Introduction
With the improvement of people’s living standard,
social aging trend will be more obvious, so senile disease rates
are on the rise. Neurodegenerative diseases is a progressive
disease associated with age in nervous system, including
Alzheimer’s disease (AD), Parkinson’s disease (PD), amyotrophic
lateral sclerosis (ALS) and multiple sclerosis (MS). Statistics
indicate that thirty-four million people will have AD in the world
by the year 2025 (1). It is
estimated that the central nervous system (CNS), such as AD, PD,
brain cancer, and stroke, will cost trillions of dollars for their
treatment (2). Alzheimer’s
disease is the most common type of dementia. The United States
statistics show that AD affects 5.4 million people and ∼200,000
people under the age 65 have younger-onset AD (3). The clinical presentation is mainly
memory loss and cognitive decline. Currently available drugs for
the treatment of AD are purely for symptoms (4) and among these drugs are most the
cholinesterase inhibitors (5).
Another type of drug available for AD patients is an
N-methyl-daspartate (NMDA) receptor antagonist named memantine
(6). Many drugs are available to
treat AD currently, but the effect is not significant or serious
side effect may be occurs. We still lack specially effective drug
treatment for the AD patients. Therefore, it is a very important
responsibility and obligation for the pharmaceutical industry to
study a new central nervous system drugs with high quality, high
efficiency and low side effects.
In recent years, a large number of research data
show that many acute and chronic neurodegenerative diseases are
association with extracellular abnormally gathered glutamic acid in
the brain (7). Glutamate, an
excitatory amino acid, is one of the major neurotransmitters in the
central nervous system (CNS). Glutamate participate in multiple
physiological pathology processes. The right amount of glutamate is
required to maintain normal cell physiological activities. However,
high concentrations of glutamate in the brain can lead to neuronal
damage. Glutamate are thought to be involved in the etiology of a
number of neurodegenerative disorders including AD, PD, ALS, MS. A
large amount of glutamate cause excessive activation of NMDA
receptors, leading to calcium overload which can trigger a cascade
of events eventually leading to apoptosis or necrosis (8).
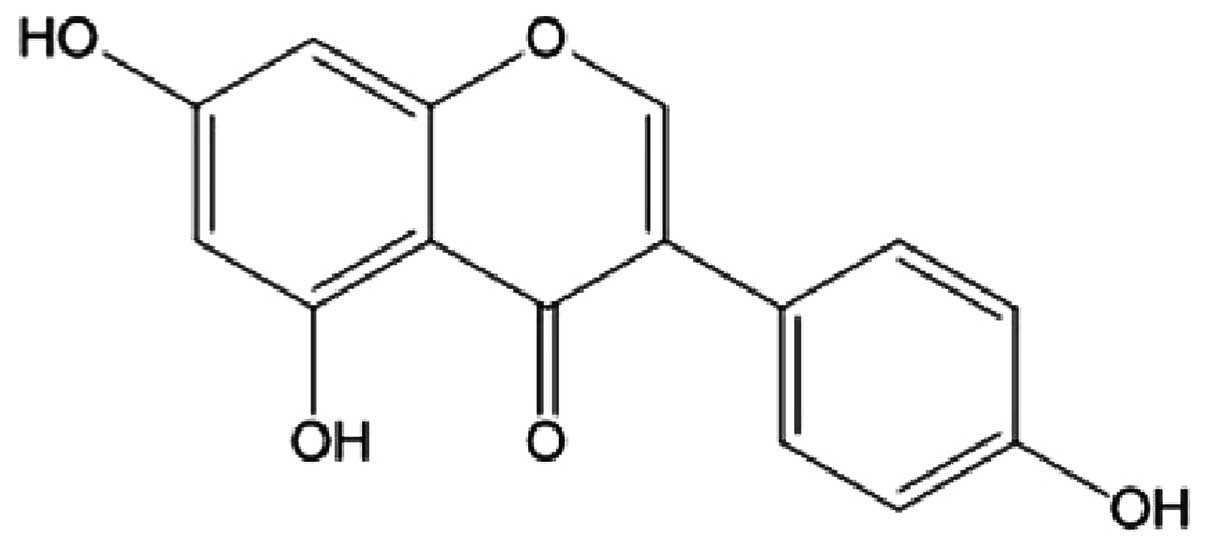
Genistein (4′,5,7-trihydroxyisoflavone) (Fig. 1), plentiful in soybeans, has
estrogenic activity. Physiological levels of estrogen have many
significant biological activities which are beneficial for
treatment of osteoporosis and menopausal symptoms (9), AD in women after menopause (10), inflammatory response (11,12), atherosclerosis (13), liver cancer (14), colon cancer (15), and it is neuro-protective
(16). Genistein has a strong
protective effect on the damage induced by oxidative stress
(17). However, the absorption of
genistein in the gastrointestinal tract is poor, resulting in low
biological activity (18). To
overcome this problem, the CHF2 group was introduced into the lead
compound genistein, which can change its chemical and physical
properties (19). After the
introduction of CF2 into genistein, Fu et al
(20) design and synthesized a
series of difluoromethyl-derivatives of genistein to screen an
effective drug with oxidative stress injury model. The results
confirmed that 7-difluoromethylyl-genistein is a protective new
chemical entity for injury model induced by oxidative stress
(21).
PC12 is a cell line derived from a rat adrenal
medulla pheochromocytoma. PC12 cells have typical characteristics
of nerve cells, which can be widely used as a nerve cell model.
There are many mechanisms of nerve cell injury, one of which is the
glutamate-induced damage. In this study, we used MTT assay to
measure cell growth and proliferation activity, flow cytometry
(FCM) with propidium iodide (PI) staining and acri-dine orange (AO)
staining to detect cell apoptosis, assay kits to detect LDH
activity, SOD activity and MDA content. Then we investigated the
effect of 7-difluoromethoxy-5,4′-Di-hydroxyl isoflvone (dFGEN) on
PC12 cells induced by glutamate.
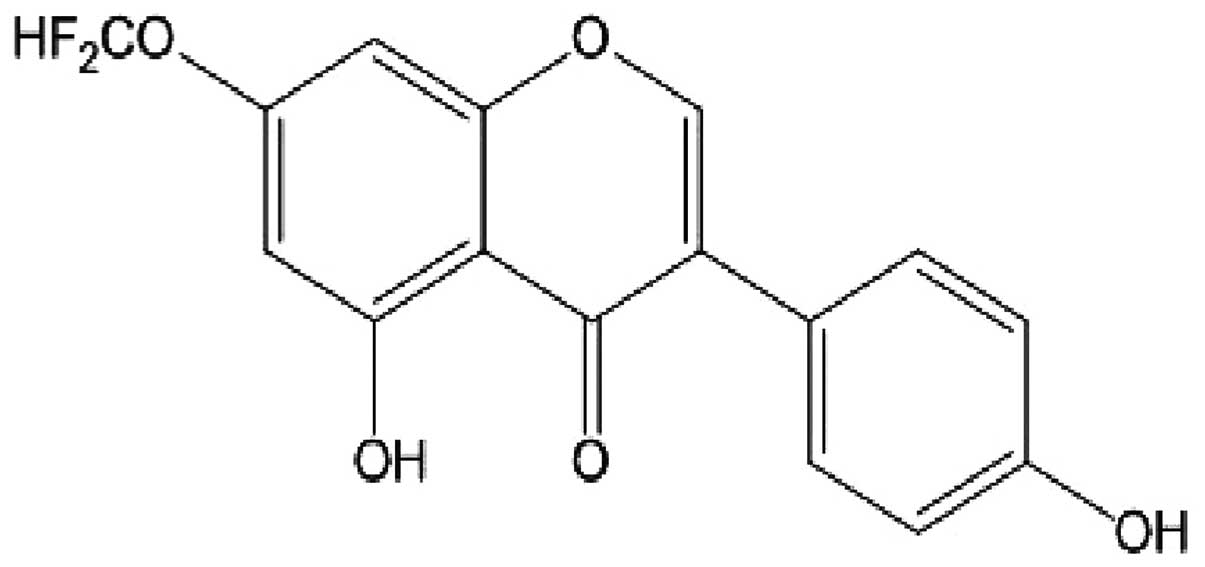
7-difluoromethoxy-5,4′-dihydroxy isoflavone (dFGEN)
is a genistein derivative (332 kDa) that had 7-OH group substituted
by -OCHF2. Its formula is shown in Fig. 2.
Materials and methods
Reagents
dFGEN (98% pure) was synthesized as reported in our
laboratoty (20). Genistein and
3-(4,5-dimethylthiazo-2-yl)-2,5-diphenyltetrazolium bromide (MTT)v
were purchased from Sigma (USA). Glutamate, vitamin E and Dimethyl
sulfoxide (DMSO) were purchased from Genview (USA). Dulbecco’s
minimum essential medium (DMEM) were obtained from Hyclone (USA).
Fetal calf serum (FBS) was purchased from Hangzhou Sijiqing
Biological Engineering Materials Co. (Hangzhou, China). Acridine
orange was purchased from Sinopharm Chemical Reagent Co., Ltd.
(China). Cell lysis buffer, trypan blue staining, penicillin and
streptomycin were purchased from Beijing Dingguo Changsheng Biotech
Co., Ltd. (China). Lactate dehydrogenase (LDH) assay kit,
superoxide dismutase (SOD) assay kit, lipid peroxidation (MDA)
assay kit were purchased from Nanjing Jiancheng Bioengineering
Institute (Nanjing, China). BCA protein assay kit and trypsin were
purchased from Beyotime Institute of Biotechnology (Shanghai,
China). Cell culture plates and cell culture dishes were purchased
from Corning Inc. (USA).
Cell culture and treatment
PC12 cells (rat adrenal pheochromocytoma cells) were
purchased from Cell Bank, Chinese Academy of Sciences (Shanghai,
China). Cells were cultured in DMEM medium supplemented with 10%
FBS, 100 U/ml penicillin and 100 μg/ml streptomycin at 37°C in a
humidified atmosphere of 5% CO2 incubator. When cells
were ∼80% confluent, new media with 1% newborn calf serum were
added before the drug treatment. Glutamate was added in final
concentrations ranging from 1 to 20 mM in the pilot study, and the
concentration (10 mM) was selected by determining dose-response
curves. When needed, cells were incubated for 30 min with genistein
(0.1, 1.0 and 10 μM), dFGEN (0.1, 1.0 and 10 μM), vitamin E (10
μM), and then exposed to 10 mM glutamate for 24 h.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
The PC12 cells were seeded in 96-well plates at a
density of 1×104 cells/well. After 24 h, the cells were
treated with various concentrations of genistein, dFGEN, vitamin E
for 30 min prior to glutamate (10 mM) treatment for 24 h. Briefly,
20 μl MTT was added to each well at a final concentration of 0.5
mg/ml, and afterwards the cells were cultured for 4 h at 37°C. The
medium was then carefully removed and 150 μl of DMSO was added to
each well. The absorbance at 490 nm wavelength (A490)
was measured with enzyme-linked immunosorbent instrument (ElX800,
Bio-Tek, USA). Experiment was divided into zero setting group,
control group, and experimental group. The cell viability was
expressed as a percentage of the viability of the control culture.
Relative cell proliferation inhibition rate (IR) = (1 - average
A490 of the experimental group/average A490
of the control group) x 100%.
Flow cytometry (FCM) with propidium
iodide (PI) staining
The PC12 cells were seeded in 6-well plates at a
density of 2×105/ml (2 ml/well) and incubated for 24 h.
Cells were treated by drugs in the same way as described above.
Cells were treated for 24 h, then colleted and harvested with 0.25%
trypsin and made into a single cell suspension, washed with cold
PBS twice. Cells were resuspended as a single cell suspension with
50 μl PBS, fixed with l ml of cold 70% ethanol, stained with
propidium iodide (PI) and cell apoptosis was detected using flow
cytometry (FC 500 American Beckman Coulter Co.).
Morphological observations and acridine
orange staining
The PC12 cells were seeded in 6-well plate at a
density of 2×105/ml (2 ml/well) and incubated for 24 h.
Cells were treated by drugs in the same way as described above.
After cells were treated for 24 h, cell morphological changes were
observed under an optical microscope. Then the cells were washed
two times with cold PBS and incubated with AO (5 μg/ml) at room
temperature for 10 min in the dark. The stained cells were observed
using fluorescence microscope (Olympus BX41, Japan Olympus Co.) and
images were taken.
Detection of LDH activity
The PC12 cells were seeded in 24-well plates at a
density of 1.2×105/ml (1 ml/well) and incubated for 24
h. Cells were treated by drugs in the same way as described above.
Cells were treated for 24 h, the culture supernatants were
collected to a 1 ml Eppendorf tube, and LDH activity was detected
at 450 nm by the assay kit.
Detection of SOD activity and MDA
content
The PC12 cells were seeded in 66-mm culture dish at
a density of 1×106/ml (4 ml/dish) and incubated for 24
h. Cells were treated by drugs in the same way as described above.
Cells were treated for 24 h, then colleted, washed two times with
cold PBS, lysed at 4°C for 30 min in lysate and then centrifuged at
12,000 x g for 10 min at 4°C. SOD activity and MDA contents were
measured according to the direction of the assay kit.
Statistical analysis
Data are presented as the mean ± SD. The database
were set up with the SPSS 16.0 software package for analysis. The
means of multiple groups were compared with one-way ANOVA, the
two-two comparisons among the means were performed with LSD t-test.
P<0.05 was considered as statistically significant.
Results
Effects of dFGEN on glutamate-induced
PC12 cell proliferation
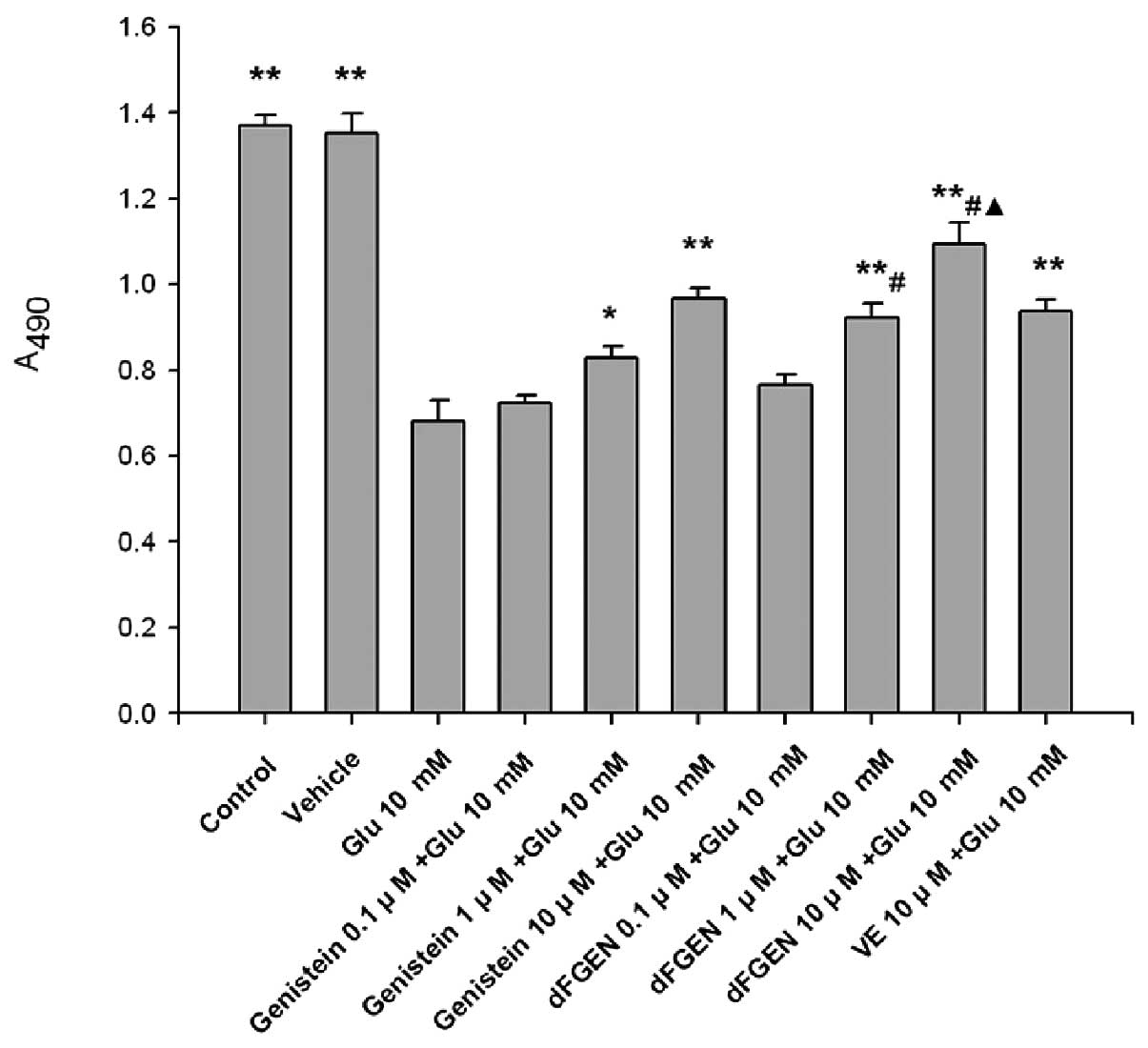
As shown in Fig.
3, the MTT assay demonstrates that glutamate obviously
inhibited proliferation of PC12 cells compared with the control
group (p<0.01). Results showed that dFGEN could effectively
increased proliferation of PC12 cells in a concentration-dependent
manner. Compared with the same concentration of genistein (1.0 and
10 μM), dFGEN (1.0 and 10 μM) could distinctly increase
proliferation of PC12 cells (p<0.05). The cell viability of 10
μM dFGEN was >10 μM vitamin E (p<0.05).
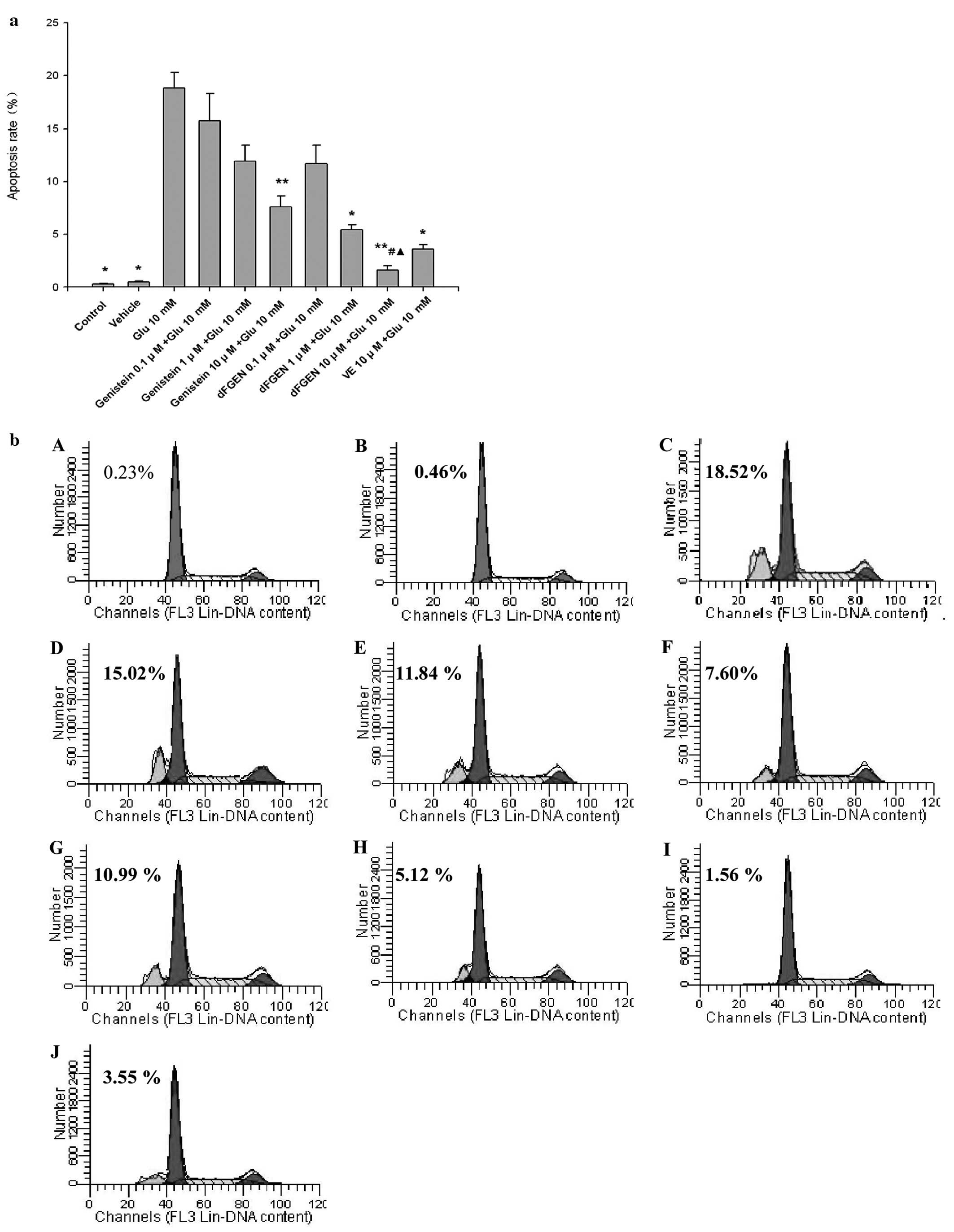
Effects of dFGEN on glutamate-induced
PC12 cell apoptosis
As shown in Fig.
4, the percentage of apoptotic cells was increased from
0.29±0.06 to 18.82±1.47% after the cells were exposed to 10 mM
glutamate for 24 h (p<0.05). dFGEN effectively decreased
apoptosis of PC12 cells in a concentration-dependent manner. dFGEN
(10 μM) distinctly decreased glutamate-induced PC12 cell apoptosis
compared to the same concentration of genistein and vitamin E
(p<0.05).
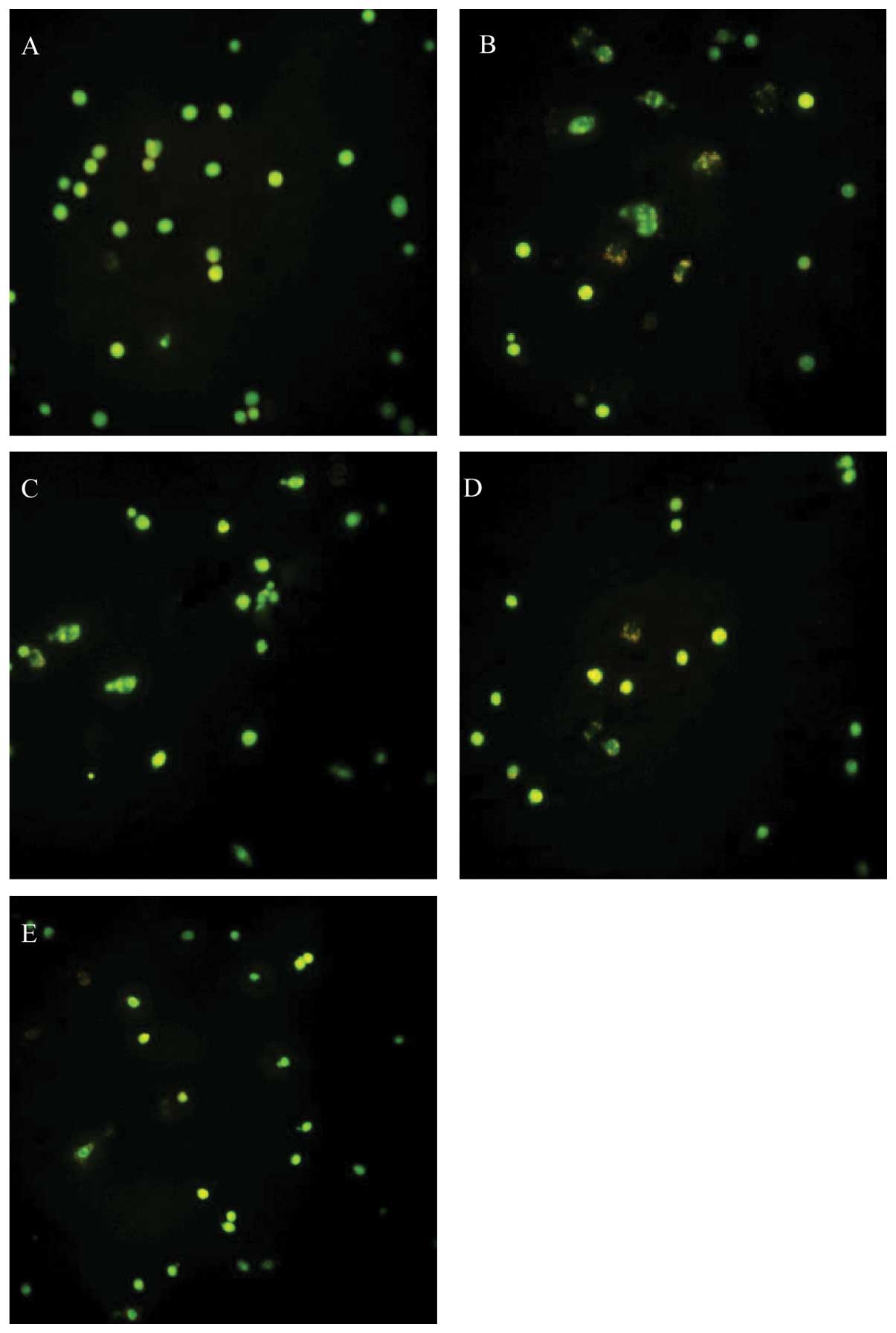
 | Figure 4.The effect of dFGEN on
glutamate-induced PC12 cell apoptosis. The PC12 cells were treated
with various concentrations of dFGEN (0.1, 1.0 and 10 μM),
genistein (0.1, 1.0 and 10 μM) or vitamin E (10 μM) for 30 min
followed by the addition of glutamate to a final concentration of
10 mM, and incubated for 24 h. Cell apoptosis was detectd by FCM
with PI staining. *p<0.05 vs glutamate 10 mM;
**p<0.01 vs glutamate 10 mM; #p<0.05 vs
the same concentration of genistein + glutamate 10 mM;
▴p<0.05 vs the same concentration of VE + glutamate
10 mM. (b) A, Control; B, Vehicle; C, Glu 10 mM; D, genistein 0.1
μM + Glu 10 mM; E, genistein 1 μM Glu 10 mM; F, genistein 10 μM +
Glu 10 mM; G, dFGEN 0.1 μM + Glu 10 mM; H, dFGEN 1 μM + Glu 10 mM;
I, dFGEN 10 μM + Glu 10 mM; J, VE 10 μM + Glu 10 mM. |
Effects of dFGEN on glutamate-induced
PC12 cell morphology and apoptotic morphology
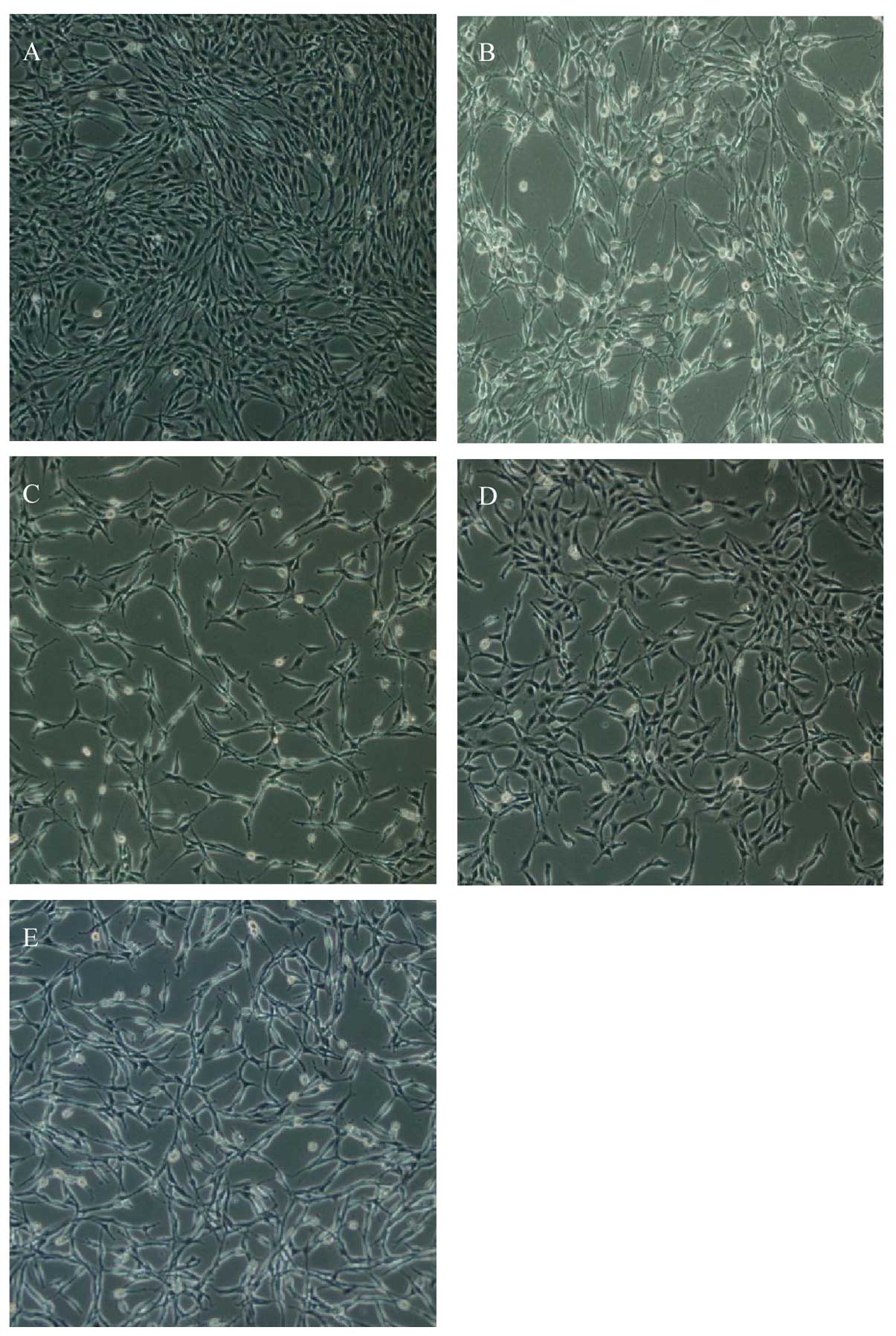
As shown in Fig.
5, glutamate-induced PC12 cells exhibited significantly
morphological alterations under microscopy and they became smaller
and irregularly shaped. dFGEN obviously improved cell morphology.
DNA-binding dye acridine orange (AO) was used to observe the
morpgological characteristic of apoptotic cells. As shown in
Fig. 6, AO staining displayed
karyopyknosis, chromatin condensation and apoptotic bodies by
fluorescence microscopy in glutamate treated group. Dense staining
yellow-green fluorescence and granules could be seen under the
fluorescence microscope. dFGEN evidently decreased the
glutamate-induced apoptosis of PC12 cells.
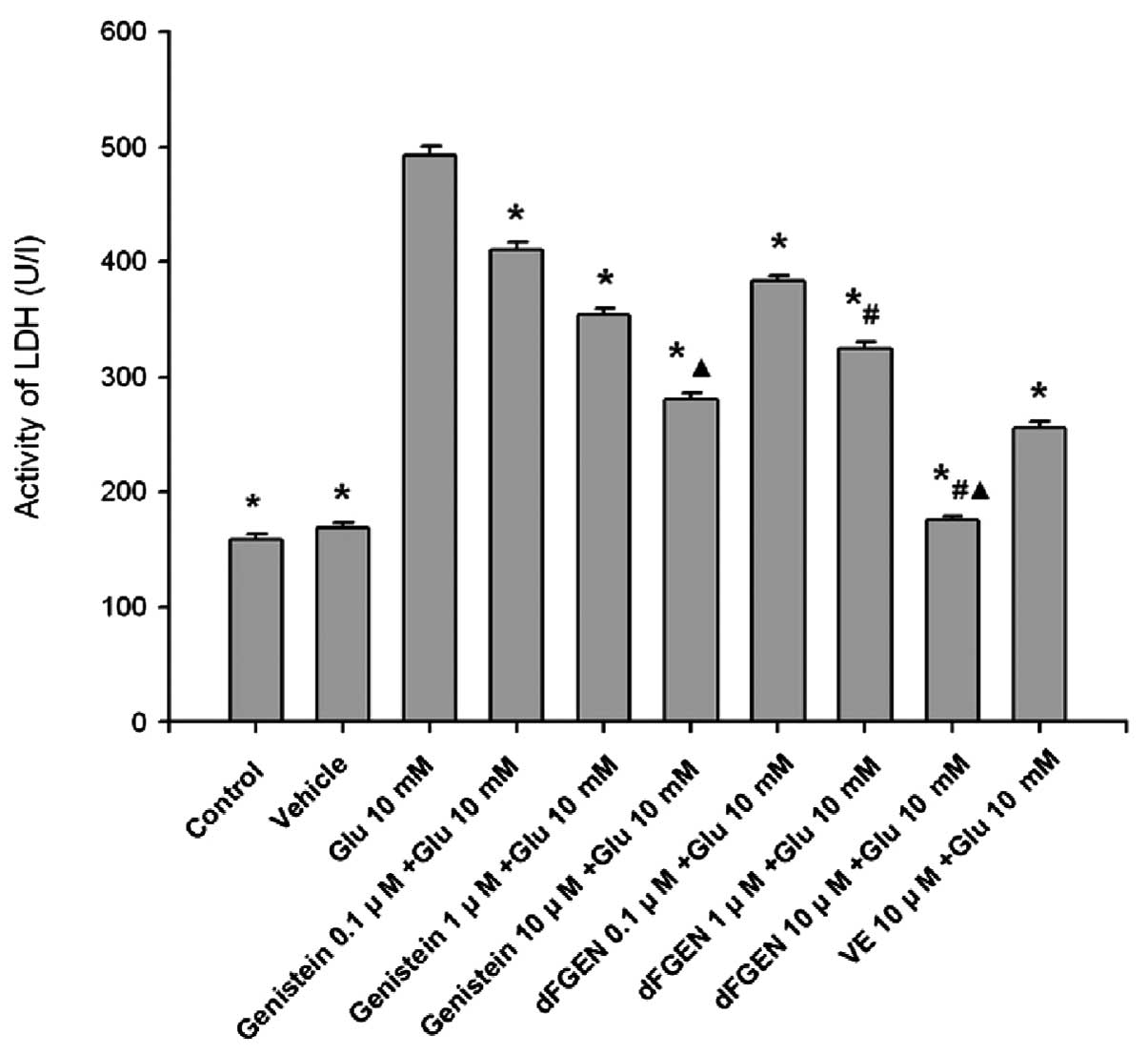
Effects of dFGEN on glutamate-induced
PC12 cell LDH activity
As shown in Fig.
7, while glutamate (10 mM) increased the release of LDH to
492.58±8.48 U/l (p<0.01), dFGEN reduced their release in a
concentration-dependent manner. Compared with the same
concentration of genistein (1.0 and 10 μM), dFGEN (1.0 and 10 μM)
distinctly decreased LDH activity of PC12 cells (p<0.01). The
LDH activity of 10 μM dFGEN group was lower than in the 10 μM
vitamin E group (p<0.01).
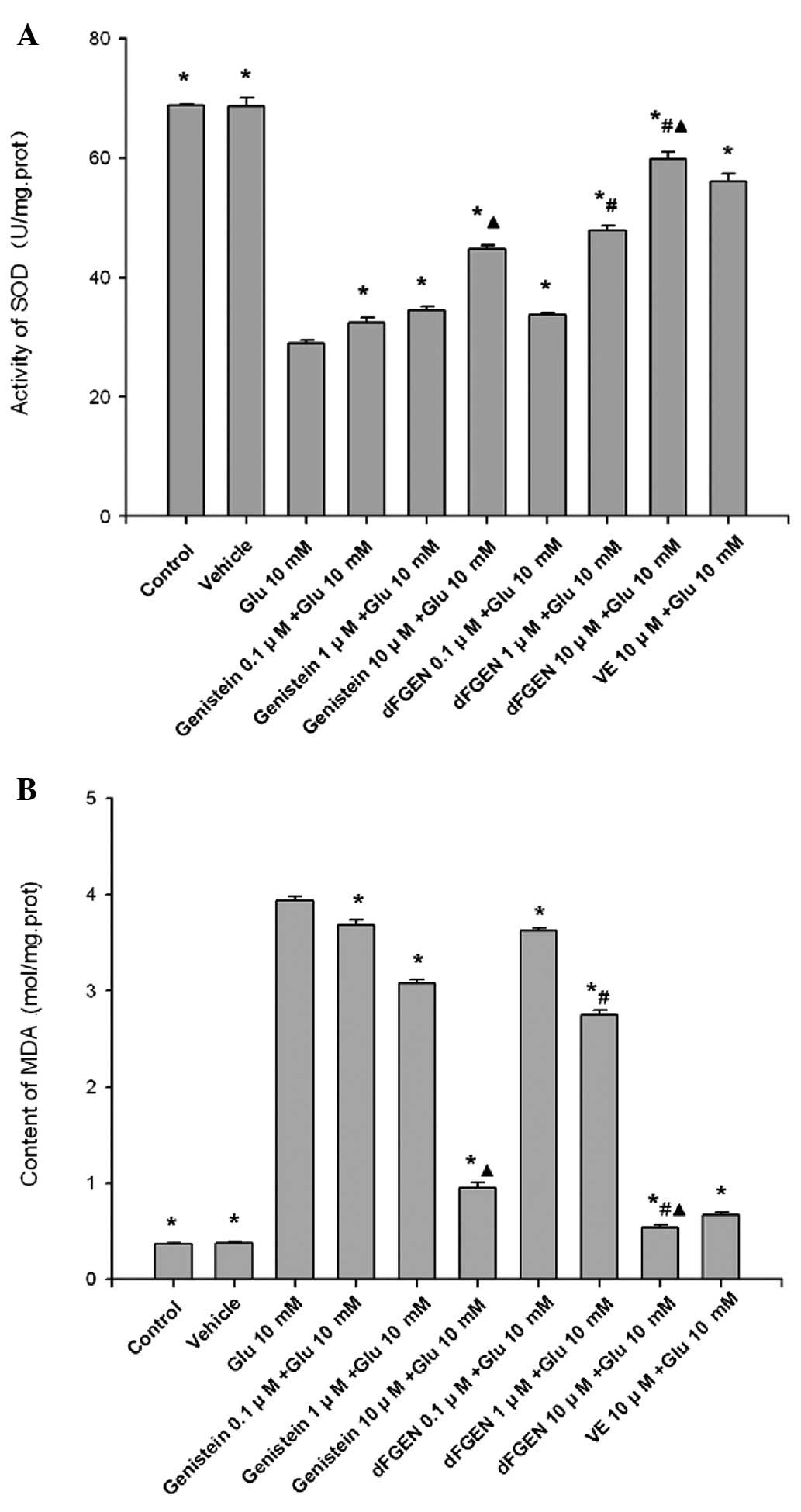
Effects of dFGEN on glutamate-induced
PC12 cell SOD activity and MDA content
As shown in Fig.
8, glutamate treatment for 24 h induced an increase in MDA
formation but decreased SOD enzyme activity in PC12 cells
(p<0.01). While dFGEN significantly attenuated lipid
peroxidation in a concentration-dependent manner. Compared with the
same concentration of genistein (1.0 and 10 μM), dFGEN (1.0 and 10
μM) distinctly improved SOD activity and decreased MDA content
(p<0.01). dFGEN attenuated lipid peroxidation (10 μM) compared
with 10 μM vitamin E (p<0.01).
Discussion
Aging is companied by many neurodegenerative
diseases. A consistent number of neurons undergo apoptosis in the
aged brain, which may lead to neurodegeneration in the long-term
(22). Glutamate is the major
excitatory neurotransmitter in the CNS. In many neurodegenerative
diseases, glutamate causes over-excited glutamate receptors,
causing glutamate excitotoxicity, which can trigger a cascade of
events eventually leading to apoptosis or necrosis (23). Data suggest that glutamate
excitotoxicity is an apoptotic process (24), while some data suggest that the
excitotoxicity death is only due to the mechanism of necrosis
(25). Although mechanism of
glutamate-induced excitotoxicity is not clear, a large number of
data suggest that the mechanism is related to glutamate-induced
oxidative stress (26,27). In this study, we used glutamate
(10 mM) to establish PC12 cell injury model, and then to explore
whether dFGEN have protective effect on damaged nerve cells
compared with genistein and vitamin E.
Mitochondria is considered to be very important
during cell apoptosis. Mitochondrial activity can be measured with
MTT assay. In this study, pretreatment of dFGEN significantly
improved cell survival and reduced the rate of proliferation
inhibition in a concentration-dependent manner, suggesting that
dFGEN could effectively maintain mitochondrial structure and
function. dFGEN antagonized the effect of glutamate on cell
proliferation inhibition rate, which is more effective than
genistein and vitamin E. This study provided the experimental basis
for inhibition of apoptosis and antioxidant function of dFGEN.
Propidium iodide (PI) is a nuclear fluorescent dye
used for staining DNA. PI can not pass through viable cell
membrane, but it can pass through the damaged membrane, used in
cell apoptosis detection, which can be used for detection of cell
apoptosis. Our study showed that dFGEN decreased the cell apoptosis
rate in a concentration-dependent manner. Acridine orange (AO)
staining showed that dFGEN evidently decreased the
glutamate-induced apoptosis of PC12 cells. We proposed that dFGEN
may play a positive role in the process of glutamate-induced PC12
cell apoptosis.
LDH activity is a classic indicator that reflects
cell function. The more LDH release into extracellular space the
greater damage to cells. Schreihofer and Redmond (28) reported that phytoestrogen
genistein can reduce LDH release of cortical cells induced by
glutamate. The results in this study showed that genistein, dFGEN
and vitamin E could reduce LDH release of PC12 cells to different
degrees. Compared with the same concentration of genistein and
vitamin E, dFGEN could distinctly decrease LDH activity of PC12
cells and reduce the degree of cell damage. Our results indicated
that dFGEN has a protective effect better than the lead compound
genistein and vitamin E in a dose-dependent manner.
Oxidative stress is an important factor in a
neurodegenerative disease. The loss of redox system balance will
lead to oxidative damage of the body. SOD activity and MDA content
are the indicators reflecting free radical damage. Our study showed
that dFGEN increase SOD activity and decrease MDA content more than
the same concentrations of genistein and vitamin E. The results
suggested that dFGEN could protect PC12 cells against oxidative
stress by increasing capacity of scavenging the free radicals,
reducing the damage of free radicals on the cells, inhibiting
changes in cell membrane permeability. This might represent one of
the mechanisms for dFGEN against glutamate induced damage.
The process of glutamate-induced PC12 cell injury
involves multiple signal transduction pathways, and the mechanism
is very complex. Numerous studies show that Bcl-2 gene family
(29) and caspase proteinase
family (30) play important roles
in the apoptotic process. Some reports have shown that apoptosis of
PC12 cells may be related to the ERK1/2 and P38 signaling pathway
(31). Our present preliminary
study shows that dFGEN protects PC12 cells against glutamate
toxicity. Its anti-apoptotic mechanism and in vitro
experiments still need further research, so that ultimately our
goal for dFGEN applied to neurodegenerative disease treatment can
be achieved.
In conclusion, dFGEN can improve cell morphology,
improve cell viability, suppress the apoptosis of cells, reduce the
release of LDH, improve SOD activity and decrease MDA content in a
concentration-dependent manner. These data demonstrated that dFGEN
provide better protection against PC12 cell injury caused by
glutamate than the lead compound genistein in a
concentration-dependent manner. The mechanism of protective effect
of dFGEN may be mainly related to its antioxidative activities.
Acknowledgements
This project was supported by
Provincial Science and Technology Plan of Hunan, China
(No2010FJ3017), Research Foundation of Education Bureau of Hunan
Province, China (No11B081), Chinese Traditional Medicine
Administration of Hunan Province, China (No 2009102), Program
Excellent Talent Hunan Normal University (2011).
References
|
1.
|
MR HyndHL ScottPR DoddGlutamate-mediated
excitotoxicity and neurodegeneration in Alzheimer’s diseaseInt J
Neurochem455835952004
|
|
2.
|
AK GhoseT HerbertzRL
HudkinsKnowledge-based, central nervous system (CNS) lead selection
and lead optimization for CNS drug discoveryACS Chem
Neurosci35068201210.1021/cn200100h22267984
|
|
3.
|
LE HebertPA ScherrJL BieniasAlzheimer
disease in the US population:prevalence estimates using the 2000
censusArch
Neurol6011191122200310.1001/archneur.60.8.111912925369
|
|
4.
|
H HampelK BroichEnrichment of MCI and
early Alzheimer’s disease treatment trials using neurochemical and
imaging candidate biomarkersJ Nutr Health Aging133733752009
|
|
5.
|
A KadirN AndreasenO AlmkvistEffect of
phenserine treatment on brain functional activity and amyloid in
Alzheimer’s diseaseAnn Neur63621631200818300284
|
|
6.
|
ED RobersonL Mucke100 years and counting:
prospects for defeating Alzheimer’s
diseaseScience314781784200617082448
|
|
7.
|
JA BobichQ ZhengA CampbellIncubation of
nerve endings with a physiological concentration of Abetal-42
activates CaV2.2 (N-Type)-voltage operated calcium channels and
acutely increases glutamate and noradrenalin releaseJ Alzheimers
Dis62432552004
|
|
8.
|
M LeistP NicoteraApoptosis, excitotoxicity
and neuropathologyExp Cell
Res239183201199810.1006/excr.1997.40269521837
|
|
9.
|
RA DixonD FerreiraMolecules of interest:
genistein phytochemistryPhytochemistry602052112002
|
|
10.
|
GG KuiperJG LemmenB CarlssonInteraction of
estrogenic chemicals and phytoestrogens with estrogen receptor
betaEndocrinology1394252426319989751507
|
|
11.
|
K PolkowskiAP MazurekBiological properties
of genisteinA review of in vitro and in vivo data Acta Pol
Pharm571351552002
|
|
12.
|
X WangS ChenG MaGenistein protects
dopaminergic neurons by inhibiting microglial
activationNeuroreport16267270200510.1097/00001756-200502280-0001315706233
|
|
13.
|
H SiD LiuPhytochemical genistein in the
regulation of vascular function: new insightsCurr Med
Chem1425812589200710.2174/09298670778202332517979711
|
|
14.
|
D ChodonN RamamurtyD
SakthisekaranPreliminary studies on induction of apoptosis by
genistein on HepG2 cell lineToxicol In Vitro21887891207
|
|
15.
|
Y SamuelsK EricsonOncogenic P13K and its
role in cancerCurr Opin
Oncol187782200610.1097/01.cco.0000198021.99347.b9
|
|
16.
|
M MalinowskaFL WilkinsonKJ
Langford-SmithGenistein improves neuropathology and corrects
behaviour in a mouse model of neurodegenerative metabolic
diseasePLoS One5e14192201010.1371/journal.pone.001419221152017
|
|
17.
|
HW LiangSF QiuJ ShenGenistein attenuates
oxidative stress and neuronal damage following transient global
cerebral ischemia in rat hippocampusNeurosci
Lett438116120200810.1016/j.neulet.2008.04.058
|
|
18.
|
PA KroomMN CliffordA CrozierHow should we
assess the effects of exposure to dietary polyphenols in vitroAm J
Clin Nutr801521200415213022
|
|
19.
|
D O’HaganC SchaffrathSL CobbBiochemistry:
biosynthesis of an organofluorine moleculeNature4162792002
|
|
20.
|
XH FuL WangH ZhaoSynthesis of genistein
derivatives and determination of their protective effects against
vascular endothelial cell damages caused by hydrogen peroxideBioorg
Med Chem Lett18513517200810.1016/j.bmcl.2007.11.09718068980
|
|
21.
|
CY WangH XiaYL TuThe protective effect of
7-difluoromethyl-genistein on oxidative stress injury induced by
H2O2 in PC12 cellsJ Hunan Normal Univ (Med
Sci)5462008
|
|
22.
|
RA FloydK HensleyOxidative stress in brain
agingImplications for therapeutics of neurodegenerative diseases
Neurobiol Aging23795807200212392783
|
|
23.
|
J SeyfriedBO EvertC RundfeldtFlupirtine
and retigabine prevent L-glutamate toxicity in rat pheochromocytoma
PC12 cellsEur J
Pharmacol400155166200010.1016/S0014-2999(00)00397-610988329
|
|
24.
|
Ya HiguchiS MarsukawaActive
oxygen-mediated chromosomal 1–2 Mbp gaint DNA fragmentation into
internucleosomal DNA fragmentation in apoptosis glioma cells
induced by glutamateFree Radic Biol Med2441842619989438554
|
|
25.
|
VA TyurinYY TyutinaPJ
QuinnGlutamate-induced cytotoxity in PC12 pheochromocytoma cells:
role of oxidation of phospholipids, glutathione and protein
sulfhydryls revealed by bcl-2 transfectionBrain Res Mol Brain
Res60270281199810.1016/S0169-328X(98)00181-8
|
|
26.
|
R PiW LiNT LeeMinocycline prevents
glutamate-induced apoptosis of cereballar granule neurons by
differential regulation of p38 and Akt pathwaysJ
Neurochem9112191230200410.1111/j.1471-4159.2004.02796.x15569265
|
|
27.
|
SR ParathathI GravanisSE TsirkaNitric
oxide synthase isoforms undertake unique roles during
excitotoxicityStroke3819381945200710.1161/STROKEAHA.106.47882617446423
|
|
28.
|
DA SchreihoferL RedmondSoy phytoestrogens
are neuroprotective against stroke-like injury in
vitroNeuroscience158602609200910.1016/j.neuroscience.2008.10.00318976694
|
|
29.
|
JH JangYL SurhPotentiation of cellular
antioxidant capacity by Bcl-2: implications for its antiapoptotic
functionBiochem
Pharmacol6613711379200310.1016/S0006-2952(03)00487-814555211
|
|
30.
|
BR PikeX ZhaoJK NewcombStretch injury
causes calpain and caspase-3 activation and necrotic and apoptotic
cell death in septo-hippocampal cell culturesJ
Neurotrauma17283298200010.1089/neu.2000.17.28310776913
|
|
31.
|
QJ SuXW ChenZB ChenSG SunInvolvement of
ERK1/2 and p38 MAPK in up-regulation of 14-3-3 protein induced by
hydrogen peroxide preconditioning in PC12 cellsNeurosci
Bull24244250200810.1007/s12264-008-0307-z18668153
|