Introduction
Melanin is synthesized in the melanosomes of
melanocytes by a process known as melanogenesis and plays a crucial
role in protecting the skin from the harmful effects of ultraviolet
(UV) radiation and diverse free radicals. Melanogenesis is
regulated by at least three melanogenic enzymes, tyrosinase,
tyrosinase-related protein (TRP)-1 and TRP-2 (1). Tyrosinase is a rate-limiting enzyme
that catalyzes the first two steps in the melanin biosynthetic
pathway: hydroxylation of tyrosine to 3,4-dihydroxyphenylalanine
(DOPA) and oxidation of DOPA to DOPAquinone (2). TRP-2, which functions as a
DOPAchrome tautomerase, catalyzes the rearrangement of DOPAchrome
to 5,6-dihydroxyindole-2-carboxylic acid (DHICA) (3), and TRP-1 oxidizes DHICA to a
carboxylated indole-quinone (4).
Understanding the regulation of melanogenesis is of
great interest pharmaceutically and cosmeceutically as
melanogenesis inhibitors can be used for the treatment of
hyper-pigmentation-related diseases, such as melasma, lentigines,
nevus, ephelis, freckles and age spots (5). Of the various signaling pathways
that regulate melanogenesis, the cyclic AMP (cAMP)-dependent
signaling pathway plays a pivotal role. cAMP-elevating agents, such
as α-melanocyte stimulating hormone (α-MSH), isobutylmethylxanthine
(IBMX) and forskolin stimulate melanogenic processes in the human
epidermis (6,7). cAMP increases the expression of
melanogenic enzymes partly through protein kinase A (PKA). PKA
phosphorylates the cAMP responsive element binding protein (CREB),
which induces the expression of microphthalmia-associated
transcription factor (MITF) (8).
MITF is known as a master regulator of melanocyte development,
survival, differentiation and melanogenesis (9). It also regulates the transcription
of three major melanogenic enzymes: tyrosinase, TRP-1 and
TRP-2.
Flavonoids are a group of polyphenolic compounds
widely distributed in plants. Their potent bioactivity and
relatively low toxicity have rendered them attractive for use as
active ingredients in functional foods and cosmetics. Guggulsterone
[4,17(20)-pregnadiene-3,16-dione], which is
the active component of gugulipid, is derived from the gum resin
(guggulu) of the tree, Commiphora mukul. This gum
resin has been used for centuries in Ayurvedic medicine to treat
obesity, arthritis and hyperlipidemia (10,11). In addition, guggulsterone has been
reported to act as a farnesoid X receptor (FXR) antagonist
(12,13). Therefore, it can effectively
regulate bile acid synthesis (11–14) and carbohydrate metabolism
(15). We have previously studied
the effects of guggulsterone on type 1 diabetes (16) and arthritis (17). However, to our knowledge, there is
no report on effect of guggulsterone on melanogenesis. During
screening for new melanogenesis-inhibiting agents from flavonoids,
we found that guggulsterone effectively inhibited melanogenesis.
Therefore, in this study, we investigated the inhibitory mechanism
of guggulsterone against IBMX-induced melanogenesis in B16 melanoma
cells.
Materials and methods
Cells and materials
The B16/F10 mouse melanoma cell line was obtained
from the Korean Cell Line Bank (Seoul, Korea). Cells were cultured
in DMEM containing 10% fetal bovine serum, 100 U/ml penicillin, 0.1
mg/ml streptomycin, and 0.25 μg/ml amphotericin B at 37°C in
a humidified 95% air/5% CO2 atmosphere. Guggulsterone
was obtained from Alexis Biochemicals (Lausen, Switzerland), and
6-ethyl chenodeoxycholic acid (CDCA), α-MSH, IBMX, and forskolin
were obtained from Sigma (St. Louis, MO, USA). Drug treatment began
24 h after seeding, and cells were harvested after two days of
incubation.
Melanin content measurement
The melanin contents of the cultured B16 cells were
measured as described previously (18). The cells were washed twice with
phosphate-buffered saline (PBS) and lysed with 20 mM Tris-0.1%
Triton X-100 (pH 7.5). Cell lysates were precipitated with the same
amount of 20% trichloroacetic acid. After washing twice with 10%
trichloroacetic acid, the pellets were treated with ethyl
alcohol:diethyl ether (3:1) and diethyl ether in succession. The
samples were air-dried, dissolved in 1 ml of 0.85 M KOH, and boiled
for 15 min. After cooling, absorbance was measured with a
spectrophotometer at 440 nm. The amount of cellular melanin was
corrected according to the DNA content of the samples. The DNA
content was determined using the fluorescence assay of bisbenzimide
H 33258 using a DNA Quantification kit (Sigma).
Tyrosinase activity assay
Tyrosinase activity was assayed as DOPA oxidase
activity with some modifications, as described previously (18). Briefly, cell lysate was obtained
after washing twice with PBS. Tyrosinase activity was then analyzed
spectrophotometrically by following the oxidation of DOPA to
DOPAchrome at 475 nm. The reaction mixture containing 100 μl
of freshly prepared substrate solution (0.1% L-DOPA in 0.1 M sodium
phosphate, pH 6.0) and 50 μl of enzyme solution was
incubated at 37°C. The absorbance change was measured during the
first 10 min of the reaction, while the increase of the absorbance
was linear. Corrections for the auto-oxidation of L-DOPA in the
controls were made. The tyrosinase activity was corrected according
to the DNA content of the samples and presented as a percentage of
IBMX-treated control cells.
MTT assay
The viability of the cultured cells was determined
by the reduction of
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
to formazan. The cells were seeded in 96-well plates and cultured
for 24 h. Following drug treatment, MTT (5 mg/ml in PBS, 100
μl) was added to each well. The cells were incubated at 37°C
for 30 min, and dimethyl sulfoxide (100 μl) was then added
to dissolve the formazan crystals. The absorbance was measured at
570 nm with a spectrophotometer.
Western blot analysis
Cells were homogenized in ice-cold lysis buffer. The
homogenates containing 10 μg of protein were separated by
SDS-PAGE with 10% resolving and 3% acrylamide stacking gel and
transferred to a nitrocellulose membrane in a western blot analysis
apparatus run at 100 V for 1.5 h. The nitrocellulose membrane was
blocked with 2% bovine serum albumin and then incubated overnight
with 1 μg/ml goat anti-murine tyrosinase IgG (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA). The binding of the
antibody was detected with anti-goat IgG conjugated with
horseradish peroxidase (Sigma). Immunoblots were developed using an
Enhanced Chemiluminescence Plus kit (Amersham Biosciences,
Buckinghamshire, UK), and the intensity of the bands was measured
by LAS-1000 (Fujifilm, Tokyo, Japan).
Real-time RT-PCR
Total RNA was prepared from the cells using TRIzol
reagent (Invitrogen, Carlsbad, CA, USA). Total RNA (2 μg)
was treated with RNase-free DNase (Invitrogen), and first-strand
cDNA was generated using random hexamer primers provided in the
first-strand cDNA synthesis kit (Applied Biosystems, Foster City,
CA, USA). Specific primers for each gene (Table I) were designed using Primer
Express software (Applied Biosystems). The real-time RT-PCR
reaction mixture consisted of 10 ng reverse transcribed total RNA,
167 nM forward and reverse primers, and 2X PCR master mixture in a
final volume of 10 μl. PCR reactions were carried out in
384-well plates using the ABI PRISM 7900HT Sequence Detection
System (Applied Biosystems). All the experiments were performed in
triplicate.
 | Table ISequences and accession numbers for
forward (F) and reverse (R) primers used in real-time RT-PCR. |
Table I
Sequences and accession numbers for
forward (F) and reverse (R) primers used in real-time RT-PCR.
| Gene | Sequences for
primers | Accession no. |
|---|
| Tyrosinase | F,
TTGCCACTTCATGTCATCATAGAATATT
R, TTTATCAAAGGTGTGACTGCTATACAAAT | NM011661 |
| TRP1 | F,
ATGCGGTCTTTGACGAATGG
R, CGTTTTCCAACGGGAAGGT | NM031202 |
| TRP2 | F,
CTCAGAGCTCGGGCTCAGTT
R, TGTTCAGCACGCCATCCA | X63349 |
| MITF | F,
CGCCTGATCTGGTGAATCG
R, CCTGGCTGCAGTTCTCAAGAA | NM008601 |
| GAPDH | F,
CGTCCCGTAGACAAAATGGT
R, TTGATGGCAACAATCTCCAC | NM008084 |
Statistical analysis
Statistical analysis of the data was performed using
ANOVA and Duncan’s test. P<0.05 was considered to indicated
statistically significant differences.
Results
Guggulsterone inhibits melanogenesis in
B16 cells
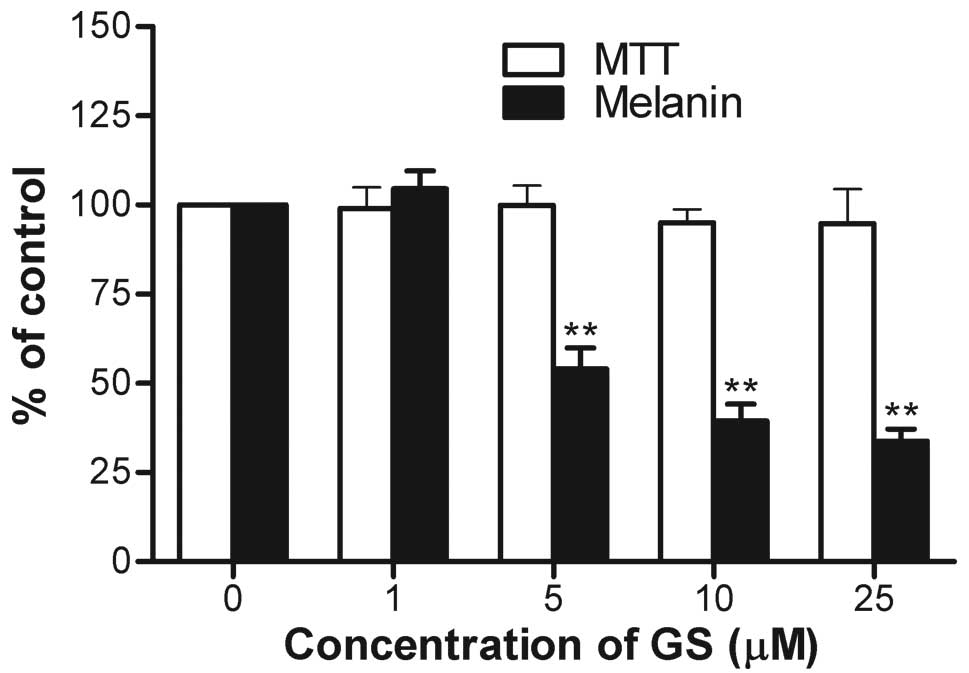
In order to investigate the effects of guggulsterone
on IBMX-induced melanogenesis, the melanin contents in the B16
cells were measured following treatment with guggulsterone. At
concentrations of 1, 5, 10, and 25 μM guggulsterone, melanin
production was compared with the untreated controls (Fig. 1). At concentrations of 1, 5, 10,
and 25 μM, guggulsterone decreased melanin production to
104.5±5.0, 54.1±5.8, 39.4±4.8 and 33.8±3.4%, respectively in a
dose-dependent manner without obvious cytotoxicity at any of the
concentrations tested.
Guggulsterone decreases the expression of
melanogenesis-related genes
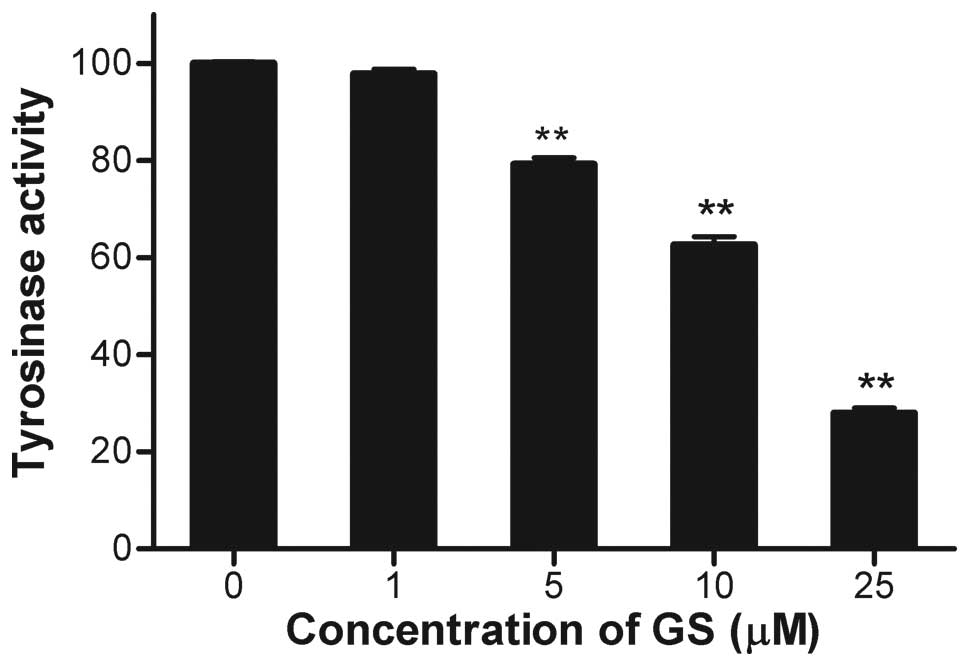
Since tyrosinase is the rate-limiting enzyme for
melanin biosynthesis, the effect of guggulsterone on tyrosinase
activity was determined. Cellular tyrosinase activity was decreased
by guggulsterone in a dose-dependent manner (Fig. 2), which was consistent with the
decreased melanin content (Fig.
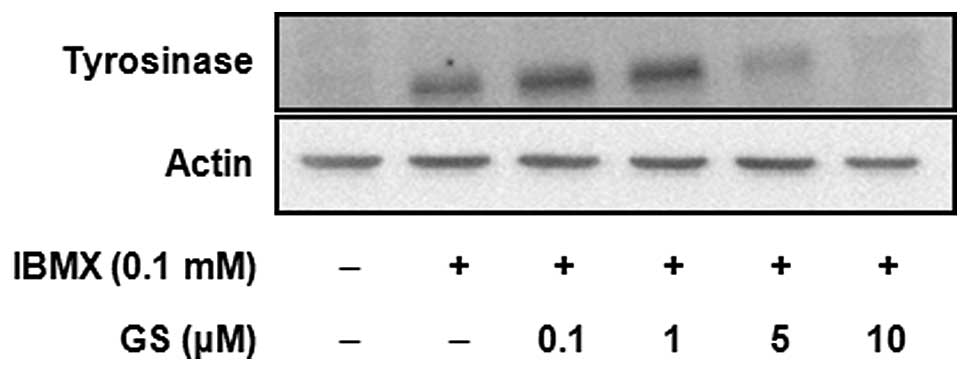
1). The expression of tyrosinase protein was determined by
western blot analysis (Fig. 3).
The results showed that the tyrosinase protein was greatly
increased by IBMX treatment, and this induction was significantly
inhibited by guggulsterone in a dose-dependent manner. The
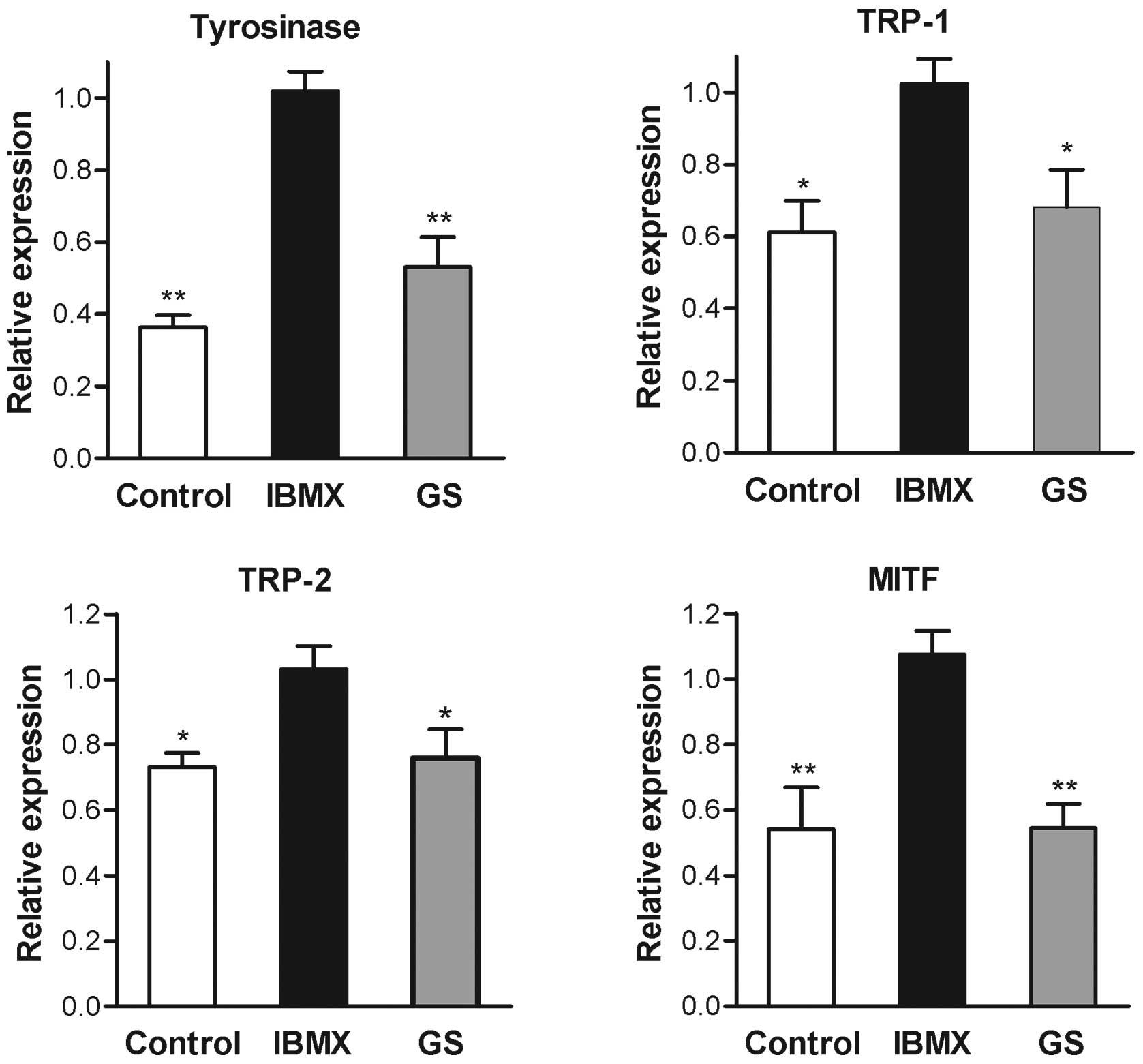
expression of tyrosinase mRNA determined by real-time RT-PCR also
exhibited a significant decrease following guggulsterone treatment
(Fig. 4). These results indicated
that the inhibition of tyrosinase by guggulsterone was exerted at
the transcriptional level. The mRNA levels of TRP-1, TRP-2 and
MITF, members of the melanogenesis-related gene family, were also
decreased by the presence of guggulsterone (Fig. 4).
Guggulsterone inhibits cAMP-elevating
agent-induced melanogenesis
When the B16 cells were incubated with IBMX, the
cell suspension turned black, indicating increased cellular
melanogenesis (Fig. 5). The
cellular melanin contents were also markedly increased in the cells
treated with 5 μM α-MSH or 5 μM forskolin. However,
the presence of guggulsterone significantly inhibited melanogenesis
induced by both α-MSH and forskolin (Fig. 5), suggesting that guggulsterone
regulates melanogenesis through the cAMP-dependent pathway.
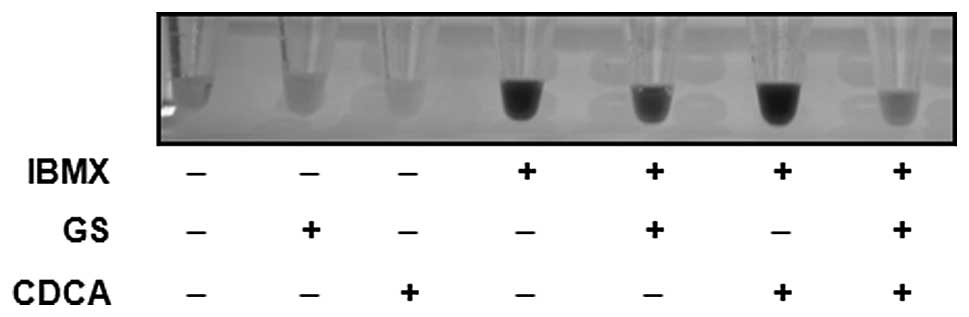
The inhibitory effect of guggulsterone on
IBMX-induced melanogenesis was not affected when the B16 cells were
treated with both guggulsterone and CDCA, an antagonist and an
agonist for FXR, respectively (Fig.
6). These results indicated that the effect of guggulsterone on
melanogenesis was not mediated by antagonizing the FXR signaling
pathway. Again, treatment with guggulsterone or CDCA alone did not
affect melanogenesis at the concentration used in this study.
Discussion
We performed this study to examine whether
guggulsterone can be used as a whitening cosmetic agent. To answer
this question, we first evaluated whether guggulsterone can inhibit
melanogenesis in IBMX-treated B16 melanoma cells. When the B16
cells were treated with guggulsterone, a dose-dependent inhibition
of melanin production was observed. This result cannot be explained
by the cytotoxicity of guggulsterone, as there was no evident
decrease in the number of viable cells up to a concentration of 25
μM.
UV-induced hyperpigmentation occurs in two stages,
an immediate darkening and a delayed tanning reaction. Immediate
pigment darkening is thought to result from the oxidation of
pre-existing melanin and redistribution of melanosomes. By
contrast, the delayed tanning response that is photoprotective
against subsequent UV injury begins as the immediate pigmentation
reaction fades and progresses for at least three to five days after
UV exposure (19). Delayed
tanning is preceded by the increase in tyrosinase activity in
melanocytes (5,19). Since tyrosinase catalyzes the
rate-limiting reaction of the melanogenic process, any reduction in
the amount of enzyme activity or expression will result in a
corresponding decrease in the amount of melanin synthesized.
Indeed, in B16 cells treated with IBMX, there were marked increases
in tyrosinase activity, namely increases in protein and mRNA
expression, which were similar to those of the delayed tanning
response after UV irradiation. Accordingly, the treatment of cells
with guggulsterone resulted in dose-dependent inhibition of the
enzymatic activity and expression of tyrosinase. These results
indicate that guggulsterone inhibits IBMX-induced melanogenesis in
B16 cells through the suppression of tyrosinase expression.
The melanocyte-keratinocyte complex of the skin
responds quickly to a wide range of environmental stimuli, often
through paracrine and/or autocrine means. IBMX is known to increase
cellular cAMP through the inhibition of the cAMP-degrading enzyme,
phosphodiesterase (20).
Guggulsterone effectively blocked the IBMX-induced increase in
melanogenesis by decreasing the expression of tyrosinase. This
effect occurred at the transcriptional level, suggesting its action
on the cAMP-dependent pathway. When the B16 cells were treated with
α-MSH, a peptide acting on melanocortin 1-receptor (MC1-R) of
melanocytes (21), or forskolin,
a direct activator of adenylate cyclase (22), the cellular melanin contents were
significantly increased. Again, guggulsterone significantly
inhibited melanogenesis induced by both α-MSH and forskolin, as in
the case of IBMX stimulation. These results also support the action
mechanism of guggulsterone on the cAMP-dependent pathway. In
addition to the cAMP/PKA pathway, increased melanogenesis after UV
irradiation was thought to occur through the activation of the
diacylglycerol/protein kinase C (PKC) and nitric oxide/protein
kinase G (PKG) pathways, and SOS response to UV-induced DNA damage
(5). The PKC-induced activation
of tyrosinase occurs through phosphorylation rather than the
synthesis of new enzymes (23).
However, PKG is known to increase the expression of tyrosinase
protein (24). The additional
effects of guggulsterone on these pathways require further
study.
Safety following long-term application is a very
important issue for therapeutic compounds. In recent years,
naturally occurring herbal extracts and flavonoids have gained
attention as putative hypopigmenting agents (18,25–27). In the case of guggulsterone, no
toxicity was observed after oral administration (75 mg/kg) for
eight weeks in laboratory rats (28). The topical application of
guggulsterone prior to 12-O-tetradecanoylphorbol-13-acetate (TPA)
application onto mouse skin resulted in a significant inhibition
against TPA-induced skin edema and hyperplasia without any
noticeable side-effects (29). In
addition, guggulsterone has long been used in traditional medicine.
This evidence suggests the possibility of guggulsterone as a safe
hypopigmenting agent.
In conclusion, to our knowledge, the present study
demonstrates for the first time that guggulsterone is an effective
inhibitor of tyrosinase and inhibits melanin biosynthesis. Even
though we have not determined its effects in in vivo
conditions, guggulsterone may have beneficial effects in the
treatment of hyperpigmentation diseases.
Acknowledgements
The present study was supported by a
National Research Foundation of Korea grant funded by the Korean
Government (no. 2011-0028222).
References
|
1.
|
Y YamaguchiVJ HearingPhysiological factors
that regulate skin
pigmentationBiofactors35193199200910.1002/biof.2919449448
|
|
2.
|
VJ HearingM JimenezMammalian tyrosinase -
the critical regulatory control point in melanocyte pigmentationInt
J Biochem1911411147198710.1016/0020-711X(87)90095-43125075
|
|
3.
|
K YokoyamaK YasumotoH SuzukiS
ShibaharaCloning of the human DOPAchrome
tautomerase/tyrosinase-related protein 2 gene and identification of
two regulatory regions required for its pigment cell-specific
expressionJ Biol Chem26927080270871994
|
|
4.
|
T KobayashiK UrabeA WinderTyrosinase
related protein 1 (TRP1) functions as a DHICA oxidase in melanin
biosynthesisEMBO J135818582519947813420
|
|
5.
|
GE CostinVJ HearingHuman skin
pigmentation: melanocytes modulate skin color in response to
stressFASEB J21976994200710.1096/fj.06-6649rev17242160
|
|
6.
|
R BuscaR BallottiCyclic AMP a key
messenger in the regulation of skin pigmentationPigment Cell
Res136069200010.1034/j.1600-0749.2000.130203.x10841026
|
|
7.
|
S ImO MoroF PengActivation of the cyclic
AMP pathway by alpha-melanotropin mediates the response of human
melanocytes to ultraviolet B radiationCancer
Res58475419989426056
|
|
8.
|
C BertolottoP AbbeTJ
HemesathMicrophthalmia gene product as a signal transducer in
cAMP-induced differentiation of melanocytesJ Cell
Biol142827835199810.1083/jcb.142.3.8279700169
|
|
9.
|
P WanY HuL HeRegulation of melanocyte
pivotal transcription factor MITF by some other transcription
factorsMol Cell
Biochem354241246201110.1007/s11010-011-0823-421519923
|
|
10.
|
CJ SinalFJ GonzalezGuggulsterone: an old
approach to a new problemTrends Endocrinol
Metab13275276200210.1016/S1043-2760(02)00640-912163224
|
|
11.
|
NL UrizarDD MooreGUGULIPID: a natural
cholesterol-lowering agentAnnu Rev
Nutr23303313200310.1146/annurev.nutr.23.011702.07310212626688
|
|
12.
|
NL UrizarAB LivermanDT DoddsA natural
product that lowers cholesterol as an antagonist ligand for
FXRScience29617031706200210.1126/science.107289111988537
|
|
13.
|
J WuC XiaJ MeierS LiX HuDS LalaThe
hypolipidemic natural product guggulsterone acts as an antagonist
of the bile acid receptorMol
Endocrinol1615901597200210.1210/mend.16.7.089412089353
|
|
14.
|
J CuiL HuangA ZhaoGuggulsterone is a
farnesoid X receptor antagonist in coactivator association assays
but acts to enhance transcription of bile salt export pumpJ Biol
Chem2781021410220200310.1074/jbc.M20932320012525500
|
|
15.
|
KR StayrookKS BramlettRS SavkurRegulation
of carbohydrate metabolism by the farnesoid X
receptorEndocrinology146984991200510.1210/en.2004-096515564327
|
|
16.
|
N LvMY SongEK KimJW ParkKB KwonBH
ParkGuggulsterone, a plant sterol, inhibits NF-κB activation and
protects pancreatic beta cells from cytokine toxicityMol Cell
Endocrinol28949592008
|
|
17.
|
YR LeeJH LeeEM NohGuggulsterone blocks
IL-1β-mediated inflammatory responses by suppressing NF-κB
activation in fibroblast-like synoviocytesLife
Sci82120312092008
|
|
18.
|
JH KooI LeeSK YunHU KimBH ParkJW
ParkSaponified evening primrose oil reduces melanogenesis in B16
melanoma cells and reduces UV-induced skin pigmentation in
humansLipids45401407201010.1007/s11745-010-3405-420352496
|
|
19.
|
MS EllerBA GilchrestTanning as part of the
eukaryotic SOS responsePigment Cell Res13Suppl
8S94S97200010.1034/j.1600-0749.13.s8.17.x11041364
|
|
20.
|
JA BeavoNL RogersOB CroffordJG HardmanEW
SutherlandEV NewmanEffects of xanthine derivatives on lipolysis and
on adenosine 3′,5′-monophosphate phosphodiesterase activityMol
Pharmacol65976031970
|
|
21.
|
K WakamatsuA GrahamD CookAJ
ThodyCharacterisation of ACTH peptides in human skin and their
activation of the melanocortin-1 receptorPigment Cell
Res10288297199710.1111/j.1600-0749.1997.tb00688.x9359624
|
|
22.
|
T TamagawaH NikiA NikiInsulin release
independent of a rise in cytosolic free Ca2+ by
forskolin and phorbol esterFEBS
Lett183430432198510.1016/0014-5793(85)80825-52985438
|
|
23.
|
HY ParkV RussakovskyS OhnoBA GilchrestThe
beta isoform of protein kinase C stimulates human melanogenesis by
activating tyrosinase in pigment cellsJ Biol
Chem268117421174919937685020
|
|
24.
|
M SasakiT HorikoshiH UchiwaY
MiyachiUp-regulation of tyrosinase gene by nitric oxide in human
melanocytesPigment Cell
Res13248252200010.1034/j.1600-0749.2000.130406.x10952392
|
|
25.
|
DS KimSH ParkSB KwonK LiSW YounKC
Park(-)-Epigallocatechin-3-gallate and hinokitiol reduce melanin
synthesis via decreased MITF productionArch Pharm
Res27334339200410.1007/BF0298006915089040
|
|
26.
|
JH KooHT KimHY YoonEffect of xanthohumol
on melanogenesis in B16 melanoma cellsExp Mol
Med40313319200810.3858/emm.2008.40.3.31318587269
|
|
27.
|
N LvJH KooHY YoonEffect of Angelica
gigas extract on melanogenesis in B16 melanoma cellsInt J Mol
Med207637672007
|
|
28.
|
B SharmaR SalunkeS SrivastavaC MajumderP
RoyEffects of guggulsterone isolated from Commiphora mukul
in high fat diet induced diabetic ratsFood Chem
Toxicol4726312639200919635521
|
|
29.
|
S SarfarazIA SiddiquiDN SyedF AfaqH
MukhtarGuggulsterone modulates MAPK and NF-κB pathways and inhibits
skin tumorigenesis in SENCAR
miceCarcinogenesis2920112018200818684729
|