Introduction
Bone marrow-derived stem cells (BMSCs) have been
widely utilized in bone tissue engineering (1,2).
They have been shown to readily attach to culture plates and
differentiate down an osteogenic pathway under appropriate chemical
stimulation (3). However, the
aspiration of bone marrow is quite painful and the concentration of
harvested cells is often low. These shortcomings have limited their
application in bone tissue engineering.
More recently, adipose-derived stem cells (ASCs)
have garnered great attention from researchers (4–7).
These cells can be isolated by simple liposuction, and the
harvested cell number is relatively high. Studies concentrating on
ASCs have demonstrated their multi-differentiation potential. With
the appropriate chemical stimulus, they can differentiate along an
osteogenic lineage, a chondrogenic lineage, and an adipogenic
lineage (6,7).
Many studies have compared the osteogenic
differentiation capacity of BMSCs and ASCs in an attempt to
determine whether ASCs could be an alternative for BMSCs in bone
tissue engineering, and thus far, the results have been
inconsistent. De Ugarte et al (8) reported that there was no difference
between BMSCs and ASCs in their osteogenic differentiation
potential. Similarly, Hattori et al (9) showed in their study that ASCs are
similar to BMSCs in their ability to differentiate into
osteoblasts. However, Im et al (10) demonstrated that the level of
mineralization and alkaline phosphatase (ALP) activity of BMSCs
were 2- to 3-fold higher than that of ASCs when undergoing
osteogenesis. In a recent study Vishnubalaji et al (11) concluded that the osteogenic
differentiation potential of BMSCs was higher than that of ASCs
when studied by qualitative methods, but when studied by the
quantitative real-time polymerase chain reaction, there was no
difference between them.
Apart from sensitivity to chemical stimuli, BMSCs
and ASCs were also found to be sensitive to mechanical stimuli
(12–14). Numerous studies have shown that
mechanical stimuli play an important role in bone tissue
engineering, which is also referred to as functional bone tissue
engineering (15,16). In an attempt to find a better seed
cell in functional bone tissue engineering, we tried to quantify
the osteogenic differentiation capacity of BMSCs and ASCs in
response to both chemical and mechanical stimuli.
In the present study, human bone marrow-derived stem
cells (hBMSCs) and human adipose-derived stem cells (hASCs) were
isolated from the same volunteers to eliminate differences caused
by age and gender. Cells were then studied for their
multi-differentiation ability and surface antigen expression
profiles. Isolated hBMSCs and hASCs were cultured in osteogenic
differentiation medium under cyclic tensile stretch (CTS) and
static controls. Results revealed that both hBMSCs and hASCs were
sensitive to CTS during the osteogenic differentiation process.
Quantitative measurement of ALP activity showed that the
early-phase osteogenic differentiation capacity of hBMSCs was
similar to hASCs in the CTS-stimulated groups. While quantitative
measurement of mineralization revealed that the late-phase
osteogenic differentiation capacity of hBMSCs was superior to that
of hASCs with statistical difference. RT-PCR revealed that the
osteogenic differentiation capacity of hBMSCs were superior to that
of hASCs both in the CTS-stimulated and unstimulated groups. This
study compared the osteogenic differentiation capacity of hBMSCs
and hASCs both in the CTS-stimulated and unstimulated groups and
has great implication in the field of functional bone tissue
engineering.
Materials and methods
Isolation and culture of hBMSCs and
hASCs
Bone marrow and subcutaneous adipose tissue were
obtained from the same healthy volunteers to exclude effects of age
and gender. The surgical procedure was performed after informed
consent was obtained from the volunteers (6 volunteers, 24–44 years
of age). All procedures were approved by the Ethics Committee of
Tongji Medical College, Huazhong University of Science and
Technology, China. In brief, bone marrow aspirates were isolated by
centrifugation at 1,200 × g for 20 min, and mononucleated cells
were then collected and suspended in growth medium which contained
low glucose-Dulbecco’s modified Eagle’s medium (DMEM), 10% fetal
bovine serum (FBS), 2 mM L-glutamine and 100 units
penicillin-streptomycin. HASCs were isolated by density and
differentiation adhesions. The adipose tissue was digested with
0.075% type I collagenase for 20 min, and the hASCs-rich fraction
was pelleted by centrifugation at 1,800 × g for 20 min. Red blood
cells were lysed and cell pellets were suspended. Cells were then
pelleted again by centrifugation at 1,800 × g for 20 min and
suspended in growth medium. Twenty-four hours later, non-adherent
cells were washed with phosphate-buffered saline (PBS).
Finally, hBMSCs and hASCs were seeded at a density
of 4×105 cells/ml in 5% CO2 and a
water-saturated atmosphere and passaged at a 1:2 dilution once they
had reached complete confluence. Cells that had passaged 3–7 times
were used in this study.
Multi-differentiation ability tests
Cells were cultured in the growth medium for 24 h
before changing into the corresponding adipogenic differentiation
medium and osteogenic differentiation medium. The adipogenic
differentiation medium contained DMEM supplemented with 10% FBS, 1
mM dexamethasone (Sigma), 0.5 mM methylisobutyl-xanthine (Sigma),
10 mg/ml insulin (Invitrogen Life Technologies, Carlsbad, CA) and
100 mM indomethacin (Sigma). The osteogenic differentiation medium
contained DMEM supplemented with 10% FBS, 10 mM β-glycerophosphate
(Sigma), 100 nM dexamethasone and 0.1 mM ascorbate-2-phosphate
(Sigma). Both adipogenesis and osteogenesis lasted for a total of 2
weeks. Adipogenesis was evaluated by oil red O staining and
osteogenesis was evaluated after staining with alizarin red.
Surface antigen characterization
Surface antigen expression profiles of hBMSCs and
hASCs at passage 4 were examined using a flow cytometer, and
isotype-matched normal IgG was used as the control. Fluorescein
isothiocyanate (FITC)-labeled anti-human CD44, CD45, CD105,
phycoerythrin (PE)-labeled anti-human CD29 antibodies were
purchased from BD Biosciences. After staining, the cells were
washed twice in PBS containing 2% FBS and analyzed using a Coulter
FC500 flow cytometer.
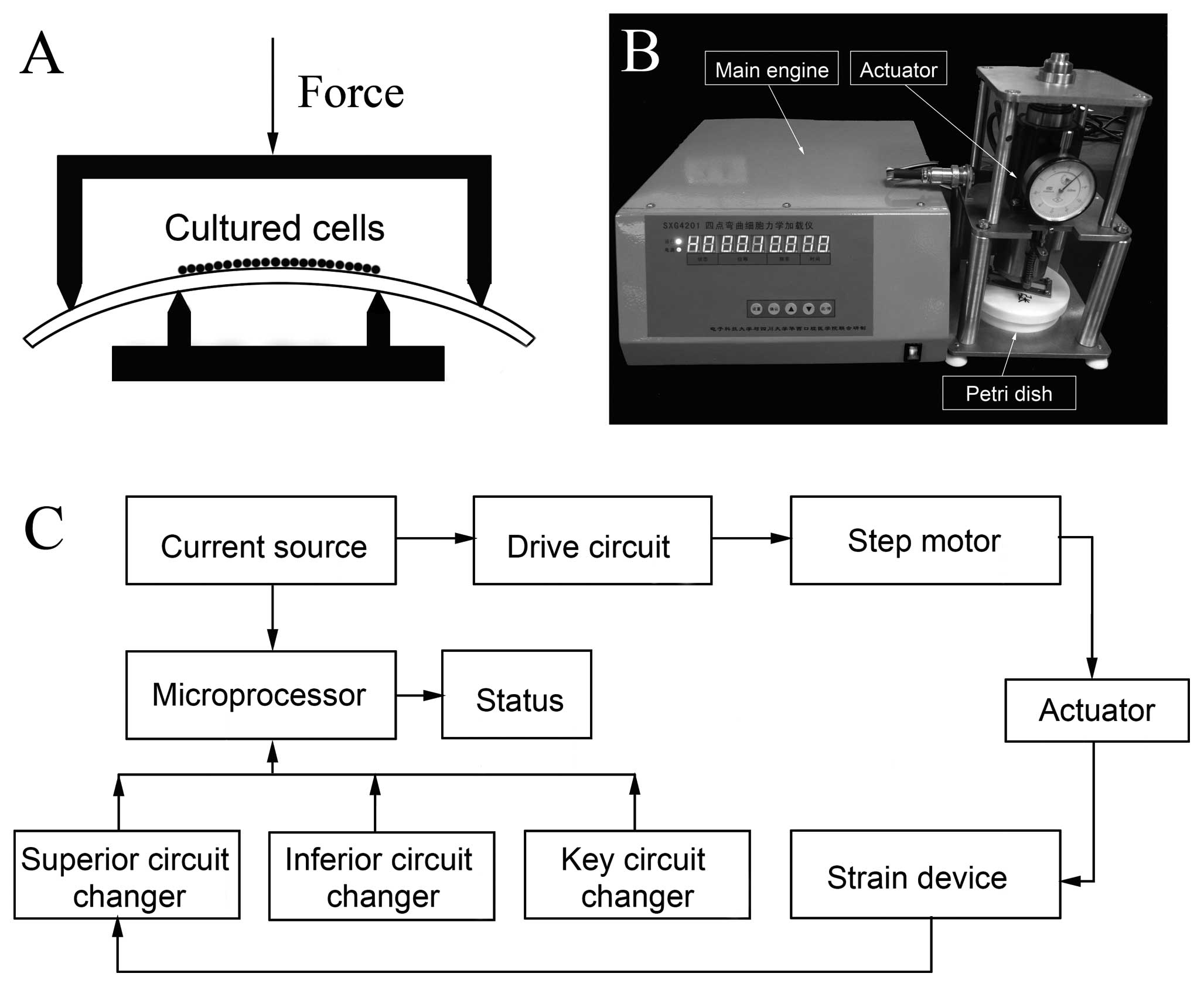
Application of CTS
The 4-point bending mechanical stimulation device
was purchased from Sichuan University at the requests of the
authors. The diagrammatic sketch of the device is shown in Fig. 1A. The device consisted of 3 main
parts: the main engine, the actuator and the cell culture Petri
dish (Fig. 1B) and the main
working process is shown in Fig.
1C. The frequency of the mechanical stimulation was 1 Hz, with
15 sec of rest after every 5-sec working duration, with a total
time of 2 h every day. The strain magnitude ranged from 0 to 3,200
μɛ (0, 800, 1,600, 2,400 and 3,200). The treatments were repeated
at least 3 times independently.
Determination of lactate dehydrogenase
(LDH)
In an attempt to properly simulate a physiological
mechanical stimulation, LDH production of hBMSCs and hASCs was
measured under different mechanical stimulation conditions and at
different time points. LDH is a cytosolic enzyme which is released
upon cell lysis. LDH production of hBMSCs and hASCs stimulated by
CTS at magnitudes ranging from 0 to 3,200 μɛ was measured 1 day
after cell culture. Subseqeently, CTS at a magnitude of 2,400 μɛ
was applied to hBMSCs and hASCs. The LDH production of hBMSCs and
hASCs was measured 1, 2, 4, 8, 12 and 14 days after cell culture.
The LDH activity was measured using an LDH cytotoxicity detection
kit. In brief, 150 μl working solution and 50 μl medium was mixed
and then cultured in the dark at room temperature for 30 min. Then
the reaction was stopped by adding 1 N HCl, after which the
absorbance at a wavelength of 570 nm was read. The results were
expressed as arbitrary units and were adjusted to the unstimulated
CTS control values.
In vitro osteogenesis under CTS and
static controls
Cells were seeded on a polyethylene plastic dish at
a density of 1.5×105 cells/plate in the above-mentioned
osteogenic differentiation medium. When cells reached 80–90%
confluence, they were subjected to the cyclic mechanical tensile
stretch. The strain magnitude was 2,400 μɛ, frequency was 1 Hz,
with a 15-sec rest after every 5-sec working duration for a total
of 2 h/day. The complete osteogenic differentiation process lasted
for 14 days. The stimulated group consisted of cells cultured in
osteogenic differentiation medium under CTS, whereas the
unstimulated group consisted of cells that were cultured in the
same differentiation medium but without exposure to CTS. The cell
culture medium was replaced every 2 days.
Quantitative measurement of ALP activity
and mineralization
Measurement of the ALP activity was performed 4 and
7 days after cell culture. ALP activity was measured by detecting
the concentration of p-nitrophenol phosphate substrate. Cultured
cells were lysed, and cell lysates were mixed with alkaline buffer
for ∼15 min. Then, ALP substrate was added to the mixture for
another 30 min, and finally the reaction was stopped by adding 0.05
N NaOH. The absorbance was read at a wavelength of 405 nm, and the
concentration was measured using a standard curve. Finally, the ALP
activity was normalized to cellular protein concentration. Cellular
protein concentration was detected as described by Lowry et
al (17). Values are
expressed as fold change over the static control, which were ASCs
unstimulated by CTS for 4 days.
Measurement of mineralization (extracellular calcium
deposition) was performed 10 and 14 days after the CTS. Cultured
cells were washed twice with PBS and incubated in ethyl alcohol for
10 min. Then, cells were washed with PBS another 3 times and
stained with alizarin red to stain the extracellular calcium
depositions. Nonspecific stained cells were washed with PBS for 15
min. Extracellular calcium deposition was extracted using 10% (w/v)
cetylpyridinium chloride in 10 mM sodium phosphate (pH 7.0) for
quantification. Absorbance at a wavelength of 562 nm was read and
the concentration was determined using a standard curve. Values are
expressed as fold change over the control, which were ASCs
unstimulated by CTS for 10 days.
Real-time quantitative PCR analysis
Five and ten days after cell culture, total RNA was
isolated with TRIzol reagent and used to synthesize cDNA with the
Super-Script II cDNA synthesis kit (all were from Invitrogen Life
Technologies). The osteogenic differentiation markers were assessed
by quantitative real-time PCR using the SYBR-Green Master mix
(ABI). Glyceraldehyde phosphate dehydrogenase (GAPDH) was selected
as an internal control. The primer information is as follows: Runx2
(accession no. NM_001024630F) forward, CCAGATGGGACTGTGGTTACTG and
reverse, TTCCGGA GCTCAGCAGAATAA; BMP-2 (accession no. NM_001200F)
forward, GCCCTTTTCCTCTGGCTGAT and reverse, TTG ACCAACGTCTGAACAATGG;
ALP (accession no. NM_013059) forward, CCTAGACACAAGCACTCCCACTA and
reverse, GTCAGTCAGGTTGTTCCGATTC; OC (accession no. NM_199173F)
forward, TGTGAGCTCAATCCGGA CTGT and reverse, CCGATAGGCCTCCTGAAAGC;
GAPDH (accession no. NM_017008) forward, TATGACTCTACCCAC GGCAAGT
and reverse, ATACTCAGCACCAGCATCACC.
Statistical analysis
All experiments were repeated at least 3 times
independently. All the data are presented as mean ± SD and analyzed
with SPSS 13.0. The significance of difference was determined using
the Student’s t-test and analysis of variance (ANOVA); differences
with P<0.05 were considered significant.
Results
Morphology, surface antigen profiles and
multi-differentiation ability of hBMSCs and hASCs
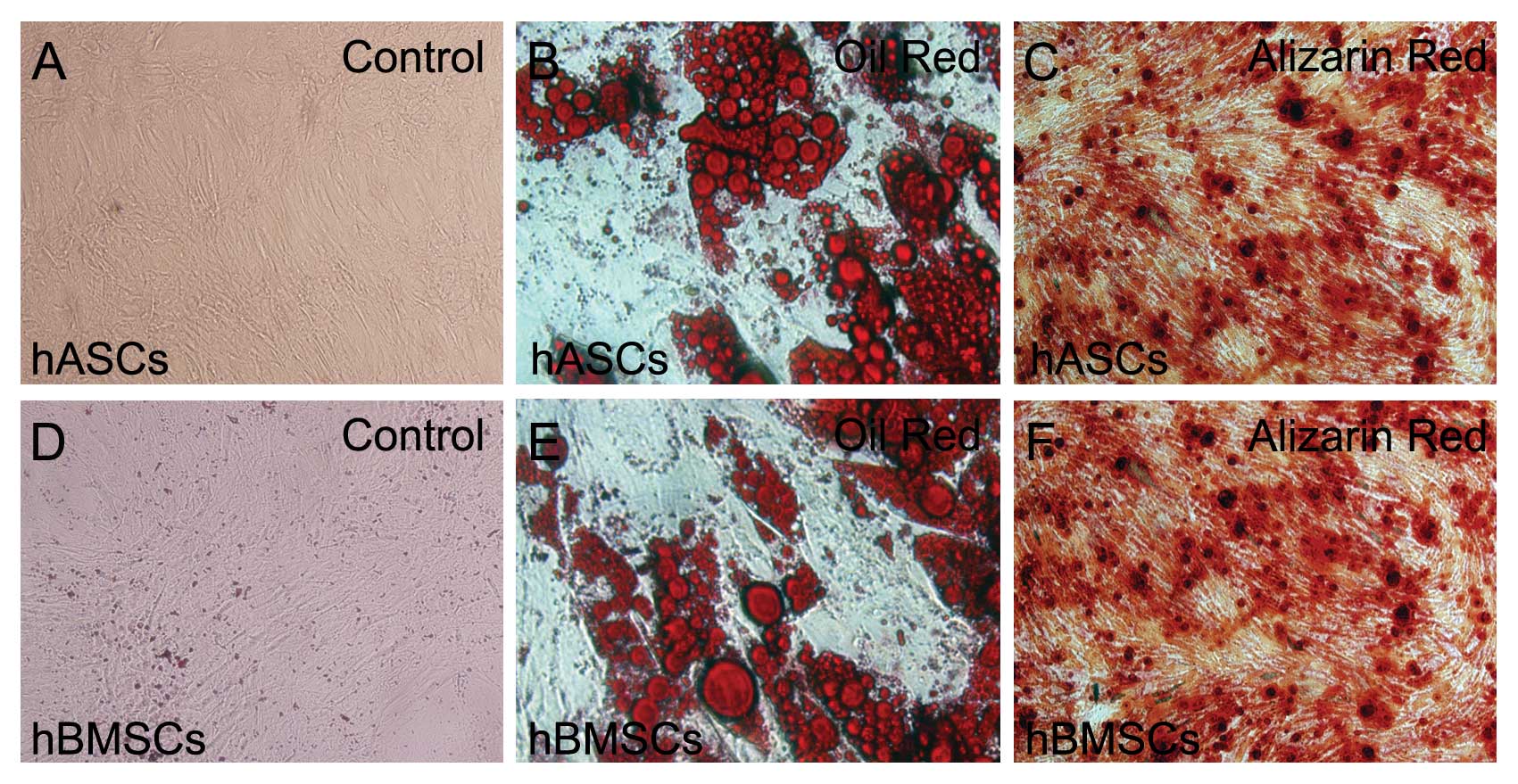
HBMSCs and hASCs passaged 3 times generally showed a
homogeneous population containing flat and fibroblast-like cells
(Fig. 2A and D). Flow cytometry
results showed that both hBMSCs and hASCs positively expressed CD29
(95.56±1.4%, 94.23±2.5%), CD44 (94.65±2.2%, 96.10±1.9%), CD105
(97.24±1.7%, 95.54±1.5%) and negatively expressed leukocyte common
antigen CD45 (6.7±0.8%, 5.4±1.2%), which was consistent with
previous studies (20,21).
To test the multi-differentiation ability of hBMSCs
and hASCs, adipogenic differentiation and osteogenic
differentiation assays were performed. Two weeks after
adipogenesis, lipid droplets were clearly detected in both hBMSCs
and hASCs, as demonstrated by oil red O staining (Fig. 2B and E). Two weeks after
osteogenesis, both hBMSCs and hASCs were positively stained with
alizarin red, and obvious calcification nodules were noted in both
groups (Fig. 2C and F). Results
of the flow cytometry and multi-differentiation test showed that
both hBMSCs and hASCs were successfully isolated. Results also
revealed that the surface antigen expression profiles and
multi-differentiation capacity of hBMSCs and hASCs were similar to
each other.
Effect of CTS on the survival of cultured
cells
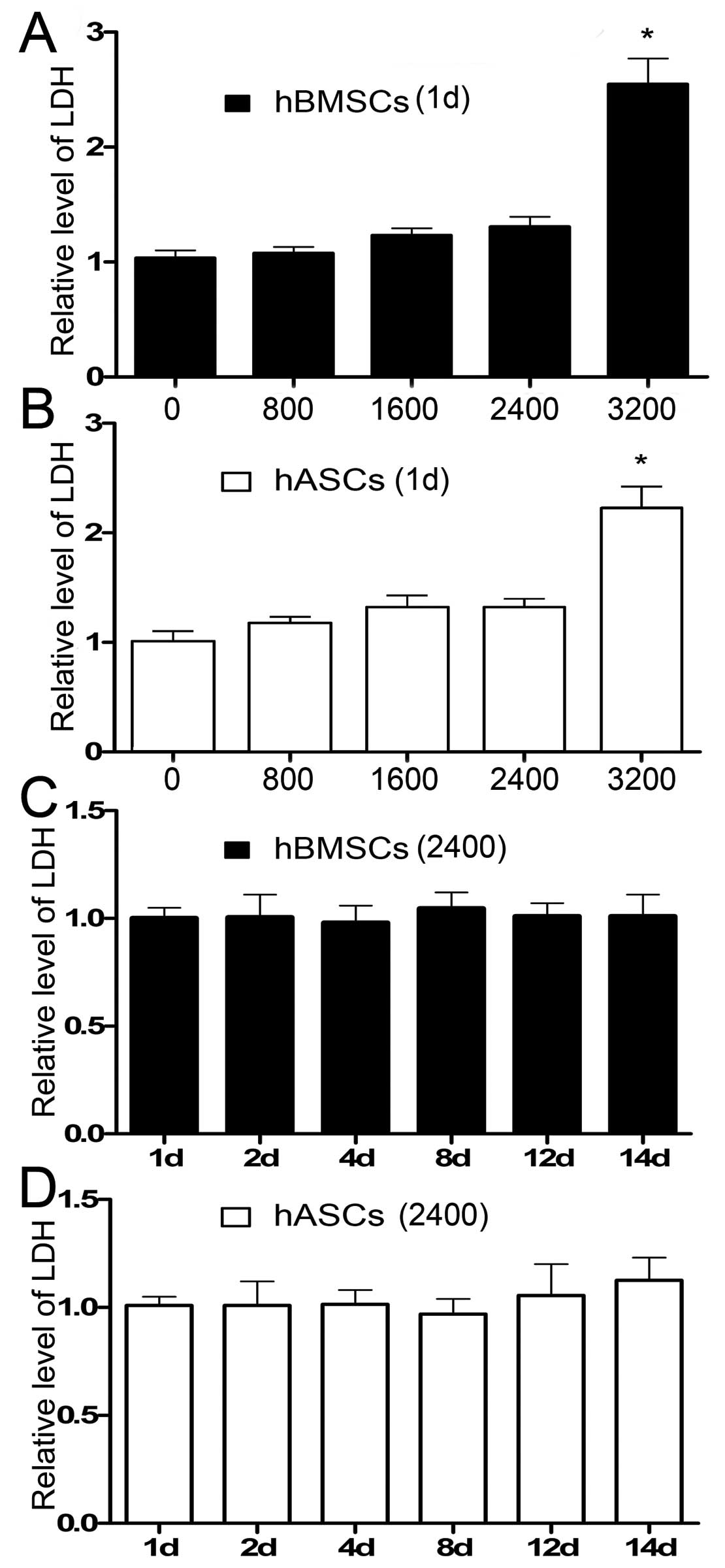
The LDH production of hBMSCs and hASCs measured 1
day after cell culture increased as the strain magnitude increased.
The only exception was that the LDH production in the hASC group
stimulated at a strain of 2,400 μɛ was slightly lower than that at
1,600 μɛ. There was no significant difference in LDH production 1
day after cell culture when the strain magnitude was ≤2,400 μɛ.
Both hBMSCs and hASCs expressed a higher LDH production when the
strain magnitude changed to 3,200 μɛ, which implied that a strain
of 3,200 μɛ was not conducive to cell survival and should not be
chosen as an ideal strain magnitude (Fig. 3A and B). The LDH production level
was normalized to the level measured 1 day after cell culture of
the unstimulated group.
A strain magnitude of 2,400 μɛ was then applied to
both hBMSCs and hASCs. The LDH production in both groups was then
measured 1, 2, 4, 8, 12 and 14 days after cell culture. The LDH
production level was normalized to the level measured 1 day after
cell culture. Results showed that LDH production in both types of
celsl remained practically equal to each other with no statistical
difference (Fig. 3C and D). Based
on these data, a strain at 2,400 μɛ was chosen to simulate the
physiological mechanical stimulus in this study.
Quantitative measurement of ALP
activity
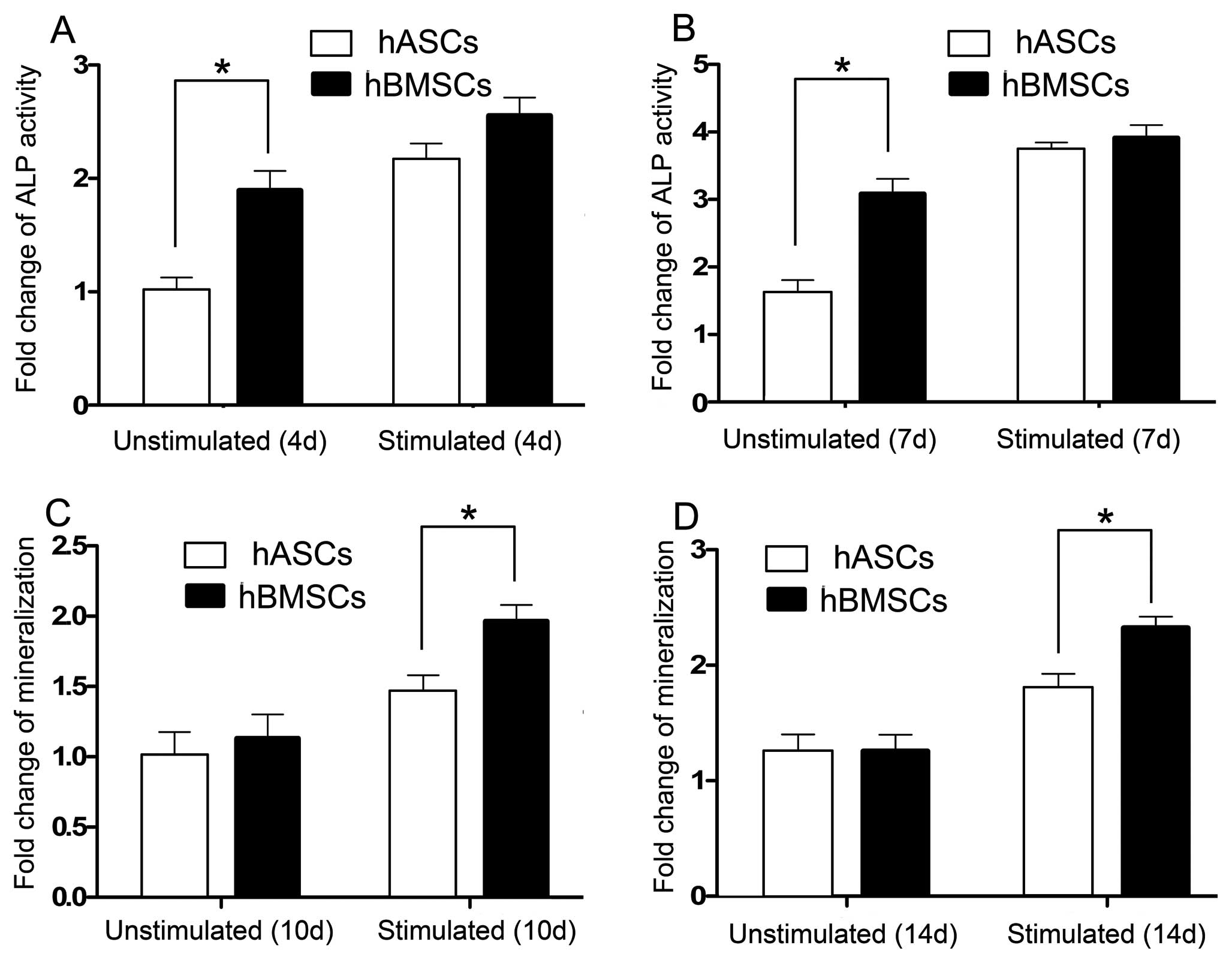
Quantitative measurement of ALP activity was
performed 4 and 7 days after cell culture. Results showed that ALP
activity of both hBMSCs and hASCs in the CTS-stimulated groups and
CTS-unstimulated groups increased with time (Fig. 4A and B). The ALP activity of
hBMSCs and hASCs unstimulated by CTS on days 4 and 7 showed a
significant difference. However, when stimulated by CTS, the ALP
activity in both the hBMSCs and hASCs increased and exhibited no
difference on days 4 and 7 (Fig. 4A
and B).
Quantitative measurement of
mineralization
The mineralization, which was assessed by measuring
extracellular matrix calcium depositions, occurred 10 and 14 days
after cell culture. Results showed that both the extracellular
matrix calcium deposition of hBMSCs and hASCs increased with time.
Mineralization of hBMSCs and hASCs as represented by calcium
depositions showed no difference in the CTS-unstimulated groups on
days 10 and 14. When stimulated by CTS, mineralization of hBMSCs
and hASCs increased and a significant difference was noted
(Fig. 4C and D).
Osteogenic differentiation-specific mRNA
expression profiles with and without exposure to CTS
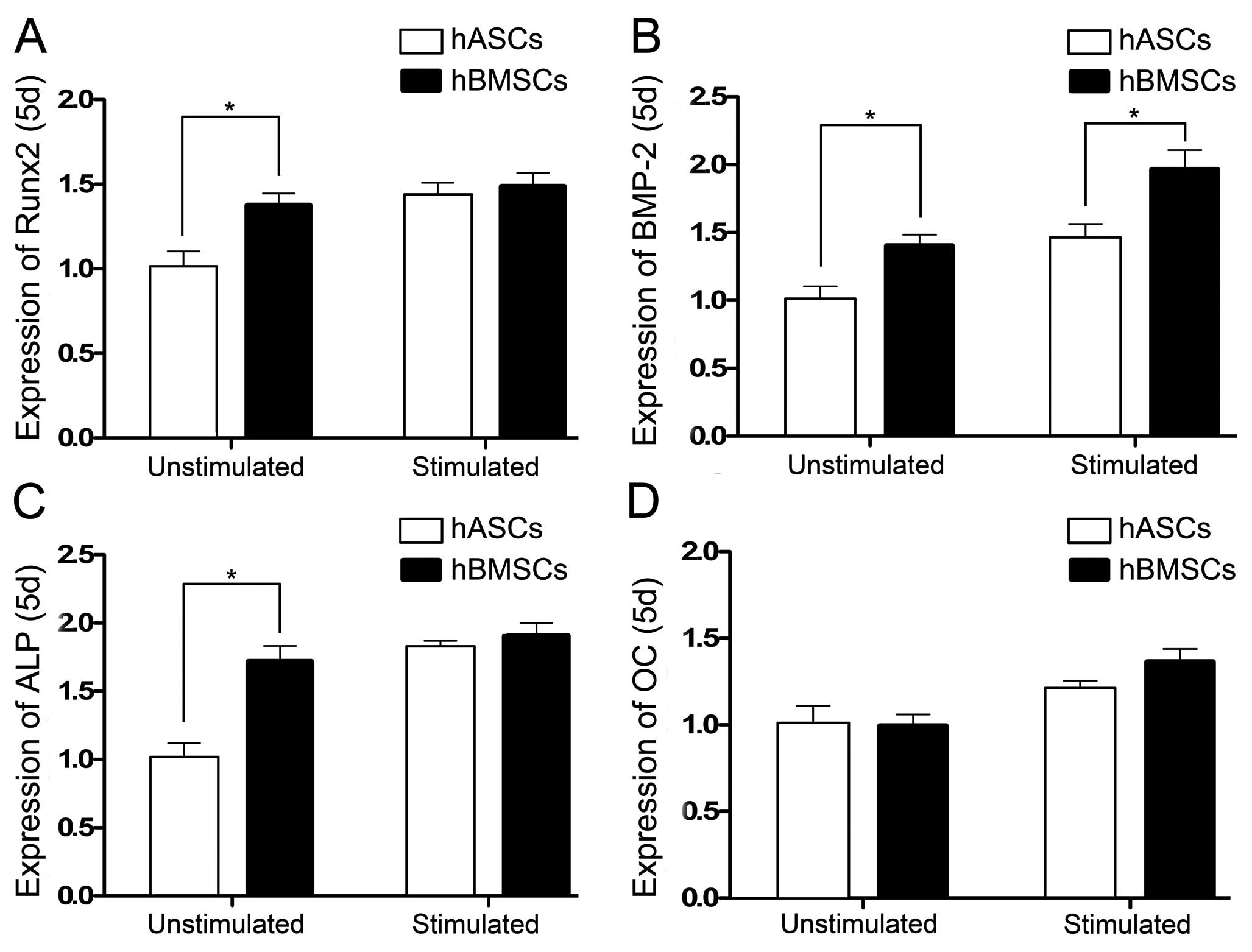
We selected Runx2, bone morphogenetic protein-2
(BMP-2), ALP and osteocalcin (OC) as osteogenic
differentiation-specific genes. The real-time PCR analysis was
performed 5 and 10 days after cell culture.
Runx2 gene expression in the hBMSCs and hASCs
unstimulated by CTS on day 5 showed a significant difference. When
stimulated by CTS, Runx2 gene expression in both the hBMSCs and
hASCs increased but the difference was not significant (Fig. 5A). Expression of the BMP-2 gene in
the hBMSCs and hASCs in the CTS-unstimulated and stimulated groups
on day 5 showed a significant difference, and the BMP-2 gene
expression was increased when stimulated by CTS (Fig. 5B). ALP gene expression in the
hBMSCs and hASCs were increased when stimulated by CTS. The ALP
gene expression in the CTS-unstimulated hBMSCs and hASCs showed a
significant difference, while no difference was noted in the
CTS-stimulated groups (Fig. 5C).
OC gene expression in the hBMSCs and hASCs in both the unstimulated
and stimulated groups showed no significant difference on day 5.
When stimulated with CTS, the OC expression of hBMSCs and hASCs
demonstrated no increase (Fig.
5D).
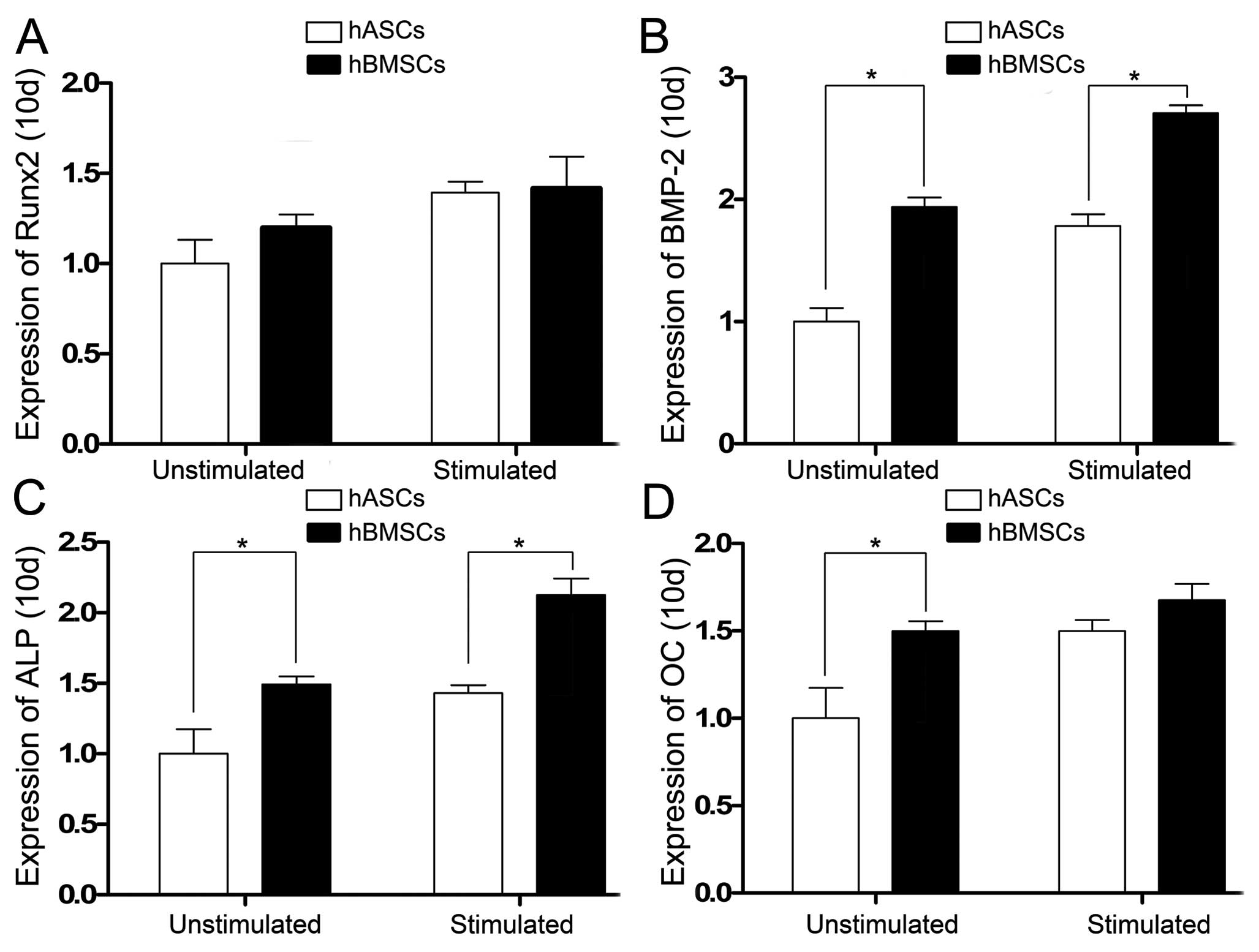
Expression of the osteogenic
differentiation-specific genes was also analyzed 10 days after cell
culture. Runx2 gene expression on day 10 in the hBMSCs and hASCs
unstimulated and stimulated by CTS showed no statistical
difference. When stimulated by CTS, Runx2 expression in both the
hBMSCs and hASCs increased (Fig.
6A). BMP-2 and ALP gene expression levels on day 10 in the
hBMSCs were greater than levels of the hASCs in the
CTS-unstimulated and stimulated groups and statistical differences
were noted. When stimulated by CTS, BMP-2 and ALP expression in the
hBMSCs and hASCs increased (Fig. 6B
and C). OC gene expression on day 10 in the hBMSCs and hASCs
unstimulated by CTS showed a statistical difference, but there was
no statistical difference in the CTS-stimulated groups. When
stimulated by CTS, OC expression in the hBMSCs and hASCs was
increased (Fig. 6D).
Discussion
Bone tissue engineering has been developed in order
to treat skeletal defects and other related clinical problems
(18). With the progress of stem
cell research, BMSCs have been widely utilized in bone tissue
engineering. However, aspiration of bone marrow is painful and the
yield in the cell concentration is relatively low. More recently,
researchers have found that ASCs can also differentiate into
mesodermal lineages such as chondrocytes and osteoblasts. The
isolation of ASCs causes little harm to an organism and yields a
large number of cells.
Numerous studies have compared the osteogenic
differentiation capacity of BMSCs and ASCs cultured in static
conditions without exposure to mechanical stimuli, yet the results
have been inconsistent. Some researchers have reported that BMSCs
possess an osteogenic differentiation capacity similar to that of
ASCs (8,9), while others have demonstrated that
BMSCs possess a stronger osteogenic differentiation capacity than
ASCs (10). A recent study
conducted by Vishnubalaji et al (11) concluded that quantitative analysis
is quite important in comparing the osteogenic potential of BMSCs
and ASCs.
BMSCs and ASCs are not only sensitive to chemical
but also mechanical stimuli. Numerous studies have shown that
mechanical stimuli promote the development and function of
engineered bone tissues (19).
BMSCs and ASCs have both been widely used in the field of
functional bone tissue engineering which includes mechanical
stimuli. However, to our knowledge, there have been no studies
aiming to compare the osteogenic differentiation capacity of hBMSCs
and hASCs in response to mechanical stimuli.
In this study, BMSCs and ASCs were isolated from the
same volunteers to eliminate differences caused by age and gender.
Isolated BMSCs and ASCs were then tested for their
multi-differentiation ability and surface antigen expression
profiles. Results showed that both BMSCs and ASCs could
differentiate along osteogenic and adipogenic pathways, formation
of calcium nodules was observed in both groups undergoing
osteogenesis and lipid droplets were also clearly detected in both
groups undergoing adipogenesis. Flow results showed that both BMSCs
and ASCs positively expressed CD29, CD44, CD105 and negatively
expressed CD45; the flow results were consistent with previous
studies (20,21).
Previous studies have shown that excessive
mechanical stress results in an elevated production of LDH
(22). In an attempt to simulate
physiological mechanical CTS but not excessive tensile stretch, the
cultured cells were tested for the LDH production under different
stretching magnitudes and time durations. Our study showed that a
strain magnitude of 2,400 μɛ is an effective physiological cyclic
tensile strain. Isolated cells were cultured in the osteogenic
differentiation medium with and without exposure to CTS.
ALP activity was quantified 4 and 7 days after cell
culture. Our results showed that both hBMSCs and hASCs were
sensitive to CTS, and the ALP activity of hBMSCs and hASCs
increased when stimulated by CTS. The ALP activity of hBMSCs and
hASCs in the unstimulated groups on days 4 and 7 was significantly
different. When stimulated by CTS, the ALP activity increased in
both cases, but no significant difference was noted.
The mineralization of the hBMSCs and hASCs was
detected by extracellular matrix calcium deposition. Results showed
that mineralization of hBMSCs and hASCs increased when stimulated
by CTS. The mineralization of hBMSCs and hASCs showed no difference
on days 10 and 14 in the unstimulated groups and showed a
significant difference in the stimulated groups on days 10 and
14.
Real-time PCR analysis was performed 5 and 10 days
after cell culture. Runx2, a master gene that controls osteogenic
differentiation (23), exhibited
a difference in expression in the unstimulated groups of hBMSCs and
hASCs 5 days after cell culture. When stimulated by CTS, the Runx2
expression increased and showed no difference between the hBMSCs
and hASCs. However, Runx2 expression in the hBMSCs and hASCs
measured 10 days after cell culture in the CTS stimulated and
unstimulated groups showed no significant difference.
BMP-2 is a low-molecular-weight glycoprotein which
functions as a morphogen and belongs to the transforming growth
factor-β (24). The gene
expression of BMP-2 was also analyzed in this study. Our study
showed that BMP-2 gene expression was elevated in the
CTS-stimulated hBMSC and hASC groups when compared to that in the
CTS-unstimulated hBMSCs and hASCs 5 and 10 days after cell culture.
The BMP-2 gene expression of hBMSCs was greater than that in the
hASCs both in the CTS-stimulated and unstimulated groups 5 and 10
days after cell culture. ALP gene expression of hBMSCs in the
CTS-unstimulated group was greater than that of hASCs in the
CTS-unstimulated group 5 and 10 days after cell culture, while ALP
gene expression of hBMSCs in the CTS-stimulated group showed a
similar expression level to that of hASCs in the CTS-stimulated
group 5 days after cell culture. Additionally, the ALP gene
expression of hBMSCs in the CTS-stimulated group was greater than
that of the hASCs in the CTS-stimulated group 10 days after cell
culture.
Finally, gene expression of OC was measured. OC is
only secreted by osteoblasts and is commonly used as a marker of
osteoblastic differentiation in the late stages of differentiation
(25). OC expression in the
hBMSCs and hASCs stimulated and unstimulated by CTS 5 days after
cell culture showed no difference, and CTS did not promote OC
expression in the hBMSCs and hASCs 5 days after cell culture.
However, OC expression in the hBMSCs and hASCs unstimulated by CTS
10 days after cell culture showed a difference, and this difference
was eliminated by the application of CTS.
In summary, this study compared the osteogenic
differentiation capacity of hBMSCs and hASCs in both CTS-stimulated
and unstimulated conditions. Our results revealed that CTS promoted
the osteogenic differentiation of both hBMSCs and hASCs. The
early-phase osteogenic differentiation capacity of hBMSCs
stimulated by CTS was similar to that of hASCs stimulated by CTS as
demonstrated by ALP activity measurement and RT-PCR analysis. The
late-phase osteogenic differentiation capacity of hBMSCs stimulated
by CTS was superior to that of hASCs stimulated by CTS as shown by
mineralization measurement and RT-PCR. This study highlights the
important role that mechanical stimuli play in functional bone
tissue engineering and also provides critical information to the
fields of functional bone tissue engineering and regenerative
medicine.
Acknowledgements
This study was supported by grants
from the National Natural Science Foundation of China (no.
31070831).
References
|
1.
|
E PotierJ NoaillyK ItoDirecting bone
marrow-derived stromal cell function with mechanicsJ
Biomech43807817201010.1016/j.jbiomech.2009.11.01919962149
|
|
2.
|
C XiaoH ZhouS GeRepair of orbital wall
defects using biocoral scaffolds combined with bone marrow stem
cells enhanced by human bone morphogenetic protein-2 in a canine
modelInt J Mol Med26517525201020818491
|
|
3.
|
NA AritaD PelaezHS CheungActivation of the
extracellular signal-regulated kinases 1 and 2 (ERK1/2) is needed
for the TGFbeta-induced chondrogenic and osteogenic differentiation
of mesenchymal stem cellsBiochem Biophys Res
Commun405564569201110.1016/j.bbrc.2011.01.068
|
|
4.
|
JC BodleAD HansonEG LoboaAdipose-derived
stem cells in functional bone tissue engineering: lessons from bone
mechanobiologyTissue Eng Part B
Rev17195211201110.1089/ten.teb.2010.073821338267
|
|
5.
|
NJ PanettaDM GuptaJK LeeDC WanGW CommonsMT
LongakerHuman adipose-derived stromal cells respond to and
elaborate bone morphogenetic protein-2 during in vitro osteogenic
differentiationPlast Reconstr
Surg125483493201010.1097/PRS.0b013e3181c82d75
|
|
6.
|
H TappEJ HanleyJC PattHE
GruberAdipose-derived stem cells: characterization and current
application in orthopaedic tissue repairExp Biol Med
(Maywood)23419200910.3181/0805-MR-17019109553
|
|
7.
|
G LiuY ChengS GuoTransplantation of
adipose-derived stem cells for peripheral nerve repairInt J Mol
Med28565572201121687931
|
|
8.
|
DA De UgarteK MorizonoA
ElbarbaryComparison of multi-lineage cells from human adipose
tissue and bone marrowCells Tissues Organs174101109200312835573
|
|
9.
|
H HattoriM SatoK MasuokaOsteogenic
potential of human adipose tissue-derived stromal cells as an
alternative stem cell sourceCells Tissues
Organs178212200415550755
|
|
10.
|
GI ImYW ShinKB LeeDo adipose
tissue-derived mesenchymal stem cells have the same osteogenic and
chondrogenic potential as bone marrow-derived cells?Osteoarthritis
Cartilage13845853200510.1016/j.joca.2005.05.00516129630
|
|
11.
|
R VishnubalajiM Al-NbaheenB KadalmaniA
AldahmashT RameshComparative investigation of the differentiation
capability of bone-marrow- and adipose-derived mesenchymal stem
cells by qualitative and quantitative analysisCell Tissue
Res347419427201210.1007/s00441-011-1306-322287041
|
|
12.
|
S GhazanfariM Tafazzoli-ShadpourMA
ShokrgozarEffects of cyclic stretch on proliferation of mesenchymal
stem cells and their differentiation to smooth muscle cellsBiochem
Biophys Res
Commun388601605200910.1016/j.bbrc.2009.08.07219695226
|
|
13.
|
F ColazzoP SarathchandraRT
SmolenskiExtracellular matrix production by adipose-derived stem
cells: implications for heart valve tissue
engineeringBiomaterials32119127201110.1016/j.biomaterials.2010.09.00321074262
|
|
14.
|
CE SarrafWR OttoM EastwoodIn vitro
mesenchymal stem cell differentiation after mechanical
stimulationCell
Prolif4499108201110.1111/j.1365-2184.2010.00740.x21199014
|
|
15.
|
RD SumanasingheSH BernackiEG
LoboaOsteogenic differentiation of human mesenchymal stem cells in
collagen matrices: effect of uniaxial cyclic tensile strain on bone
morphogenetic protein (BMP-2) mRNA expressionTissue
Eng1234593465200610.1089/ten.2006.12.3459
|
|
16.
|
Z Goli-MalekabadiM Tafazzoli-ShadpourM
RabbaniM JanmalekiEffect of uniaxial stretch on morphology and
cytoskeleton of human mesenchymal stem cells: static vsdynamic
loading Biomed Tech
(Berl)56259265201110.1515/BMT.2011.10921988158
|
|
17.
|
OH LowryNJ RosebroughAL FarrRJ
RandallProtein measurement with the Folin phenol reagentJ Biol
Chem193265275195114907713
|
|
18.
|
HA ElSH CartmellBioreactors for bone
tissue engineeringProc Inst Mech Eng
H22415231532201010.1243/09544119JEIM80221287835
|
|
19.
|
L TirkkonenH HalonenJ HyttinenThe effects
of vibration loading on adipose stem cell number, viability and
differentiation towards bone-forming cellsJ R Soc
Interface817361747201110.1098/rsif.2011.021121613288
|
|
20.
|
M DominiciK Le BlancI MuellerMinimal
criteria for defining multipotent mesenchymal stromal cells. The
International Society for Cellular Therapy position
statementCytotherapy8315317200610.1080/14653240600855905
|
|
21.
|
JB MitchellK McIntoshS
ZvonicImmunophenotype of human adipose-derived cells: temporal
changes in stromal-associated and stem cell-associated markersStem
Cells24376385200610.1634/stemcells.2005-023416322640
|
|
22.
|
MN KangHH YoonYK SeoJK ParkEffect of
mechanical stimulation on the differentiation of cord stem
cellsConnect Tissue
Res53149159201210.3109/03008207.2011.61928422149641
|
|
23.
|
F OttoAP ThornellT CromptonCbfa1, a
candidate gene for cleidocranial dysplasia syndrome, is essential
for osteoblast differentiation and bone
developmentCell89765771199710.1016/S0092-8674(00)80259-79182764
|
|
24.
|
C ColnotCell sources for bone tissue
engineering: insights from basic scienceTissue Eng Part B
Rev17449457201121902612
|
|
25.
|
RT FranceschiBS IyerRelationship between
collagen synthesis and expression of the osteoblast phenotype in
MC3T3-E1 cellsJ Bone Miner
Res7235246199210.1002/jbmr.56500702161373931
|