Introduction
UV irradiation leads to cell aging, senescence,
apoptosis and cancer in human keratinocytes by inducing reactive
oxygen species (ROS), DNA damage and inflammatory and immunological
reactions (1). Members of the
mitogen-activated protein kinase (MAPK) family, including ERK1/2,
p38 MAPK and JNK, are phosphorylated (activated) by UV irradiation
(1). A low-dose of UVB
(ultraviolet B) (0.1 kJ/cm2) significantly produces
tumor necrosis factor α (TNF-α), which is involved in the
suppression of contact hypersensitivity and in the decreased
immunosurveillance in UV-damaged keratinocytes (2). In addition, the protein half-life of
nuclear factor-κB (NF-κB) is prolonged by the inhibitor of NF-κBα
(IκBα) phosphorylation and proteasomal degradation, and activated
NF-κB may induce both anti- and pro-apoptotic pathways (3–7).
Furthermore, UVB irradiation induces cytokine IL-21 and nitric
oxide (NO), which are closely related to keratinocyte immune
responses (8,9). Collectively, UVB irradiation
activates multiple signaling cascades in keratinocytes.
microRNAs (miRNAs) are small, non-coding RNA
molecules that directly regulate the expression of target mRNA
transcripts (10). miRNAs have
been reported to be involved in almost all cellular processes,
including development, proliferation, immune response, metabolism
and cell death (11–13). In human skin, miRNA-based studies
first investigated miRNA expression patterns in normal human skin
and melanocytic nevi (14,15).
A functional relationship between keratinocyte miRNAs and psoriasis
was reported, and it was determined that miR-125b modulates
abnormal keratinocyte proliferation in psoriasis by targeting FGFR2
(16,17). The miRNA expression pattern during
human keratinocyte differentiation has also been investigated
(18). One recent study analyzed
UVB-dependent miRNA expression profile changes in keratinocytes
(19). Overall, these reports
indicate that miRNAs may be key regulators of multiple cellular
processes in keratinocytes and further suggest that miRNAs may have
protective functions in response to UVB.
Centella asiatica (C. asiatica, also
known as gotu kola) is a plant used in traditional herbal medicine
with pharmacological effects on skin wound healing (20,21). It also shows anti-oxidant,
anti-microbial and anticancer properties (22,23). We demonstrated that
H2O2-induced cell senescence was inhibited by
treating human dermal fibroblasts with a titrated extract of C.
asiatica (TECA) (24).
Although there was a recent report regarding the UVB protective
effect of TECA treatment on dermal fibroblasts (25), the molecular mechanisms of UV
protection have not been elucidated. Furthermore, a possible UV
protective effect on human keratinocytes has not been studied. In
this current study, we demonstrate that TECA exerts novel UVB
protection in human keratinocytes and characterized miRNA
expression profiles that correspond to TECA-mediated UVB
protection.
Materials and methods
Cell culture
Normal human HaCaT keratinocytes were maintained in
Dulbecco’s modified Eagle’s medium (DMEM; Gibco-Invitrogen Life
Technologies, Carlsbad, CA, USA) supplemented with 10% fetal bovine
serum (FBS; Sigma-Aldrich, St. Louis, MO, USA) with
penicillin/streptomycin.
UVB irradiation and TECA treatment
A day before UVB irradiation, HaCaT cells
(4×103) were seeded into 96-well plates. For RNA
purification, 7×105 cells were seeded into 60-mm dishes.
Before UVB irradiation, cells were pre-treated with the control
dimethyl sulfoxide (DMSO; Sigma-Aldrich) or TECA (Bayer Health
Care, Berlin, Germany) for 3 h. Cells were washed with
phosphate-buffered saline (PBS) and exposed to 50 mJ/cm2
UVB without dish covers. After irradiation, the cells were cultured
in DMEM media containing 10% FBS with DMSO or TECA for 24 h.
RNA purification and qualification
Total RNA was extracted and purified with TRIzol
reagent (Invitrogen Life Technologies) according to the
manufacturer’s instructions. The integrity of each RNA sample was
verified with an Agilent 2100 Bioanalyzer (Agilent Technologies,
Santa Clara, CA, USA). The quality and concentration of each RNA
sample were determined using MaestroNano (Maestrogen, Las Vegas,
NV, USA). RNA quality parameters for miRNA microarray analysis were
A260/280 and A260/A230 values >1.8 and an RNA integrity no.
(RIN) >8.0.
Microarray analysis of miRNA
profiles
The miRNA profiling analysis was performed using
SurePrint G3 Human V16 miRNA 8 × 60K (Agilent Technologies)
containing a probe for 1,205 and 144 human viral miRNAs. Each
qualified RNA sample (100 ng) was dephosphorylated with calf
intestinal alkaline phosphatase (CIP) at 37°C for 30 min. Then, the
dephosphorylated RNA samples were labeled with cyanine 3-pCp using
T4 RNA ligase by incubating at 16°C for 2 h. After the labeling
reaction, the samples were completely dried using a vacuum
concentrator at 55°C for 4 h. The dried samples were treated with
GE Blocking Agent (Agilent Technologies) and hybridized to the
probes on the microarray at 55°C, at 20 rpm in the Agilent
Microarray Hybridization Chamber (Agilent Technologies) for 20 h.
The microarray slide was washed and scanned with the Agilent
scanner to obtain the microarray image. The numerical data for the
miRNA profiles were extracted from the image with the Feature
Extraction program (Agilent Technologies). These data were analyzed
with GeneSpring GX software version 7.3 (Agilent Technologies).
miRNAs whose flags were present in at least one sample were
filtered and applied to the fold-change analysis, which was
conducted by a factor of 1.5-fold between two groups:
UVB-exposed/DMSO-treated control and UVB-exposed/2 μg/ml
TECA-treated HaCaT keratinocytes.
Bioinformatical analysis of miRNAs
Meaningful altered miRNAs were selected, and their
putative cellular target genes were determined using MicroCosm
Target version 5 (www.ebi.ac.uk/enright-srv/microcosm/thdoc/targets/v5/).
The target genes were categorized into four groups (aging,
apoptosis, cell proliferation and skin development) using the Gene
Ontology (GO) analysis tool AmiGO (amigo.geneontology.org/cgi-bin/amigo/browse.cgi).
Further GO analysis was performed for several categories, such as
anti-apoptosis, MAPKK activity, Ras protein signal transduction,
small GTPase-mediated signal transduction, positive or negative
regulation of cell growth, cell proliferation, cell cycle, immune
response and positive regulation of p53-mediated signaling.
Results
TECA protects HaCaT cells against UVB
damage
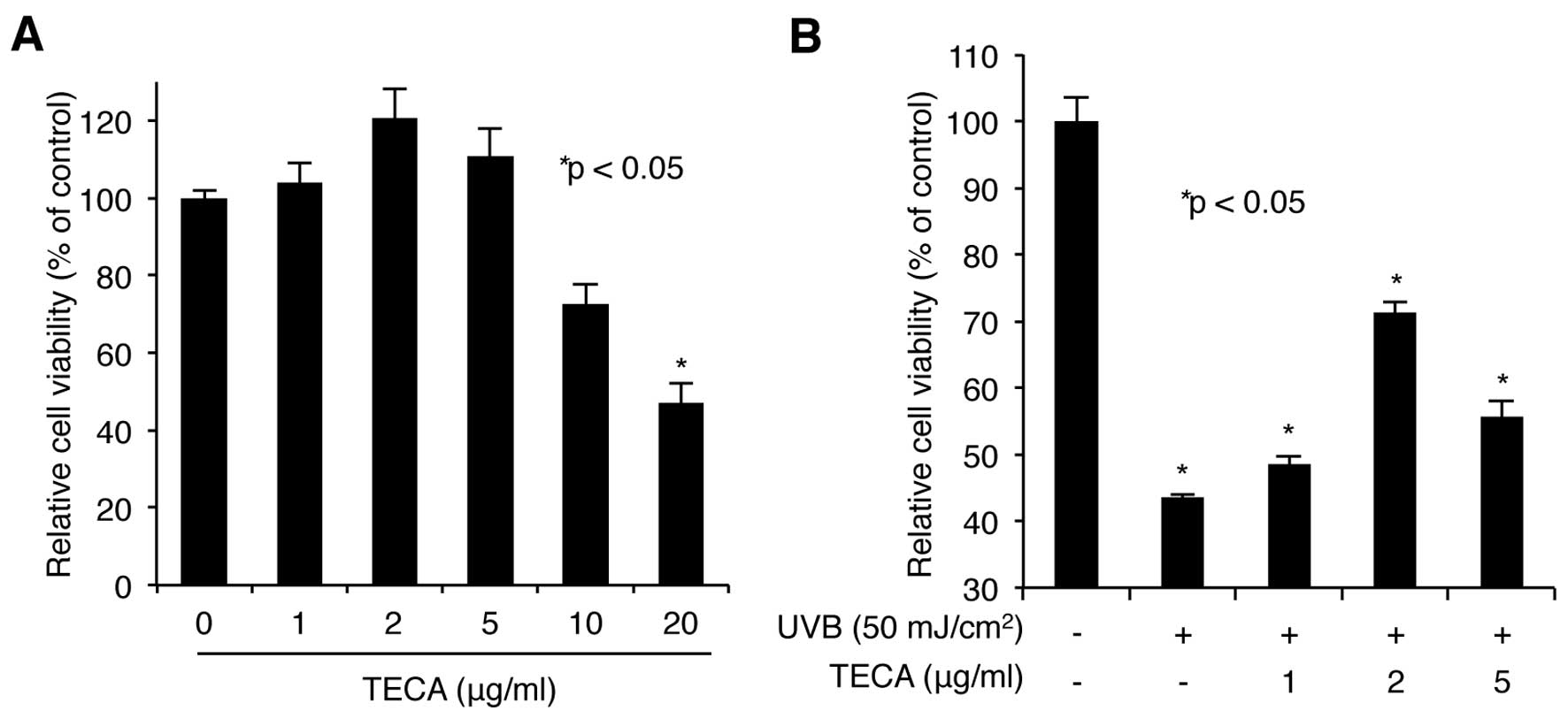
We first assessed the cytotoxicity of TECA. HaCaT
cells were treated with increasing doses of TECA (1, 2, 5, 10 or 20
μg/ml) for 24 h, and the WST-1-based cellular toxicity assay was
used to determine cell viability. As shown in Fig. 1A, low doses (up to 5 μg/ml) of
TECA had no significant cytotoxic effect on HaCaT cells, while
higher doses (10 and 20 μg/ml) were more cytotoxic. Based on these
results, we used 1, 2 and 5 μg/ml treatments in further
experiments. Next, the protective activity of TECA on keratinocytes
was determined. HaCaT cells pre-treated with TECA were exposed to
50 mJ/cm2 of UVB without any protective covers. After
UVB-irradiation, the cells were incubated with the indicated doses
of TECA for 24 h. The WST-1 assay showed that the irradiated cells
without TECA displayed a 43.52% cell survival rate, compared to
non-irradiated control cells and TECA treatment (2 μg/ml) markedly
improved the cell survival rate to 71.25% (Fig. 1B). Overall, TECA prevented
UVB-mediated keratinocyte cell death.
TECA alters miRNA expression profiles in
UVB-treated keratinocytes
We further determined the protective effect of TECA
with a miRNA expression profiling analysis and observed different
miRNA expression patterns in response to TECA treatment in
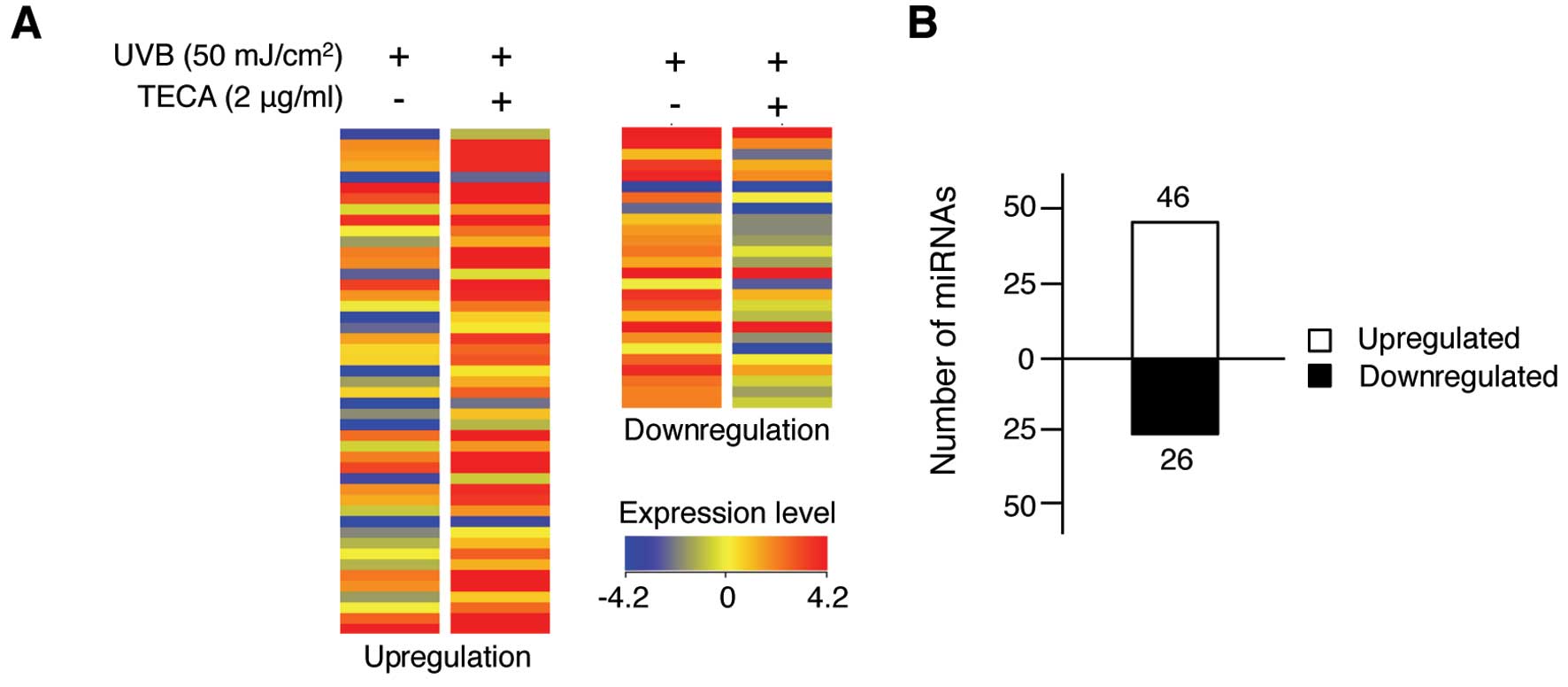
UVB-irradiated HaCaT cells. As shown in Fig. 2A, 72 human miRNAs were altered
>1.5-fold in the TECA-treated, UVB-irradiated HaCaT cells,
compared to those that were only exposed to UVB. The full list of
72 miRNAs is shown in Table I.
TECA treatment affected miRNA expression levels, but the fold
change was not >4.0. Among the altered miRNAs, 46 miRNAs were
upregulated after TECA treatment and 26 miRNAs were downregulated
(Fig. 2B). However, the extent of
changes varied among miRNAs; the expression levels of miR-636,
miR-3620 and miR-296-5p were significantly increased by 3.51-,
3.60- and 2.54-fold, respectively, whereas the expression of
miR-622 and miR-455-5p was significantly decreased by 2.82- and
2.07-fold, respectively. Overall, TECA treatment influenced certain
miRNA expression levels, suggesting that specific cellular response
mechanisms may be involved in TECA-mediated UVB protection of
keratinocytes.
 | Table ImiRNAs altered by TECA treatment in
UVB-exposed HaCaT keratinocytes. |
Table I
miRNAs altered by TECA treatment in
UVB-exposed HaCaT keratinocytes.
| miR name | FC | Chromosome | miR name | FC | Chromosome |
|---|
|
ebv-miR-BHRF1-1 | 1.57 | - | hsa-miR-636 | 3.51 | Chr17 |
|
hsa-let-7b* | 1.53 | Chr22 | hsa-miR-664 | 1.54 | Chr1 |
| hsa-let-7f-1 | 1.59 | Chr9 | hsa-miR-767-3p | 1.56 | ChrX |
|
hsa-miR-1225-3p | 1.70 | Chr16 | hsa-miR-92b | 1.71 | Chr1 |
| hsa-miR-1227 | 1.57 | Chr19 | hsa-miR-933 | 1.63 | Chr2 |
| hsa-miR-1228 | 1.55 | Chr12 | hsv1-miR-H6-3p | 1.61 | - |
| hsa-miR-1234 | 1.66 | Chr8 |
hsv1-miR-H7* | 1.65 | - |
| hsa-miR-1237 | 1.54 | Chr11 | hsv1-miR-H20 | 1.61 | - |
| hsa-miR-1238 | 1.58 | Chr19 | hsv1-miR-H7-3p | 1.66 | - |
|
hsa-miR-129* | 1.61 | Chr7 |
kshv-miR-K12-8* | 1.63 | - |
| hsa-miR-1470 | 1.80 | Chr8 | hsa-miR-192 | −1.78 | Chr11 |
| hsa-miR-1539 | 1.62 | Chr18 | hsa-miR-182 | −1.84 | Chr7 |
| hsa-miR-1825 | 1.66 | Chr20 | hsa-miR-210 | −2.06 | Chr11 |
|
hsa-miR-18b* | 1.55 | ChrX | hsa-miR-132 | −2.00 | Chr17 |
|
hsa-miR-191* | 1.53 | Chr3 |
hsa-miR-9* | −2.08 | Chr1 |
|
hsa-miR-2116* | 1.59 | Chr15 |
hsa-miR-125a-5p | −1.53 | Chr19 |
| hsa-miR-223 | 1.64 | ChrX | hsa-miR-155 | −1.58 | Chr21 |
| hsa-miR-296-5p | 2.54 | Chr20 | hsa-miR-99b | −1.80 | Chr19 |
|
hsa-miR-3180-5p | 1.72 | Chr16 | hsa-miR-362-5p | −1.72 | ChrX |
|
hsa-miR-33b* | 1.54 | Chr17 | hsa-miR-374a | −1.54 | ChrX |
|
hsa-miR-3613-3p | 1.52 | Chr13 | hsa-miR-494 | −1.53 | Chr14 |
|
hsa-miR-3614-5p | 1.61 | Chr17 | hsa-miR-181d | −1.70 | Chr19 |
| hsa-miR-3620 | 3.60 | Chr1 | hsa-miR-532-5p | −2.07 | ChrX |
|
hsa-miR-3675-3p | 1.78 | Chr1 | hsa-miR-455-5p | −2.07 | Chr9 |
| hsa-miR-3676 | 1.53 | Chr17 | hsa-miR-622 | −2.82 | Chr13 |
|
hsa-miR-3679-3p | 1.54 | Chr2 | hsa-miR-625 | −1.65 | Chr14 |
| hsa-miR-3940 | 1.77 | Chr19 | hsa-miR-660 | −1.63 | ChrX |
| hsa-miR-423-3p | 2.04 | Chr17 |
ebv-miR-7-1* | −1.53 | - |
|
hsa-miR-425* | 1.59 | Chr3 |
hsa-miR-7-1* | −1.88 | Chr9 |
| hsa-miR-4274 | 1.61 | Chr4 |
hsa-miR-181a-2* | −1.51 | Chr9 |
| hsa-miR-4310 | 1.52 | Chr15 | hsa-miR-140-3p | −1.56 | Chr16 |
| hsa-miR-4313 | 1.58 | Chr15 | hsa-miR-362-3p | −2.06 | ChrX |
| hsa-miR-4323 | 1.62 | Chr19 | hsa-miR-423-5p | −1.65 | Chr17 |
| hsa-miR-550a | 1.51 | Chr17 | hsa-miR-483-5p | −1.54 | Chr11 |
| hsa-miR-602 | 1.57 | Chr9 | hsa-miR-3652 | −1.78 | Chr12 |
| hsa-miR-634 | 1.69 | Chr17 | | | |
Bioinformatical analysis of TECA-specific
miRNAs and their putative targets
We next assessed the biological meaning of the
altered miRNA expression in UVB protection. miRNAs
post-transcriptionally regulate gene expression by binding to
target mRNAs, indicating that the biological functions of miRNAs
are dependent on that of their target genes (26).
First, we analyzed the putative target genes of
miRNAs that were meaningfully altered by TECA treatment using the
miRNA target prediction bioinformatical tool MicroCosm. We observed
that 1,354 and 1,975 genes were putatively targeted by the
upregulated and downregulated miRNAs, respectively (p<0.05, data
not shown). We next analyzed the biological functions for each
target gene with the GO analytical tool AmiGO. Since UV irradiation
induces cell aging and apoptosis (1), we sorted the target genes into
several categories, including aging, apoptosis, cell proliferation
and skin development (Tables II
and III). We revealed that a
number of target genes were involved in these four processes,
suggesting that the effects of TECA may be functionally related to
UV protective properties by affecting the protein products of those
genes. For example, miR-636, which was increased by 3.51-fold by
TECA, putatively targets genes such as suppressor of cytokine
signaling 3 (SOCS3), microphthalmia-associated transcription factor
(MITF), empty spiracles homeobox 2 (EMX2) and transcription factor
7-like 2 (TCF7L2). Conversely, miR-622 was decreased by 2.82-fold
by TECA and putatively targets genes included nucleophosmin (NPM1),
E2F transcription factor 1 (E2F1) and peroxisome
proliferator-activated receptor δ (PPARD).
 | Table IIPredicted target genes of the miRNAs
upregulated in response to TECA treatment in UVB-exposed HaCaT
keratinocytes. |
Table II
Predicted target genes of the miRNAs
upregulated in response to TECA treatment in UVB-exposed HaCaT
keratinocytes.
| Target gene
functions
|
|---|
| miRNA | Aging | Apoptosis | Cell
proliferation | Skin
development |
|---|
| hsa-miR-1228 | TP53 | TP53, CEBPG,
PLAGL2, TJP1 | TP53, ATP8A2, CD47,
MKI67, SSTR1 | - |
| hsa-miR-1237 | ID2 | BCL6, ERBB3, PAK7,
ANKRD13C, SGPL1, UBE2Z | ID2, BCL6, ERBB3,
PAK7, NFIB, CCND2 | - |
| hsa-miR-1825 | SERPINE1, ULK3 | SERPINE1, ITCH,
OSR1, CECR2, PREX1, ROCK1, TIAM1, BCL11B, GPI, MITF, PKN2 | SERPINE1, ITCH,
OSR1, CHRNB2, EPS15, HHIP, KIT, MAB21L1, R3H1, FOXO4, BCL11B,
MITF | COL5A3, BCL11B |
| hsa-miR-223 | F3, RPS6KB1 | F3, RPS6KB1, APC,
FGFR2, FOXO1, FOXO3, IGF1R, ECT2, ATP7A, HSP90B1, NLRP3, RASA1,
RHOB, RNF34, SNCA, SYNGAP1 | F3, RPS6KBI, APC,
FGFR2, FOXO1, FOXO3, IGF1R, ACVR2A, CBLB, MYH10, PDS5B, SCARB1,
WDR77, NFIB | APC, ATP7A |
| hsa-miR-296-5p | BBC3, HMGA1,
PRELP | BBC3, CNTFR, FGFR1,
NUAK2, MEF2D | HMGA1, CNTFR,
FGFR1, CXCL10, MLL2 | - |
| hsa-miR-550a | CDK6, TERF2 | ARHGEF12, MTDH,
PSME1, UNC5A, SOX4 | CDK6, APPL2,
PDGFRA, TRIM27, SOX4, SOX11 | - |
| hsa-miR-634 | P2RY2, TGFBR1 | ADRB2, ERBB4, VAV3,
CSDA, IAPP, NEUROD1, RASSF5, YWHAE, KCNMA1, TGFBR1, KPNA1, MAP3K1,
BLOC1S2, PDCD6IP | ADRB2, ERBB4, VAV3,
CKS1B, FOXP2, GOLPH3, INSR, JAG1, CCND2, TGFBR1, FBXW7, TBC1D8,
EVI1, SSR1 | - |
| hsa-miR-636 | SOCS3 | TCF7L2, RPS6KA2,
SFRP2, TGFBR2, TRAF5, ACTN1, ARF6, GRIK2, ITSN1, PCGF2, PROC,
RPS6KA3, RTN3, CBL, SENP1, YWHAZ, SOCS3, MITF, PKN2, PRKCE | TCF7L2, RPS6KA2,
SFRP2, TGFBR2, TRAF5, BCAT1, EMX2, LIFR, RNF139, MITF, TOB1 | TCF7L2 |
| hsa-miR-664 | - | CUL3, ZMYND11, BMX,
C1D, CYCS, NET1, PDPK1, TAF9, TNFAIP1, UBE2D3, PAX3 | CUL3, ZMYND11,
ACSL6, BAP1, CDC14A, MTCP1, SMAD4, IRF2, PHOX2B, PAX3, FOXO4 | - |
| hsa-miR-767-3p | AGT, HTR2A | AGT, CSNK2A1,
CSNK2A2, HSPA9, SET, TRAF7, UBE2B, BLOC1S2, PDCD6IP | AGT, HTR2A, ASPH,
CDC25C, FGF7, JARID2, MLXIPL, NPR1,PDPN, PTCH1, ST8SIA1, TENC1,
TIMP2, UBR5, BLOC1S2, CNOT8, EVI1, SSR1 | - |
| hsa-miR-92b | ADRB1, MORC3, NOX4,
HCN2, PTEN | ADRB1, APPL1, BTG2,
GPI, CDK5R1, HAND2, ITGAV, KLF4, PTPRJ, SGK3, USP28, ARHGEF17,
BCL2L11, DYRK2, FXR1, HIPK3, ITGA6, LYST, MAP2K4, NR4A3, RAD21,
ROBO2, TRAF3, PTEN, SOX4, DAB2IP, BCL11B, PAX3 | MORC3, NOX4, APPL1,
BTG2, CDK5R1, HAND2, ITGAV, KLF4, BMPR2, EVI5, CDC27, CDCA7L, TSC1,
CHRM5, CDKN1C, SGK3, FOSL2, PTPRJ, CXCL5, GATA2, GDF11, MS4A2,
NKX2-3, PTPRU, S1PR1, TACC2, TGIF1, TOB2, DAB2IP, ZEB2, PTEN, ODZ1,
BCL11B, SOX4, SOX11, PAX3 | COL1A2, BCL11B |
 | Table IIIPredicted target genes of the miRNAs
downregulated in response to TECA treatment in UVB-exposed HaCaT
keratinocytes. |
Table III
Predicted target genes of the miRNAs
downregulated in response to TECA treatment in UVB-exposed HaCaT
keratinocytes.
| Target gene
functions
|
|---|
| miRNA | Aging | Apoptosis | Cell
proliferation | Skin
development |
|---|
| hsa-miR-192 | SMC5 | DICER1, GDNF | DICER1, EREG | - |
| hsa-miR-182 | TMEM115, RTN4 | RTN4, CITED2, EGR3,
MEF2C, BCL2L13, ELMO1, NF1, MLL, ARHGEF3, BCL2L12, CREB1, MITF,
SOX2, AATK, ACVR1, RAC1, TNFAIP8, TOX3 | TMEM115, CITED2,
NF1, EGR3, MEF2C, TNFSF11, MITF, SOX2, CBFA2T3, EVI1, MTSS1, PYGO2,
CDV3, TOB1, FGF9 | - |
| hsa-miR-132 | CTGF | FOXO3, MAPT, APAF1,
RB1, SYNGAP1, MAPK3, CTGF | ZEB2, FOXO3, HBEGF,
CTGF, RB1, SPRY1 | - |
|
hsa-miR-125a-5p | BAK1, VDR, CASP2,
MAPK14 | SGPL1, SYVN1,
TAF9N, TIAF1, BAK1, VDR, CASP2, MAPK2, IER3IP1, KCNIP3, MYO18A,
TRIAP1, MCL1, MAP3K11, SSTR3, ECE1, HK2, APC, BCL11B, COL4A3,
IL6R, | BCL11B, COL4A3,
GPC4, BCAT1, CDH5, CGREF1, CYP27B1, DIS3L2, LIPA, BAK1, VDR,
APC,IL6R, PRDM1, ENPEP, ESRRA, FBW4, TNFAIP3, MAP3K11, TNFSF4S | COL4A3, BCL11B,
APC |
| hsa-miR-155 | FOS | MAP3K10, RPS6KA3,
VAV3, NKX3-1, TRIM32, MEF2A, PHF17, PKN2, SGK3, CBL, TP53INP1,
YWHAZ | NKX3-1, TRIM32,
VAV3, SMAD2, SMAD1, SGK3, CSF1R, FGF7, JARID2 | GNAS |
| hsa-miR-374a | CCL2, ADRB1, WRN,
WNT16 | NTF3, PDPK1, PSMF1,
SMAD6, CEBPB, NMT1, FGFR2, TGFA, DUSP6, EDAR, SOX4, WNT5A, ABR,
CCL2, ADRB1, WRN, AKT1, BMP2, IL10, MSX1, MAP2K4, MEF2D, | WNT5A, ADAM10,
CD47, NCK1, PELI1, IL10, ING1, NUMB, CEBPB, FGFR2, ASCL1, BMP2,
CNOT8, DLG3, EIF2S2, TFDP1, HES1, MMP14, CCL2, WNT16, AKT1, PITX2,
TGFA, NR2F2, SOX4, | - |
| hsa-miR-494 | PTEN, BBC3, CHEK2,
CNR1, SLC1A2, SIRT1 | PTEN, BBC3, CHEK2,
CNR1, CLI3, IL12B, KLF11, ACVR1C, GULP1, MTDH, PPARGC1A, ROCK1,
UACA, IGF1R | PTEN, GLI3, IL12B,
NFIB, TACC2, IGF1R, DNAJA2, KLF11, CKS1B, GPNMB, PBRM1, EVI5,
RAP1B, HHIP, SIRT1 LIF | - |
| hsa-miR-181d | ATM, PRKCD,
SERPINE1, TGFBR1, ADRBK1, TIMP3 | NOTCH2, PAWR,
SRPK2, TNF, BAG4, CBX4, CCNG1, DDIT4, PRKCD, SERPINE1, TGFBR1, ATM,
RAD21, RNF34, BMP7, GATA6, HEY2, IL1A, INSL3, ITSN1, HSP90B1,
TNFAIP1, TRIM22, USP47 | ATM, PRKCD,
SERPINE1, TNF, CBLB, CDON, ING5, CARD11, GATA6, HEY2, INSL3,
NOTCH2, PAWR, PLAU, PRDM4, PROX1, TGFBR1, BIRC6, IL1A, RBBP7,
TNS3 | - |
| hsa-miR-532-5p | NUAK1 | HSPA9, MED1, MBD4,
IRS2, LEP, CAPN3, CYC5, EYA2 | LEP, NDP, PURA,
SKAP2, MED1, DDX11, IRS2, KRAS, FRS2 | - |
| hsa-miR-622 | NPM1 | NPM1, APPL1, E2F1,
PPARD, SATB1, BTK, SORT1, RARG, DYRK2 | PPARD, SATB1 FBXW7,
RXRB, TGIF1, RARG, NPM1, APPL1, E2F1, SALL1, MBD2 | - |
| hsa-miR-625 | - | GHRH, IGF1,
ISL1 | GHRH, ARIH2,
HOXD13, SPARC, IGF1, ISL1 | - |
| hsa-miR-660 | - | TFAP2B, CDH13,
HIPK1 | TFAP2B, CDH13,
LIFR | TFAP2B |
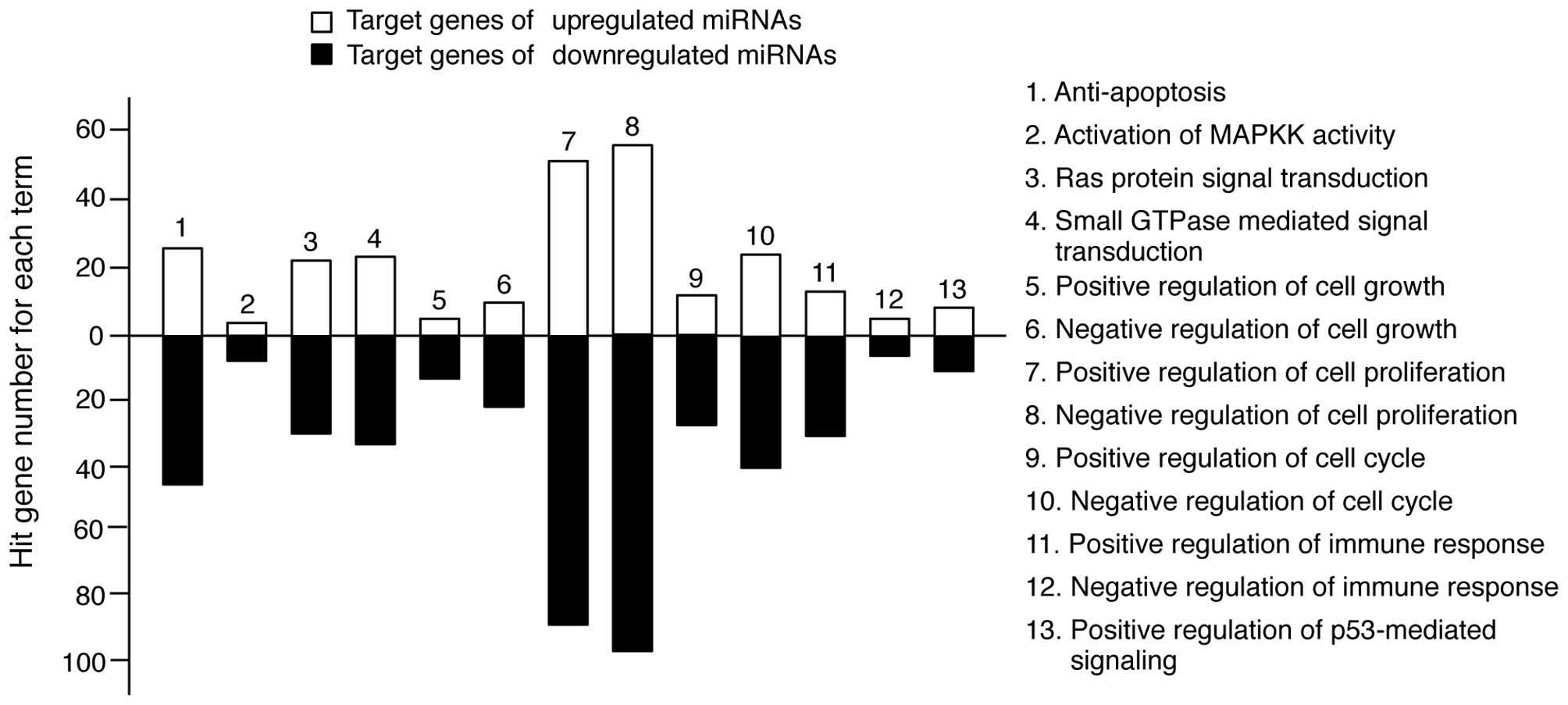
In keratinocytes, UV irradiation induces several
molecular responses, such as pro-apoptotic signaling pathways;
Ras-, MAPK- and small GTPase-mediated signal transduction and
immune responses (1). We
re-sorted the target genes in Tables
II and III into categories of
molecular responses. As shown in Fig.
3, the miRNA target genes were highly involved in these types
of responses. However, the level of involvement varied. The
majority of target genes was functionally related to anti-apoptosis
and cell proliferation regulation, whereas a limited number of
target genes was related to MAPKK activity. Collectively, these
findings suggest that TECA-mediated UV protective properties may
regulate molecular interplay between miRNAs and their target genes
to influence apoptosis and cell proliferation.
Discussion
In the present study, we verified that C.
asiatica protects keratinocytes against UVB-induced damage.
WST-1 assays demonstrated that C. asiatica-induced effects
were dose-dependent. Low-doses of TECA induced proliferation, while
high-doses of TECA induced cell death, indicating that C.
asiatica has a dichotomous role in cell growth. The medical and
pharmaceutical uses of C. asiatica are diverse. In human
dermal fibroblasts, C. asiatica induces cell proliferation,
collagen synthesis and anti-senescence (24,27). Additionally, C. asiatica
induced gastric ulcers and promoted epithelial cell proliferation
in rats (28). However, C.
asiatica has also shown anti-proliferative properties in solid
cancers, such as melanoma, breast, liver and gastric cancers, as
well as in keratinocytes (29–33). An anti-proliferative effect on
keratinocytes was shown to be highest following treatment with
18.4±0.6 μg/ml C. asiatica extract (33). We determined that >10 μg/ml of
TECA was anti-proliferative, however, the lower doses of TECA
(<5 μg/ml) led to increased cell proliferation. This was
recently confirmed by another group, which demonstrated that C.
asiatica has concentration-dependent, reciprocal proliferative
effects (34).
Hashim et al (25) reported that C. asiatica
protects against UVB damage in human dermal fibroblasts. However,
we demonstrated that the protective effects also extended to
keratinocytes and investigated the impact on miRNA. Although
protecting keratinocytes from UVB-induced damage has been widely
researched using other molecules and gene-based molecular studies,
a limited number of miRNA-based molecular studies have been
undertaken. Recently, p63-miRNA feedback was identified as an
important signaling pathway in keratinocyte senescence (35). Another study determined that the
protective effect of baicalin on UVB-treated keratinocytes was
mediated by miRNA expression modulation (36), indicating that miRNAs have
important roles in keratinocyte proliferation. Therefore, our
miRNA-based study regarding keratinocytes may provide important
information regarding anti-UV therapeutics.
Zhou et al (19) recently characterized the miRNA
profile in UVB-irradiated normal human keratinocytes.
Interestingly, they displayed that miR-296-5p and miR-423-5p were
down-regulated and upregulated by UVB irradiation (30
mJ/cm2, for 4 and 24 h) respectively. We found that the
expression levels of these miRNAs showed the opposite responses to
C. asiatica treatment; miR-296-5p was significantly
increased by 2.54-fold and miR-423-5p was decreased by 1.65-fold.
These results indicate that these miRNAs may be novel C.
asiatica target miRNAs that protect keratinocytes against
UVB-induced damage.
The miRNA target prediction and GO analysis revealed
that a number of the target genes were involved in the apoptosis
and cell proliferation pathways. MAPK-mediated signaling pathways
are reportedly involved in UVB responses in keratinocytes (1). However, our data demonstrated that
the target genes were less involved in MAPK-related signal
transduction, suggesting that miRNA-based UVB protection pathways
in keratinocytes may be mediated via anti-apoptosis and positive
regulation of cell growth pathways, rather than through a MAPK
pathway. In fact, although UV irradiation increases
phosphorylation-mediated activation of MAPK proteins, including p38
MAPK, JNK and ERK1/2, the transcription and translation levels of
these proteins are not altered by UVB irradiation (1), indicating that the MAPK genes do not
interplay with C. asiatica-specific miRNAs involved in
protecting keratinocytes from UVB-induced damage.
In conclusion, our findings suggest that C.
asiatica-mediated protective mechanisms are mediated by
alterations in miRNA expression. Although further confirmative
studies are required to verify miRNA alterations and their putative
targets, these data provide meaningful information to further our
understanding of the cellular responses in TECA-mediated UVB
protection in human keratinocytes.
Acknowledgements
We are grateful to the members of our
research group for their support and advice regarding this study.
This study was supported by the Ministry of Education, Science and
Technology (grant 20110028646 to S.A.) of the Republic of
Korea.
References
|
1.
|
V MuthusamyTJ PivaThe UV response of the
skin: a review of the MAPK, NFkappaB and TNFalpha signal
transduction pathwaysArch Dermatol
Res302517201010.1007/s00403-009-0994-y19756672
|
|
2.
|
GJ ClydesdaleGW DandieHK MullerUltraviolet
light induced injury: immunological and inflammatory effectsImmunol
Cell Biol79547568200110.1046/j.1440-1711.2001.01047.x11903614
|
|
3.
|
K BenderM GottlicherS WhitesideHJ
RahmsdorfP HerrlichSequential DNA damage-independent and -dependent
activation of NF-kappaB by UVEMBO
J1751705181199810.1093/emboj/17.17.51709724653
|
|
4.
|
DA LewisDF SpandauUVB activation of
NF-kappaB in normal human keratinocytes occurs via a unique
mechanismArch Dermatol
Res29993101200710.1007/s00403-006-0729-217256146
|
|
5.
|
N LiM KarinIonizing radiation and short
wavelength UV activate NF-kappaB through two distinct
mechanismsProc Natl Acad Sci
USA951301213017199810.1073/pnas.95.22.130129789032
|
|
6.
|
ZQ YuanRI FeldmanM SunInhibition of JNK by
cellular stress- and tumor necrosis factor alpha-induced AKT2
through activation of the NF kappa B pathway in human epithelial
CellsJ Biol Chem2772997329982200210.1074/jbc.M20363620012048203
|
|
7.
|
J LiuD YangY MinemotoM LeitgesMR RosnerA
LinNF-kappaB is required for UV-induced JNK activation via
induction of PKCdeltaMol
Cell21467480200610.1016/j.molcel.2005.12.02016483929
|
|
8.
|
H KimJS KangWJ LeeThe production IL-21 and
VEGF in UVB-irradiated human keratinocyte cell line, HaCaTImmune
Netw107580201010.4110/in.2010.10.2.7520532127
|
|
9.
|
DS ChangSJ SeoCK HongThe effect of
amniotic membrane extract on the expression of iNOS mRNA and
generation of NO in HaCaT cell by ultraviolet B
irradiationPhotodermatol Photoimmunol
Photomed18280286200210.1034/j.1600-0781.2002.02752.x12535023
|
|
10.
|
V AmbrosRC LeeIdentification of microRNAs
and other tiny noncoding RNAs by cDNA cloningMethods Mol
Biol265131158200415103073
|
|
11.
|
AM ChengMW ByromJ SheltonLP FordAntisense
inhibition of human miRNAs and indications for an involvement of
miRNA in cell growth and apoptosisNucleic Acids
Res3312901297200510.1093/nar/gki20015741182
|
|
12.
|
JF ChenEM MandelJM ThomsonThe role of
microRNA-1 and microRNA-133 in skeletal muscle proliferation and
differentiationNat Genet38228233200610.1038/ng172516380711
|
|
13.
|
WJ ChoJM ShinJS KimmiR-372 regulates cell
cycle and apoptosis of ags human gastric cancer cell line through
direct regulation of LATS2Mol
Cells28521527200910.1007/s10059-009-0158-019937137
|
|
14.
|
LM HolstB KaczkowskiR
GniadeckiReproducible pattern of microRNA in normal human skinExp
Dermatol19e201e205201010.1111/j.1600-0625.2009.01049.x20201961
|
|
15.
|
LM HolstB KaczkowskiM GludE
Futoma-KazmierczakLF HansenR GniadeckiThe microRNA molecular
signature of atypic and common acquired melanocytic nevi:
differential expression of miR-125b and let-7cExp
Dermatol20278280201110.1111/j.1600-0625.2010.01163.x21166724
|
|
16.
|
A IchiharaM JinninK
YamanemicroRNA-mediated keratinocyte hyperproliferation in
Psoriasis vulgarisBr J Dermatol16510031010201121711342
|
|
17.
|
N XuP BrodinT WeiMiR-125b, a microRNA
down-regulated in psoriasis, modulates keratinocyte proliferation
by targeting FGFR2J Invest
Dermatol13115211529201110.1038/jid.2011.5521412257
|
|
18.
|
J HildebrandM RutzeN WalzA comprehensive
analysis of microRNA expression during human keratinocyte
differentiation in vitro and in vivoJ Invest
Dermatol1312029201110.1038/jid.2010.26820827281
|
|
19.
|
BR ZhouY XuF PermatasariCharacterization
of the miRNA profile in UVB-irradiated normal human
keratinocytesExp
Dermatol21317319201210.1111/j.1600-0625.2012.01465.x22417313
|
|
20.
|
B BrinkhausM LindnerD SchuppanEG
HahnChemical, pharmacological and clinical profile of the East
Asian medical plant Centella
asiaticaPhytomedicine7427448200010.1016/S0944-7113(00)80065-311081995
|
|
21.
|
FX MaquartF ChastangA SimeonP BirembautP
GilleryY WegrowskiTriterpenes from Centella asiatica
stimulate extracellular matrix accumulation in rat experimental
woundsEur J Dermatol92892961999
|
|
22.
|
G JayashreeG Kurup MuraleedharaS
SudarslalVB JacobAnti-oxidant activity of Centella asiatica
on lymphoma-bearing miceFitoterapia744314342003
|
|
23.
|
TD BabuG KuttanJ PadikkalaCytotoxic and
anti-tumour properties of certain taxa of Umbelliferae with special
reference to Centella asiatica (L.) UrbanJ
Ethnopharmacol485357199510.1016/0378-8741(95)01284-K8569247
|
|
24.
|
YJ KimHJ ChaKH NamY YoonH LeeS
AnCentella asiatica extracts modulate hydrogen
peroxide-induced senescence in human dermal fibroblastsExp
Dermatol209981003201110.1111/j.1600-0625.2011.01388.x
|
|
25.
|
P HashimH SidekMH HelanA SaberyUD
PalanisamyM IlhamTriterpene composition and bioactivities of
Centella
asiaticaMolecules1613101322201110.3390/molecules1602131021278681
|
|
26.
|
RS PillaiSN BhattacharyyaW
FilipowiczRepression of protein synthesis by miRNAs: how many
mechanisms?Trends Cell
Biol17118126200710.1016/j.tcb.2006.12.00717197185
|
|
27.
|
L LuK YingS WeiAsiaticoside induction for
cell-cycle progression, proliferation and collagen synthesis in
human dermal fibroblastsInt J
Dermatol43801807200410.1111/j.1365-4632.2004.02047.x15533060
|
|
28.
|
JS GuoCL ChengMW KooInhibitory effects of
Centella asiatica water extract and asiaticoside on
inducible nitric oxide synthase during gastric ulcer healing in
ratsPlanta Med70115011542004
|
|
29.
|
BC ParkKO BosireES LeeYS LeeJA KimAsiatic
acid induces apoptosis in SK-MEL-2 human melanoma cellsCancer
Lett2188190200510.1016/j.canlet.2004.06.03915639343
|
|
30.
|
S BabykuttyJ PadikkalaPP
SathiadevanApoptosis induction of Centella asiatica on human
breast cancer cellsAfr J Tradit Complement Altern Med69162008
|
|
31.
|
LT LinLT LiuLC ChiangCC LinIn vitro
anti-hepatoma activity of fifteen natural medicines from
CanadaPhytother Res16440444200210.1002/ptr.93712203264
|
|
32.
|
M YoshidaM FuchigamiT
NagaoAntiproliferative constituents from Umbelliferae plants
VIIActive triterpenes and rosmarinic acid from Centella
asiatica Biol Pharm Bull28173175200515635187
|
|
33.
|
JH SampsonA RamanG KarlsenH NavsariaIM
LeighIn vitro keratinocyte antiproliferant effect of Centella
asiatica extract and triterpenoid
saponinsPhytomedicine8230235200110.1078/0944-7113-0003211417919
|
|
34.
|
BH RuszymahSR ChowdhuryNA MananOS FongMI
AdenanAB SaimAqueous extract of Centella asiatica promotes
corneal epithelium wound healing in vitroJ
Ethnopharmacol1403333382012
|
|
35.
|
P Rivetti di Val CervoAM LenaM
Nicolosop63-microRNA feedback in keratinocyte senescenceProc Natl
Acad Sci USA10911331138201222228303
|
|
36.
|
Y XuB ZhouDi WuZ YinD LuoBaicalin
modulates microRNA expression in UVB irradiated mouse skinJ Biomed
Res26125134201210.1016/S1674-8301(12)60022-023554741
|