Introduction
Three inositol 1,4,5-trisphosphate receptor
(IP3R) subtypes, IP3R1, IP3R2, and
IP3R3, are differentially expressed among tissues
(1–5) and function as the Ca2+
release channel on endoplasmic reticulum membranes (6–10).
IP3R is regulated by many intracellular modulators,
phosphorylation by kinases, and associated proteins (11–15).
KRAS-induced actin-interacting protein (KRAP)
was originally identified as one of the deregulated expression gene
in the colorectal cancer cell line, HCT116 (16). The previous studies using
KRAP-knockout (KRAP-KO) mice demonstrate that KRAP
participates in the regulation of systemic energy homeostasis
(17) and of exocrine system
(18). Among the adult mouse
tissues, KRAP is ubiquitously expressed, with high levels in the
pancreas, liver, and brown adipose tissues, and KRAP localizes in
the restricted apical regions of the liver parenchymal cells and of
the pancreatic exocrine acinar cells (19). Our recent findings indicate that
KRAP associates with IP3R to regulate its proper
subcellular localization in the mouse liver and the pancreas
(20) as well as in immortalized
cultured cell lines (21).
Despite these advances, it remains largely unknown which cell types
express KRAP among the other tissues including stomach and
kidneys.
Herein, we performed immunohistological analysis and
identified the exact KRAP-expressing cells in the stomach and the
kidneys, and demonstrated that KRAP plays critical role in the
regulation of the precise subcellular localization of
IP3R in the mucous and the chief cells of the stomach
and in the proximal tubular cells of the kidneys.
Materials and methods
Animals
All animals used in this study were treated in
accordance with the guidelines of Fukuoka University. KRAP-knockout
mice were generated as described previously (17).
Immunohistochemical staining
Immunohistochemical staining was performed as
described previously (19,20).
Specific signals were detected by using rabbit polyclonal anti-KRAP
antibody (19), mouse monoclonal
anti-ZO-1 antibody (ZYMED), mouse monoclonal anti-IP3R3
antibody (610313) from BD Transduction Laboratories, rabbit
polyclonal anti-IP3R2 antibody (AB3000) from Millipore,
and rabbit polyclonal anti-IP3R1 antibody (ab5840) from
Abcam.
Immunoprecipitations and western
blotting
Immunoprecipitations and western blotting were
performed as described previously (19,20).
Results
Localization of KRAP protein in the adult
mouse stomach
To examine the cellular distribution of KRAP protein
in the adult mouse tissues, we performed immunohistochemical
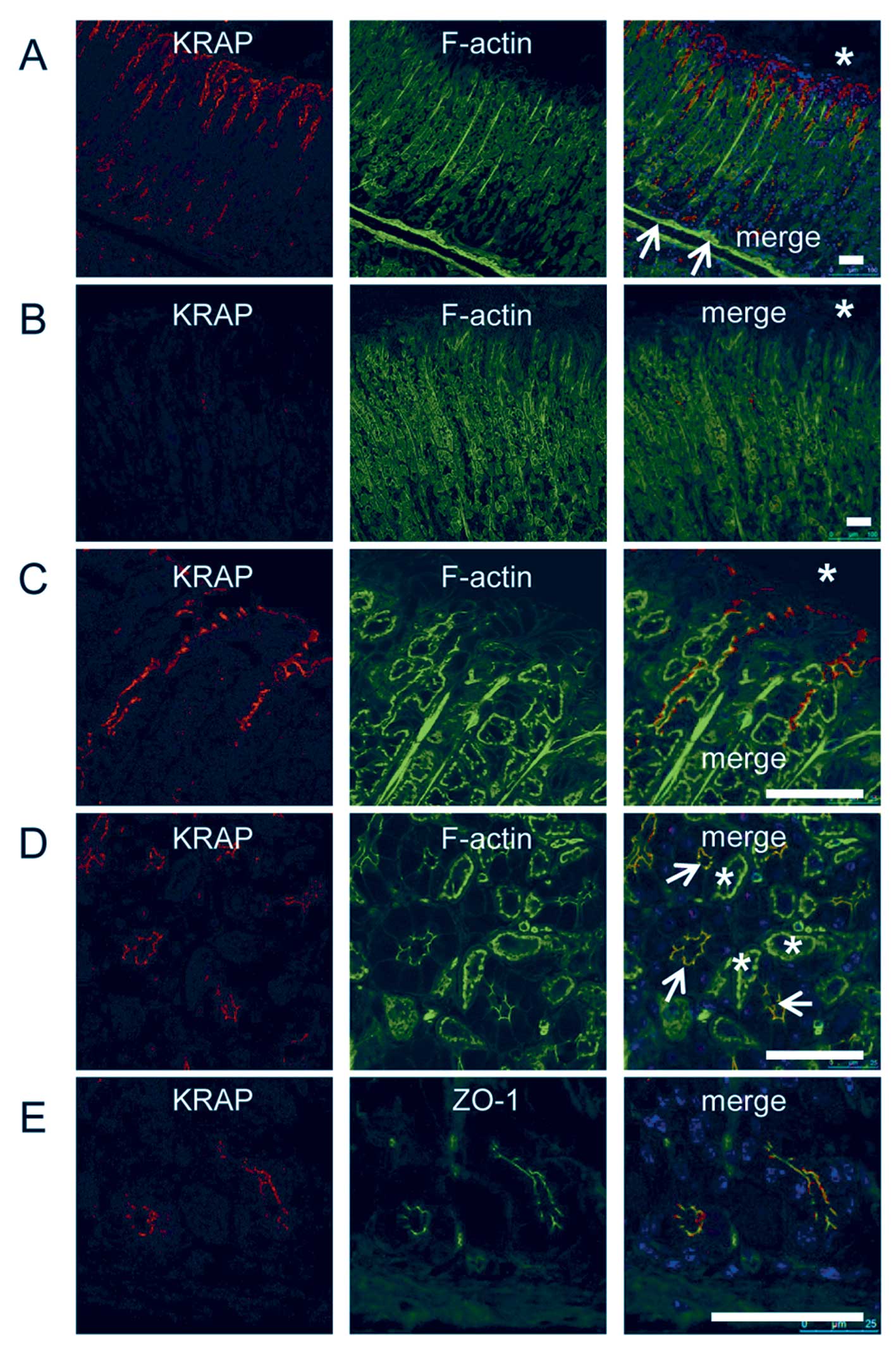
staining by using anti-KRAP antibody. In the stomach, strong KRAP
immunoreactivity was restricted to the pit regions of gastric
glands (Fig. 1A), whereas
significant expression of KRAP was not detected in the muscularis
mucosae beneath the gastric glands (Fig. 1A, arrows). The specificity of KRAP
expression in the stomach was confirmed by using KRAP-KO
tissue as a control (Fig. 1B). In
the pit region of the gastric gland, where columnar surface mucous
cells mainly exist (22), KRAP
was localized beneath the apical membranes of the mucous cells
(Fig. 1C). In the base region of
the gastric glands, where zymogenic chief cells mainly exist,
coronal plane of deeper gastric glands showed that KRAP was
restricted to the apical regions of the chief cells (Fig. 1D, arrowheads), whereas KRAP was
not detected in the parietal cells (Fig. 1D, asterisks). The distinction
between the chief and the parietal cells was validated by ZO-1
staining as described (23),
indicating that KRAP was expressed in the ZO-1-positive chief cells
but not in the ZO-1-negative parietal cells (Fig. 1E).
KRAP co-localized with IP3R in
the stomach
Since we previously reported that KRAP associates
with particular subtypes of IP3R in the liver and the
pancreas (20), we examined
whether KRAP in the stomach is also co-localized with
IP3R. Double-immunostaining of the stomach for KRAP and
IP3R3 revealed that KRAP was co-localized with
IP3R3 in the apical regions of both the chief cells
(Fig. 2A, arrows) and the mucous
cells (Fig. 2B, arrows). Of note,
IP3R2 co-existed with IP3R3 in the chief
cells (Fig. 2C, arrow) but not in
the parietal cells (Fig. 2C,
asterisks). Furthermore, IP3R2 was not detected in
the mucous cells (Fig. 2D,
arrows). These results indicated that KRAP was co-localized
with IP3R2 and IP3R3 in the chief cells and
with IP3R3 in the mucous cells.
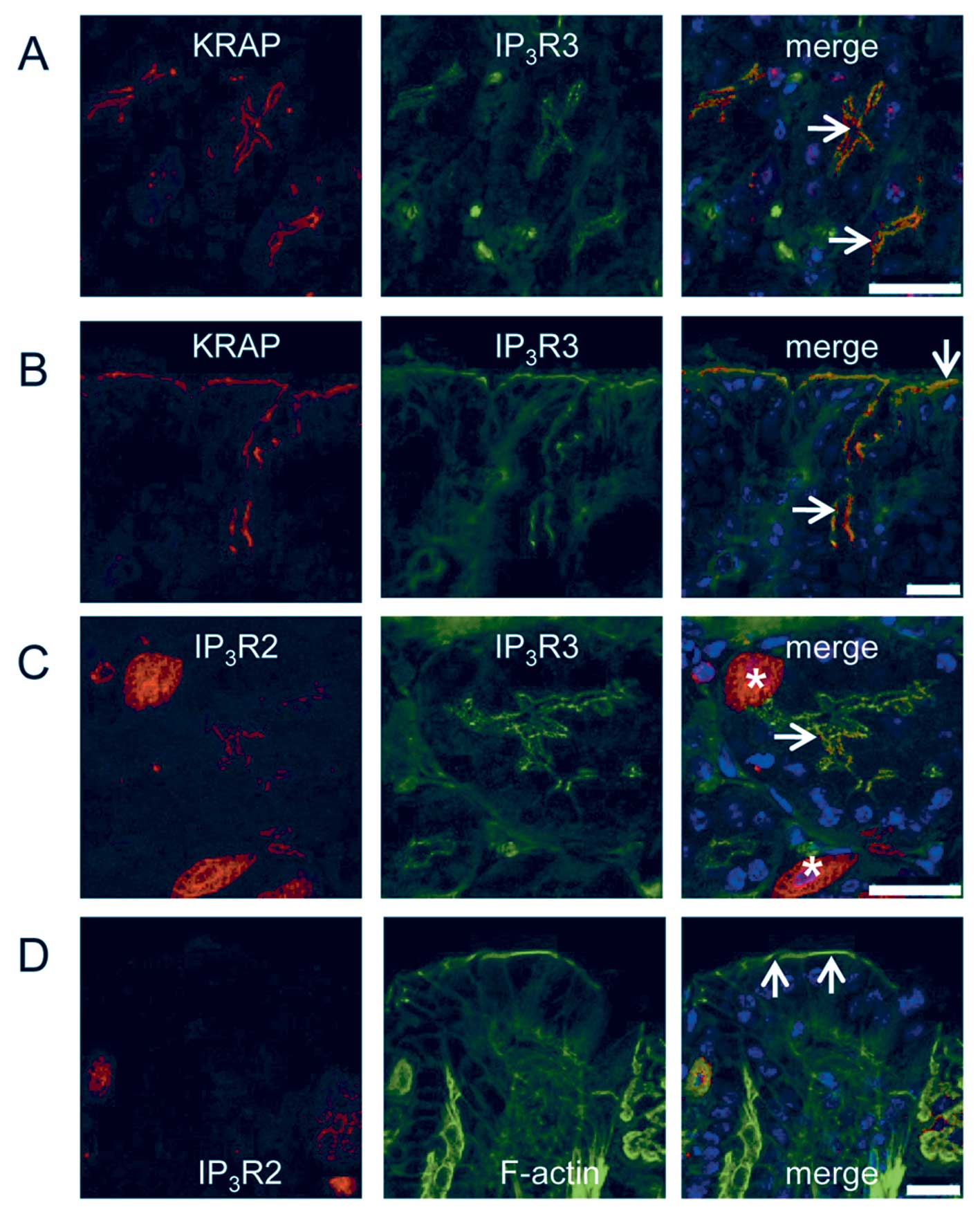 | Figure 2Colocalization of KRAP with
IP3Rs in the chief cells and the mucous cells of the
mouse stomach. (A) Fluorescent confocal images of the base region
of gastric glands for KRAP (red), IP3R3 (green), and the
merged photo. Arrows indicate the apical membranes of the chief
cells. (B) Fluorescent confocal images of the pit region of gastric
glands for KRAP (red), IP3R3 (green), and the merged
photo. Arrows indicate the apical membranes of the mucous cells.
(C) Fluorescent confocal images of the base region of gastric
glands for IP3R2 (red), IP3R3 (green), and
the merged photo. Asterisks and arrow indicate the parietal cells
and the apical membranes of the chief cells, respectively. (D)
Fluorescent confocal images of the pit region of gastric glands for
IP3R2 (red), IP3R3 (green), and the merged
photo. Arrows indicate the apical membranes of the mucous cells.
Blue, 4′,6-diamidino-2-phenylindole (DAPI) staining; scale bar, 25
μm. |
Impaired localization of IP3R
in the KRAP-deficient chief cells and the mucous cells
We addressed the functional relevance of KRAP to the
proper localization of IP3R by using KRAP-KO
mice. IP3R3 was located in the apical region of the
chief cells (Fig. 3A, arrow) and
of the mucous cells (Fig. 3C,
arrows) in the wild-type (WT) mouse stomach, whereas the
restricted localization of IP3R3 appeared to be
diminished in the KRAP-KO stomach (Fig. 3B, arrow; 3D, arrows). Furthermore,
IP3R2 was detected in both the chief cells (Fig. 3E, arrows) and the parietal cells
(Fig. 3E, asterisks) in the WT
stomach, whereas the localization of IP3R2 in the
KRAP-KO stomach was impaired in the chief cells (Fig. 3F, arrows) but not in the parietal
cells (Fig. 3F, asterisks). Thus,
KRAP plays critical role in the regulation of the proper
localization of IP3R2 and IP3R3 in the chief
cells and of IP3R3 in the mucous cells.
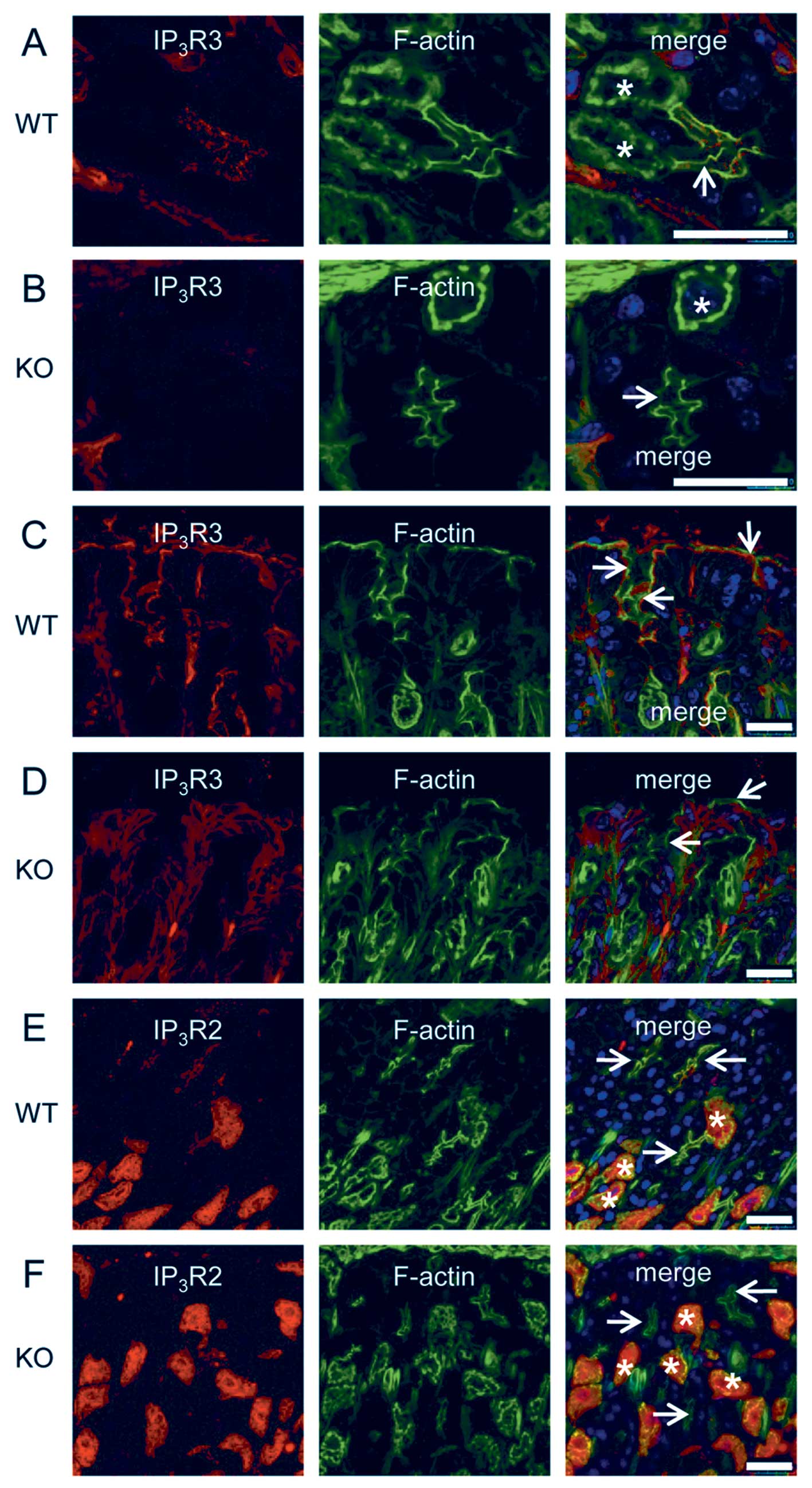 | Figure 3Impaired localization of
IP3Rs in the KRAP-deficient chief cells and the
mucous cells. (A and B) Fluorescent confocal images of the base
region of gastric glands for IP3R3 (red), F-actin with
phalloidin (green), and the merged photo from wild-type (WT) (A) or
KRAP-deficient (KO) (B) mice. Asterisks and arrows indicate
the parietal cells and the apical membranes of the chief cells,
respectively. (C and D) Fluorescent confocal images of the pit
region of gastric glands for IP3R3 (red), F-actin
(green), and the merged photo from WT (C) or KO (D) mice. Arrows
indicate the apical membranes of the mucous cells. (E and F)
Fluorescent confocal images of the base region of gastric glands
for IP3R2 (red), F-actin (green), and the merged photo
from WT (E) or KO (F) mice. Asterisks and arrows indicate the
parietal cells and the apical membranes of the chief cells,
respectively. Blue, 4′,6-diamidino-2-phenylindole (DAPI) staining;
scale bar, 25 μm. |
KRAP expression and its contribution to
the localization of IP3R1 in the proximal tubules of the
mouse kidney
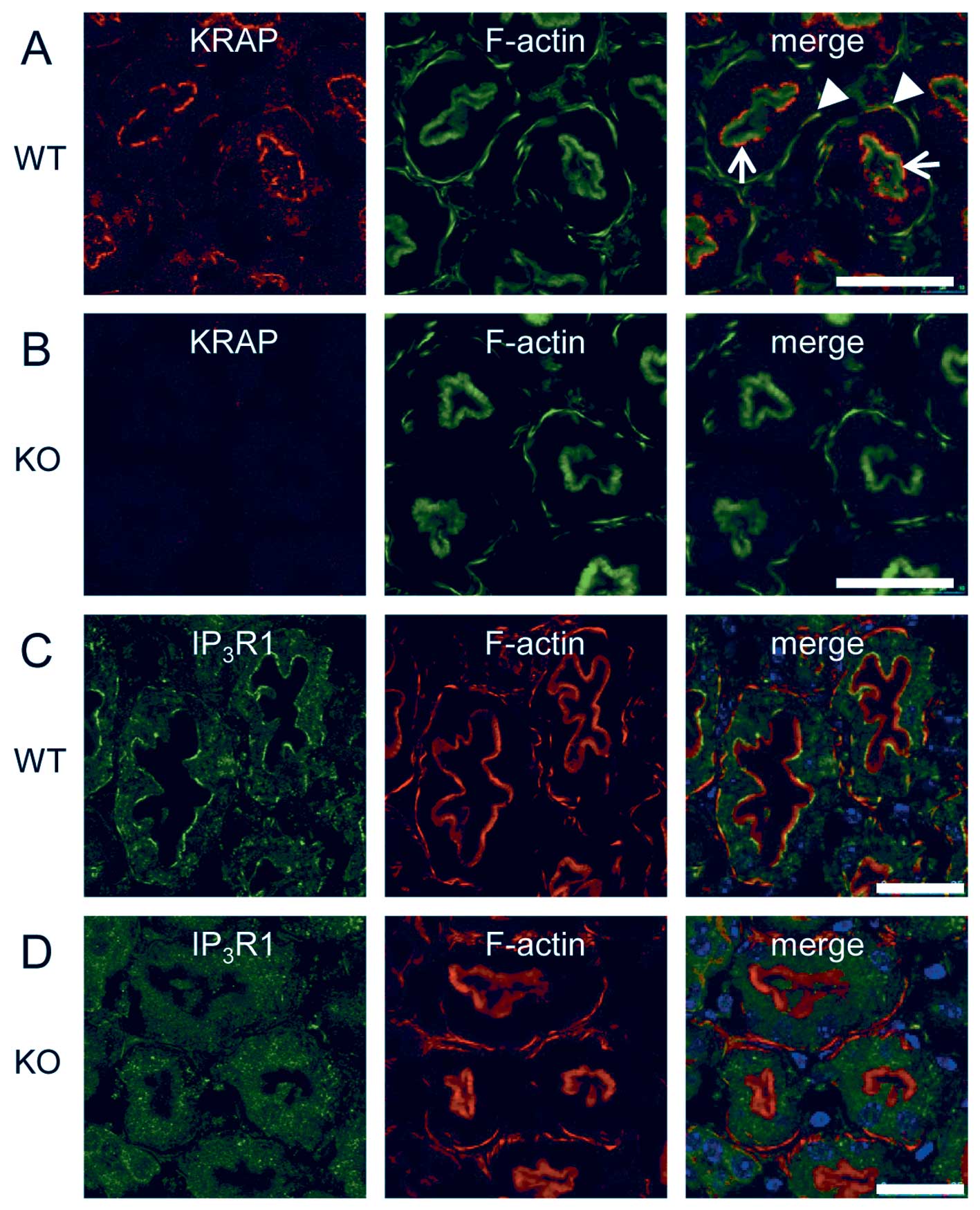
To examine the cellular distribution of KRAP protein
in the adult mouse kidneys, we performed immunohistochemical
staining by using anti-KRAP antibody. The specificities of the
signals were validated by comparing the immunoreactivities of WT
and KRAP-KO mouse tissues. In the WT kidneys, intense
immunoreactivities were observed in the renal proximal tubules
(Fig. 4A) but not in the renal
distal tubules (data not shown). On the other hand, significant
immunoreactive signal was not detected in the proximal tubules in
the KRAP-KO mice (Fig.
4B). Taken together, these results indicate that KRAP was
exactly expressed in the proximal tubules. The proximal tubules
were identified by the presence of the brush-border stained with
phalloidin (Fig. 4A and B).
Immunostaining in the proximal region showed that KRAP was
accumulated beneath the brush-border (Fig. 4A, arrows) and KRAP was also
detected in the basolateral actin bundles (Fig. 4A, arrowheads). We next examined
which subtypes of IP3R, IP3R1,
IP3R2, and IP3R3, expressed in the proximal
tubular cells, revealing that IP3R1 (Fig. 4C) but not IP3R 2 or
IP3R3 (data not shown) was detected in the beneath the
brush-border and in the basolateral actin bundles. Finally, we
addressed the functional relevance of KRAP expression in the
proximal tubular cells to the regulation of IP3R
localization. It is of note that the restricted localization of
IP3R1 detected in the WT mouse kidney (Fig. 4C) was disturbed in the
KRAP-KO mouse kidney (Fig.
4D). Thus, KRAP plays critical role in the regulation of the
proper localization of IP3R1 in the proximal tubular
cells.
KRAP interacts with IP3R1 in
the kidneys and with IP3R3 in the stomach
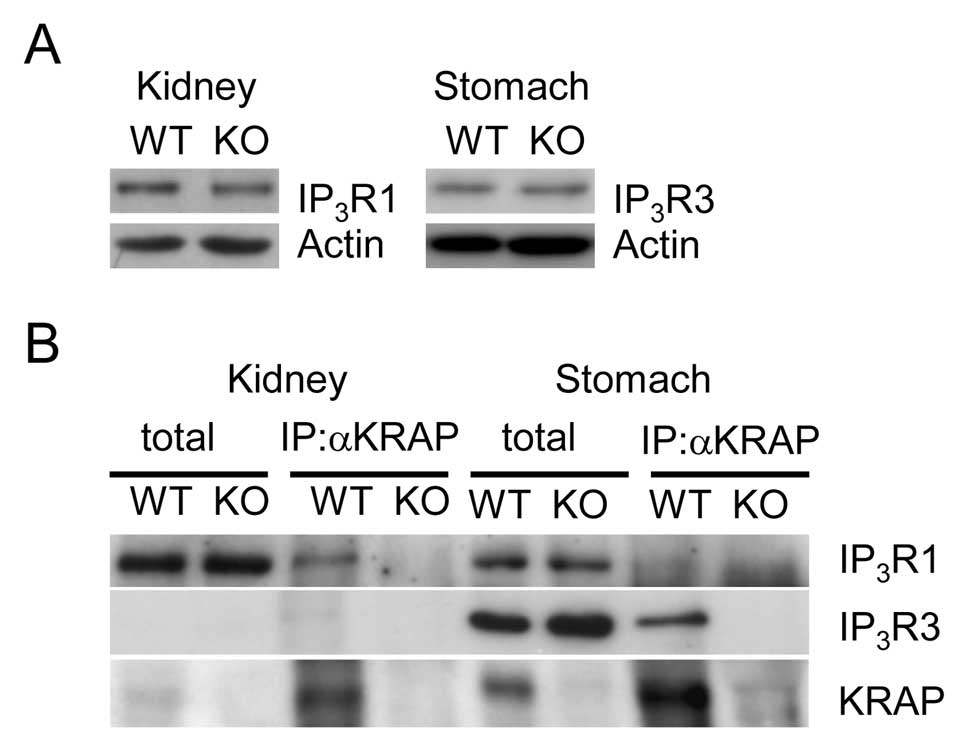
As described above, immunohistochemical signals for
particular IP3R subtypes in the KRAP-KO mouse
kidneys or the stomach were abrogated, leading us to check the
expression levels of IP3R between the WT and
KRAP-KO mouse tissues. Normal expression levels of
IP3R1 and IP3R3 were detected in the
KRAP-KO mouse kidney and the stomach, respectively, compared
with the WT mouse tissues (Fig.
5A), suggesting that mislocalizations but not deregulated
expressions of IP3R occur in the KRAP-KO mouse
kidneys and the stomach. Next, to examine the physical association
of KRAP with IP3R, we performed co-immunoprecipitations
by anti-KRAP antibody in the kidneys or the stomach, in which we
could not evaluate the specific association of IP3R2
with KRAP due to lack of IP3R2-specific antibody
available for western blotting. In the preparations from the WT
mouse tissues, KRAP precipitates IP3R1 and
IP3R3 in the kidney and the stomach, respectively
(Fig. 5B). The specificity of
co-immunoprecipitations of IP3R was confirmed by using
KRAP-KO mouse tissue as a control (Fig. 5B). Thus, KRAP physically interacts
with IP3R1 in the kidneys and with IP3R3 in
the stomach.
Discussion
In this study, we demonstrated that KRAP protein
expression and the subcellular localization was restricted beneath
the apical and/or basolateral membranes in specific cell types of
the stomach and the kidneys, in which KRAP physically associated
with particular IP3R subtype(s). In the KRAP-KO
mouse stomach and the kidneys, the polarized localization of
IP3R was impaired, indicating that KRAP plays critical
roles in the regulation of the proper subcellular localization of
IP3R in the stomach and the kidneys.
Notably, KRAP as well as IP3R3 proteins
were polarized beneath the apical membranes facing the gastric
gland lumen and were absent in the parietal cells (Fig. 1), suggesting an association of
these proteins with chief cell functions including pepsinogen
secretion (22–24). From this view point, KRAP
expression and the localization beneath the apical membranes of the
pancreatic acinar cells (19),
another type of zymogen cells, may suggest a similar role for KRAP
in the stomach and the pancreas. Considering the fact that KRAP
physically interacts with IP3R to regulate its proper
localization in these tissues, stomach (Figs. 2, 3 and 5) and pancreas (20), and that double-knockout of
IP3R2 and IP3R3 in mice revealed a failure in
secretory function in the pancreas (25), KRAP seems to be involved in the
exocrine systems. Actually, the pancreatic acinar cells in
KRAP-KO mice showed an increased amount of zymogen granules,
although they seemed to maintain the proper physiological
agonist-induced exocytosis (18).
Thus, exact functional relevance of KRAP and its interaction with
IP3R to the exocrine systems in the pancreas and the
stomach should await future studies.
It is of note that KRAP was restricted to both the
apical region and the basolateral region of the proximal tubular
cells of the kidneys (Fig. 4),
and that KRAP physically associated with IP3R1 in the
kidneys (Fig. 5). Furthermore,
our previous study showed that KRAP was distributed along the bile
canaliculi of hepatocytes and underneath the apical membrane of
pancreatic acinar cells (19).
All these KRAP localizations in the distinct tissues examined are
restricted to epithelial cell types bearing well-developed cell
polarity, cell-cell junction and microvilli, where transports of
various substances between epithelial cells and extracellular
spaces, exocrine space or blood stream occur (22,26–28). Since KRAP-KO mice displayed
profound metabolic disorders after birth without developmental
defects, and certain systemic inter-tissue dysregulations appeared
to underlie the metabolic phenotypes (17), KRAP might play physiological roles
in secretion and/or absorption functions after birth rather than in
developmental events.
Renal proximal tubules serve the reabsorption of the
bulk of substances filtered in the glomeruli and the excretion
(26,29). These two opposite transports are
accomplished by the coordinated action of ion channels and
transporters located in the brush border membrane and basolateral
membrane (29–31). Thus, the polarized expression of
these membrane proteins is crucial for the function of the proximal
tubules. Based on the findings that KRAP protein possesses
characteristic features like scaffolding protein, such as polarized
localization and transporting of IP3R, potential
functional relevance of KRAP to these processes would be
suspected.
In conclusion, we identified the exact
KRAP-expressing cells in the stomach and the kidneys, and found
that KRAP physically associates with IP3R to regulate
its proper subcellular localization in vivo. Considering the
KRAP function as an IP3R regulator and the importance of
KRAP in energy homeostasis in vivo, further research on the
exact relevance of the association between KRAP and IP3R
to the biological phenomena will lead to a better understanding of
physiological metabolic processes.
Acknowledgements
This work was supported in part by the
Ministry of Education, Culture, Sports, Science and Technology
(MEXT)-Supported Program for the Strategic Research Foundation at
Private Universities, a Grant-in-Aid for Scientific Research from
the Japan Society for the Promotion of Science. We thank Takami
Danno and Yoko Tanaka for their technical assistance.
References
|
1
|
Ross CA, Danoff SK, Schell MJ, Snyder SH
and Ullrich A: Three additional inositol 1,4,5-trisphosphate
receptors: molecular cloning and differential localization in brain
and peripheral tissues. Proc Natl Acad Sci USA. 89:4265–4269. 1992.
View Article : Google Scholar
|
|
2
|
Sharp AH, McPherson PS, Dawson TM, Aoki C,
Campbell KP and Snyder SH: Differential immunohistochemical
localization of inositol 1,4,5-trisphosphate- and
ryanodine-sensitive Ca2+ release channels in rat brain.
J Neurosci. 13:3051–3063. 1993.PubMed/NCBI
|
|
3
|
Sugiyama T, Yamamoto-Hino M, Miyawaki A,
Furuichi T, Mikoshiba K and Hasegawa M: Subtypes of inositol
1,4,5-trisphosphate receptor in human hematopoietic cell lines:
dynamic aspects of their cell-type specific expression. FEBS Lett.
349:191–196. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Newton CL, Mignery GA and Südhof TC:
Co-expression in vertebrate tissues and cell lines of multiple
inositol 1,4,5-trisphosphate (InsP3) receptors with distinct
affinities for InsP3. J Biol Chem. 269:28613–28619. 1994.PubMed/NCBI
|
|
5
|
Wojcikiewicz RJ: Type I, II, and III
inositol 1,4,5-trisphosphate receptors are unequally susceptible to
down-regulation and are expressed in markedly different proportions
in different cell types. J Biol Chem. 270:11678–11683. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jayaraman T, Ondriasová E, Ondrias K,
Harnick DJ and Marks AR: The inositol 1,4,5-trisphosphate receptor
is essential for T-cell receptor signaling. Proc Natl Acad Sci USA.
92:6007–6011. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Khan AA, Soloski MJ, Sharp AH, Schilling
G, Sabatini DM, Li SH, Ross CA and Snyder SH: Lymphocyte apoptosis:
mediation by increased type 3 inositol 1,4,5-trisphosphate
receptor. Science. 273:503–507. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sugawara H, Kurosaki M, Takata M and
Kurosaki T: Genetic evidence for involvement of type 1, type 2 and
type 3 inositol 1,4,5-trisphosphate receptors in signal
transduction through the B-cell antigen receptor. EMBO J.
16:3078–3088. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Scharenberg AM, Humphries LA and Rawlings
DJ: Calcium signalling and cell-fate choice in B cells. Nat Rev
Immunol. 7:778–789. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
deSouza N, Cui J, Dura M, McDonald TV and
Marks AR: A function for tyrosine phosphorylation of type 1
inositol 1,4,5-trisphosphate receptor in lymphocyte activation. J
Cell Biol. 179:923–934. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Patterson RL, Boehning D and Snyder SH:
Inositol 1,4,5-trisphosphate receptors as signal integrators. Annu
Rev Biochem. 73:437–465. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bezprozvanny I: The inositol
1,4,5-trisphosphate receptors. Cell Calcium. 38:261–272. 2005.
View Article : Google Scholar
|
|
13
|
Foskett JK, White C, Cheung KH and Mak DO:
Inositol trisphosphate receptor Ca2+ release channels.
Physiol Rev. 87:593–658. 2007. View Article : Google Scholar
|
|
14
|
Mikoshiba K: IP3 receptor/Ca2+
channel: from discovery to new signaling concepts. J Neurochem.
102:1426–1446. 2007.PubMed/NCBI
|
|
15
|
Zhang S, Fritz N, Ibarra C and Uhlén P:
Inositol 1,4,5-trisphosphate receptor subtype-specific regulation
of calcium oscillations. Neurochem Res. 36:1175–1185. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Inokuchi J, Komiya M, Baba I, Naito S,
Sasazuki T and Shirasawa S: Deregulated expression of KRAP, a novel
gene encoding actin-interacting protein, in human colon cancer
cells. J Hum Genet. 49:46–52. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fujimoto T, Miyasaka K, Koyanagi M,
Tsunoda T, Baba I, Doi K, Ohta M, et al: Altered energy homeostasis
and resistance to diet-induced obesity in KRAP-deficient mice. PLoS
One. 4:e42402009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Miyasaka K, Fujimoto T, Kawanami T,
Takiguchi S, Jimi A, Funakoshi A and Shirasawa S: Pancreatic
hypertrophy in Ki-ras-induced actin-interacting protein gene
knockout mice. Pancreas. 40:79–83. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fujimoto T, Koyanagi M, Baba I,
Nakabayashi K, Kato N, Sasazuki T and Shirasawa S: Analysis of KRAP
expression and localization, and genes regulated by KRAP in a human
colon cancer cell line. J Hum Genet. 52:978–984. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fujimoto T, Machida T, Tanaka Y, Tsunoda
T, Doi K, Ota T, Okamura T, et al: KRAS-induced actin-interacting
protein is required for the proper localization of inositol
1,4,5-trisphosphate receptor in the epithelial cells. Biochem
Biophys Res Commun. 407:438–443. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fujimoto T, Machida T, Tsunoda T, Doi K,
Ota T, Kuroki M and Shirasawa S: Determination of the critical
region of KRAS-induced actin-interacting protein for the
interaction with inositol 1,4,5-trisphosphate receptor. Biochem
Biophys Res Commun. 408:282–286. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mills JC, Andersson N, Stappenbeck TS,
Chen CC and Gordon JI: Molecular characterization of mouse gastric
zymogenic cells. J Biol Chem. 278:46138–46145. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu L, Hatakeyama J, Zhang B, Makdisi J,
Ender C and Forte JG: Novel insights of the gastric gland
organization revealed by chief cell specific expression of moesin.
Am J Physiol Gastrointest Liver Physiol. 296:G185–G195. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schubert ML: Gastric exocrine and
endocrine secretion. Curr Opin Gastroenterol. 25:529–536. 2009.
View Article : Google Scholar
|
|
25
|
Futatsugi A, Nakamura T, Yamada MK, Ebisui
E, Nakamura K, Uchida K, Kitaguchi T, et al: IP3 receptor types 2
and 3 mediate exocrine secretion underlying energy metabolism.
Science. 309:2232–2234. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wright SH and Dantzler WH: Molecular and
cellular physiology of renal organic cation and anion transport.
Physiol Rev. 84:987–1049. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Alrefai WA and Gill RK: Bile acid
transporters: structure, function, regulation and
pathophysiological implications. Pharm Res. 24:1803–1823. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Husain S and Thrower E: Molecular and
cellular regulation of pancreatic acinar cell function. Curr Opin
Gastroenterol. 25:466–471. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Anzai N, Jutabha P, Kanai Y and Endou H:
Integrated physiology of proximal tubular organic anion transport.
Curr Opin Nephrol Hypertens. 14:472–479. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hernando N, Wagner CA, Gisler SM, Biber J
and Murer H: PDZ proteins and proximal ion transport. Curr Opin
Nephrol Hypertens. 13:569–574. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Brône B and Eggermont J: PDZ proteins
retain and regulate membrane transporters in polarized epithelial
cell membranes. Am J Physiol Cell Physiol. 288:C20–C29.
2005.PubMed/NCBI
|