Introduction
Breast cancer is a global health problem,
representing the primary cause of cancer-related female mortality
in developing countries (1).
Treatment for breast cancer varies depending on tumor stage and
molecular characteristics. However, only 50–70% of patients
receiving chemotherapy respond to first-line treatment (2). Almost all chemotherapeutic agents
used in the treatment of breast cancer develop resistance
mechanisms that are responsible for recurrence. Thus, discovering
new chemotherapeutic drugs and treatment strategies is a
challenging issue for the management of breast cancer.
Some natural plant compounds provide a potential
source of chemotherapeutic agents and display potent anticancer
activity; artemisinin (ART) and its derivatives are such promising
natural compounds. ART is the active principle of the herbal drug
Artemisia annua L. (commonly known as Qing Hao) and
has been used in traditional Chinese medicine for centuries. ART
and its main active metabolites dihydroartemisinin (DHA) are highly
effective anti-malarial drugs used as first-line therapeutics
against malaria falciparum worldwide (3–7).
Both ART and DHA are well-tolerated in human and animals with fewer
adverse side-effects than any other antimalarial drug (8). DHA is a sesquiterpene lactone
containing an endoperoxide trioxane moiety and has been widely used
to treat malaria owing to its ability to generate reactive oxygen
species (ROS) or carbon-centered radicals through cleavage of the
endoperoxide bridge (8–11) and its extremely potent inhibition
of the SERCA orthologue (PfATP6) of Plasmodium falciparum
(12–14). Apart from the use in the treatment
of malaria, more recent studies have shown that DHA also has
profound antitumor activity both in vitro and in
vivo, including in lung, ovarian, pancreatic, colon, breast,
prostate, liver and brain cancer (15–22). It is believed that DHA exerts its
cytotoxic and apoptotic effects by the generation of organic free
radicals, resulting in the induction of ROS from the iron-or
heme-mediated cleavage of endoperoxide bridge contained in DHA
(11,23). Some studies have also shown that
DHA-induced apoptosis may be related to the p38 MAPK, NF-κB,
hypoxia inducible factor-1α (HIF-1α), transferrin receptor, MEK/ERK
inactivation and Bcl-2 family signaling pathway (16,22,24). In addition, DHA seems to be able
to bypass the multi-drug resistance (MDR) and presents similar
anticancer potential in the parent and the resistant MDR cancer
cells (25). However, the exact
molecular mechanism by which DHA executes its anticancer effect is
not fully understood.
The present study was designed to further explore
the underlying mechanism of DHA treatment against breast cancer
cells in vitro from the standpoint of the proliferation
inhibition and apoptosis signaling pathway. We found that DHA
displayed cytotoxicities against the T-47D breast cancer cells by
inhibiting the growth of cancer cells, arresting cell-cycle
progression and inducing the mitochondrial pathway of apoptosis.
Furthermore, Bcl-2-interacting mediator of cell death (Bim), a
pro-apoptotic BH3-only member of the Bcl-2 family, may be involved
in the regulation of apoptotic signaling.
Materials and methods
Reagents
DHA and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl
tetrazolium bromide (MTT) were purchased from Sigma (Beijing,
China). Stock solution of 100 mM DHA was prepared in
dimethylsulfoxide (DMSO) and diluted with complete DMEM medium
before the experiments. The final concentration of DMSO was
<0.1%. Rabbit anti-caspase-8 polyclonal antibody was obtained
from NeoMarkers, Inc. (Fremont, CA, USA). Antibodies against
truncated Bid (tBid), cleaved-caspase-9 and cytochrome c
were purchased from Cell Signaling Technology (Beverly, MA, USA).
Anti-human β-actin antibody was purchased from Sigma-Aldrich (St.
Louis, MO, USA). Annexin V-FITC Apoptosis Detection kit was a
product of BD Biosciences (Shenzhen, China).
Cell line and cell culture
Cell culture reagents were purchased from Invitrogen
Corporation (Beijing, China) unless otherwise stated. The T-47D
human breast carcinoma cell line was obtained from China Center for
Type Culture Collection (CCTCC) and was maintained in DMEM
supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin
and 100 mg/ml streptomycin in a humidified incubator with 5%
CO2 at 37°C.
Cell growth assay
Effect of DHA on cell growth was measured by MTT
assay. Briefly, T-47D cells were seeded in triplicate in 96-well
plates at 5×103 cells/well and left overnight to adhere.
Subsequently, the medium was replaced with 200 μl of fresh medium
containing different concentrations of DHA (0–100 μM), followed by
incubation at 37°C and 5% CO2 for 24, 48 and 72 h. After
treatment, 10 μl of MTT solution (5 g/l) were added to each well
for the last 4 h. After 15 min of centrifuging at 2,000 rpm,
culture medium was discarded and then replaced with 150 μl
DMSO/well to dissolve the resultant formazan crystals. Absorbance
(A) was measured with an enzyme-linked immunosorbent assay reader
(Bio-Rad, USA) using 570 nm as test wavelength and 630 nm as
reference wavelength. Results were representative of three
individual experiments. Inhibition ratio (%) = (1-experimental
group A570–630/control group A570–630)
×100%.
Cell cycle analysis
After treatment for 48 h, cells were washed twice
with cold phosphate buffered saline (PBS). Subsequently, cells were
treated with PBS (pH 7.4) containing 1% RNase, and were stained
with propidium iodide (PI) at 100 mg/ml (final concentration). The
percentages of cells in the G0/G1, S or G2/M phase were calculated
from a contour plot obtained for the flow cytometric analysis. The
experiments were repeated at least three times independently.
Annexin V-FITC and PI apoptosis assay by
flow cytometry
T-47D cells were plated at 2×105 in 60-mm
tissue culture dishes. Twenty-four hours later, the cells were
treated with various concentrations of DHA for 48 h. At the end of
48 h, the cells were trypsinized and stained with the Annexin V-PI
apoptosis detection kit as per the manufacturer’s protocol.
Finally, the stained cells were analyzed with a Beckman-Coulter
flow cytometer. This assay distinguishes four groups of cells: dead
cells (Annexin-/PI+), normal living cells
(Annexin-/PI-), early apoptosis cells
(Annexin+/PI-), and late apoptosis cells
(Annexin+/PI+). The apoptosis of T-47D cells
was estimated by the relative amount of
Annexin+/PI- of cell populations. Triplicate
assays were performed.
Western blot analysis
T-47D cells from different treated groups were
washed twice in ice-cold PBS and lysed in complete cell lysis
buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% Triton X-100, 0.25%
Na-deoxycholate, 1 mM EDTA, 1 mM NaF, 1 mM DTT, 1 mM PMSF, 1 mM
activated Na3VO4, 1 μg/ml aprotinin, 1 μg/ml
leupeptin, and 1 μg/ml pepstatin). Protein concentrations were
determined using BCA assay (Hyclone-Pierce, USA). Protein samples
were resolved on 10% SDS-PAGE and transferred to nitrocellulose
membranes. The membranes were blocked in 5% nonfat dry milk
containing 0.1% Tween-20 at room temperature for 1 h, and then
probed with primary antibodies at 4°C overnight. After washing, the
membranes were incubated with horseradish peroxidase
(HRP)-conjugated secondary antibody (1:8,000) followed by ECL
detection (Amersham Pharmacia Biotech, Inc., USA). The membranes
were scanned with a LAS-4000 luminescent image analyzer (Fujifilm,
Japan). In the detection of cytochrome c, proteins in
cytosolic fraction of cells were concentrated according to the
method of Yuan et al (26)
and the release of cytochrome c from mitochondria to cytosol
in apoptosis was detected.
Semi-quantitative RT-PCR detection
Total RNA was extracted from the cultured cells with
TRIzol reagent (Invitrogen Corporation) according to the
manufacturer’s protocol. First-strand cDNA was synthesized by MMLV
Reverse Transcriptase (Promega Corporation). PCR was performed in
50 μl volume. The primers were: Bim, forward, 5′-A TCT CAG AGC AAT
GGC TT-3′ and reverse, 5′-A TTC GTG GGT GGT CTT CG-3′; its
amplification product was 163 bp. Bcl-2, forward, 5′-CGA CGA CTT
CTC CCG CCG CTA CCG C-3′ and reverse, 5′-CCG CAT GCT GGG GCC GTA
CAG TTC C-3′; its amplification product was 318 bp. Human β-actin
was used as internal control, forward, 5′-GTG GGG CGC CCC AGG CAC
C-3′ and reverse, 5′-CTC CTT AAT GTC ACG CAC GAT TT-3′; its
amplification product was 506 bp. Amplification was performed under
the following conditions: 95°C for 10 min, followed by 30 cycles of
94°C for 30 sec, 60°C for 30 sec and 72°C for 1 min, with a final
extension of 10 min at 72°C. DNA marker DL2000 (Takara Bio, Inc.,
Dalian, China) was used as standard. The relative mRNA expression
of Bim, Bcl-2 was determined by normalizing to β-actin mRNA
expression.
Statistical analysis
All experiments were performed in triplicate and
data are presented as the means ± standard deviation. Differences
between groups were examined using the one-way ANOVA or Student’s
t-test, when appropriate. All statistical tests were two-sided and
a P-value of <0.05 was considered to indicate statistically
significant differences.
Results
Cytotoxicity of DHA toward human breast
cancer cells
We first evaluated the in vitro antitumor
effect of DHA on the T-47D human breast cancer cells using the MTT
assay. Cells were exposed to various concentrations (0–100 μM) of
DHA for 24, 48 and 72 h and results showed that treatment with DHA
had an obvious inhibitory effect in a dose-dependent (r=0.911,
P<0.01) and time-dependent manner (r=0.918, P<0.01) (Fig. 1). The half maximal inhibitory
concentration (IC50) of DHA was detected to be 60.03,
33.86 and 17.18 μM for 24, 48 and 72 h, respectively. These data
indicated that DHA attenuated the in vitro proliferation of
breast cancer cells.
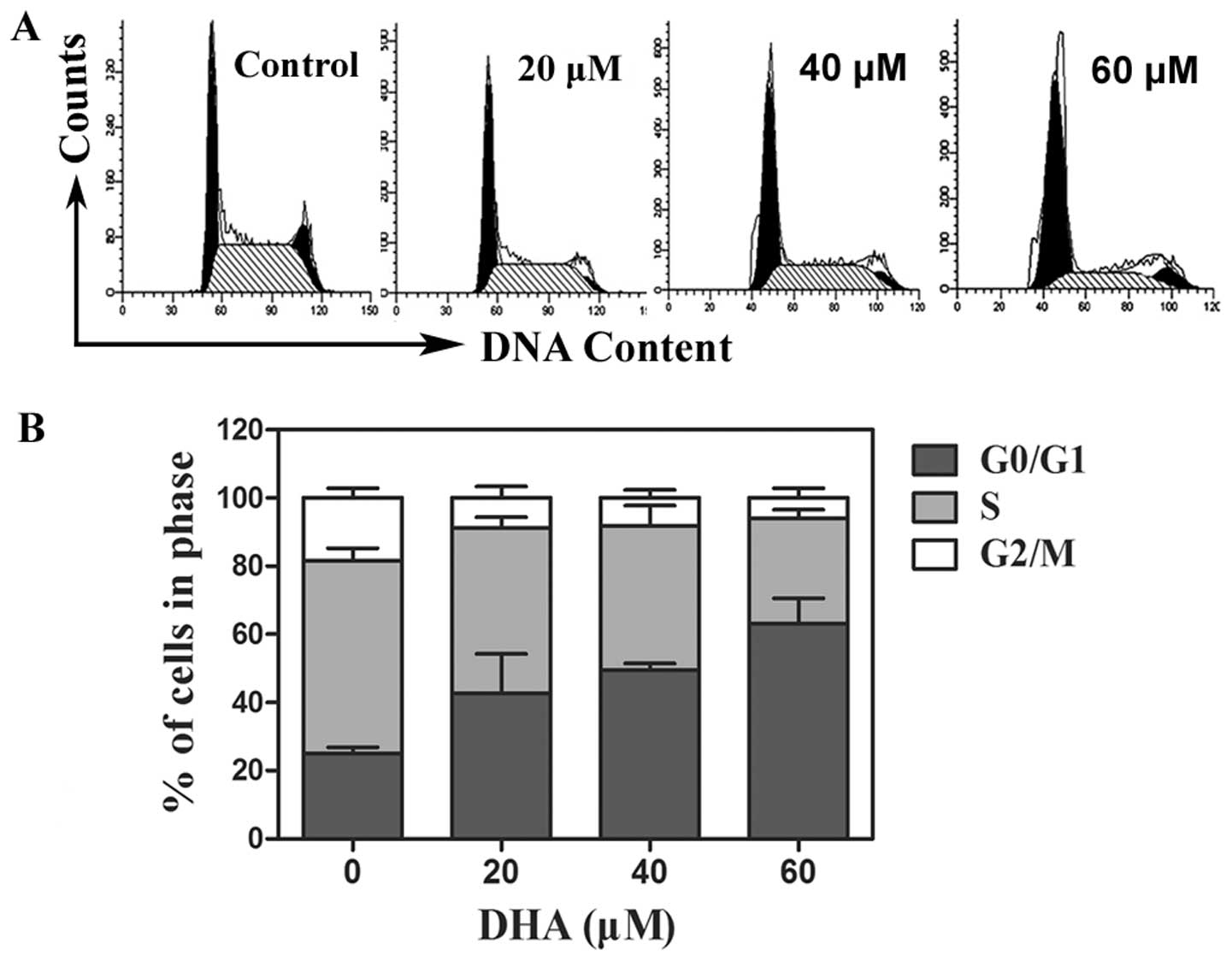
DHA arrests cell cycle in breast cancer
cells
To examine whether the cell growth inhibitory effect
of DHA is induced via perturbation in cell cycle progression, we
performed cellular DNA content distribution analysis by flow
cytometric analysis. There were significant differences in
proportions of G0/G1 and S phase between T-47D cells treated with
DHA and no treatment control (Fig.
2A). The DHA treatment of different concentrations in breast
cancer cells markedly increased the proportion of G0/G1 phase and
reduced the proportion of S phase, thereby preventing tumor cells
entering DNA synthesis phase (Fig.
2B).
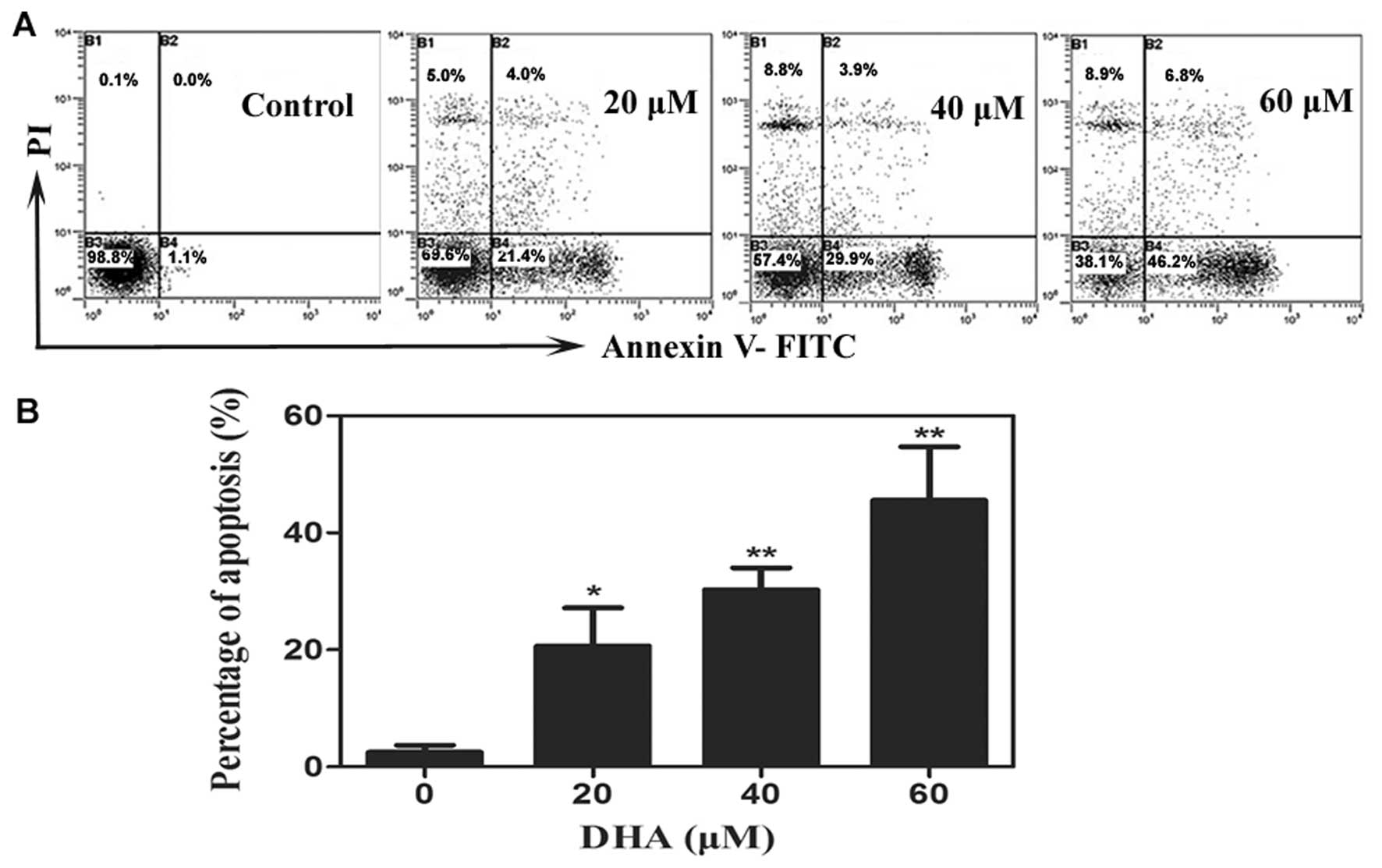
DHA induces apoptosis in breast cancer
cells
Based on the above results, we further evaluated the
pro-apoptotic activity of DHA. Results from Annexin V-PI analysis
revealed that T-47D cells treated with DHA underwent obvious
apoptosis compared to the control group in a dose-dependent manner.
The apoptotic ratio was increased to 20.67±6.53%, 30.30±3.71% and
45.57±9.16%, respectively, after DHA treatment, at the
concentration of 20, 40 and 60 μM for 48 h (Fig 3). There were significant
differences compared with control of 2.47±1.21% (P<0.01,
P<0.001 and P<0.001, respectively).
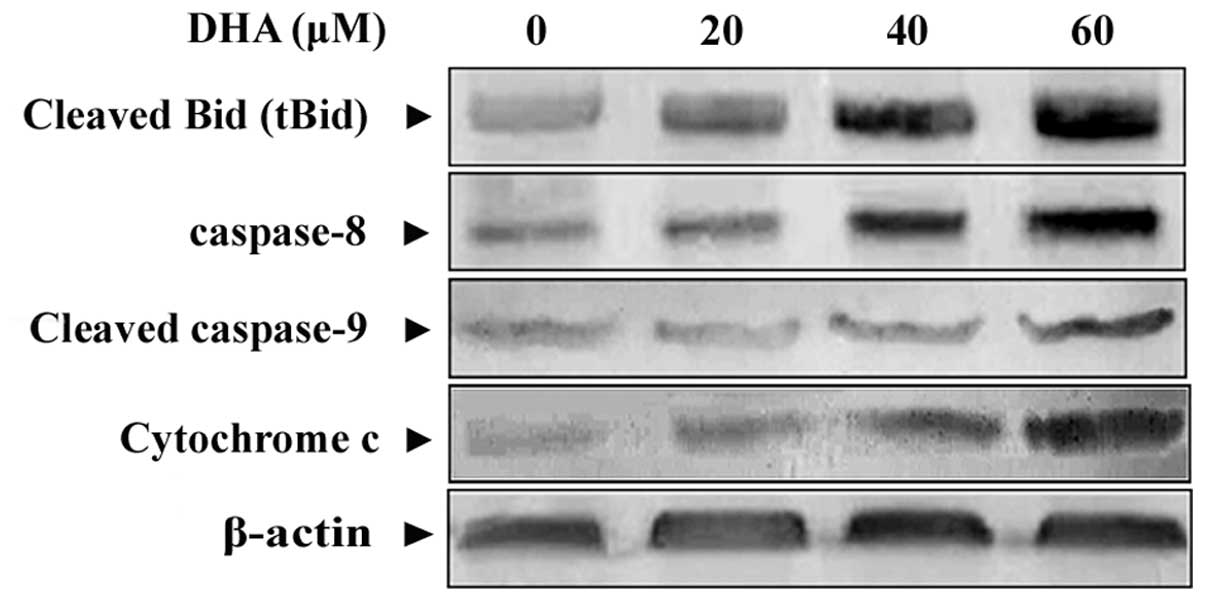
DHA increases the expression of
apoptosis-related proteins
To define the apoptotic pathway(s) being activated
by DHA, we examined the status of activation of several critical
apoptosis-related factors. Western immunoblotting was employed to
detect the activation of caspase-8, and -9, Bid and cytochrome
c released into the cytosolic fractions. Fig. 4 shows that caspase-8, cleaved
caspase-9 and the active, truncated form of Bid (tBid) were
activated in response to DHA. Moreover, following stimulation with
DHA, cytochrome c was released from mitochondria into
cytosol and resulted in a dramatic increase of cytochrome c
protein concentration, indicating the involvement of the
mitochondrial apoptotic pathway.
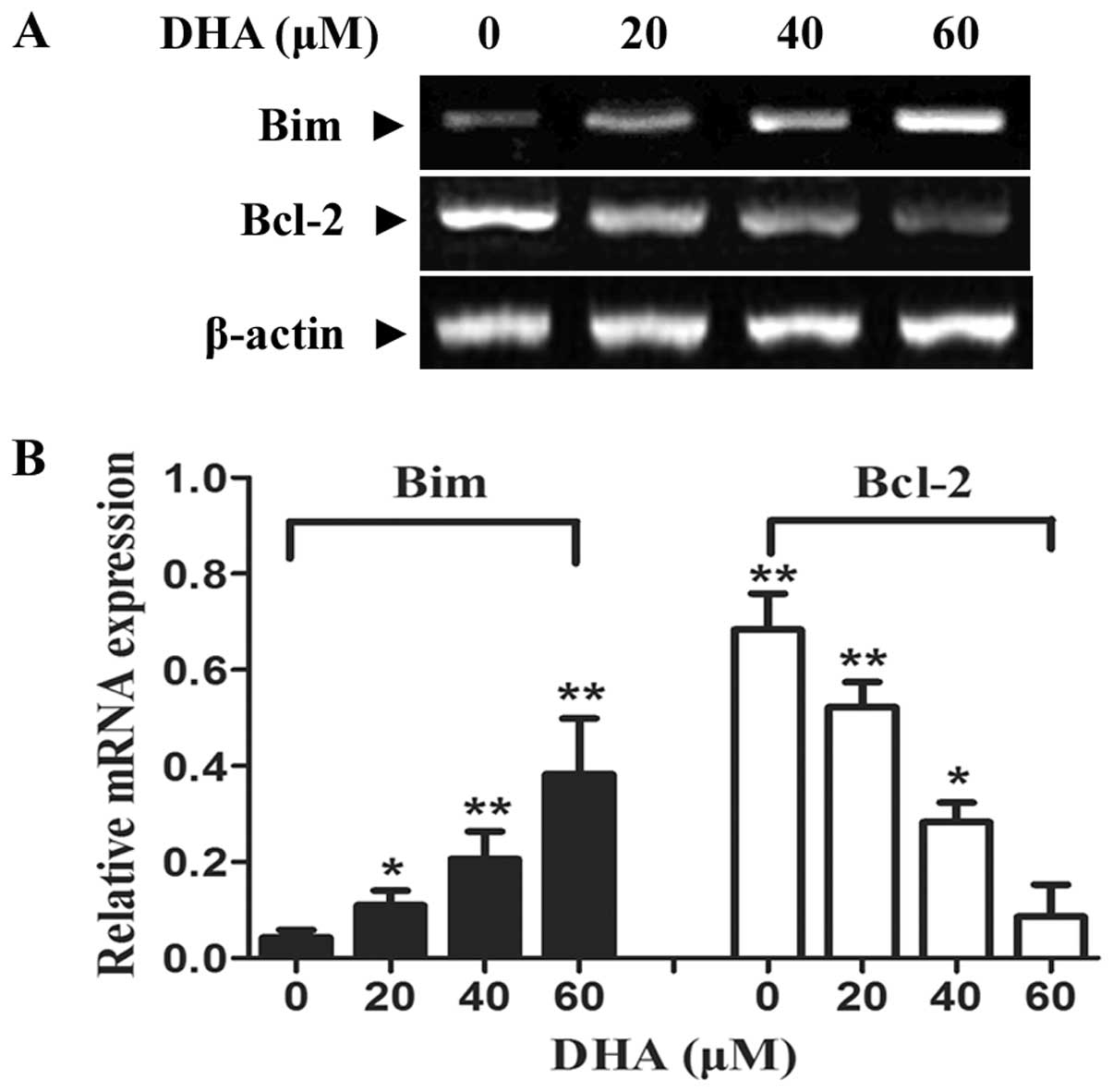
DHA regulates Bim and Bcl-2
expression
Bim, as a pro-apoptotic gene of the Bcl-2 family,
has been considered to play an important role in initiating the
mitochondrial apoptotic pathway by binding to anti-apoptotic Bcl-2
and sequestering it from pro-apoptotic proteins. To further
investigate the underlying mechanism of the apoptosis mediated by
DHA, we performed RT-PCR analysis to detect the change of Bim and
Bcl-2 mRNA level. There was increased Bim expression in DHA
treatment groups of different concentrations compared to no
treatment control in a dose-dependent manner (Fig. 5A). Furthermore, the marked
decrease of mRNA expression at the Bcl-2 gene was induced
(P<0.05 and P<0.01, respectively).
Discussion
Among various anticancer candidates, the natural
product artemisinin and its derivatives, particularly DHA, have
been found to display powerful anticancer activity in several types
of tumors. Cancer cells have been shown to be much more sensitive
to DHA than their normal counterparts (8,19,27). However, the molecular details of
DHA-induced cytotoxic effects remain unclear. The present in
vitro study was conducted in an effort to explore the potential
mechanisms of DHA treatment as a novel anticancer drug for breast
cancer. The growth assay of T-47D breast cancer cells treated with
DHA was detected in this study. The results from MTT assay
suggested that DHA was able to suppress the proliferation of breast
cancer cells in a dose- and time-dependent manner. The molecular
mechanism behind this inhibition has been explored in previous
studies. It has been reported that DHA inhibited Akt and ERK
activation and, thus, appeared to mediate its effect partly via
inhibition of the PI3-K/Akt and ERK pathways, the two major cell
proliferation and survival pathways (13). Another study found that the
proliferation inhibitory effect of DHA was related to the
expression of proliferating cell nuclear antigen (PCNA) (28). PCNA is synthesized during the
early G1 and S phases of the cell cycle and is involved in the
uncontrollable proliferation of cancer cells by assisting DNA
replication and base excision repair (29). PCNA was clearly downregulated by
DHA in pancreatic cancer in a dose-dependent manner (28). This result was also supported by
our own observation and other studies (30,31) that the treatment of DHA delayed
the cell cycle and induced a marked reduction of S phase and
accumulation of G0/G1 phase.
In addition to the antiproliferative effect and cell
cycle arrest on breast cancer cells, DHA was also shown to induce
apoptosis in a dose-dependent manner in our study. Apoptosis is one
of the major mechanisms of cell death in response to cancer
therapies. Two major apoptosis pathways have been defined in a
number of different cell types; the first is the death receptor
pathway initiated mainly by tumor necrosis factor receptors (TNFRs)
and Fas and the second is termed the mitochondrial apoptotic
pathway and involves mitochondria and Bcl-2 family members.
Caspase-8 is an important apoptosis protein that is activated
initially in both the death receptor and the mitochondrial pathway.
In the current study, caspase-8 was strongly upregulated by DHA
treatment. Sequentially, caspase-8 activated Bid, a pro-apoptotic
Bcl-2 family protein. As shown in this study, the DHA action
enhanced the activation of tBid (active form of Bid that is capable
of triggering apoptosis) significantly and induced mitochondrial
damage, cytochrome c release and caspase-9 activation.
Activation of caspase-9 then triggered the downstream effector
caspase cascade, resulting in the apoptosis of cells. As an
apo-protein, cytochrome c is nearly undetectable in the
cytosol in normal cells. However, the release of cytochrome
c from mitochondria to cytosol can be induced in apoptosis
when the mitochondrial pathway is involved (32). We measured the expression of
cytochrome c in cytosolic fractions and the data revealed a
significant upregulation of cytochrome c expression in
cytoplasm in DHA-exposed breast cancer cells. A previous study also
demonstrated that DHA treatment markedly lowered the mitochondrial
transmembrane potential, resulting from mitochondrial membrane
depolarization and the release of cytochrome c from
mitochondria to cytoplasm in human hepatocellular carcinoma cells
(21). Based on the above data,
we concluded that DHA induced apoptosis in breast cancer cells via
the mitochondrial pathway.
To date, two protein families are known to be
crucial in the process of apoptosis: one is the Bcl-2 family as a
decision-maker of apoptosis; the other is the caspase family, which
is an executor of apoptosis. Bcl-2 family members include
anti-apoptotic (Bcl-2, Bcl-xL) and pro-apoptotic proteins (Bax,
Bak, Bid, Bim and Bad) and they tightly control the activation of
the mitochondrial apoptotic pathway by regulating mitochondrial
homeostasis and permeability (33). As a pro-apoptotic BH3-only member
of the Bcl-2 family, Bim plays a key role in the initiation of
apoptosis induced by a broad range of cytotoxic stimuli. Bim is
required for apoptosis of autoreactive thymocytes and neurons and a
Bim/Bcl-2 balance is critical for controlling normal homeostasis of
naïve and memory T cells (34–36). It has been established that Bim
can induce apoptosis by engaging both anti- and pro-apoptotic
family members at mitochondria. Recent studies have also confirmed
that Bim interacts with and embeds Bcl-2 in mitochondrial
membranes. Subsequently, Bcl-2 and Bim form oligomers that
permeabilize the mitochondrial outer membrane to release cytochrome
c and activate caspases (35,37). In the current study, we further
investigated whether Bim and Bcl-2 were involved in DHA-induced
apoptosis as an upstream messenger, so that we could better
elucidate the molecular mechanisms of the observed cell apoptosis.
Here, we found that DHA-induced apoptosis was accompanied by an
increase of Bim and a decrease of Bcl-2. Based on previous studies
and our own observations, we deduced that the Bim/Bcl-2 interaction
may be involved in the apoptotic effect induced by DHA. DHA
treatment upregulated pro-apoptotic Bim expression and
downregulated anti-apoptotic Bcl-2 expression, and thereby led to
the imbalance of the Bim/Bcl-2 interaction. Abundant Bim either
bound to Bcl-2 proteins and formed oligomers to induce cytochrome
c release, or activated Bax/Bak directly to initiate the
mitochondrial cell death pathway. The validity of this assumption
and the mechanism underlying the upregulation of Bim remain to be
verified in future studies.
In summary, we have confirmed that the natural plant
drug DHA exerts its anticancer effects by inhibiting proliferation,
arresting cell cycle and promoting the apoptosis of tumor cells.
Moreover, the mitochondrial pathway is involved in the apoptosis of
breast cancer cells induced by DHA and Bim/Bcl-2 may be responsible
for DHA-induced apoptosis. These cellular effects, in combination
with relatively low toxicity in humans, make DHA an attractive
candidate drug for the treatment of breast cancer.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (31270970), the Science and Technology
Project of Shandong, China (2008GG10002035, 2012G0021821) and the
Science and Technology Project of Jinan, China (201202197).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Antoon JW, Lai R, Struckhoff AP, et al:
Altered death receptor signaling promotes epithelial-to-mesenchymal
transition and acquired chemoresistance. Sci Rep. 2:5392012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
White NJ: Qinghaosu (artemisinin): the
price of success. Science. 320:330–334. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
German PI and Aweeka FT: Clinical
pharmacology of artemisinin-based combination therapies. Clin
Pharmacokinet. 47:91–102. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Efferth T: Willmar Schwabe Award 2006:
antiplasmodial and antitumor activity of artemisinin - from bench
to bedside. Planta Med. 73:299–309. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Efferth T, Dunstan H, Sauerbrey A, Miyachi
H and Chitambar CR: The anti-malarial artesunate is also active
against cancer. Int J Oncol. 18:767–773. 2001.PubMed/NCBI
|
|
7
|
Klayman DL: Qinghaosu (artemisinin): an
antimalarial drug from China. Science. 228:1049–1055. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Disbrow GL, Baege AC, Kierpiec KA, et al:
Dihydroartemisinin is cytotoxic to papillomavirus-expressing
epithelial cells in vitro and in vivo. Cancer Res. 65:10854–10861.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hosoya K, Murahari S, Laio A, London CA,
Couto CG and Kisseberth WC: Biological activity of
dihydroartemisinin in canine osteosarcoma cell lines. Am J Vet Res.
69:519–526. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mercer AE, Copple IM, Maggs JL, O’Neill PM
and Park BK: The role of heme and the mitochondrion in the chemical
and molecular mechanisms of mammalian cell death induced by the
artemisinin antimalarials. J Biol Chem. 286:987–996. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang S and Gerhard GS: Heme mediates
cytotoxicity from artemisinin and serves as a general
anti-proliferation target. PLoS One. 4:e74722009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Toovey S, Bustamante LY, Uhlemann AC, East
JM and Krishna S: Effect of artemisinins and amino alcohol partner
antimalarials on mammalian sarcoendoplasmic reticulum calcium
adenosine triphosphatase activity. Basic Clin Pharmacol Toxicol.
103:209–213. 2008. View Article : Google Scholar
|
|
13
|
Handrick R, Ontikatze T, Bauer KD, et al:
Dihydroartemisinin induces apoptosis by a Bak-dependent intrinsic
pathway. Mol Cancer Ther. 9:2497–2510. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Eckstein-Ludwig U, Webb RJ, Van Goethem
ID, et al: Artemisinins target the SERCA of Plasmodium
falciparum. Nature. 424:957–961. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen T, Li M, Zhang R and Wang H:
Dihydroartemisinin induces apoptosis and sensitizes human ovarian
cancer cells to carboplatin therapy. J Cell Mol Med. 13:1358–1370.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang SJ, Gao Y, Chen H, et al:
Dihydroartemisinin inactivates NF-kappaB and potentiates the
anti-tumor effect of gemcitabine on pancreatic cancer both in vitro
and in vivo. Cancer Lett. 293:99–108. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen M, Chen TS, Lu YY, Liu CY and Qu JL:
Dihydroarteminsinin-induced apoptosis is not dependent on the
translocation of Bim to the endoplasmic reticulum in human lung
adenocarcinoma cells. Pathol Oncol Res. 18:809–816. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
He Q, Shi J, Shen XL, et al:
Dihydroartemisinin upregulates death receptor 5 expression and
cooperates with TRAIL to induce apoptosis in human prostate cancer
cells. Cancer Biol Ther. 9:819–824. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Singh NP and Lai H: Selective toxicity of
dihydroartemisinin and holotransferrin toward human breast cancer
cells. Life Sci. 70:49–56. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Singh NP and Lai HC: Synergistic
cytotoxicity of artemisinin and sodium butyrate on human cancer
cells. Anticancer Res. 25:4325–4331. 2005.PubMed/NCBI
|
|
21
|
Zhang CZ, Zhang H, Yun J, Chen GG and Lai
PB: Dihydroartemisinin exhibits antitumor activity toward
hepatocellular carcinoma in vitro and in vivo. Biochem Pharmacol.
83:1278–1289. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang XJ, Ma ZQ, Zhang WP, Lu YB and Wei
EQ: Dihydroartemisinin exerts cytotoxic effects and inhibits
hypoxia inducible factor-1alpha activation in C6 glioma cells. J
Pharm Pharmacol. 59:849–856. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mercer AE, Maggs JL, Sun XM, et al:
Evidence for the involvement of carbon-centered radicals in the
induction of apoptotic cell death by artemisinin compounds. J Biol
Chem. 282:9372–9382. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gao N, Budhraja A, Cheng S, et al:
Interruption of the MEK/ERK signaling cascade promotes
dihydroartemisinin-induced apoptosis in vitro and in vivo.
Apoptosis. 16:511–523. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Reungpatthanaphong P and Mankhetkorn S:
Modulation of multidrug resistance by artemisinin, artesunate and
dihydroartemisinin in K562/adr and GLC4/adr resistant cell lines.
Biol Pharm Bull. 25:1555–1561. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yuan J, Murrell GA, Trickett A and Wang
MX: Involvement of cytochrome c release and caspase-3 activation in
the oxidative stress-induced apoptosis in human tendon fibroblasts.
Biochim Biophys Acta. 1641:35–41. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Noori S and Hassan ZM: Dihydroartemisinin
shift the immune response towards Th1, inhibit the tumor growth in
vitro and in vivo. Cell Immunol. 271:67–72. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Aung W, Sogawa C, Furukawa T and Saga T:
Anticancer effect of dihydroartemisinin (DHA) in a pancreatic tumor
model evaluated by conventional methods and optical imaging.
Anticancer Res. 31:1549–1558. 2011.PubMed/NCBI
|
|
29
|
Kelman Z: PCNA: structure, functions and
interactions. Oncogene. 14:629–640. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lu JJ, Chen SM, Ding J and Meng LH:
Characterization of dihydroartemisinin-resistant colon carcinoma
HCT116/R cell line. Mol Cell Biochem. 360:329–337. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Morrissey C, Gallis B, Solazzi JW, et al:
Effect of artemisinin derivatives on apoptosis and cell cycle in
prostate cancer cells. Anticancer Drugs. 21:423–432. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Renz A, Berdel WE, Kreuter M, Belka C,
Schulze-Osthoff K and Los M: Rapid extracellular release of
cytochrome c is specific for apoptosis and marks cell death in
vivo. Blood. 98:1542–1548. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Youle RJ and Strasser A: The BCL-2 protein
family: opposing activities that mediate cell death. Nat Rev Mol
Cell Biol. 9:47–59. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bouillet P, Purton JF, Godfrey DI, et al:
BH3-only Bcl-2 family member Bim is required for apoptosis of
autoreactive thymocytes. Nature. 415:922–926. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhao L, He F, Liu H, et al: Natural
diterpenoid compound elevates expression of Bim protein, which
interacts with antiapoptotic protein Bcl-2, converting it to
proapoptotic Bax-like molecule. J Biol Chem. 287:1054–1065. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wojciechowski S, Tripathi P, Bourdeau T,
et al: Bim/Bcl-2 balance is critical for maintaining naive and
memory T cell homeostasis. J Exp Med. 204:1665–1675.
2007.PubMed/NCBI
|
|
37
|
Willis SN, Fletcher JI, Kaufmann T, et al:
Apoptosis initiated when BH3 ligands engage multiple Bcl-2
homologs, not Bax or Bak. Science. 315:856–859. 2007. View Article : Google Scholar : PubMed/NCBI
|