Introduction
Pathological cardiac hypertrophy is a common heart
disease occurring in 20–50% of patients with mild to moderate
hypertension and up to 90% of patients with severe hypertension
(1). Although cardiac hypertrophy
represents an adaptive response, prolonged hypertrophic status has
been reported to be associated with decompensation of heart
function, development of heart failure and sudden death in humans
(2). Both conditions are
independent risk factors for morbidity and mortality (3). The regression of cardiovascular
hypertrophy is currently regarded as an important therapeutic
target in reducing complications of hypertension (4).
The angiotensin-II-regulated downstream insulin-like
growth factor II receptor (IGF-IIR) signaling pathway plays an
important role in regulating the development of cardiac hypertrophy
(5,6). Insulin-like growth factor II
(IGF-II) is synthesized in most mammalian tissues. When IGF-II
activates the IGF-IIR signaling pathway, IGF-II may activate
calcineurin through Gi protein signaling transduction and stimulate
cardiac cell hypertrophy (6). An
additional pathway that has received attention is mediated by
Ca2+-calmodulin activated phosphatase calcineurin. Once
activated, calcineurin directly binds to and dephosphorylates the
nuclear factor of the activated T-cells, cytoplasmic 3 (NFATc-3)
transcription factor in the cytoplasm, permitting its translocation
to the nucleus where dephosphorylated NFATc-3 further interacts
with the GATA-4 transcription factor to form the complex that
participates in the development of concentric hypertrophy and in
the expression levels of hypertrophy responsive genes, such as
atrial natriuretic peptide (ANP) and B-type natriuretic peptide
(BNP) (7,8).
γ-aminobutyric acid (GABA) is a non-protein amino
acid widely distributed in nature from microorganisms to plants and
animals. It has several physiological functions, such as
neurotransmission and the induction of hypotensive diuretic and
tranquilizer effects (9–11). GABA may be obtained from a number
of fruits and vegetables, but it is scarce in nature ranging from
0.03 to 2.00 μmol/g fresh weight (12). GABA is synthesized by glutamate
decarboxylase (GAD; EC 4.1.1.15), an enzyme that catalyzes the
irreversible decarboxylation of L-glutamate to GABA. It has been
reported that GAD is present in the mammalian brain, plants
(13,14) and in lactic acid bacteria (LAB)
(11). Therefore, many studies
have focused on GABA production by using LAB as bacterial cell
factories (10,12,15). The consumption of GABA-enriched
foods has been reported to depress the elevation of systolic blood
pressure in spontaneously hypertensive rats (SHRs) and mildly
hypertensive humans (16,17). Vascular hypertrophy of the
thoracic aorta, coronary and renal interlobular arteries has been
show to be reduced in SHRs with the oral administration of
GABA-rich soy sauce, which is produced from moromi fermented with
Lactobacillus rennini (18).
The purple sweet potato [Ipomoea batatas (L.)
Lam.] (PSP) can be easily grown in tropical areas, such as Taiwan,
Japan and China. It is rich in vitamins, minerals, dietary fiber
and non-fibrous carbohydrates, as well as an excellent source of
the antioxidant, anthocyanin (19,20). The aqueous extracts of
anthocyanin-producing sweet potato have higher antiproliferative
and antimutagenic potential than other crops (21), in which the bioactive compounds
provide sensorial characteristics and are involved in
cardiovascular disease risk protection (22). Previous studies have shown that
treatment with antioxidants inhibits the hypertrophic response of
cardiac myocytes (23–25). Thus, PSP may be used in the food
industry as an antioxidant to improve human health.
In our previous study, we found that purple sweet
potato yogurt (PSPY) fermented with Lactobacillus
acidophilus, L. delbrueckii subsp. lactis and
L. gasseri has high GABA activity (26). To the best of our knowledge,
previous studies regarding the potential role of GABA-enriched
yogurt in hypertension-induced cardiac hypertrophy have not been
conducted. Therefore, the purpose of this study was to determine
the potency of PSPY to attenuate cardiac hypertrophy in SHRs and
elucidate the anti-hypertrophic effects of PSPY on the
intracellular transduction pathways in heart tissue in vivo.
We anticipated that PSPY would be capable of providing dietary
assistance in regulating blood pressure and preventing the
development of cardiac hypertrophy.
Materials and methods
Bacteria strains and their growth
conditions
The 3 LAB strains were purchased from the Food
Industry Research and Development Institute of Biological Resources
Conservation and Research Center, Hsinchu, Taiwan: L.
acidophilus BCRC 14065 (LA), L. delbrueckii subsp.
lactis BCRC 12256 (LDL) and L. gasseri BCRC 14619
(LGA). The stock culture was maintained at −80°C in 20% glycerol
prior to usage. The bacteria were propagated twice in
Lactobacilli MRS Broth (DIFCO, Baltimore, MD, USA)
containing 0.05% L-cysteine overnight at 37°C before experimental
procedure.
Preparation of PSPY
The PSPs were acquired from the Taiwan Agricultural
Research Institute and stored at 4°C after being washed with tap
water. To make PSPY, the potatoes were first peeled, cut into 1-cm
slices and steamed at 100°C for 20 min. The cooked spuds were then
homogenized and before pasteurization (121°C, 15 min), a mixture of
0.05% α-amylase, 10% skimmed milk powder, 0.05% protease and 3%
whey protein was added. The 3 different LAB strains were inoculated
to the PSP milk and incubated at 37°C for 24 h until the fermented
PSPY was obtained. The final product was stored at 4°C in the
refrigerator for later experimental usage.
Animals and experimental groups
Twenty-two male SHRs and 12 male Wistar-Kyoto rats
(WKYs) were purchased from BioLASCO Taiwan Co., Ltd. (Taipei,
Taiwan). These animals, aged 6 weeks, were housed individually in a
temperature (20±2°C)- and humidity (55±5%)-controlled environment.
The rats were maintained on a 12-h dark-light cycle with lights on
from 8 a.m. to 8 p.m. They were fed with chow pellets (MF-18;
Oriental Yeast Co., Ltd., Tokyo, Japan) and were allowed access to
water ad libitum. An acclimatization period of 1 week after
delivery by the supplier was allowed before the SHRs were randomly
distributed into 4 groups: SHR control (2.5 ml distilled water),
antihypertensive captopril medicine (15.6 mg/kg, body weight/day),
10% PSPY (150 μg/2.5 ml) and 100% PSPY (1500 μg/2.5 ml). The WKY
rats were used as the negative control. The rats were sacrificed
after 8 weeks of the experimental period. The entire experimental
procedure was performed according to the NIH Guide for the Care and
Use of Laboratory Animals, and the protocol was approved by the
Institutional Animal Care and Use Committee of HungKuang
University, Taichung, Taiwan (approval no. 96027).
Body weight and cardiac
characteristics
The rats were weighed first and then sacrificed by
decapitation. The hearts of the rats were removed and cleaned with
double distilled H2O before dehydration. The left and
right atrium and ventricle were separated. The dry weight of the
whole heart and left ventricle was obtained, and ratios of the 2
measurements to rat body weight plus the ratio of left ventricle
weight to the whole heart weight were calculated independently.
Cross-section and hematoxylin and eosin
(H&E) staining
The heart was soaked in formalin and covered with
wax after removal. Cross-sections of the whole heart were sliced
and the maximal cross-section was selected. Slides were prepared by
first soaking them for dehydration. They were passed through a
series of graded alcohols (100, 95 and 75%), 15 min for each. The
slides were dyed with Mayer’s hematoxylin for 5–10 min and then
washed with tap water for 10–20 min. Each slide was soaked in mild
warm water until it turned bright violet and then placed into eosin
solution for 3–5 min. After gently rinsing with water, each slide
was then soaked with 85% alcohol, 100% alcohol I and II for 15 min
each. Finally, each slide was soaked with Xylene I–Xylene II.
Photomicrographs were obtained using Zeiss Axiophot microscopes
(magnification, ×200) (Olympus, Japan).
Tissue extraction
The left ventricle was cut into 8 parts. One part of
the left ventricle was minced with scissors, added to lysis buffer
(20 mM Tris, 2.0 mM EDTA, 50 mM 2-mercaptoethanol, 10% glycerol, pH
7.4), proteinase inhibitor cocktail tablet and phosphatase
inhibitor cocktail (Roche Diagnostics, Mannheim, Germany) at a
concentration of 100 mg tissue/ml buffer and homogenized at ice
temperature with a Model PT l0/35 Polytron homogenizer for 2 cycles
of l0 sec each. The homogenate was placed on ice for 10 min and
then centrifuged at 12,000 × g for 40 min. The supernatant was
collected and stored at −70°C for further western blot
analysis.
Protein contents
The protein contents of the left ventricle extract
were determined using the Bradford protein assay 14 using the
protein-dye kit (Bio-Rad, Hercules, CA, USA). A commercially
available bovine serum albumin (Sigma Chemical, St. Louis, MO, USA)
was used as the standard. Changes in absorption were monitored at
595 nm.
Electrophoresis and western blot
analysis
The left ventricle extract samples were prepared as
described above. Sodium dodecyl sulphate-polyacrylamide gel
electrophoresis was performed using 10% polyacrylamide gels. Equal
amounts (20 mg) of the samples were electrophoresed at 100 V for 3
h and equilibrated for 15 min in transfer buffer [25 mM Tris-HCl,
pH 8.3, containing 192 mM glycine and 20% (v/v) methanol]. The
electrophoresed proteins were then transferred onto polyvinylidene
difluoride (PVDF) membranes (Millipore, Bedford, MA, 0.45 μm pore
size) using a Bio-Rad Scientific Instruments Transphor Unit at 100
V with transfer buffer for 3 h. PVDF membranes were incubated at
room temperature for 1 h in the blocking buffer containing 100 mM
Tris-Base, 0.9% (w/v) NaCl, 0.1% (v/v) Tween-20 (pH 7.4) and 5%
non-fat milk. Monoclonal antibodies of protein kinase Cα (PKCα),
Ca2+/calmodulin-dependent protein kinase II (CaMKII),
phosphorylated extracellular signal-regulated kinase 5 (p-ERK5)
(Millipore-Upstate, Billerica, MA, USA), calcineurin (BD
Pharmingen, San Diego, CA, USA) and polyclonal antibodies of ANP,
BNP, IGF II, phosphorylated PKCα (p-PKCα), guanine
nucleotide-binding protein G(q) subunit α (Gαq), phosphorylated
NFATc3 (p-NFATc3) and interleukin 6 (IL-6) (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) were diluted in an
antibody-binding buffer containing 100 mM Tris-Base, pH 7.5, 0.9%
(w/v) NaCl and 0.1% (v/v) Tween-20. The immunoblots were washed 3
times in binding buffer for 10 min and then immersed in the second
antibody solution containing goat anti-mouse IgG-HRP, goat
anti-rabbit IgG-HRP, or donkey anti-goat IgG-HRP (Santa Cruz
Biotechnology, Inc.) for 1 h and diluted 500-fold in binding
buffer. The filters were then washed 3 times (10 min each) in
blotting buffer. The immunoblotted proteins were visualized using
an enhanced chemiluminescence ECL western blotting luminol reagent
(Santa Cruz Biotechnology, Inc.) and quantified using a Fujifilm
LAS-3000 chemiluminescence detection system (Tokyo, Japan). The
color was developed in a 20 ml mixture consisting of 7 mg nitro
blue tetrazolium, 5 mg 5-bromo-4-chloro-3-indolyl-phosphate, 100 mM
NaCl and 5 mM MgCl2 in 100 mM Tris-HCl, pH 9.5. The
immunoblot with antibody against α-tubulin, which was prepared with
the same procedure, was used as the internal control.
Statistical analysis
Statistical analyses were performed using SPSS 17.0
software (SPSS, Inc., Chicago, IL, USA). The data were compared
between groups of animals, using one-way analysis of variance
(ANOVA). Dunnett’s test was used to determine significant
differences. P-values <0.05 were considered to indicate
statistically significant differences. The significant differences
are indicated with symbols as shown in the tables and figures.
Results
Body weight and cardiac
characteristics
There was no significant difference in body weight
(P<0.05) among the SHR-control, SHR-captopril, SHR-PSPY (both
doses, 10 and 100%) and WKY groups (Table I). Whole heart weight, left
ventricular weight, the ratio of the whole heart weight to body
weight, left ventricular weight to body weight and left ventricular
weight to whole heart weight were significantly higher in the
SHR-control and SHR-PSPY (both doses, 10 and 100%) groups, whereas
the whole heart weight and left ventricular weight were lower in
the SHR-captopril group, compared with those of the WKY normal
controls. However, the ratio of the whole heart weight to the body
weight, which was traditionally regarded as an index of cardiac
hypertrophy, was lower both in the SHR-captopril, SHR-10 and 100%
PSPY groups than in the SHR-control group (Table I).
 | Table ICardiac characteristics of WKYs and
SHRs treated with captopril and PSPY. |
Table I
Cardiac characteristics of WKYs and
SHRs treated with captopril and PSPY.
| No. of animals | WKYs
| SHRs
|
|---|
| Control (n=10) | Control (n=3) | Captopril
(n=4) | 10% PSPY (n=3) | 100% PSPY
(n=4) |
|---|
| Body weight (BW),
g | 302.20±11.71 | 287.33±15.54 | 279.00±16.53 | 303.33±23.86 | 296.50±15.52 |
| Whole heart weight
(WHW), g | 1.11±0.28b | 1.24±0.04a | 1.12±0.01b | 1.24±0.05a | 1.23±0.05a |
| Left ventricle
weight (LVW), g | 0.81±0.21b | 1.02±0.02a | 0.91±0.05b | 1.00±0.03a | 1.02±0.03a |
| WHG/BW
×103 | 3.66±0.91b | 4.32±0.21a | 4.01±0.20 | 4.09±0.17a | 4.14±0.09a |
| LVW/BW
×103 | 2.67±0.70b | 3.55±0.12a | 3.28±0.23a | 3.29±0.22a | 3.44±0.12a |
| LVW/WHW | 0.73±0.04b | 0.82±0.02a | 0.82±0.05a | 0.80±0.02 | 0.83±0.01a |
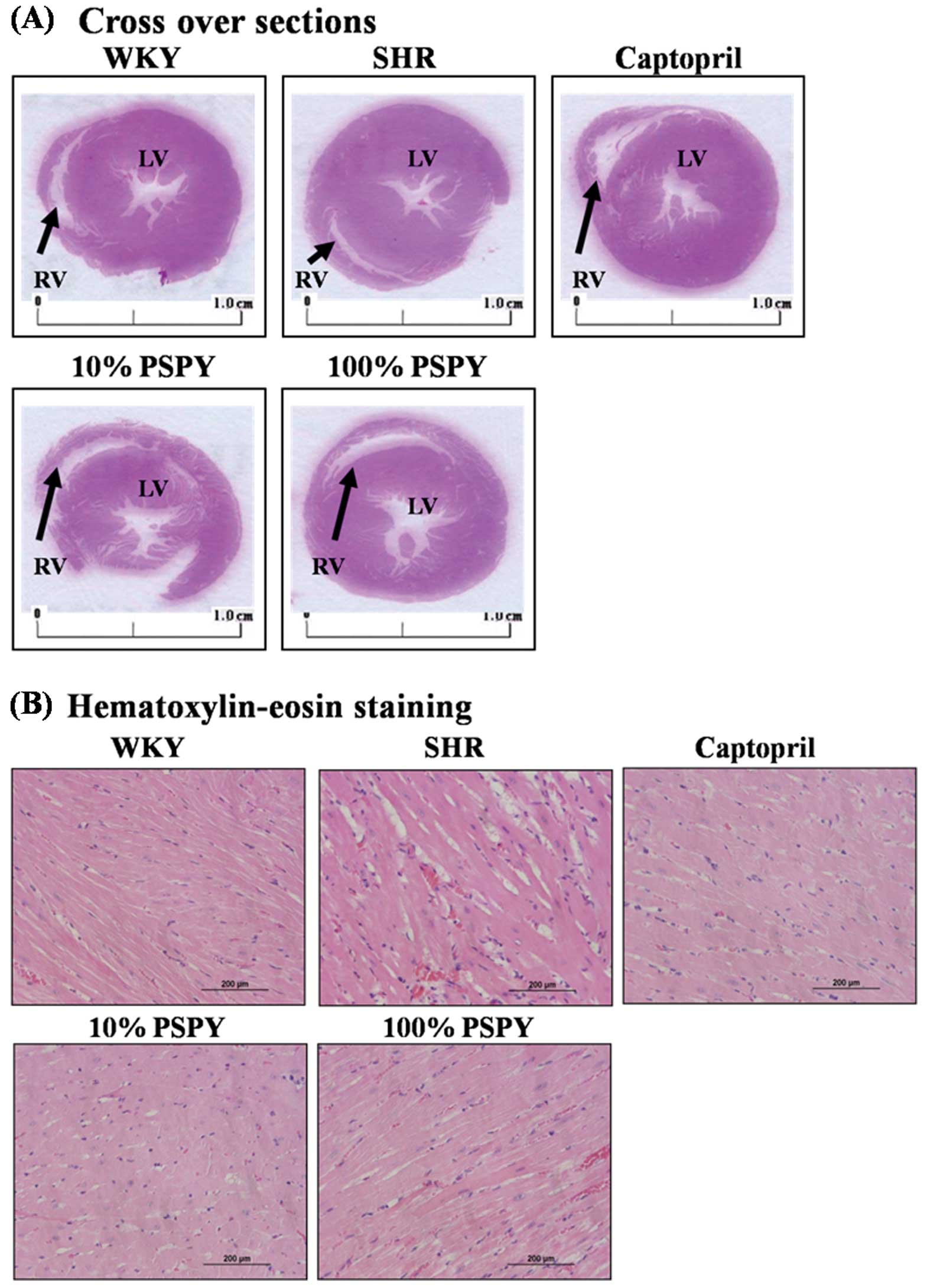
Cardiac architectural changes
To further define the characteristics of cardiac
hypertrophy, we made a cross-section of the whole heart and carried
out a histopathological analysis of the ventricular tissue stained
with H&E. We found that the SHR controls presented no
significant difference in ventricular wall thickness and in the
ratio of wall thickness to cavity diameter compared to the WKY
control group. However, the ventricular wall thickness was
significantly reduced (P<0.05 and 0.01) only in the SHR-100%
PSPY group, compared with those of the SHR-control and WKY-control
groups (Table II and Fig. 1A). Similarly, the ratio of wall
thickness to cavity diameter was significantly (P<0.05)
decreased in the SHR-100% PSPY group (Table II and Fig. 1A). Although the SHR-captopril and
10% PSPY groups presented no significant difference in either
ventricular wall thickness or ratio of wall thickness to cavity
diameter compared to the control groups, the SHR-captopril and 10%
PSPY groups had an enhanced diameter of the left ventricle.
Moreover, the ventricular myocardium in the WKY group showed normal
architecture with normal interstitial space. By contrast, abnormal
myocardial architecture, such as cardiomyocyte disarray and
increased interstitial space were observed in the SHR-control group
(Fig. 1B). Nevertheless,
restorations of myocardial architecture were observed in the
SHR-captopril, SHR-10% and 100% PSPY groups, presenting a normal
interstitial space as observed in the WKY control group (Fig. 1B).
 | Table IICardiac characteristics by tissue
cross-sections of WKYs and SHRs with captopril and PSPY. |
Table II
Cardiac characteristics by tissue
cross-sections of WKYs and SHRs with captopril and PSPY.
| No. of animals | WKY
| SHR
|
|---|
| Control (n=3) | Control (n=3) | Captopril
(n=3) | 10% PSPY (n=3) | 100% PSPY n=3 |
|---|
| Diameter of LV
(mm) | 0.98±0.02 | 0.94±0.01 |
1.03±0.01a,d | 1.04±0.04c |
0.91±0.01b,c |
| Thickness of LV
(mm) | 0.45±0.01 | 0.44±0.01 | 0.46±0.01 | 0.48±0.02 |
0.39±0.02b,c |
| Thickness/diameter
(mm) | 0.46±0.01 | 0.47±0.01 | 0.45±0.01 | 0.46±0.01 |
0.43±0.01a,c |
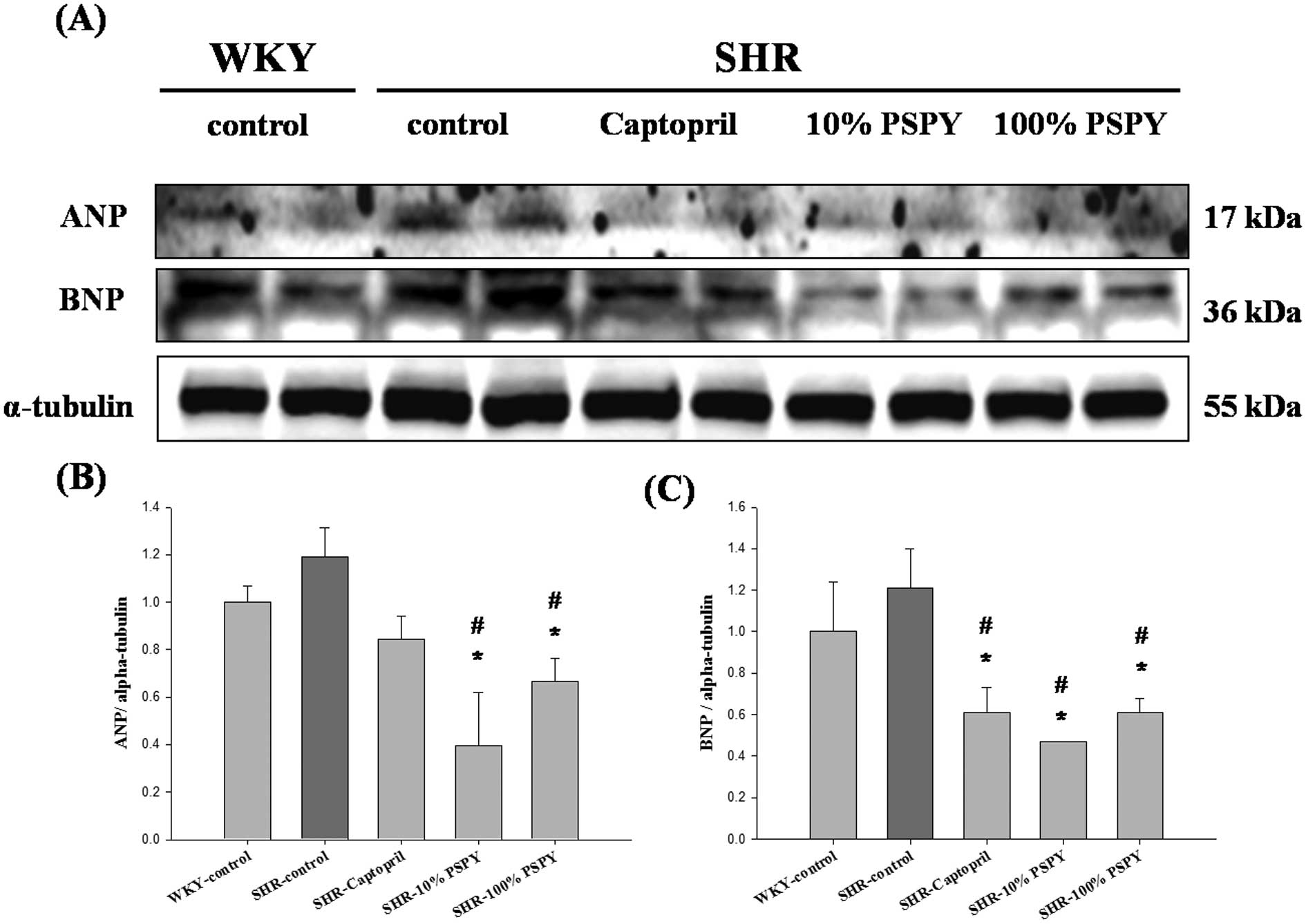
Changes in ANP and BNP pathological
hypertrophy markers of the rat left ventricle
To further investigate whether the proteins
associated with cardiac pathological hypertrophy are influenced by
PSPY, the pathological hypertrophy markers such as ANP and BNP in
the left ventricle were analyzed by western blotting. The levels of
ANP and BNP, markers of left ventricular remodeling associated with
cardiac hypertrophy, were higher in the SHR-control than in the WKY
group (Fig. 2), without reaching
significant levels. Moreover, ANP and BNP protein levels were lower
in the SHR-10% and 100% PSPY groups than in the SHR-control rats
(P<0.05) (Fig. 2B and C).
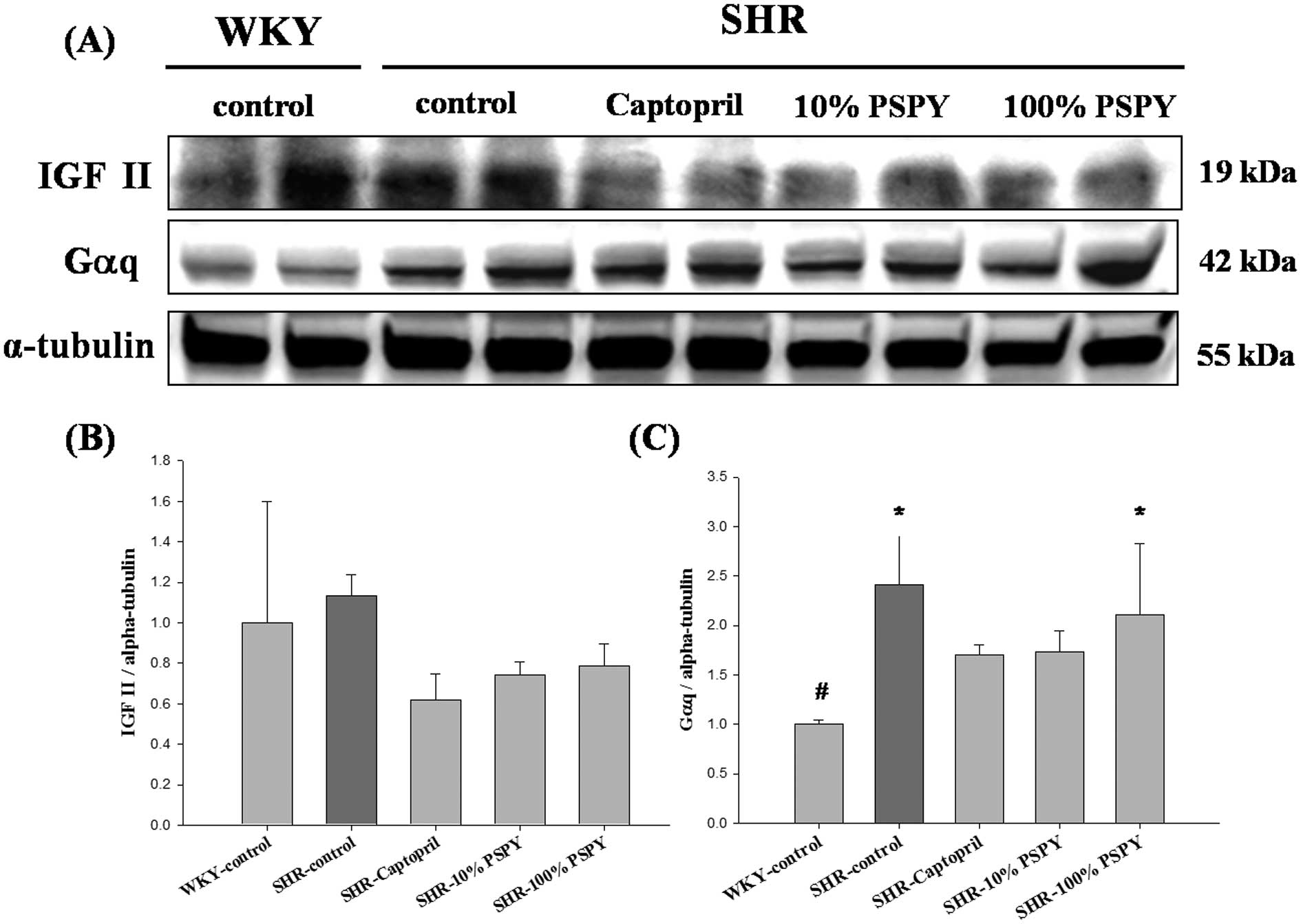
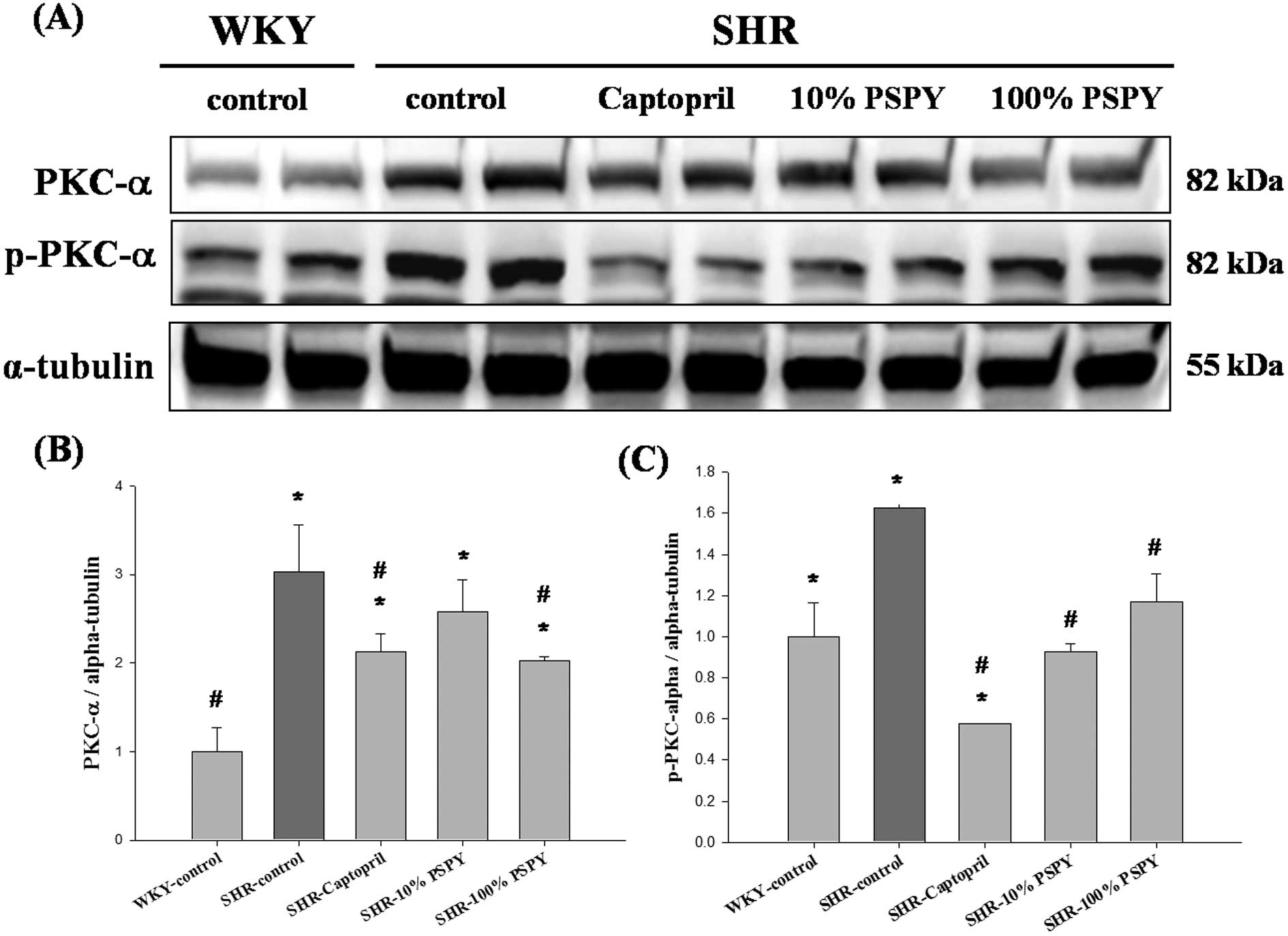
Changes in IGF-II pathway-related
proteins of the rat left ventricle
To investigate the hypertrophic factor, IGF-II, and
its cardiac hypertrophy-associated downstream signaling pathway
that was influenced by PSPY, the protein products of IGF-II were
measured by western blot analysis. However, the protein products of
IGF-II were lower in the SHR-captopril, 10% and 100% PSPY groups,
compared with the SHR-control, although all the SHR groups showed
no significant difference compared to the WKY group (Fig. 3B). The downstream signaling
pathway IGF-II-related protein levels, including those of Gαq,
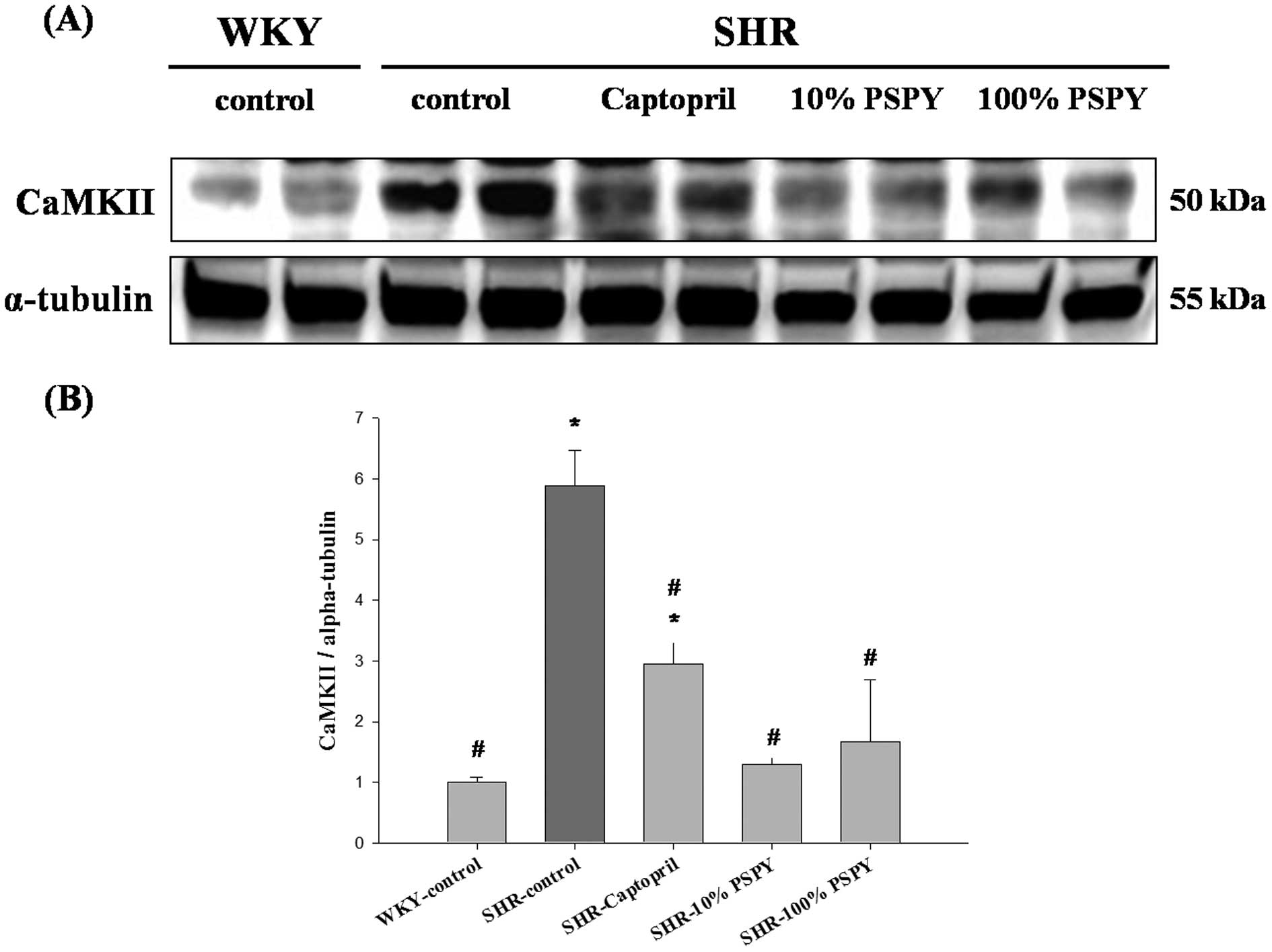
PKCα, p-PKCα and CaMKII were significantly increased in the
SHR-control compared to the WKY group (P<0.05) (Figs. 3C, 4 and 5). Compared with the SHR-control group,
the levels of the p-PKCα and CaMKII proteins were decreased
significantly in the SHR-captopril, 10% and 100% PSPY groups
(P<0.05) (Figs. 4C and
5B). The PKCα protein level was
decreased significantly in the SHR-captopril and 100% PSPY groups
compared to the SHR group.
Changes in calcineurin/NFATc-3 pathway
proteins of the rat left ventricle
The results summarized in Figs. 3–5 suggest that PSPY regulates the levels
of the IGF-II- and downstream-related proteins of myocardiac
hypertrophy in SHR. Therefore, we used western blot analysis to
extend the analysis on calcineurin and NFATc-3, which are both
indicators of the development of cardiac hypertrophy. In the
calcineurin/NFATc-3 pathway, the calcineurin protein levels were
slightly lower in the WKY and SHR-captopril groups than in the
SHR-control group (Fig. 6A),
whereas no changes in the SHR-10% and 100% PSPY groups were
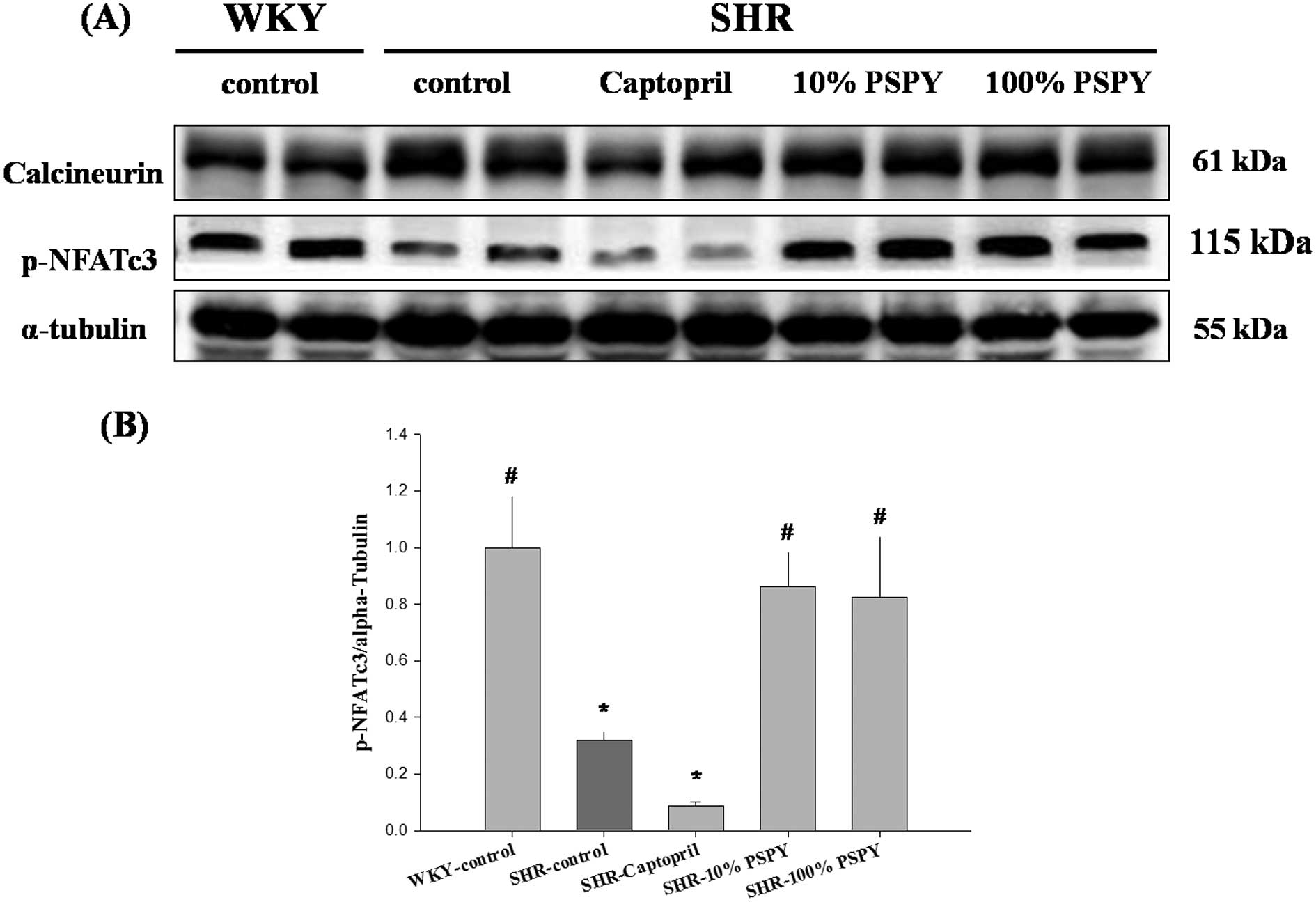
observed. However, the p-NFATc-3 levels were significantly
decreased in the SHR-control group compared with the WKY group, but
increased both in the SHR-10% and 100% PSPY groups (Fig. 6). Quantitative results of the
p-NFATc-3 protein in both PSPY groups were normalized close to the
levels presented in the WKY group (P<0.05) (Fig. 6B). These results suggest that PSPY
affects ANP and BNP protein levels by inhibiting the
dephosphorylation of NFATc-3 and therefore impedes the development
of myocardiac hypertrophy.
Changes in IL-6 and p-ERK5 protein levels
of the rat left ventricle
In order to identify the hypertrophic factor, IL-6,
and cardiac hypertrophy-associated mitogen-activated protein kinase
5 (MEK5)/ERK5 signaling pathways that were influenced by PSPY, the
protein products of IL-6 and p-ERK5 were measured by western blot
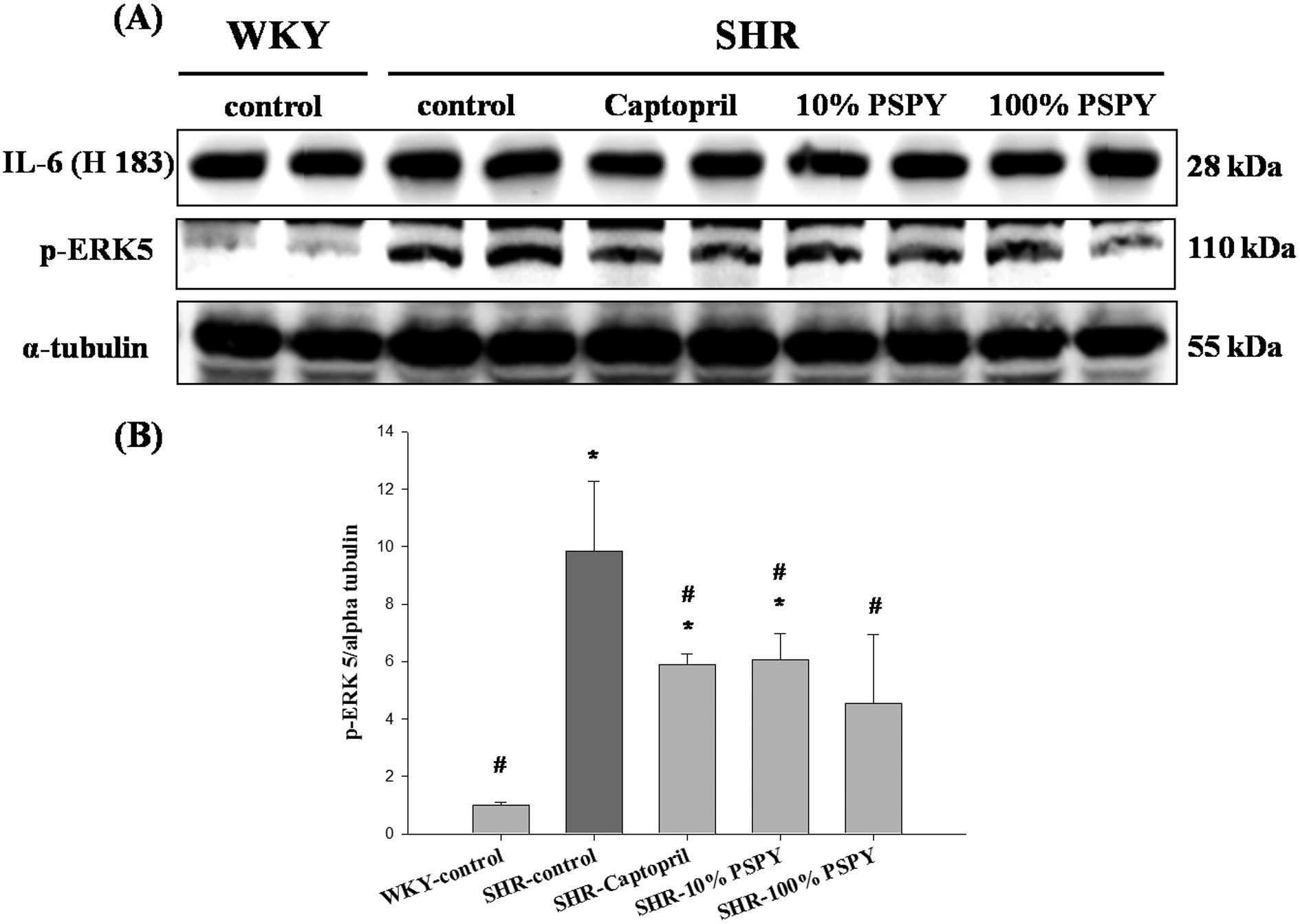
analysis (Fig. 7). The protein
level of IL-6 was not significantly different in all SHR groups
compared to the WKY group, whereas p-ERK5 was increased markedly in
the SHR-control group and significantly decreased in the
SHR-captopril, 10% and 100% PSPY groups, when compared to the WKY
group (P<0.05) (Fig. 7B).
Discussion
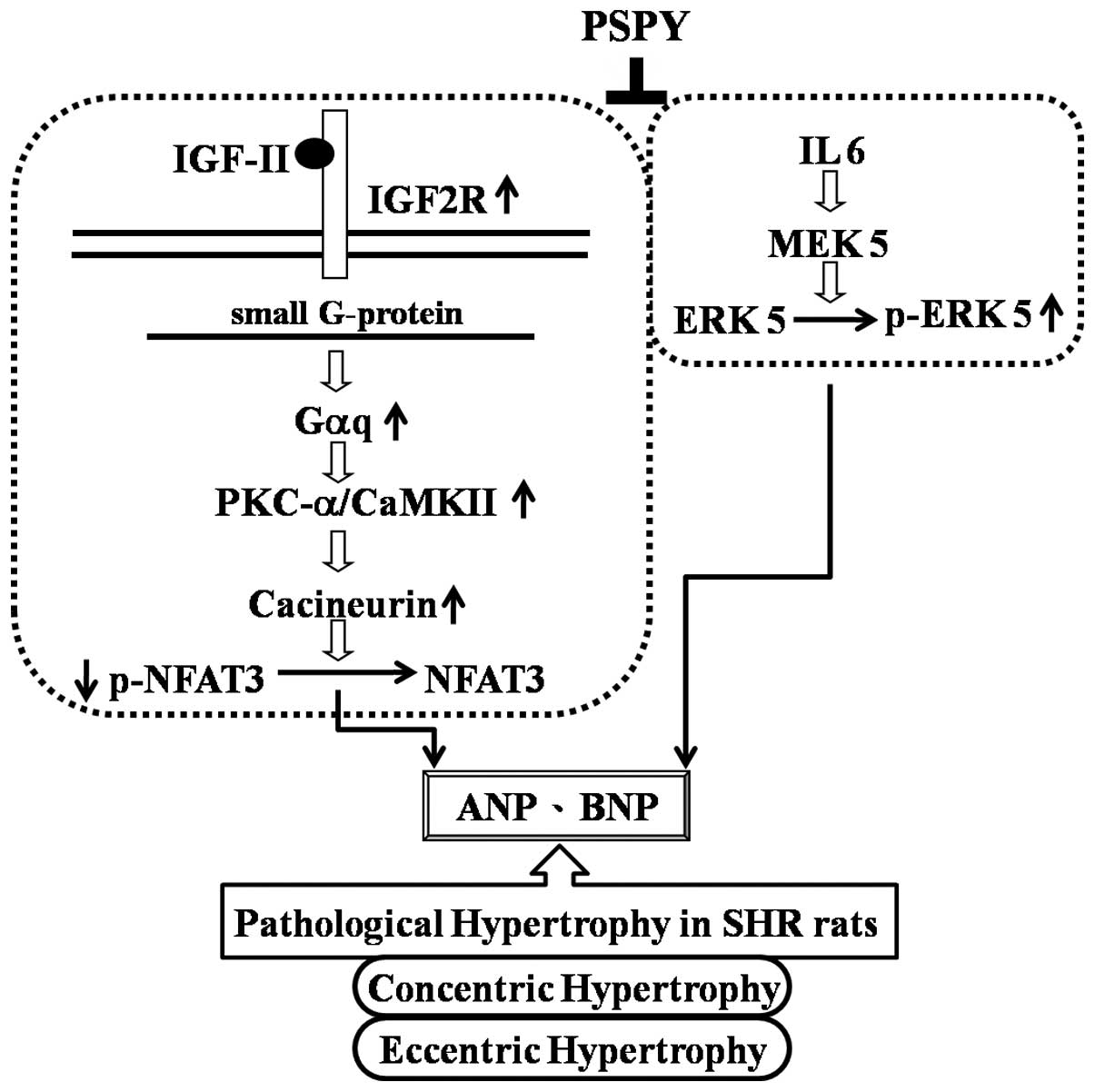
The main findings of the present study may be
summarized as follows: i) the ratio of the whole heart weight to
body weight and myocardial architecture with the interstitial space
were increased in the SHRs that were associated with significantly
increased protein levels of Gαq, PKCα, p-PKCα, CaMKII, as well as
p-ERK5 and decreased protein levels of p-NFATc3; ii) the
SHR-captopril and SHR-PSPY (both doses, 10 and 100%) groups showed
normal myocardial architecture with normal interstitial space
similar to the WKY controls and a decreased ratio of whole heart
weight to body weight. Furthermore, the pathological hypertrophy
markers, ANP and BNP, as well as the protein levels of p-PKCα,
CaMKII and p-ERK5 were decreased. However, the SHR-PSPY group had a
higher level of p-NFATc3 similar to that in the WKY controls. iii)
In conclusion, we hypothesized that hypertension caused cardiac
pathological hypertrophy that was associated with the increase in
the levels of the hypertrophic factors, ANP, BNP and IGF-II, and
the signaling molecules, Gαq, PKCα, p-PKCα, CaMKII. However, the
captopril and PSPY groups may suppress the hypertrophic factors and
pathological hypertrophic effect. Moreover, PSPY inhibited both the
concentric and eccentric hypertrophy determining molecules and
enhanced the p-NFATc-3 and reduced p-ERK5 protein levels (Fig. 8).
Pathological cardiac hypertrophy is associated with
ventricular remodeling through alterations in the extracellular
matrix that eventually impact the cardiac function and energy
utilization (27) and increase
the myocyte cell death rate through apoptotic and necrotic
mechanisms (28). Hypertrophy is
associated with disease-inducing stimuli, such as chronic
hypertension that may produce concentric hypertrophy in which the
ventricular wall and septum thicken with a net decrease in
ventricular chamber dimensions. This remodeling is associated with
a greater increase in cardiac myocyte width than length. In
addition, pathological cardiac hypertrophy may also produce a
phenotype of eccentric and dilatory cardiac growth. Cardiac
dilation, although not typically referred to as hypertrophy, may
result from a growth response in which sarcomeres are predominantly
added in a series to individual myocytes (29). Therefore, cardiac hypertrophy may
be characterized by quantitative effects on cell size and
width.
We observed that the captopril, 10 and 100% PSPY
groups had reduced cardiac hypertrophy index and whole heart weight
to body weight ratio. In addition, the ventricular wall thickness
and wall thickness to cavity diameter ratio were significantly
suppressed in the 100% PSPY group. The captopril and 10% PSPY
groups had an enhanced diameter of the left ventricle. Moreover, an
abnormal myocardial architecture and increased interstitial space
were observed in the SHRs; however, the PSPY groups (10 and 100%
doses) appeared more normal in architecture. Similarly, a recent
study showed that L. paracasei NTU 101 (101FM) and L.
plantarum NTU 102 fermented milk had antihypertensive effects,
possibly due to ACEI and GABA activities in SHRs, and also reduced
the disorganization of the aortic media layer (30). Liu et al (30) suggested that these hypotensive
effects depend on decreased peripheral vascular resistance.
Accordingly, we speculated that hypertension could induce cardiac
hypertrophy and abnormal myocardial architecture. However,
captopril and PSPY may somewhat ameliorate this situation. Further
investigation on the mechanisms of the involved proteins influenced
by PSPY in hypertrophic signaling pathways is required.
ANP and BNP are circulating hormones of cardiac
origin that play an important role in regulating the permeability
of systemic vasculature, cellular growth, cellular proliferation
and, as previously recently, cardiac hypertrophy (31). In a previous study conducted by
Chu et al (32), treatment
with angiotensin-II and IGF-II induced H9c2 cardiomyoblast cell
hypertrophy and an increase in the protein levels ANP and BNP
(6). As shown in Fig. 2, PSPY inhibited NFATc3 activation
which subsequently repressed the reactivation of ANP and BNP in
hypertensive rats. These observations indicate that PSPY has a
positive effect on reducing the levels of protein markers of
cardiac hypertrophy.
Previous studies have shown that IGF II directly
induces the hypertrophy of adult rat ventricular cardiomyocytes in
serum-free medium, as demonstrated by their increased size, total
protein synthesis and transcription of muscle-specific genes
(5), suggesting that IGF-II and
IGF-II-mediated pathways are possibly involved in the IGF-IIR
pathway and stimulate the hypertrophy of cardiomyocytes (6,33).
Moreover, specifically-activated IGF2R signaling by Leu27IGF-II has
been shown to trigger the phosphorylation of PKCα/CaMKII signaling
in order to induce cell hypertrophy (32). Although IGF-II and Gαq were not
significantly increased in all the SHR groups compared to the WKY
group, the downstream IGF-II signaling pathway protein levels,
including those of p-PKCα and CaMKII were significantly increased
in the hypertensive rats. However, we found that p-PKCα/CaMKII
through Gαq were activated in the hypertensive rats and were
inhibited by the administration of captopril and PSPY.
Calcineurin is a serine/threonine-specific
phosphatase activated by a sustained increase in calcium. A large
number of studies have demonstrated that the calcineurin/NFATc-3
signaling pathway plays an important role in the development of
cardiac hypertrophy. Calcineurin directly binds to and
dephosphorylates multiple conserved serine residues in the
N-terminus of NFAT-3 transcription factors, permitting their
translocation to the nucleus and promoting the subsequent induction
of the hypertrophic gene program (7,34).
It has also been demonstrated that calcineurin activity is
increased in compensated hypertrophied human myocardium and
end-stage heart failure (35). In
the present study, we observed that the protein level of
calcineurin was not reduced in the SHR-10% and 100% PSPY groups.
However, the downstream phosphorylation of NFATc-3 was
significantly decreased in the SHRs, but highly increased in both
10% and 100% PSPY groups. PSPY may regulate phosphorylated
calcineurin and lead to the direct decrease in the the nuclear
localization of NFATc-3 in SHR hearts. The transcription factor of
p-NFAT-3 increased in the cytoplasm in order to suppress the
expressions of hypertrophy response markers, such as ANP and
BNP.
Interleukin-6 (IL-6), a typical cytokine, was found
to have a potent hypertrophic effect on cardiomyocytes (36). The concomitant overexpression of
both IL-6 and the IL-6 receptor (IL-6R) in mice induces concentric
hypertrophy typical of that occurring in a hypertensive heart.
IL-6/IL-6R does this by interacting with a membrane-bound
glycoprotein (gp130), which in turn leads to the phosphorylation of
the downstream second messengers, such as Janus kinase, to induce
signal transduction and activators of transcription, causing the
stimulation of various cellular events (37). IL-6 is involved in multiple
intracellular signaling pathways, including p38 MAPK, STAT-1-STAT-3
heterodimer, STAT-3 homodimer and ERKs pathways (38,39). The ERK5 and its upstream
MAPK-kinase 5 (MEK5) reveal a specific role in the transduction of
cytokine signals that regulate serial sarcomere assembly and play a
role in the induction of eccentric cardiac hypertrophy that
progresses to dilated cardiomyopathy and sudden death (40). The MEK5-ERK5 pathway plays a
critical role in the induction of eccentric cardiac hypertrophy
that progresses to dilated cardiomyopathy and sudden death
(40,41). In the present study, we observed
the IL-6 was not significantly different among all the SHR groups
whereas p-ERK5 was highly activated in the SHR-control group, but
significantly suppressed in the SHR-captopril, SHR-10 and 100% PSPY
groups. These observations suggest that the inhibitory effect of
PSPY on the activation of ERK5 is regulated by additional upstream
messengers, such as Janus kinase or MEK5, rather than IL-6.
Therefore, we strongly suggest that PSPY possibly via the ERK5
inactivation, blocks the IL-6-MEK5-ERK5 pathway-related eccentric
dilated cardiac hypertrophic effects.
Our previous experimental data showed that the
concentrations of GABA in PSPY with a mixture of 3 LAB strains (LA,
LGA and LDL) and a control (PSP without LAB strains) were
1068.8±21.3 μg/ml and 250.0±10.1 μg/ml, respectively (26). The findings indicate that the
probiotics fermentation process may enhance the production of GABA
in PSPY. Our previous animal experimentation showed that continuous
PSPY feedings for 8 weeks significantly reduced the blood pressure
of SHRs (data not shown). Interestingly, our results from
histopathological analysis and analysis of key molecules of the
cardiac hypertrophy-related intracellular signaling pathway
demonstrated that the oral administration of low-dose PSPY (10%
dosage) was sufficient in order to prevent cardiac hypertrophy.
There was no significant dose-response effect observed between
10-fold differences in PSPY concentration (10 and 100%). The
mechanisms underlying the low- and high-dose hypotensive effects of
GABA may account for this difference in effect. The administration
of GABA at a low dose was considered to decrease blood pressure
through a peripheral mechanism (without crossing the brain blood
barrier). However, a high dose of GABA is considered to occur via a
central mechanism (16). The
majority of efforts have concentrated on designing highly
hydrophobic derivatives of GABA that may easily permeate brain
tissue (42).
Other substances in PSPY may contribute to the
anti-hypertrophic effects, such as antioxidant activity. Current
research on the antioxidant ability of LAB has shown that some LAB
strains cannot reduce the risk of reactive oxygen species
accumulation through food ingestion but may degrade the superoxide
anion and hydrogen peroxide (43). PSPs are also an excellent source
of anthocyanins which account for their antioxidant activity. In
our previous study, we indicated that the fermentation procedure
may further elevate anthocyanin antioxidative activity
significantly in PSPY with LAB strains (26). Li et al (25) showed that the natural antioxidant,
epigallocatechin-3-gallate (EGCG), inhibited angiotensin-II-induced
NF-κB and AP-1 activation, which subsequently repressed the
reactivation of ANP and BNP, and ultimately prevented the progress
of cardiac hypertrophy. Thus, the combination of these factors may
result in interfering with the hypertrophy-related intracellular
signaling pathway.
In conclusion, PSPY may mediate the
anti-hypertrophic effect in the SHR hearts by interfering with
different intracellular signaling pathways. The results from the
present study map aid in the understanding of the effects of PSPY
on cardiac hypertrophy and may shed light on the related molecular
mechanisms. PSPY may also suppress cellular signaling associated
with angiotensin-II-regulated IGF-II signaling pathways and the
IL-6-related-ERK5 pathway and may provide a novel insight into the
prevention and treatment of the pathological hypertrophic process.
However, extensive clinical trials or studies in vivo on the
human system are required to determine the long-term treatment
effects of PSPY and the half-life period and optimum dosage for
beneficial effects.
Acknowledgements
This study was supported in part by
the Taiwan Department of Health Clinical Trial and Research Center
of Excellence (DOH101-TD-B-111-004), the Taiwan Department of
Health Cancer Research Center of Excellence (DOH101-TD-C-111-005)
and the National Science Council, Taiwan, R.O.C.
(NSC97-2313-B-241-004-MY3).
References
|
1.
|
WW KuoCY ChuCH WuJA LinJY LiuYH HsiehKC
UengSD LeeDJ HsiehHH HsuImpaired IGF-I signalling of hypertrophic
hearts in the developmental phase of hypertension in genetically
hypertensive ratsCell Biochem
Funct23325331200510.1002/cbf.124415996002
|
|
2.
|
JA TowbinNE BowlesThe failing
heartNature415227233200210.1038/415227a11805847
|
|
3.
|
D DebloisBS TeaD BeaudryP HametRegulation
of therapeutic apoptosis: a potential target in controlling
hypertensive organ damageCan J Physiol
Pharmacol832941200510.1139/y05-00115759048
|
|
4.
|
SE KjeldsenB DahlofRB DevereuxS JuliusP
AurupJ EdelmanEffects of losartan on cardiovascular morbidity and
mortality in patients with isolated systolic hypertension and left
ventricular hypertrophy: a Losartan Intervention for Endpoint
Reduction (LIFE)
substudyJAMA28814911498200210.1001/jama.288.12.1491
|
|
5.
|
CY HuangLY HaoDE BuetowInsulin-like growth
factor-induced hypertrophy of cultured adult rat cardiomyocytes is
L-type calcium-channel-dependentMol Cell
Biochem2315159200210.1023/A:101443292322011952165
|
|
6.
|
SD LeeCH ChuEJ HuangMC LuJY LiuCJ LiuHH
HsuJA LinWW KuoCY HuangRoles of insulin-like growth factor II in
cardiomyoblast apoptosis and in hypertensive rat heart with
abdominal aorta ligationAm J Physiol Endocrinol
Metab291E306E314200610.1152/ajpendo.00127.200516825605
|
|
7.
|
JD MolkentinJR LuCL AntosA
calcineurin-dependent transcriptional pathway for cardiac
hypertrophyCell93215228199810.1016/S0092-8674(00)81573-19568714
|
|
8.
|
B WilkinsLJ De WindtOF BuenoJC BrazBJ
GlascockTF KimballJD MolkentinTargeted disruption of NFATc3, but
not NFATc4, reveals an intrinsic defect in calcineurin-mediated
cardiac hypertrophy growthMol Cell
Biol2276037613200210.1128/MCB.22.21.7603-7613.200212370307
|
|
9.
|
N KomatsuzakiT NakamuraT KimuraJ
ShimaCharacterization of glutamate decarboxylase from a high
gamma-aminobutyric acid (GABA)-producer, Lactobacillus
paracaseiBiosci Biotechnol
Biochem72278285200810.1271/bbb.7016318256502
|
|
10.
|
H LiY CaoLactic acid bacterial cell
factories for gamma-aminobutyric acidAmino
Acids3911071116201010.1007/s00726-010-0582-720364279
|
|
11.
|
Y UenoK HayakawaS TakahashiK
OdaPurification and characterization of glutamate decarboxylase
from Lactobacillus brevis IFO 12005Biosci Biotechnol
Biochem6111681171199710.1271/bbb.61.11689255981
|
|
12.
|
JY KimMY LeeGE JiYS LeeKT HwangProduction
of gamma-aminobutyric acid in black raspberry juice during
fermentation by Lactobacillus brevis GABA100Int J Food
Microbiol1301216200910.1016/j.ijfoodmicro.2008.12.02819167126
|
|
13.
|
LA DennerJY WuTwo forms of rat brain
glutamic acid decarboxylase differ in their dependence on free
pyridoxal phosphateJ
Neurochem44957965198510.1111/j.1471-4159.1985.tb12910.x3882886
|
|
14.
|
SH OhWG ChoiIT LeeSJ YunCloning and
characterization of a rice cDNA encoding glutamate decarboxylaseJ
Biochem Mol
Biol38595601200510.5483/BMBRep.2005.38.5.59516202241
|
|
15.
|
S YokoyamaJ HiramatsuK HayakawaProduction
of gamma-aminobutyric acid from alcohol distillery lees by
Lactobacillus brevis IFO-12005J Biosci
Bioeng939597200210.1016/S1389-1723(02)80061-516233172
|
|
16.
|
K HayakawaM KimuraK KasahaK MatsumotoH
SansawaY YamoriEffect of a gamma-aminobutyric acid-enriched dairy
product on the blood pressure of spontaneously hypertensive and
normotensive Wistar-Kyoto ratsBr J
Nutr92411417200410.1079/BJN2004122115469644
|
|
17.
|
K InoueT ShiraiH OchiaiM KasaoK HayakawaM
KimuraH SansawaBlood-pressure-lowering effect of a novel fermented
milk containing gamma-aminobutyric acid (GABA) in mild
hypertensivesEur J Clin
Nutr57490495200310.1038/sj.ejcn.160155512627188
|
|
18.
|
J YamakoshiS FukudaT SatohR TsujiM SaitoA
ObataA MatsuyamaM KikuchiT KawasakiAntihypertensive and natriuretic
effects of less-sodium soy sauce containing gamma-aminobutyric acid
in spontaneously hypertensive ratsBiosci Biotechnol
Biochem71165173200710.1271/bbb.6042417213662
|
|
19.
|
M PhilpottCC LimLR FergusonDietary
protection against free radicals: A case for multiple testing to
establish structure-activity relationships for antioxidant
potential of anthocyanic plant speciesInt J Mol
Sci101081103200910.3390/ijms10031081
|
|
20.
|
M PhilpottKS GouldC LimLR FergusonIn situ
and in vitro antioxidant activity of sweetpotato anthocyaninsJ
Agric Food Chem5215111513200410.1021/jf034593j15030203
|
|
21.
|
I Konczak-IslamM YoshimotoDX HouN
TeraharaO YamakawaPotential chemopreventive properties of
anthocyanin-rich aqueous extracts from in vitro produced tissue of
sweet potato (Ipomoea batatas L.)J Agr Food
Chem5159165922200310.1021/jf030066o13129295
|
|
22.
|
S de Pascual-TeresaDA MorenoC
García-VigueraFlavanols and anthocyanins in cardiovascular health:
A review of current evidenceInt J Mol Sci1116791703201020480037
|
|
23.
|
K NakamuraK FushimiH KouchiK MiharaM
MiyazakiT OheM NambaInhibitory effects of antioxidants on neonatal
rat cardiac myocyte hypertrophy induced by tumor necrosis
factor-alpha and angiotensin
IICirculation98794799199810.1161/01.CIR.98.8.7949727550
|
|
24.
|
HL LiAB WangY HuangDP LiuG LiuCN ZhangC
WeiYQ LiuRT HuiCC LiangIsorhapontigenin, a new resveratrol analog,
attenuates cardiac hypertrophy via blocking signaling transduction
pathwaysFree Radic Biol
Med38243257200510.1016/j.freeradbiomed.2004.10.02015607907
|
|
25.
|
HL LiY HuangCN ZhangG LiuYS WeiAB WangYQ
LiuRT HuiC WeiGM WilliamsEpigallocathechin-3 gallate inhibits
cardiac hypertrophy through blocking reactive oxidative
species-dependent and -independent signal pathwaysFree Radic Biol
Med4017561775200610.1016/j.freeradbiomed.2006.01.005
|
|
26.
|
TY WuCC TsaiYT HwangTH ChiuEffect of
antioxidant activity and functional properties of Chingshey purple
sweet potato fermented milk by Lactobacillus acidophilus,
L. delbrueckii subsp lactis, and L gasseri
strainsJ Food
Sci77M2M8201210.1111/j.1750-3841.2011.02507.x22182227
|
|
27.
|
AM DeschampsFG SpinalePathways of matrix
metalloproteinase induction in heart failure: bioactive molecules
and transcriptional regulationCardiovasc
Res69666676200610.1016/j.cardiores.2005.10.00416426590
|
|
28.
|
PM KangS IzumoApoptosis in heart: basic
mechanisms and implications in cardiovascular diseasesTrends Mol
Med9177182200310.1016/S1471-4914(03)00025-X12727144
|
|
29.
|
J HeinekeJD MolkentinRegulation of cardiac
hypertrophy by intracellular signalling pathwaysNat Rev Mol Cell
Biol7589600200610.1038/nrm198316936699
|
|
30.
|
CF LiuYT TungCL WuBH LeeWH HsuTM
PanAntihypertensive effects of Lactobacillus-fermented milk
orally administered to spontaneously hypertensive ratsJ Agric Food
Chem59453745432011
|
|
31.
|
T NishikimiN MaedaH MatsuokaThe role of
natriuretic peptides in cardioprotectionCardiovasc
Res69318328200610.1016/j.cardiores.2005.10.00116289003
|
|
32.
|
CH ChuBS TzangLM ChenCH KuoYC ChengLY
ChenFJ TsaiCH TsaiWW KuoCY HuangIGF-II/mannose-6-phosphate receptor
signaling induced cell hypertrophy and atrial natriuretic
peptide/BNP expression via Gαq interaction and protein kinase
C-α/CaMKII activation in H9c2 cardiomyoblast cellsJ
Endocrinol197381390200818434368
|
|
33.
|
CY HuangLY HaoDE BuetowHypertrophy of
cultured adult rat ventricular cardiomyocytes induced by antibodies
against the insulin-like growth factor (IGF)-I or the IGF-I
receptor is IGF-II-dependentMol Cell
Biochem2336572200210.1023/A:101551432432812083381
|
|
34.
|
H OkamuraJ AramburuC
Garcia-RodriguezConcerted dephosphorylation of the transcription
factor NFAT1 induces a conformational switch that regulates
transcriptional activityMol
Cell6539550200010.1016/S1097-2765(00)00053-8
|
|
35.
|
HW LimJD MolkentinCalcineurin and human
heart failureNat Med5246247199910.1038/6430
|
|
36.
|
T KandaT TakahashiInterleukin-6 and
cardiovascular diseasesJpn Heart
J45183193200410.1536/jhj.45.183
|
|
37.
|
GC MeléndezJL McLartySP LevickY DuJS
JanickiGL BrowerInterleukin 6 mediates myocardial fibrosis,
concentric hypertrophy, and diastolic dysfunction in
ratsHypertension56225231201020606113
|
|
38.
|
T HiranoInterleukin 6 and its receptor:
ten years laterInt Rev Immunol1624928419989505191
|
|
39.
|
H HirotaK YoshidaT KishimotoT
TagaContinuous activation of gp130, a signal-transducing receptor
component for interleukin 6-related cytokines, causes myocardial
hypertrophy in miceProc Natl Acad Sci
USA9248624866199510.1073/pnas.92.11.48627539136
|
|
40.
|
RL NicolN FreyG PearsonM CobbJ
RichardsonEN OlsonActivated MEK5 induces serial assembly of
sarcomeres and eccentric cardiac hypertrophyEMBO
J2027572767200110.1093/emboj/20.11.275711387209
|
|
41.
|
SJ CameronS ItohCP BainesC ZhangS OhtaW
CheM GlassmanJD LeeC YanJ YangJ AbeActivation of big MAP kinase 1
(BMK1/ERK5) inhibits cardiac injury after myocardial ischemia and
reperfusionFEBS
Lett566255260200410.1016/j.febslet.2004.03.12015147905
|
|
42.
|
M KimuraK HayakawaH SansawaInvolvement of
γ-aminobutyric acid (GABA) B receptors in the hypotensive effect of
systemically administered GABA in spontaneously hypertensive
ratsJpn J Pharmacol893883942002
|
|
43.
|
MY LinCL YenAntioxidative ability of
lactic acid bacteriaJ Agric Food
Chem4714601466199910.1021/jf981149l10563999
|