Introduction
Right ventricular (RV) function may be impaired in
pulmonary hypertension (PH), congenital heart disease (CHD), and
coronary artery disease (CAD) and in patients with left-sided heart
failure or valvular heart disease. The right ventricle (RV) and
left ventricle (LV) of the mammalian heart originate from different
progenitor cells during cardiac morphogenesis (1,2)
and undertake distinct functions in the circulation (3,4).
Animal studies have also demonstrated that the RV and LV exhibit
distinct responses to neurohormone stimulation and hypoxia
(5,6). Current therapies on RV dysfunctions
focus mainly on protopathy, with little attention being paid to the
RV. Hence, the evidence that guides the management of isolated RV
failure is not nearly as well established as the evidence that
guides the management of chronic heart failure resulting from LV
dysfunction. Hence, investigating the mechanism of right heart
failure and choosing therapy is important.
Trimetazidine (TMZ) is a first-line anti-anginal
agent. The mechanism of action of TMZ can be attributed to the
optimization of energy metabolism. A number of clinical studies
have shown the efficacy of TMZ in the treatment of various forms of
ischemia, including angina pectoris (7,8)
and acute coronary syndromes (9).
These beneficial effects have been attributed to the inhibition of
fatty acid oxidation by TMZ. The mechanism of action of TMZ is
believed to be a based on metabolic changes; however, its molecular
opus moderandi and non-metabolic effects remain to be
elucidated. Liu et al showed that TMZ effectively inhibited
myocardial fibrosis (10). In
vitro evidence suggests that TMZ can also modulate a transition
in mitochondrial permeability and protect myocardial cells (MCs)
against ischemia-reperfusion injury (11,12). Whether TMZ can protect MCs in the
RV against hypoxic injury is not known.
miRNAs are small, non-coding RNAs of 18–25 nt that
regulate gene expression by forming base-pair interactions with the
3′ untranslated regions (3′UTR) of target genes, thereby
suppressing target mRNA stability or translation into proteins
(13). Previous studies have
shown that miRNAs play particularly important roles in the
development of the cardiovascular system and cardiovascular disease
in humans (14). miRNA-21
(miR-21) is highly expressed in the cardiovascular system and has
been linked to many forms of heart disease (15,16). Furthermore, one of the most
important functions of miR-21 is its anti-apoptotic role. Evidence
has shown that miR-21 can protect MCs from hypoxia and
H2O2-induced apoptosis via targeting Fas
ligand (FasL) and programmed cell death 4 (PDCD4) (17,18).
In the present study, we investigated the protective
role of TMZ in right heart failure, and ascertained whether the
protective role of TMZ is associated with regulation of miR-21
expression.
Materials and methods
The study protocol was approved by the Ethics
Committee of the Second Affiliated Hospital of Harbin Medical
University (Harbin, China). All animal procedures were carried out
in accordance with the guidelines specified by the Johns Hopkins
Animal Care and Use Committee.
Cell culture and treatment
Hearts were isolated from 1- or 2-day-old
Sprague-Dawley (SD) rats and separated into the right and left
heart plus atrioventricular septum using a microscope. The chambers
were separated by first removing the left and right atria from the
base of the heart. The RV was then dissected from the LV and septum
by inserting iridectomy scissors into the opening of the tricuspid
valve and cutting around the interface of the RV and septum,
leaving a little ‘rim’ of RV tissue at the margins, then removing
excess tissue around the LV and septum. This procedure resulted in
an oblong portion of the RV and a globular section of LV and
septum. MCs from the LV and RV were isolated and cultured according
to a well-established method. Briefly, the 2 ventricles were minced
and trypsinized at 37°C. Cells were centrifuged (800 x g for 5 min)
and resuspended in Dulbecco’s modified Eagle’s medium/F-12 (Gibco,
Billings, MT, USA) supplemented with 10% fetal bovine serum (FBS;
HyClone-Thermo Scientific, Jülich, Germany), 100 U/ml penicillin
and 100 μg/ml streptomycin. After cell panning at 37°C for
90 min, unattached MCs were harvested and seeded at a density of
5x104 cells/cm2. 5-Bromo-2′-deoxyuridine
(BrdU; 0.1 mM; Sigma-Aldrich, St. Louis, MO, USA) was added to the
culture for 36 h to inhibit the proliferation of non-MCs. To ensure
that the following experiment involved the same number of MCs, we
also used flow cytometry to count MCs and non-MCs. The number of
MCs in the RV and LV were identical (95.77±0.012 and 95.57±0.007,
respectively; P>0.05, data not shown). The MCs were then treated
with 10 μM TMZ.
Animal model of right heart failure
A model of right heart failure was created as
described previously (19). Male
SD rats (150–200 g) received a single subcutaneious (s.c.)
injection of the vascular endothelial growth factor receptor
(VEGFR) blocker, Su5416 (20 mg/kg). The rats were then placed in a
normobaric hypoxia (10% O2) chamber for 4 weeks in order
to cause RV failure (SuHx group), whereas the control rats (CON
group) were reared in room air for the same period of time. The
SuHx group was randomly subdivided into 2 groups: 1 group received
TMZ (10 mg/kg/day, SuHx + TMZ group) and the other group (SuHx
group) received placebo treatment. Animals were sacrificed 4 weeks
later after echo-cardiographic assessment. All animals were
anesthetized with an intraperitoneal (i.p.) injection of sodium
pentobarbital (130 mg/kg) prior to removing the heart. RV
hypertrophy was created by separating the RV from the LV plus
septum (LV + S), weighing these components, and calculating the
ratio of RV/(LV + S).
Isolation of MCs in the RV from rats
The adult rat ventricle MC isolation was carried out
using an adaptation of the method described previously (20). The hearts were removed quickly
from the rats and enzymatically dissociated. Rectangular, trypan
blue-excluding cells constituted approximately 80% of all myocytes.
The myocytes were plated into the 6-well plates coated with 0.5
μg/cm2 of laminin (Sigma), at a density of
2x104 cells/cm2. The cultures were incubated
in serum-free medium (SFM). The SFM was changed 30 min after
plating to remove the myocytes that had not attached to the dish.
All the myocytes were cultivated for 24 h before the
experiments.
Echocardiographic assessment
Echocardiographic measurements were taken of the
inner diameter of the RV and tricuspid annular plane systolic
excursion (TAPSE) as previously reported (21).
Histological measurements
Histological examinations were performed in 3 groups
of 5 rats that were submitted to the same exposure protocol. After
sacrifice, each heart was immediately dissected, and the RV and LV
were placed in a formalin solution, and cut into slices (40
μm, long axis) for Masson staining. Structural changes were
evaluated qualitatively by an investigator blinded to the treatment
protocol.
Quantitative reverse
transcription-polymerase chain reaction (RT-PCR)
miR-21 was detected by qRT-PCR as described
previously (19). Briefly, total
RNA was harvested from the cells and reverse-transcribed into cDNA.
For miR-21, a miScript Reverse Transcription kit and miScript
SYBR-Green PCR kit were used (Qiagen, Hilden, Germany). As the
internal control, U6 was used for the normalization of the miR-21
templates. The threshold cycle (Ct) was set within the exponential
phase of the PCR. Relative gene expression was calculated by
comparing cycle times for each target PCR. The target PCR Ct values
were normalized by subtracting the U6 Ct value, which provided the
ΔCt value.
Transient transfection
miR-21 inhibitors were synthesized as unconjugated
and fully phosphorothioate mixed DNA oligonuleotides with a
6-carboxyfluorescein (FAM) moiety at the 5′ terminus (Shanghai
GenePharma Co., Shanghai, China). Cells were transfected with 2
μg inhibitor in 6-well plates using a commercial
transfection reagent (X-tremeGene siRNA Transfection Reagent;
Roche, Basel, Switzerland) according to the manufacturer’s
instructions.
Western blot analysis
For western blot analyses, 60 g of total protein
were extracted from the harvested cells. Samples were loaded onto
12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE) gels. These were separated by electrophoresis, and
transferred onto Immobilon-P polyvinylidene fluoride nylon
membranes. Membranes were incubated with antibodies against
caspase-3 (Cell Signaling Technology, Inc., Danvers, MA, USA), and
β-actin (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA).
Detection of caspase-3 activity
Caspase-3 activity was measured using a commercial
spectroscopic assay based on the ability of caspase-3 to convert
acetyl-Asp-Glu-Val-Asp p-nitroanilide (Ac-DEVD-pNA) into a yellow
formazan product, p-nitroaniline (pNA). The lysates were
centrifuged at 12,000 x g for 10 min at 37°C, and the supernatants
incubated in 96-well microtiter plates with 20 ng Ac-DEVD-pNA for 4
h at 37°C. The optical density (OD) at an absorbance of 405 nm
(OD405), which is indicative of caspase-3 activity, was
read on a 96-well plate reader (Infinite M200; Tecan, Männedorf,
Switzerland). Each assay was performed in triplicate.
Terminal
deoxynucleotidyl-transferase-mediated dUTP nick end-labeling
(TUNEL) assay
TUNEL staining was carried out using an In
Situ Cell Death Detection kit (Roche) according to the
manufacturer’s instructions. RVMCs and LVMCs cultured on coverslips
in 24-well plates were fixed in 4% paraformaldehyde and stained.
TUNEL-positive cells were imaged under a confocal laser scanning
microscope (Fluo View v5.0 FV300; Olympus, Tokyo, Japan) and
counted in 10 randomly chosen fields. The results were expressed as
the percentage of TUNEL-positive cells relative to total number of
cells counted. 4′,6-Diamidino-2-phenylindole dihydrochloride DAPI
(1 μg/ml; Sigma-Aldrich) was used for nuclear
counterstaining.
Statistical analyses
Data are the means ± standard error (SE). Group
comparisons were made using an analysis of variance (ANOVA) with
Fisher’s test. P<0.01 or P<0.05 were considered to indicated
statistically significant differences.
Results
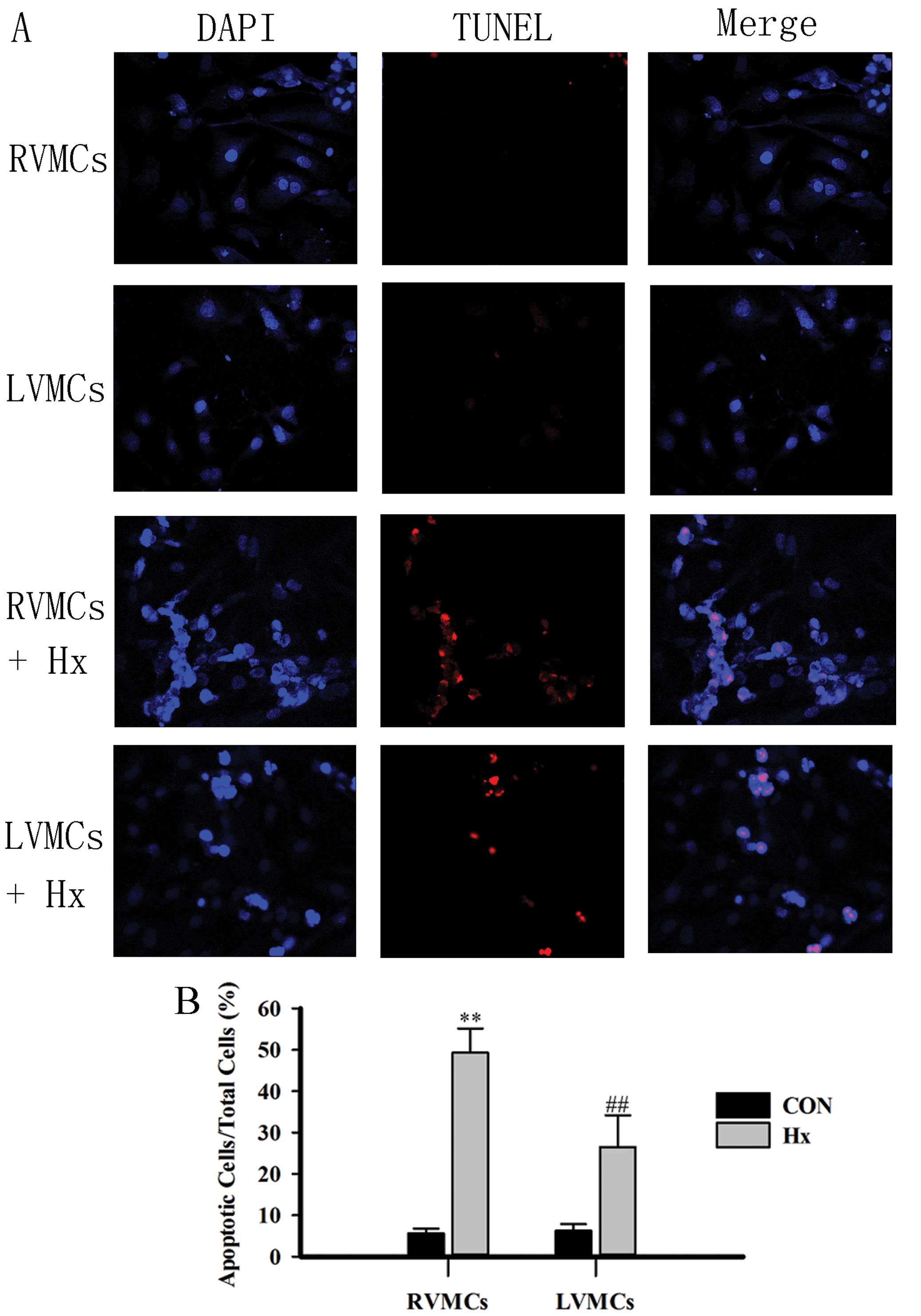
To compare apoptosis between RVMCs and LVMCs after
hypoxia, we used separate cultures of neonatal rat MCs from the RV
and LV (see Materials and methods). TUNEL staining was used to
evaluate apoptosis in the MCs. The RVMCs and LVMCs exhibited a
significantly greater proportion of TUNEL-positive nuclei compared
with those from the control group (Fig. 1A and B). In addition, there were
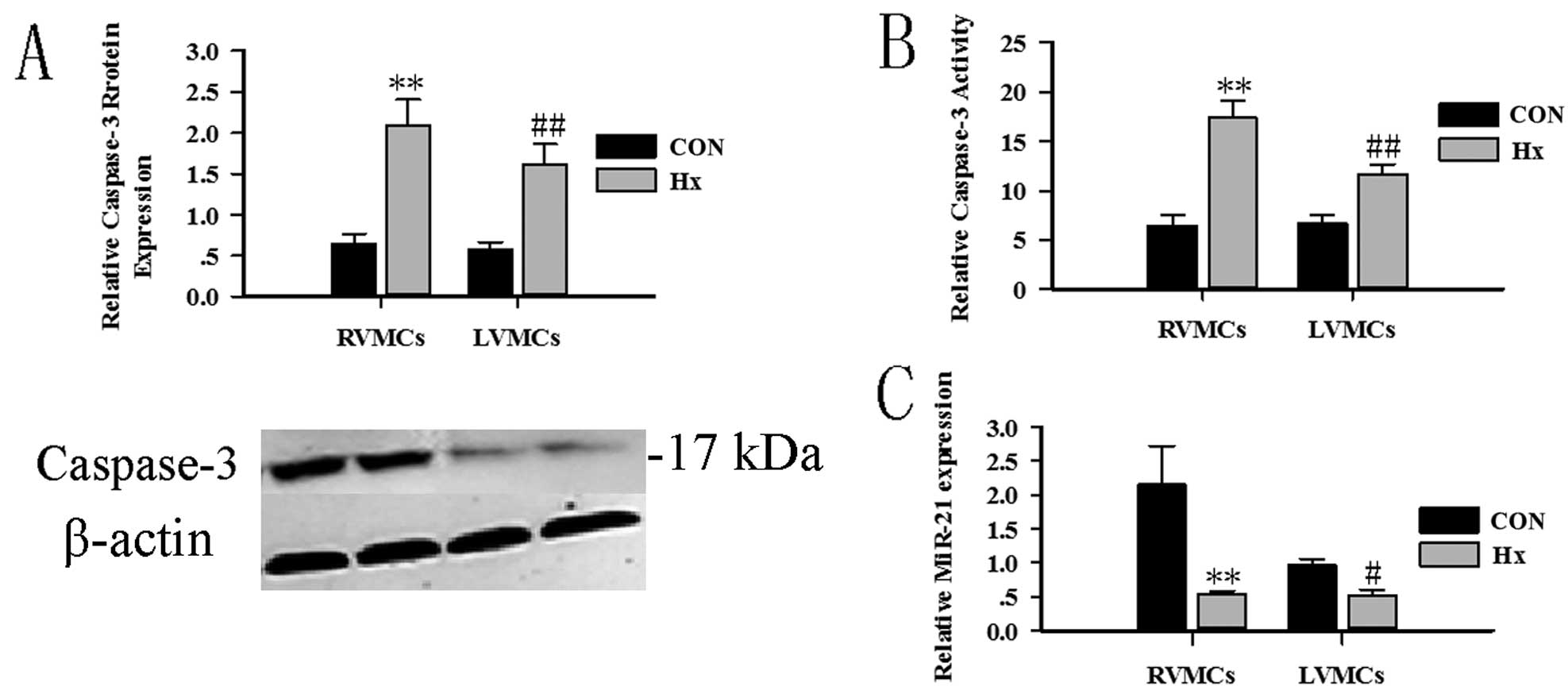
slightly more RVMCs than LVMCs. The caspase-3 protein assay and
activity assay were used to evaluate MC apoptosis (Fig. 2A and B). miR-21 expression was
evaluated at the same time. Twenty-four hours after hypoxia
treatment, RVMCs and LVMCs showed a decreased expression of miR-21
(Fig. 2C).
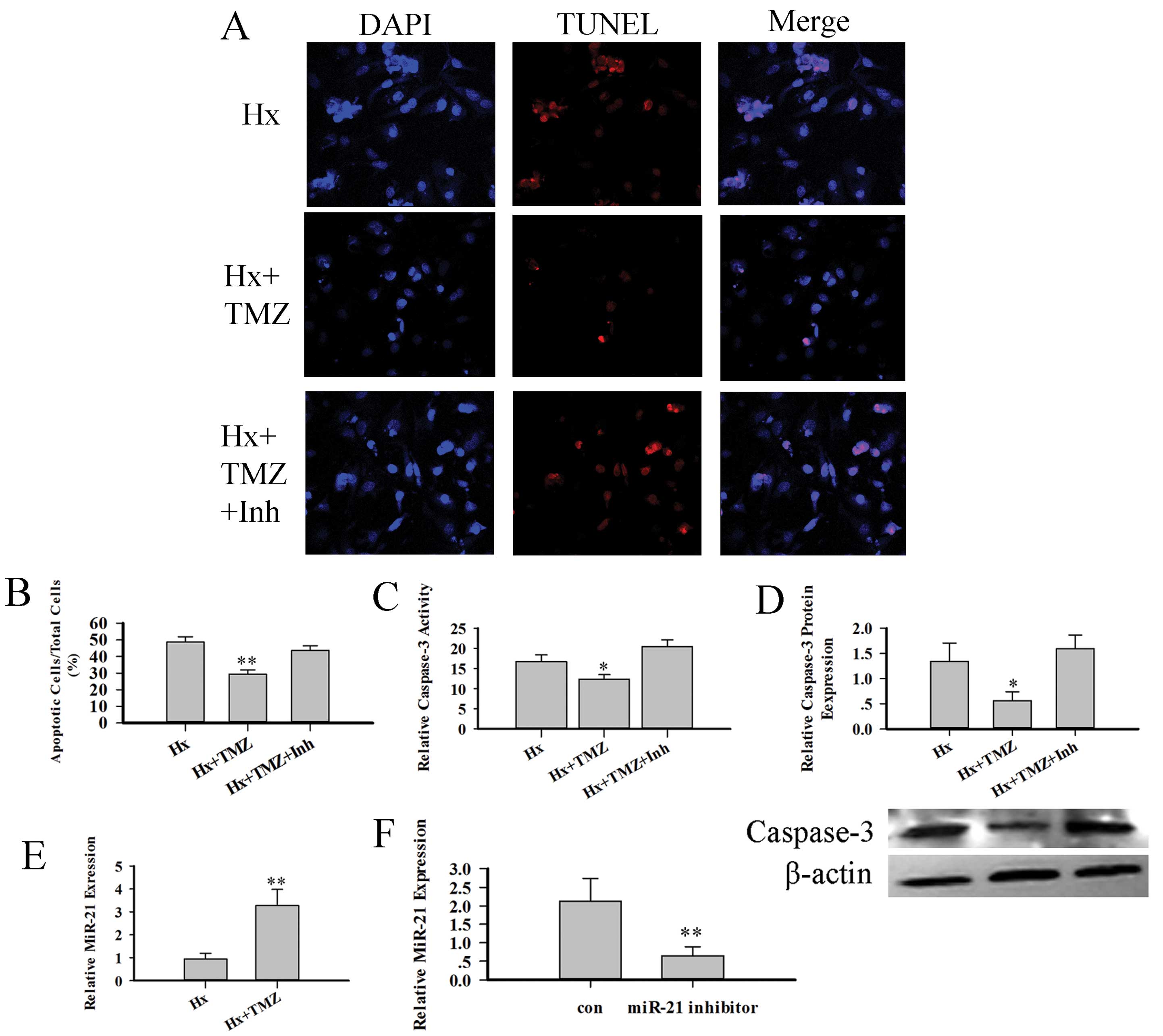
We then investigated whether TMZ can protect RVMCs
from hypoxia-induced injury: the RVMCs were treated with 10
μM TMZ prior to hypoxia treatment. The TUNEL assay (Fig. 3A and B) showed that TMZ decreased
the number of TUNEL-positive nuclei. The TMZ-treated RVMCs
exhibited a reduced expression of caspase-3 protein and reduced
activity (Fig. 3C and D). We then
detected anti-apoptotic miRNA and miR-21 expression with RT-PCR.
The TMZ-treated RVMCs demonstrated an increased expression of
miR-21 (Fig. 3E). To discover
whether the increases in miR-21 expression were associated with the
protective role of TMZ, we used a synthesized miR-21-specific
inhibitor (Shanghai GenePharma Co) to decrease miR-21 expression.
This miR-21 inhibitor decreased miR-21 expression by almost 50%
(Fig. 3F). We then investigated
the protective role of TMZ again using TUNEL staining, the
caspase-3 protein assay and caspase-3 activity assay. The amount of
caspase-3 protein and the activity of caspase-3 decreased following
miR-21 inhibition (Fig. 3C and
D). TUNEL staining also showed that this miR-21 inhibitor
weakened the protective role of TMZ (Fig. 3A and B).
Our results demonstrate (at least in part) that TMZ
can protect RVMCs against hypoxic injury by regulating miR-21
expression on a cellular level. We then wished to elucidate whether
TMZ can be effective in an animal model of right heart failure.
In this study, we used a stable model of right heart
failure by employing combination treatment with Su5416 and hypoxia.
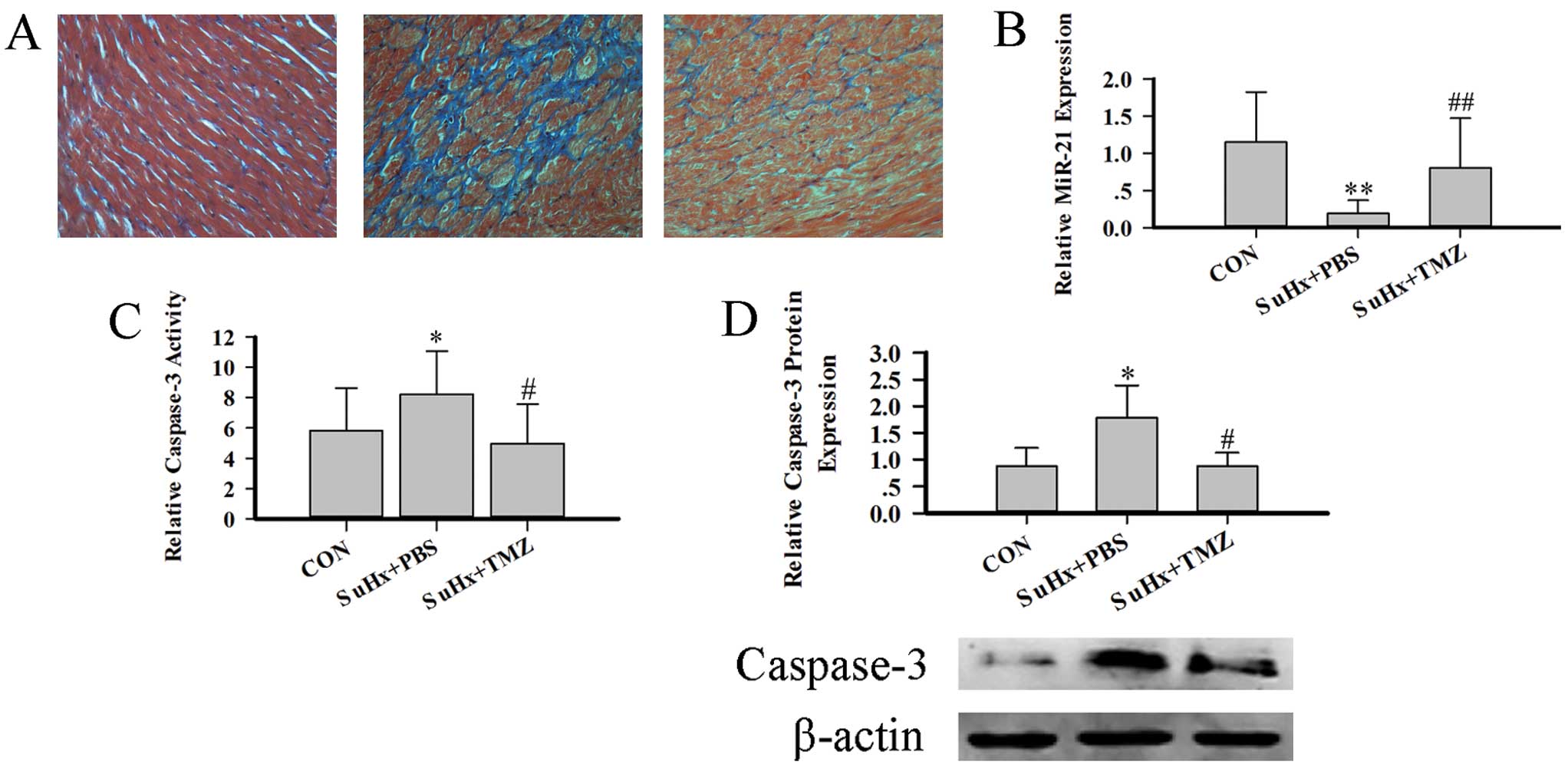
Table I shows the characteristics
of all 3 groups of rats. The SuHx group showed a dramatic decrease
in TAPSE and dilation of the inner diameter of the RV. TMZ produced
a slight increase in TAPSE, and decreased the size of the RV,
indicating that TMZ improves RV function. Fibrosis is another
important feature of RV function. The TMZ-treated group exhibited a
low level of fibrosis (Fig. 4A).
Furthermore, the amount of caspase-3 protein, the activity of
caspase-3, and miR-21 expression are indices of right heart
function. miR-21 is highly expressed in MCs and fibroblasts. Hence,
we isolated RVMCs and then detected the amount of caspase-3
protein, the activity of caspase-3 and miR-21 expression. The SuHx
group showed a decreased expression of miR-21, increased levels of
caspase-3 protein and increased activity of caspase-3. In the
TMZ-treated group, the MCapoptosis was interrupted and heart
function improved (Fig. 4B–D).
These results demonstrate (at least in part) that TMZ improved RV
function by increasing miR-21 expression and decreasing the extent
of RVMC apoptosis.
 | Table IRat characteristics, ultrasound
findings in control rats, 6 weeks after Su5416 treatment and
hypoxia, and 6 weeks after Su5416 treatment, hypoxia and TMZ
treatment. |
Table I
Rat characteristics, ultrasound
findings in control rats, 6 weeks after Su5416 treatment and
hypoxia, and 6 weeks after Su5416 treatment, hypoxia and TMZ
treatment.
| BW (g) | RV (mg) | RV (cm) | RV/LV + S | TAPSE (mm) |
|---|
| CON (n=6) | 380±15 | 230±18 | 1.82±0.45 | 0.36±0.05 | 3.5±0.2 |
| SuHx + PBS
(n=8) | 317±24a | 324±36a | 3.05±0.47a | 0.60±0.1a | 1.6±0.5a |
| SuHx + TMZ
(n=8) | 352±19b | 287±47b | 2.6±0.34b | 0.51±0.07b | 2.6±0.7b |
Discussion
The present study demonstrates that the beneficial
effect of TMZ on right heart failure and hypoxia-induced apoptosis
in RVMCs occurs through an anti-apoptotic function. The
anti-apoptotic mechanism is associated with the increased
expression of miR-21.
Clinical evidence has shown that the 3-ketoacyl-CoA
thiolase (3-KAT) inhibitor, TMZ, improves LV function in elderly
patients or those with previous myocardial infarction (22,23). The mechanism of action for this
phenomenon is a decrease in the oxidation of fatty acids and
stimulation of glucose oxidation (24). These functions directly modify the
use of energy substrates by the heart. However, whether this
metabolic shift is beneficial or detrimental is controversial. A
number of clinical studies have shown that TMZ improves the
ejection fraction of individuals with heart failure with or without
ischemic cardiomyopathy (25,26). Hence, researchers have begun to
pay attention to the non-metabolic functions of TMZ. Liu et
al showed that TMZ effectively inhibited myocardial fibrosis
through the NADPH oxidase-ROS-CGF signaling pathway (10). The present study shows another
non-metabolic effect of TMZ, the inhibition of MC apoptosis. We
also demonstrate that the anti-apoptotic function of TMZ os based
upon the regulation of miR-21 expression. However, we could not
clarify through which pathway TMZ regulates miR-21 expression in
RVMCs.
miR-21 is highly expressed in the cardiovascular
system, where it regulates the growth and elimination of cardiac
cells, as well as the functions of cardiac fibroblasts. Increased
miR-21 expression has also been linked to MC hypertrophy (27). Recently, miR-21 was shown to be an
important anti-apoptotic factor in MCs under several pathological
conditions. miR-21 upregulation has been shown to protect MCs
against hypoxiaor H2O2-induced apoptosis, and
phosphatase and tensin homolog (PTEN), FasL and PDCD4 have been
identified as targets of miR-21-mediated suppression (28,29). The expression of miR-21 has been
shown to be decreased in infarcted regions of the heart, and that
the upregulation of miR-21 can decrease the infarcted area
(30). The present study also
shows the decreased expression of miR-21 in RVMCs after hypoxia.
Moreover, we also evaluated miR-21 expression after brief periods
of hypoxia (15 min) (data not shown). miR-21 expression increased,
which was in accordance with the increase observed during ischemic
preconditioning (31). During
this period, MCs are decompensated. Hence, MCs can directly
regulate miR-21 expression, and their own miR-21 expression may be
considered to be a regulator of protection. Hence, we speculate
that the mechanism by which TMZ regulates miR-21 cannot be
separated from its energy optimization role.
We also demonstrate that TMZ improves heart function
during right heart failure. Furthermore, such effectiveness may not
be due only to energy optimization, but also to anti-apoptotic
actions and the inhibition of fibrosis. In recent years, a number
of studies have shown that RV function is an important predictor of
survival in patients with heart failure (32). In 2006, the National Heart, Lung
and Blood Institute in the USA identified the function and failure
of the RV to be a research priority in cardiovascular disease
(33). However, an effective
therapy for RV failure is lacking. Clinical research has shown that
many effective therapies which apparently improve the prognosis of
patients with left heart failure have little merit in those with
right heart failure. Animal-based research has shown that MC
apoptosis is the major reason for right heart failure. Chronic PH
cannot lead to right heart failure, whereas the inhibition of
cardiac capillary density with the VEGFR blocker, Su5416, can lead
to severe RV failure in rats with RV pressure-overload (19). The present study also demonstrates
that, after hypoxia, RVMCs show more severe apoptosis than LVMCs.
Hence, we believe that the main issue of right heart failure may be
‘running out of fuel’, so that the ‘hungry’ MCs eventually die.
Hence, we chose TMZ to treat right heart failure. As expected, TMZ
effectively improved RV function, decreased apoptosis and inhibited
fibrosis. The results from the present study present strong
evidence that TMZ can be used in subjects with right heart
failure.
Acknowledgements
This study was supported in part by
the National Basic Research Program (973 Program) of China (grant
no. 2007CB512005) and the Key Laboratory of Myocardial Ischemia
Mechanism and Treatment (Harbin Medical University), Ministry of
Education, China (KF201010/KF201002).
References
|
1.
|
MP VerziDJ McCulleyS De ValThe right
ventricle, outflow tract, and ventricular septum comprise a
restricted expression domain within the secondary/anterior heart
fieldDev Biol287134145200510.1016/j.ydbio.2005.08.041
|
|
2.
|
S ZaffranRG KellySM MeilhacRight
ventricular myocardium derives from the anterior heart fieldCirc
Res95261268200410.1161/01.RES.0000136815.73623.BE15217909
|
|
3.
|
F HaddadSA HuntDN RosenthalDJ MurphyRight
ventricular function in cardiovascular disease, part I: Anatomy,
physiology, aging, and functional assessment of the right
ventricleCirculation11714361448200810.1161/CIRCULATIONAHA.107.65357618347220
|
|
4.
|
F HaddadR DoyleDJ MurphySA HuntRight
ventricular function in cardiovascular disease, part II:
pathophysiology, clinical importance, and management of right
ventricular
failureCirculation11717171731200810.1161/CIRCULATIONAHA.107.65358418378625
|
|
5.
|
M StrniskovaT RavingerovaJ NeckarChanges
in the expression and/or activation of regulatory proteins in rat
hearts adapted to chronic hypoxiaGen Physiol
Biophys252541200616714773
|
|
6.
|
GY WangDT McCloskeyS TurcatoContrasting
inotropic responses to alpha1-adrenergic receptor stimulation in
left versus right ventricular myocardiumAm J Physiol Heart Circ
Physiol291H2013H2017200610.1152/ajpheart.00167.200616731650
|
|
7.
|
SC ManchandaS KrishnaswamiCombination
treatment with trimetazidine and diltiazem in stable angina
pectorisHeart78353357199710.1136/hrt.78.4.3539404250
|
|
8.
|
H SzwedZ SadowskiW ElikowskiCombination
treatment in stable effort angina using trimetazidine and
metoprolol: results of a randomized, double-blind, multicentre
study (TRIMPOL II). Trimetazidine in POLandEur Heart
J2222672274200110.1053/euhj.2001.2896
|
|
9.
|
G KoberT BuckH SievertC
VallbrachtMyocardial protection during percutaneous transluminal
coronary angioplasty: effects of trimetazidineEur Heart
J13110911151992
|
|
10.
|
X LiuY GaiF LiuTrimetazidine inhibits
pressure overload-induced cardiac fibrosis through NADPH
oxidase-ROS-CTGF pathwayCardiovasc
Res88150158201010.1093/cvr/cvq18120534773
|
|
11.
|
JN WeissP KorgeHM HondaP PingRole of the
mitochondrial permeability transition in myocardial diseaseCirc
Res93292301200310.1161/01.RES.0000087542.26971.D412933700
|
|
12.
|
AP HalestrapSJ ClarkeSA
JavadovMitochondrial permeability transition pore opening during
myocardial reperfusion - a target for cardioprotectionCardiovasc
Res61372385200410.1016/S0008-6363(03)00533-914962470
|
|
13.
|
LP LimNC LauP Garrett-EngeleMicroarray
analysis shows that some microRNAs downregulate large numbers of
target mRNAsNature433769773200510.1038/nature0331515685193
|
|
14.
|
EM SmallRJ FrostEN OlsonMicroRNAs add a
new dimension to cardiovascular
diseaseCirculation12110221032201010.1161/CIRCULATIONAHA.109.88904820194875
|
|
15.
|
Y ChengC ZhangMicroRNA-21 in
cardiovascular diseaseJ Cardiovasc Transl
Res3251255201010.1007/s12265-010-9169-720560046
|
|
16.
|
PA da Costa MartinsLJ De WindtMiR-21: A
miRaculous Socratic paradoxCardiovasc Res87397400201020562424
|
|
17.
|
B OzpolatU AkarM SteinerProgrammed cell
death-4 tumor suppressor protein contributes to retinoic
acid-induced terminal granulocytic differentiation of human myeloid
leukemia cellsMol Cancer
Res595108200710.1158/1541-7786.MCR-06-0125
|
|
18.
|
H AllgayerPdcd4, a colon cancer prognostic
that is regulated by a microRNACrit Rev Oncol
Hematol73185191201010.1016/j.critrevonc.2009.09.00119836969
|
|
19.
|
HJ BogaardR NatarajanSC HendersonChronic
pulmonary artery pressure elevation is insufficient to explain
right heart
failureCirculation12019511960200910.1161/CIRCULATIONAHA.109.88384319884466
|
|
20.
|
J KajsturaE CigolaA MalhotraAngiotensin II
induces apoptosis of adult ventricular myocytes in vitroJ Mol Cell
Cardiol29859870199710.1006/jmcc.1996.03339152847
|
|
21.
|
F CacciapuotiEchocardiographic evaluation
of right heart function and pulmonary vascular bedInt J Cardiovasc
Imaging25689697200910.1007/s10554-009-9478-619634000
|
|
22.
|
C VitaleM WajngatenB SposatoTrimetazidine
improves left ventricular function and quality of life in elderly
patients with coronary artery diseaseEur Heart
J2518141821200410.1016/j.ehj.2004.06.03415474696
|
|
23.
|
P Di NapoliAA TaccardiA BarsottiLong term
cardio-protective action of trimetazidine and potential effect on
the inflammatory process in patients with ischaemic dilated
cardiomyopathyHeart91161165200515657223
|
|
24.
|
PF KantorA LucienR KozakGD LopaschukThe
antianginal drug trimetazidine shifts cardiac energy metabolism
from fatty acid oxidation to glucose oxidation by inhibiting
mitochondrial long-chain 3-ketoacyl coenzyme A thiolaseCirc
Res86580588200010.1161/01.RES.86.5.580
|
|
25.
|
G FragassoA PalloshiP PuccettiA randomized
clinical trial of trimetazidine, a partial free fatty acid
oxidation inhibitor, in patients with heart failureJ Am Coll
Cardiol48992998200610.1016/j.jacc.2006.03.06016949492
|
|
26.
|
H TuunanenE EngblomA NaumTrimetazidine, a
metabolic modulator, has cardiac and extracardiac benefits in
idiopathic dilated
cardiomyopathyCirculation11812501258200810.1161/CIRCULATIONAHA.108.77801918765391
|
|
27.
|
M TatsuguchiHY SeokTE CallisExpression of
microRNAs is dynamically regulated during cardiomyocyte
hypertrophyJ Mol Cell
Cardiol4211371141200710.1016/j.yjmcc.2007.04.00417498736
|
|
28.
|
D SayedM HeC HongMicroRNA-21 is a
downstream effector of AKT that mediates its antiapoptotic effects
via suppression of Fas ligandJ Biol
Chem2852028120290201010.1074/jbc.M110.10920720404348
|
|
29.
|
Y ChengX LiuS ZhangMicroRNA-21 protects
against the H(2)O(2)-induced injury on
cardiac myocytes via its target gene PDCD4J Mol Cell
Cardiol475142009
|
|
30.
|
S DongY ChengJ YangMicroRNA expression
signature and the role of microRNA-21 in the early phase of acute
myocardial infarctionJ Biol
Chem2842951429525200910.1074/jbc.M109.02789619706597
|
|
31.
|
J QianX RenX WangBlockade of Hsp20
phosphorylation exacerbates cardiac ischemia/reperfusion injury by
suppressed autophagy and increased cell deathCirc
Res10512231231200910.1161/CIRCRESAHA.109.20037819850943
|
|
32.
|
P MeyerRV DesaiM MujibRight ventricular
ejection fraction <20% is an independent predictor of mortality
but not of hospitalization in older systolic heart failure
patientsInt J Cardiol1551201252012
|
|
33.
|
NF VoelkelRA QuaifeLA LeinwandRight
ventricular function and failure: report of a National Heart, Lung,
and Blood Institute working group on cellular and molecular
mechanisms of right heart
failureCirculation11418831891200610.1161/CIRCULATIONAHA.106.632208
|