutrophil activation in miceGrowth
Horm IGF Res19136145200910.1016/j.ghir.2008.08.00318809348
|
|
23.
|
P HuangZQ ZhouRH HuangB ZhouQW WeiFX
ShiAge-dependent expression of forkhead box O proteins in the
duodenum of ratsJ Zhejiang Univ Sci
B12730735201110.1631/jzus.B100029821887848
|
|
24.
|
ZQ ZhouT WangLM PanRH HuangFX ShiFoxO4 is
the main forkhead transcriptional factor localized in the
gastrointestinal tracts of pigsJ Zhejiang Univ Sci
B83944200710.1631/jzus.2007.B003917173361
|
|
25.
|
N ShimozawaK OkajimaN HaradaContribution
of sensory neurons to sex difference in the development of
stress-induced gastric mucosal injury in
miceGastroenterology13118261834200610.1053/j.gastro.2006.09.00517087955
|
|
26.
|
KJ KellyRM SandovalKW DunnBA MolitorisPC
DagherA novel method to determine specificity and sensitivity of
the TUNEL reaction in the quantitation of apoptosisAm J Physiol
Cell Physiol284C1309C1318200310.1152/ajpcell.00353.200212676658
|
|
27.
|
W WangJ XuL LiNeuroprotective effect of
morroniside on focal cerebral ischemia in ratsBrain Res
Bull83196201201010.1016/j.brainresbull.2010.07.00320637265
|
|
28.
|
L SongB ZhangY FengX LuoX WeiX XiaoA role
for forkhead box A1 in acute lung
injuryInflammation32322332200910.1007/s10753-009-9139-x19649697
|
|
29.
|
W DingW WangB ZhouFormation of primordial
follicles and immunolocalization of PTEN, PKB and FOXO3A proteins
in the ovaries of fetal and neonatal pigsJ Reprod
Dev56162168201010.1262/jrd.09-094H19996554
|
|
30.
|
WM LiuFX ShiLZ LuEffects of linoleic acid
and eicosapentaenoic acid on cell proliferation and
lipid-metabolism gene expression in primary duck hepatocytesMol
Cell Biochem3521924201110.1007/s11010-011-0735-321274596
|
|
31.
|
T BrzozowskiPC KonturekS
ChlopickiTherapeutic potential of 1-methylnicotinamide against
acute gastric lesions induced by stress: role of endogenous
prostacyclin and sensory nervesJ Pharmacol Exp
Ther326105116200810.1124/jpet.108.136457
|
|
32.
|
T BrzozowskiK Zwirska-KorczalaPC
KonturekRole of circadian rhythm and endogenous melatonin in
pathogenesis of acute gastric bleeding erosions induced by stressJ
Physiol Pharmacol58Suppl 6S53S64200718212400
|
|
33.
|
P CeranowiczZ WarzechaA DembinskiTreatment
with ghrelin accelerates the healing of acetic acid-induced gastric
and duodenal ulcers in ratsJ Physiol
Pharmacol608798200919439811
|
|
34.
|
CY ChenTL KuoSY SheuTF KuoPreventive
effects of Chinese herb chai-hu-gui-zhi-tang extract on water
immersion restraint stress-induced acute gastric ulceration in
ratsJ Vet Med Sci72679685201010.1292/jvms.09-028420086327
|
|
35.
|
Z SunX WangR WallenThe influence of
apoptosis on intestinal barrier integrity in ratsScand J
Gastroenterol33415422199810.1080/003655298501710539605264
|
|
36.
|
PC KonturekT BrzozowskiA DudaEpidermal
growth factor and prostaglandin E(2) accelerate mucosal recovery
from stress-induced gastric lesions via inhibition of apoptosisJ
Physiol Paris95361367200110.1016/S0928-4257(01)00049-311595461
|
|
37.
|
PC KonturekT BrzozowskiSJ
KonturekApoptosis in gastric mucosa with stress-induced gastric
ulcersJ Physiol Pharmacol50211225199910424718
|
|
38.
|
EM CreaghH ConroySJ
MartinCaspase-activation pathways in apoptosis and immunityImmunol
Rev1931021200310.1034/j.1600-065X.2003.00048.x12752666
|
|
39.
|
N HaradaK OkajimaM UchibaT
KatsuragiContribution of capsaicin-sensitive sensory neurons to
stress-induced increases in gastric tissue levels of prostaglandins
in ratsAm J Physiol Gastrointest Liver
Physiol285G1214G1224200310.1152/ajpgi.00364.200212893632
|
|
40.
|
ED RabinovskyThe multifunctional role of
IGF-1 in peripheral nerve regenerationNeurol
Res26204210200410.1179/01616410422501385115072640
|
|
41.
|
OP ChaurasiaSP MarcuardER
SeidelInsulin-like growth factor I in human gastrointestinal
exocrine secretionsRegul
Pept50113119199410.1016/0167-0115(94)90026-48190912
|
|
42.
|
RP KorolkiewiczK TashimaA FujitaS KatoK
TakeuchiExogenous insulin-like growth factor (IGF)-1 improves the
impaired healing of gastric mucosal lesions in diabetic
ratsPharmacol Res41221229200010.1006/phrs.1999.058110623490
|
|
43.
|
S KatoA TanakaY OgawaEffect of polaprezinc
on impaired healing of chronic gastric ulcers in adjuvant-induced
arthritic rats - role of insulin-like growth factors (IGF)-1Med Sci
Monit72025200111208487
|
|
44.
|
S CoerperS WolfS von KiparskiInsulin-like
growth factor I accelerates gastric ulcer healing by stimulating
cell proliferation and by inhibiting gastric acid secretionScand J
Gastroenterol36921927200110.1080/00365520175030542211521981
|
|
45.
|
D AlvaroVD MetalliG AlpiniThe intrahepatic
biliary epithelium is a target of the growth hormone/insulin-like
growth factor 1 axisJ
Hepatol43875883200510.1016/j.jhep.2005.04.01116083987
|
|
46.
|
X XiongHZ RenMH LiJH MeiJF WenCL
ZhengDown-regulated miRNA-214 induces a cell cycle G1 arrest in
gastric cancer cells by up-regulating the PTEN proteinPathol Oncol
Res17931937201110.1007/s12253-011-9406-721688200
|
|
47.
|
X WangY XieQ XiaoLentivirus-mediated RNA
interference targeting E2F-1 inhibits human gastric cancer MGC-803
cell growth in vivoExp Mol
Med43638645201110.3858/emm.2011.43.11.07221869593
|
|
48.
|
X LiuWJ GuoXW ZhangX CaiS TianJ
LiCetuximab enhances the activities of irinotecan on gastric cancer
cell lines through downregulating the EGFR pathway upregulated by
irinotecanCancer Chemother
Pharmacol68871878201110.1007/s00280-011-1559-221286718
|
|
49.
|
J NakaeWH Biggs IIIT KitamuraRegulation of
insulin action and pancreatic beta-cell function by mutated alleles
of the gene encoding forkhead transcription factor Foxo1Nat
Genet32245253200210.1038/ng89012219087
|
|
50.
|
Y LiuY WangT ShanJ GuoC XuJ LiuThe
tissue-specific and developmental expression patterns of the
forkhead transcription factor FoxO1 gene in pigsJ Anim Feed
Sci171821902008
|