Introduction
Osteosarcoma is the most common primary malignancy
of bone in children and adolescents. Clinical data show that this
tumor has a poor prognosis, even with the current treatment
including amputation and chemotherapy (1,2).
During the past 30 years, surgery and neo-adjuvant chemotherapy
have been considered as effective treatment approaches for
osteosarcoma and have greatly increased limb salvage rate and
considerably raised the survival to 65–75%. However, approximately
30% of patients develop lung metastasis, which is the leading cause
of mortality (3). Therefore, it
is essential to identify metastasis-associated molecules and to
better understand the mechanism behind the lung metastasis of
osteosarcoma.
microRNAs are a class of small non-coding regulatory
RNA molecules, with a profound impact on various biological
processes (4–6). It has been reported that microRNAs
are aberrantly expressed in most types of cancer where they are
considered to play significant roles by regulating the expression
of various tumor suppressors and oncogenes (7–9).
However, the role of miRNAs in mediating tumor metastasis has only
recently been investigated and still remains largely ambiguous.
miR-183 is a member of a miRNA family (miR-183,
miR-182 and miR-96) that are clustered within 2–4 kb at chromosome
7q32. miRNAs from this locus are dysregulated in a variety of
tumors such as hepatic and colorectal, as well as in leukaemia,
lung, and breast cancer (10–13). Furthermore, it has been shown that
downregulation of miR-183 is associated with lung cancer metastasis
and ectopic overexpression of it inhibits the invasiveness of lung
cancer cells (14). Taken
together, this suggests that miR-183 plays a significant role in
the carcinogenesis or the metastatic cascade, possibly having a
tumor suppressor role.
The aim of this study was to investigate the
potential role of miR-183 in the invasion and metastasis of
osteosarcoma. In this study, we investigated the expression level
and functional pattern of miR-183 in osteosarcoma cells. This was
performed by quantitation of miR-183 in paired high-metastatic
human osteosarcoma F5M2 and low-metastatic human osteosarcoma F4
cells. Functional analysis was then carried out by transfection of
miR-183 mimics or inhibitors into the high-metastasis osteosarcoma
F5M2 cell line with low endogenous miR-183 expression. The results
of the transfection were subsequently assessed on cell viability
patterns, cell migration and alterations in gene expression by
real-time PCR and in protein levels by western blotting and
immunocytochemistry (ICC).
It has been demonstrated that miR-183 regulates the
expression of the Ezrin protein, which is involved in controlling
actin cytoskeleton, cell adhesion and motility. This is consistent
with the cellular function of Ezrin. Taken together, our results
suggest that miR-183 plays a significant regulatory role in
osteosarcoma cell metastasis, indicating that it might be a novel
potential diagnostic and therapeutic target in osteosarcoma.
Materials and methods
Cell culture
A pair of human osteosarcoma cell lines with
different pulmonary metastatic potentials, high-metastatic F5M2 and
low-metastatic F4 cells originating from the human osteosarcoma
cell line SOSP-9607 were established in our laboratory (15). The F4 and F5M2 cell lines were
maintained in complete RPMI-1640 medium (HyClone) supplemented with
10% fetal calf serum (Sijiqing Co., China) at 37°C with 5%
CO2.
Real-time PCR analysis
miR-183, Ezrin mRNA expression was measured by
real-time PCR. Total-RNA was extracted by TRIzol reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA) according to the
manufacturer’s protocol. For miR-183 quantitative real-time PCR,
total-RNA was re-transcribed with a miR-specific primer (RiboBio,
Guangzhou, China) and then quantitative real-time PCR was performed
with a miR-specific primer on the ABI PRISM 7500 real-time PCR
system (Applied Biosciences, USA), compared with normalization
control U6. Quantitative real-time PCR for Ezrin was performed with
primers for Ezrin (forward, 5′-tgggatgctcaaagataatgc-3′ and
reverse, 5′-actccaagc caaaggtctgtt-3′) and the relative expression
level compared with GAPDH was calculated using the comparative Ct
method.
Transwell insert
We used the Transwell insert (24-well insert; pore
size, 8 μm; Corning) to explore the effect of miR-183 on the
migration and invasion of F5M2 and F4 cells. Cells suspended in
RPMI-1640 medium without fetal bovine serum (FBS) were added to the
insert. RPMI-1640 medium with 20% FBS were added to the well out of
the insert. After 48 h, the cells on the lower surface of the
insert were fixed with 95% ethanol and stained with crystal violet.
The invasion assay was performed as the migration assay with the
addition of the inserts precoated with 40 μl BD Matrigel (dilution;
1:3; BD Biosciences, San Jose, CA, USA). Then, 6 random visual
fields of each insert were counted under a microscope (×40).
Wound healing assay
Adhered cell monolayers were scratched with a 20 μl
pipette tip (Eppendorf) and grown in RPMI-1640 medium with 10% FBS
(Sijiqing Co.) at 37°C with 5% CO2. Wound healing
capacity was monitored by microscopy after 0, 12, 24 and 36 h.
Apoptosis test
The cells were stained with FITC-conjugated
anti-Annexin V antibody. The annexin V-FITC apoptosis detection kit
(BD Pharmingen, San Diego, CA, USA) was used to analyze cell
apoptosis with flow cytometry (BD Aria; BD Biosciences).
Western blot analysis and
immunocytochemistry
The Ezrin protein was analyzed by western blot
analysis using Ezrin Rabbit Monoclonal Antibody and anti-β-actin
mouse monoclonal antibody (Epitomics Inc., USA).
Immunocytochemistry (ICC) was performed with Ezrin Rabbit
Monoclonal Antibody (Epitomics Inc.) and Envision™ Detection kit
(Gene Tech, Co., Ltd., Shanghai, China) as standard method.
Transfection
The transfection was performed with Lipofectamine™
2000 Reagent (Invitrogen Life Technologies) according to the
manufacturer’s instructions. miR-183 mimics, and their negative
controls (NCs) and miR-183 inhibitor were purchased from RiboBio. A
low concentration of 20 nM or a high concentration of 50 nM of
mimics were used for each transfection in the migration, invasion
and apoptosis assays, compared with F5M2 cells transfected with NC
or with the miR-183 inhibitors. Efficiency of miR-183 transfection
was measured by real-time PCR.
Statistical analysis
All statistical analyses were performed using SPSS
17.0. All data were expressed as the mean ± SD of at least 3
independent experiments. The differences between groups were
analyzed using the Student’s t-test; P<0.05 was considered to
indicate statistically significant differences.
Results
Expression of miR-183 in F5M2 cells is
lower than in F4 cells
In this study, the F5M2 and F4 cell lines were
selected as the objectives since they originate from the same
maternal cell line of human osteosarcoma cell SOSP-9607, but
display notable differences in metastatic ability (15).
Of the most potential miRNAs, we focused on miR-183
as it is one of the clearly altered miRNAs and is under-expressed
in high-metastatic human pulmonary giant cell carcinoma and
colorectal cancer (14,16). However, the functional role of
miR-183 in these types of cancer remains unclear.
To study the differential expression of miR-183 in
different metastatic potential osteosarcoma cell lines, we employed
real-time PCR to compare miR-183 expression between F5M2 and F4.
Consistent with the results in pulmonary giant cell carcinoma and
colorectal cancer, real-time PCR demonstrated that miR-183
expression in F5M2 was lower than in F4 cells. The difference was
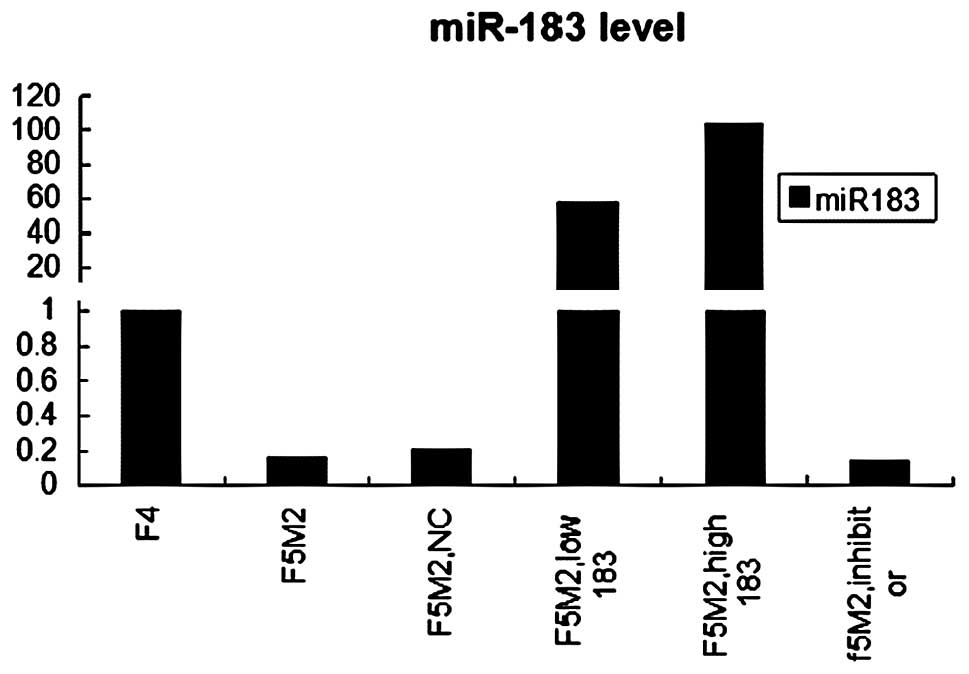
statistically significant (P<0.05) (Fig. 1).
F5M2 significantly overexpresses miR-183
following miR-183 mimic transfection
F5M2 cells were transfected with the miR-183 mimics
at a low concentration of 20 nM or a high concentration of 50 nM.
Control groups included F5M2 cells that were untreated or
transfected with the miR-183 NC or with the miR-183 inhibitors. To
examine the efficiency of the transfection, total-RNA was extracted
and the miR-183 level was measured by real-time PCR 48 h after
transfection.
Real-time PCR showed that miR-183 was significantly
over-expressed in F5M2 cells after transfection with the miR-183
mimics, compared with the untreated or treated with mimics NC or
the miR-183 inhibitor groups (P<0.05) (Fig. 1). It also showed that miR-183
levels in F5M2 transfection with 50 nM mimics was significantly
higher than that of F5M2 20 nM mimics. Real-time PCR demonstrated
that the transfection was effective; a higher concentration of
miR-183 mimics led to a higher expression of the miR-183.
miR-183 significantly decreases the
migratory and invasive ability of F5M2
In our study, F5M2 cells displayed significantly
higher migratory and invasive abilities than F4 cells in
vitro in a transwell insert experiment, which is in accordance
with their metastatic potential (15).
To investigate the possible role of miR-183 in
osteosarcoma cell metastasis, we examined the impact on cell
motility and invasive ability after ectopic expression of miR-183
in F5M2 cells, which had been verified under expression of
endogenous miR-183.
To examine the migration ability, Transwell insert
tests without Matrigel were employed. Cells that penetrated this
membrane and reached the underside of the Transwell were counted
after 48 h of incubation. The results showed that ectopic
expression of miR-183 repressed chemotaxis of F5M2 cells
significantly, compared with the NC groups or the untransfected
F5M2 cells (Fig. 2A and B)
(P<0.05).
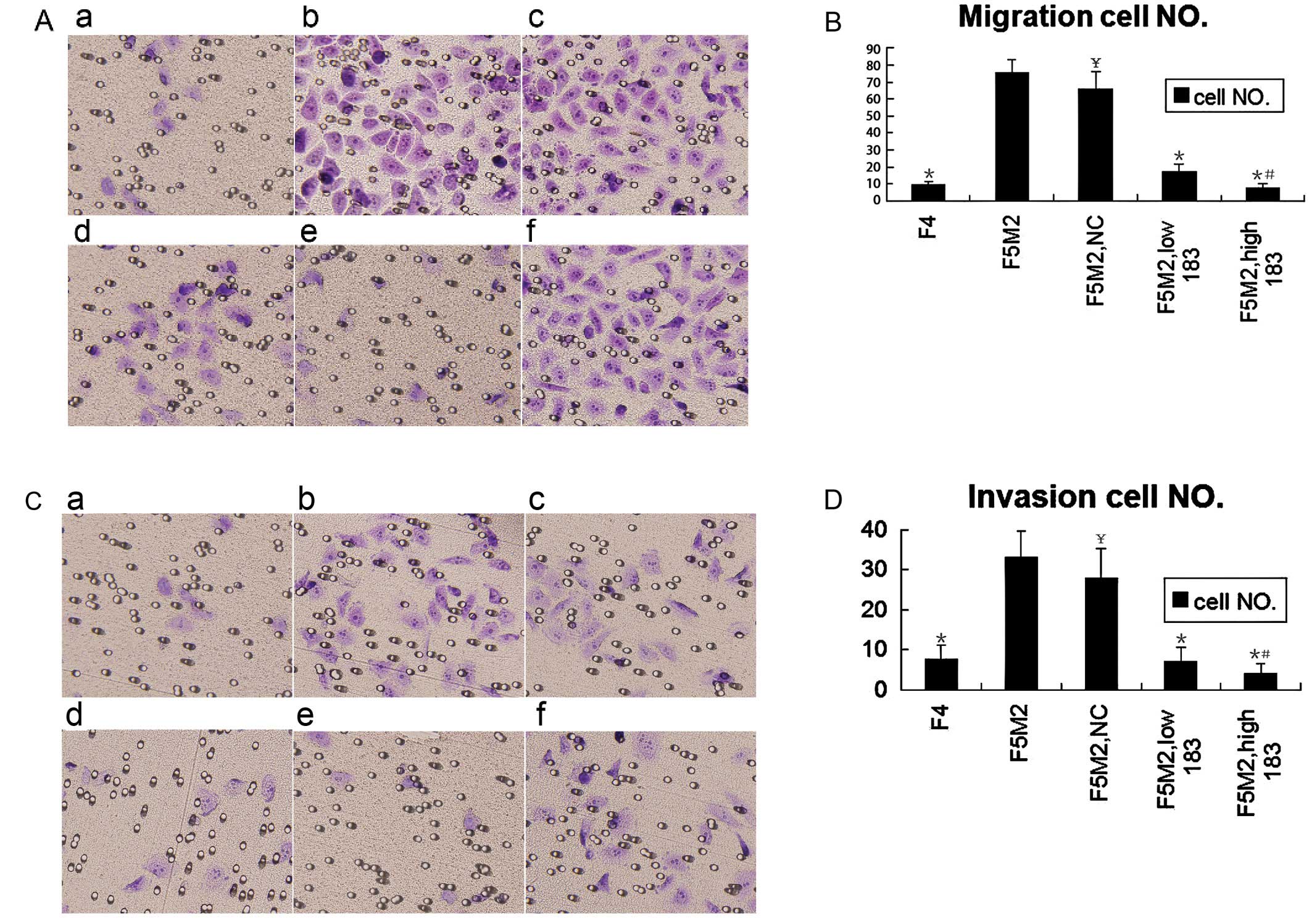 | Figure 2.Transwell trials showed that miR-183
regulated F5M2 migration and invasion negatively in vitro.
(A) Following transfection with or without miR-183 or NC, F5M2
cells suspended in RPMI-1640 medium without fetal bovine serum
(FBS) were added to the insert. RPMI 1640 medium with 20% FBS were
added to the well out of the insert. After 48 h, the cells on the
lower surface of the insert were fixed with 95% ethanol and stained
with crystal violet. a, F4; b, F5M2; c, F5M2 with NC; d, F5M2 with
low miR-183; e, F5M2 with high miR-183; f, F5M2 with miR-183
inhibitor. (B) The migratory cell number of F4 or F5M2 transfected
with miR-183 mimics was lower than that of F5M2 cells untreated or
treated with NC. (C) The invasion assay was performed as the
migration assay with the addition of the inserts coated with BD
Matrigel. a, F4; b, F5M2; c, F5M2 with NC; d, F5M2 with low
miR-183; e, F5M2 with high miR-183; f, F5M2 with miR-183 inhibitor.
(D) The invasive cell number of F4 or F5M2 transfected with miR-183
mimics was lower than that of F5M2 cells untreated or treated with
NC. *P<0.05, compared with F5M2.
#P<0.05, compared high miR-183 with low miR-183.
¥P>0.05, compared with F5M2. |
To examine the invasion ability, Transwell insert
tests with a layer of Matrigel on top of the insert were employed.
Cells that penetrated both the Matrigel and membrane were recorded
following incubation for 48 h. It showed that miR-183 in F5M2 cells
significantly inhibited their invasion (Fig. 2C and D) (P<0.05), which was
consistent with the results of migration. Taken together, our
results demonstrate that miR-183 inhibited F5M2 cell migration and
invasion potential in vitro.
Our findings also reveal that the cell motility
ability of F5M2 transfection with high concentration mimics was
significantly weaker than that of F5M2 with low concentration
mimics in both the migration and invasion assay.
miR-183 significantly decreases the wound
healing capacity of F5M2 cells
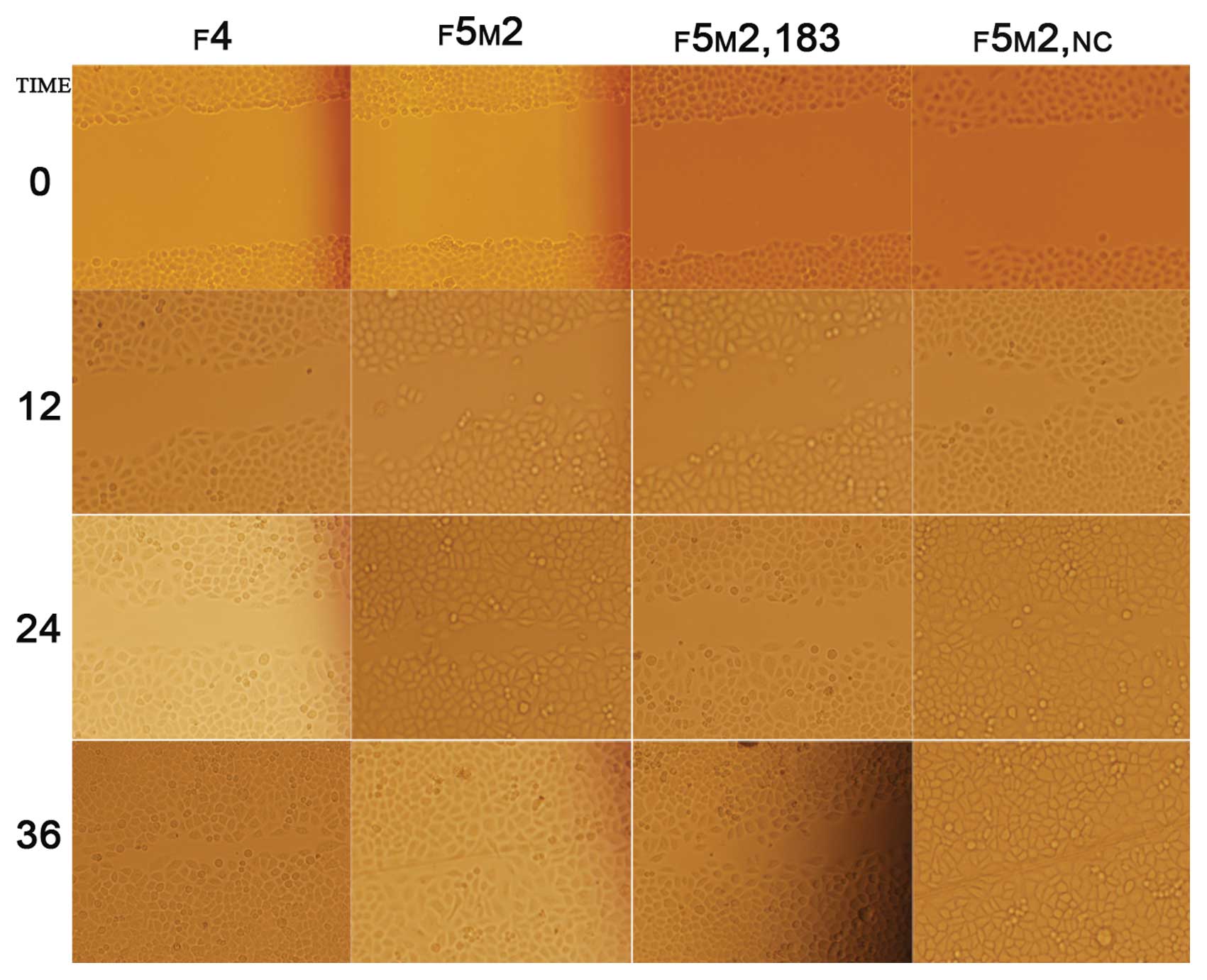
We employed the scratch wound cell model to compare
the polarized migration of F5M2 and F4 cells. The results revealed
that F5M2 cells closed the scratch wounds faster than F4 cells
(Fig. 3) (P<0.05). This model
showed that ectogenic miR-183 significantly decreased the wound
healing capacity of F5M2 cells when compared with those cells
untreated or transfected with NC (Fig. 3 and Table I). It also showed that inhibition
of miR-183 was concentration-dependent.
 | Table I.Wound closure rate of F4 or F5M2
transfected with miR-183 mimics was significantly lower than that
of F5M2 cells untreated or treated with NC (P<0.05). There was
no significant difference between F5M2 cells untreated or treated
with NC (P>0.05). Wound closure rate was defined as closed
width/0 h width (means ± SD). |
Table I.
Wound closure rate of F4 or F5M2
transfected with miR-183 mimics was significantly lower than that
of F5M2 cells untreated or treated with NC (P<0.05). There was
no significant difference between F5M2 cells untreated or treated
with NC (P>0.05). Wound closure rate was defined as closed
width/0 h width (means ± SD).
| Time | 0 h | 12 h | 24 h | 36 h |
|---|
| F4 | 0 | 0.44±0.08 | 0.60±0.02a | 0.90±0.03a |
| F5M2 | 0 | 0.42±0.11 | 0.82±0.06 | 0.97±0.03 |
| F5M2, with 183 | 0 | 0.41±0.07 | 0.60±0.08a | 0.77±0.11a |
| F5M2, NC | 0 | 0.45±0.12b | 0.85±0.06b | 0.97±0.03b |
miR-183 does not affect apoptosis in F5M2
cells
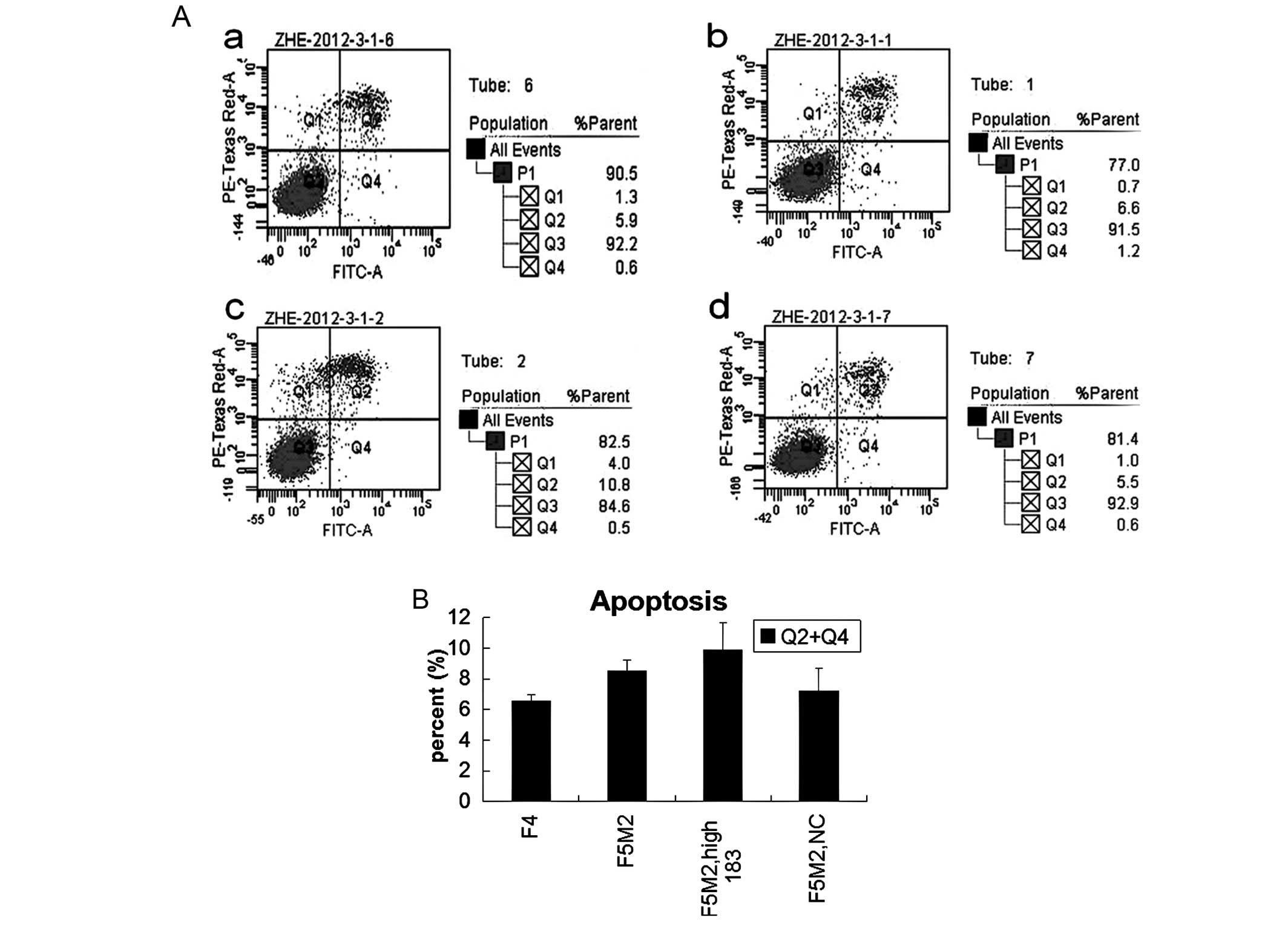
The results of apoptosis with flow cytometry showed
that there was no statistically significant difference either
between F5M2 and F4 cells, or between F5M2 cells transfected with
miR-183 mimics and NC or untreated (Fig. 4). Thus, miR-183 has little effect
on cell apoptosis and viability.
miR-183 inhibits the expression of
Ezrin
We used 3 miRNA target prediction programs
(TargetScan, PicTar and miRanda) to predict the targets of miR-183.
Markedly, all 3 programs predicted that VIL2/Ezrin, was the target
of miR-183. Therefore, we speculated that miR-183 might alter F5M2
cell migration and invasion by regulating the expression of Ezrin.
To verify this speculation, we examined the expression level of
Ezrin by western blotting and ICC.
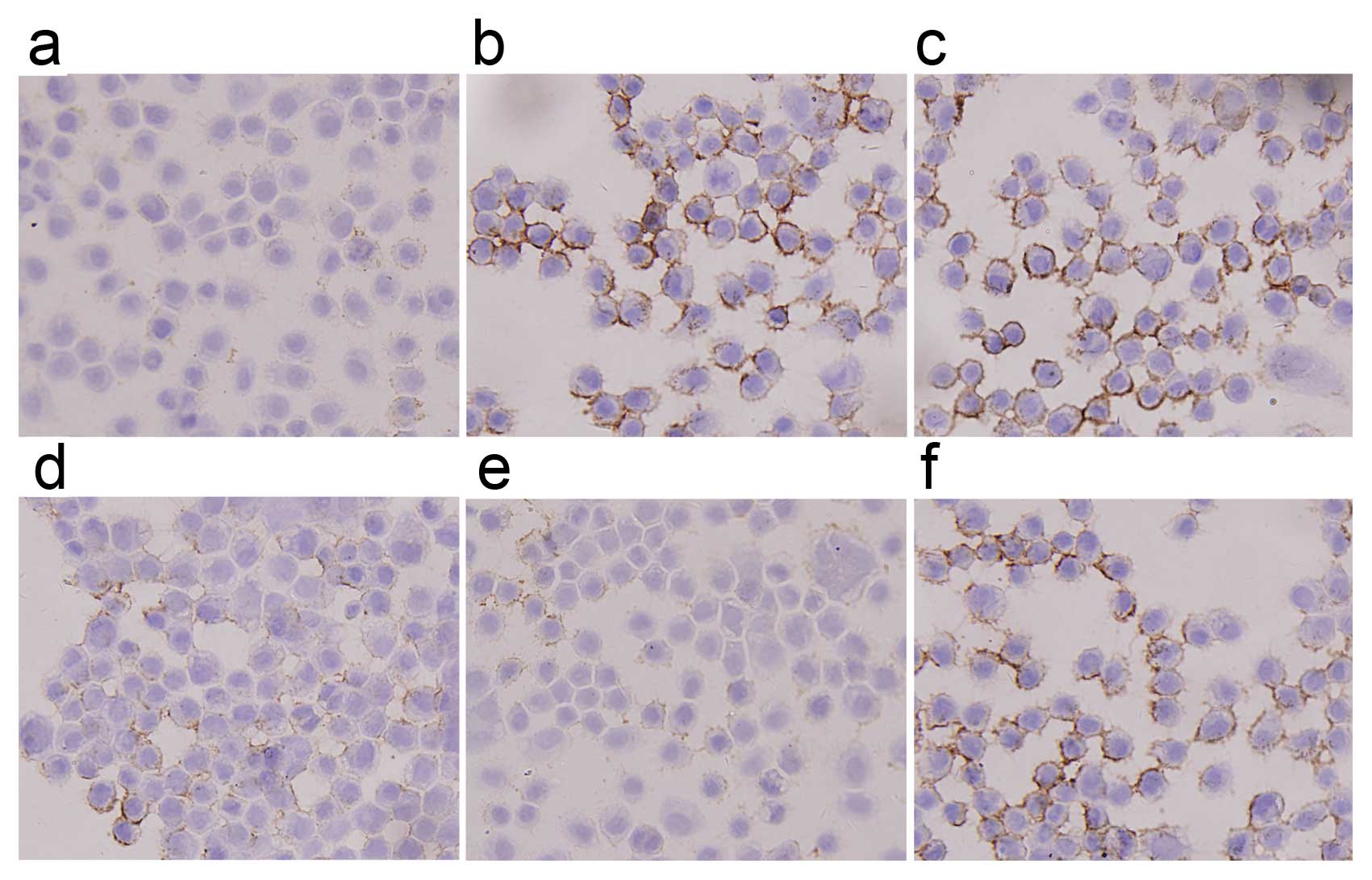
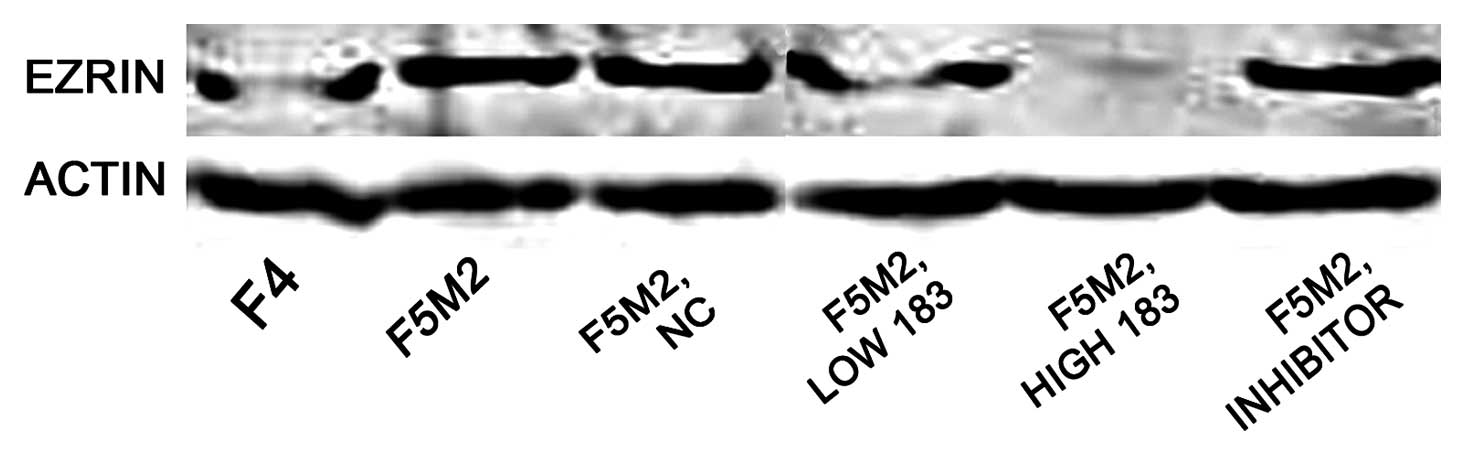
ICC analysis revealed that staining intensity of
Ezrin in F5M2 cells was stronger than in F4 cells and that it
decreased greatly after transfection with miR-183 mimics (Fig. 5). Western blotting also showed
that the expression level of Ezrin in F5M2 cells was significantly
higher than that in F4 cells. Expression of Ezrin in F5M2 cells
decreased markedly after transfection with miR-183 mimics, compared
with cells untreated or treated with NC (Fig. 6). Both in western blotting and
ICC, the expression of Ezrin took on the same tendency; F5M2 cells
treated with 50 nM miR-183 mimics expressed less Ezrin than F5M2
cells treated with 20 nM miR-183 mimics (P<0.05). There was an
inverse correlation between Ezrin production and miR-183
levels.
Discussion
miR-183 family members have been shown to be
upregulated in colorectal and hepatic tumors, as well as in
leukaemia and breast cancer (10–13). By contrast, miR-183 has been shown
to be downregulated and inversely correlated with invasive and
metastatic ability in pulmonary giant cell cancer (14) and breast cancer (17). Previous studies have demonstrated
that the expression profiling of miR-183 was tissue-specific and
that it might have divergent functions depending on the tumor
tissue or cell type. Previous studies have reported that miRNA
repression of mRNA is dependent on the conditions of specific
cellular targets (18).
To identify the potential role of miR-183 in
osteosarcoma metastasis, we compared miR-183 expression levels in
F5M2 and F4 cells, which are high and low metastatic cell lines of
osteosarcoma SOSP9607, respectively. We employed multiple
approaches to evaluate the inhibitory role of miR-183 in the
motility and invasion of F5M2 cells. Consistent with the results in
pulmonary giant cell carcinoma and colorectal cancer, real-time PCR
demonstrated that miR-183 expression in F5M2 was lower than in F4
cells. Following transfection with miR-183 mimics, the results
indicated that overexpression of miR-183 mainly inhibited the
migration and invasion of F5M2 cells. Therefore it is possible that
miR-183 exerts a suppressing effect on osteosarcoma metastasis, not
apoptosis.
It has been reported that several miRNAs, such as
miR-335, miR-126, let-7 family, miR-100, miR-218, miR-125, miR-375,
miR-142 and miR-198, appear to be metastasis suppressors. Reduced
expression of miR-335 and miR-126 were found in breast cancer
characterized by poor metastatic-free survival (19), while expression of miR-let7c,
miR-100 and miR-218 were significantly decreased in metastatic
prostate cancer compared with localized prostate cancer (20). Moreover, ectopic enforced
expression of miR-125 impaired cell migration and invasion in a
breast cancer cell line and reduction of miR-125 expression
enhanced migration of cells (21,22). Ectopic expression of miR-375
induced changes in cell morphology and inhibited melanoma cell
invasion and wound healing, strongly suggesting a functional role
of miR-375 in cytoskeletal architecture and migration (23). In hepatocellular carcinoma cell
lines, the overexpression of miR-142-3p was suppressed, while
blocking of miR-142-3p increased migration and invasion. This
demonstrates that miR-142-3p expression was downregulated in HCC
cells and that miR-142-3p inhibited HCC cell migration and invasion
by targeting RAC1 (24). miR-198
was downregulated in hepatocellular carcinoma and forced expression
of miR-198 inhibited HCC cell migration and invasion in a c-MET
dependent manner (25).
The functional study of miR-183 in malignancy was
previously reported in lung and breast cancer cells. Wang et
al (14) postulated that
miR-183 was a potential metastasis inhibitor in lung cancer and
reported that upregulation of miR-183 inhibited migration of cancer
cells. They demonstrated that miR-183 induced dysregulation of
genes related to migration and invasion, including Ezrin. Li et
al (26) demonstrated that
upregulation of miR-183 repressed migration and invasion in HeLa
cells, however, this was shown to be mediated via direct targeting
of ITGB1. It was indicated that miR-183 was likely to have a number
of targets through which it regulated biological functions on
cancer cells.
Identification of cancer-specific miRNAs and their
targets is pivotal for understanding their role in tumorigenesis
and might be important for discovering novel therapeutic targets.
To investigate the suppressing mechanism of miR-183 in the
metastasis of osteosarcoma, we employed 3 miRNA target prediction
programs (TargetScan, PicTar and miRanda) to identify the direct
targets of miR-183 (27).
Markedly, all the programs predicted that Ezrin was one of the
targets of miR-183. Ezrin, which contained the corresponding
binding site of miR-183 in Ezrin 3′UTR, could be regulated by
miR-183. Several previous studies have demonstrated that miR-183
regulates Ezrin expression in lung cancer cells and Ezrin
expression has been associated with tumor invasion and metastasis
(14,28). Our study revealed that Ezrin
expression was inversely correlated with miR-183, which was
consistent with this hypothesis. Our findings also demonstrated
that Ezrin levels were positively correlated with osteosarcoma
invasion and metastasis. It is reasonable to conclude that
alteration of miR-183 might regulate cell migration and invasion
targeting the downregulation of Ezrin expression.
Ezrin (also known as cytovillin or villin2) belongs
to the ERM family that acts as membrane organizers and linkers
between cytoskeleton and plasma membrane (29). Ezrin is a component of cell
surface structures that are involved in cell-extracellular matrix
interactions as well as in cell-cell interactions (30,31). High expression of the Ezrin
protein has been reported to correlate with the metastatic
potential of several malignant tumors (32,33).
Ezrin is an interesting molecular marker in
osteosarcoma. It has been reported that Ezrin is required for
metastasis and recurrence of osteosarcoma (32). Khanna et al (34) suggested that Ezrin is a molecule
significantly involved in the metastasis of human osteosarcoma and
there was a significant association beetween high Ezrin expression
and poor outcome in pediatric osteosarcoma. They also reported that
a high expression of Ezrin was necessary for metastasis in a mouse
osteosarcoma model and high expression of Ezrin was also associated
with early pulmonary metastasis in dog osteosarcoma (35,36). Wang et al (37) found that expression change of
Ezrin was a positive prognostic factor for overall survival and
event-free survival in a recent clinical trial. They also concluded
that downregulation of Ezrin might be a potential new therapeutic
strategy for the treatment of osteosarcoma.
To verify the inhibition of miR-183 on Ezrin
expression, we conducted a trial to transfect miR-183 into F5M2
cells, which expressed more Ezrin protein and presented more
pulmonary metastatic potential than the paired F4 cells. Notably,
following transfection with miR-183 mimics, Ezrin expression in
F5M2 cell was almost eradicated either by western blotting or by
ICC. By contrast, F5M2 cells following transfection with NC or
miR-183 inhibitors changed weakly on Ezrin expression. Our study
also showed that ectopic overexpression of miR-183 repressed the
motility and invasion of F5M2 cells significantly and acted on the
apoptosis or proliferation of F5M2 cells weakly. Taken together,
this suggests that miR-183 expression was inversely related to
migration and invasion of osteosarcoma cells. Ezrin expression was
positively correlated to migration and invasion of osteosarcoma
cells. By downregulating the Ezrin expression level, miR-183 might
act as a significant inhibitory factor in the progression of
osteosarcoma cells.
There have recently been reports regarding miRNAs,
identifying miR-20a, miR-222, miR-223, miR-195 and miR-219 as
tumor-associated miRNAs, further supporting the hypothesis that
miRNAs are involved in tumor metastasis. miR-20a could promote
migration and invasion of cervical cancer cells through the direct
upregulation of TNKS2, which induced colony formation, migration
and invasion of cervical cancer cells (38). Overexpression of miR-223 inhibited
proliferation of the cells greatly via the regulation of FOXO1
expression (39). miR-219-5p
exerted tumor-suppressive effects in hepatic carcinogenesis through
negative regulation of GPC3 expression in vitro (40).
To our knowledge, this is the first in vitro
study to regulate metastasis and progression of osteosarcoma, by
upregulation of miR-183 to target the expression of Ezrin in F5M2
cells. The findings of this study illustrate that by downregulating
the Ezrin expression level, miR-183 plays a suppressing role in
cell migration and invasion of osteosarcoma. Our study might
provide an important avenue for further analysis in vivo
with the aim to develop a new potential diagnostic and therapeutic
target for the screening and treatment of high metastatic
osteosarcoma. Further studies are required to fully understand the
regulation mechanisms of miR-183 and Ezrin in osteosarcoma in
vitro and in vivo.
References
|
1.
|
SM BentzenHS PoulsenS KaaeOM JensenH
JohansenHT MouridsenPrognostic factors in osteosarcomas. A
regression
analysisCancer62194202198810.1002/1097-0142(19880701)62:1%3C194::AID-CNCR2820620129%3E3.0.CO;2-83164231
|
|
2.
|
AM DavisRS BellPJ GoodwinPrognostic
factors in osteosarcoma: a critical reviewJ Clin
Oncol1242343119948113851
|
|
3.
|
HJ MankinFJ HornicekAE RosenbergDC
HarmonMC GebhardtSurvival data for 648 patients with osteosarcoma
treated at one institutionClin Orthop Relat
Res429286291200410.1097/01.blo.0000145991.65770.e615577500
|
|
4.
|
V AmbrosRC LeeIdentification of microRNAs
and other tiny noncoding RNAs by cDNA cloningMethods Mol
Biol265131158200415103073
|
|
5.
|
RS PillaiSN BhattacharyyaW
FilipowiczRepression of protein synthesis by miRNAs: how many
mechanisms?Trends Cell
Biol17118126200710.1016/j.tcb.2006.12.00717197185
|
|
6.
|
DP BartelMicroRNAs: genomics, biogenesis,
mechanism and
functionCell116281297200410.1016/S0092-8674(04)00045-514744438
|
|
7.
|
C CaldasJD BrentonSizing up miRNAs as
cancer genesNat Med11712714200510.1038/nm0705-71216015356
|
|
8.
|
GA CalinCM CroceMicroRNA signatures in
human cancersNat Rev Cancer6857866200610.1038/nrc199717060945
|
|
9.
|
B KefasJ GodlewskiL ComeauY LiR AbounaderM
HawkinsonmicroRNA-7 inhibits the epidermal growth factor receptor
and the Akt pathway and is downregulated in glioblastomaCancer
Res6835663572200810.1158/0008-5472.CAN-07-663918483236
|
|
10.
|
Y LadeiroG CouchyC BalabaudP Bioulac-SageL
PelletierJ Zucman-RossiMicroRNA profiling in hepatocellular tumors
is associated with clinical features and oncogene/tumor suppressor
gene mutationsHepatology4719551963200810.1002/hep.2225618433021
|
|
11.
|
K MotoyamaH InoueY TakatsunoOver- and
under-expressed microRNAs in human colorectal cancerInt J
Oncol3410691075200919287964
|
|
12.
|
X AgirreA Jimenez-VelascoE San
Jose-EnerizL GarateE BandresDown-regulation of hsa-miR-10a in
chronic myeloid leukemia CD34+ cells increases
USF2-mediated cell growthMol Cancer
Res618301840200810.1158/1541-7786.MCR-08-016719074828
|
|
13.
|
MD MattieCC BenzJ BowersK
SensingerOptimized high-throughput microRNA expression profiling
provides novel biomarker assessment of clinical prostate and breast
cancer biopsiesMol Cancer524200610.1186/1476-4598-5-24
|
|
14.
|
G WangW MaoS ZhengMicroRNA-183 regulates
Ezrin expression in lung cancer cellsFEBS
Lett58236633668200810.1016/j.febslet.2008.09.05118840437
|
|
15.
|
X ChenTT YangW WangHH SunEstablishment and
characterization of human osteosarcoma cell lines with different
pulmonary metastatic
potentialsCytotechnology613744200910.1007/s10616-009-9239-320016965
|
|
16.
|
E BandresE CubedoX AgirreR MalumbresR
ZarateIdentification by real-time PCR of 13 mature microRNAs
differentially expressed in colorectal cancer and non-tumoral
tissuesMol Cancer529200610.1186/1476-4598-5-2916854228
|
|
17.
|
AJ LoweryN MillerRM DwyerMJ
KerinDysregulated miR-183 inhibits migration in breast cancer
cellsBMC Cancer10502201010.1186/1471-2407-10-50220858276
|
|
18.
|
JG DoenchPA SharpSpecificity of microRNA
target selection in translational repressionGenes
Dev18504511200410.1101/gad.118440415014042
|
|
19.
|
SF TavazoieC AlarconT OskarssonEndogenous
human microRNAs that suppress breast cancer
metastasisNature451147152200810.1038/nature0648718185580
|
|
20.
|
KR LeiteJM Sousa-CanavezST ReisAH
TomiyamaLH Camara-LopesA SañudoAA AntunesM SrouqiChange in
expression of miR-let7c, miR-100 and miR-218 from high grade
localized prostate cancer to metastasisUrol
Oncol29265269201110.1016/j.urolonc.2009.02.00219372056
|
|
21.
|
GK ScottA GogaD BhaumikCE BergerCS
SullivanCC BenzCoordinate suppression of ERBB2 and ERBB3 by
enforced expression of micro-RNA, miR-125a or miR-125bJ Biol
Chem28214791486200710.1074/jbc.M60938320017110380
|
|
22.
|
MS KumarJ LuKL MercerTR GolubT
JacksImpaired microRNA processing enhances cellular transformation
and tumorigenesisNat Genet39673677200710.1038/ng200317401365
|
|
23.
|
J MazarD DeBlasioSS GovindarajanS ZhangRJ
PereraEpigenetic regulation of microRNA-375 and its role in
melanoma development in humansFEBS
Lett58524672476201110.1016/j.febslet.2011.06.02521723283
|
|
24.
|
L WuC CaiX WangM LiuX LiH
TangMicroRNA-142-3p, a new regulator of RAC1, suppresses the
migration and invasion of hepatocellular carcinoma cellsFEBS
Lett58513221330201110.1016/j.febslet.2011.03.06721482222
|
|
25.
|
S TanR LiK DingPE LobieT ZhumiR-198
inhibits migration and invasion of hepatocellular carcinoma cells
by targeting the HGF/c-MET pathwayFEBS
Lett58522292234201110.1016/j.febslet.2011.05.04221658389
|
|
26.
|
G LiC LunaJ QiuDL EpsteinP
GonzalezTargeting of integrin beta1 and kinesin 2alpha by microRNA
183J Biol Chem28554615471201010.1074/jbc.M109.03712719940135
|
|
27.
|
A KrekD GrunMN PoyR WolfL RosenbergEJ
EpsteinP MacMenaminI da PiedadeKC GunsalusM StoffelN
RajewskyCombinatorial microRNA target predictionsNat
Genet37495500200510.1038/ng1536
|
|
28.
|
Y YuJ KhanC KhannaL HelmanPS MeltzerG
MerlinomExpression profiling identifies the cytoskeletal organizer
ezrin and the developmental homeoprotein Six21 as key metastatic
regulatorsNat Med10175181200410.1038/nm96614704789
|
|
29.
|
P MangeatC RoyM MartinERM proteins in cell
adhesion and membrane dynamicsTrends Cell
Biol9187192199910.1016/S0962-8924(99)01544-510322453
|
|
30.
|
A BretscherK EdwardsRG FehonERM proteins
and merlin: integrators at the cell cortexNat Rev Mol Cell
Biol3586599200210.1038/nrm88212154370
|
|
31.
|
JW WangSY PengJT LiY WangZP ZhangY ChengDQ
ChengWH WengXS WuXZ FeiIdentification of metastasis-associated
proteins involved in gallbladder carcinoma metastasis by proteomic
analysis and functional exploration of chloride intracellular
channel 1Cancer Lett2817181200910.1016/j.canlet.2009.02.020
|
|
32.
|
KW HunterEzrin, a key component in tumor
metastasisTrends Mol
Med10201204200410.1016/j.molmed.2004.03.00115121044
|
|
33.
|
WH WengJ AhlenK AstromWO LuiC
LarssonPrognostic impact of immunohistochemical expression of ezrin
in highly malignant soft tissue sarcomasClin Cancer
Res1161986204200510.1158/1078-0432.CCR-05-054816144921
|
|
34.
|
C KhannaX WanS BoseR CassadayO OlomuA
MendozaC YeungR GorlickSM HewittLJ HelmanThe membrane cytoskeleton
linker ezrin is necessary for osteosarcoma metastasisNat
Med10182186200410.1038/nm98214704791
|
|
35.
|
MS KimWS SongWH ChoSY LeeDG JeonEzrin
expression predicts survival in stage IIB osteosarcomasClin Orthop
Relat Res459229236200710.1097/BLO.0b013e3180413dbf17353802
|
|
36.
|
S Xu-DongS ZanZ Shui-ErT Li-NaY Wen-XiL
FengY YangExpression of ezrin correlates with lung metastasis in
Chinese patients with osteosarcomaClin Invest
Med32E180E188200919331807
|
|
37.
|
YF WangJN ShenExpression change of ezrin
as a prognostic factor in primary osteosarcomaMed
Oncol28S636S643201110.1007/s12032-010-9684-z20859706
|
|
38.
|
HW KangF WangmiR-20a promotes migration
and invasion by regulating TNKS2 in human cervical cancer cellsFEBS
Lett586897904201210.1016/j.febslet.2012.02.02022449978
|
|
39.
|
L WuH LiCY JiaW ChengMicroRNA-223
regulates FOXO1 expression and cell proliferationFEBS
Lett58610381043201210.1016/j.febslet.2012.02.05022569260
|
|
40.
|
N HuangJ LinJ RuanN SuMiR-219-5p inhibits
hepatocellular carcinoma cell proliferation by targeting
glypican-3FEBS
Lett586884891201210.1016/j.febslet.2012.02.01722449976
|