Introduction
As the largest insulin-sensitive tissue, skeletal
muscle is considered the most important tissue for insulin-mediated
glucose disposal, accounting for approximately 80% of
insulin-stimulated glucose uptake (1). Defects in insulin-induced glucose
uptake in the tissue are strongly linked to insulin resistance (IR)
(2), a hallmark of metabolic
diseases including type 2 diabetes and the metabolic syndrome. The
prevalence of these conditions continues to rise thus imposing an
enormous healthcare burden worldwide (3,4).
IR is simply the inability of insulin to stimulate
insulin signaling. Under insulin stimulation, the activated insulin
receptor (InsR) recruits and phosphorylates insulin receptor
substrate proteins (IRS), causing activation of the
phosphatidylinositol-3 kinase (PI3K) that phosphorylates
phosphatidylinositol-4,5-bisphosphate (PIP2) to generate
phosphatidylinositol-3,4,5-triphosphate (PIP3) which
recruits phosphoinositide-dependent kinase 1/2 (PDK1/2) and the
three known isoforms of Akt/protein kinase B (PKB), and then PDK1
and PDK2 phosphorylate Akt/PKB on Thr308/Ser473 respectively.
Activated Akt/PKB regulates downstream targets such as glucose
transporter-4 (Glut4), glycogen synthase kinase 3 (GSK3), forkhead
box O1 (Foxo1), hormone sensitive lipase (HSL), and mTOR, thus
being responsible for most of the metabolic actions of insulin to
maintain glucose, fat and protein homeostasis, including glucose
uptake, glucose synthesis, and gluconeogenesis (5–9).
It is well known that the inactivation of the PI3K pathway leads to
IR, showing a decrease in insulin-induced glucose uptake and
disposal (10,11). Recently, a novel insulin signaling
dependent on β-arrestin-2 has been discovered (12). Upon stimulation by insulin,
β-arrestin-2 scaffolds Src and Akt to InsR, causing the formation
of a new β-arrestin-2 signal complex, which allows Src to
phosphorylate Akt, thus enhancing the phosphorylation of Akt at
Thr308/Ser473, and subsequently regulating insulin-mediated
phosphorylation of GSK3 and Foxo1, promoting insulin-stimulated
translocation of Glut4 from intracellular organelles (endosomes) to
the cell surface within insulin-responsive tissues, where Glut4
binds glucose and is in charge of glucose uptake (12,13). Loss or dysfunction of β-arrestin-2
leads to deficiency of this complex and disturbance of the
signaling, resulting in IR and progression of type 2 diabetes. On
the contrary, overexpression of β-arrestin-2 promotes the formation
of the complex, and improves insulin sensitivity in insulin
resistance model animals (12,14–16). β-arrestin-2 and the
β-arrestin-2-mediated signaling display a potential role in
preventing IR. Therefore, a strategy for enhancing glucose uptake
is to preserve or strengthen the β-arrestin-2-mediated
signaling.
Pollen Typhae is the pollen of several species of
the genus Typha (Typhaceae) including T. angustifolia
L., T. orientalis Presl., T. davidiana Hand.-Mazz.
and T. minima Funk. It has been widely used to treat trauma,
haematemesis, metrorrhagia, dysmenorrhea, hematuria and stranguria
in Chinese medical clinical practice. Increasing evidence also
indicates that Pollen Typhae performs a series of pharmacological
functions. For example, Pollen Typhae improves the
microcirculation, ameliorates dyslipidemia, and prevents and
controls coronary heart diseases and myocardial infarction
(17,18). Moreover, it shows cytotoxicity
against tumor cells (19), and
regulates immune activity (20).
Studies have proven that the main constituents of Pollen Typhae
contain flavones, linoleic acid, and other unsaturated fatty acids
(21,22). Pollen Typhae total flavone (PTF),
the extract of Pollen Typhae, possesses anti-inflammatory and
anticoagulant activities (23,24). In clinical research and animal
studies, the Chinese herbal medicine ‘Yiqi Sanju Formula’ chiefly
consisting of Pollen Typhae and several other Chinese herbs has
been used to treat type 2 diabetes, central obesity, and
non-alcoholic fatty liver disease, characterized by IR, showing
anti-IR activity (25–27). We have also reported that PTF
ameliorated high-glucose- and high-insulin-induced impairment of
glucose uptake in 3T3-L1 adipocytes (28), suggesting the ability of PTF to
improve IR, but the potential molecular mechanisms remain
unclear.
The aim of this study was to investigate the effects
of PTF on glucose uptake, and to explore the underlying molecular
mechanisms in C2C12 myotubes.
Materials and methods
Reagents
Dulbecco’s modified Eagle’s medium (DMEM), fetal
calf serum (FCS), and horse serum were purchased from Gibco (Grand
Island, NY). PP2, wortmannin, insulin solution, bovine serum
albumin (BSA), palmitic acid,
2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)
carbonyl]-2H-tetrazolium hydroxide (XTT), and cytochalasin B were
obtained from Sigma (St. Louis, MO), fatty acid-free BSA was from
Roche (Mannheim, Germany). Culture plates were from Corning (New
York, NY). 2-Deoxy-D-[2,6-3H] glucose (2-DOG) was
obtained from Amersham (Buckinghamshire, UK). Antibodies directed
against Akt (total), Akt (phosphorylated Thr308), Akt
(phosphorylated Ser473), and PI3K-p85 were from Cell Signaling
Technology (Danvers, MA). Secondary HRP-conjugated antibodies and
antibodies to InsR-β (phosphorylated Tyr1150/1151), Src, and
β-arrestin-2 were purchased from Santa Cruz Biotechnology, Inc.
(Santa Cruz, CA). The PI3K ELISA kit was from Echelon Biosciences
and BCA protein assay kit was from Pierce (Rockford, IL). TRIzol
reagents were purchased from Invitrogen Life Technologies
(Carlsbad, CA).
Preparation of PTF
The pollen of T. angustifolia L. was
collected in Anguo, Hebei, China, in June 2004, and authenticated
by W.-J.W. based on microscopic and macroscopic characteristics.
The fresh pollen of T. angustifolia L. was dried at 37°C
with protection from light, and ground into powder. The extract of
pollen of T. angustifolia L. was obtained as described with
some modifications (20,21). Briefly, the material (500 g) was
extracted with 70% ethanol three times under reflux for 1 h. After
filtration and concentration in rotavapour at 45°C until there was
no flavor of ethanol, the solution was extracted with n-butyl
alcohol three times. The combined solution was then concentrated
under reduced pressure in rotavapor at 45°C to evaporate the
solvent, and finally dried in high vacuum. The dried extract
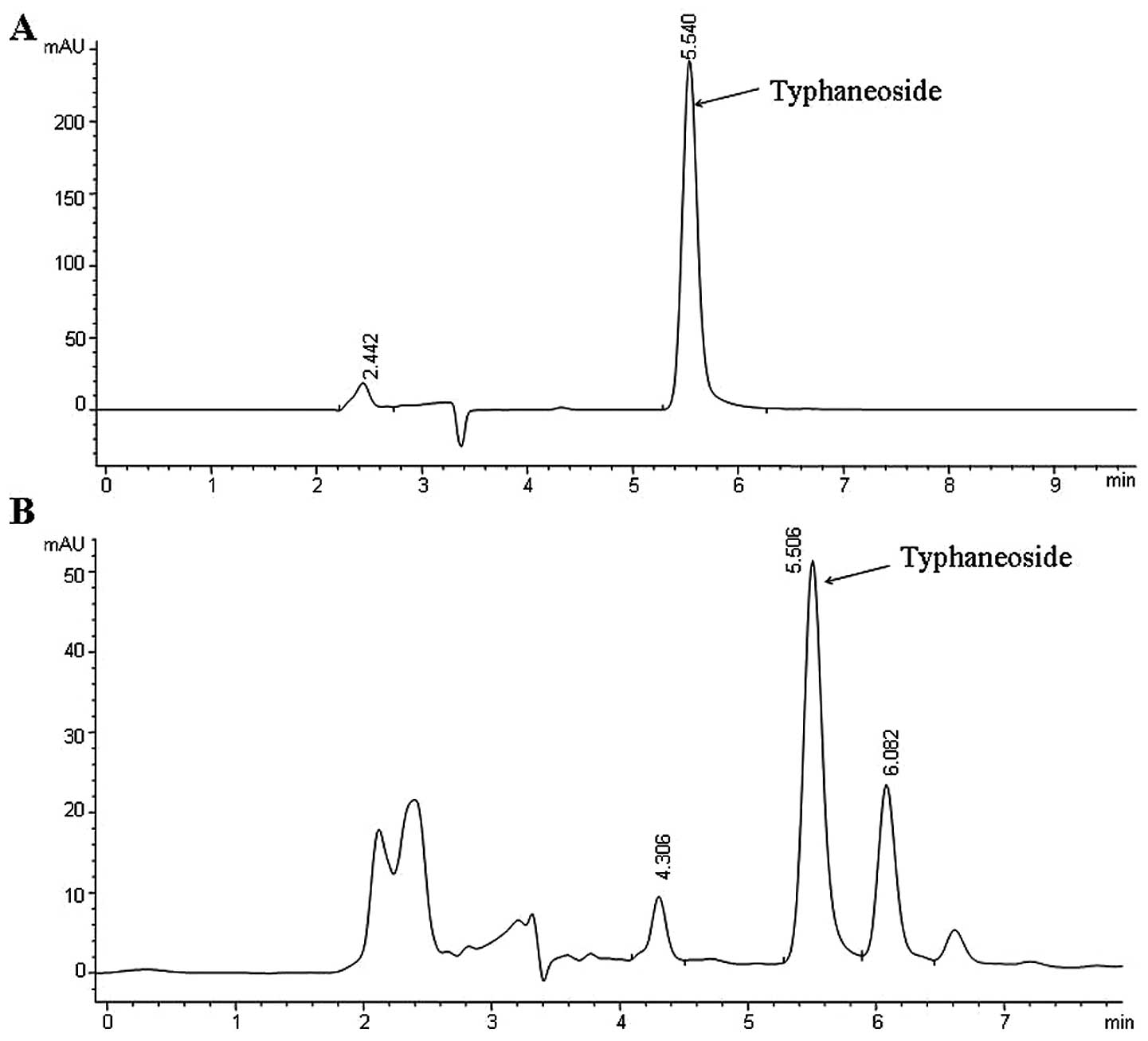
weighed 8.78 g (yield 1.76%, w/w), which was determined to be PTF
by HPLC according to the study by Yang et al (21), which contains Typhaneoside and
undefined components (Fig. 1).
The specimens of dried pollen of T. angustifolia L. and
extract were deposited in the Institute of Chinese Integrative
Medicine, Huashan Hospital, Fudan University, Shanghai, China.
Cell culture and differentiation
C2C12 myoblasts were obtained from the American Type
Culture Collection (Manassas, VA). The cells were maintained in
DMEM supplemented with 10% (vol/vol) FCS and antibiotics
(penicillin 100 U/ml, streptomycin 100 μg/ml) for growth at
37°C under a humidified atmosphere containing 5% CO2.
For differentiation of myotubes, C2C12 myoblasts were transferred
to DMEM containing 2% horse serum when the cells reached
confluence. After an additional 4 days, the cells fused into
myotubes, which were used in the subsequent experiments. The medium
was changed every other day.
Cytotoxicity analysis
The cytotoxicity of PTF was determined by the XTT
assay (29). C2C12 myoblasts were
cultured in 96-well culture plates. After differentiation, the
medium was replaced by serum-free DMEM supplemented with 1% BSA,
and the cells were cultured for 12 h. C2C12 myotubes in each well
were then treated with 100 μl serum-free 1% BSA-DMEM
containing different concentrations of PTF (0, 0.05, 0.1, 0.25,
0.5, 1.0 and 2.0 mg/ml). After treatment for 24 h, 50 μl of
0.1% XTT dissolved in serum-free DMEM was added directly into each
well. After incubation for 4 h at 37°C, the optical absorbance was
read on a microplate reader (ELX 800; Bio-Tek Instruments,
Winooski, VT) at 490 nm.
Glucose uptake assay
Glucose uptake was determined by measuring the
transport of 2-DOG into the cells as previously described with some
modifications (30). C2C12
myoblasts were differentiated in 24-well culture plates. After 12-h
serum deprivation and then PTF treatment for different times (8, 16
and 24 h), the cells were washed three times with Krebs-Ringer
phosphate buffer (KRPB) (10 mM HEPES, 131.2 mM NaCl, 4.7 mM KCl,
1.2 mM MgSO4, 2.5 mM CaCl2, 2.5 mM
NaH2PO4, pH 7.4), and then incubated in KRPB
with or without 100 nM insulin at 37°C. After 20 min, 0.5
μCi/ml 2-DOG was added to the cells. After 10 min
incubation, the cells were washed three times with ice-cold PBS
containing 10 mM glucose to terminate the reaction. Finally, the
cells were lysed with 0.1 N NaOH for 2 h, and the radioactivity
taken up by the cells was measured by a scintillation counter
(Beckman Instruments). Non-specific glucose uptake was determined
in the presence of 20 μM cytochalasin B, and this was
subtracted from the total uptake to get specific glucose
uptake.
Western blotting and ELISA assay
After 12-h serum deprivation, C2C12 myotubes in
6-well plates were treated with PTF for 16 h, and then incubated
with or without 100 nM insulin for 30 min. The cells were washed
three times with ice-cold PBS, and then lysed in cell lysis buffer
(RIPA, 50 mM Tris-HCl, 150 mM NaCl, 1 mM EDTA, 1% NP-40, 1 mM PMSF,
2 μg/ml pepstatin, 10 μg/ml leupeptin, pH 7.4). The
insoluble lysate material was removed by centrifugation (12,000 rpm
for 30 min, 4°C), and the protein concentrations of supernatants
were determined using a BCA protein assay kit, which were utilized
for western blotting and ELISA as previously described (31). The cell lysates were boiled for 5
min, and then separated via SDS-PAGE. The protein was
electrophoretically transferred to polyvinylidene difluoride
membranes. The membranes were incubated with the first antibody for
1–2 h and then the secondary antibody for 1–2 h. Immunoreactive
bands were visualized by incubation with ECL Plus detection
reagents (Amersham Biosciences). For the measurement of PI3K
activity, lysates were measured by ELISA per the manufacturer’s
instructions. The detection limits were 12.5 to 200 pmol in 100
μl detection volume.
Quantification of mRNA levels by
real-time PCR
The mRNA levels of β-arrestin-2, Src, and Akt2 were
identified by real-time PCR after reverse transcription as
previously described (32). After
12-h serum deprivation, C2C12 myotubes in 6-well culture plates
were treated with PTF for 16 h, and then washed three times with
ice-cold PBS. Total-RNA was extracted from the cells using TRIzol
reagents, and then transcribed into cDNA with a superscript
first-strand cDNA synthesis system. The relative gene abundance was
quantified by real-time PCR, the reactions were performed in an ABI
7500 sequence detection system. The sequences of primers used were:
β-arrestin-2, forward, 5′-TCC CTA GGG CGG CAA GCT GT-3′ and
reverse, 5′-ACT GGG GGC GAG TTG GTG TGA-3′; Src, forward, 5′-TCG
GAC ACC GTC ACC TCC CC-3′ and reverse, 5′-GAC AAT CTG CAG CCG CTC
CCC-3′; Akt2, forward, 5′-AAA AAG TGG CTC TGG TGT GTG GAG C-3′ and
reverse, 5′-GAC TGT GGT CCA CTG CAG GCA-3′; GAPDH, forward, 5′-CCC
CAG CAA GGA CAC TGA GCA AGA G-3′ and reverse, 5′-GCC CCT CCT GTT
ATT ATG GGG GTC-3′.
Statistical analysis
The data are presented as means ± standard error
(SE). Changes in 2-DOG uptake between baseline and insulin
stimulation (known as 2-DOG uptake induced by insulin) are
expressed as δ. All data were analyzed with SPSS 16.0 for Windows.
To identify significant differences between the two groups,
comparisons were analyzed by the Student’s t-test. When multiple
comparisons were performed, the significance was analyzed by
one-way analysis of variance (ANOVA). A value of P<0.05 was
regarded as statistically significant.
Results
Cytotoxicity of PTF in C2C12
myotubes
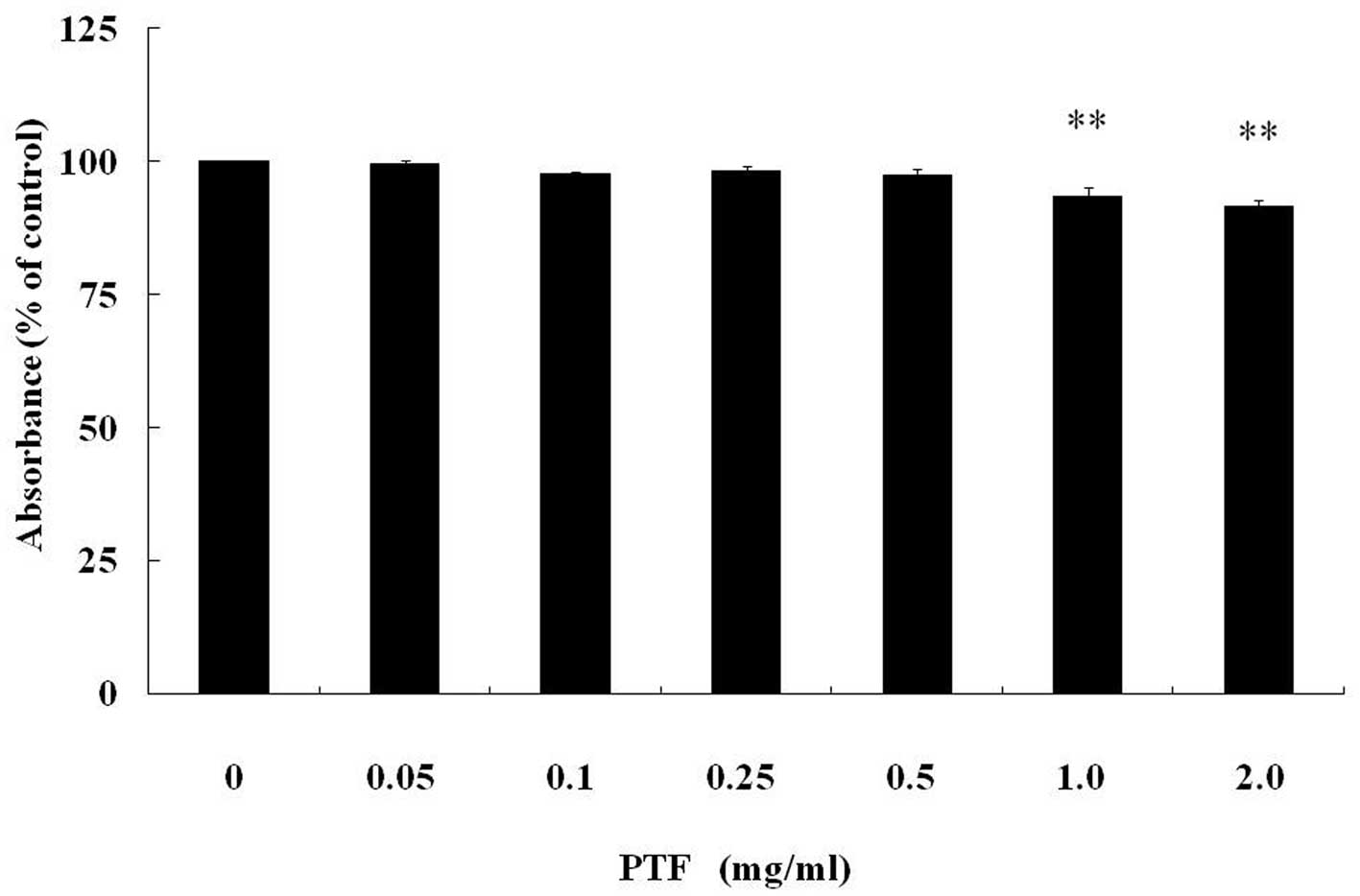
The cytotoxicity of PTF in C2C12 myotubes was
examined after PTF treatment for 24 h (Fig. 2). In C2C12 myotubes, PTF treatment
decreased cell activity in a dose-dependent manner. PTF doses of
0.05–0.5 mg/ml, were not cytotoxic, but PTF was cytotoxic
(P<0.01) at the concentration of 1.0 mg/ml or above compared
with the control (0 mg/ml).
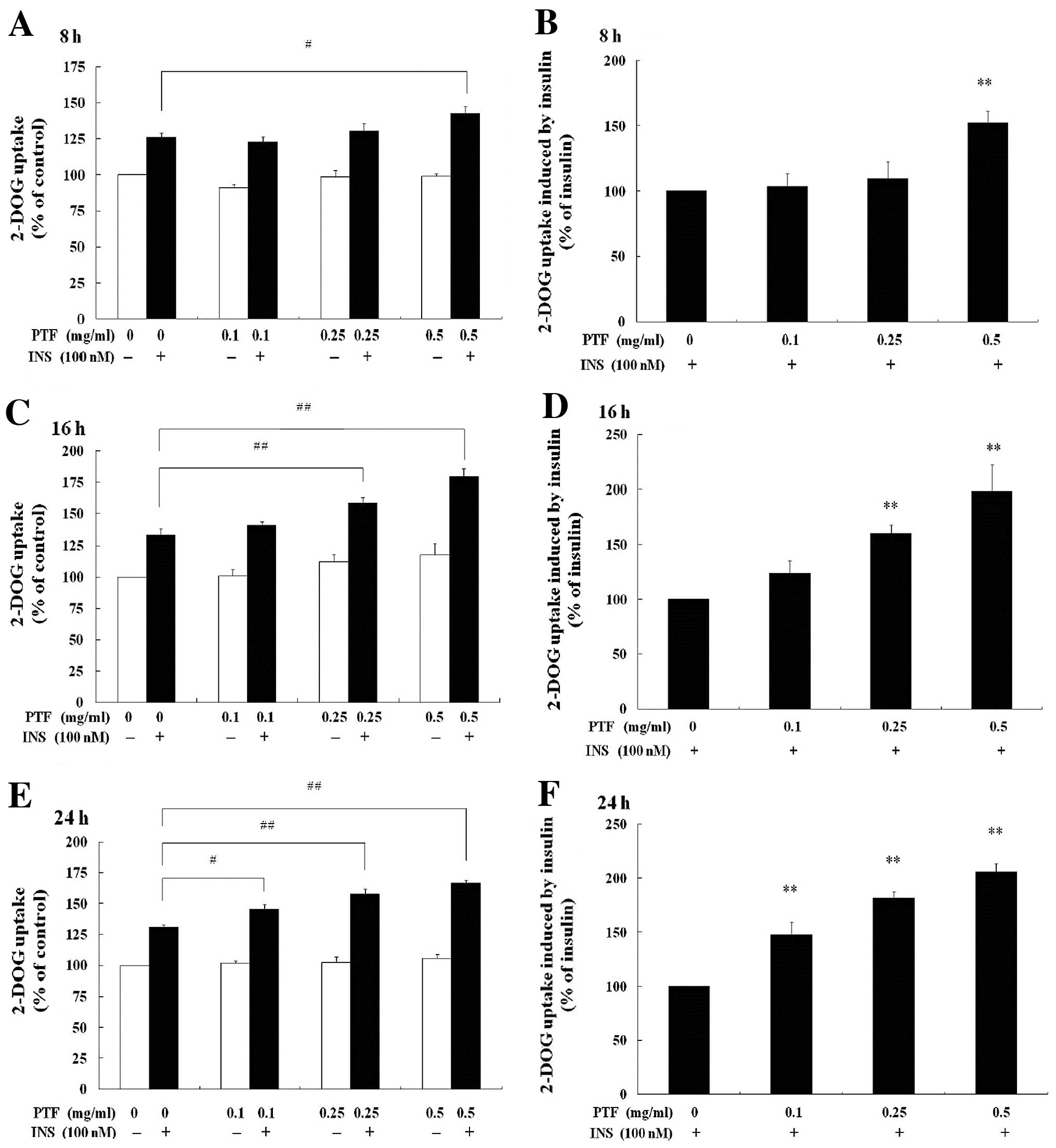
PTF ameliorates insulin-stimulated
glucose uptake in a dose-and time-dependent manner in C2C12
myotubes
Glucose uptake was investigated for PTF in C2C12
myotubes with 2-DOG. In the absence of insulin, 2-DOG uptake was
not promoted with PTF treatment for 8, 16 or 24 h (Fig. 3A, C and E) at all concentrations
(0.1, 0.25 or 0.5 mg/ml) compared with the control (0 mg/ml PTF).
In the presence of insulin, compared with insulin alone, only 0.5
mg/ml PTF increased insulin-induced 2-DOG uptake by 52.26%
(P<0.01) after PTF pre-treatment for 8 h (Fig. 3B). After PTF pre-treatment for 16
h (Fig. 3D), both 0.25 and 0.5
mg/ml PTF dramatically improved insulin-induced 2-DOG uptake by
60.00% (P<0.01) and 98.76% (P<0.01) respectively.
Additionally, after PTF pre-treatment for 24 h (Fig. 3F), PTF at all concentrations of
0.1, 0.25 and 0.5 mg/ml prominently enhanced insulin-induced 2-DOG
uptake by 47.60% (P<0.01), 81.46% (P<0.01) and 105.82%
(P<0.01), respectively. These results indicate that PTF improves
glucose uptake in an insulin-dependent manner in C2C12 myotubes,
suggesting the potential ability of PTF to ameliorate IR.
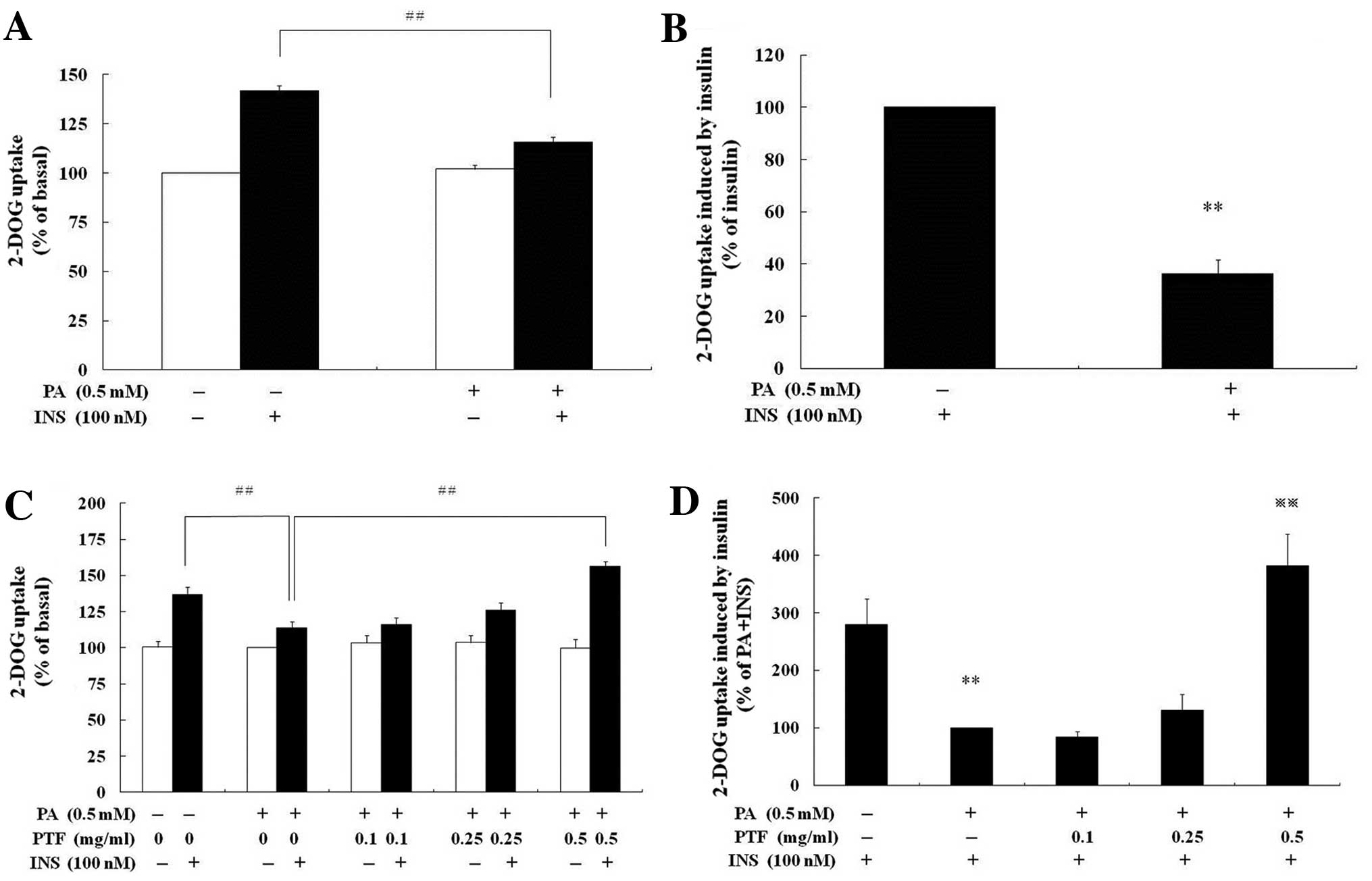
PTF prevents palmitate-induced IR in
C2C12 myotubes
According to Reaven’s initial studies (33), IR is defined as a decrease in
insulin-mediated glucose uptake. In our previous study (28), PTF was established to improve
high-glucose- and high insulin-induced IR in 3T3-L1 adipocytes. In
order to determine whether PTF ameliorate IR in C2C12 myotubes,
palmitate (PA) was used to induce IR according to the study by Senn
(31). PA (0.5 mM) pre-treatment
for 16 h resulted in a reduction in insulin-induced glucose uptake
by 63.69% (P<0.01) in C2C12 myotubes compared with insulin alone
(Fig. 4B). After both PTF and PA
pre-treatment for 16 h, PTF (0.5 mg/ml) notably increased
insulin-mediated glucose uptake (P<0.01) compared with PA +
insulin (Fig. 4D), which was
similar to insulin alone (P>0.05).
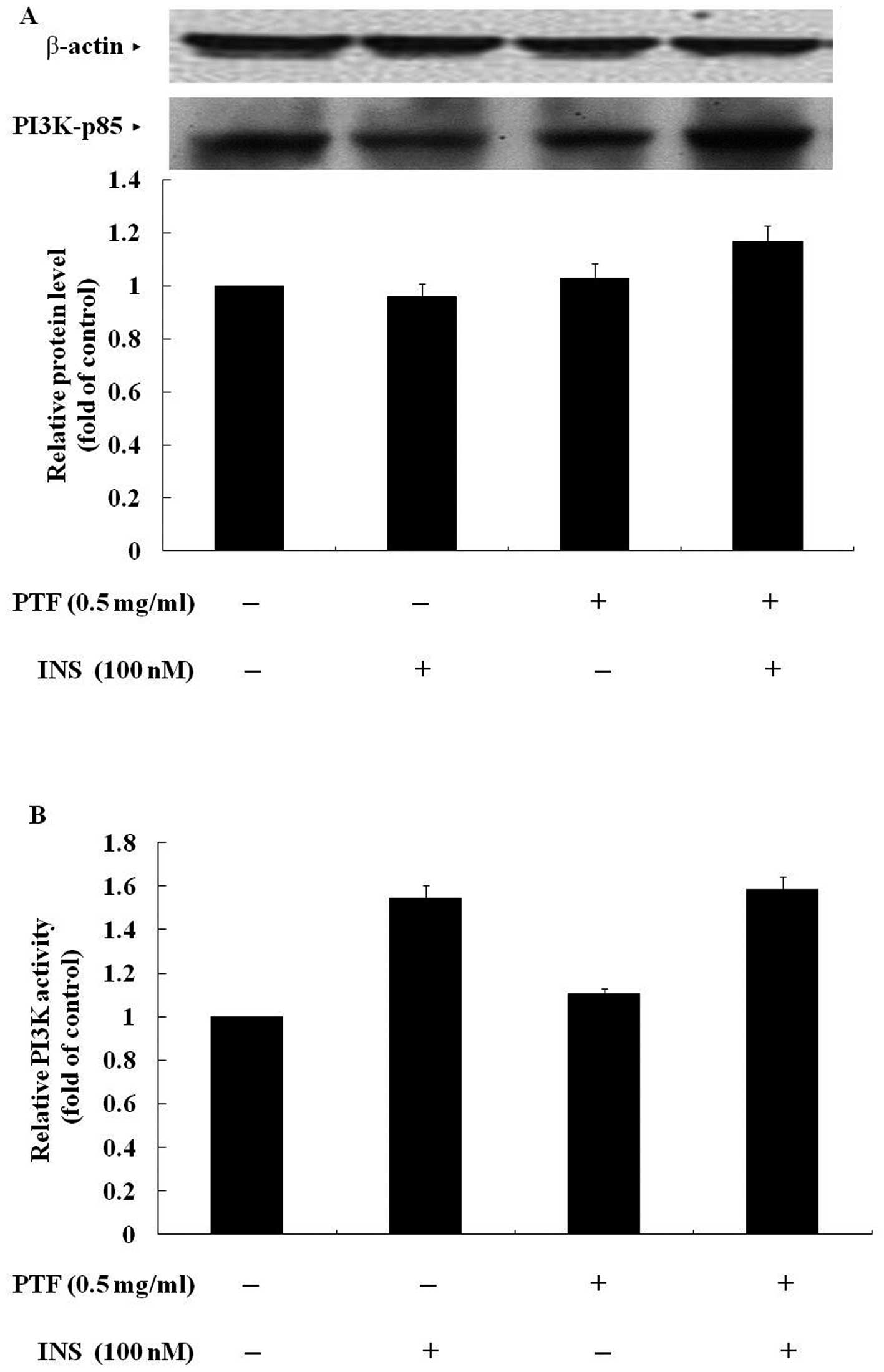
PTF has no effects on the protein
expression of p85 or the activity of PI3K in C2C12 myotubes
To gain insight into the molecular mechanisms, we
observed the effects of PTF on insulin signaling. PI3K, which
consists of a regulatory (p85) and a catalytic subunit (p110), and
plays a key role in the PI3K pathway, was firstly investigated.
Western blotting showed that C2C12 myotubes were pre-treated with
PTF (0.5 mg/ml) for 16 h, the protein expression of p85 was not
obviously changed whether there was insulin to stimulate the cells
for 30 min or not (Fig. 5A). The
ELISA assay revealed that the activity of PI3K was not induced with
PTF treatment for 16 h in the absence of insulin. In the presence
of insulin, insulin obviously increased the activity of PI3K, and
PTF pre-treatment for 16 h did not further enhance the activity of
PI3K (Fig. 5B). These data
indicate that PTF exhibits few effects on the protein expression of
p85 and the activity of PI3K, suggesting that PTF improves
insulin-induced glucose uptake independent of PI3K.
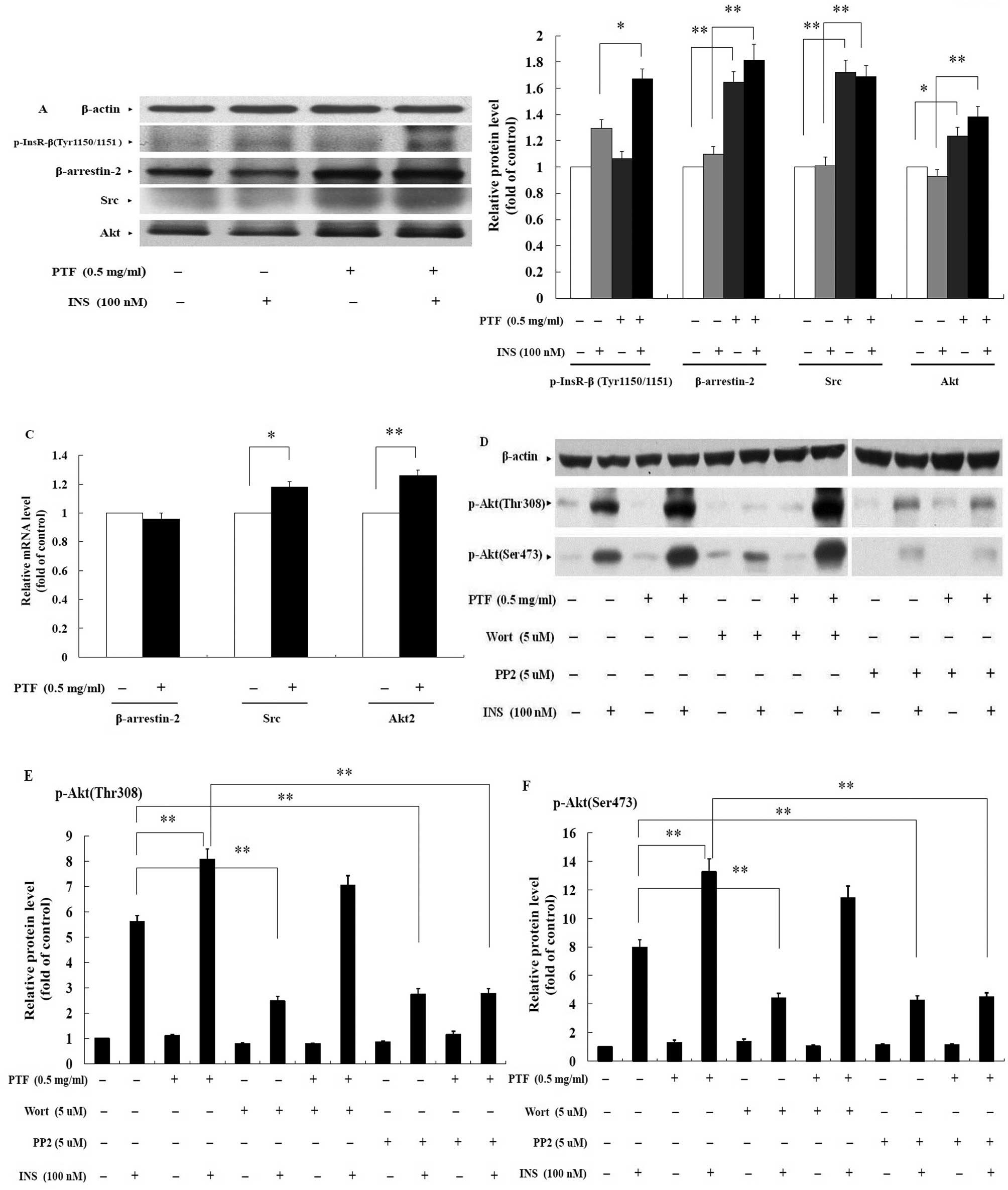
PTF improves the phosphorylation of
InsR-β in an insulin-dependent manner in C2C12 myotubes
Tyrosine autophosphorylation of InsR, comprised of
two extracellular α subunits and two intracellular β subunits, is
one of the earliest cellular responses to insulin stimulation.
Autophosphorylation begins with the phosphorylation of β subunits
at Tyr1146 and either Tyr1150/1151, which are required for full
InsR activation (34). In the
absence of insulin, PTF treatment for 16 h did not increase the
phosphorylation of InsR-β at Tyr1150/1151 compared with the
control. In the presence of insulin, however, insulin increased
InsR-β phosphorylation, and PTF pre-treatment for 16 h further
enhanced insulin-induced phosphorylation by 28.93% (P<0.05)
compared with insulin alone (Fig. 6A
and B). These results suggest that PTF increases the
phosphorylation of InsR-β in an insulin-dependent manner, and that
PTF improves insulin-induced glucose uptake through another pathway
independent of PI3K.
PTF enhances the protein expression of
β-arrestin-2 with no effect on the basal gene expression in C2C12
myotubes
In addition to the PI3K pathway, the second pathway
downstream of InsR is required for full Akt activation (12,15,16). In the β-arrestin-2-mediated
signaling, β-arrestin-2 scaffolds Akt and Src to InsR upon insulin
stimulation, causing the formation of the β-arrestin-2 signal
complex, thereby regulating insulin-stimulated glucose disposal
including glucose uptake. In view of the results, it was likely
that PTF enhanced insulin-mediated glucose uptake via this
signaling. Compared with the control and insulin alone, PTF
pre-treatment of C2C12 myotubes for 16 h prominently increased the
protein expression of β-arrestin-2 by 64.60% (P<0.01) and 65.51%
(P<0.01) respectively (Fig. 6A and
B). However, real-time PCR assay revealed that mRNA levels of
β-arrestin-2 were not enhanced with PTF treatment for 16 h
(Fig. 6C).
PTF increases the protein and the basal
gene expression of Src in C2C12 myotubes
In the β-arrestin-2 complex (12,15,16), Src phosphorylates Akt on
Tyr315/326 which is required for the subsequent phosphorylation of
Akt at Thr308/Ser473. In this study, the protein expression of Src
in C2C12 myotubes was elevated by 72.23% (P<0.01) and 67.48%
(P<0.01) with PTF pre-treatment for 16 h compared with the
control and insulin alone, respectively (Fig. 6A and B). Moreover, PTF increased
mRNA expression of Src compared with the control (P<0.05)
(Fig. 6C).
PTF elevates the protein and the basal
gene expression of Akt, and improves the phosphorylation of Akt in
an insulin-dependent manner in C2C12 myotubes
Akt is the downstream target of Src in the
β-arrestin-2 complex, and has three isoforms in mammals, each
encoded by a different gene (Akt1-3), where Akt2 is closely related
with the insulin-induced glucose uptake (35,36). Phosphorylation of Akt at
Thr308/Ser473 stands for its full activation (6–8).
In C2C12 myotubes, compared with the control and insulin alone, PTF
pre-treatment for 16 h markedly increased the protein expression of
Akt by 23.61% (P<0.05) and 48.83% (P<0.01), respectively
(Fig. 6A and B). Similarly, mRNA
levels of Akt2 with PTF treatment for 16 h were enhanced
(P<0.01) compared with the control (Fig. 6C).
In the absence of insulin, PTF did not elevate the
phosphorylation of Akt at Thr308 or Ser473. The phosphorylation was
significantly increased with insulin stimulation for 30 min, and
PTF further enhanced the insulin-induced phosphorylation at Thr308
by 44.02% (P<0.01) (Fig. 6D and
E) and Ser473 by 66.10% (P<0.01) (Fig. 6D and F) after PTF pre-treatment
for 16 h.
Wortmannin, an inhibitor of PI3K, used to pre-treat
C2C12 myotubes for 16 h, remarkably inhibited insulin-induced
phosphorylation of Akt at Thr308 by 55.95% (P<0.01) (Fig. 6D and E) and Ser473 by 44.56%
(P<0.01) (Fig. 6D and F)
compared with insulin alone, but failed to inhibit PTF-induced
phosphorylation (P>0.05) (Fig. 6D,
E and F).
Similarly, PP2, an inhibitor of Src, significantly
reduced insulin-induced phosphorylation of Akt at Thr308 by 51.18%
(P<0.01) (Fig. 6D and E) and
Ser473 by 46.83% (P<0.01) (Fig. 6D
and F) compared with insulin alone. Furthermore, PP2 also
reduced PTF-induced phosphorylation at Thr308 by 65.74% (P<0.01)
(Fig. 6D and E) and Ser473 by
66.06% (P<0.01) (Fig. 6D and
F) compared with PTF + insulin.
Together, PTF increased the protein and the basal
gene expression of Akt, and improved insulin-induced
phosphorylation of Akt, which was inhibited by PP2 but not
wortmannin. The data support the idea that PTF improves
insulin-induced glucose uptake through the β-arrestin-2-mediated
signaling in C2C12 myotubes.
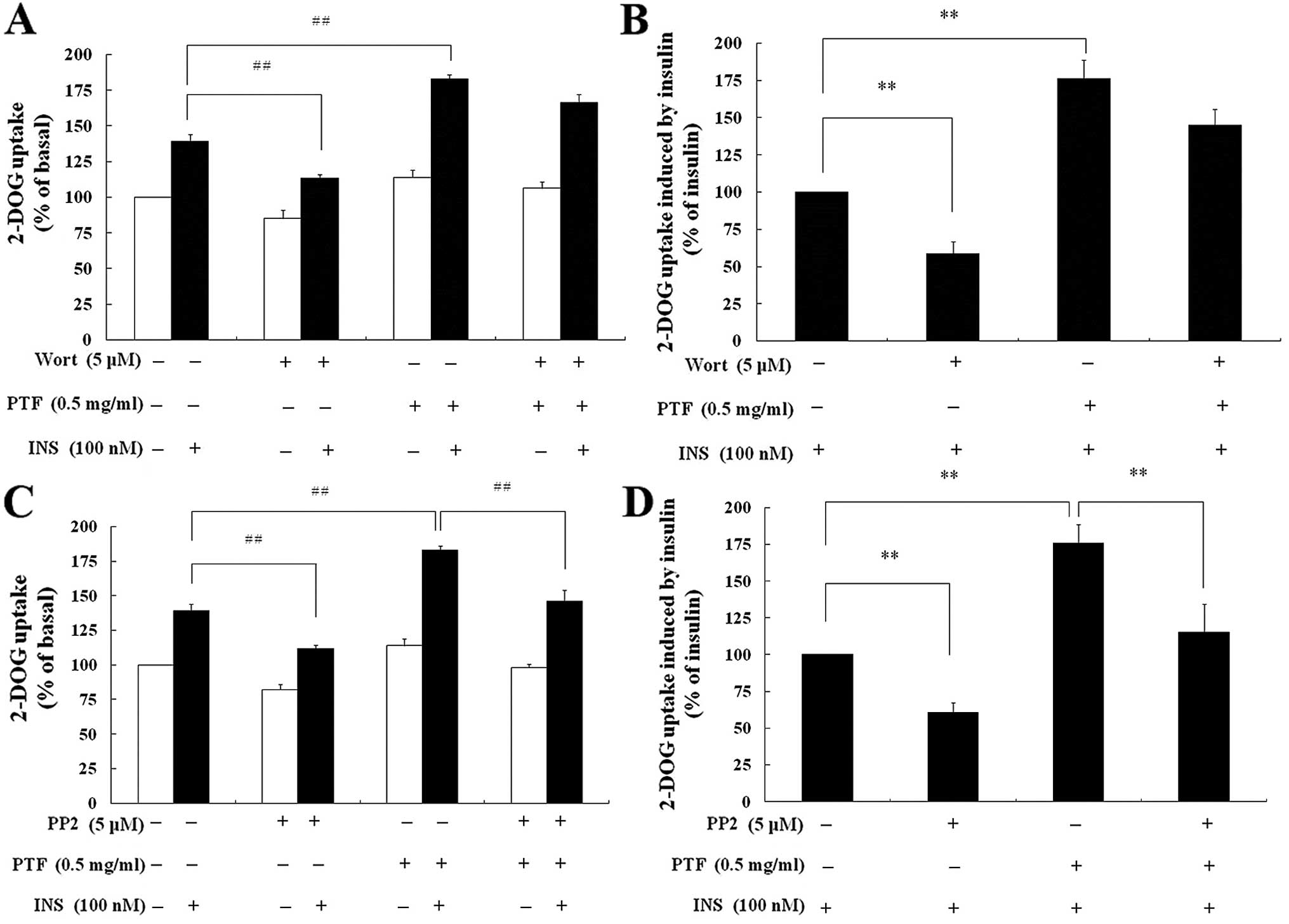
Effects of wortmannin and PP2 on
PTF-induced glucose uptake in C2C12 myotubes
Compared with insulin alone, wortmannin
pre-treatment for 16 h resulted in a decrease in insulin-mediated
2-DOG uptake by 42.81% (P<0.01) (Fig. 7B), and PP2 by 38.33% (P<0.01)
(Fig. 7D). Compared with PTF +
insulin, wortmannin + PTF + insulin only inhibited insulin-induced
2-DOG uptake by 15.79% (P>0.05) (Fig. 7B), but PP2 by 35.29% (P<0.01)
(Fig. 7D).
Discussion
Chinese herbs have been used to treat diabetes for
thousands of years in Chinese clinical practice, and have become
popular complementary and alternative medicine in the treatment of
metabolic diseases by improving IR (37,38). It is worthwhile to screen insulin
sensitizing reagents from Chinese herbs, and to elucidate their
molecular mechanisms. In this study, we found that PTF increased
insulin-stimulated glucose uptake in a dose-dependent manner, and
prevented PA-induced IR in C2C12 myotubes, which conform to our
previous report (28). Insulin
signaling mainly refers to two pathways: the Ras-mitogen-activated
protein kinase (MAPK) pathway, and the PI3K signaling. The former
regulates expression of some genes, and controls growth and
differentiation of cells (5,6,8).
The latter mediates most of the metabolic actions of insulin
including insulin-induced glucose uptake. It is agreed that PA
results in IR by restraining the PI3K pathway (39,40), and that enhancing the activity of
the PI3K pathway is the key strategy for improving glucose uptake
and IR. In order to determine whether PTF has some relationship
with the PI3K pathway in improving insulin-induced glucose uptake,
PI3K was hereby assayed, which consists of a regulatory (p85) and a
catalytic subunit (p110) and plays a key role in the PI3K pathway.
In the basal state, p85 binds to p110, thus inhibiting the activity
of p110. Upon insulin stimulation, p85 associates with IRS and sets
p110 free. In turn p110 catalyzes the production of
PIP3, which recruits PDK1/2 and Akt (8,41).
In this study, PTF had no effects on the protein expression of p85
or the activity of PI3K. Interestingly, PTF prompted
insulin-induced tyrosine phosphorylation of InsR-β at Tyr1150/1151,
the feature of InsR standing for the earliest activation, which is
required for full kinase activation of InsR in triggering insulin
signaling. The results indicate that PTF increases insulin-induced
glucose uptake dependent on InsR but not PI3K, suggesting that PTF
improves insulin-stimulated glucose uptake through another
pathway.
During 2009, Luan et al (12) found another novel insulin
signaling involving β-arrestin-2, Src, InsR, and Akt, which provide
new insight into the molecular pathogenesis of IR, and implicate a
new strategy for screening insulin-sensitizing reagents to improve
IR (42). Interestingly, PTF
significantly enhanced the protein expression of β-arrestin-2 in
C2C12 myotubes. It is generally accepted that β-arrestin-2 is an
important adaptor in modulating the strength and duration of
cellular signaling by scaffolding and interacting with a lot of
cytoplasmic proteins including InsR, Src and Akt (15,16,43). Additionally, allowing for the
improvement in the phosphorylation of InsR-β by PTF, we inferred
that PTF had some relationship with the β-arrestin-2-mediated
signaling. Western blotting and real-time PCR assay revealed that
PTF not only increased the protein expression of Src and Akt, but
also prompted the gene expression of Src and Akt. According to the
novel signaling, β-arrestin-2 scaffolds Src and Akt to InsR upon
insulin stimulation, causing the formation of the β-arrestin-2
signal complex. Following autophosphorylation of InsR-β, Src
phosphorylates Akt on Tyr315/326, which enhances the
phosphorylation of Akt at Thr308 by PDK1 and at Ser473 by PDK2
(5,6,8).
To our surprise, PTF increased the phosphorylation of Akt at Thr308
and Ser473.
In order to further confirm the results, PP2, the
inhibitor of Src, was used to inhibit the activity of the
β-arrestin-2-mediated signaling. The results exhibited that PP2 not
only inhibited the insulin-induced phosphorylation of Akt, but also
depressed the PTF-induced phosphorylation of Akt. Together, PTF had
beneficial effects on the β-arrestin-2-mediated signaling. Since,
PTF had no effects on PI3K, wortmannin, the inhibitor of PI3K, was
used to inhibit the activity of the PI3K pathway. The results
showed that wortmannin inhibited insulin-induced phosphorylation of
Akt, but did not depress PTF-induced phosphorylation. The present
study does not prove that the PI3K pathway is not required for the
PTF-induced increase in insulin-mediated phosphorylation of Akt,
because the phosphorylation of Akt at Thr308 and Ser473 is
dependent on PI3K, and Src only affects the phosphorylation at
Thr308 and Ser473 via phosphorylating Akt on Tyr315/326. PTF had
beneficial effects on the β-arrestin-2-mediated signaling, thus to
some extent compensating for the loss of the phosphorylation of Akt
by wortmannin. On the other hand, PTF contains a number of
constituents including typhaneoside, isorhamnetin,
isorhamnetin-3-O-neohesperidoside, kaempferol, quercetin and so on
(21,44). It has been reported that
isorhamnetin increases Akt activity in PC12 cells (45), kaempferol regulates
lipopolysaccharide-induced phosphorylation of Akt in BV2 microglial
cells (46), and quercetin
protects oligodendrocyte precursor cells from oxygen/glucose
deprivation injury in vitro via upregulating phosphorylation
of Akt (47). It is expected that
a certain ingredient or a specific constituent directly represses
wortmannin, thus relieving the inhibition of wortmannin on the
phosphorylation of Akt at Thr308/Ser473, which needs further
studying. Activated Akt promotes insulin-stimulated translocation
of Glut4 from intracellular organelles (endosomes) to cell surface
within insulin-responsive tissues including muscle and fat, where
Glut4 binds glucose and is in charge of glucose uptake (13). After transport of glucose into the
skeletal muscle cells, glucose is mainly oxidized for energy, and
used for glycogen synthesis. Skeletal muscle is not the site of
gluconeogenesis due to a lack of gluconeogenesis enzyme. Consistent
with our results involving Akt, glucose uptake induced by PTF was
blocked by PP2 but not wortmannin, which further proved the
beneficial effects of PTF on the β-arrestin-2-mediated
signaling.
Furthermore, western blotting and real-time PCR
analysis showed that PTF enhanced the protein expression of
β-arrestin-2, but did not increase the gene expression. The
ubiquitin system-induced ubiquitination and degradation of protein
is an essential cellular mechanism. As previously reported
(48–50), β-arrestin-2 is also an important
regulator in the ubiquitin system-mediated ubiquitination of target
proteins. β-arrestin-2 not only scaffolds and recruits ubiquitin
ligase substrates, such as GPCRs, to the ubiquitin ligase Mdm2,
thereby augmenting E3-mediated ubiquitination of target proteins
and blocking relevant cellular signaling, but also interacts with
Mdm2, causing β-arrestin-2 itself degradation by Mdm2 but
competitively inhibiting ubiquitination of target proteins.
Therefore, we propose that PTF may inhibit ubiquitination and
degradation of β-arrestin-2 to enhance its protein levels, which
merits further investigation.
In conclusion, PTF increases insulin-induced glucose
uptake through the β-arrestin-2-mediated signaling in C2C12
myotubes. The findings suggest the potential uses of PTF, or
compounds derived thereof, against type 2 diabetes and metabolic
syndrome.
Acknowledgements
We thank Wei-Hua Chen and Jian Ying
for providing technical assistance. This study was supported by the
PhD Programs Foundation of the Ministry of Education of China (no.
20090071120047), the Leading Academic Discipline Project of
Shanghai Municipal Education Commission (no. J50307), and the State
Administration of Traditional Chinese Medicine.
References
|
1.
|
AD BaronG BrechtelP WallaceSV EdelmanRates
and tissue sites of non-insulin- and insulin-mediated glucose
uptake in humansAm J Physiol255E769E77419883059816
|
|
2.
|
C FrøsigEA RichterImproved insulin
sensitivity after exercise: focus on insulin signalingObesity
(Silver Spring)17Suppl 3S15S20200919927140
|
|
3.
|
W YangJ LuJ WengPrevalence of diabetes
among men and women in ChinaN Engl J
Med36210901101201010.1056/NEJMoa090829220335585
|
|
4.
|
J FernandesIE LofgrenPrevalence of
metabolic syndrome and individual criteria in college studentsJ Am
Coll Health59313321201110.1080/07448481.2010.50808421308592
|
|
5.
|
BM BurgeringPJ CofferProtein kinase B
(c-Akt) in phosphatidylinositol-3-OH kinase signal
transductionNature376599602199510.1038/376599a07637810
|
|
6.
|
DR AlessiM AndjelkovicB CaudwellMechanism
of activation of protein kinase B by insulin and IGF-1EMBO
J156541655119968978681
|
|
7.
|
DD SarbassovDA GuertinSM AliDM
SabatiniPhosphorylation and regulation of Akt/PKB by the
rictor-mTOR
complexScience30710981101200510.1126/science.110614815718470
|
|
8.
|
CM TaniguchiB EmanuelliCR KahnCritical
nodes in signalling pathways: insights into insulin actionNat Rev
Mol Cell Biol78596200610.1038/nrm183716493415
|
|
9.
|
E JacintoV FacchinettiD LiuSIN1/MIP1
maintains rictor-mTOR complex integrity and regulates Akt
phosphorylation and substrate
specificityCell127125137200610.1016/j.cell.2006.08.03316962653
|
|
10.
|
T KadowakiInsights into insulin resistance
and type 2 diabetes from knockout mouse modelsJ Clin
Invest106459465200010.1172/JCI1083010953020
|
|
11.
|
K CusiK MaezonoA OsmanInsulin resistance
differentially affects the PI3-kanase-and MAP kinase-mediated
signaling in human muscleJ Clin
Invest105311320200010.1172/JCI753510675357
|
|
12.
|
B LuanJ ZhaoH WuDeficiency of a
β-arrestin-2 signal complex contributes to insulin
resistanceNature457114611492009
|
|
13.
|
A MaretteJM RichardsonT RamlalAbundance,
localization, and insulin-induced translocation of glucose
transporters in red and white muscleAm J
Physiol263C443C45219921514590
|
|
14.
|
T JiangY QiuInteraction between Src and a
C-terminal proline-rich motif of Akt is required for Akt
activationJ Biol
Chem2781578915793200310.1074/jbc.M21252520012600984
|
|
15.
|
JM BeaulieuTD SotnikovaS MarionAn
Akt/β-arrestin 2/PP2A signaling complex mediates dopaminergic
neurotransmission and behaviorCell1222612732005
|
|
16.
|
M LodeiroM TheodorpoulouM Pardoc-Src
regulates Akt signaling in response to ghrelin via beta-arrestin
signaling-independent and -dependent mechanismsPLoS
One4e4686200910.1371/journal.pone.000468619262695
|
|
17.
|
A GibbsC GreenVM DoctorIsolation and
anticoagulant properties of polysaccharides of Typha
augustata and Daemonoropes speciesThromb
Res3297108198310.1016/0049-3848(83)90021-X6658717
|
|
18.
|
J ZhaoCY ZhangDM XuThe antiatherogenic
effects of components isolated from pollen typhaeThromb
Res57957966199010.1016/0049-3848(90)90162-62116685
|
|
19.
|
S ChungS ParkCH YangUnsaturated fatty
acids bind Myc-Max transcription factor and inhibit Myc-Max-DNA
complex formationCancer
Lett188153162200210.1016/S0304-3835(02)00455-X12406560
|
|
20.
|
F QinHX SunImmunosuppressive activity of
Pollen Typhae ethanol extract on the immune responses in miceJ
Ethnopharmacol102424429200510.1016/j.jep.2005.06.02716095855
|
|
21.
|
Y YangS WangS ZhangDetermination of
flavonoids in pollen typhae (Puhuang) by HPCE and HPLCZhongguo
Zhong Yao Za Zhi246826847031999(In Chinese)
|
|
22.
|
HF MaB LiuGY ZhangGC-MS analysis of the
fatty components of Pollen Typhae before and after being
carbonizedZhongguo Zhong Yao Za Zhi312002022006(In Chinese)
|
|
23.
|
SY LouY LiuWH ChenPollen Typhae total
flavones inhibit expression of interleukin-6 in C2C12 skeletal
muscle cells cultured with palmitateZhong Xi Yi Jie He Xue
Bao64884922008(In Chinese)
|
|
24.
|
N OhkuraK TamuraA TanakaExperimental study
on the hemostatc activity of Pollen Typhae: a traditional folk
medicine used by external and oral applicationBlood Coagul
Fibrinolysis22631636201110.1097/MBC.0b013e328349a22c21934490
|
|
25.
|
SY LouY LiuYY MaEffects of Yiqi Sanju
Formula on non-alcoholic fatty liver disease: a randomized
controlled trialZhong Xi Yi Jie He Xue Bao67937982008(In
Chinese)
|
|
26.
|
CY HeWJ WangB LiClinical research of Yiqi
Sanju Formula in treating central obese men at high risk of
metabolic syndromeZhong Xi Yi Jie He Xue Bao52632672007(In
Chinese)
|
|
27.
|
Z ZhangHL XueY LiuWJ
WangYi-Qi-Zeng-Min-Tang, a Chinese medicine, ameliorates insulin
resistance in type 2 diabetic ratsWorld J
Gastroenterol17987995201121448349
|
|
28.
|
YM HeWJ WangWH ChenEffects of Pollen
Typhae total flavone on glucose and lipid metabolism in 3T3-L1
adipocytesZhong Xi Yi Jie He Xue Bao45935952006(In Chinese)
|
|
29.
|
EA KoulaouzidouN EconomidesP BeltesIn
vitro evaluation of the cytotoxicity of ProRoot MTA and MTA
AngelusJ Oral Sci50397402200810.2334/josnusd.50.39719106466
|
|
30.
|
B JiangY YangH JinAstragaloside IV
attenuates lipolysis and improves insulin resistance induced by
TNFalpha in 3T3-L1 adipocytesPhytother
Res2214341439200810.1002/ptr.243418972582
|
|
31.
|
JJ SennToll-like receptor-2 is essential
for the development of palmitate-induced insulin resistance in
myotubesJ Biol
Chem2812686526875200610.1074/jbc.M51330420016798732
|
|
32.
|
A XuH WangRL HooSelective elevation of
adiponectin production by the natural compounds derived from a
medicinal herb alleviates insulin resistance and glucose
intolerance in obese
miceEndocrinology150625633200910.1210/en.2008-099918927219
|
|
33.
|
GM ReavenRole of insulin resistance in
human
diseaseDiabetes3715951607198810.2337/diab.37.12.15953056758
|
|
34.
|
MF WhiteSE ShoelsonH KeutmannCR KahnA
cascade of tyrosine autophosphorylation in the beta-subunit
activates the phosphotransferase of the insulin receptorJ Biol
Chem2632969298019882449432
|
|
35.
|
S GeorgeJJ RochfordC WolfrumA family with
severe insulin resistance and diabetes due to a mutation in
AKT2Science30413251328200410.1126/science.109670615166380
|
|
36.
|
P LauRL FitzsimmonsMA PearenHomozygous
staggerer (sg/sg) mice display improved insulin sensitivity and
enhanced glucose uptake in skeletal
muscleDiabetologia5411691180201110.1007/s00125-011-2046-321279323
|
|
37.
|
M ChaoD ZouY ZhangImproving insulin
resistance with traditional Chinese medicine in type 2 diabetic
patientsEndocrine36268274200910.1007/s12020-009-9222-y19728183
|
|
38.
|
W XieL DuDiabetes is an inflammatory
disease: evidence from traditional Chinese medicinesDiabetes Obes
Metab13289301201110.1111/j.1463-1326.2010.01336.x21205111
|
|
39.
|
A DresnerD LaurentM MarcucciEffects of
free fatty acids on glucose transport and IRS-1-associated
phosphatidylinositol 3-kinase activityJ Clin
Invest103253259199910.1172/JCI50019916137
|
|
40.
|
R BelfortL MandarinoS KashyapDose response
effect of elevated plasma FFA on insulin
signalingDiabetes5416401648200510.2337/diabetes.54.6.164015919784
|
|
41.
|
LM NeriP BorgattiS CapitaniAM MartelliThe
nuclear phosphoinositide 3-kinase/AKT pathway: a new second
messenger systemBiochim Biophys
Acta15847380200210.1016/S1388-1981(02)00300-112385889
|
|
42.
|
X FengW WangJ LiuY Liuβ-Arrestins:
multifunctional signaling adaptors in type 2 diabetesMol Biol
Rep38251725282011
|
|
43.
|
VV GurevichEV GurevichThe structural basis
of arrestin-mediated regulation of G protein coupled
receptorPharmacol
Ther110465502200610.1016/j.pharmthera.2005.09.00816460808
|
|
44.
|
B LiuY LuHPLC determination of two
flavonoids in pollen Typhae (puhuang) and its different processed
productsZhongguo Zhong Yao Za Zhi234024044471983(In Chinese)
|
|
45.
|
SL HwanqGC YenModulation of Akt, JNK, and
p38 activation is involved in citrus flavonoid-mediated
cytoprotection of PC12 cells challenged by hydrogen peroxideJ Agric
Food Chem5725762582200910.1021/jf803360719222219
|
|
46.
|
SE ParkK SapkotaS KimKaempferol acts
through mitogen-activated protein kinases and protein kinase B/AKT
to elicit protection in a model of neuroinflammation in BV2
microglial cellsBr J
Pharmacol16410081025201110.1111/j.1476-5381.2011.01389.x21449918
|
|
47.
|
XQ WangRQ YaoX LiuQuercetin protects
oligodendrocyte precursor cells from oxygen/glucose deprivation
injury in vitro via the activation of the PI3K/Akt signaling
pathwayBrain Res
Bull86277284201110.1016/j.brainresbull.2011.07.014
|
|
48.
|
UI IsaoI TakeshiH Jieβ-Arrestin-1
competitively inhibits insulin-induced ubiquitination and
degradation of insulin receptor substrate 1Mol Cell
Biol24892989372004
|
|
49.
|
X LiGS BaillieMD HouslayMdm2 directs the
ubiquitination of beta-arrestin-sequestered cAMP
phosphodiesterase-4D5J Biol
Chem2841617016182200910.1074/jbc.M109.00807819372219
|
|
50.
|
L NoguésA SalcedoF Mayor JrP
PenelaMultiple scaffolding functions of (beta)-arrestins in the
degradation of G protein-coupled receptor kinase 2J Biol
Chem28611651173201121081496
|