Introduction
Esophageal cancer patients have a survival rate
lower than 40% in the first year due to late diagnosis, high
incidence of post-surgical locoregional recurrence and frequent
distant metastasis. For patients undergoing potentially curative
surgery, 5-year survival rates are lower than 25% (1–3).
Therefore, new strategies with better antitumor activity, such as
gene therapy are required (4–6).
Oncolytic viruses (OVs) are members of a class of
adenoviruses (Ads) that selectively replicate in tumor cells and
lyse infected cells. OVs have been extensively investigated as
novel anticancer agents. A variety of strategies have been
developed to establish selectivity to transformed cells, including
deleting the viral genome or replacing the viral endogenous
promoters through cancer-selective promoters (7,8).
Collectively, these approaches produce viral agents which have the
ability to replicate in target tumor cells and kill these cells by
virtue of replicating in them. Viral oncolysis may also be achieved
through infecting adjacent tumor cells through the release of
progeny virions and the activation of antitumoral immune responses.
Theoretically, the infecting OVs may spread through a solid tumor
and eliminate the tumor (9). The
H101 virus produced by Shanghai Sunway Biotech also contains a
deletion in the E1B 55 gene and E3 region. This recombinant
Ad has a replication-selective property and replicates only in
tumor cells. Although there are several promising advances
involving OVs, their clinical efficacy in human trials has failed
to fulfill high expectations, and single-agent efficacy of OVs
remains to be improved. The coxsackievirus and adenovirus receptor
(CAR) is considered a surrogate marker to monitor the outcome of
OVs (10).
CAR, which functions as a primary receptor for both
the coxsackie B virus (CVB) and Ads, plays a crucial role in gene
transfer efficacy (11). Recent
studies have indicated that CAR levels are closely related with Ad
attachment, infection or transgene expression (12). Furthermore, CAR is also considered
a surrogate marker for monitoring and/or predicting the outcome of
Ad-mediated gene therapy. Accumulating evidence indicates that CAR
expression levels are lower in several types of tumors, such as
ovarian, lung, breast and bladder when compared to their normal
counterpart (13–16).
Histone deacetylation is an important component of
the epigenetic regulation of gene expression (17). Histone deacetylation also
regulates CAR expression. Kitazono et al (18) demonstrated that the histone
deacetylase inhibitors (HDACi) FR901228 (depsipeptide),
trichostatin A (TSA) and valproic acid (VPA) increased the protein
levels of CAR in 6 cancer cell lines, they found that the small
molecular inhibitors regulate CAR expression through histone
deacetylation modification.
In the present study, the protein level of CAR in
esophageal squamous cell carcinoma (ESCC) cell lines and its
correlation to the antitumor efficiency of oncolytic Ad H101 were
investigated. The effect of TSA on the protein level of CAR and the
related signaling pathway were also evaluated.
Materials and methods
Cell culture
Positive control, HeLa cells and ESCC cell lines,
EC9706 and EC1, were propagated in a monolayer culture in RPMI-1640
medium supplemented with 10% fetal inactived bovine serum (56°C, 30
min), 1×105 U/l penicillin and 100 mg/l streptomycin in
an incubator with a humidified atmosphere of 5% CO2 at
37°C.
Compounds
TSA was purchased from Sigma Corporation. Cell lines
were treated with 0.1, 0.3, 0.5 and 1.0 μmol/l TSA for 24
and 48 h. The protein levels of CAR in EC1 cells were detected with
immunocytochemical analysis, flow cytometry and western blot
analysis. The positive rate of CAR protein expression evoked by
different concentrations of TSA in EC9706 and EC1 cells was
analyzed using flow cytometry (FCM).
Immunocytochemical analysis
Cells (5×104) were seeded on glass
coverslips in 24-well plates. After a 24-h-incubation, the cells
were treated with different concentrations of drugs for 48 h, then
fixed with 4% paraformaldehyde for 30 min. Cells were then treated
with 3% H2O2 for 30 min to remove the
endogenous peroxidase activity. Cells were incubated with rabbit
anti-CAR monoclonal antibody (1:200; Santa Cruz Biotechnology,
Inc.) overnight at 4°C. After washing 3 times with PBS, the cells
were incubated at room temperature with biotin-labeled secondary
antibody for 1 h. The resultant immune activity was developed in
0.5% 3,3′-diaminobenzi-dine hydrochloride (DAB) for 3 min.
Appropriate negative controls were performed by omitting the
primary antibody and/or substituting it with an irrelevant
antiserum. HeLa cells were used as the positive control (19).
Flow cytometric analysis
Cells were incubated with different concentrations
of TSA for 48 h, and were then washed with PBS and harvested with
2.5 g/l trypsin and resuspended in PBS containing 1% BSA. The cells
were resuspended in PBS with a concentration of
1×106/ml. The rabbit against human anti-CAR monoclonal
antibody was incubated with the cells for 30 min in the dark at
4°C. After being washed with PBS-BSA, cells were incubated in a
1:100 dilution of FITC-labeled goat anti-rabbit IgG (Sigma) for 1 h
at 4°C. Cells (1×105/ml) were analyzed immediately using
FACS.
Western blotting
Cells were washed twice in cold PBS, collected and
then lysed in lysis buffer (50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 1%
Nonidet P-40, 0.25% sodium deoxycholate, 0.1% SDS, 1 mM EDTA and
protease inhibitor mixture). After 20 min on ice, the lysates were
spun at 14,000 rpm in a microcentrifuge at 4°C for 10 min. The
supernatants were used as whole cell extracts. Cell lysates (50
μg) were separated on a 10% SDS-polyacrylamide gel and
transferred on polyvinylidene fluoride (PVDF) membranes. The
membranes were incubated with 5% non-fat dried milk and were
incubated with the primary antibodies against CAR (1:200), or
anti-p44/42 ERK1/2 Ab (1:1,000, Thr202/Tyr204; Cell Signaling
Technology, Inc.) at 4°C overnight. HRP-IgG secondary antibody was
added and incubation was carried out for 2 h at room temperature.
An ECL chemiluminescent detection kit (ECL; Pierce Biotechnology,
Inc.) was used to detect target proteins. The bands were subjected
to densitometry for quantitation using Bio-Rad Quantity One
software.
Viral replication
Cells were seeded in 6-well plates
(5×105/well) for 24 h before the infection and were then
infected with H101 Ads at a multiplicity of infection (MOI) of 1.
The cells and the supernatants were collected 48 h after the
infection and freeze-thawed thrice, serially diluted and titered by
the limiting dilution method [determination of the 50% tissue
culture infective dose (TC ID50) using HEK293
cells].
Cell viability assay
Cells were seeded in 96-well plates
(5×103/well). After a 24-h incubation, cells were
infected with H101 at a MOI of 0.01, 0.1, 1, 10 and 100. Cells
treated with PBS were used as the negative control. Each data point
was obtained in quintuple. After a 72-h infection, the cells were
washed with PBS to remove the free virus in the medium. Cell
viability was measured using the Cell Counting Kit-8 (CCK-8).
Control absorbance was designated as 100%, and cell viability was
expressed as a percentage of the control absorbance.
MTT assay
Cells were seeded in 96-well plates at a density of
5×103/well. After incubation for 24 h, the medium was
removed and replaced with medium containing various concentrations
of TSA. Cells treated with identical concentrations of DMSO
(diluent for depsipeptide) were used as the control. After 24 or 48
h of incubation with TSA, cell viability was measured using MTT
[3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide]. The
number of cells was determined using an enzyme-linked immunosorbent
assay at 490 nm wavelength using an enzyme-labeling instrument.
Data was obtained in triplicate.
Statistical analysis
All results are presented as the means ± standard
deviation (SD). Statistical analysis was performed by ANOVA using
SPSS 11.0 software. P<0.05 was considered to indicate a
statistically significant difference.
Results
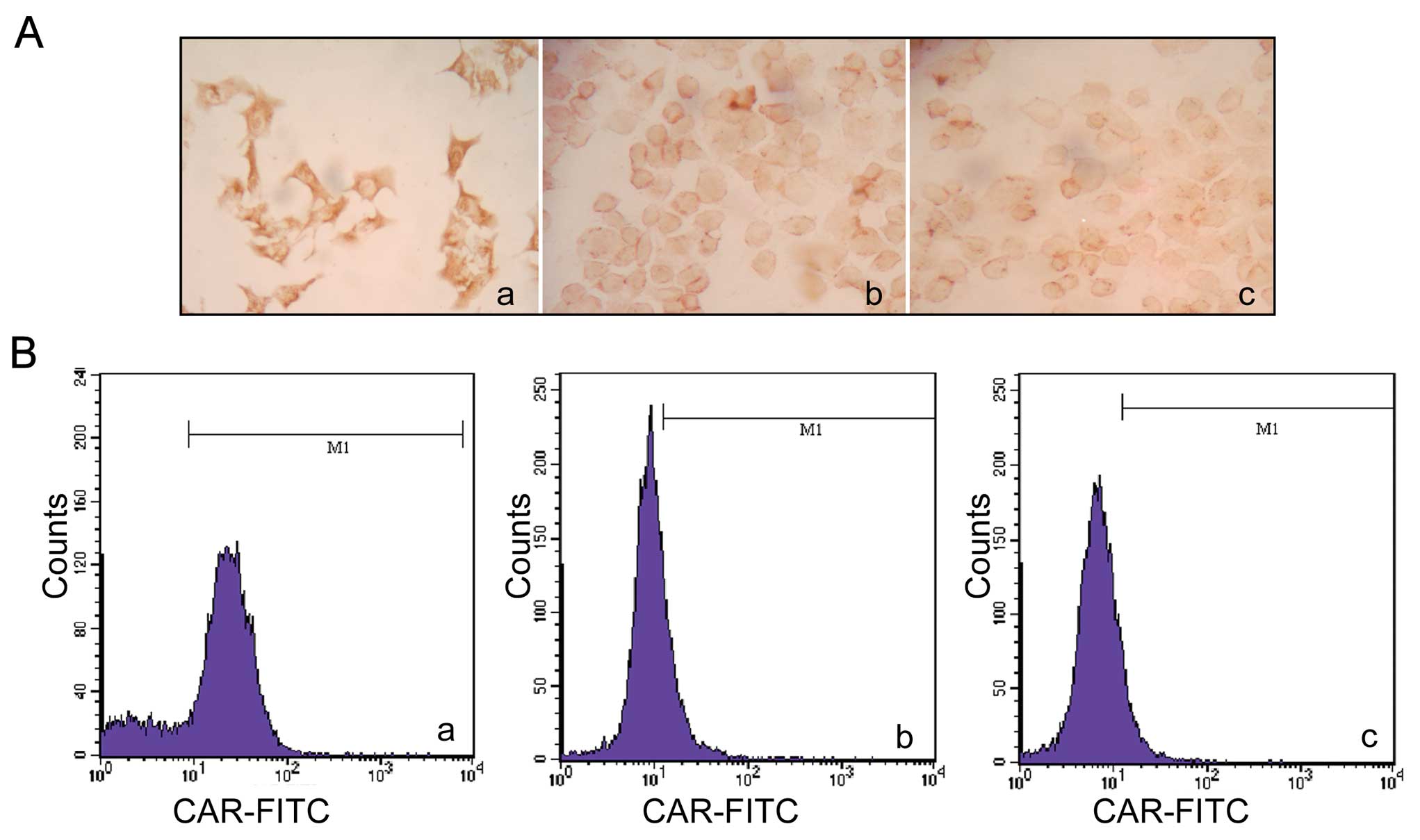
CAR expression in ESCC cell lines
CAR expression levels have been found to be lower in
several types of tumors. To investigate the expression levels of
CAR in ESCC cell lines, we performed immunocytochemistry assay,
flow cytometry and western blot analysis. EC9706 and EC1 cell lines
were evaluated with HeLa cells used as the positive control. CAR
was expressed in the 3 cell lines examined. In the
immunocytochemistry assay, brown coloration was not noted in the
EC9706 and EC1 cells compared with the positive control cells
(Fig. 1A). The positive rate of
CAR expression in the 2 ESCC cell lines was lower compared to the
HeLa cells as detected with FCM (Fig.
1B). The percentages of CAR expression were 21.00±2.00,
9.67±3.05 and 74.67±9.45% in the EC9706, EC1 and HeLa cells,
respectively. Western blot analysis also demonstrated that the CAR
protein level decreased in the ESCC cell lines, compared to the
positive control (Fig. 1C).
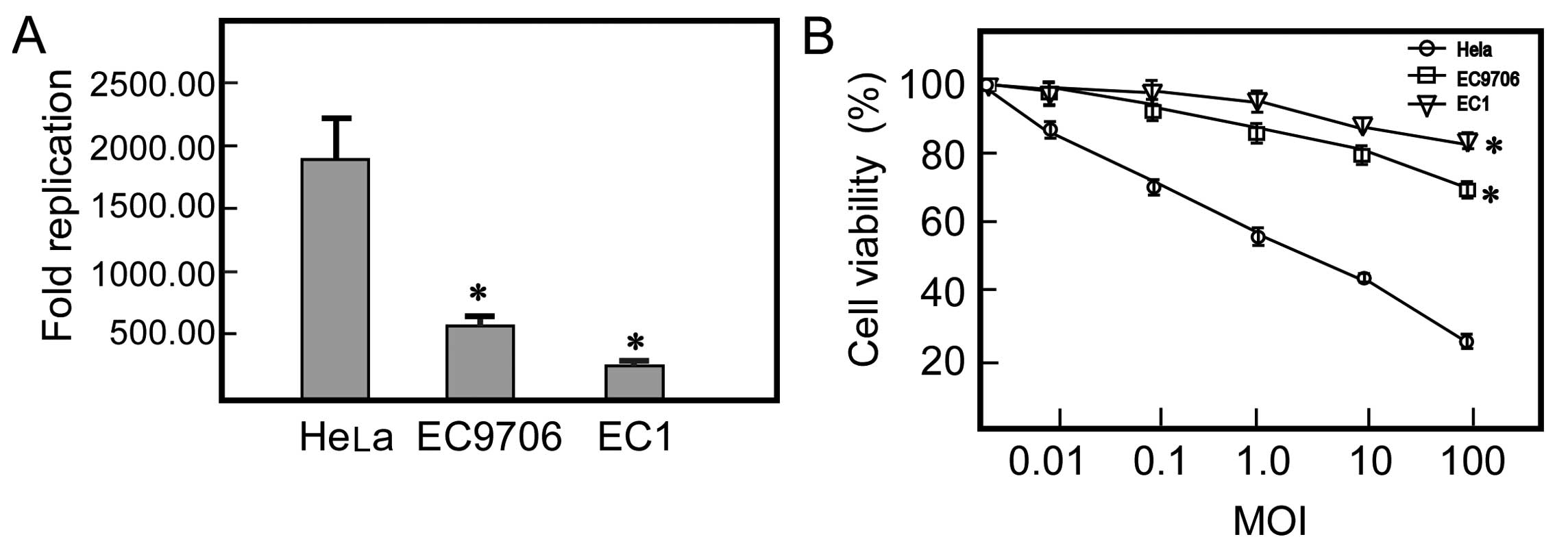
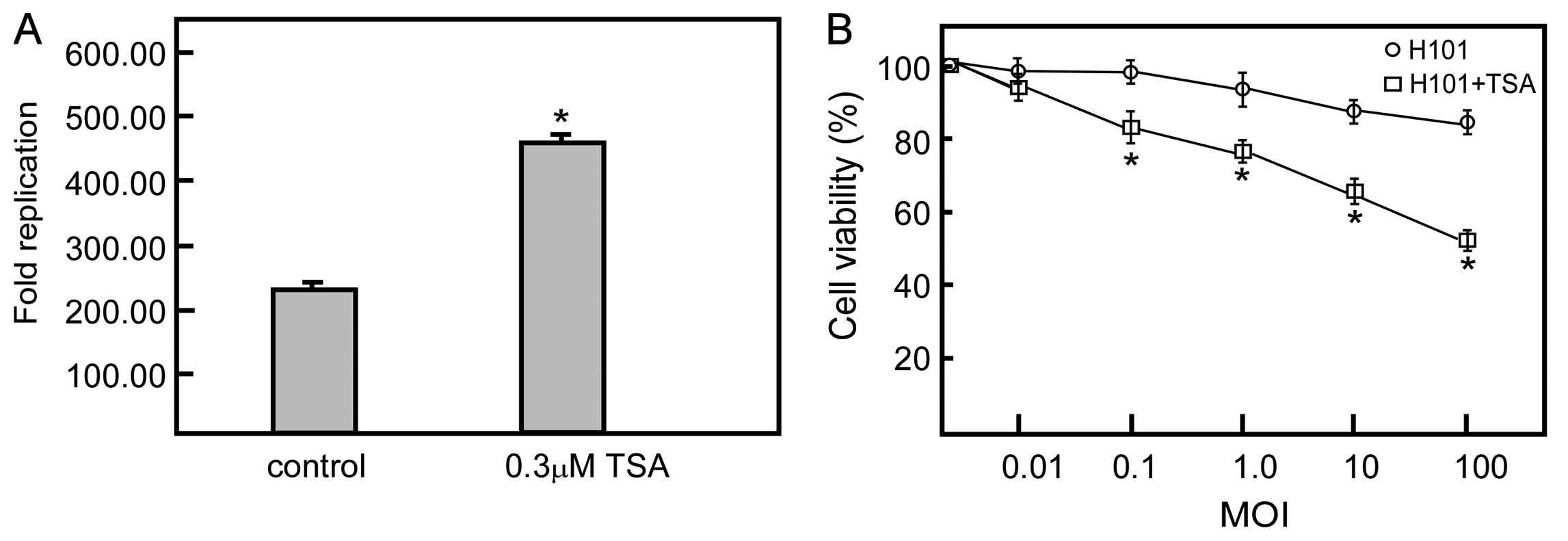
Replication of H101 and cytotoxicity of
H101 in ESCC cell lines
Decreased expression of the CAR protein on the
surface of cancer cells, which is required for efficient virus
entry into target cells, is a potential explanation for the limited
activity. We examined the replication of H101 in the 2 ESCC cell
lines and the HeLa cells. In the CAR-deficient ESCC cell lines,
H101 displayed poor replication ability. After 48 h of infection,
titers increased 538.33- and 240.55-fold in the EC9706 and EC1
cells, respectively. By contrast, the replication of H101 increased
1,825.00-fold when compared to the initial titer in the
CAR-positive cells. To evaluate the correlation between the
expression levels of CAR in the human ESCC cells and the oncolytic
activity of H101, we treated ESCC and HeLa cells with H101 at
various levels of MOI ranging from 0.01 to 100 pfu/cell. The cell
viability rate of the EC9706 and EC1 cells was respectively 69.47
and 84.35% at a MOI of 100. Whereas, HeLa cells treated with H101
dramatically reduced cell viability compared to the ESCC cells at
the same MOIs. These results indicated that a low CAR expression in
the 2 ESCC cell lines decreased the antitumor activity of oncolytic
Ad H101 (Fig. 2).
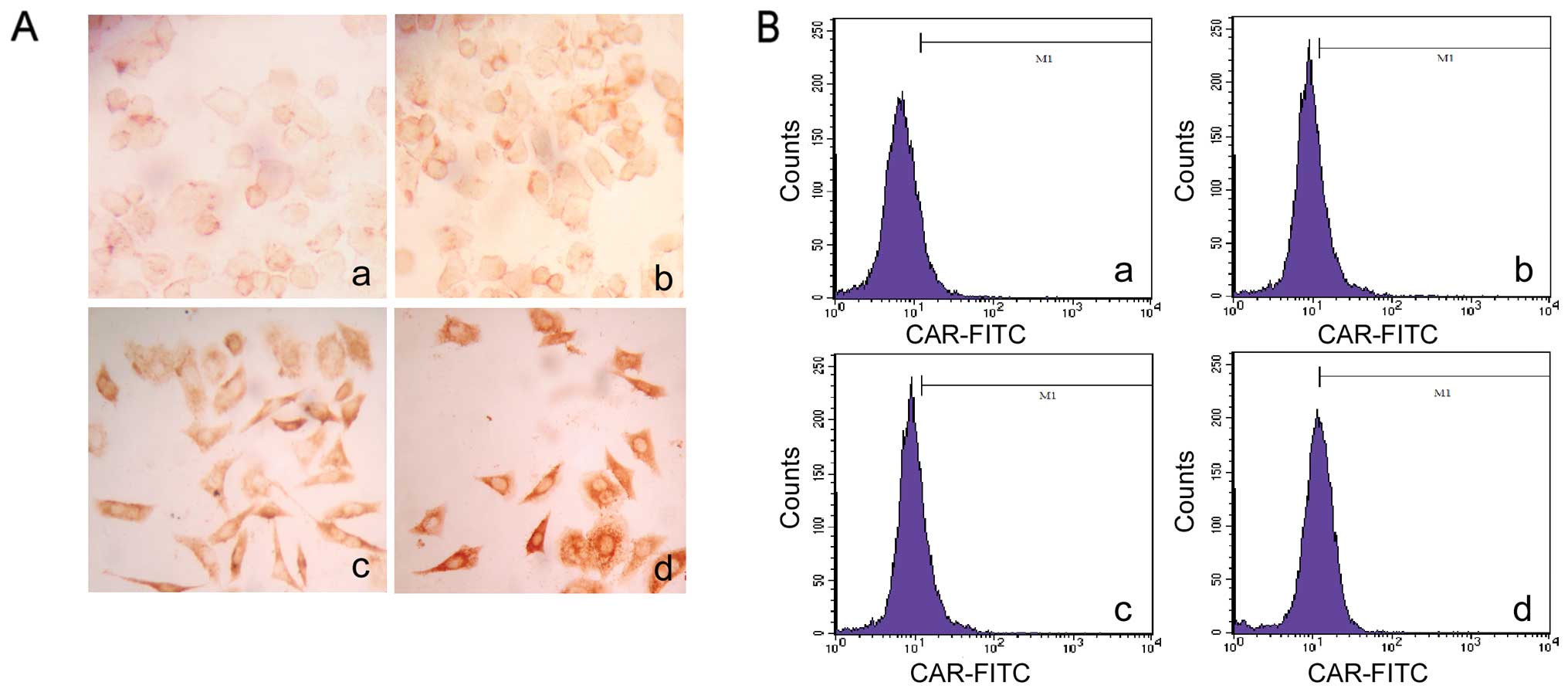
Effect of TSA on CAR expression in
ESCC
TSA is an HDAC inhibitor which blocks the activity
of histone deacetylases (HDACs), leading to increased acetylation
of histones and other proteins (20). The positive rate of CAR expression
in the EC1 cells was lower compared to the rate in the EC9706 cells
thus affecting the antitumor activity of H101. Therefore, we
modulated CAR expression in the EC1 cells to enhance the efficacy
of H101. To investigate whether TSA regulates CAR expression, we
first used FCM to detect the expression of CAR in the EC1 cells
induced by TSA. After different doses of TSA treatment, the
percentages of CAR-positive cells were 20.93, 27.77 and 40.63% in
the 0.3, 0.5 and 1.0 μmol/l treatment groups.
Immunocytochemical, flow cytometric and western blot analyses
demonstrated that the protein level of CAR increased in a
dose-dependent manner in the EC1 cells after TSA treatment
(Fig. 3).
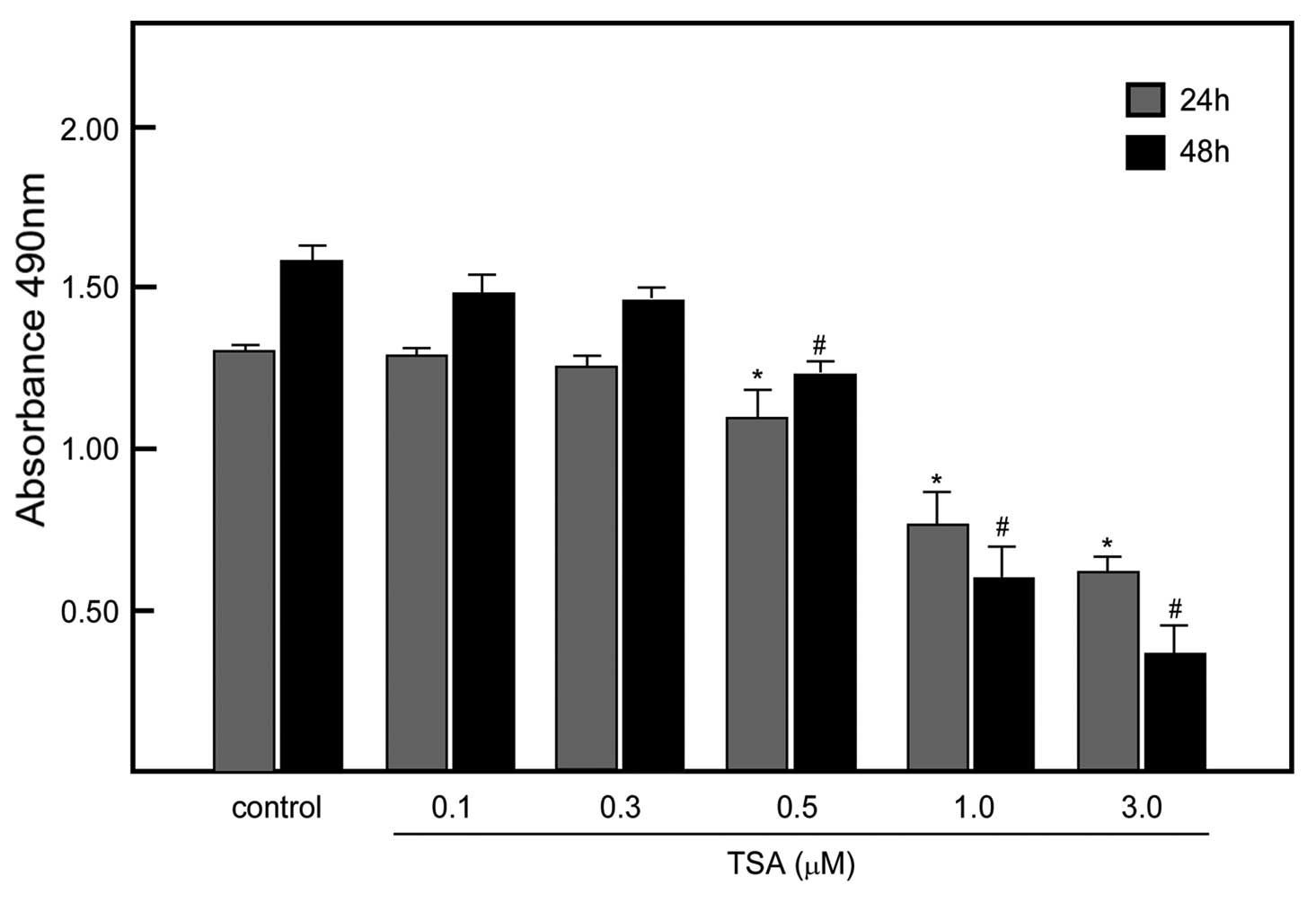
TSA at a low concentration did not affect
tumor cell viability
TSA has been observed to have antitumor activity as
well as alter the growth of tumors. Our purpose was to use TSA to
modulate the expression of CAR, but not affect cell viability. We
treated EC1 cells with various concentrations of TSA and assessed
the cell viability. The data indicated that 96% of cells survived
after 0.3 μmol/l TSA treatment for 48 h. Therefore, we
conducted the subsequent experiments using 0.3 μmol/l of TSA
(Fig. 4).
Replication of H101 after TSA treatment
and cytotoxicity of H101 in ESCC cell lines
We next examined the effect of TSA on OV H101
replication. The titer increased 527.46-fold in the EC1 cells
treated with 0.3 μmol/l of TSA; the replication of H101
increased in the EC1 cells treated with TSA compared to the
untreated control cells. We then examined whether TSA had an effect
on the antitumor ability of H101 in EC1 cells. The cell viability
of the EC1 cells treated with different doses of H101 was assessed
using MTT assay 72 h after treatment with 0.3 μmol/l TSA.
Based on a dose-time response curve (Fig. 5), the cell viability was 52.29% in
the 0.3 μmol/l TSA and H101 infection (MOI 100) group.
However, the cell viability in the H101 infection (MOI 100) group
was 84.35%. Furthermore, EC1 cells treated with H101 at the other
MOI levels combined with TSA treatment also reduced cell viability
(Fig. 5).
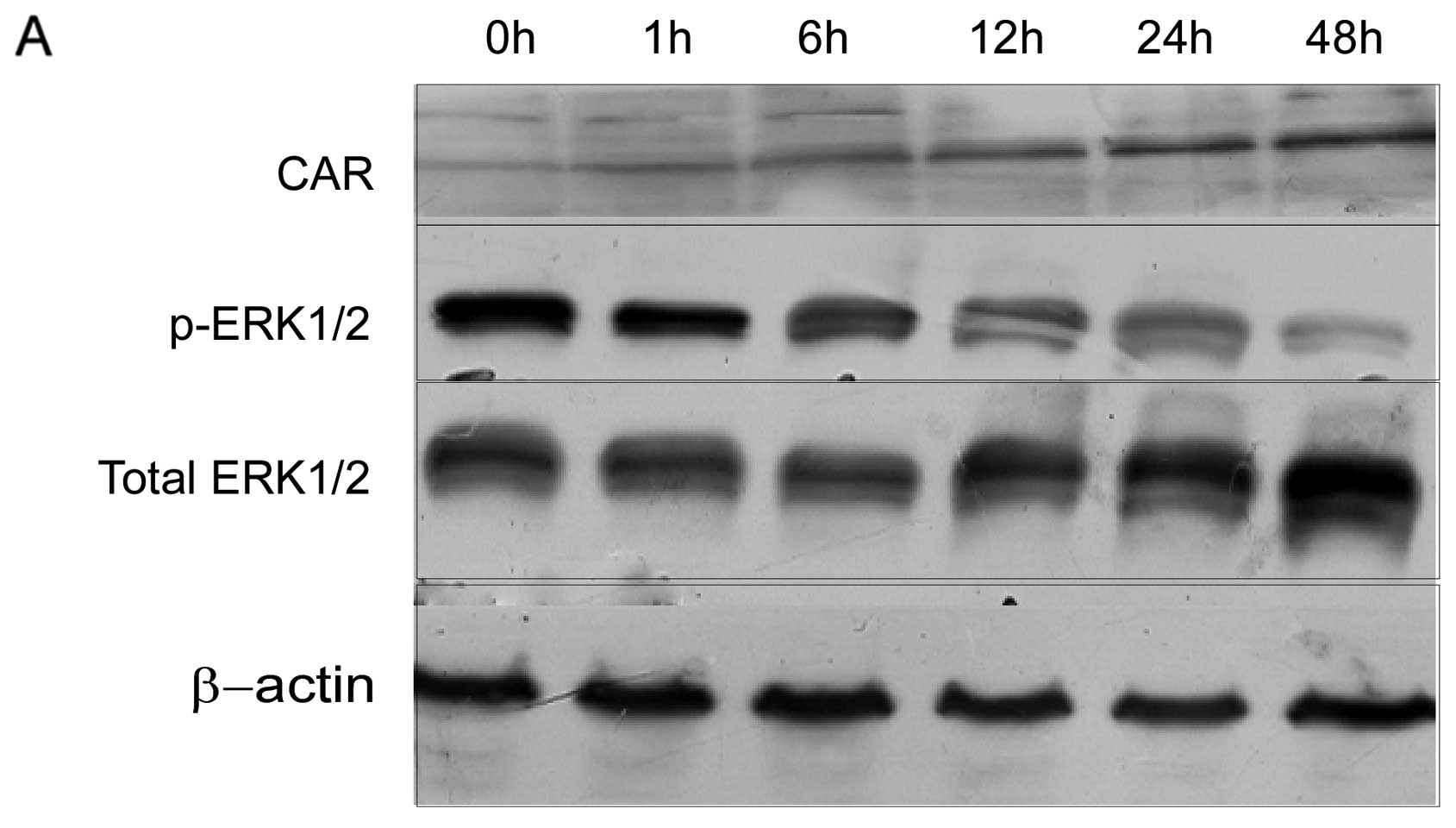
Inhibition of ERK1/2 signaling increased
the expression of CAR
Disruption of MAPK pathway signaling by
pharmacological inhibition of MEK has been found to upregulate CAR
protein level, resulting in enhanced Ad entry (21). To determine the role of
MAPK/ERK1/2 signaling in CAR expression induced by TSA, we examined
the correlation between the phosphorylation activity of ERK1/2 and
the expression of CAR. Western blotting results demonstrated that
the expression of the CAR protein increased and the level of
phosphorylation of ERK1/2 decreased in a time-dependent manner.
There was a significant negative correlation between the activity
of p-ERK1/2 and the expression of CAR (Fig. 6)
Discussion
OVs are designed or selected to grow preferentially
in tumor cells, and are a promising novel therapeutic platform for
cancer. H101, a recombinant human 5 Ad, lacks the E1B 55
gene and also contains a deletion in the E3 region. Before
modification, the E1B region of the wild-type Ad 5 expresses early
gene products that bind to and inhibit the function of p53, a tumor
suppressor. The deletion produces p53-selective replication of OVs
which infect tumor cells and induce massive accumulation of normal
p53. As a result, the Ad causes direct cytotoxicity only to tumor
cells during replication (22).
Clinical trials using attenuated strains of these viruses have
demonstrated limited toxicity. However, the use of Ad vectors in
gene therapy faces a critical prerequisite that single-agent
efficacy requires improvement. The relatively low efficiency of
recombinant gene transfer to tumor cells contributes partially to
the low therapy efficiency. CAR is a surface protein which is
involved in Ad binding to the cell surface. Accumulating evidence
indicates that CAR expression levels in target cells are a major
determinant of gene transfer efficacy with Ads (23–25).
CAR is ubiquitously expressed in most benign
epithelial tissues. However, CAR expression is lower in several
types of advanced clinical tumors. The level of CAR in ESCC cell
lines has not been reported. By using flow cytometric analysis, we
discovered that, compared to HeLa cells, EC1 cells had lower
surface CAR protein content (Fig.
1). The low CAR protein content resulted in poor adenoviral
transduction efficiency. Decreased CAR expression observed in the
ESCC cells was parallel with a decreased replication and oncolytic
activity of H101 (Fig. 2).
Therefore, decreased CAR expression in EC1 cells may also impose an
obstacle for Ad-based gene therapy. Thus, restoring the surface
expression of CAR will benefit the antitumor effects of OVs.
Histone acetylation plays an important role in
epigenetic gene regulation. In general, histone acetylation may
cause chromatin relaxation and transcription activation whereas
histone deacetylation causes chromatin structure condensation and
repression of transcription. Histone deacetylase inhibitors (HDACi)
block the activity of histone deacetylases (HDACs) and affect the
transcription of specific genes (26,27). CAR transcriptional regulation is
modulated mainly through histone acetylation. TSA is one of the
most promising HDACi that may increase the levels of CAR in various
types of tumor cells such as breast, lung and ovarian (28). The present study demonstrated that
TSA regulates the protein content of CAR in a dose-dependent
manner. We discovered that 0.3 μmol/l of TSA increases the
protein levels of CAR in EC1 cells but does not affect cell
viability. The reproductive multiple of H101 was higher in the 0.3
μmol/l TSA and the H101 group compared to the H101 group
alone. The cytotoxic activity of H101 increased in the EC1 cells
induced by 0.3 μmol/l of TSA compared with the control
group. This result may indicate that TSA increases the anti-tumor
activity of oncolytic Ad H101 through the modulation of CAR in EC1
cells.
It was reported that HDACi may induce CAR on the
tumor cell surface via accumulation of hyperacetylated nucleosome
core histones resulting in transcriptional activation of genes
(29). However, the signaling
pathway involved in HDACi-induced expression of CAR in tumor cells
needs further investigation. The MAPK/ERK1/2 signaling pathway
plays a key role in mediating signals from membrane receptors to
the nucleus, and is involved in multiple physiological processes,
including cell growth, cell differentiation, cell proliferation and
apoptosis (30,31). Once activated, ERK1/2 translocates
onto the nucleus to phosphorylate related transcription factors and
regulates their activity. Disrupting MAPK pathway signaling by
pharmacological inhibition of MEK upregulates CAR expression
(32,33). In this study, we observed that
there were negative correlations between the phosphorylation of
ERK1/2 and the protein level of CAR induced by TSA. This
observation is consistent with the report that constitutively
active MEK may direct the activation of HDAC4. TSA may decrease the
activity of HDAC4 and in turn upregulate the acetylation level of
histone H4 through inhibiting the activation of ERK1/2.
In conclusion, human ESCC cell lines have low CAR
protein levels. TSA may increase CAR protein levels in human ESCC
cells, which may benefit the antitumor activity of oncolytic Ad
H101 in ESCC cells. The signaling transduction involved in
TSA-induced CAR occurs possibly through interrupting the MAPK/ERK
pathway.
Acknowledgements
This study was supported by the
National Basic Research Program of China (no. 2012CB933300), the
Natural Science Foundation of Henan Province of China (nos.
12B310023, 2011A310009, 104200510009 and 112106000039) and the
National Sciences Foundation of China (no. 81101731).
References
|
1.
|
K ShigemitsuY NaomotoY ShirakawaM HaisaM
GunduzN TanakaA case of advanced esophageal cancer with extensive
lymph node metastases successfully treated with multimodal
therapyJpn J Clin Oncol32310314200210.1093/jjco/hyf06712411570
|
|
2.
|
SH BaxiB BurmeisterJA HarveyM SmithersJ
ThomasSalvage definitive chemo-radiotherapy for locally recurrent
oesophageal carcinoma after primary surgery: retrospective reviewJ
Med Imaging Radiat
Oncol52583587200810.1111/j.1440-1673.2008.02023.x
|
|
3.
|
W HartwigO StrobelF LordickMW BuchlerJ
WernerMultimodal therapy of esophageal cancerZ
Gastroenterol46120712132008(In German)
|
|
4.
|
W GuoYG JiangCurrent gene expression
studies in esophageal carcinomaCurr
Genomics10534539200910.2174/13892020978950388820514215
|
|
5.
|
CC LinKP PapadopoulosNovel targeted
therapies for advanced esophageal cancerDis
Esophagus20365371200710.1111/j.1442-2050.2007.00730.x17760648
|
|
6.
|
WP TewDP KelsenDH IlsonTargeted therapies
for esophageal
cancerOncologist10590601200510.1634/theoncologist.10-8-59016177283
|
|
7.
|
DM NettelbeckV JeromeR MullerGene therapy:
designer promoters for tumour targetingTrends
Genet16174181200010.1016/S0168-9525(99)01950-210729833
|
|
8.
|
D KoL HawkinsDC YuDevelopment of
transcriptionally regulated oncolytic
adenovirusesOncogene2477637774200510.1038/sj.onc.120904816299536
|
|
9.
|
DL LichtensteinWS WoldExperimental
infections of humans with wild-type adenoviruses and with
replication-competent adenovirus vectors: replication, safety, and
transmissionCancer Gene Ther11819829200410.1038/sj.cgt.7700765
|
|
10.
|
H JiangC Gomez-ManzanoFF LangR AlemanyJ
FueyoOncolytic adenovirus: preclinical and clinical studies in
patients with human malignant gliomasCurr Gene
Ther9422427200910.2174/15665230978975335619860656
|
|
11.
|
RW WaltersP FreimuthTO MoningerI GanskeJ
ZabnerMJ WelshAdenovirus fiber disrupts CAR-mediated intercellular
adhesion allowing virus
escapeCell110789799200210.1016/S0092-8674(02)00912-112297051
|
|
12.
|
HS PandhaLH StockwinJ EatonCoxsackie B and
adenovirus receptor, integrin and major histocompatibility complex
class I expression in human prostate cancer cell lines:
implications for gene therapy strategiesProstate Cancer Prostatic
Dis6611200310.1038/sj.pcan.4500611
|
|
13.
|
Y AbdolazimiM MojarradM PedramMH
ModarressiAnalysis of the expression of coxsackievirus and
adenovirus receptor in five colon cancer cell linesWorld J
Gastroenterol1363656369200710.3748/wjg.v13.i47.636518081225
|
|
14.
|
M YamashitaA InoK KawabataF SakuraiH
MizuguchiExpression of coxsackie and adenovirus receptor reduces
the lung metastatic potential of murine tumor cellsInt J
Cancer12116901696200710.1002/ijc.2285217546646
|
|
15.
|
Y WangS ThorneJ HannockA novel assay to
assess primary human cancer infectibility by replication-selective
oncolytic adenovirusesClin Cancer Res11351360200515671566
|
|
16.
|
M AndersM ViethC RockenLoss of the
coxsackie and adenovirus receptor contributes to gastric cancer
progressionBr J
Cancer100352359200910.1038/sj.bjc.660487619142187
|
|
17.
|
JH LeePA MarksHistone deacetylase
inhibitors in the therapy of cancer: much to
learnEpigenomics2723725201010.2217/epi.10.5922122077
|
|
18.
|
M KitazonoME GoldsmithT AikouS BatesT
FojoEnhanced adenovirus transgene expression in malignant cells
treated with the histone deacetylase inhibitor FR901228Cancer
Res6163286330200111522619
|
|
19.
|
B Segura-PachecoB AvalosE RangelD
VelazquezG CabreraHDAC inhibitor valproic acid upregulates CAR in
vitro and in vivoGenet Vaccines
Ther510200710.1186/1479-0556-5-1017892546
|
|
20.
|
R SomechS IzraeliA J SimonHistone
deacetylase inhibitors - a new tool to treat cancerCancer Treat
Rev30461472200410.1016/j.ctrv.2004.04.00615245778
|
|
21.
|
JW RamosThe regulation of extracellular
signal-regulated kinase (ERK) in mammalian cellsInt J Biochem Cell
Biol4027072719200810.1016/j.biocel.2008.04.00918562239
|
|
22.
|
CA BenedictPS NorrisTI PrigozyThree
adenovirus E3 proteins cooperate to evade apoptosis by tumor
necrosis factor-related apoptosis-inducing ligand receptor-1 and
-2J Biol Chem27632703278200110.1074/jbc.M00821820011050095
|
|
23.
|
W LuS ZhengXF LiJJ HuangX ZhengZ
LiIntra-tumor injection of H101, a recombinant adenovirus, in
combination with chemotherapy in patients with advanced cancers: a
pilot phase II clinical trialWorld J
Gastroenterol1036343638200415534920
|
|
24.
|
K TothWS WoldIncreasing the efficacy of
oncolytic adenovirus
vectorsViruses218441866201010.3390/v209184421994711
|
|
25.
|
Q HeY LiuQ ZouYS GuanTransarterial
injection of H101 in combination with chemoembolization overcomes
recurrent hepatocellular carcinomaWorld J
Gastroenterol1723532355201110.3748/wjg.v17.i18.235321633603
|
|
26.
|
D MarchionP MunsterDevelopment of histone
deacetylase inhibitors for cancer treatmentExpert Rev Anticancer
Ther7583598200710.1586/14737140.7.4.58317428177
|
|
27.
|
W WeichertHDAC expression and clinical
prognosis in human malignanciesCancer
Lett280168176200910.1016/j.canlet.2008.10.04719103471
|
|
28.
|
G ChenBB WangFJ LiEnhancive effect of
histone deacetylase inhibitor trichostatin a on transfection
efficiency of adenovirus in ovarian carcinoma cell line A2780Ai
Zheng24119612002005(In Chinese)
|
|
29.
|
RC PongYJ LaiH ChenEpigenetic regulation
of coxsackie and adenovirus receptor (CAR) gene promoter in
urogenital cancer cellsCancer Res6386808686200314695181
|
|
30.
|
JA McCubreyLS SteelmanWH ChappellRoles of
the Raf/MEK/ERK pathway in cell growth, malignant transformation
and drug resistanceBiochim Biophys
Acta177312631284200710.1016/j.bbamcr.2006.10.00117126425
|
|
31.
|
S BoldtUH WeidleW KolchThe kinase domain
of MEKK1 induces apoptosis by dysregulation of MAP kinase
pathwaysExp Cell
Res2838090200310.1016/S0014-4827(02)00018-612565821
|
|
32.
|
M AndersC ChristianM McMahonF McCormickWM
KornInhibition of the Raf/MEK/ERK pathway up-regulates expression
of the coxsackievirus and adenovirus receptor in cancer cellsCancer
Res6320882095200312727824
|
|
33.
|
N BagheriM ShiinaDA LauffenburgerWM KornA
dynamical systems model for combinatorial cancer therapy enhances
oncolytic adenovirus efficacy by MEK-inhibitionPLoS Comput
Biol7e1001085201110.1371/journal.pcbi.100108521379332
|