Introduction
Oncolytic virotherapy is a promising approach for
patients who are resistant to traditional cancer therapies. A
number of strategies have been evaluated in pre-clinical and
clinical trials (1–3), including treatment with conditional
replication-competent adenovirus (CRCA) (4–6).
However, insufficient efficacy and poor specificity of treatment
remain the major challenges for this targeted cancer gene therapy
(4,5) and highlight the need for the
development of novel cancer therapies.
The human telomerase reverse transcriptase (hTERT)
promoter is highly activated in immortalized cell lines and in over
85% of human cancers and has therefore been used widely for the
construction of CRCAs (4,7). In these studies, early region 1a
(E1a) gene, which plays a central role in viral infection by
regulation of viral replication and the cell cycle, was the gene
used most frequently in the constructs and was essential for
adenovirus replication (8,9).
Apoptin, a protein of 13.6 kDa in mass that was
derived from the chicken anemia virus (CAV) VP3 gene and is thought
to induce apoptosis selectively in a variety of tumor cells, such
as hepatomas, lymphomas, cholangiocarcinomas, melanomas, as well as
breast, lung, oral and colon carcinomas (10–12). Preliminary studies have
demonstrated that more than 70 different transformed and malignant
cells of human origin are sensitive to apoptin-induced apoptosis
(11). However, apoptin does not
act on normal non-transformed human cells, such as primary
fibroblasts, smooth muscle cells, T cells, hepatocytes,
hematopoietic stem cells, keratinocytes, or endothelial cells
(10,13,14). Furthermore, the long-term
expression of apoptin in normal human fibroblasts has shown that it
has no toxic or transforming activity in these cells (11,15). All these factors make apoptin an
attractive candidate for cancer gene therapy and numerous studies
have demonstrated the effects of apoptin inserted into various
vectors on the restriction of manifold tumors (4,16,17).
In the present study, we show the significant
antitumor activity of a novel dual cancer-specific oncolytic
adenovirus construct, designated as Ad-hTERT-E1a-apoptin (4), which consists of a cancer-specific
promoter (hTERT promoter) and a cancer cell-selective
apoptosis-inducing gene (apoptin). We found that the
Ad-hTERT-E1a-apoptin construct exhibited selective replication and
specific induction of apoptosis in human gastric cancer cells.
Furthermore, we also show that Ad-hTERT-E1a-apoptin induces
apoptosis in human gastric cancer cells specifically and induces
apoptosis more rapidly when compared with the control viruses.
These results suggest that the Ad-hTERT-E1a-apoptin construct is a
potentially applicable anti-cancer agent for the treatment of human
gastric carcinoma and may have clinical use in other neoplastic
diseases.
Materials and methods
Cell lines, viruses and animals
The human gastric cancer cells, SGC7901, the human
gastric epithelial cells, GES-1, and the human embryonic kidney
cells, HEK-293, were obtained from the Type Culture Collection of
the Chinese Academy of Sciences (Shanghai, China). The cells were
maintained in Dulbecco’s modified Eagle’s medium (DMEM; HyClone,
Beijing, China) that contained 10% heat-inactivated fetal bovine
serum (FBS; HyClone), penicillin (100 U/ml) and streptomycin (100
mg/ml) at 37°C under an atmosphere of 95% air and 5%
CO2. All cell lines were passaged for no more than 6
months after receipt. The cells were routinely subcultured every
2–3 days, and were all taken from the logarithmic phase of growth.
Female BALB/c nude mice (nu/nu, 6–8-week-old) were purchased from
the Experimental Animal Center of the Academy of Military Medical
Sciences (Beijing, China) and were housed in a pathogen-free
facility for the duration of all experiments, following institute
guidelines.
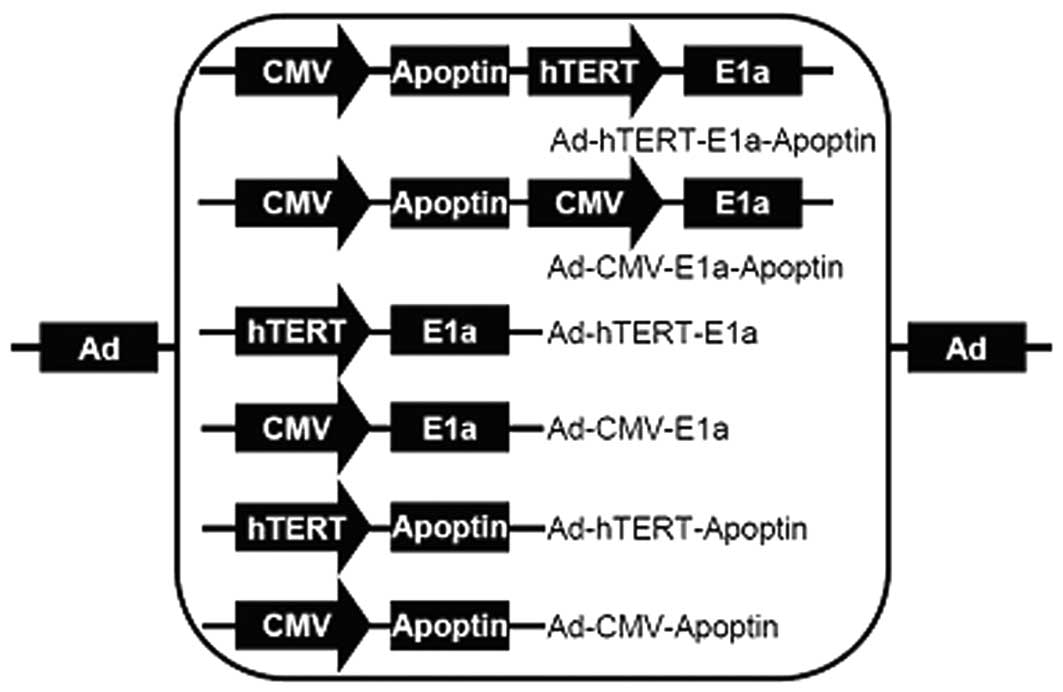
Recombinant adenoviruses
The construction and characterization of the dual
cancer-specific oncolytic adenovirus, Ad-hTERT-E1a-apoptin, and the
control viruses, Ad-mock, Ad-CMV-apoptin, Ad-hTERT-apoptin,
Ad-CMV-E1a, Ad-hTERT-E1a and Ad-CMV-E1a-apoptin, used in this study
have been described previously (4). Briefly, shuttle plasmids were
constructed to generate the recombinant adenoviruses (Fig. 1). Packaging and production of the
recombinant adenoviruses were performed in HEK-293 cells.
Purification and titration of the virus stock were performed using
the Adeno-X Virus Purification kit and Adeno-X Rapid titer kit
(both from BD Biosciences Clontech).
MTT viability assay
Cell viability was determined by standard
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT;
Sigma, St. Louis, MO, USA) assays as described previously (4,18).
In brief, cells were seeded in 96-well plates (1×104
cells/well) and 1 day later the cells were infected with various
concentrations [1 multiplicity of infection (MOI), 10 and 100 MOI]
of the recombinant adenoviruses. Cell viability was measured every
day over a 4-day period. The percentage of cell death was expressed
with respect to the control values using the following formula:
[100× (control cells − experimental cells)/(control cells)]
(4). Untreated cells were used as
the controls and all measurements were repeated in triplicate.
Acridine orange (AO)/ethidium bromide
(EB) staining assay
The AO/EB staining assay was performed as described
previously to determine the relative percentage of live, necrotic
and apoptotic cells (16,19). Cells that had been infected with
the recombinant adenoviruses were trypsinized and washed 3 times in
Hank’s balanced salt solution (HBSS). A 250-μl aliquot that
contained 1×106 cells was incubated with 2 μl of
EB and 2 μl of AO. The cells were then observed under a
fluorescence microscope (BX51) and the images of representative
cells were captured with a charged-coupled device (CCD) camera
(DP71) (all were from Olympus). Samples were processed
simultaneously, and all images were captured using the same
parameters. Tests were performed in triplicate and at least 500
cells were measured from each sample to determine the presence of
normal, apoptotic, or necrotic chromatin.
Western blot analysis
Cells were infected with the recombinant
adenoviruses; target proteins were analyzed by western blot
analysis as described previously (4). All antibodies were purchased from
Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Protein bands
were visualized with the Pierce ECL Western Blotting Substrate
(Pierce, Shanghai, China). Extracts of Ad-mock-infected cells were
used as the negative control; detection of glyceraldehyde
3-phosphate dehydrogenase (GAPDH) was used as the internal
control.
Tumor xenograft experiments
In vivo antitumor experiments were performed
in 2 independent models. Briefly, 1×106 SGC7901 cells
were implanted subcutaneously into the right flanks of BALB/c nude
mice. Two weeks later, after establishment of the tumors, the
tumor-bearing mice were divided randomly into 8 groups (5
mice/group). The mice in the first model received intratumoral
injections of various recombinant adenoviruses at a dose of
1×109 plaque-forming units (pfu) in 50 μl of
saline, and the control group received 50 μl of saline
alone. In the second model, the injections were administered via
the tail vein. All injections were given every 2 days for the first
week (days 12, 14 and 16 after implantation) and once weekly for 2
more weeks (days 23 and 30 after implantation). Tumor size was
measured using calipers twice a week and calculated with a formula
of [0.52 (smallest diameter)2 × (largest diameter)]
(4,16,17). During the tumor study, all animals
were monitored daily and sacrificed at the end of the
experiment.
Statistical analysis
Statistical significance of the results was
calculated using one-way analysis of variance (ANOVA) and results
were deemed to be statistical significant if the P-value was
<0.05. Log-rank tests were used to calculate survival rates.
Data from all animals are represented as Kaplan-Meier plots.
Results
Selectively inhibitory effects of
Ad-hTERT-E1a-apoptin on gastric cancer cells
The correlation between infection time and MOI was
complex and synergistic and consistent with the results of previous
studies (4,16) (Fig.
2). At longer infection times, the growth rates of SCG7901 or
GES-1 cells that were infected with replication-competent
adenoviruses (Ad-CMV-E1a, Ad-hTERT-E1a, Ad-CMV-E1a-apoptin and
Ad-hTERTE1a-apoptin) were inhibited (Fig. 2A). However, cells treated with
Ad-CMV-apoptin, Ad-hTERT-apoptin and Ad-mock gradually resumed
their growth rate after 2 days (Fig.
2A). By contrast, the growth of GES-1 cells infected only with
non-specific replication-competent adenoviruses (Ad-CMV-E1a or
Ad-CMV-E1a-apoptin) was suppressed (Fig. 2B). Furthermore, cell viabilities
were dependent to a certain extent on the MOI of the recombinant
adenoviruses. In the SGC7901 human gastric cancer cells, infection
with Ad-CMV-E1a-apoptin or Ad-hTERT-E1a-apoptin at a MOI of 1 or 10
induced a cell growth inhibition of 10-25 or 35-55% after 4 days,
respectively, and the growth of cells infected with 100 MOI was
80-85%. When infected with 1 or 10 MOI of Ad-CMV-E1a or
Ad-hTERT-E1a, cell growth was inhibited by 30–50 or 60–70% after 4
days, respectively. However, in the GES-1 human gastric epithelial
cells, dose-dependent inhibition was only observed in cells in the
Ad-CMV-E1a and Ad-CMV-E1a-apoptin experimental groups. Therefore
replication-competent adenoviruses were much more potent than the
replication-incompetent adenoviruses in gastric cancer cell
suppression, and adenoviruses with the dual cancer-specific genes
were more effective than the normal replication-incompetent
adenoviruses. In addition, Ad-hTERT-E1a-apoptin induced growth
suppression selectively in cancer cells without harming the normal
counterparts. That is, Ad-hTERT-E1a-apoptin replicated specifically
in gastric cells (SGC7901) and restricted the cell growth
selectively, exhibiting higher tumor-specific killing activity than
the control viruses.
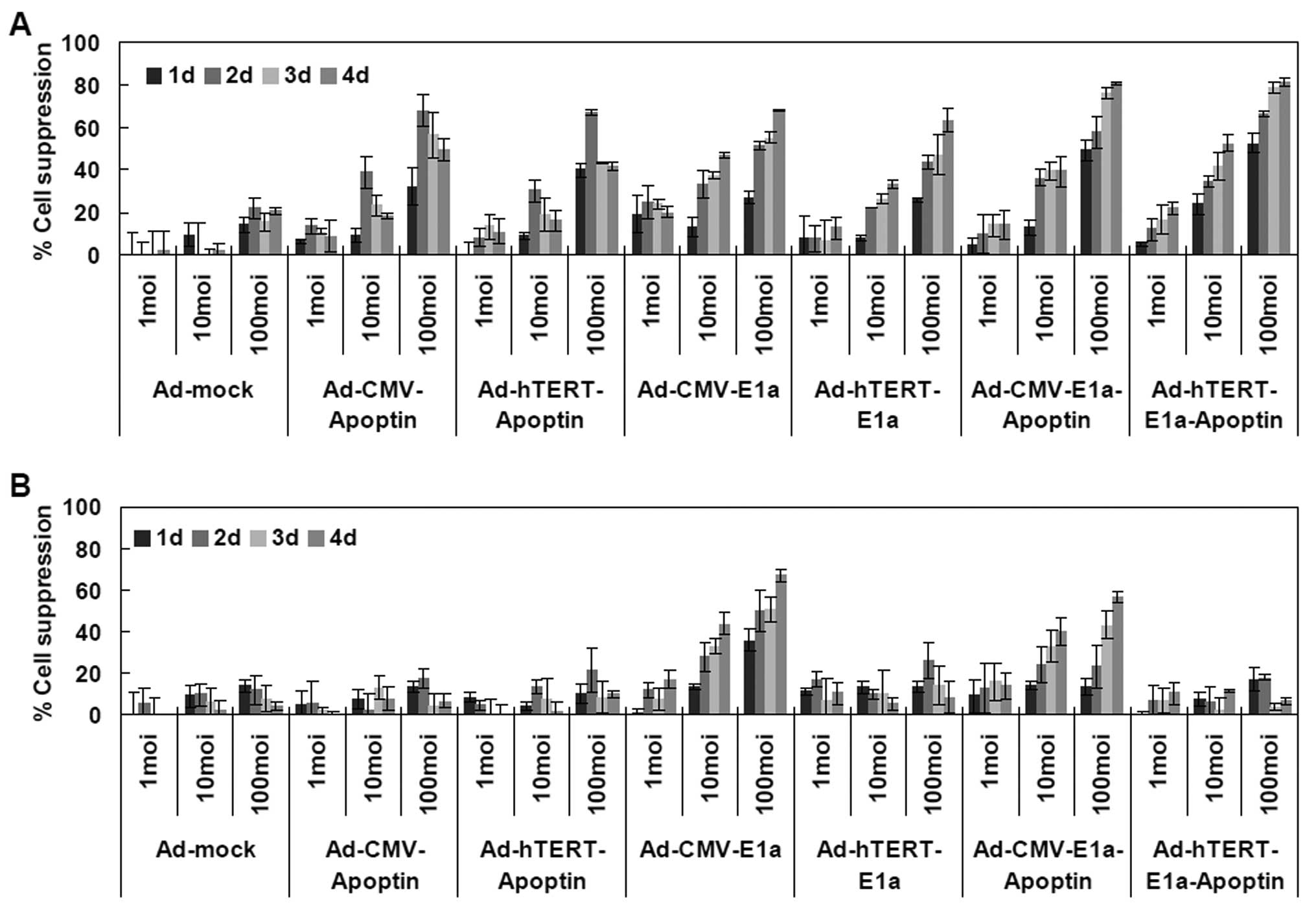 | Figure 2Assessment of the selective
inhibitory effect of Ad-hTERT-E1a-apoptin on gastric cancer cells.
Effects of the different multiplicities of infection (MOIs) and
infection times on (A) SGC7901 cell viability; and (B) GES-1 cell
viability. Cells were seeded into 96-well plates (1×104
cells/well) 1 day before cells were infected with various
concentrations (1, 10 and 100 MOI) of the indicated adenoviruses.
Tumor viability was measured every day over a 4-day period by
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT)
colorimetric assay and all measurements were performed in
triplicate. Data are presented as the means ± standard deviation
(SD). (A) In SGC7901 cells, infection with Ad-hTERT-E1a-apoptin,
Ad-hTERT-E1a-apoptin, Ad-CMV-E1a and Ad-hTERT-E1a induced growth
inhibition. (B) By contrast, in GES-1 cells, infection with
Ad-CMV-E1a or Ad-CMV-E1a-apoptin, but not Ad-CMV-apoptin,
Ad-hTERT-apoptin, Ad-hTERT-E1a or Ad-hTERT-E1a-apoptin resulted in
significant growth inhibition. |
Ability of Ad-hTERT-E1a-apoptin to induce
tumor-specific apoptosis
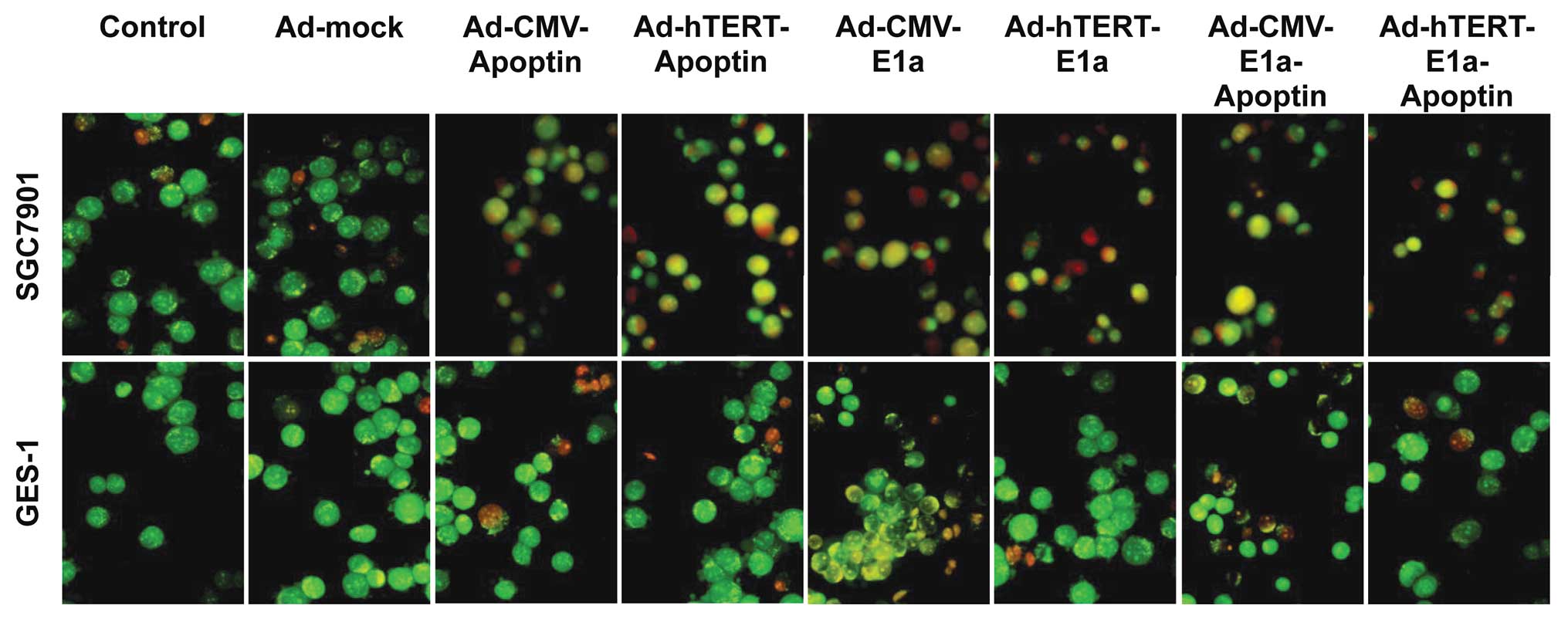
Using the AO/EB method, we compared and quantified
the percentage of live, necrotic and apoptotic cells of the control
and recombinant adenovirus-treated SGC7901 and GES-1 cells. Live
cells have a normal green nucleus; early apoptotic cells have a
bright green nucleus with condensed or fragmented chromatin; late
apoptotic cells display condensed and fragmented orange chromatin;
and cells that have died from direct necrosis have a structurally
normal orange nucleus (20)
(Fig. 3A). Infection with all
recombinant adenoviruses resulted in apoptosis of SGC7901 cells,
whereas, in GES-1 cells, only infection with Ad-CMV-E1a or
Ad-CMV-E1a-apoptin was cytotoxic (Fig. 3A). Furthermore, the proportion of
live, necrotic and apoptotic cell populations in the control and
treated SGC7901 or GES-1 cells was significantly different
(Fig. 3B). In SGC7901 cells,
infection with adenoviruses that expressed apoptin predominantly
caused apoptosis; however infection with Ad-CMV-E1a or Ad-hTERT-E1a
mainly caused necrosis. In GES-1 cells, due to the loss of the
apoptosis-inducing effect of apoptin, infection with
Ad-CMV-E1a-apoptin mainly caused necrosis, similar to the
Ad-CMV-E1a-infected cells. These results indicated that although
the recombinant adenoviruses had a significant in vitro
antitumor effect via the induction of necrosis,
Ad-hTERT-E1a-apoptin significantly restrained the growth of SGC7901
cells by the induction of apoptosis.
Effects of Ad-hTERT-E1a-apoptin on
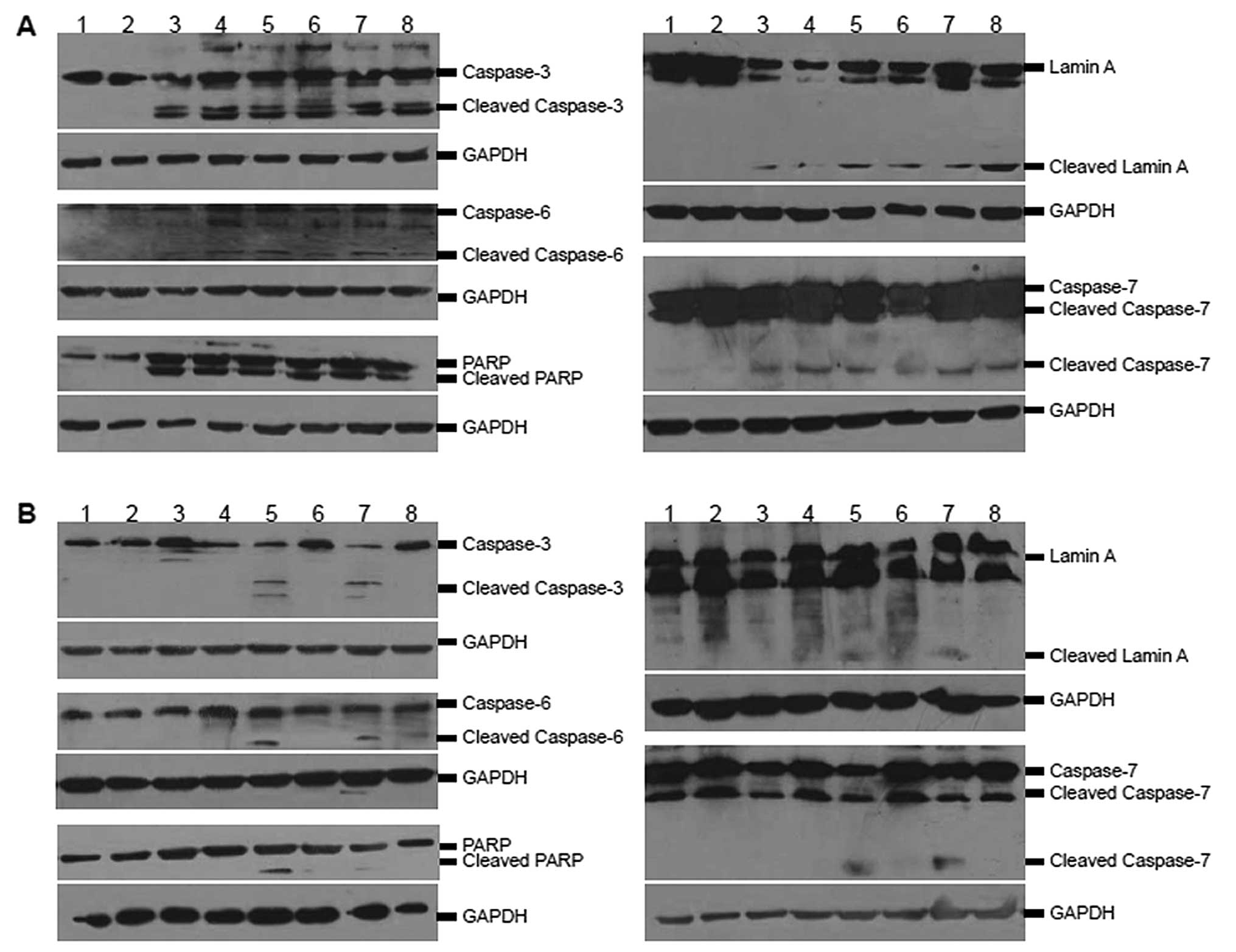
caspases in SGC7901 cells
The activation of caspases and corresponding markers
detected by antibodies specific for the activated form of these
proteins was determined by western blot analysis. Infection of
SGC7901 cells with the recombinant adenoviruses caused a marked
increase in the active cleaved subunit of caspase-3 (Fig. 4A). Furthermore, the recombinant
adenovirus infection resulted in detectable expression of cleaved
lamin A, a marker for caspase-6 activation. Cleaved poly ADP-ribose
polymerase (PARP) was also detected in the infected SGC7901 cells,
which not only indicated the activation of caspase-7 but also
recon-firmed the existence of an active caspase-3. By contrast, the
activation of the caspases and the corresponding substrates was
detected at only a limited degree in GES-1 cells infected with the
recombinant adenoviruses except for Ad-CMV-E1a and
Ad-CMV-E1a-apoptin (Fig. 4B).
These results suggested that the specific activation of caspases-3,
-6 and -7 in SGC7901 cells was associated with apoptin expression
and viral replication.
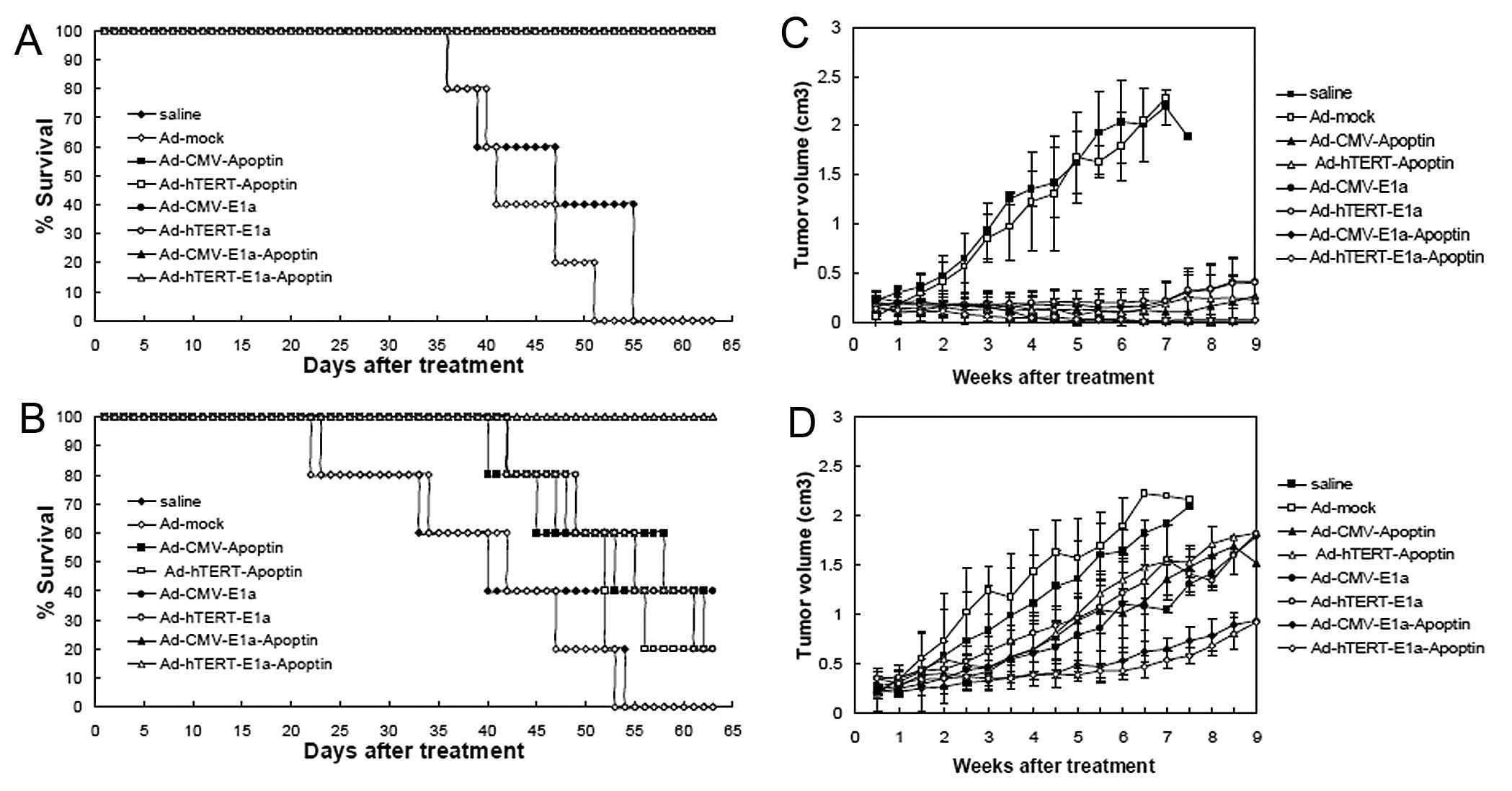
Growth tendency and life span analysis in
vivo
For ethical reasons, all animals in the study were
sacrificed by day 63 before the tumors grew to a volume of 2,500
mm3. Therefore, only the mean survival curve of each
group of mice was obtained. When the injections were performed
intratumorally, the saline-treated and Ad-mock-infected groups had
the worst survival rate, while infection with the recombinant
adenoviruses significantly improved the survival time (Fig. 5A). The mean survival times were
47.5 days for the saline-treated nude mice, 41.5 days for the
Ad-mock-infected nude mice and 63 days for mice infected with the
other recombinant adenoviruses. This observation demonstrates that
the intratumoral injection of apoptin-expressing recombinant
adenoviruses effectively improves survival. In the second model,
the intravenous injection of Ad-hTERT-E1a-apoptin or
Ad-CMV-E1a-apoptin significantly increased the survival of nude
mice in comparison with the other recombinant adenovirus-infected
or saline-treated mice (Fig. 5B).
At the end of the experiment, the survival rates of the treated
mice were 0% (saline), 0% (Ad-mock), 80% (Ad-CMV-apoptin), 40%
(Ad-hTERT-apoptin), 60% (Ad-CMV-E1A), 40% (Ad-hTERT-E1a), 100%
(Ad-CMV-E1a-apoptin) and 100% (Ad-hTERT-E1a-apoptin). The mean
survival times of the groups that received an injection in the tail
vein were 40.5 days for the saline-treated mice, 42.5 days for the
Ad-mock-infected mice, 58.5 days for the Ad-CMV-apoptin-infected
mice, 52.5 days for the Ad-hTERT-apoptin-infected mice, 53.5 days
for the Ad-CMV-E1a-infected mice and 55.5 days for the
Ad-hTERT-E1a-infected mice. These results indicate that the
intravenous injection of the dual cancer-specific oncolytic
adenovirus confers significant survival benefits in
vivo.
We also evaluated the growth kinetics of the tumors
following treatment. The tumors in the recombinant adenovirus
groups were suppressed after the infection in the first model,
compared with the control and Ad-mock-infected groups (Fig. 5C). However, the Ad-CMV-E1a- and
Ad-hTERTE1a-infected tumors gradually resumed their growth at 6
weeks after the first injection, whereas the growth of the majority
of the Ad-CMV-E1a-apoptin-infected or Ad-hTERTE1a-apoptin-infected
tumors was markedly reduced. These results indicate that the
intratumoral injection of Ad-CMVE1a-apoptin or Ad-hTERT-E1a-apoptin
induces a powerful antitumor response. In the systemic delivery
model (Fig. 5D), the tumors
infected with Ad-CMV-E1a-apoptin or Ad-hTERTE1a-apoptin began to
show growth impairment after 2 weeks, consistent with the results
of the intratumoral injection experiment. Significant differences
between the other groups and Ad-CMV-E1a-apoptin or the
Ad-hTERT-E1a-apoptin groups were confirmed during follow-up. The
growth of the Ad-CMV-apoptin-, Ad-hTERT-apoptin-, Ad-CMV-E1a- or
Ad-hTERT-E1a-infected tumors was suppressed compared with the
saline-treated and Ad-mock infected mice; however, the differences
in size impairment of the tumors in these groups were slight. These
results demonstrate that the systemic delivery of
Ad-hTERT-E1a-apoptin or Ad-CMV-E1a-apoptin significantly reduces
tumor burdens and that the dual cancer-specific oncolytic
adenoviruses are a much more potent tool than
replication-incompetent or non-specific replication-competent
adenoviruses for cancer cell suppression.
Discussion
Gastric cancer is a worldwide health burden.
Treatment has limited progress and 75% of patients are diagnosed
only at the unresectable stage (21). Following curative resection alone
or even after adjuvant therapy, approximately 60% of affected
patients succumb to gastric cancer (22). All these factors pose the
challenge of whether to choose a strictly supportive approach or to
expose patients to the side-effects of a potentially ineffective
treatment.
Cancer gene therapy based on adenoviruses has been
studied extensively in pre-clinical and clinical trials (2,3).
In particular, use of CRCAs has gained increased attention for a
number of reasons (5,23). Importantly, as the promoters in
these vectors are selective for cancer cells (5,23),
these oncolytic viruses have the ability to replicate and spread to
adjacent tumor cells (24).
Furthermore, infection with CRCA generates antitumoral immune
responses (25) that can
complement chemotherapy and radiotherapy (24). Additionally, CRCAs are capable of
achieving the destruction of primary and distant tumors given the
proper therapeutic transgenes (5).
In the present study, we describe the antitumor
effects of a dual cancer-specific oncolytic adenovirus,
Ad-hTERTE1a-apoptin, in which replication was driven by the hTERT
promoter that replicates selectively and induces apoptosis
specifically in tumor cells. The antitumor effects were evident
within 24 h when administered to the gastric cancer cells in
vitro. Treatment with 100 or 10 MOI doses completely inhibited
the growth of SGC7901 cells 4 days later (Fig. 2A), although a single
Ad-hTERT-E1a-apoptin treatment at 1 MOI was less effective. By
contrast, growth inhibition was not observed in GES-1 cells after
treatment with Ad-hTERT-E1aapoptin at any MOI dose (Fig. 2B). These findings indicate that
Ad-hTERT-E1a-apoptin replicates specifically and induces growth
suppression selectively in SGC7901 cells compared with the normal
counterparts.
The applicability of cancer therapies is not only
determined by their efficiency as specificity is an equally
important prerequisite (26).
Various studies have indicated that more than 70 tumor cell lines
are susceptible to apoptin, whereas apoptin does not affect a
variety of normal and non-transformed cells (13,27). In contrast to our findings, Guelen
et al (28) showed the
toxicity of apoptin towards non-cancerous cells; however, this
study demonstrated cell death only in a fetal cell type.
Furthermore, the ability of apoptin to induce tumor-specific
apoptosis is independent of p53 (13,29). Thus, apoptin is similarly
effective at killing tumor cells that are p53-deficient or that
express either wild-type or mutant p53 (13,29). The role of anti-apoptotic
molecules in apoptin-induced apoptosis is still a matter of debate.
In certain tumor cell lines, apoptin-mediated cell death is
independent of the Bcl-2 status and is even stimulated by Bcl-2 or
is insensitive to Bcr-Abl and Bcl-xL (13,30). Based on these concepts, apoptin
can be used to complement radiotherapeutic and chemotherapeutic
approaches.
Chromatin condensation and nuclear fragmentation
remain the hallmarks of apoptotic cells (16,20). Fluorescence light microscopy with
differential uptake of fluorescent DNA-binding dyes (such as EB/AO
staining) is the method of choice due to its simplicity. In this
study, using the AO/EB method, we quantified the percentage of
live, necrotic and apoptotic cells after recombinant adenovirus
treatment (Fig. 3). The infection
of SGC7901 cells with the recombinant adenoviruses may, in part,
inhibit the cells by causing necrosis. We expected, therefore, that
the effects of the apoptin gene may be masked. However, the effects
of the recombinant virus did not impede apoptin-induced apoptosis.
After Ad-hTERT-E1a-apoptin infection, 38.6% of apoptin-expressing
transformed cells became apoptotic after only 48 h, and 27.1% of
infected cells exhibited necrosis. By contrast, although 24.3% of
cells were apoptotic 48 h after Ad-hTERT-E1a infection, 25.5% of
cells died as a result of necrosis. These results indicate that,
although replication-component adenovirus infection stimulates
necrosis, this event does not appear to render tumor cells
sensitive to apoptin. Furthermore, classification of cell death
should always include morphological examination coupled with at
least one other assay. Apoptin induces apoptosis in a wide variety
of human cancer cell lines via the classical apoptotic pathways
(31). Caspases-3, -6 and -7 are
the important downstream effecter caspases that cleave major
cellular substrates in apoptotic cells and amplify these substrates
via intrinsic or extrinsic pathways (32). In the present study, a western
blot analysis of total protein extracts showed activation of
caspases-3, -6 and-7 in recombinant virus-infected SGC7901 cells.
However, no caspase activation was observed in GES-1 cells, except
for non-specific replication-competent adenovirus infection. GAPDH
expression was used to confirm equal loading of the gel. These
results validate the specific apoptosis-inducing effects of the
dual cancer-specific oncolytic adenovirus Ad-hTERT-E1a-apoptin
construct.
We also evaluated antitumor activity in vivo
in a human gastric cancer xenograft mouse model, which confirmed
and extended the results of the in vitro studies. In this
study, tumors derived from mouse gastric cancer cells transplanted
into BALB/c nude mice were infected with various recombinant
adenoviruses. We found that all recombinant adenoviruses had a
significant antitumor effect, despite the fact that the
replication-defective adenoviruses infected only part of the
tumors. The injection of Ad-hTERT-E1a-apoptin resulted in a
complete response to treatment (Fig.
5). However, infection with Ad-hTERT-apoptin, Ad-CMV-apoptin,
Ad-hTERT-E1a or Ad-CMV-E1a was less effective. It is plausible that
the application of replication-component adenoviruses allows virus
dispersion into any tumor tissues in the animal and that apoptin
expression enhances the capability of the oncolytic adenovirus.
When infection was carried out intravenously, however, treatment
with Ad-hTERT-E1a-apoptin did not lead to complete tumor
regression, although the tumor growth was significantly delayed
(Fig. 5). The suppression of the
indirectly injected tumor may also reflect a secondary viral
infection by the CRCA. The oncolytic activity of Ad-hTERT-apoptin,
Ad-CMV-apoptin, Ad-hTERT-E1a or Ad-CMV-E1a was limited, which was
similar to that of intratumoral injection groups. No tumor
regression was observed in mice that had SGC7901 tumors when
injected intravenously with saline and Ad-mock. Furthermore, we did
not observe any toxic effects after injection of
Ad-hTERT-E1a-apoptin in the in vivo experiments described in
this study. Thus, our data indicate that there is great potential
for improvement in the safety and efficacy of adenovirus vectors
for wide application in the treatment of neoplastic diseases.
In conclusion, gene therapy with apoptin offers
unique advantages over current approaches for cancer therapy.
Factors such as: i) apoptin does not need a functional p53 pathway;
ii) it is not hindered by the commonly found blockage of apoptosis
by Bcl-2 or Bcr-Abl, iii) it acts downstream of most other factors,
and iv) it has unparalleled potency, suggest that apoptin is
applicable for the treatment of a wide range of tumors. In
addition, the CRCA we describe in this study selectively induces
apoptosis in various cancer cells without adverse effects on normal
cells. In vivo and in vitro data described in this
study show that the expression of apoptin increases the
effectiveness of CRCAs and that an adenovirus vector under the
control of a hTERT promoter does not reduce the efficacy of the
construct but improves the global safety of CRCAs. All these
factors highlight the need for further evaluation of this strategy
for clinical trials.
Acknowledgements
This study was supported in part by
grants from the National Science and Technology Major Projects for
‘Major New Drugs Innovation and Development’ (no.
2010ZX09401-305-14), the National Natural Science Foundation of
China (nos. 81072210 and 81101140), the Key Technologies R&D
Programme of Jilin Province (nos. 10ZDGG007 and 201015166) and the
China Postdoctoral Science Foundation (no. 20100481057).
References
|
1.
|
Y XuZ LiuH KongCo-expression of
interleukin 12 enhances antitumor effects of a novel chimeric
promoter-mediated suicide gene therapy in an immunocompetent mouse
modelBiochem Biophys Res
Commun412763768201110.1016/j.bbrc.2011.08.07721875574
|
|
2.
|
R AlemanyCancer selective adenovirusesMol
Aspects Med284258200710.1016/j.mam.2006.12.002
|
|
3.
|
YS HavivDT CurielConditional gene
targeting for cancer gene therapyAdv Drug Deliv
Rev53135154200110.1016/S0169-409X(01)00225-311731024
|
|
4.
|
X LiY LiuZ WenPotent antitumor effects of
a dual specific oncolytic adenovirus expressing apoptin in vitro
and in vivoMol Cancer910201010.1186/1476-4598-9-1020085660
|
|
5.
|
D SarkarZZ SuN VozhillaES ParkP GuptaPB
FisherDual cancer-specific targeting strategy cures primary and
distant breast carcinomas in nude miceProc Natl Acad Sci
USA1021403414039200510.1073/pnas.050683710216172403
|
|
6.
|
DE PostEM SandbergMM KyleTargeted cancer
gene therapy using a hypoxia inducible factor dependent oncolytic
adenovirus armed with interleukin-4Cancer
Res6768726881200710.1158/0008-5472.CAN-06-324417638898
|
|
7.
|
OA KovalenkoJ KaplunovU HerbigS DetoledoEI
AzzamJH SantosExpression of (NES-)hTERT in cancer cells delays cell
cycle progression and increases sensitivity to genotoxic stressPLoS
One5e10812201010.1371/journal.pone.001081220520826
|
|
8.
|
JM WojciakMA Martinez-YamoutHJ DysonPE
WrightStructural basis for recruitment of CBP/p300 coactivators by
STAT1 and STAT2 transactivation domainsEMBO
J28948958200910.1038/emboj.2009.3019214187
|
|
9.
|
N HotiWH ChowdhuryS MustafaArmoring CRAds
with p21/Waf-1 shRNAs: the next generation of oncolytic
adenovirusesCancer Gene
Ther17585597201010.1038/cgt.2010.1520448671
|
|
10.
|
AA Danen-Van OorschotDF FischerJM
GrimbergenApoptin induces apoptosis in human transformed and
malignant cells but not in normal cellsProc Natl Acad Sci
USA945843584719979159162
|
|
11.
|
C BackendorfAE VisserAG de BoerApoptin:
therapeutic potential of an early sensor of carcinogenic
transformationAnnu Rev Pharmacol
Toxicol48143169200810.1146/annurev.pharmtox.48.121806.15491017848136
|
|
12.
|
RA SchoopRJ Baatenburg de JongMH
NotebornApoptin induces apoptosis in an oral cancer mouse
modelCancer Biol Ther713681373200810.4161/cbt.7.9.641918708764
|
|
13.
|
M LosS PanigrahiI RashediApoptin, a
tumor-selective killerBiochim Biophys
Acta179313351342200910.1016/j.bbamcr.2009.04.00219374922
|
|
14.
|
JL RohnMH NotebornThe viral death effector
Apoptin reveals tumor-specific
processesApoptosis9315322200410.1023/B:APPT.0000025808.48885.9c15258463
|
|
15.
|
YH ZhangSR LeliveldK KooistraRecombinant
Apoptin multimers kill tumor cells but are nontoxic and
epitope-shielded in a normal-cell-specific fashionExp Cell
Res2893646200310.1016/S0014-4827(03)00188-512941602
|
|
16.
|
X LiN JinZ MiAntitumor effects of a
recombinant fowlpox virus expressing Apoptin in vivo and in
vitroInt J Cancer11929482957200610.1002/ijc.2221517036330
|
|
17.
|
H LianN JinX LiInduction of an effective
antitumor immune response and tumor regression by combined
administration of IL-18 and ApoptinCancer Immunol
Immunother56181192200710.1007/s00262-006-0178-y16767432
|
|
18.
|
Q LiJ ZhuF SunL LiuX LiuY YueOncostatin M
promotes proliferation of ovarian cancer cells through signal
transducer and activator of transcription 3Int J Mol
Med28101108201121399864
|
|
19.
|
SH Zainal AriffinWH Wan OmarZ Zainal
AriffinMF SafianS SenafiR Megat Abdul WahabIntrinsic
anticarcinogenic effects of Piper sarmentosum ethanolic
extract on a human hepatoma cell lineCancer Cell
Int96200919257877
|
|
20.
|
E CandalR AnadonWJ DeGripI
Rodriguez-MoldesPatterns of cell proliferation and cell death in
the developing retina and optic tectum of the brown troutBrain Res
Dev Brain
Res154101119200510.1016/j.devbrainres.2004.10.00815617760
|
|
21.
|
F PatrascuA CroitoruI GramaticuM AndreiA
TeiutanuM DiculescuLocally advanced or metastatic gastric cancer -
new therapeutic solutionsRev Med Chir Soc Med Nat
Iasi11520262011(In Romanian)
|
|
22.
|
AN MilneF CarneiroC O’MorainGJ
OfferhausNature meets nurture: molecular genetics of gastric
cancerHum Genet126615628200910.1007/s00439-009-0722-x19657673
|
|
23.
|
CL WuGS ShiehCC ChangTumor-selective
replication of an oncolytic adenovirus carrying oct-3/4 response
elements in murine metastatic bladder cancer modelsClin Cancer
Res1412281238200810.1158/1078-0432.CCR-07-104718281558
|
|
24.
|
DH KirnF McCormickReplicating viruses as
selective cancer therapeuticsMol Med
Today2519527199610.1016/S1357-4310(97)81456-69015793
|
|
25.
|
J ShislerP Duerksen-HughesTM HermistonWS
WoldLR GoodingInduction of susceptibility to tumor necrosis factor
by E1A is dependent on binding to either p300 or p105-Rb and
induction of DNA synthesisJ Virol70687719968523594
|
|
26.
|
U FischerK Schulze-OsthoffNew approaches
and therapeutics targeting apoptosis in diseasePharmacol
Rev57187215200510.1124/pr.57.2.615914467
|
|
27.
|
C OroDA JansThe tumour specific
pro-apoptotic factor apoptin (Vp3) from chicken anaemia virusCurr
Drug Targets5179190200410.2174/138945004349063115011951
|
|
28.
|
L GuelenH PatersonJ GakenM MeyersF
FarzanehM TavassoliTAT-apoptin is efficiently delivered and induces
apoptosis in cancer
cellsOncogene2311531165200410.1038/sj.onc.120722414691460
|
|
29.
|
SM ZhuangA ShvartsH van OrmondtAG
JochemsenAJ van der EbMH NotebornApoptin, a protein derived from
chicken anemia virus, induces p53-independent apoptosis in human
osteosarcoma cellsCancer Res5548648919957834613
|
|
30.
|
MH NotebornProteins selectively killing
tumor cellsEur J
Pharmacol625165173200910.1016/j.ejphar.2009.06.06819836376
|
|
31.
|
V PavetMM PortalJC MoulinR HerbrechtH
GronemeyerTowards novel paradigms for cancer
therapyOncogene30120201110.1038/onc.2010.46020935674
|
|
32.
|
I ChowdhuryB TharakanGK BhatCaspases - an
updateComp Biochem Physiol B Biochem Mol
Biol1511027200810.1016/j.cbpb.2008.05.01018602321
|