Introduction
Platelets play a central role in thrombus formation,
a pathological process associated with vascular diseases (1). Thrombosis is a critical event
associated with myocardial infarction and stroke, major concerns of
high mortality (2). Platelet
activation is triggered by initial tethering at injured vascular
sites, mediated by the formation of glycoprotein Ib/IX/V and von
Willebrand factor complex. The release of adenosine diphosphate
(ADP), thrombin, epinephrine and thromboxane A2. from activated
platelets are rapidly generated to repair vascular injury (1). The release of inflammatory and
mitogenic mediators including CD40 ligand (CD40L) and
platelet-derived growth factor-AB (PDGF-AB) from platelets alters
the vascular endothelial-cell function, resulting in
atherosclerosis (1).
Type 2 diabetes mellitus (DM) is a major global
health problem (3). These
patients have an increased risk of vascular complications due to
atherosclerosis such as cardiovascular disease (4), so the control of platelet
aggregation is a clinical issue to improve the prognosis of DM
patients. Although anti-platelet therapy, such as aspirin, is
widely used in DM patients for the prevention of ischemic
cardiovascular diseases (5), the
existence of ‘aspirin-resistance’ is well known, but its mechanism
remains to be clarified (6).
Regarding platelet functions, we previously reported that
irreversible platelet microaggregation could be induced by a low
dose of ADP (1 μM) in the majority of type 2 DM patients, and that
the P2Y12 receptor plays a key role in the hypersensitivity of
platelet aggregation (7). We also
reported that the collagen-induced activation of p44/p42
mitogen-activated protein (MAP) kinase and p38 MAP kinase are
related to the platelet hyper-aggregation in type 2 DM patients
(8).
In response to biological stress, heat shock
proteins (HSPs) are induced in both prokaryotic and eukaryotic
cells (9). Among them,
low-molecular-weight HSPs including HSP27 and αB crystallin possess
high homology in their amino acid sequences, α-crystallin domain,
and are known to function as molecular chaperones (10,11). Human HSP27 is reportedly
phosphorylated at three serine residues (Ser-15, Ser-78 and Ser-82)
(12). HSP27 in a resting state
exists in an aggregated form. Once phosphorylated, HSP27 is rapidly
dissociated, resulting in the loss of its molecular chaperone
activity (13,14). We previously reported that the
ADP-induced HSP27 phosphorylation by p44/p42 MAP kinase and p38 MAP
kinase is correlated with the secretion of granules, such as
PDGF-AB, from human platelets (15). However, the clinical relevance of
HSP27 phosphorylation in platelets has not yet been clarified.
Herein, we investigated the relationship between
HSP27 phosphorylation and the platelet activation induced by ADP in
type 2 DM patients. Our results strongly suggest that the
phosphorylation of HSP27 is closely related to the acceleration of
platelet aggregation induced by ADP in type 2 DM patients.
Materials and methods
Materials
ADP was purchased from Sigma Chemical Co. (St.
Louis, MO, USA). PD98059 and SB203580 were purchased from
Calbiochem-Novabiochem Corporation (La Jolla, CA, USA). Anti-HSP27,
anti-phospho-HSP27 (Ser-15) and anti-phospho-HSP27 (Ser-78)
antibodies were purchased from Stressgen Biotechnologies (Victoria,
BC, Canada). Anti-phospho-HSP27 (Ser-82) antibodies were purchased
from BioMol Research Laboratories (Plymouth Meeting, PA, USA). The
PDGF-AB enzyme-linked immunosorbent assay (ELISA) kit and sCD40L
ELISA kit were purchased from R&D Systems (Minneapolis, MN,
USA). The other materials and chemicals were obtained from
commercial sources.
Subjects
The inclusion criteria for the study were the
presence of type 2 DM according to the criteria of the World Health
Organization. We excluded the patients who were being treated with
non-steroidal anti-inflammatory drugs, statins,
angiotensin-receptor blockers or angiotensin-converting enzyme
inhibitors, which could affect their platelet functions. We also
excluded the patients who were complicated with a malignancy,
infectious diseases including hepatitis B and C, or autoimmune
disorders. All participants were advised to avoid sleep deprivation
or blood donation. The study was approved by the Committee of
Ethics at the National Center for Geriatrics and Gerontology and
Gifu University Graduate School of Medicine.
Blood sampling
Ten millilitres of blood was drawn from the vein
between 8:00 and 9:00 after at least 15 min of bed rest to preserve
steady state conditions. Sodium citrate (14 μM) was added to the
blood immediately as an anticoagulant, and platelet-rich plasma
(PRP) was obtained by centrifugation at 155 × g for 12 min at room
temperature. Platelet-poor plasma (PPP) was prepared from the
residual blood by centrifugation at 2,500 × g for 5 min.
Platelet aggregation
Platelet aggregation was measured using an
aggregometer (PA-200 apparatus; Kowa Co., Ltd., Tokyo, Japan) with
a laser-scattering (LS) system as described previously (7,15).
In brief, PRP was preincubated at 37°C for 1 min with a stirring
speed of 800 rpm. Platelet aggregation was monitored for 4 min
after the addition of various doses of ADP (0, 0.3, 1 and 3 μM).
The percentage of transmittance of the isolated platelets was
recorded as 0%, and that of the appropriate PPP (blank) was
recorded as 100%. Platelet aggregation was then terminated by the
addition of ice-cold EDTA (10 mM). The conditioned mixture was
collected and centrifuged at 10,000 × g at 4°C for 2 min. The
supernatant was collected and stored at −80°C. The pellet was
washed twice with PBS and then lysed immediately by boiling in a
lysis buffer containing 62.5 mM Tris-HCl, pH 6.8, 2% sodium dodecyl
sulfate (SDS), 50 mM dithiothreitol and 10% glycerol for western
blot analysis. When indicated, the PRP was pretreated with 20 μM
SB203580 or 50 μM PD98059 for 15 min prior to the stimulation with
ADP.
Determination of the individual
ED50 value of ADP
To evaluate individual platelet aggregation, we used
an ED50 value for ADP-induced aggregation according to
the assessment by an aggregometer with the LS system. The
percentage of aggregation in each subject was analyzed at a dose of
0, 0.3, 1 and 3 μM ADP. Using the ALOKA curve software included in
the ALOKA RIA programs (ALOKA, Tokyo, Japan), a dose-response curve
was plotted. From the regression equation, the ADP dose
corresponding to 50% aggregation was calculated and identified as
the individual ED50 value for each subject.
Western blot analysis
Western blot analysis was performed as previously
described (16). In brief,
SDS-polyacrylamide gel electrophoresis (PAGE) was performed by the
Laemmli method (17) in a 12.5%
polyacrylamide gel. The proteins fractioned in the gels were
transferred onto polyvinylidene fluoride (PVDF) membranes, and then
the membranes were blocked with 5% fat-free dry milk in
Tris-buffered saline with 0.1% Tween-20 (TBS-T, 20 mM Tris, pH 7.6,
137 mM NaCl, 0.1% Tween-20) for 2 h before incubation with the
indicated primary antibody. A peroxidase-labeled antibody raised in
a goat against rabbit IgG (KPL, Gaithersburg, MD, USA) was used as
the secondary antibody. The primary and secondary antibodies were
diluted to their optimal concentrations with 5% fat-free dry milk
in TBS-T. The peroxidase activity on the PVDF membrane was
visualized with X-ray film by means of an ECL western blotting
detection system (GE Healthcare, Buckinghamshire, UK) following the
manufacturer’s protocol. The bands were analyzed by densitometry
using the ImageJ software program (National Institutes of Health,
USA).
ELISA for sCD40 ligand (sCD40L) or
PDGF-AB
The levels of sCD40L and PDGF-AB in the supernatant
of the conditioned mixture after platelet aggregation were
determined using specific ELISA kits.
Statistical analysis
The statistical significance of the differences
between two groups was assessed using an unpaired Student’s t-test
or the Chi square test. To analyze the correlation between two
variables, the Spearman’s rank-order correlation test was adopted.
A probability of >5% was considered to be statistically
significant.
Results
Characterization of the study groups
We first determined the standard range of the ADP
ED50 value for platelet aggregation analyzed by an
aggregometer with the LS system in non-DM healthy control subjects
(n=52), and found that it was 1.778±0.244 μM (mean ± 2 SEM). Based
on the normal range identified in non-DM controls, the subjects
were divided into two groups: Group 1, a hyper-aggregate group
(ED50 <1.534 μM) and Group 2, a normal or
hypo-aggregate group (ED50 ≥1.534 μM). The clinical and
biochemical characteristics of the subjects are presented in
Table I. The HbA1c levels were
significantly higher in Group 1 than in Group 2. However, the
anthropometric indices were within the normal limits in both
groups, and the differences in the metabolic variables were not
significant between the two groups.
 | Table ICharacteristics of the study
subjects. |
Table I
Characteristics of the study
subjects.
| Group 1 | Group 2 | P-value |
|---|
| Total number | 13 | 10 | |
| Gender (F/M) | 6/7 | 3/7 | |
| Age (years) | 68.1±7.7 | 67.0±4.2 | 0.695 |
| DM duration
(years) | 11.1±11.0 | 5.2±5.5 | 0.139 |
| Height (cm) | 156.3±8.7 | 163.3±7.9 | 0.059 |
| Weight (kg) | 58.0±9.8 | 62.6±13.6 | 0.349 |
| BMI | 23.7±3.2 | 23.3±3.8 | 0.786 |
| sBP (mmHg) | 121.8±16.9 | 131.4±18.7 | 0.213 |
| dBP (mmHg) | 69.2±8.4 | 78.2±10.2 | 0.030a |
| HbA1c
(%) | 9.4±2.1 | 7.8±1.1 | 0.023a |
| Glu (mg/dl) | 173.1±70.0 | 131.9±26.4 | 0.069 |
| TC (mg/dl) | 235.8±46.2 | 218.4±34.7 | 0.333 |
| TG (mg/dl) | 120.1±36.9 | 174.0±95.8 | 0.120 |
| HDL (mg/dl) | 54.5±11.5 | 53.3±14.0 | 0.818 |
| Plt
(×104) | 22.3±6.1 | 21.6±6.1 | 0.766 |
| ADP ED50
(μM) | 0.706±0.2 | 1.963±0.2 | <0.001b |
Platelet aggregation of the study
groups
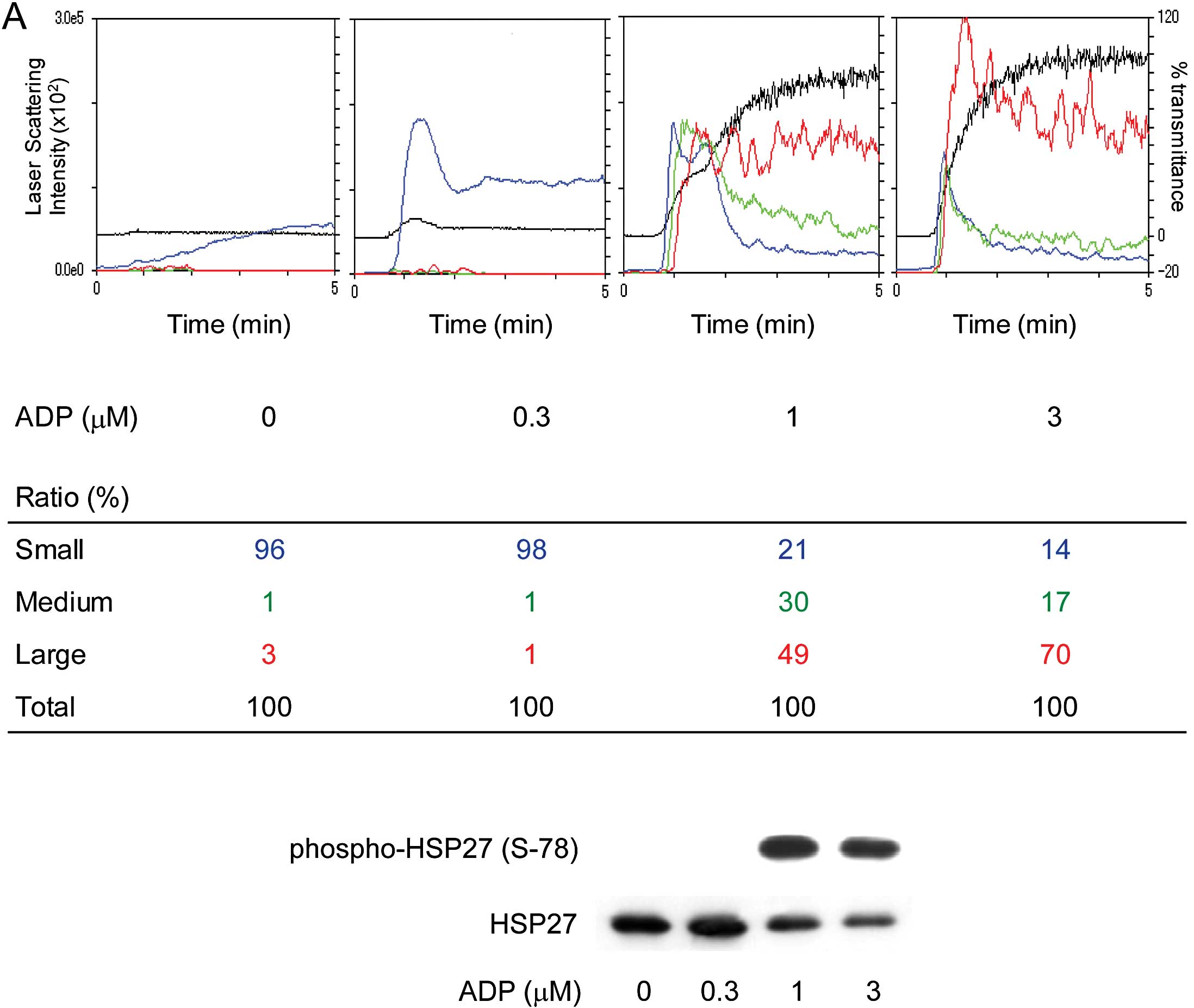
Representative patterns of ADP-induced platelet
aggregation in the study groups analyzed by an aggregometer with
the LS system are shown in Fig.
1A. ADP dose-dependently elicited platelet aggregation in both
groups, however, irreversible formation of large aggregates induced
by 1 μM ADP was observed only in Group 1. In addition, as
previously reported (7),
spontaneous microaggregation without ADP stimulation was observed
in several cases from Group 1.
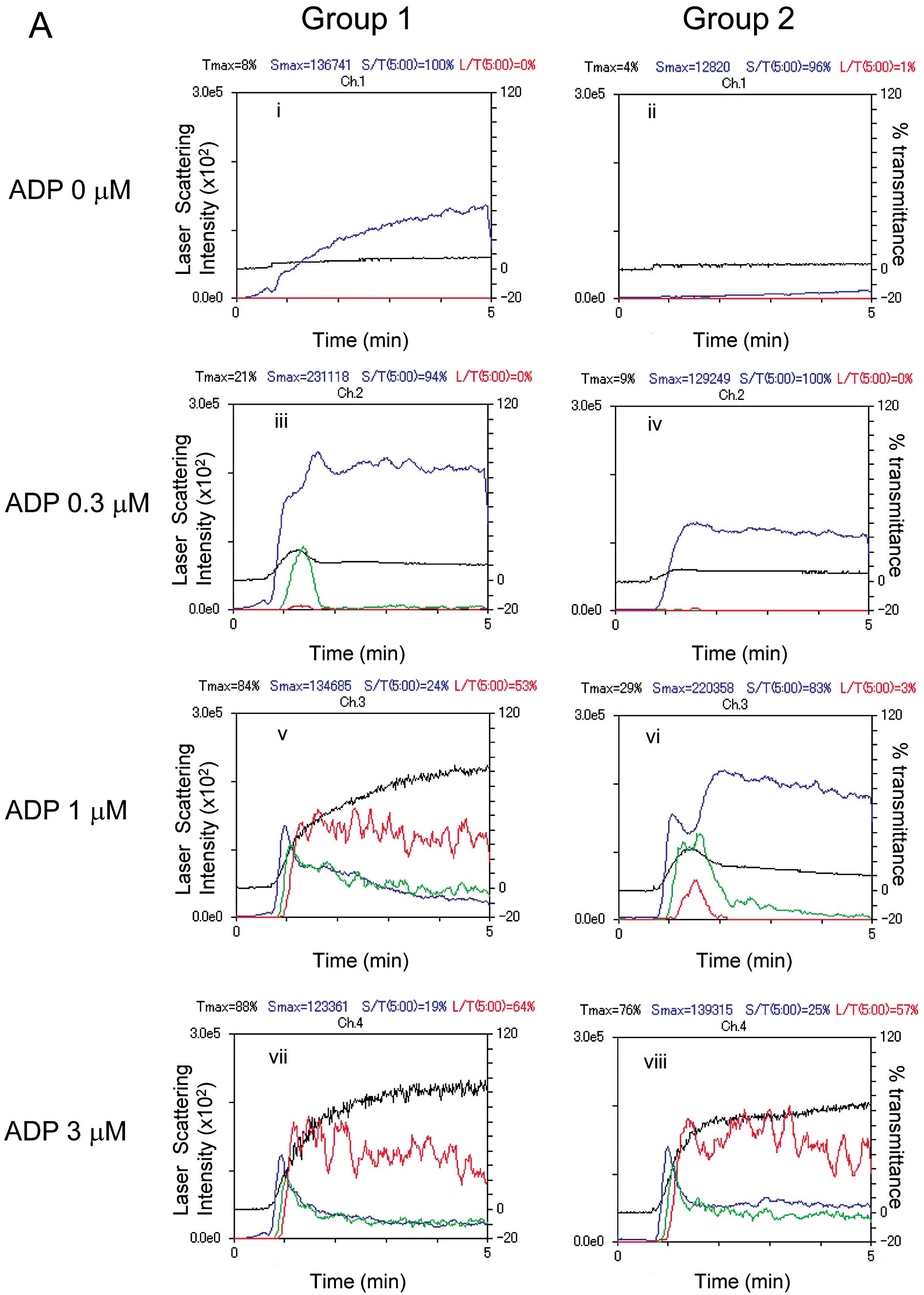 | Figure 1Representative patterns of platelet
aggregation induced by various doses of ADP as detected by an
aggregometer with the LS system and representative data showing the
ADP-induced HSP27 phosphorylation in platelets from type 2 DM
patients. PRP from type 2 DM patients was stimulated by various
doses of ADP (0, 0.3, 1 and 3 μM) in an aggregometer at 37°C for 4
min with a stirring speed of 800 rpm. (A) Time-dependent changes in
the platelet aggregation after stimulation with 0 (i and ii), 0.3
(iii and iv), 1 (v and vi) and 3 μM (vii and viii) are shown. The
black line indicates the percentage of transmittance of each sample
(the isolated platelets were recorded as 0%, and platelet-free
plasma was recorded as 100%). The blue line indicates small
aggregates (9–25 μm); green line, medium aggregates (25–50 μm); red
line, large aggregates (50–70 μm). The distributions (%) of the
aggregated particle size were measured with the LS methods. The DM
patients were divided into groups based on a platelet
aggregation-accelerated state (Group 1, ED50 <1.534
μM) and a platelet aggregation-non-accelerated state (Group 2,
ED50 ≥1.534 μM) on the basis of the normal range
calculated from the mean ED50 value of the non-DM
control group (mean ± 2 SEM range; 1.778±0.244 μM). (B) The
reaction was terminated by the addition of an ice-cold EDTA (10 mM)
solution. The extracts of platelets were subjected to western blot
analysis using antibodies against total HSP27 and phospho-specific
HSP27 (Ser-15, Ser-78 and Ser-82). The bands of phospho-HSP27 were
quantified using the ImageJ software program and normalized to the
total HSP27 band, and the ratio (phospho-HSP27/total HSP27) is
presented for each value. Hatched bars indicate the phosphorylation
ratio of HSP27 (Ser-78), and filled bars indicate the
phosphorylation ratio of HSP27 (Ser-82). |
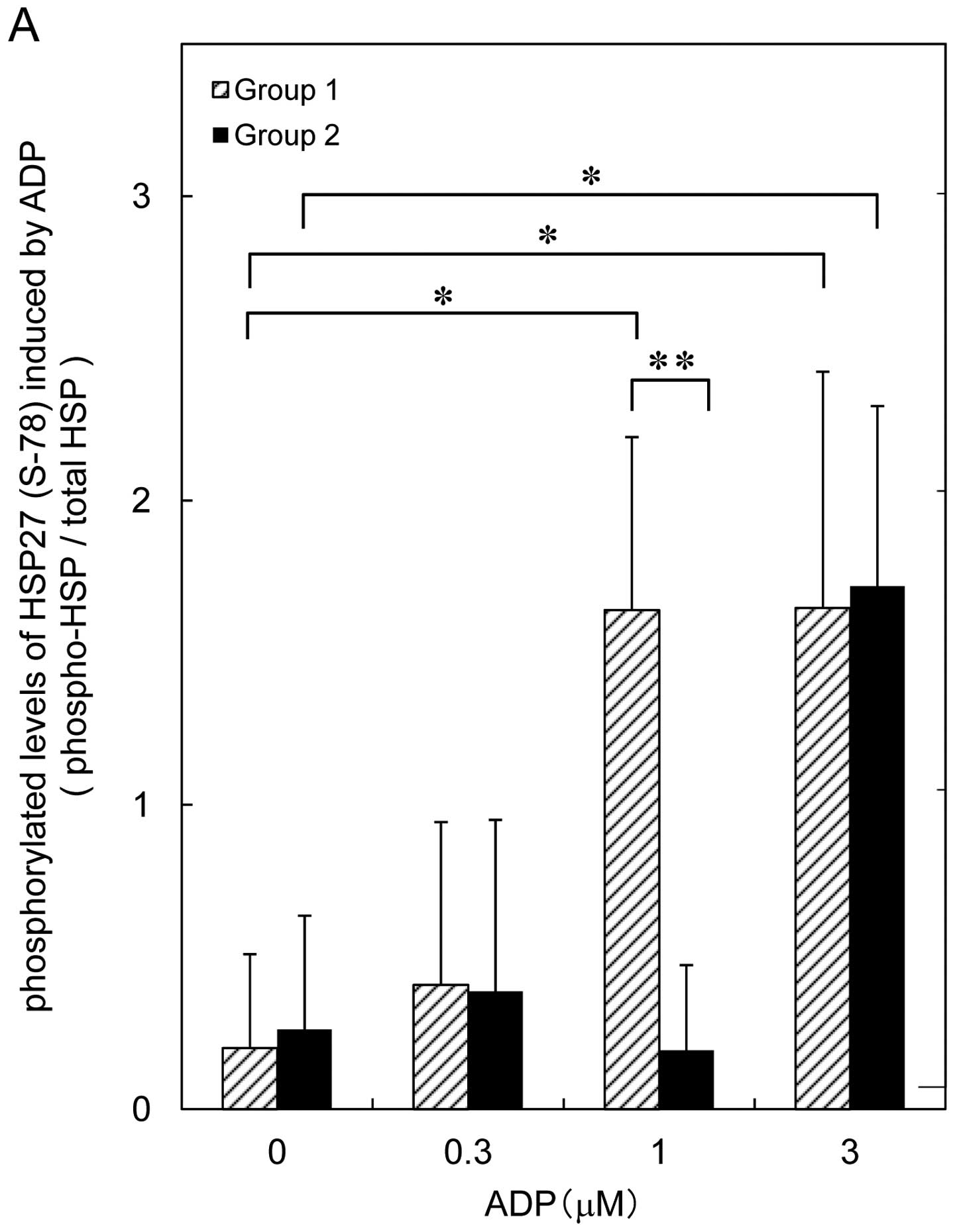
Comparison of the ADP-induced HSP27
phosphorylation levels of platelets from type 2 DM patients in
Groups 1 and 2
ADP has been reported to induce HSP27
phosphorylation in human platelets (18). We previously reported that
ADP-induced platelet granule secretion is correlated with the
phosphorylation of HSP27 in healthy donors (15). It is well known that human HSP27
is phosphorylated at three serine residues (Ser-15, Ser-78 and
Ser-82) (11). Thus, we first
examined the effects of various doses of ADP (0.3, 1 and 3 μM) on
the phosphorylation of HSP27 (Ser-15, Ser-78 and Ser-82) by western
blot analysis. In both groups, ADP dose-dependently induced the
phosphorylation of HSP27 at Ser-78 and Ser-82, without affecting
the phosphorylation at Ser-15 (Fig.
1B). The ADP-induced levels of HSP27 phosphorylation (Ser-78)
as quantified by western blotting using the ImageJ software program
are shown in Fig. 2A. In Group 1,
ADP (1 or 3 μM) caused a significant increase in HSP27 (Ser-78). On
the other hand, only 3 μM of ADP, but not 1 μM, increased the
phosphorylation level of HSP27 (Ser-78) in Group 2 (Fig. 2A). The ADP-induced levels of HSP27
phosphorylation (Ser-82) are shown in Fig. 2B. Similarly, the same doses of ADP
(1 or 3 μM) caused a significant increase in the phosphorylation of
HSP27 (Ser-82) in Group 1, however, ADP increased the
phosphorylation level of HSP27 (Ser-82) in Group 2 only at a dose
of 3 μM (Fig. 2B). The effect of
ADP on the phosphorylation of HSP27 (Ser-78) was also more
extensive than that on the phosphorylation of HSP27 (Ser-82).
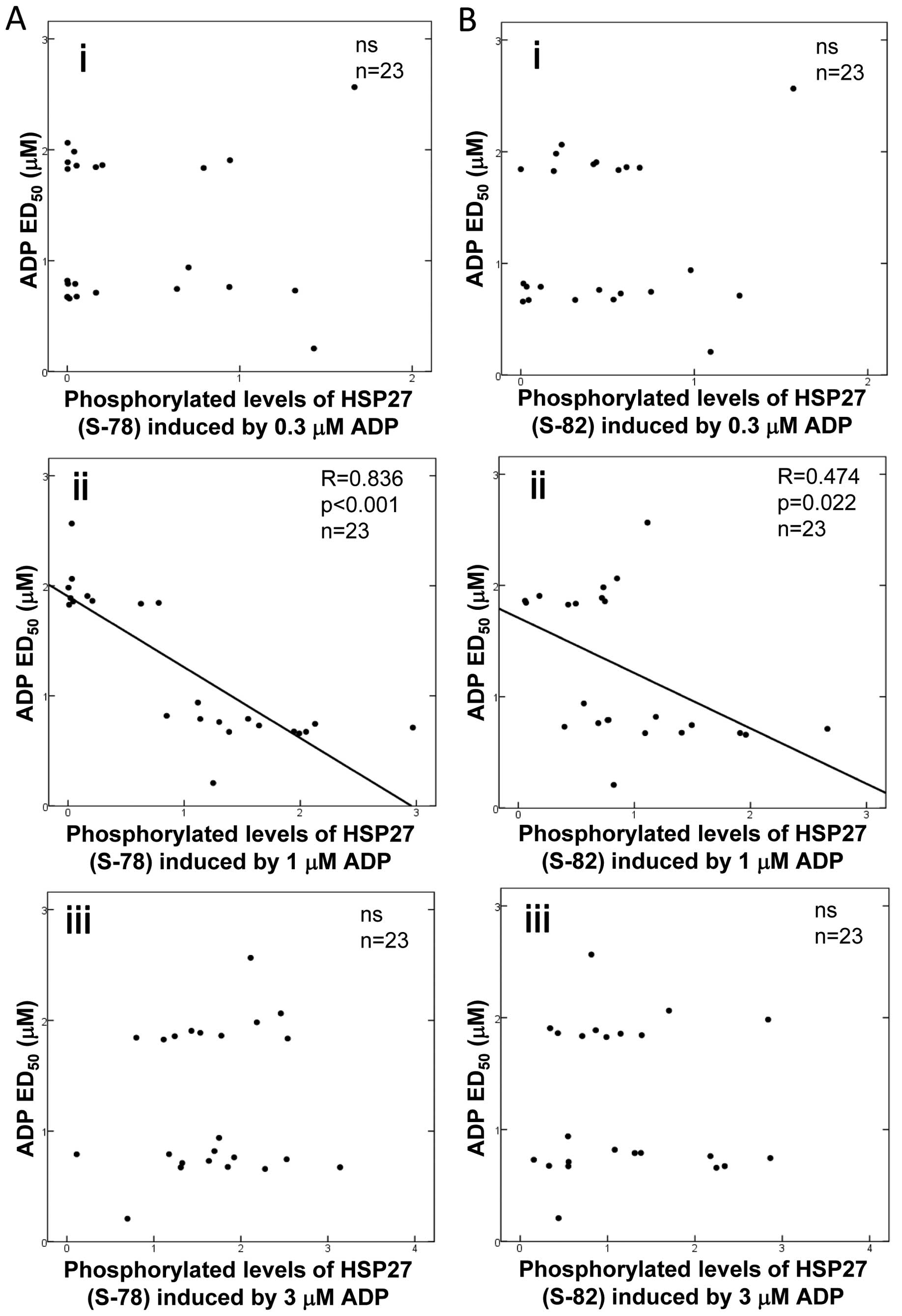
Relationship between individual ADP
ED50 values and levels of HSP27 phosphorylation induced
by ADP in type 2 DM patients
To clarify whether there is a relationship between
the platelet aggregation induced by ADP and the phosphorylation
levels of HSP27 in platelets from type 2 DM patients, we plotted
the levels of HSP27 phosphorylation (Ser-78 and Ser-82) induced by
various doses of ADP against the corresponding individual ADP
ED50 values (Fig. 3).
For the phosphorylation of HSP27 at Ser-78, we observed a
significant negative correlation for ADP at a concentration of 1 μM
(Fig. 3ii, R=0.836, P<0.001,
n=23). There was no significant relationship between the individual
ADP ED50 values and the HSP27 (Ser-78) phosphorylation
levels induced by 0.3 or 3 μM of ADP (Fig. 3A-i and iii).
In regards to HSP27 (Ser-82) phosphorylation levels
(Fig. 3B), there was a
significant negative correlation with ADP at a concentration of 1
μM (Fig. 3B-ii, R=0.474, P=0.022,
n=23). However, the correlation was weaker than that observed for
the phosphorylation of HSP27 at Ser-78. No relationship was
observed between the individual ADP ED50 values and
HSP27 (Ser-82) phosphorylation levels induced by 0.3 or 3 μM of ADP
(Fig. 3B-i and iii).
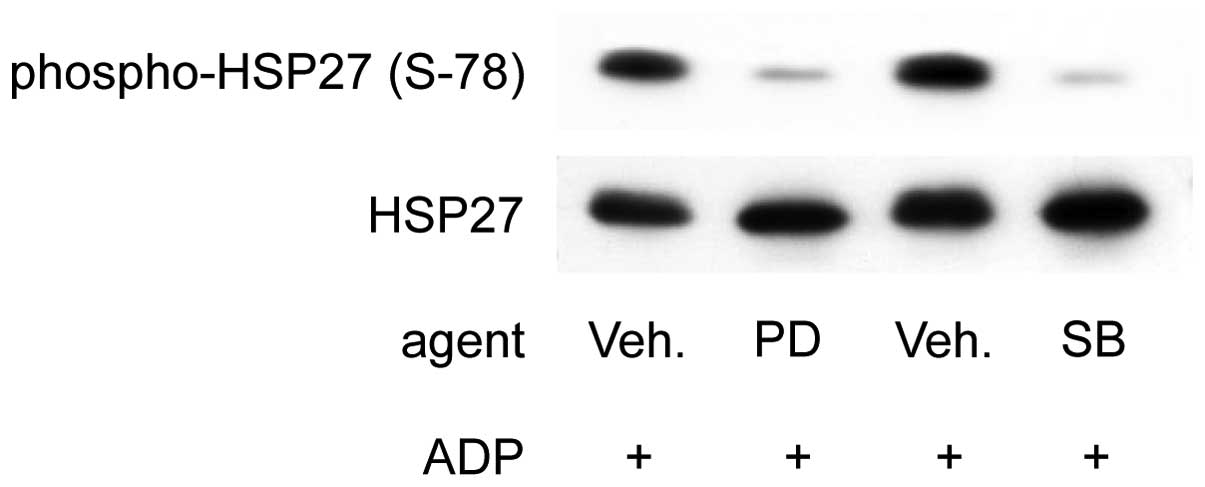
Effects of PD98059 or SB203580 on the
phosphorylation of HSP27 (Ser-78) induced by 1 μM ADP in the
platelets of type 2 DM patients classified into Group 1
We previously reported that PD98059, a specific
inhibitor of MEK1/2 (19), or
SB203580, a specific inhibitor of p38 MAP kinase (20), inhibits the phosphorylation of
HSP27 induced by 3 μM of ADP in the platelets from healthy donors
and that this is related to granule secretion (15). We next examined the effects of
PD98059 or SB203580 on the phosphorylation of HSP27 (Ser-78)
induced by 1 μM of ADP in the platelets of Group 1 type 2 DM
patients. We previously confirmed that PD98059 (50 μM) or SB203580
(20 μM) potently suppresses the ADP-induced phosphorylation of
p44/p42 MAP kinase or that of p38 MAP kinase, respectively, in
human platelets (15). In several
cases from Group 1, PD98059 (50 μM) significantly suppressed the
phosphorylation of HSP27 (Ser-78) in the platelets induced by 1 μM
of ADP (Fig. 4). SB203580 also
inhibited ADP (1 μM)-induced HSP27 (Ser-78) phosphorylation
(Fig. 4).
Comparison of the release of sCD40L and
the secretion of PDGF-AB induced by ADP from platelets of type 2 DM
patients in Groups 1 and 2
It has been reported that sCD40L, generated from
CD40L, appears on the cell surface of activated platelets and is
elevated in the plasma of type 2 DM patients (21). We therefore examined the effects
of ADP on the release of sCD40L from the platelets of DM patients
in Groups 1 and 2. The highest concentration of ADP (3 μM) induced
the release of sCD40L in both groups; however, a lower dose of ADP
(1 μM) caused the release in Group 1 but not in 2 (Fig. 5A).
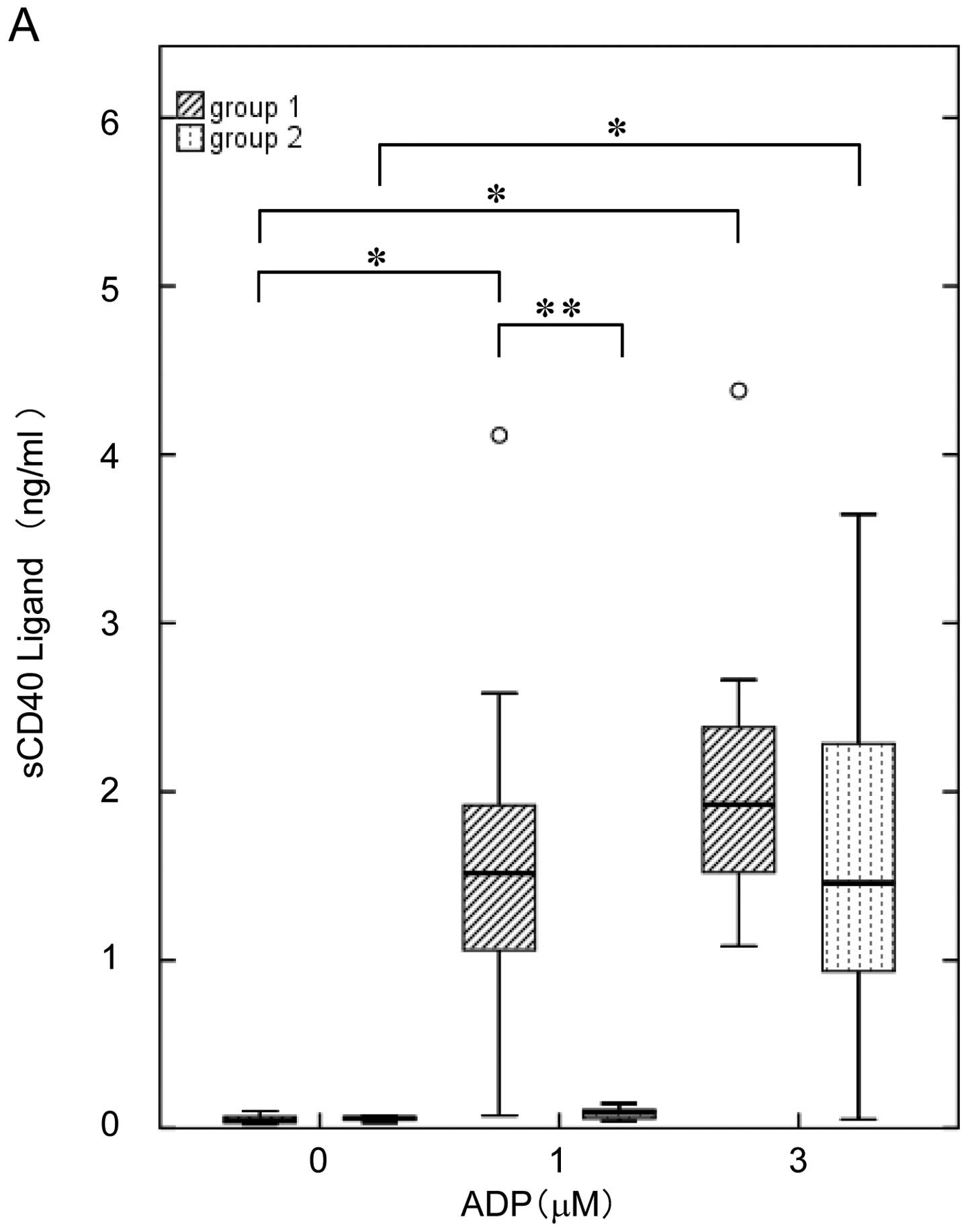 | Figure 5Comparison of the ADP-induced release
of sCD40L and PDGF-AB between Groups 1 and 2 DM patients. The
subjects were divided into Group 1 (ED50 <1.534 μM,
hatched bars, n=13) and Group 2 (ED50 ≥1.534 μM, dotted
bars, n=10) on the basis of the normal range calculated from the
mean ED50 value of the non-DM group (mean ± 2 SEM range;
1.778±0.244 μM). The platelet-rich plasma was stimulated by various
doses of ADP (0, 1 and 3 μM) in an aggregometer at 37°C for 30 min
with a stirring speed of 800 × g, and the reaction was terminated
by the addition of an ice-cold EDTA (10 mM) solution. The level of
(A) sCD40L and (B) PDGF-AB in the supernatant of the conditioned
mixture after platelet aggregation was determined using specific
ELISA kits. The thick horizontal lines correspond to the median
values, the rectangles span the 25th to 75th percentiles, and the
error bars indicate the range of the standard deviations.
*P<0.05, compared to the value without ADP,
**P<0.05, for the value of Group 1 compared with that
of Group 2 by Student’s t-test. |
We previously reported that the ADP-induced
phosphorylation of HSP27 via p44/p42 MAP kinase and p38 MAP kinase
is correlated with the secretion of PDGF-AB from the platelets of
healthy donors (15). Therefore,
we also compared the effects of ADP on the secretion of PDGF-AB
from the platelets of Groups 1 and 2. We observed that 3 μM of ADP
elicited PDGF-AB secretion from the platelets in both groups, but
that the 1 μM concentration of ADP significantly stimulated the
release only in Group 1 (Fig.
5B).
Suppressive effect of aspirin on
ADP-induced HSP27 phosphorylation (Ser-78) in Group 1 type 2 DM
patients
Aspirin is widely used as an anti-platelet agent,
and the adequate treatment with aspirin in DM patients for the
prevention of cardiovascular diseases is recommended by the
American Diabetes Association (5). We further examined the effects of
aspirin therapy for 4 weeks on the phosphorylation of HSP27
(Ser-78) induced by ADP in several patients with type 2 DM who were
classified into Group 1. The representative data are shown in
Fig. 6. According to the results
of the aggregometer, 1 μM of ADP caused significant platelet
aggregation in the case and almost 50% of the aggregates were of a
large size (50–70 μm) (Fig. 6A).
Before aspirin therapy, the individual ADP ED50 value in
the case was calculated to be 0.658 μM (classified as Group 1). The
phosphorylation of HSP27 (Ser-78) was significantly induced by ADP
even at 1 μM in this patient (Fig.
6A). Treatment with an anti-platelet agent was proposed and the
patient started to take aspirin at a dose of 100 mg daily. After 4
weeks, the acceleration of ADP-induced platelet aggregation was
significantly ameliorated. The platelet aggregates induced by 1 μM
of ADP were still observed, but 84% of them were microaggregates
(9–25 μm) (Fig. 6B). The
individual ADP ED50 value was calculated to be 2.421 μM.
In parallel with the amelioration of platelet aggregation, the
phosphorylation of HSP27 (Ser-78) induced by ADP was markedly
decreased and 1 μM of ADP hardly elicited any HSP phosphorylation
(Fig. 6B).
Discussion
In the present study, we investigated the
relationship between HSP27 phosphorylation and platelet aggregation
induced by ADP in type 2 DM patients. According to the individual
ED50 value of ADP for platelet aggregation analyzed by
the LS aggregometer, type 2 DM patients were classified into two
groups, hyper-aggregate group (Group 1) and normo- or
hypo-aggregate group (Group 2). We found that a low dose of ADP (1
μM) caused significant phosphorylation of HSP27 (Ser-78 and Ser-82)
in the patients with hyper-aggregation (Group 1), and that the
effect of ADP on the phosphorylation of HSP27 (Ser-78) was more
evident than that on HSP27 (Ser-82). We further investigated the
relationship between the levels of HSP27 phosphorylation induced by
various doses of ADP and the individual ED50 values for
platelet aggregation in the study subjects, and showed that the
phosphorylation of HSP27 only occurred when the platelets were
stimulated by ADP (1 μM), and that the extent of phosphorylation
closely correlated with the individual ED50 values.
Moreover, the relationship was more significant for the
phosphorylation at Ser-78 than for that at Ser-82. These results
strongly suggest that the phosphorylation of HSP27, especially at
Ser-78, plays a role in the platelet hypersensitivity to ADP in
type 2 DM patients. To the best of our knowledge, this is the first
report clearly indicating the clinical and pathological
significance of HSP27 phosphorylation in human platelets.
Phosphorylated HSP27 is reportedly associated with the
activation-dependent cytoskeleton in human platelets (18). The phosphorylation-mimicking
mutants of HSP27 (Ser-15, Ser-78 and Ser-82) have been reported to
lead to faster and stronger actin polymerization than the wild-type
protein in human platelets (22).
The conformational changes in the cytoskeleton and the
actinpolymerization caused by HSP27 phosphorylation may be involved
in the pathogenesis of platelet hyper-aggregation in type 2 DM
patients.
In addition, we previously reported that a low dose
of ADP (1 μM) induced microaggregation, and that this was
significantly correlated with the HbA1c value, a clinical indicator
of DM control, and that P2Y12 receptors (not P2Y1) play a central
role in the microaggregation (7).
In the present study subjects, the individual HbA1c values were
higher in the subjects of Group 1 than in Group 2, which was
consistent with our previous findings. Based on our findings, it is
probable that P2Y12 receptor-mediated signaling is involved in the
low-dose ADP (1 μM)-induced phosphorylation of HSP27 in the
platelets derived from type 2 DM patients with a hyper-aggregated
status.
It has been previously reported that HSP27
phosphorylation is catalyzed by the MAP kinase superfamily
(11,23,24). In our present study, we also
demonstrated that PD98059 (19)
and SB203580 (20) both markedly
inhibited the phosphorylation of HSP27 (Ser-78) induced by low-dose
ADP (1 μM) in Group 1 subjects, suggesting that the phosphorylation
is dependent upon the activation of p44/p42 MAP kinase and p38 MAP
kinase. Among the MAP kinase family members, we have found that
only p44/p42 MAP kinase and p38 MAP kinase are involved in the
phosphorylation of HSP27 induced by 3 μM of ADP in human platelets
from healthy donors, and that HSP27 phosphorylation is sufficient
to induce granular secretion, but not platelet aggregation
(15). In addition, we
demonstrated that a low-dose of ADP (1 μM) hardly elicits
phosphorylation of HSP27 or the aggregation of platelets in healthy
donors (15). In the present
study, the relationship between the individual ADP ED50
value of platelet aggregation and the phosphorylated levels of
HSP27 induced by ADP in the Group 1 DM patients was not significant
at 3 μM, but was at 1 μM. Therefore, it seems likely that the
involvement of HSP27 phosphorylation via p44/p42 MAP kinase and p38
MAP kinase in platelet aggregation is limited to situations of
pathological hyper-aggregated status, such as type 2 DM.
We next examined the effect of ADP on the release of
sCD40L and the secretion of PDGF-AB from the platelets and compared
the results between Groups 1 and 2. We observed that a low dose of
ADP (1 μM) significantly induced the release of both molecules in
Group 1, but not in 2. This release induced by a low-dose of ADP
seems to occur in parallel with the phosphorylation of HSP27. The
CD40L released from platelets is known to be immediately cleaved
into sCD40L, which induces inflammatory responses in the
endothelium, resulting in the production of reactive oxygen
species, adhesion molecules, chemokines and tissue factors, major
components of inflammatory responses promoting atherosclerosis
(1). PDGF-AB is stored in the
α-granules of platelets and is released when the platelets are
activated, after which it becomes involved in vascular
atherosclerosis (25). Thus, it
is likely that the low-dose ADP-induced phosphorylation of HSP27
plays significant roles in both the release of inflammatory or
atherosclerogenic factors and the enhancement of platelet
aggregation, resulting in an increased risk of vascular diseases in
type 2 DM patients.
In the present study, we examined the effect of
aspirin on the phosphorylation of HSP27 (Ser-78) induced by ADP in
several cases of type 2 DM classified into Group 1. We confirmed
that 4 weeks of treatment with aspirin at a dose of 100 mg daily
caused significant improvement in the platelet hyper-aggregation,
in parallel with the suppression of the low-dose ADP (1
μM)-stimulated HSP27 (Ser-78) phosphorylation levels in the
platelets of these patients. Aspirin is a well known irreversible
cyclooxygenase inhibitor which causes the inhibition of thromboxane
A2 synthesis in human platelets (1), and its use is widely recommended for
the prevention of cardiovascular diseases in type 2 DM patients
(5). The significant effect of
aspirin on the phosphorylation of HSP27 induced by low-dose ADP
might indicate that the regulation of HSP27 phosphorylation is a
novel therapeutic target for platelet hyper-aggregation in type 2
DM patients. Moreover, the detection of HSP27 phosphorylation could
be developed as a method for monitoring platelets for a
hyper-aggregation status, particularly in the patients with
vascular diseases associated with atherosclerosis. Further
investigations would be required to clarify the detailed mechanisms
underlying the functions of HSP27 in human platelets.
In conclusion, the phosphorylation of HSP27,
particularly at Ser-78, is closely related to the acceleration of
platelet microaggregation induced by ADP in type 2 DM patients.
Acknowledgements
We are very grateful to Yoko Kawamura
for her skillful technical assistance. This study was supported in
part by the Grant-in-Aid for Scientific Research (20590565) from
the Ministry of Education, Science, Sports and Culture of Japan and
the Research Funding for Longevity Sciences (22-4) from National
Center for Geriatrics and Gerontology (NCGG), Japan.
References
|
1.
|
G DaviC PatronoPlatelet activation and
atherothrombosisNew Engl J
Med35724822494200710.1056/NEJMra07101418077812
|
|
2.
|
B FurieBC FurieMechanism of thrombus
formationNew Engl J Med359938949200810.1056/NEJMra0801082
|
|
3.
|
P ZimmetKG AlbertJ ShawGlobal and social
implications of the diabetes
epidemicNature414782787200110.1038/414782a
|
|
4.
|
SM GrundyIJ BenjaminGL BrurkeA ChaitRH
EckelBV HowardW MitchSC Smith JrJR SowersDiabetes and
cardiovasucular disease: a statement for healthcare professionals
from the American Heart
AssociationCirculation10011341146199910.1161/01.CIR.100.10.113410477542
|
|
5.
|
JA ColwellAmerican Diabetes Association.
Aspirin therapy in diabetesDiabetes Care26Suppl
1S87S88200310.2337/diacare.26.2007.S87
|
|
6.
|
GJ HankeyJW EikelboomAspirin
resistanceLancet367606617200610.1016/S0140-6736(06)68040-916488805
|
|
7.
|
H MatsunoH TokudaA IshisakiY ZhouY
KitajimaO KozawaP2Y12 receptors play a significant role in the
development of platelet microaggregation in patients with diabetesJ
Clin Endocrinol Metab90920927200510.1210/jc.2004-013715483100
|
|
8.
|
Y HanaiS AdachiI YasudaS TakaiR
Matsushima-NishiwakiH KatoY EnomotoS AkamatsuS SakakibaraS
OguraCollagen-induced p38 MAP kinase activation is a biomarker of
platelet hyper-aggregation in patients with diabetes mellitusLife
Sci85386394200910.1016/j.lfs.2009.07.00319631227
|
|
9.
|
JP HendricFU HartlMolecular chaperone
functions of heat-shock proteinsAnn Rev
Biochem62349384199310.1146/annurev.bi.62.070193.0020258102520
|
|
10.
|
Y InagumaS GotoH ShinoharaK HasegawaK
OhshimaK KatoPhysiological and pathological changes in levels of
the two small stress proteins, HSP27 and αB crystallin, in rat
hindlimb musclesJ Biochem11437838419938282729
|
|
11.
|
IJ BenjaminDR McMillanStress (heat shock)
proteins: molecular chaperones in cardiovascular biology and
diseaseCirc Res83117132199810.1161/01.RES.83.2.1179686751
|
|
12.
|
J LandryH LambertM ZhouJN LavoieE HickeyLA
WeberCW AndersonHuman HSP27 is phosphorylated at serines 78 and 82
by heat shock and mitogen-activated kinases that recognize the same
amino acid motif as S6 kinase IIJ Biol Chem26779480319921730670
|
|
13.
|
K KatoK HasegawaS GotoY
InagumaDissociation as a result of phosphorylation of an aggregated
form or the small stress protein, hsp27J Biol
Chem269112741127819948157658
|
|
14.
|
T RogallaM EhrnspergerX PrevilleA
KotlyarovG LutschC DucasseC PaulM WieskeAP ArrigoJ BuchnerM
GaestelRegulation of Hsp27 oligomerization, chaperone function, and
protective activity against oxidative stress/tumor necrosis factor
α by phosphorylationJ Biol Chem2741894718956199910383393
|
|
15.
|
H KatoS TakaiR Matsushima-NishiwakiS
AdachiC MinamitaniT OtsukaH TokudaS AkamatsuT DoiS OguraO
KozawaHSP27 phosphorylation is correlated with ADP-induced platelet
granule secretionArch Biochem
Biophys4758086200810.1016/j.abb.2008.04.02318471985
|
|
16.
|
K KatoH ItoK HasegawaY InagumaO KozawaT
AsanoModulation of the stress-induced synthesis of hsp27 and αB
crystallin by cyclic AMP in C6 rat glioma cellsJ
Neurochem669469501996
|
|
17.
|
UK LaemmliCleavage of structural proteins
during the assembly of the head of bacteriophage
T4Nature227680685197010.1038/227680a05432063
|
|
18.
|
Y ZhuS O’NeillJ SaklatvalaL TassiME
MendelsohnPhosphorylated HSP27 associates with the
activation-dependent cytoskeleton in human
plateletsBlood843715372319947949127
|
|
19.
|
DR AlessiA CuendaP CohenDY DudleyAR
SaltielPD98059 is a specific inhititor of the activation of
mitogen-activated protein kinase in vitro and in vivoJ Biol
Chem2702748927494199510.1074/jbc.270.46.274897499206
|
|
20.
|
A CuendaJ RouseYN DozaR MeierP CohenTF
GallagherPR YoungJC LeeSB203580 is a specific inhibitor of a MAP
kinase homologue which is stimulated by cellular stresses and
interleukin-1FEBS
Lett364229233199510.1016/0014-5793(95)00357-F7750577
|
|
21.
|
F SantilliG DaviA ConsoliF CipolloneA
MezzettiA FalcoT TarborelliE DevangelioG CiabattoniS BasiliC
PatronoThromboxane-dependent CD40 ligand release in type 2 diabetes
mellitusJ Am Coll
Cardiol47391397200610.1016/j.jacc.2005.03.07916412866
|
|
22.
|
E ButtD ImmlerHE MeyerA KotlyarovK LaassM
GaestelHeat shock protein 27 is a substrate of cGMP-dependent
protein kinase in intact human platelets: phosphorylation-induced
actin polymerization caused by HSP27 mutantsJ Biol
Chem27671087113200110.1074/jbc.M009234200
|
|
23.
|
JM KyriakisJ AvruchSounding the alarm:
protein kinase cascades activated by stress and inflammationJ Biol
Chem2712431324316199610.1074/jbc.271.40.243138798679
|
|
24.
|
J GuayH LambertG Gingras-BretonJN LavoieJ
HuotJ LandryRegulation of actin filament dynamics by p38 map
kinase-mediated phosphorylation of heat shock protein 27J Cell
Sci11035736819979057088
|
|
25.
|
F RenduB Brohard-BohnThe platelet release
reaction: granules’ constituents, secretion and
functionsPlatelets122612732001
|