Introduction
Opium, the substance derived from the poppy plant,
Papaver somniferum, has been used in medicine as an
analgesic agent of pain reliever in different parts of the world
for over 6000 years. Morphine is one of the highly potent and
abundant alkaloids present in the opium, and is responsible for
analgesic property. As early as eighteenth century, morphine was
used in surgical procedures and pain management. The World Health
Organization (WHO) recommended its use for controlling pain in
cancer patients (1). Since
morphine also produces euphoric feeling, it has become one of the
highly abused drugs in the world currently. Long-term or chronic
use of morphine is shown to associate with drug tolerance (2). Drug tolerance not only limits the
use of morphine in clinical application but also involves tragic
circumstances in drug addicts. In vivo studies indicated
that morphine alters gene transcription in the brain (3) and spinal cord after acute and
chronic administration. Previous studies have demonstrated that
morphine induces long-term changes in neurons (4).
It is widely believed that the behavioral changes in
drug addicts could be due to the altered gene expression in central
nervous system (CNS). Studies demonstrated that μ-opioid
receptor (MOR-1) is the primary site of action for morphine and the
other most commonly used opioids (5,6).
The process of morphine tolerance is very complex (7), but from the clinical point of view,
it is important to understand the mechanism of its tolerance,
because it may lead to treatment and prevention of opiate
addiction. The MOR-1 gene expression is regulated at the level of
DNA transcription or post-transcription. Since the short-term
morphine treatment does not downregulate the MOR-1 receptor
(8), in the present work, we
studied the long-term chronic morphine treatment for drug tolerance
mechanism on the regulation of MOR-1 in SH-SY5Y cells and CHO cells
at the post-transcriptional level. In addition, we also
investigated the effect of morphine on the regulation of MOR-1
receptor mRNA levels in the presence of receptor antagonist
naloxone.
Materials and methods
Materials
Morphine sulfate, naloxone hydrochloride and
all-trans-retinoic acid were obtained from
Sigma-Aldrich® (St. Louis, MO, USA). All other routine
chemicals and reagents used were of analytical grade.
Cell cultures
The human neuroblastoma cells (SH-SY5Y) were
purchased from the American Type Culture Collection (Manassas, VA,
USA). The recombinant Chinese hamster ovary (CHO) cells,
transfected with human μ-opioid receptor gene (hMOR), were a
kind gift from Dr Richard Rothman, NIDA-NIH Addiction Research
Center (Baltimore, MD, USA). Both cell-types were maintained
separately as adherent monolayer cultures. The SH-SY5Y cells were
grown in the media without phenol-red, in a ratio of 1:1 mixture of
Dulbecco’s modified Eagle’s medium (DMEM) and Ham’s F12 medium
(Invitrogen, Molecular Probes, Eugene, OR, USA), with 2.5 mM
L-glutamine, 0.5 mM sodium pyruvate, and 1200 mg/l sodium
bicarbonate, supplemented with 10% FBS, penicillin (100
μg/ml) and streptomycin (100 U/ml). The recombinant CHO
cells, transfected with hMOR-1 gene, were grown in the same media
in a ratio of 1:1 as described above, containing phenol-red. The
medium was supplemented with 10% FBS, penicillin (100 μg/ml)
and streptomycin (200–250 U/ml). During experimental studies with
CHO cells, the phenol-red free medium was employed, supplemented
with all components as mentioned above. The cultures were
maintained in an atmosphere of humidified air with 5%
CO2 at 37°C in an incubator.
Differentiation of SH-SY5Y cells
The neuroblastoma cells (5x105) were
seeded in culture dishes in complete medium (30 ml), and allowed to
grow until the cells reached 70–80% confluence. All-trans-retinoic
acid (RA) was dissolved in 95% ethanol as a stock of 10 mM. A known
volume of RA stock was added to the cultures to attain a final
concentration of 10 μM (9). Control cells received an equal
volume of the vehicle (0.1%). All culture dishes were incubated for
72 h continuously without further renewal of growth medium in the
incubator.
Treatments with morphine in SH-SY5Y
cells
Morphine sulfate was dissolved in deionized water as
a 10 mM stock and added to the cultures to achieve a final
concentration of 10 μM (10). Control cells received an equal
volume of the vehicle. In some of the experiments, the cells were
pre-treated with 10 μM naloxone hydrochloride
(MOR-antagonist) for 1 h, followed by treatment with 10 μM
morphine sulfate for 24 h.
Treatments with morphine in recombinant
CHO cells
The CHO cells were seeded in culture dishes in
complete medium devoid of phenol-red. To the cells, a known volume
of morphine stock was added to the cultures to attain a final
concentration of 10 μM (10). Control cells received an equal
volume of the vehicle. All culture dishes were incubated for 24 h
continuously without further renewal of growth medium in the
incubator.
RNA isolation
At the end of 24 h treatment, cells were washed
three times with PBS to remove the drug compounds and the serum
proteins. Then the cells were harvested using cell scrapers, and
centrifuged at 1500 rpm for 3 min. The cell-pellets were
re-suspended in 1 ml PBS, and transferred into eppendorf tubes, and
subjected to centrifugation at 1000 rpm for 3 min. Finally, the
pellets were homogenized in 1 ml TRIzol reagent with VirTishear
polytron homogenizer (Virtis Company, Inc., Cardiner, NY, USA).
Total RNA was extracted with chloroform and isopropanol according
to the manufacturer’s instructions (Invitrogen, Carlsbad, CA, USA).
Following ethanol precipitation, the vacuum-dried RNA was dissolved
in 100 μl of DEPC-water. The quantity of total RNA was
measured by the Nanodrop ND-1000 spectrophotometer (NanoDrop
Technologies, Wilmington, DE, USA). RNA was subjected to DNAase
treatment for 30 min at 37°C using DNase Treatment and Removal
Reagent (Ambion, Austin, TX, USA). The purified RNA with A260/A280
ratio of ≥1.8 was subsequently used for cDNA synthesis.
cDNA synthesis
The cDNA synthesis was performed with an iScript
cDNA synthesis kit (Bio-Rad, Hercules, CA, USA) using 10 μg
of total RNA according to the manufacturer’s instructions.
Data analyses
Data were presented as mean ± standard error of the
mean (SEM). Differences between the means were compared by
Student’s t-test. Statistical significance is ascribed for
P<0.05. Curve fitting was conducted using GraphPad Prism 3.02
(GraphPad Software Inc., San Diego, CA).
Results
Morphological differentiation of SH-SY5Y
cells
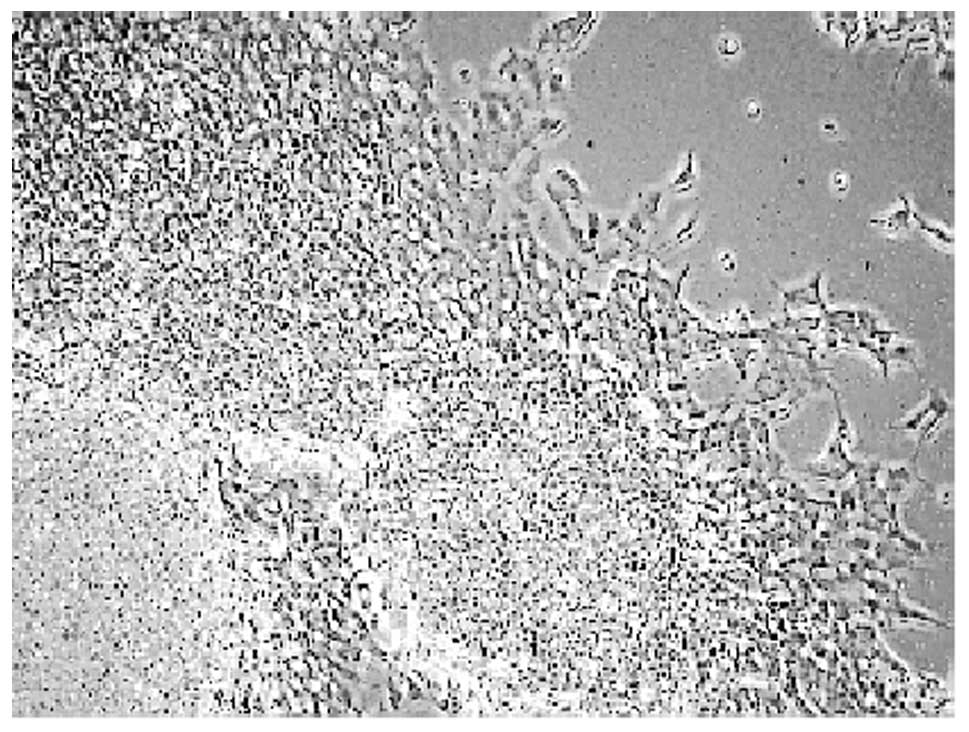
It was observed that the undifferentiated
neuroblastoma cells grew mostly together in the form of clumps
(Fig. 1A). On the other hand,
cells treated with all-trans-retinoic acid (RA) for 72 h resulted
in a significant cellular differentiation, characterized with
elongated neurites (Fig. 1B). The
concentration of RA was based on previous studies (10). Our results clearly demonstrate
that RA promotes SH-SY5Y cell differentiation.
Regulation of MOR-1 gene expression in
SH-SY5Y cells
The mRNA levels of MOR-1 were quantitated by
real-time RT-PCR using MOR-1 specific primers listed in Table I. β-actin, a housekeeping gene,
was used for the normalization of gene expression. It was observed
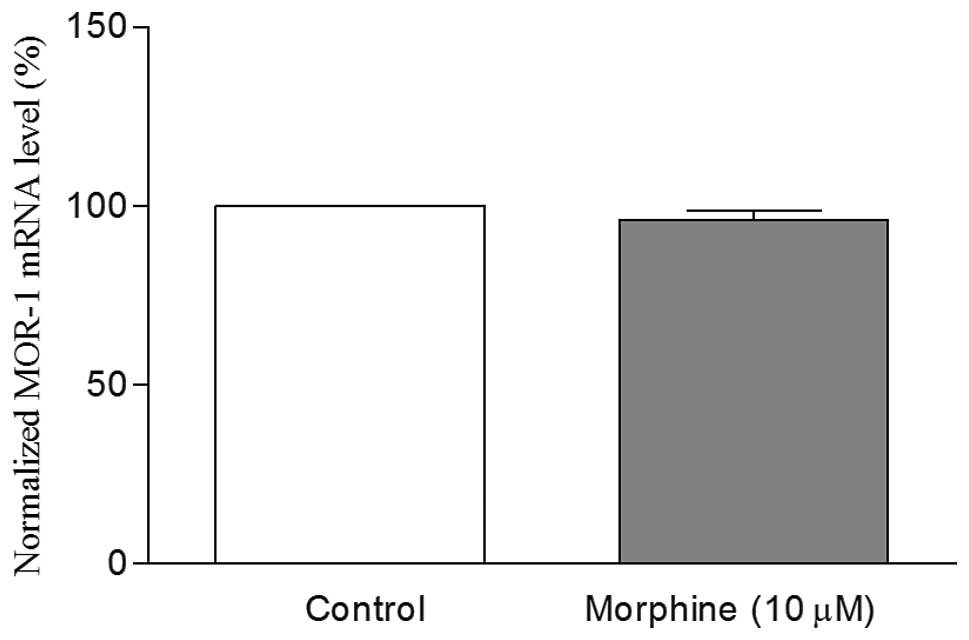
that the MOR-1 mRNA levels in undifferentiated cells with 10
μM morphine treatment for 24 h remained the same as those of
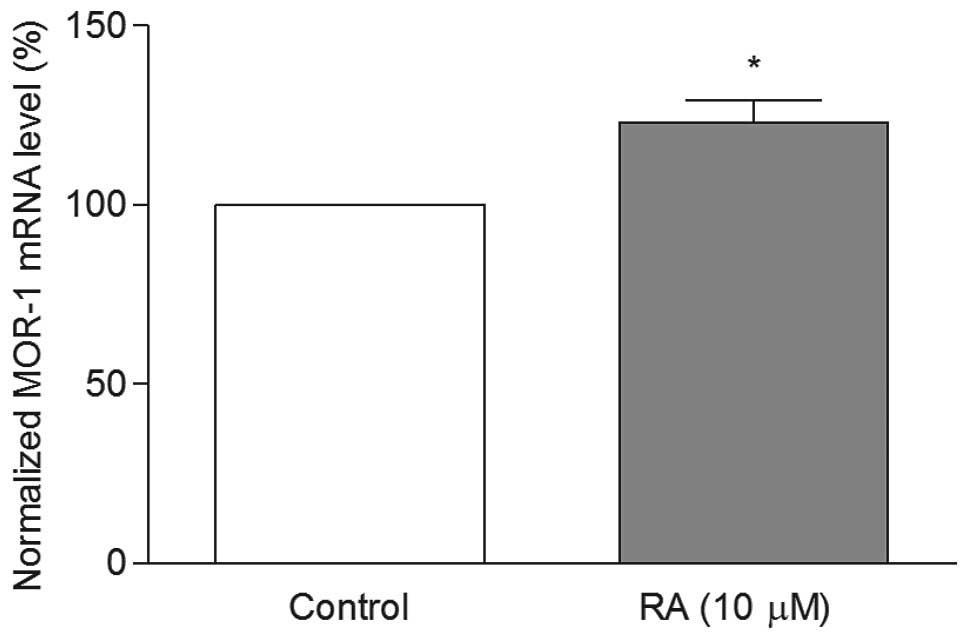
the undifferentiated control cells (Fig. 2). However, in the RA
differentiated cells, these levels were significantly increased in
comparison to the undifferentiated control cells (Fig. 3). These results clearly show that
the MOR-1 mRNA levels depend on the cellular differentiation. When
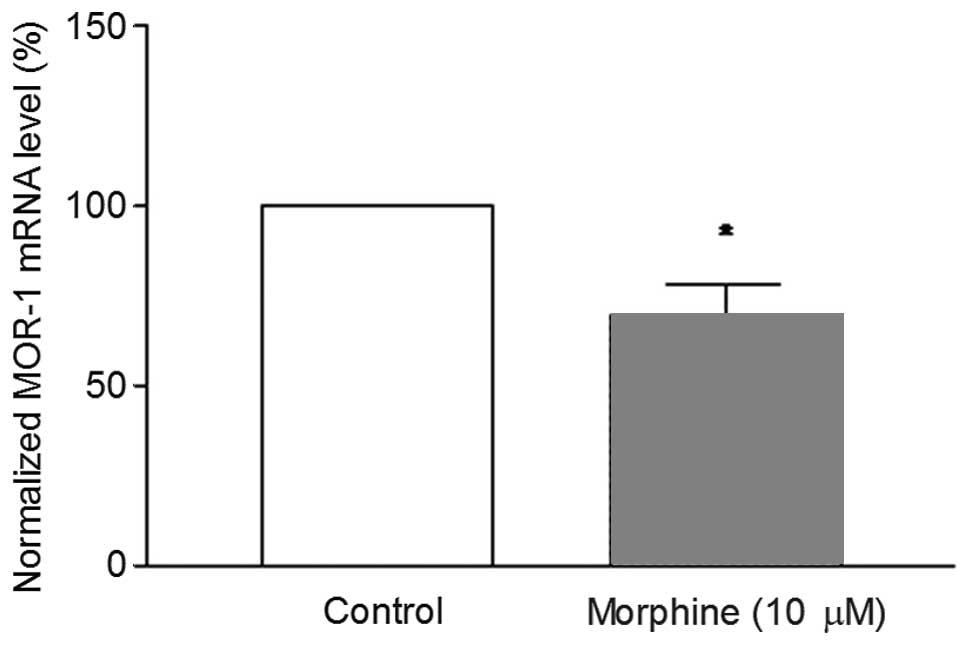
the differentiated cells were treated with 10 μM morphine
for 24 h, the MOR-1 mRNA levels were significantly reduced compared
to differentiated control cells (Fig.
4). The results indicate that the MOR-1 gene regulation with
morphine treatment depends on the cellular differentiation.
 | Table ISequence of the primers used in
real-time PCR for human SH-SY5Y cells. |
Table I
Sequence of the primers used in
real-time PCR for human SH-SY5Y cells.
| mRNA | Primers |
|---|
| MOR-1 | F:
5′-ATGCCAGTGCTCATCATTAC-3′ |
| R:
5′-GATCCTTCGAAGATTCCTGTCCT-3′ |
| β-actin | F:
5′-GATGAGATTGGCATGGCTTT-3′ |
| R:
5′-CACCTTCACCGTTCCAGTTT-3′ |
Regulation of MOR-1 gene expression in
hMOR-CHO cloned cells
In an effort to understand the extent of MOR-1 gene
regulation in a different cell system, we used Chinese hamster
ovary (CHO) cells that were transfected with hMOR which stably
express the MOR protein. The cells were treated with 10 μM
morphine for 24 h, and the mRNA levels were measured by real-time
RT-PCR. It was found that morphine treatment caused decrease in the
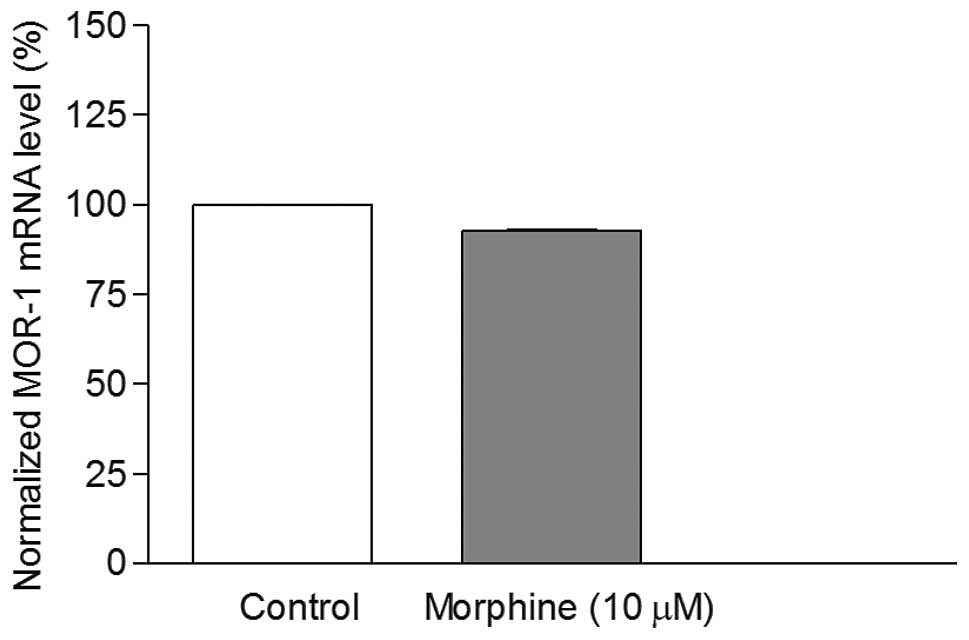
mRNA levels (Fig. 5). However,
this decrease was not significant (P>0.05). Therefore, the CHO
cells were not utilized in our further studies.
Reversal of MOR-1 mRNA downregulation by
naloxone
The effect of naloxone, which is an antagonist for
MOR, was studied on the regulation of MOR-1 gene expression in
morphine-treated cells. For this purpose, the differentiated human
SH-SY5Y cells were pretreated with 10 μM naloxone for 1 h,
followed by co-treatment with 10 μM morphine for 24 h. It
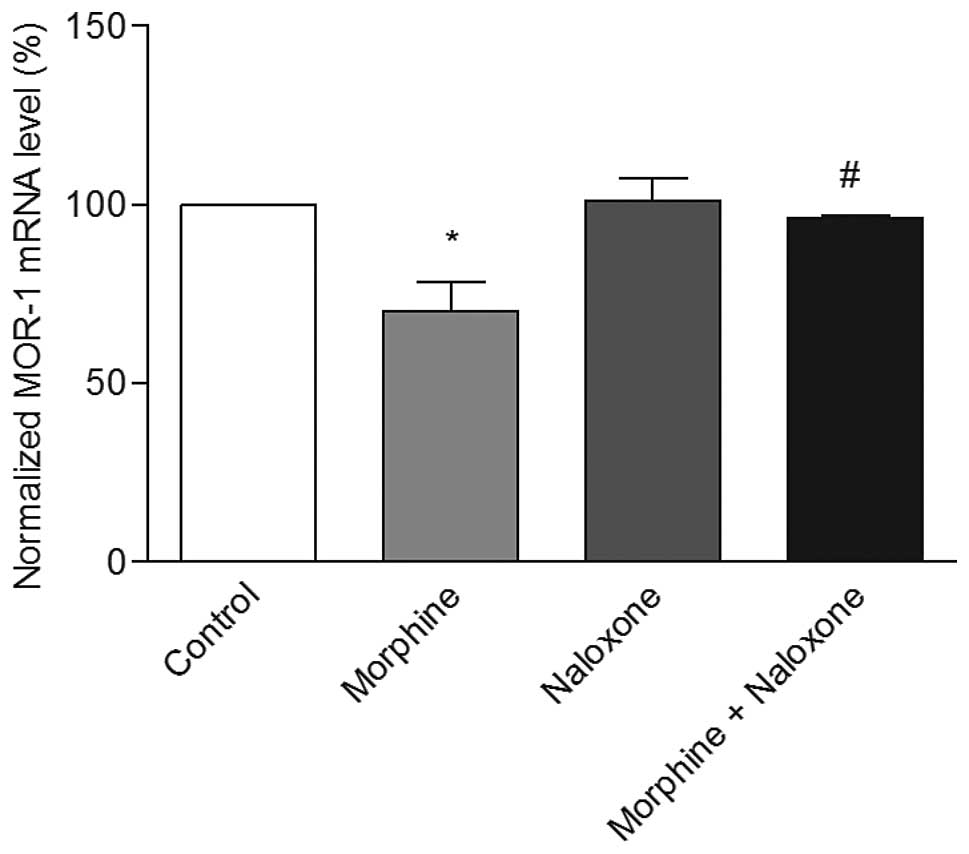
was observed that morphine treatment caused a significant decrease
in the MOR-1 mRNA levels, while naloxone alone did not alter these
levels. However, in naloxone pretreated cells, morphine treatment
did not decrease the MOR-1 mRNA levels (Fig. 6), and remained almost the same
levels as those of the control. These results clearly show that
naloxone reverses the morphine-induced downregulation of MOR-1 gene
expression.
Discussion
In the present study, the SH-SY5Y neuroblastoma
cells were used as an in vitro model to investigate the
effect of morphine on MOR-1 gene expression. This cell line was
originally derived from neuroblastoma SK-N-SH clone, and expresses
μ and δ receptors (11). Usually,
the SH-SY5Y cells are differentiated by all-trans-retinoic acid
(RA) to achieve neurite-outgrowth and morphological features
(12). Both differentiated and
undiffer entiated SH-SY5Y cells were used as model cultures in
neuroscience research (13–16). This posed a selection problem
between differentiated and undifferentiated SH-SY5Y cells for our
studies.
In order to find a suitable answer for this
question, we first compared the MOR-1 gene expression levels in
undifferentiated SH-SY5Y cells with morphine treatment. It was
observed that morphine treatment did not alter the MOR-1 mRNA
levels in these cells compared to undifferentiated control cells
(Fig. 2). However, the levels
were significantly upregulated in RA differentiated SH-SY5Y cells
compared to the undifferentiated cells (Fig. 3). The results highlight that the
process of differentiation appears to modulate the response to
morphine treatment. These observations were consistent with
previous report, where MOR-1 mRNA levels were shown to upregulate
in RA differentiated SH-SY5Y cells (17). Since the MOR-1 levels were higher
in the differentiated cells than the undifferentiated cells, we
preferred to differentiate the cells with RA for further
studies.
The morphological features of differentiated cells
clearly showed that the cells have elongated neurite extensions
(Fig. 1), which are in agreement
with previous reports (18,19). We next studied the effect of
morphine on MOR-1 mRNA levels in the differentiated cells. It was
found that morphine downregulated the MOR-1 levels significantly
(Fig. 4). The downregulation of
MOR-1 with morphine treatment was also observed earlier in
different cell lines (20–22).
We further studied the effect of morphine in
recombinant CHO cells for MOR-1 mRNA levels. Morphine treatment did
not alter the mRNA levels significantly in these cells (Fig. 5). The results clearly suggest that
regulation of MOR-1 gene expression is cell-type specific. Earlier
studies on recombinant CHO cells confirmed our results in terms of
having no alteration in mu opoid receptor protein with morphine
treatment (23).
Since morphine treatment caused downregulation of
MOR-1 mRNA levels in our study, we investigated the compounds that
act as antagonist to MOR-1 receptor to prevent the down-regulation
of MOR-1 gene. Naloxone, an opioid antagonist, was employed in our
studies with 1 h pretreatment, prior to morphine treatment. It was
observed that naloxone-pretreatment blocked the downregulation of
MOR-1 gene expression significantly (Fig. 6). Similar observation was reported
earlier, where naloxone was shown to block the downregulation of
receptor protein with morphine treatment (10).
In conclusion, chronic morphine treatment caused the
downregulation of MOR-1 gene expression in human differentiated
SH-SY5Y cells, while naloxone reversed this process. The results
clearly demonstrate that antagonists have a potential role in the
treatment against morphine drug addiction.
Abbreviations:
|
CNS
|
central nervous system
|
|
FBS
|
fetal bovine serum
|
|
MOR-1
|
μ-opioid receptor
|
|
PBS
|
phosphate-buffered saline
|
|
RA
|
all-trans-retinoic acid
|
|
WHO
|
World Health Organization
|
Acknowledgements
This study was supported by the
NCRR/RCMI G12 RR03020, the NIGMS/MBRS/SCORE GM08111, and the HRSA
SD34HP0 4018 grants.
References
|
1.
|
World Health OrganizationCancer Pain
ReliefGeneva1986
|
|
2.
|
E CollinF CesselinNeurobiological
mechanisms of opioid tolerance and dependenceClin
Neuropharmacol14465488199110.1097/00002826-199112000-000011663419
|
|
3.
|
Z ZhuRB BadisaDE PalmRegulation of rat
MOR-1 gene expression after chronic ICV administration of
morphineMol Med Rep5513516201222089925
|
|
4.
|
MT OliveiraAC RegoMT MorgadinhoMacedo:
Toxic effects of opioid and stimulant drugs on undifferentiated
PC12 cellsAnn NY Acad Sci
USA965487496200210.1111/j.1749-6632.2002.tb04190.x12105124
|
|
5.
|
HH LohAP SmithMolecular characterization
of opioid receptorsAnnu Rev Pharmacol
Toxicol30123147199010.1146/annurev.pa.30.040190.001011
|
|
6.
|
MJ KreekOpioid receptors: some
perspectives from early studies of their role in normal physiology,
stress responsivity, and in specific addictive diseasesNeurochem
Res2114691488199610.1007/BF025323878947936
|
|
7.
|
SL BorglandAcute opioid receptor
desensitization and tolerance: is there a link?Clin Exp Pharmacol
Physiol28147154200110.1046/j.1440-1681.2001.03418.x11207668
|
|
8.
|
MP CastelliM MelisM MameliChronic morphine
and naltrexone fail to modify mu-opioid receptor mRNA levels in the
rat brainBrain Res Mol Brain
Res45149153199710.1016/S0169-328X(96)00305-19105683
|
|
9.
|
Y-T CheungWK-W LauM-S YuEffects of
all-transretinoic acid on human SH-SY5Y neuroblastoma as in vitro
model in neurotoxicity
researchNeurotoxicology30127135200910.1016/j.neuro.2008.11.00119056420
|
|
10.
|
JE ZadinaSL ChangLJ GeMu opiate receptor
down-regulation by morphine and up-regulation by naloxone in
SH-SY5Y human neuroblastoma cellsJ Pharmacol Exp
Ther26525426219938097244
|
|
11.
|
VC YuG HochhausFG ChangDifferentiation of
human neuroblastoma cells: Marked potentiation of prostaglandin
E-stimulated accumulation of cyclic AMP by retinoic acidJ
Neurochem5118921899198810.1111/j.1471-4159.1988.tb01174.x2903224
|
|
12.
|
S PahlmanAI RuusalaL AbrahamssonRetinoic
acid-induced differentiation of cultured human neuroblastoma cells:
a comparison with phorbolester-induced differentiationCell
Differ14135144198410.1016/0045-6039(84)90038-16467378
|
|
13.
|
Y LevitesMB YoudimG MaorAttenuation of
6-hydroxydopamine (6-OHDA)-induced nuclear factor-kappaB
(NF-kappaB) activation and cell death by tea extracts in neuronal
culturesBiochem
Pharmacol632129200210.1016/S0006-2952(01)00813-911754870
|
|
14.
|
Y LevitesT AmitS MandelNeuroprotection and
neurorescue against Abeta toxicity and PKC-dependent release of
nonamyloidogenic soluble precursor protein by green tea polyphenol
(−)-epigallocatechin-3-gallateFASEB J17952954200312670874
|
|
15.
|
S XueL JiaJ JiaHypoxia and reoxygenation
increased BACE1 mRNA and protein levels in human neuroblastoma
SH-SY5Y cellsNeurosci
Lett405231235200610.1016/j.neulet.2006.07.01316901640
|
|
16.
|
JH LeeSY ShinS KimSuppression of PTEN
expression during aggregation with retinoic acid in P19 mouse
embryonal carcinoma cellsBiochem Biophys Res
Commun347715722200610.1016/j.bbrc.2006.06.16116842746
|
|
17.
|
X YuX MaoAD BlakeMorphine and endomorphins
differentially regulate μ-opioid receptor mRNA in SH-SY5Y
human neuroblastoma cellsJ Pharmacol Exp
Ther306447454200310.1124/jpet.103.04869412754318
|
|
18.
|
M VesanenM SalminenM WessmanMorphological
differentiation of human SH-SY5Y neuroblastoma cells inhibits human
immunodeficiency virus type 1 infectionJ Gen
Virol75201206199410.1099/0022-1317-75-1-201
|
|
19.
|
M Clagett-DameEM McNeillPD MuleyRole of
all-trans retinoic acid in neurite outgrowth and axonal elongationJ
Neurobiol66739756200610.1002/neu.2024116688769
|
|
20.
|
N YabaluriF MedzihradskyDown-regulation of
mu-opioid receptor by full but not partial agonists is independent
of G protein couplingMol
Pharmacol52896902199710.1124/mol.52.5.8969351981
|
|
21.
|
PL TaoKF HanSD WangImmunohistochemical
evidence of down-regulation of mu-opioid receptor after chronic
PL-017 in ratsEur J
Pharmacol344137142199810.1016/S0014-2999(97)01596-39600647
|
|
22.
|
J YamamotoT KawamataY
NiiyamaDownregulation of mu opioid receptor expression within
distinct subpopulations of dorsal root ganglion neurons in a murine
model of bone cancer
painNeuroscience151843853200810.1016/j.neuroscience.2007.11.02518178319
|
|
23.
|
H XuX WangD ZimmermanChronic morphine
up-regulates Gα12 and cytoskeletal proteins in Chinese hamster
ovary cells expressing the cloned μ-opioid receptorJ
Pharmacol Exp Ther3152482552005
|