Introduction
Crystallins are the major structural proteins of the
vertebrate eye lens. There are 2 superfamilies: α- and
βγ-crystallins (1), which account
for approximately 90% of total soluble proteins. β-crystallins have
been reported to function as stress proteins, playing a crucial
role in maintaining lens transparency, a high refractive index and
solubility of the adult lens (2).
βB2-crystallin (Crybb2 in mice) is the most abundant and the
most thermally stable β-crystallin of the lens, and is resistant to
modification (2–4).
The Crybb2 gene in mice is located on
chromosome 5 within a cluster containing an additional 3
Cryb genes consisting of 4 Greek key motifs, which is the
common character of all members of the β- and γ-crystallin
superfamilies (2). Certain
studies have reported that α-crystallins are capable of functioning
as molecular chaperones (5,6),
while βγ-crystallins play a structural role in the mammalian eye
lens. However, certain other studies have proposed that
βγ-crystallins play unknown and unconceived non-crystallin roles
(7), and that Crybb2 is expressed
in some extralenticular tissues, such as the retina, brain and
testis (4). Previous studies have
shown that Crybb2 is localized in the retinal ganglion cells (RGCs)
(8) and that the protein is
secreted and is responsible for neurite outgrowth during retinal
regeneration (9).
In our previous study, mice with a targeted deletion
of the Crybb2 gene were used to investigate the role of
Crybb2 in mice suffering from cataract (10). In our present study, we found that
the fertility of male mice with Crybb2 deficiency was
reduced. Crybb2 has also been implicated in the subfertility of
mice exhibiting mutant Crybb2 (11); however, the actual mechanism
remains elusive. In this study, we discovered that Crybb2 was
mainly expressed in the spermatogonia from the testes of mice with
normal fertility. The proliferation of Crybb2−/−
mouse germ cells was enhanced significantly, and apoptosis was also
increased compared with the wild-type (WT) mice. In addition, Bcl-2
and Ca2+-calmodulin-dependent protein kinase IV (CaMKIV)
levels were decreased in Crybb2−/− mouse testis.
A previous study has shown that the function of CaMKIV is
regulating Bcl-2 levels and rescuing proliferation defects
(12). Our data reveal that the
disordered proliferation and apoptosis of
Crybb2−/− germ cells may result from the
decreased expression of Bcl-2, possibly due to reduced CaMKIV from
the loss of Crybb2.
Materials and methods
Animals and mouse models
Using Crybb2 target vector construction,
Crybb2−/− mice were generated by deleting the
first and second exons of Crybb2 and the 2 known transcription
initiation sites (13).
Genotyping was performed as described previously (13). Both Crybb2−/−
and WT mice were of C57BL/C genetic background, and were housed and
maintained in the Laboratory Animal Center of the Second Military
Medical University (Shanghai, China) under a 12-h light/dark cycle.
Food and water were provided ad libitum, and complete care
was given in compliance within the National Institutes of Health
and the institutional guidelines on the use of laboratory and
experimental animals.
Fertility test
Fertility was assessed by setting up natural matings
between a single male and female. The number of litters and the
number of pups/litter produced by each pair were calculated over a
3-month period. Fecundity was calculated as the total number of
pups produced/mating/30-day period.
Histological and immunofluorescence
analysis of testis
The testes isolated from 4-week-old mice were fixed
in fresh 4% paraformaldehyde for 24 h, washed in 70% ethanol, and
decalcified for 72 h. Glycol methacrylate infiltration and
embedding were performed using a JB-4 embedding kit (Polysciences,
Warrington, PA, USA). Sections of 4 μm were prepared and stained
with hematoxylin and eosin (H&E).
For immunofluorescence analysis of Crybb2 expression
in the mouse testis, paraffin sections were blocked with 2% BSA for
1 h, followed by incubation in anti-Crybb2 antibody (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) at 4°C overnight. The
samples were then washed and incubated with FITC-conjugated
anti-goat IgG (Jackson Immuno Research Laboratories, Inc.) and
analyzed under a Nikon TE2000 microscope.
Quantitative real-time RT-PCR
analysis
For qRT-PCR analysis, a 4-week-old male mouse testis
was removed and the tissue was homogenized with an electric
homogenizer. Total-RNA was isolated by using the TRIzol reagent
kit, and reverse transcription was performed using the PrimeScript
RT reagent kit (Takara Bio, Inc.), according to manufacturer’s
directions. Quantitative real-time PCR-based gene expression
analysis was performed on a Real-Time PCR machine (7300; Applied
Biosystems, USA) using a standard SYBR-Green PCR kit. Reactions
were conducted at 95°C for 2 min, followed by 40 cycles of 95°C for
15 sec, and 60°C for 30 sec, The relative expression of each target
gene compared with β-actin was calculated using the
2−ΔΔct method. Primers used were as follows: mouse
β-actin (5′-AGCCATGTACGTAGCCATCC-3′ and
5′-CTCTCAGCTGTGGTGGTGAA-3′); Crybb2 (5′-CAG ACACAGGCGGGCAAGCC-3′
and 5′-CTCGTAGCCCACCCAGGGTCC-3′); CaMKIV
(5′-TGGAGTCAGAGCTGGGACGGG-3′ and 5′-TTCGGGTGTGAGAGACGCAGGAG-3′);
Bcl-2 (5′-GGATAACGGAGGCTGGGATGCCT-3′ and
5′-CAGAGTGATGCAGGCCCCGAC-3′); Bax (5′-CAGGATGCGTCCACCAAGAA-3′ and
5′-GTTGAAGTTGCCATCAGCAAACA-3′).
Western blot analysis
For the whole protein extracts, the testicular
tissue was homogenized in lysis buffer (Promega, Madison, WI, USA),
then abraded with an electric homogenizer and centrifuged at 12,000
x g for 15 min. All buffers received a protease inhibitor cocktail
(Konchem, China) prior to use.
The protein concentration of each sample was
determined. Equal amounts of protein were loaded and separated
discontinuously on 12% sodium dodecyl sulfate-polyacrylamide gels
(SDS-PAGE), and subsequently transferred onto a nitrocellulose
membrane (Amersham Pharmacia, UK). The membrane was then incubated
in TBST blocking solution (Tris-buffered saline including 0.1%
Tween-20) containing 5% skim milk for 2 h at room temperature,
followed by incubation with primary antibodies containing
anti-CaMKIV (Abcam), anti-Crybb2 (Santa Cruz Biotechnology, Inc.),
anti-Bax, anti-Bcl-2 (Cell Signaling), anti-β-actin (Beyotime,
Jiangsu, China) at 4°C overnight. After washing, the membrane was
reacted with secondary antibodies, HRP-conjugated anti-mouse,
anti-rabbit or anti-goat secondary antibodies for 2 h. After
several washes, the immunoblot was detected with enhanced
chemiluminescence (Pierce Biotechnology), which was performed
according to the manufacturer’s instructions.
Proliferation analysis by
bromodeoxyuridine (BrdU) assay
For in vivo BrdU labeling assays, 3 mice in
each group were injected intraperitoneally with 100 μg/g BrdU
(Sigma) 2 h before sacrifice. The testis samples were then excised
quickly and fixed with 4% paraformaldehyde overnight at 4°C. After
fixation, 4-μm sections were prepared and washed in 0.1 M PBS
containing 1% Triton X-100. The testis was then treated with 2 N
HCl for 20 min at 37°C. After neutralization in 0.1 M borate
buffer, the testis was washed in PBST 3 times and blocked by PBST
with 5% normal goat serum for 1 h and stained with anti-BrdU
antibody.
Apoptosis analysis by TUNEL and Annexin V
assay
Terminal deoxynucleotidyl-transferase-mediated dUTP
nick end-labeling (TUNEL) assays were carried out using the
DeadEnd™ Colorimetric TUNEL System kit (Promega) following the
manufacturer’s instructions. The apoptotic index of TUNEL-positive
cells was calculated using the total number of positive cells/field
of sight at 5 random locations in each testis under light
microscopy (x200).
The percentage of apoptotic cells in the mouse germ
cells was also quantitated using the Annexin V fluorescein (FITC)
kit (Bender MedSystem, Vienna, Austria) according to the
manufacturer’s instructions. Stained cells were analyzed by flow
cytometry within 30 min.
Statistical analysis
For all the analyses, measurements obtained from the
groups were expressed as the means ± SD for all parameters
determined. Statistical analysis was performed using an unpaired
Student’s t-test followed by Tukey’s test. P-values <0.05 were
considered to indicated statistically significant differences.
Results
Patterns of Crybb2 expression in
Crybb2−/− and WT C57BL/C mouse testis
To investigate the role of Crybb2, Crybb2
knockout mice were produced with the assistance of the Ingenious
Targeting Laboratory, Inc. (Stony Brook, NY, USA) (13). To investigate the Crybb2
expression in WT mouse testis and verify that Crybb2 was not
produced by the knockout mice, RNA samples were first extracted
from the Crybb2−/− and WT mouse testis, and the
Crybb2 mRNA expression was analyzed by semi-quantitative
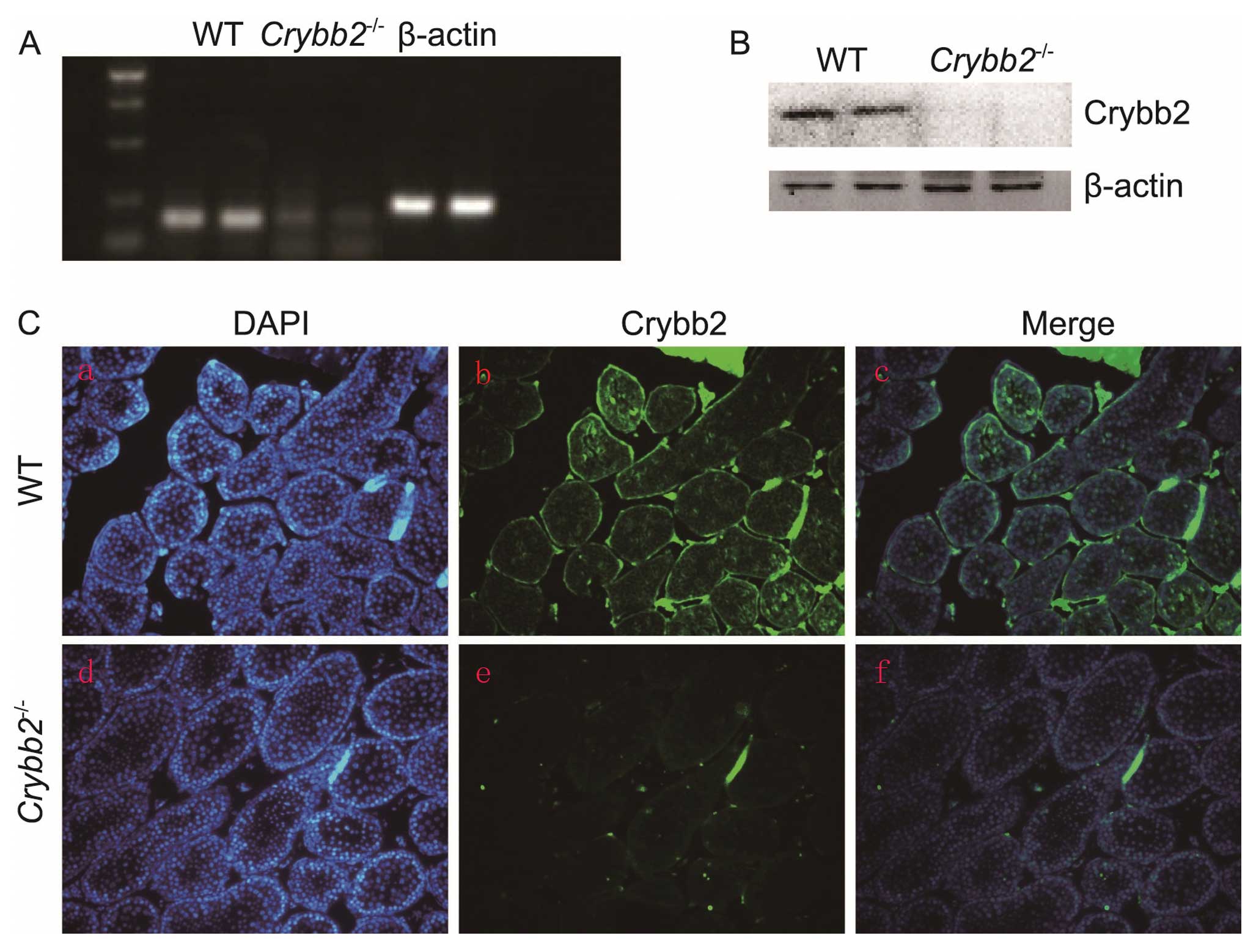
RT-PCR using Crybb2 specific primers. The results indicated
that the Crybb2 gene expression was not detected in the
testis of the knockout mice (Fig.
1A). Crybb2 protein expression in the testis was then analyzed
by western blot analysis. The Crybb2 protein was not produced in
the Crybb2−/− testis while it was expressed in WT
mouse testis (Fig. 1B). The
results were further confirmed by immunofluorescence using a
polyclonal antibody raised against Crybb2 (Fig. 1C), showing that Crybb2 was mainly
expressed in the spermatogonia of WT mouse seminiferous tubules,
which was consistent with the results of a previous study (11). Additionally, Crybb2 expression was
not detected in the Crybb2−/− mouse testis. These
data suggest that the intended alteration of Crybb2 was
successful in eliminating Crybb2 from the testis.
Subfertility of male Crybb2−/−
mice and abnormal development of testis
During the study, we intended to generate a large
number of Crybb2−/− mice for various pathological
and physiological studies by interbreeding between
Crybb2−/− mice. Surprisingly, the reproductive
performance of male Crybb2−/− mice was inferior
to that of male WT mice. To further understand this unexpected
observation, the reproductive performance of the male mice was
closely observed (Table I). Five
couples of WT mice gave birth to 13 litters (mean litter size,
9.1±1.4) in a 3-month period, only 7 litters (mean litter size,
4.8±1.5) were yielded from an equal number of
Crybb2−/− male and normal female mice.
 | Table I.Number of progeny from breeding male
Crybb2 knockout mice. |
Table I.
Number of progeny from breeding male
Crybb2 knockout mice.
| Male | Female | No. of litters | Total no. of
pups | Litter sizea |
|---|
| +/+ | +/+ | 13 | 114 | 9.1±1.4 |
| −/− | +/+ | 7 | 33 | 4.8±1.5b |
To further understand this unexpected observation,
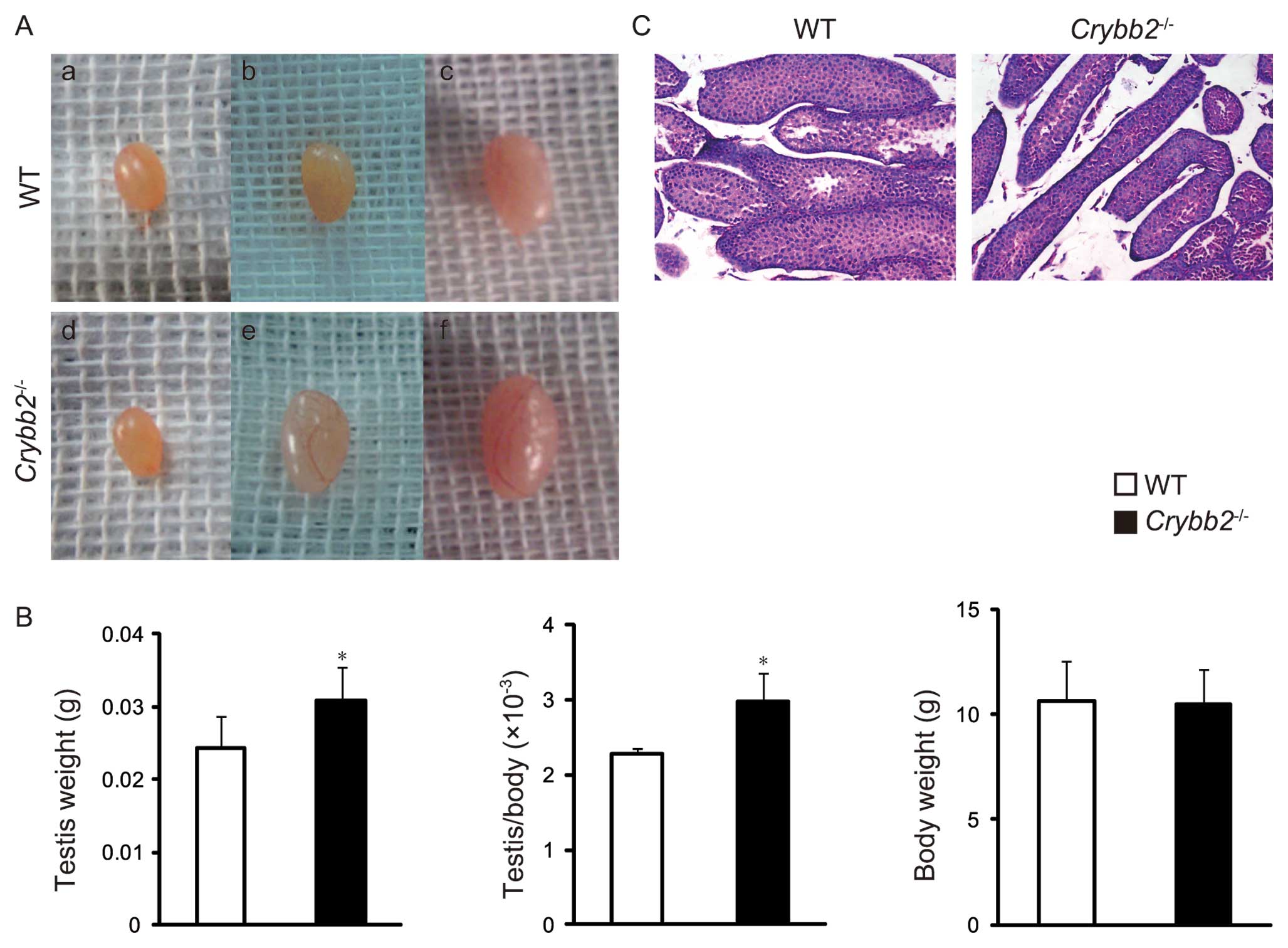
the testis morphology of the male mice were closely followed for 4
weeks postpartum. It was found that the testes of the 4-week-old
Crybb2−/− mice were significantly larger compared
to the age-matched WT mice, while no significant difference was
observed between them within 3 weeks postpartum (Fig. 2A). Accordingly, the testis weight
was increased in 4-week-old Crybb2−/− mice and
the organ mass of testis was also higher, while there was no
significant difference in body weight (Fig. 2B). We then performed histological
analysis of the testis and found that the seminiferous tubules in
Crybb2−/− mouse testis were thinner and scattered
compared with those of the WT mouse testis (Fig. 2C).
Enhanced proliferation and apoptosis of
germ cells in Crybb2−/− mouse testis
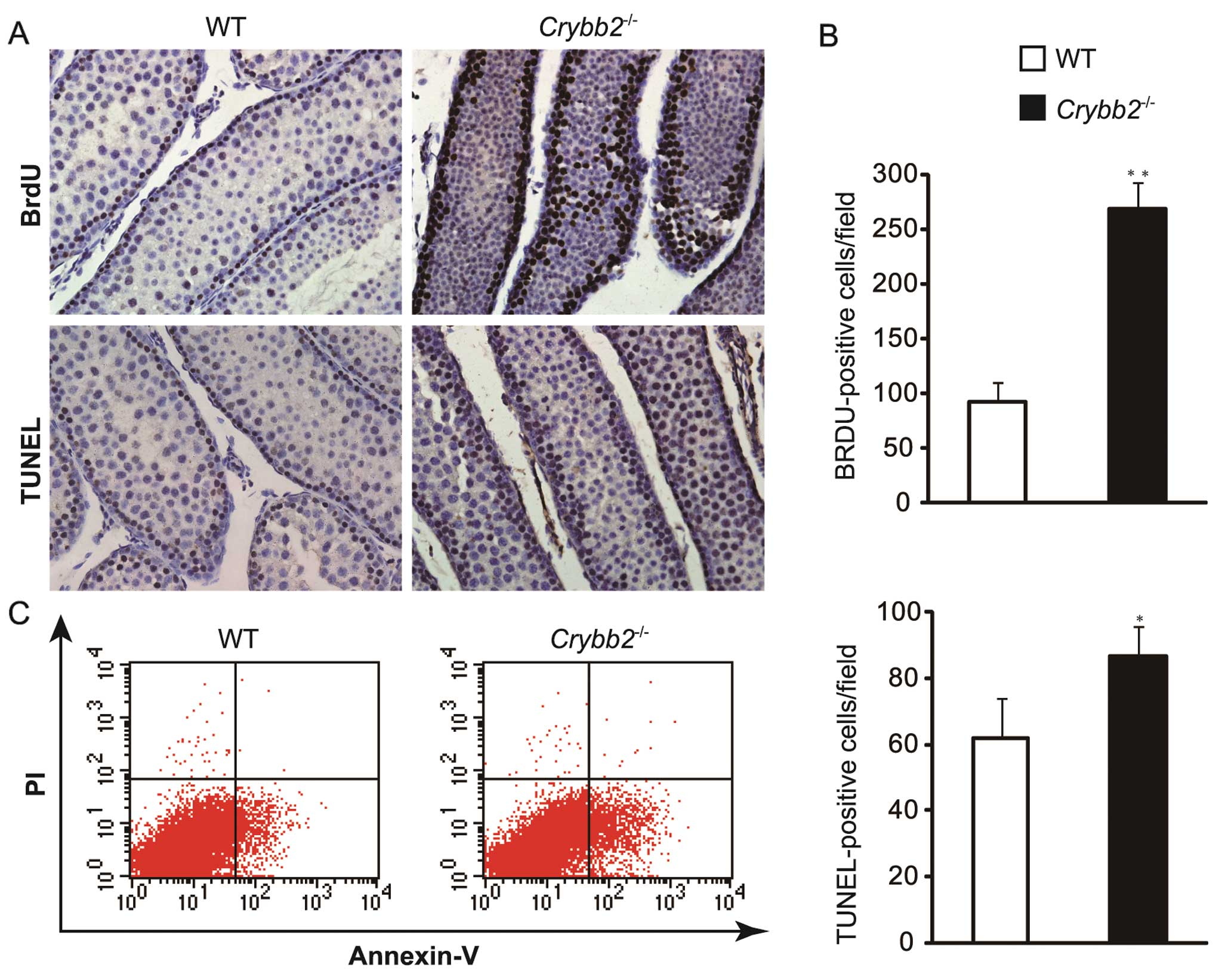
To investigate the reasons for the increased testis
size and weight in Crybb2−/− mice, the testis
sections were examined by BrdU assay. The results showed that the
number of BrdU-positive cells increased significantly in
Crybb2−/− mouse testis compared with those in WT
mouse testis (Fig. 3A and B)
(P<0.01), indicating that the proliferation of mutant cells was
increased.
The testis sections were also examined by TUNEL
assay based on the specific binding of TdT to 3′-OH ends of DNA.
Compared with WT mice, apoptotic cells were increased in
Crybb2−/− mouse testis (Fig. 3A and B) (P<0.05). In addition,
the apoptotic rate of Crybb2−/− and WT mouse
testis was analyzed by flow cytometry, while the Annexin
V-positive: propidium iodide (PI)-negative population included
cells that were in the early stages of apoptosis. The results also
indicated that there were more dead cells in
Crybb2−/− testis as compared with WT mouse testis
(Fig. 3C).
Reduction of Bcl-2 and CaMKIV in the
testis of Crybb2−/− mice
Knowing that Bcl-2 may inhibit both apoptosis and
proliferation, and that the balance of Bcl-2 family and the
pro-apoptotic Bax protein is important for normal spermatogenesis,
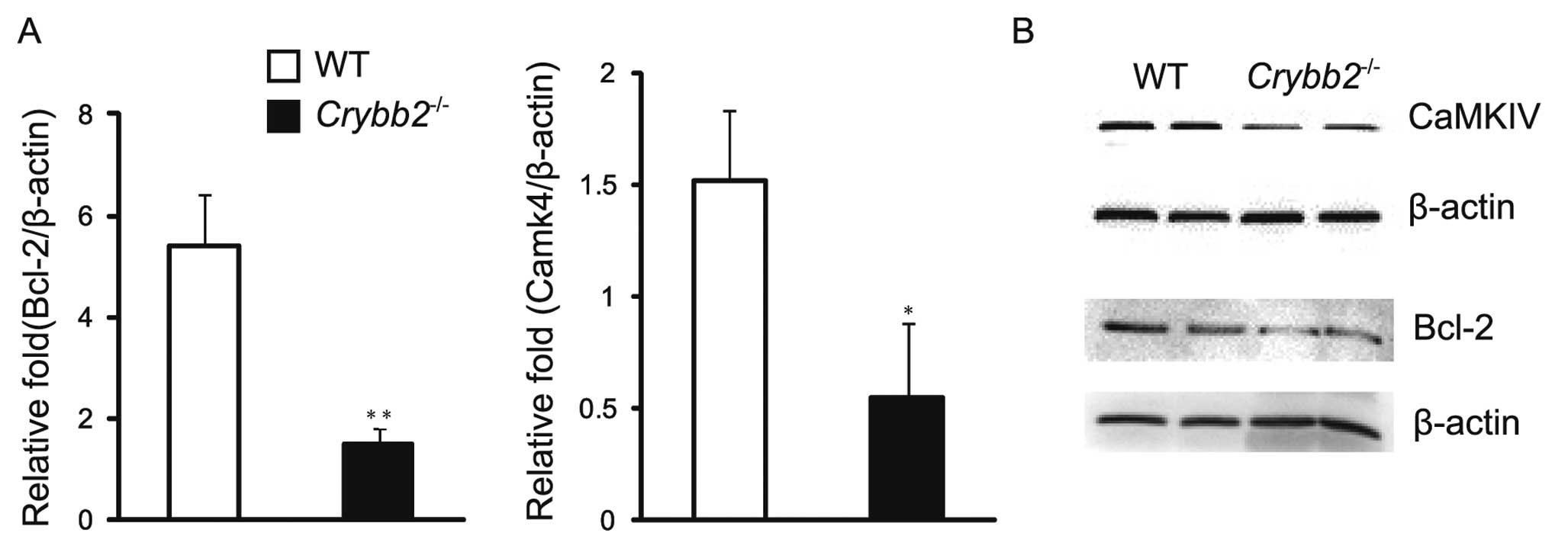
the levels of Bcl-2 mRNA and protein were investigated in the mouse
testis. The results indicated that the Bcl-2 mRNA level was higher
in the Crybb2−/− testis compared to those in the
WT mouse testis (P<0.05) (Fig.
4A). In addition, the protein level of Bcl-2 was also higher in
Crybb2−/− testis (Fig. 4B). It was also found that Bax
protein levels were increased in Crybb2−/− mouse
testis compared with those in WT mouse testis (data not shown),
while the Bax mRNA level was also higher, although there was no
significant difference between them (P>0.05) (data not shown).
The results indicated that the specific value of Bax/Bcl-2 in
Crybb2−/− testis was abnormal, and that the lower
level of Bcl-2 may be the reason for the excessive proliferation
and apoptosis of Crybb2−/− germ cells.
CaMKIV is a multifunctional serine/threonine
(Ser/Thr) protein kinase, expressed primarily in the brain, thymus,
testis, ovary, bone marrow and adrenal glands (14). It has been reported that mice
absent in CaMKIV are more prone to infertility (15), and that CaMKIV may regulate the
normal proliferation of the cells, whose effect may be in part
mediated via the regulation of Bcl-2 (12,16). Therefore, we analyzed the
expression patterns of CaMKIV mRNA and protein in the mouse testis
to further clarify the possible mechanism of the poor reproductive
performance of Crybb2−/− males. The results
indicated that both CaMKIV mRNA (Fig.
4A) and protein levels were decreased compared to WT mouse
testis (Fig. 4B).
Discussion
Since the discovery of Crybb2 in extralenticular
tissues (4), a number of studies
have reported on the function of Crybb2 in these tissues (8,9,11).
It was discovered in our study that male mice lacking Crybb2 had
reduced fertility, which was apparently associated with Crybb2
deficiency, as its expression was detected in WT mouse testis.
Immunofluorescence assay showed that Crybb2 was mainly expressed in
the spermatogonia of seminiferous tubules in testis but it was not
detected in Crybb2−/− mouse testis by qRT-PCR,
western blot analysis and immunofluorescence analysis, suggesting
that the intended alteration of Crybb2 was successful in
eliminating Crybb2 from the testis.
Crybb2 begins to be expressed after birth in
rodents; therefore, it does not contribute to the development of
the fetal testis. Our finding that Crybb2−/−
testis developed normally for weeks after birth conforms to this
later function of Crybb2. We found that the testis size and weight
of 4-week-old Crybb2−/− mice were markedly
increased, and the seminiferous tubules in
Crybb2−/− mice testes were thinner and scattered
compared with those of WT mice. However, these changes were not
observed within 3 weeks postpartum.
To determine the cause of the change in
Crybb2−/− testis morphology, the proliferation
and apoptosis of testicular germ cells were detected by BrdU and
TUNEL assays. It was found that the proliferation and apoptosis of
germ cells were markedly increased in Crybb2−/−
testes, compared with those in WT mice. The Crybb2 gene was
mainly expressed in the spermatogonia of the mouse testis, which
perhaps reflected the early defect in spermatogonial proliferation
and apoptosis, and finally resulted in the overall reduction in
spermatogenesis, thus contributing to the subfertility of the male
mice. Considering the restricted tissue distribution of Crybb2, a
change in the expression level of Crybb2 may be a therapeutic
option for defective spermatogenesis, during which germ cell
proliferation and apoptosis play a significant role (17).
A balance of anti-apoptotic members of the Bcl-2
family and the pro-apoptotic Bax protein is extremely important for
normal spermatogenesis in the regulation of germ cell survival
(18,19). Any absence of Bcl-2, Bcl-x, Bcl6
and Bax may cause defective fertility as a result of subfertility
or infertility (20–22). In addition to its role in cell
survival, Bcl-2 has also been reported to play a role in
maintaining cellular quiescence (23,24). However, our results indicated that
both Bcl-2 mRNA and protein levels were decreased, which may be the
reason for the hyperproliferative phenotype of the germ cells due
to the reduced function of inhibiting proliferation and apoptosis
of Bcl-2.
Calcium (Ca2+) is a pervasive
intracellular second messenger that initiates signaling cascades,
leading to essential biological processes, such as secretion, cell
proliferation, differentiation and migratory movement (25). However, many of the
Ca2+ effects are mediated via Ca2+-induced
activation of the ubiquitous Ca2+ receptor calmodulin
(CaM) (26). In turn,
Ca2+/CaM stimulates the increase of certain enzymes
including those that comprise the family of multifunctional,
Ser/Thr kinases (CaMKs), one of which is CaMKIV (27) and it is also expressed in the
spermatogonia of the male mouse testis (28). In our study, we found that the
level of CaMKIV was decreased in Crybb2−/−
testis, which may be the reason for Crybb2−/−
subfertility, as CaMKIV deficiency may result in the infertility of
male mice as a profound impairment to spermiogenesis (14). It has been discussed that Crybb2
is a Ca2+-binding protein (29), proposing the 4-Greek key
crystallin fold as a Ca2+-binding motif (30). We speculate that Crybb2 may not be
regulated by Ca2+ due to the loss of the Crybb2 function
of Ca2+ binding, resulting in the change of
Ca2+ signaling, which further induces a decrease in the
CaMKIV level.
However, several studies have shown that the
transcription of the pro-survival Bcl-2 gene may be stimulated by
Ca2+ (31,32). CaMKIV plays an important role in
supporting the survival of dendritic cells by regulating the
expression of Bcl-2 (15). In
addition, re-expression of CaMKIV can restore the Bcl-2 levels and
rescue both the hyperproliferation and the rapid exhaustion
phenotypes characteristic of Camk4−/− KLS cells
(11). These results indicate
that the decreased level of CaMKIV of Crybb2−/−
mice, may affect the expression of Bcl-2, which further disturbs
the proliferation and apoptosis of germ cells in
Crybb2−/− testis.
Collectively, our data indicate that a correlation
exists between the presence of Crybb2, Ca2+, CaMKIV and
Bcl-2 in testicular germ cells. Clarification of the potential role
of Crybb2 in regulating the CaMKIV expression may provide new
insights into the mechanism of male fertility.
Acknowledgements
This study was supported by grants
from the National Natural Science Foundation of China (no.
81170834) and the Shanghai Municipal Science and Technology
Commission of China (no. 11ZR1447500).
References
|
1.
|
GJ WistowJ PiatigorskyLens crystallins:
the evolution and expression of proteins for a highly specialized
tissueAnnu Rev
Biochem57479504198810.1146/annurev.bi.57.070188.0024033052280
|
|
2.
|
KS MagaboJ HorwitzJ PiatigorskyM
KantorowExpression of betaB(2)-crystallin mRNA and protein in
retina, brain, and testisInvest Ophthalmol Vis
Sci4130563060200010967064
|
|
3.
|
J FengDL SmithJB SmithHuman lens
beta-crystallin solubilityJ Biol
Chem2751158511590200010.1074/jbc.275.16.1158510766773
|
|
4.
|
Z ZhangLL DavidDL SmithJB SmithResistance
of human betaB2-crystallin to in vivo modificationExp Eye
Res73203211200110.1006/exer.2001.102311446770
|
|
5.
|
J PeschekN BraunTM FranzmannThe eye lens
chaperone alpha-crystallin forms defined globular assembliesProc
Natl Acad Sci
USA1061327213277200910.1073/pnas.090265110619651604
|
|
6.
|
PA KumarMS KumarGB ReddyEffect of
glycation on alpha-crystallin structure and chaperone-like
functionBiochem J408251258200710.1042/BJ2007098917696877
|
|
7.
|
SP BhatTransparency and non-refractive
functions of crystallins - a proposalExp Eye
Res79809816200410.1016/j.exer.2004.08.02015642317
|
|
8.
|
N PiriM SongJM KwongJ CaprioliModulation
of alpha and beta crystallin expression in rat retinas with ocular
hypertension-induced ganglion cell degenerationBrain
Res114119200710.1016/j.brainres.2006.11.09517316577
|
|
9.
|
T LiedtkeJC SchwambornU SchroerS
ThanosElongation of axons during regeneration involves retinal
crystallin beta b2 (crybb2)Mol Cell
Proteomics6895907200710.1074/mcp.M600245-MCP20017264069
|
|
10.
|
J ZhangJ LiC HuangTargeted knockout of the
mouse betaB2-crystallin gene (Crybb2) induces age-related
cataractInvest Ophthalmol Vis
Sci4954765483200810.1167/iovs.08-217918719080
|
|
11.
|
KM DupreyKM RobinsonY WangJR TaubeMK
DuncanSubfertility in mice harboring a mutation in
betaB2-crystallinMol Vis13366373200717392687
|
|
12.
|
CM KitsosU SankarM
IllarioCalmodulin-dependent protein kinase IV regulates
hematopoietic stem cell maintenanceJ Biol
Chem2803310133108200510.1074/jbc.M50520820016020540
|
|
13.
|
J ZhangCG HuangWJ LiW WengJQ
WangEstablishment of a βB2 crystallin gene knockout mice modelAcad
J Sec Mil Med Univ27124612482006
|
|
14.
|
SL WangTJ RibarAR MeansExpression of
Ca(2+)/calmodulin-dependent protein kinase IV (caMKIV) messenger
RNA during murine embryogenesisCell Growth Differ123513612001
|
|
15.
|
JY WuTJ RibarDE CummingsKA BurtonGS
McKnightAR MeansSpermiogenesis and exchange of basic nuclear
proteins are impaired in male germ cells lacking Camk4Nat
Genet25448452200010.1038/7815310932193
|
|
16.
|
M IllarioML Giardino-TorchiaU
SankarCalmodulin-dependent kinase IV links Toll-like receptor 4
signaling with survival pathway of activated dendritic
cellsBlood111723731200810.1182/blood-2007-05-09117317909078
|
|
17.
|
A CorrieroA MedinaCC MylonasProliferation
and apoptosis of male germ cells in captive Atlantic bluefin tuna
(Thunnus thynnus L.) treated with gonadotropin-releasing
hormone agonist (GnRHa)Anim Reprod
Sci116346357200910.1016/j.anireprosci.2009.02.01319304415
|
|
18.
|
RJ AitkenJK FindlayKJ HuttJB KerrApoptosis
in the germ lineReproduction141139150201110.1530/REP-10-0232
|
|
19.
|
C ShahaR TripathiDP MishraMale germ cell
apoptosis: regulation and biologyPhilos Trans R Soc Lond B Biol
Sci36515011515201010.1098/rstb.2009.012420403866
|
|
20.
|
MM MatzukDJ LambGenetic dissection of
mammalian fertility pathwaysNat Cell
Biol4S41S49200210.1038/ncb-nm-fertilityS4112479614
|
|
21.
|
MC HuNC HsuNB El HadjSteroid deficiency
syndromes in mice with targeted disruption of Cyp11a1Mol
Endocrinol1619431950200210.1210/me.2002-005512145347
|
|
22.
|
KM RobertsonL O’DonnellME JonesImpairment
of spermatogenesis in mice lacking a functional aromatase (cyp 19)
geneProc Natl Acad Sci
USA9679867991199910.1073/pnas.96.14.798610393934
|
|
23.
|
C GreiderA ChattopadhyayC ParkhurstE
YangBCL-x(L) and BCL2 delay Myc-induced cell cycle entry through
elevation of p27 and inhibition of G1 cyclin-dependent
kinasesOncogene2177657775200210.1038/sj.onc.120592812420213
|
|
24.
|
YM JanumyanCG SansamA
ChattopadhyayBcl-xL/Bcl-2 coordinately regulates apoptosis, cell
cycle arrest and cell cycle entryEMBO
J2254595470200310.1093/emboj/cdg53314532118
|
|
25.
|
MJ BerridgeMD BootmanHL RoderickCalcium
signalling: dynamics, homeostasis and remodellingNat Rev Mol Cell
Biol4517529200310.1038/nrm115512838335
|
|
26.
|
D ChinAR MeansCalmodulin: a prototypical
calcium sensorTrends Cell
Biol10322328200010.1016/S0962-8924(00)01800-610884684
|
|
27.
|
SS HookAR MeansCa(2+)/CaM-dependent
kinases: from activation to functionAnnu Rev Pharmacol
Toxicol414715052001
|
|
28.
|
JY WuAR MeansCa(2+)/calmodulin-dependent
protein kinase IV is expressed in spermatids and targeted to
chromatin and the nuclear matrixJ Biol Chem275799479992000
|
|
29.
|
MK JobbyY SharmaCalcium-binding to lens
betaB2- and betaA3-crystallins suggests that all beta-crystallins
are calcium-binding proteinsFEBS
J27441354147200710.1111/j.1742-4658.2007.05941.x17651443
|
|
30.
|
B RajiniP ShridasCS SundariCalcium binding
properties of gamma-crystallin: calcium ion binds at the Greek key
beta gamma-crystallin foldJ Biol
Chem2763846438471200110.1074/jbc.M10216420011502736
|
|
31.
|
A ApatiJ JanossyA BrozikPI BauerM
MagocsiCalcium induces cell survival and proliferation through the
activation of the MAPK pathway in a human hormone-dependent
leukemia cell line, TF-1J Biol
Chem27892359243200310.1074/jbc.M20552820012643264
|
|
32.
|
A RiccioS AhnCM DavenportJA BlendyDD
GintyMediation by a CREB family transcription factor of
NGF-dependent survival of sympathetic
neuronsScience28623582361199910.1126/science.286.5448.235810600750
|