Introduction
Paraquat (trade name Gramoxone®;
1,1′-dimethyl-4,4′-bipyridinium dichloride; PQ) is a potent
herbicide widely used throughout the world (1). PQ intoxication by accidental and
intentional ingestion has been a public health concern for several
decades due to the severe morbidity and mortality rates in both
developing and developed countries. A variety of clinical
approaches for treatment of PQ intoxication have been employed, but
highly effective treatment methods have yet to be defined.
The mechanism of PQ poisoning is based on excessive
generation of reactive oxygen species (ROS). PQ is reduced by NADPH
and then conveys electrons to molecular oxygen generating ROS. This
can disrupt the balance of intracellular redox cycling and lead to
cellular toxicity (1,2). Ingested PQ directly targets the
lungs, kidneys, and liver tissues, causing severe primary cellular
damage. PQ toxicity can lead to acute lung phenomena including
infiltration of the lungs by inflammatory cells, alveolar
hemorrhage, increased collagen deposition and sequential
development of mortal lung fibrosis (1,3).
Clinical features of PQ ingestion depend on the quantity ingested
and the time elapsed from ingestion. Upon ingestion, PQ is rapidly
absorbed, distributed to various tissues, and within 12–24 h most
of the absorbed PQ is secreted through urine (4,5).
Within a few days, patients may develop severe lung damage such as
fibrosis, the main cause of mortality with PQ toxicity. Prompt
diagnosis and prognosis of pathophysiological progression is
crucial for survival. There is, however, a lack of effective
diagnostic methods for PQ intoxication due to considerable
variations between patients such as age, gender, susceptibility,
time elapsed from PQ exposure, and amount of PQ ingested.
Proteomic analysis is one of the most widely used
techniques for defining functional proteins and crosstalk between
proteins and DNA (6–8). Furthermore, proteomics is a powerful
research tool for the identification of biomarkers and therapeutic
targets for toxicology, pharmacology, cancer biology, and other
biomedical research (8). In
pulmonary-related disease research, proteomics is a highly
effective method for identifying diagnostic and prognostic
biomarkers using various biomaterials such as cell lines, lung
bronchoalveolar lavage fluid (BALF), and tissues from animals
(9,10). However, blood samples have not
been considered useful for proteomic analysis despite the fact that
blood possesses a highly-enriched information source due to the
absence of genomic information (11). Recent developments of advanced
proteomic techniques have resulted in the recognition of blood as
an important source of information for various biomedical studies
such as toxicology (8).
In the present study, we employed a conventional
proteomics approach using rat serum to investigate and identify
diagnostic biomarkers in PQ poisoning. 2D-PAGE and MALDI-TOF
analysis revealed PQ treatment altered protein expression. The
expression of apolipoprotein E (ApoE), complement component 3 (C3),
and preprohaptoglobin (Pphg), a precursor of haptoglobin (Hp), were
greatly induced while the expression of fibrinogen γ-chain (FGG)
and Ac1-581, a precursor of fibrinogen β-chain (FGB), were reduced.
Furthermore, alteration of the protein expression of ApoE, Hp and
FGG was confirmed in PQ-exposed cell lines and sera from patients.
Therefore, our data suggest that Apo E, Hp and FGG may beneficial
diagnostic biomarkers for the early detection of acute PQ
intoxication.
Materials and methods
Chemicals and reagents
Paraquat dichloride (1,1′-dimethyl-4,4′-bipyridinium
dichloride) was purchased from Sigma-Aldrich (St. Louis, MO, USA).
The stock solution of PQ was made using distilled water. Stock
solutions were then diluted in 1xPBS to the desired concentrations
prior to treatment of animals. Stock solutions were diluted in cell
culture media for the treatment of cells.
Cell culture
Macrophage, Raw264.7, and lung adenocarcinoma, A549,
cell lines were obtained from the Korean Cell Line Bank (Seoul,
Korea) and maintained in DMEM and DMEM/HF-12 medium, respectively,
supplemented with 10% (v/v) heat-inactivated FBS and 1% (v/v)
antibiotic/antimycotic cocktail (100 U/ml penicillin, 100
μg/ml streptomycin, and 0.25 μg/ml amphotericin B;
Invitrogen, Carlsbad, CA) at 37°C under a humidified atmosphere
containing 5% CO2.
Animals
Six five-week-old male CD(SD)IGS rats were purchased
from Orient Bio, Inc. (Seongnam, Korea) and acclimated for 7 days
prior to the experiment. Rats were housed with free access to
standard rodent food in compliance with the standards set forth by
Soon Chun Hyang’s Institutional Animal Care and Use Committee.
Ethics statement
Serum samples from patients intixicated with PQ were
obtained at the department of internal medicine, Soon Chun Hyang
University Cheonan Hospital, and the use of patient serum in this
study was approved by Soon Chun Hyang University Cheonan Hospital’s
institutional review board (IRB approval no. 2011-85).
Proteomics analysis
Serum samples were collected from rats following the
guidelines for care and use of laboratory animals approved by Soon
Chun Hyang’s Institutional Animal Care and Use Committee. Untreated
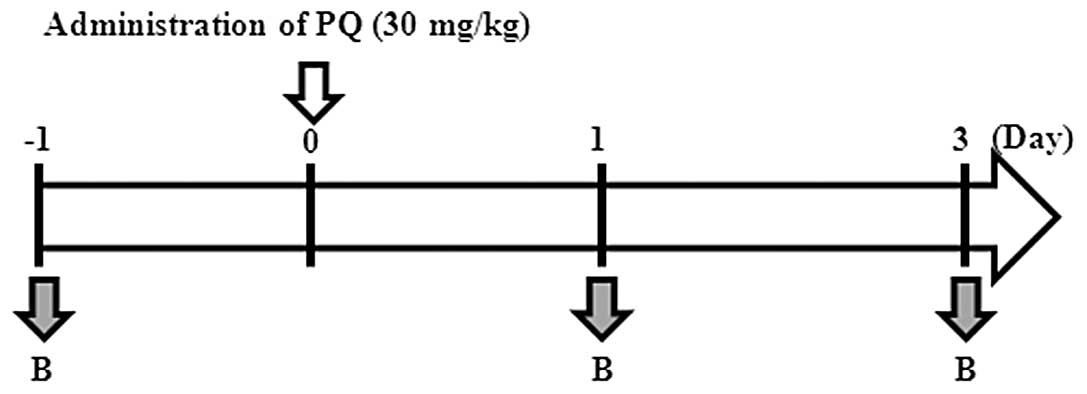
normal blood was initially collected one day prior to PQ treatment
(noted as −1 in the text and figures) from the rat tail vein. Blood
samples from the tail vein were then collected at 6 h following
intraperitoneal injection of PQ (noted as 1). At Day 3, a final
blood collection was performed. Serum samples were immediately
collected via 1,500 x g centrifugation at room temperature and
samples were stored at 4°C.
For 2D-PAGE separation, 200 μg of serum was
used for isoelectric focusing (IEF) using a multiphor II
electrophoresis kit following the manufacturer’s instructions. For
2D-PAGE, strips were equilibrated in equilibration buffer (50 mM
Tris-Cl, pH 6.8 containing 6 M urea, 2% SDS and 30% glycerol) for
10 min, and the equilibrated strips were inserted and run on 20×24
cm (10–16% gradient) SDS-PAGE gels. 2D gels were then stained with
modified silver staining according to Oakley et al (12) for image analysis. Quantitative
analysis of visualized images was executed using PDQuest (version
7.0; Bio-Rad, Hercules, CA, USA) software following the
manufacturer’s instructions. We selected the spots with >2-fold
alteration, normalized by total valid spot intensity. In order to
characterize the selected spots, the in-gel digestion was performed
using porcine trypsin (Promega; Madison, WI, USA) with modified
methods as previously described (13). Digested gel pieces were
sequentially washed with 50% acetonitrile to remove chemicals and
dye, and were then dehydrated. Spots were rehydrated with trypsin
(8–10 ng/μl in 50 mM ammonium bicarbonate, pH 8.7), and
incubated for 8–10 h at 37°C.
To identify proteins, MALDI-TOF/TOF analysis was
conducted. In brief, each spot was verified and analyzed using the
TOF/TOF™ ion optics installed in Applied Biosystems 4700 proteomics
analyzer (Applied Biosystems; Carlsbad, CA, USA). Both MS and MS/MS
data were acquired with an Nd:YAG laser with 200 Hz repetition
rate, and up to 4,000 shots were accumulated for each spectrum.
Operation MS/MS mode was 2 keV collision energy. Air was used as
the collision gas with nominally single collision conditions. For
these analyses, a resolution of 100 was applied although the prec
ursor resolution selection was 200. Both MS and MS/MS data were
acquired using the default instrument calibration without applying
internal or external calibration. Sequence tag searches were
performed with the program MASCOT (http://www.matrixscience.com).
Quantitative RT-PCR (qRT-PCR)
Cells were treated with 166 nM PQ for 24 h and
harvested. Total-RNA was purified and converted to cDNA using a
first strand cDNA synthesis kit (Intron; Daejeon, Korea). qRT-PCR
was performed with human ApoE primer set (forward,
5′-GTGGAGCAAGCGG TGGAGAC-3′ and reverse, 5′-GAGCTGAGCAGCTCCTCC
TG-3′). As an internal expression control, a GAPDH primer
set (forward, 5′-TCCCATCACCATCTTCCA-3′ and reverse, 5′-CA
TCACGCCACAGTTTCC-3′) was used. Amplicon sizes were 158 and 380 bp,
respectively.
Western blot analysis
In order to measure the Hp and ApoE protein
expression from rat and human patient serum, serum albumin was
depleted using an albumin depletion kit (Millipore; Billerica, MA,
USA) according to the manufacturer’s instructions. In brief, 25
μl of serum was pre-diluted with 275 μl of 1xPBS and
diluted serum was incubated with pre-washed albumin magnetic beads
for 6 h at room temperature. Following incubation, supernatant was
isolated and collected for further experiments. Serum protein
concentration was measured using the Pierce BCA protein assay kit
(Thermo Scientific; Rockford, IL, USA) and mixed with 2XSDS-PAGE
sample buffer (Sigma-Aldrich). Subsequently, 35 μg serum
samples were separated on gradient SDS-PAGE gel and transferred to
PVDF membranes. Although we measured the concentration of proteins,
gels were stained with the MemCode™ Reversible Protein stain kit
(Thermo Scientific) to check the concentration of serum proteins
after gel running on the SDS-PAGE (Fig. 2), and by transfering to PVDF
membranes for antibody incubation. Anti-Hp and anti-ApoE antibodies
were diluted at 1:1,000 and 1:5,000, respectively.
Results
Differentially expressed proteins in
serum from rats exposed to PQ
It is generally difficult to obtain normal serum (as
a control) from PQ-intoxicated patients since patients are
hospitalized after PQ ingestion, be it intentional or accidental
ingestion. Therefore, we mimicked the conditions of PQ poisoning
using a rat animal model. Six rats were acclimated at least 7 days
prior to the experiments. Normal serum was then collected from the
tail following sedation with ether (Fig. 1). Rats were weighed and their
general physical condition were checked. After administering 30
mg/kg (166 nM) PQ via intraperitoneal injection, body weight was
found to be slightly reduced but recovered to normal by Day 3 (data
not shown). To determine the alteration of functional protein
expression in acute PQ-exposed rats, we performed proteomic
analysis using 200 μg serum samples at 1 day prior to PQ
treatment and 1–3 days post-PQ exposure from 2 individual mice.
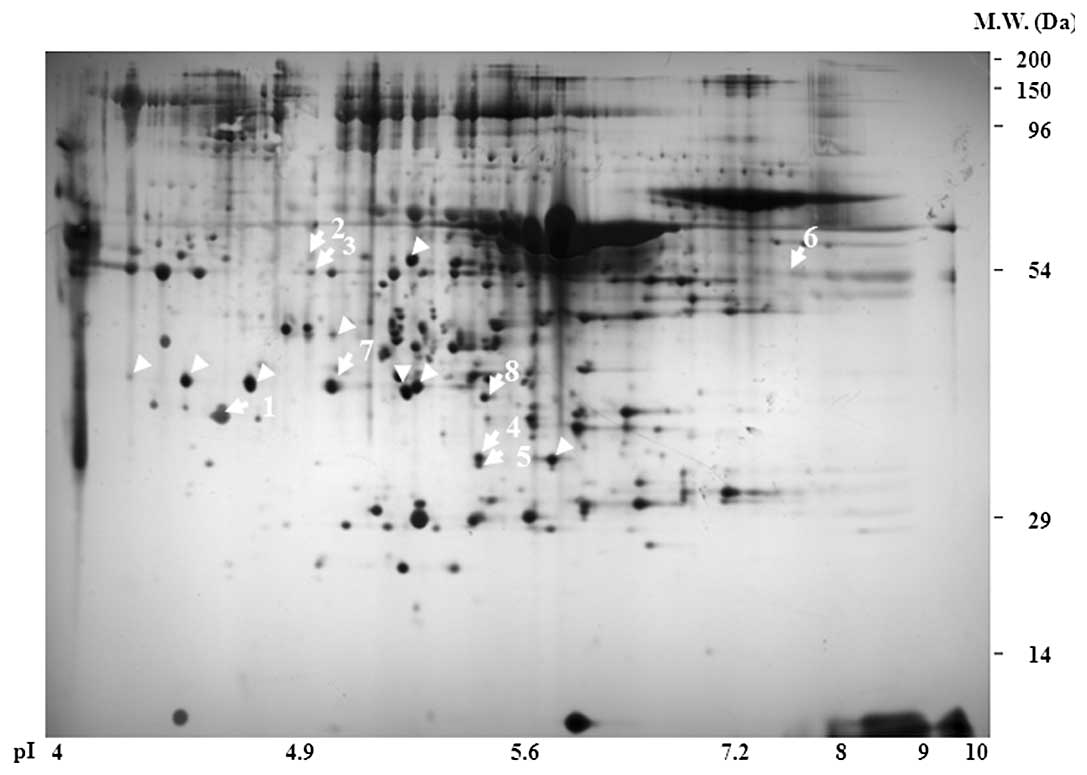
Representative 2D-PAGE image showed that >500
spots clearly altered their expression (Fig. 2). For further characterization of
each spot, 8 spots which showed >2-fold alteration were
collected and MALDI-TOF MS/MS analysis was performed. We identified
8 proteins of interest including inflammatory-related C3, FGG,
antioxidant related Pphg, ApoE and Ac1-581 (FGB) via the MASCOT
program governed by the National Resource for the Mass Spectrometic
Analysis of Biological Macromolecules and the Matrix Science
Company (Table I). The MASCOT
search program revealed that the mass fingerprint of spot 104 was a
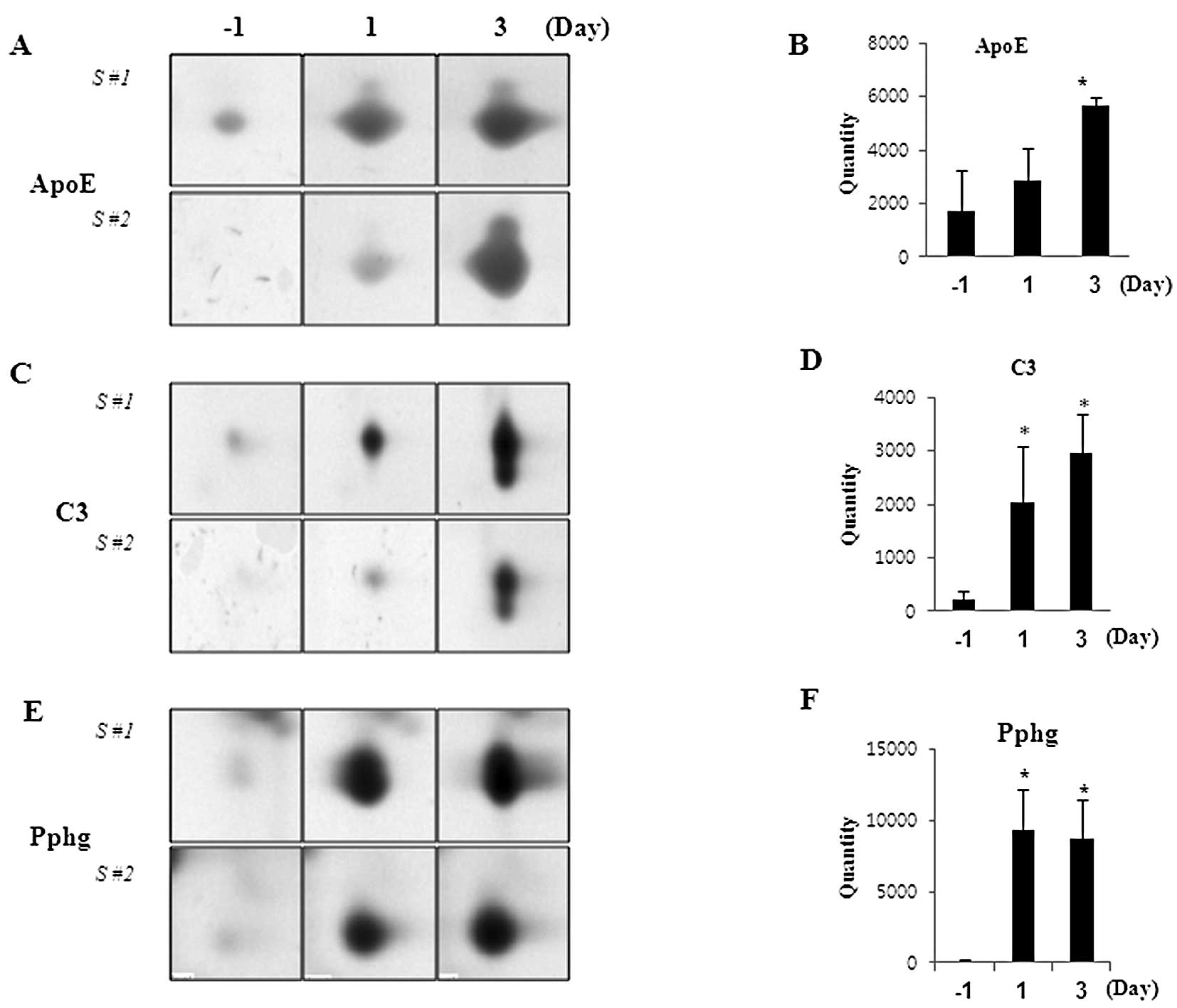
45% polypeptide match with ApoE protein. The expression of ApoE was
significantly upregulated with PQ exposure (Fig. 3A and B). Two individual spots,
4103 and 4104, were covered with 2 and 4% C3, respectively, and the
expression of C3 was significantly upregulated in PQ-exposed rat
serum in a time-dependent manner (Fig. 3C and D). Of note, 2 spots, 1103
and 4105, were matched with antioxidant-related Pphg with 10 and
16% peptide coverage, respectively, and the expression was
dramatically induced by PQ treatment (Fig. 3E and F).
 | Table IThe characterized proteins from
PQ-exposed rat serum using MALDI-TOF analysis. |
Table I
The characterized proteins from
PQ-exposed rat serum using MALDI-TOF analysis.
| Spot no. | Sample | GeneBank | MASCOT search
results file name (ms/ms)c | Identified
proteins | Score | Cov (%) | Changed
pattern |
|---|
| 1 | 104 | gi|37805241 | 104 msms.htm | Apolipoprotein
E | 236 | 45 | Up |
| 2 | 1305 | gi|61098186 | 1305 msms.htm | Fibrinogen
γ-chain | 233 | 18 | Down |
| 3 | 1306 | gi|61098186 | 1306 msms.htm | Fibrinogen
γ-chain | 121 | 20 | Down |
| 4 | 4103 | gi|116597 | 4103 msms.htm | Complement C3 | 121 | 2 | Up |
| 5 | 4104 | gi|116597 | 4104 msms.htm | Complement C3 | 205 | 4 | Up |
| 6 | 8406 | gi|32527707 | 8406 msms.htm | Ac1-581 (Fibrinogen
β-chain precursor) | 182 | 45 | Down |
| 7 | 1103 | gi|204657 | 1103 msms.htm |
Preprohaptoglobin | 44 | 10 | Up |
| 8 | 4105 | gi|204657 | 4105 msms.htm |
Preprohaptoglobin | 67 | 16 | Up |
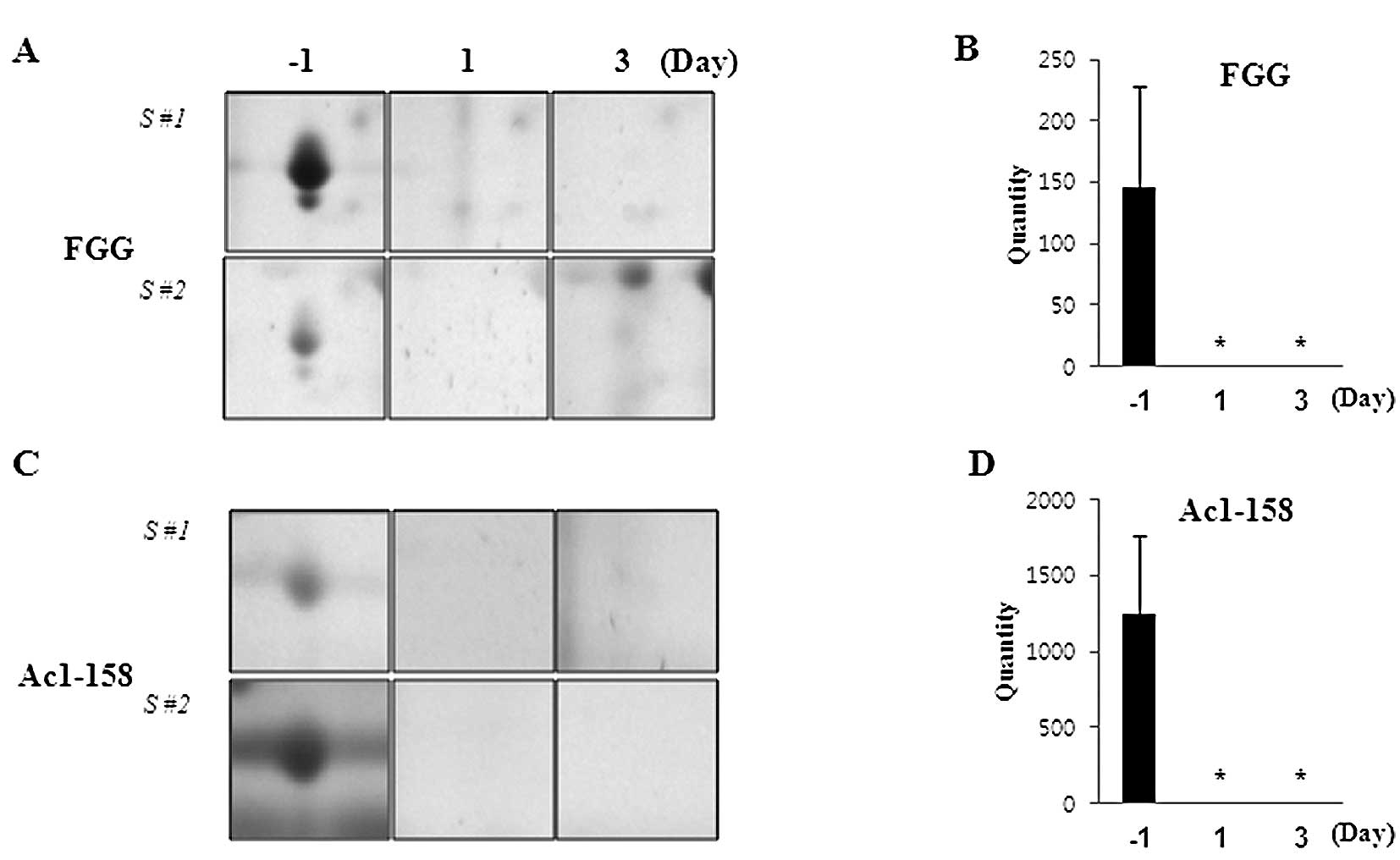
The expression of other proteins was found to be
down-regulated in PQ-exposed rat serum. Spots 1305 and 1306 were
covered with 18 and 20% FGG polypeptide, respectively, and the
expression of FGG was drastically downregulated by PQ (Fig. 4A and B). Markedly, Ac1-581 (spot
8406) which is the preproprotein precursor of FGB was identified,
with 45% coverage, using the MASCOT search engine, and found to be
downregulated by PQ exposure in a time-dependent manner (Fig. 4C and D).
Verification of the expression of APOE,
Hp and FGG in PQ-exposed cell lines, rat and patient sera
To verify the change in mRNA and protein expression
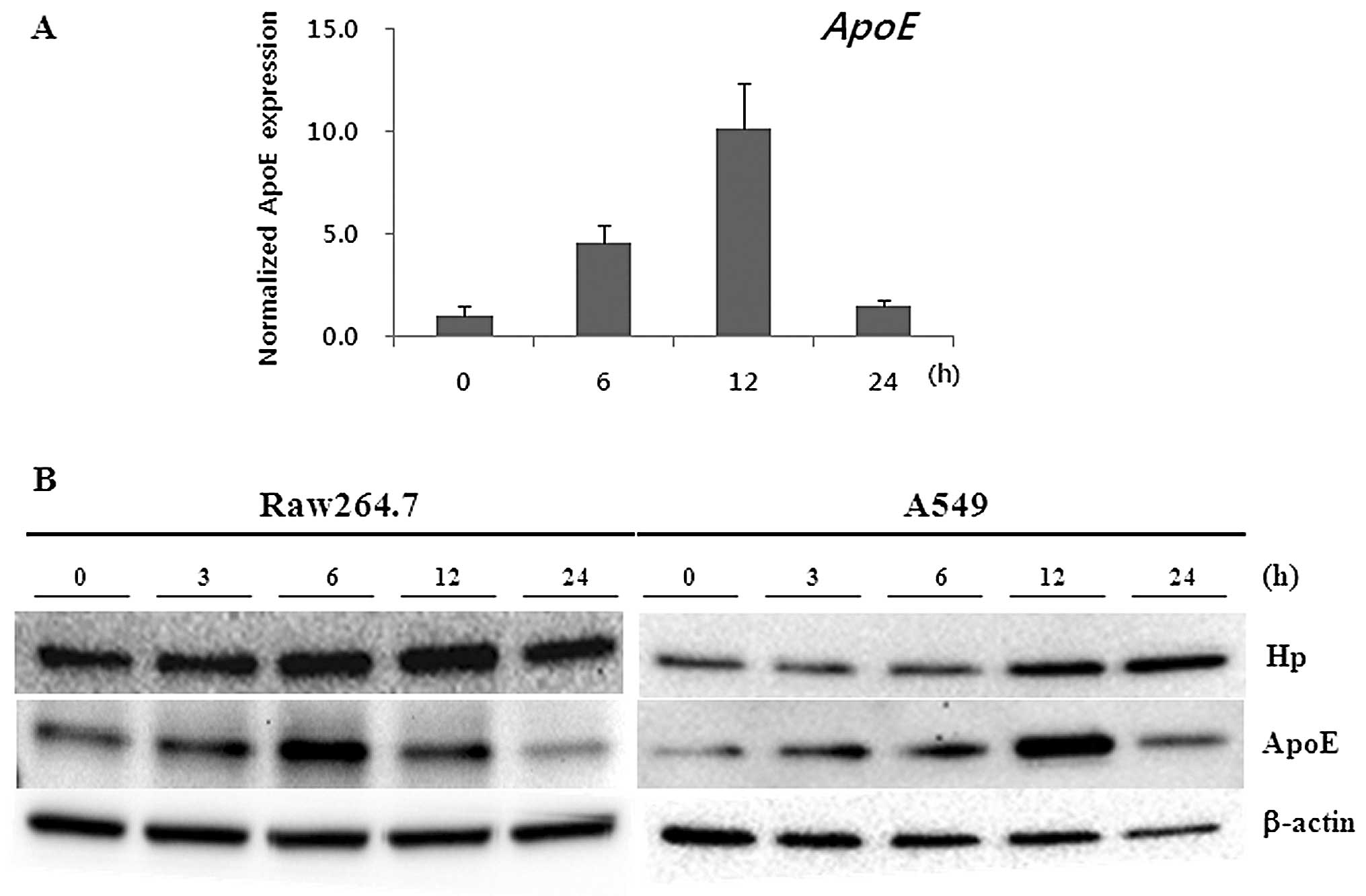
with PQ treatment, in vitro analysis was performed. In order
to measure the change in mRNA expression, the Raw264.7 cell line
was treated with 166 nM PQ for the indicated times (0, 1 and 3
days). Total-RNA was then collected and cDNA was synthesized using
1 μg of total-RNA. qRT-PCR was performed with the
ApoE gene specific primer set (described in Materials and
methods). ApoE mRNA was drastically upregulated at 6 and 12
h with PQ treatment (Fig. 5A).
However, the expression was downregulated at 24 h. In addition, we
investigated the expression of the Hp and ApoE proteins in the
PQ-treated Raw264.7 and A549 cell lines (Fig. 5B). As we expected, the expression
of the Hp and ApoE proteins was upregulated at 6 and 12 h in
Raw264.7 cells and A549 cells after PQ treatment, respectively
(Fig. 5B).
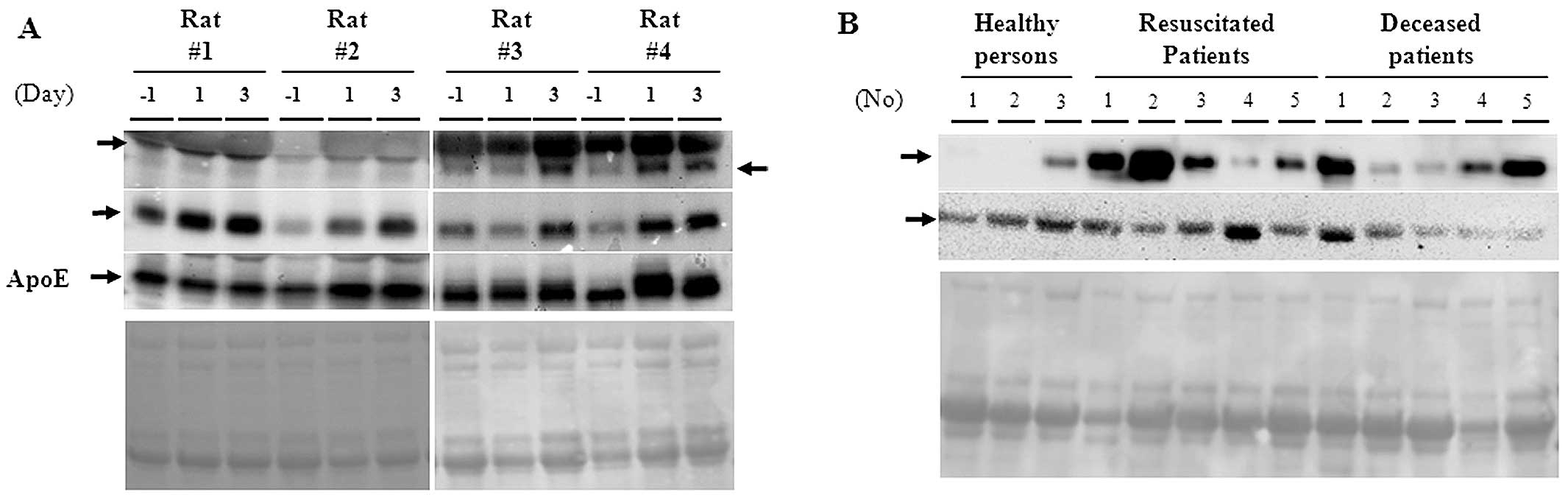
In order to investigate any possible implication for
clinical relevance, we measured the expression of both ApoE and Hp
proteins in rat and human serum. In Fig. 6A, the Hp-α, -β proteins, and ApoE
were dramatically induced by PQ treatment in a time-dependent
manner. Hp-β proteins were also prominently induced in both
resuscitated and deceased PQ patients compared to healthy
individuals (Fig. 6B). By
contrast, expression of FGG was downregulated in PQ patients.
Discussion
Due to the high rate of mortality with poisoning,
prompt prognosis and diagnosis of acute PQ poisoning is an absolute
requirement for survival. Serum uric acid and the acute-phase
response gene pentraxin 3 (PTX3) were recently
characterized and highlighted as putative biomarkers for PQ
poisoning in human serum (14,15). However, the protocols for
diagnosis, prognosis, and treatment of PQ-poisoned patients are
limited. These limitations led us to investigate the existence of
potentially more effective diagnostic biomarkers from PQ-treated
rat serum using conventional proteomics analysis. Although there
was concern of protein spots being masked by high-abundance serum
proteins such as albumin, immunoglobulin, and complement on 2D-PAGE
images, we were able to perform proteomic analysis with total serum
to identify putative biomarkers. More than 500 altered protein
spots were gained, and we identified 8 differentially expressed
proteins. The expression of ApoE, C3 and Pphg, a precursor of
haptoglobin (Hp), were induced by PQ treatment while the FGG and
Ac1-581 (FGB) proteins were downregulated. The altered protein
expressions were further verified by qRT-PCR and western blot
analysis. In addition, we detected the induction of ApoE and
Hp mRNA expression in the A549 lung carcinoma cell line.
This data suggested that these proteins may be beneficial candidate
markers for the diagnosis of acute PQ poisoning.
Haptoglobin is an acute-phase response glycoprotein
and it plays an antioxidative role due to its binding activity to
hemoglobin (16). Haptoglobin is
also a good diagnostic marker of lung cancer and is involved in
angiogenesis and cell migration (17,18). Li et al (20) also demonstrated that Hp is
upregulated in acute lung damage after pulmonary embolism (19). A human study demonstrated that
serum Hp concentration was significantly increased from
approximately 824.37 to 2063 mg/l in patients with pulmonary
embolism and deep vein thrombosis (PE/DVT) (20). Based on these results, studies
have suggested that increasing concentrations of serum Hp may
attenuate lung injury that occurs following PE. Karthik et
al (21) demonstrated that
the expression of an Hp precursor, Pphg, was greatly altered in the
alloxan-induced diabetes model. In addition, the expression of Pphg
and Hp in patient urine was dramatically increased in the presence
of passive Heymann nephritis and suggested that Pphg and Hp are
appropriate candidates for therapeutic targeting and potentially
novel biomarkers in membranous nephropathy (MN) and high altitude
pulmonary edema (22,23). We identified Pphg via proteomic
analysis and observed that its expression was dramatically induced
in PQ-treated rat serum. Prior to treatment with PQ, Pphg
expression was almost undetectable but expression was significantly
induced at Days 1 and 3 post-PQ administration. Furthermore, the
expression of Hp-α and Hp-β was detected in PQ-treated rat and
human serum. In accordance with previous studies, we speculated
that the induction of Pphg and Hp expression was a response to PQ
treatment, therefore, Pphg and Hp serum levels may be effective
diagnostic biomarkers for PQ poisoning.
Accumulating evidence from animal studies has
revealed that ApoE is a key protein in atherosclerosis via its role
in inflammation, control of cholesterol, and blood pressure
(24). A previous study
demonstrated that Hp can interact with ApoE in a mechanism by which
inflammation affects atherosclerotic progression (25). Cigliano et al (25) suggested that the interaction
between Hp and ApoE represents a novel link between the acute phase
of inflammation and ApoE function. In addition, the ApoE
knockout mice, a lack of the antioxidant enzyme, and glutathione
peroxidase-1 (GPx1), accelerate diabetes-associated atherosclerosis
through induction of inflammation and fibrosis (19). However, Cuthbert et al
(26) demonstrated that the level
of expression of the major protein component of high-density
lipoprotein (HDL), apolipoprotein A-I (ApoA-I) but not ApoB and E,
is regulated by PQ exposure in the HepG2 cell line. In this study,
we observed that the expression of the ApoE protein was
dramatically induced by PQ treatment. Based on our observation, we
speculated that early acute PQ exposure may induce ApoE which leads
to the regulation of the acute inflammatory response and
antioxidant-related genes. The dynamic expression among the Apo
family of proteins requires further investigation in order to
better understand the regulatory network between PQ and the early
immune response.
Although fibrinogen γ-chain (FGG) is known to be a
cofactor in blood clots, FGG and fibrinogen β-chain (FGB) are also
known as biomarkers for the detection of occurrence and progression
of coronary artery disease (CAD) in
ApoE−/− mice (27). FGG is also involved in aging
through the regulation of oxidative stress and inflammation
(28). In this study, FGG was
dramatically downregulated by PQ exposure. To date, there has been
no data regarding the role of FGG in PQ intoxication. Therefore, we
speculated that the antagonistic expression between ApoE and FGG is
in direct response to acute PQ poisoning.
It has been well established that PQ induces ROS
formation, and, consequently, development of acute lung injury such
as lung fibrosis via induction of the NF-κB regulated
pro-inflammatory pathway (1).
Complements have been involved in early immune reaction but
complement component 3 (C3) levels in serum were not altered with
long-term PQ exposure in Balb/c mice (29). Sun et al (30) demonstrated that blocking the
complement pathway ameliorates acute lung injury (ALI) induced by
PQ treatment. The treatment of complement C3 inhibitor, CR2-Crry,
has been shown to be particularly effective in reducing PQ-induced
inflammation, pathology, and mortality (30). Data suggest that C3 may play an
important role in acute, early inflammatory reactions induced by
PQ. In the present study, PQ induced expression of C3 which was
shown to activate the acute pro-inflammatory response and
subsequent inflammation. Therefore, we propose that the detection
of C3 expression in serum is a beneficial diagnostic tool for
PQ-induced acute lung inflammation.
In conclusion, based on our proteomics profiling
data we suggest that Hp, ApoE, C3 and FGG in PQ-exposed serum might
be appropriate diagnostic biomarkers for the early detection and
diagnosis of acute PQ poisoning.
Acknowledgements
This study was partially supported by
a grant from the Rural Development Administration, Republic of
Korea (project no. PJ008246).
References
|
1.
|
IB GawarammanaNA BuckleyMedical management
of paraquat ingestionBr J Clin
Pharmacol72745757201110.1111/j.1365-2125.2011.04026.x21615775
|
|
2.
|
I AhmadA KumarS ShuklaH Prasad PandeyC
SinghThe involvement of nitric oxide in maneb- and paraquat-induced
oxidative stress in rat polymorphonuclear leukocytesFree Radic
Res42849862200810.1080/1071576080251373318985485
|
|
3.
|
ZE SuntresRole of antioxidants in paraquat
toxicityToxicology1806577200210.1016/S0300-483X(02)00382-712324200
|
|
4.
|
P HouzeFJ BaudR MouyC BismuthR BourdonJM
ScherrmannToxicokinetics of paraquat in humansHum Exp
Toxicol9512199010.1177/096032719000900103
|
|
5.
|
SM PondLP RivoryEC HampsonMS
RobertsKinetics of toxic doses of paraquat and the effects of
hemoperfusion in the dogJ Toxicol Clin
Toxicol31229246199310.3109/155636593090003918492337
|
|
6.
|
P IndovinaE MarcelliP MarantaG TarroLung
cancer proteomics: recent advances in biomarker discoveryInt J
Proteomics2011726869201110.1155/2011/72686922229091
|
|
7.
|
AT LauJF ChiuBiomarkers of lung-related
diseases: current knowledge by proteomic approachesJ Cell
Physiol221535543200910.1002/jcp.2189319681054
|
|
8.
|
A PlymothP HainautProteomics beyond
proteomics: toward clinical applicationsCurr Opin
Oncol237782201110.1097/CCO.0b013e32834179c121107258
|
|
9.
|
P GovenderMJ DunnSC DonnellyProteomics and
the lung: Analysis of bronchoalveolar lavage fluidProteomics Clin
Appl310441051200910.1002/prca.20090003221137005
|
|
10.
|
T OkamotoY MiyazakiR ShirahamaM TamaokaN
InaseProteome analysis of bronchoalveolar lavage fluid in chronic
hypersensitivity pneumonitisAllergol
Int618392201210.2332/allergolint.11-OA-031522015564
|
|
11.
|
HJ IssaqZ XiaoTD VeenstraSerum and plasma
proteomicsChem Rev10736013620200710.1021/cr068287r17636887
|
|
12.
|
BR OakleyDR KirschNR MorrisA simplified
ultrasensitive silver stain for detecting proteins in
polyacrylamide gelsAnal
Biochem105361363198010.1016/0003-2697(80)90470-46161559
|
|
13.
|
A ShevchenkoM WilmO VormM MannMass
spectrometric sequencing of proteins silver-stained polyacrylamide
gelsAnal Chem68850858199610.1021/ac950914h8779443
|
|
14.
|
JH KimHW GilJO YangEY LeeSY HongSerum uric
acid level as a marker for mortality and acute kidney injury in
patients with acute paraquat intoxicationNephrol Dial
Transplant2618461852201110.1093/ndt/gfq63220966188
|
|
15.
|
CD YeoJW KimYO KimSA YoonKH KimYS KimThe
role of pentraxin-3 as a prognostic biomarker in paraquat
poisoningToxicol Lett212157160201122210019
|
|
16.
|
F YangAJ GhioDC HerbertFJ WeakerCA
WalterJJ CoalsonPulmonary expression of the human haptoglobin
geneAm J Respir Cell Mol
Biol23277282200010.1165/ajrcmb.23.3.4069
|
|
17.
|
E SalomonssonVL ThijssenAW GriffioenUJ
NilssonH LefflerThe anti-angiogenic peptide anginex greatly
enhances galectin-1 binding affinity for glycoproteinsJ Biol
Chem2861380113804201110.1074/jbc.C111.22909621372130
|
|
18.
|
HY TsaiK BoonyapranaiS
SriyamGlycoproteomics analysis to identify a glycoform on
haptoglobin associated with lung
cancerProteomics1121622170201110.1002/pmic.20100031921538882
|
|
19.
|
P LewisN StefanovicJ PeteLack of the
antioxidant enzyme glutathione peroxidase-1 accelerates
atherosclerosis in diabetic apolipoprotein E-deficient
miceCirculation11521782187200710.1161/CIRCULATIONAHA.106.66425017420349
|
|
20.
|
SQ LiJ YunFB XueComparative proteome
analysis of serum from acute pulmonary embolism rat model for
biomarker discoveryJ Proteome
Res6150159200710.1021/pr060310217203959
|
|
21.
|
D KarthikS IlavenilB KaleeswaranS SunilS
RavikumarProteomic analysis of plasma proteins in diabetic rats by
2D electrophoresis and MALDI-TOF-MSAppl Biochem
Biotechnol16615071519201210.1007/s12010-012-9544-822258647
|
|
22.
|
Y AhmadD ShuklaI GargIdentification of
haptoglobin and apolipoprotein A–I as biomarkers for high altitude
pulmonary edemaFunct Integr Genomics114074172011
|
|
23.
|
HH NgaiWH SitPP JiangV ThongboonkerdJM
WanMarkedly increased urinary preprohaptoglobin and haptoglobin in
passive Heymann nephritis: a differential proteomics approachJ
Proteome Res633133320200710.1021/pr070245b17616219
|
|
24.
|
K ImaizumiDiet and atherosclerosis in
apolipoprotein E-deficient miceBiosci Biotechnol
Biochem7510231035201110.1271/bbb.11005921670535
|
|
25.
|
L CiglianoCR PuglieseMS SpagnuoloR
PalumboP AbresciaHaptoglobin binds the antiatherogenic protein
apolipoprotein E - impairment of apolipoprotein E stimulation of
both lecithin: cholesterol acyltransferase activity and cholesterol
uptake by hepatocytesFEBS
J27661586171200910.1111/j.1742-4658.2009.07319.x
|
|
26.
|
C CuthbertZ WangX ZhangSP TamRegulation of
human apolipoprotein A–I gene expression by gramoxoneJ Biol
Chem27214954149601997
|
|
27.
|
L JingCE ParkerD SeoDiscovery of biomarker
candidates for coronary artery disease from an APOE-knock out mouse
model using iTRAQ-based multiplex quantitative
proteomicsProteomics1127632776201110.1002/pmic.20100020221681990
|
|
28.
|
M Santos-GonzalezC Gomez DiazP NavasJM
VillalbaModifications of plasma proteome in long-lived rats fed on
a coenzyme Q10-supplemented dietExp
Gerontol42798806200710.1016/j.exger.2007.04.01317587521
|
|
29.
|
B RiahiH RafatpanahM MahmoudiEvaluation of
suppressive effects of paraquat on innate immunity in Balb/c miceJ
Immunotoxicol83945201110.3109/1547691X.2010.54309521299353
|
|
30.
|
S SunH WangG ZhaoComplement inhibition
alleviates paraquat-induced acute lung injuryAm J Respir Cell Mol
Biol45834842201110.1165/rcmb.2010-0444OC21421909
|