Introduction
Phenolic phytochemicals such as flavonoids which are
widely found in vegetables, fruits, juices, and tea exert positive
health effects in chronic disorders (1). Quercetin
(3,5,7,3′,4′-pentahydroxyflavone) is the most common flavonoid in
nature, existing primarily in its glycoside form as quercitrin
(3-rhamnoside) (2). Several of
its bioactivities have been reported, including anti-inflammatory,
scavenging oxygen radicals, anticancer, and anti-allergy effects
(1,3,4).
On account of the wide sources and fewer side-effects compared to
drugs, quercetin presents opportunities for the development of
novel therapies for diabetes.
β-cell death in type 1 diabetes is mediated by an
autoimmune attack with inflammatory process, and the subsequent
cytokine release is thought to be responsible for β-cell
destruction (5). Cytokine-induced
β-cell apoptosis is considered the main form of β-cell death,
although necrosis may also occur (6,7).
The precise molecular mechanisms that transduce the cytokine
signals to evoke destructive gene expression in β-cells remain
unclear. However, cytokines may trigger β-cell death via an
NF-κB-dependent path resulting in caspase activation and finally
causing cell failure (8).
Oxidative stress in β-cells reduces the mitochondrial transmembrane
potential, promoting the release of cytochrome c into
cytosol and activating caspase-3, and this apoptotic process is
regulated by the Bcl-2 family (9). Antioxidant chemicals blocking
cytokine signaling pathways and protecting β-cells from subsequent
cytotoxicity are a promising approach to the treatment of diabetes
(10).
Previous studies demonstrated that quercetin was
able to protect pancreas β-cells from oxidant damage and promote
insulin generation both in vivo and in vitro
(11–13). However, the majority of studies
focused on the aglycone form (quercetin), while its rhamnose
glycoside (quercitrin) attracted little attention due to the lack
of commercial standards (14).
The comparative results of quercetin and quercitrin remain
controversial: Rattanajarasroj and Unchern (16) support that quercitrin may be a
more potent antioxidant and neuroprotective agent than its aglycone
derivative due to its high bioavailability in the digestive tract
(15), while other studies
considered quercitrin possessed equal or even weaker antioxidant
ability than quercetin (1,17).
Current knowledge of the effects of
quercetin/quercitrin on β-cells is mostly limited to the
antioxidant ability and the suppression of inflammation. Quercetin
protects against β-cell damage as it blocks inflammatory signal
molecules in β-cells (12,13).
Little is known about the effect of quercetin on cytokine-induced
mitochondrial alteration and the downstream signaling that leads to
apoptosis. Further research on this potential anti-diabetic
component is required. We aimed to investigate the role of
quercetin and its glycoside derivative quercitrin in
cytokine-induced RINm5F β-cell injuries involving mitochondrial
apoptosis and NF-κB signaling. Furthermore, comparison of the
differences between quercetin and quercitrin may provide insight
into their structures and transportation into cells.
Materials and methods
Chemicals
Quercetin and quercitrin were purchased from Sigma
(St. Louis, MO, USA). Tumor necrosis factor-α (TNF-α),
interleukin-1β (IL-1β) and interferon-γ (IFN-γ) were obtained from
PeproTech, Inc. (Rocky Hill, NJ, USA). Fetal bovine serum (FBS) was
purchased from Gibco (Auckland, New Zealand). Cytochrome c,
Bax, caspase-3, polyadenosine diphosphate-ribose polymerase (PARP),
GAPDH, NF-κB, IκBα, and inducible nitric oxide synthases (iNOS)
were obtained from Santa Cruz Biotechnology, Inc. (CA, USA) or Cell
Signaling Technology (Beverly, MA, USA). Dichlorodihydrofluorescein
diacetate (DCFH-DA) was purchased from Molecular Probes (Eugene,
OR, USA). Quercetin and quercitrin were dissolved in dimethyl
sulfoxide (DMSO).
Culture and treatment of RINm5F
cells
The insulin-secreting cell line RINm5F, donated by
Professor C.Y. Zhou (Department of Biochemistry and Molecular
Biology, Peking University Health Science Center), was cultured in
DMEM with 10% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, 2
mmol/l in a humidified atmosphere (5% CO2, 37°C). After
starvation for 24 h, cells were treated with quercetin or
quercitrin (0, 5, 10 and 20 μM) for 2 h, then co-incubated with
TNF-α (10 ng/ml), IL-1β (5 ng/ml) and IFN-γ (1,000 U/ml) for 24
h.
Cell viability of RINm5F
The viability of RINm5F was evaluated by the
3-(4,5-dimethylthiazol-2-y1)-2,5-diphenyltetrazolium bromide (MTT)
assay. Following incubation for 24 h, cells were washed twice with
Krebs-Ringer bicarbonate (KRB) buffer containing 0.1% bovine serum
albumin (BSA) and then incubated with the same KRB/BSA supplemented
with 100 mg/ml MTT for 4 h in the dark (5% CO2, 37°C).
The precipitates in the cells (formazan) were dissolved in 200 μl
DMSO, and the absorbance was measured at 570 nm on a microplate
reader. The experiments were performed in triplicate.
Glucose-stimulated insulin secretion
(GSIS) assay
After a 24-h incubation period, cells were changed
to KRB/BSA containing 2.8 mM glucose for 1 h at 37°C, and the
supernatant was collected for basal insulin secretion measurement.
Next, the medium was replaced with KRB/BSA supplemented with 16.7
mM glucose for 1 h at 37°C, and the supernatant was collected for
GSIS measurement. For the detection of insulin we employed radio
immunoassay with rat insulin as a standard, and the data were
adjusted by the cell protein content of each group.
Nitrite measurement
Following 24 h of cell treatment, the supernatant
was collected for nitric oxide (NO) determination using a
colorimetric assay. Fifty microliters of culture supernatant were
mixed with 50 μl of Griess reagent for 5 min at room temperature,
and then the absorbance at 540 nm was measured on a plate reader.
Subsequently, the concentrations of nitrite were determined by a
linear standard curve from serial dilutions of sodium nitrite.
Reactive oxygen species (ROS)
determination
Cellular ROS was measured using
2,7-dichlorodihydrofluorescein diacetate (DCFH-DA) and its
oxidation to yield fluorescent dichlorofluorescein could be
promoted by ROS in cells. After treatment for 24 h, cells were
incubated with 5 μM DCFH-DA for 30 min. Then, the cells were washed
twice with KRB, trypsinized, re-suspended and transferred to a flow
cytometer for fluorescence intensity detection.
Separation of nuclei and mitochondria
extracts from cytoplasm
Mitochondria and cytosol fractions were obtained as
previously described (18).
Briefly, after 24 h of treatment, RINm5F cells plated on 25
cm2 petri dishes (5×106) were suspended and
homogenized. Unbroken cells and nuclei were discarded by
centrifugation at 10,000 × g for 20 min at 4°C, while the
supernatant was further centrifuged at 10,000 × g for 20 min. The
new supernatant was then moved to clean tubes and underwent further
centrifugation of 100,000 × g for 1 h at 4°C. The supernatant of
100,000 × g spin was collected as cytosolic fraction, and the
pellet of 10,000 × g spin was resuspended in isolation buffer
supplemented with 0.5% Nonidet P-40 (NP-40) as mitochondrial
fraction.
Nuclear and cytoplasmic proteins were extracted by
ProteoJET™ Cytoplasmic and Nuclear Protein Extraction kit
(Fermentas International Inc., Canada). In brief, cells were
scraped, pelleted by centrifugation at 250 × g for 5 min, mixed
with cell lysis buffer, homogenized, and set on ice for 10 min.
Then, the mixture was centrifuged at 500 × g for 7 min at 4°C, and
then the supernatant was further centrifuged at 20,000 × g for 15
min at 4°C. The supernatant of 20,000 × g spin was collected as
cytoplasmic protein extract, and the pellet of 500 × g spin was
washed and lysed with reagent of the kit and centrifuged at 20,000
× g for 5 min at 4°C; this final supernatant was collected as
nuclei protein extract.
Western blotting
Aside from the mitochondria and nuclei proteins
mentioned above, other protein samples were prepared as follows:
harvested cells were homogenized in lysis buffer (10 mM Tris, pH
8.0, 120 mM NaCl, 0.5% NP-40, 1 mM EDTA, and protease inhibitors)
for 30 min on ice and centrifuged at 10,000 × g at 4°C for 30 min.
The supernatants were then collected as samples. Protein content
was examined by a BCA protein assay kit (Pierce).
The protein samples from each fraction (40 μg) were
mixed with 5× sample loading buffer, and separated via sodium
dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE).
Proteins were transferred to polyvinylidene fluoride (PVDF)
membranes, which were then blocked with 5% skim milk for 1 h at
room temperature and incubated at 4°C overnight with primary
antibodies. Then, the membranes were incubated with HRP-conjugated
second anti-IgG for 2 h and visualized by ECL. The integrated
optical density (OPTDI) of each band was normalized with the
internal control GAPDH band for comparison.
Statistical analysis
All data are presented as the means ± SEM and
compared with one-way ANOVA for differences between groups, and
with the Bonferroni-Dunnett T3 procedure for multiple comparisons.
Statistical analysis was performed using SPSS 18.0 statistics
software, and a value of P<0.05 was considered to indicate
statistically significant differences.
Results
Quercetin/quercitrin ameliorate
cytokine-impaired viability of RINm5F cells
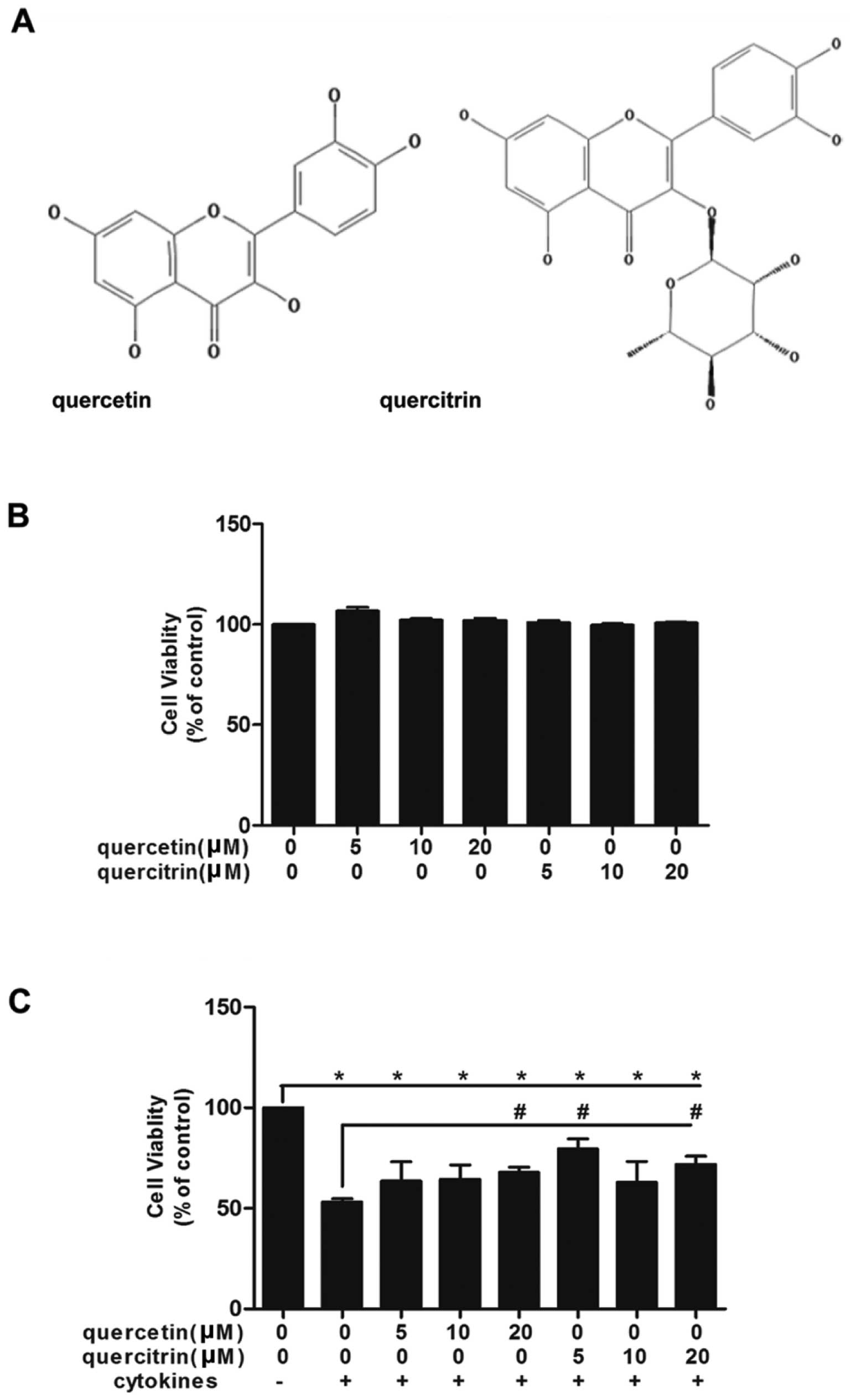
First, we confirmed the nontoxicity dose of 5, 10
and 20 μM for quercetin/quercitrin (Fig. 1B). Then, we applied these doses to
cells whose viabilities were inhibited by cytokines (Fig. 1C). The association of cytokines
significantly led to the demise of cells, however, we observed a
rise in the viability of cells in the quercetin/quercitrin
pretreated groups vs. cytokine treatment alone.
Quercetin/quercitrin improve GSIS in
cytokine-treated RINm5F cells
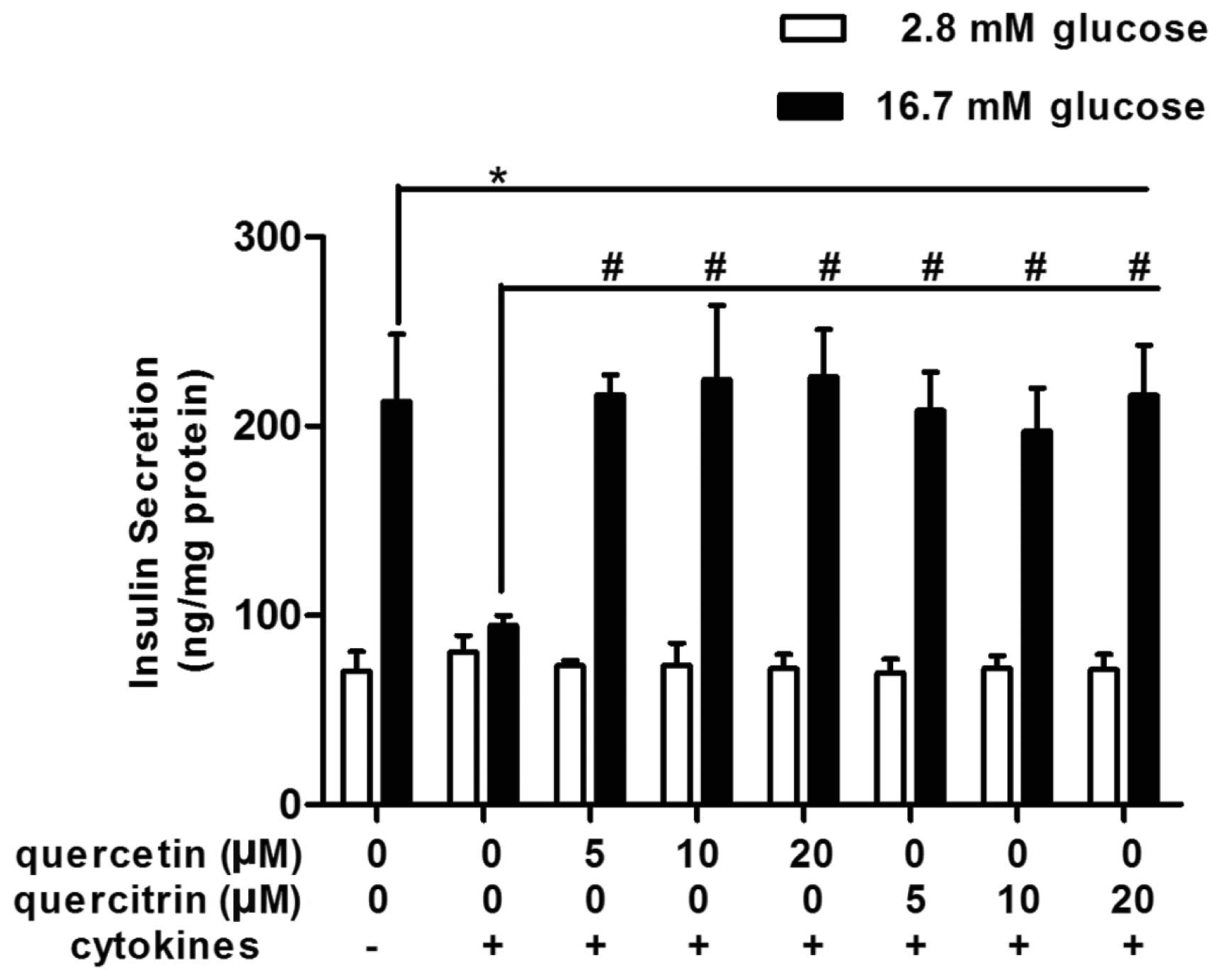
When exposed to a high concentration of glucose
(16.7 mM), normal RINm5F cells (untreated control) raised their
insulin secretion up to approximately 3-fold, compared with that of
the low glucose environment (2.8 mM) (Fig. 2). However, cytokines disturbed
this physiological reaction, and glucose-stimulated (16.7 mM)
insulin secretion was not better than the basal secretion in the
cytokine group (Fig. 2).
Quercetin/quercitrin ameliorated cytokine-induced numbness to
glucose stimulation in RINm5F cells. Quercetin/quercitrin (5, 10
and 20 μM) treatment resumed cell insulin secretion evoked by high
glucose (16.7 mM) (Fig. 2), and
the highest dose of both quercetin and quercitrin (20 μM) had the
most marked effect on restoring GSIS in RINm5F.
Quercetin/quercitrin reduce production of
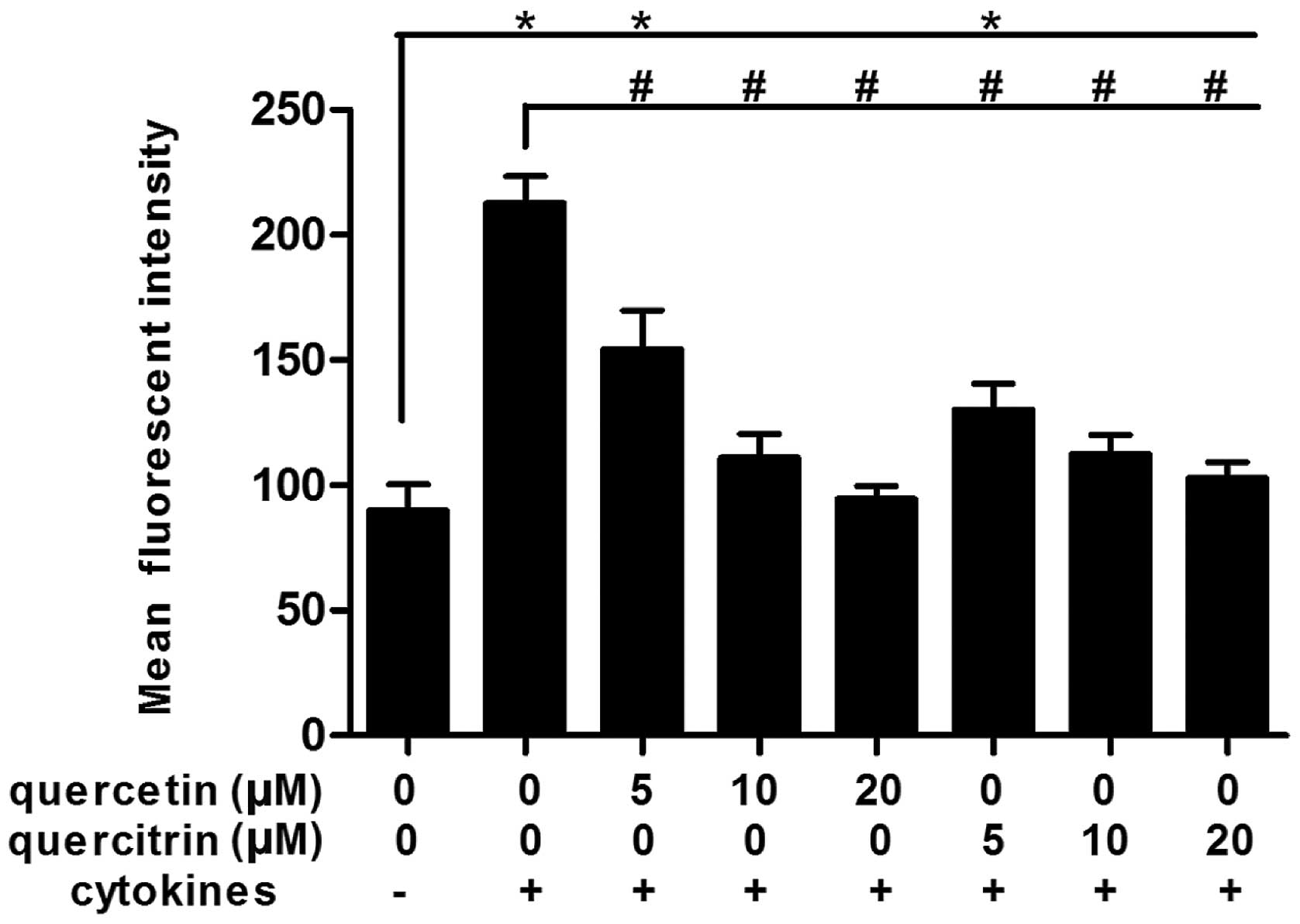
cellular ROS from cytokines in RINm5F cells
Cytokines rapidly boosted the accumulation of ROS in
RINm5F cells (Fig. 3). When
RINm5F cells were incubated with cytokines for 24 h, the mean
fluorescence intensity (DCFH-DA→DCF) for ROS increased markedly,
>2-fold that of the untreated control. However, the mean
fluorescence intensity of the quercetin/quercitrin treatment groups
decreased significantly compared to the cytokine alone group,
rendering a dose-dependent effect.
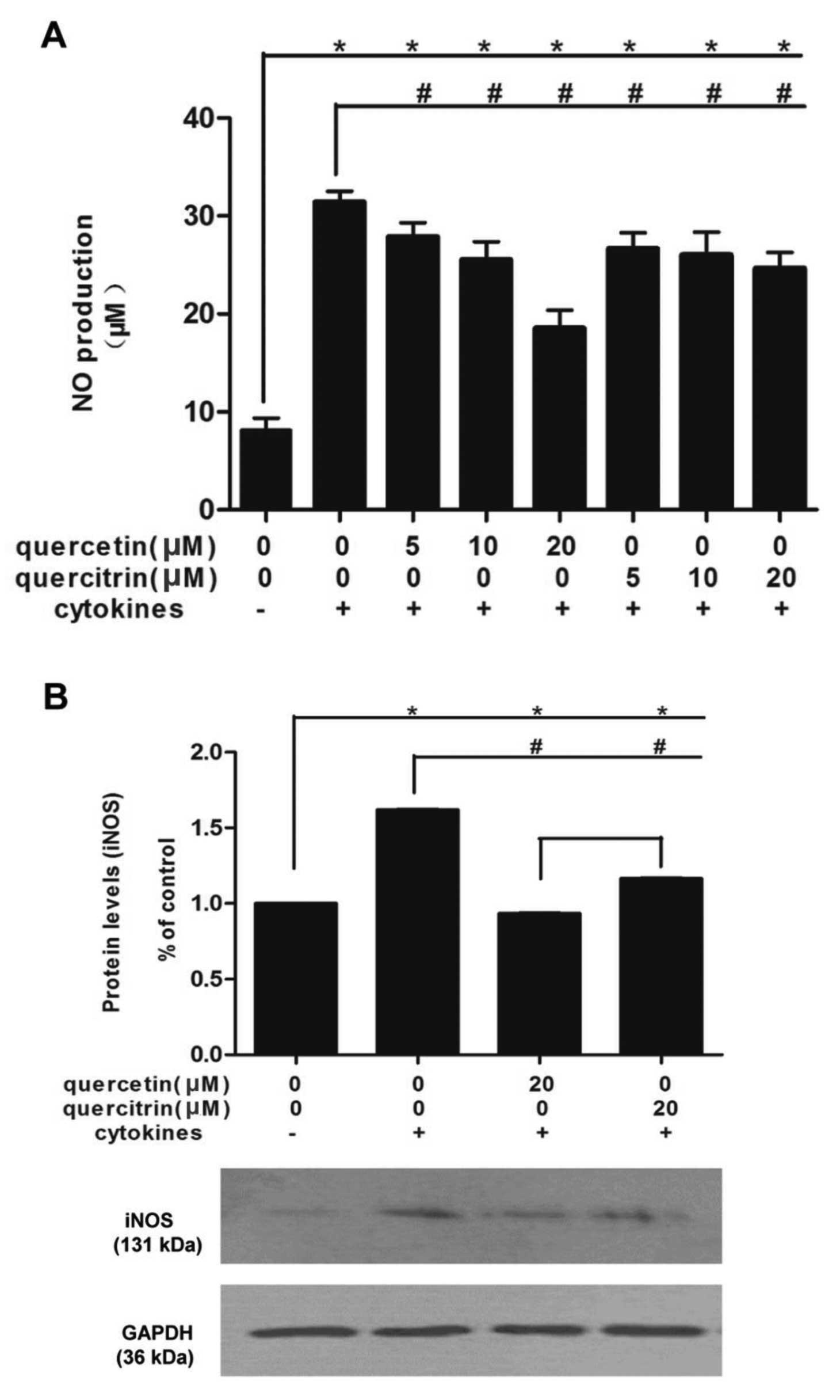
Quercetin/quercitrin restrain the
enhancement of NO production caused by cytokines in RINm5F cells
via inhibition of iNOS expression
Combined cytokines rapidly promoted NO production in
RINm5F cells (Fig. 4A). NO from
cells incubated with cytokines was >3-fold that of untreated
cells. However, preincubation of quercetin/quercitrin (5, 10 and 20
μM) for 2 h significantly reduced these boosts, in a dose-dependent
manner. Moreover, quercetin (20 μM) had stronger inhibitory effects
compared to quercitrin (20 μM).
At the dose of 20 μM, both quercetin and quercitrin
showed the most significant effect, so we applied this
concentration for the rest of the study. Cytokines markedly
increased the cellular expression of iNOS in RINm5F cells, however,
quercetin/quercitrin pretreatment (20 μM) reduced iNOS protein
levels in cells (Fig. 4B). Also,
quercetin showed a greater inhibition of iNOS expression than
quercitrin (20 μM).
Quercetin/quercitrin repress
apoptosis-associated protein in cytokine-induced RINm5F cells
Since quercetin and quercitrin mitigated the
dysfunctional insulin secretion, enhancement of ROS, or abnormal NO
production in RINm5F cells induced by cytokines, we investigated
the underlying mechanisms.
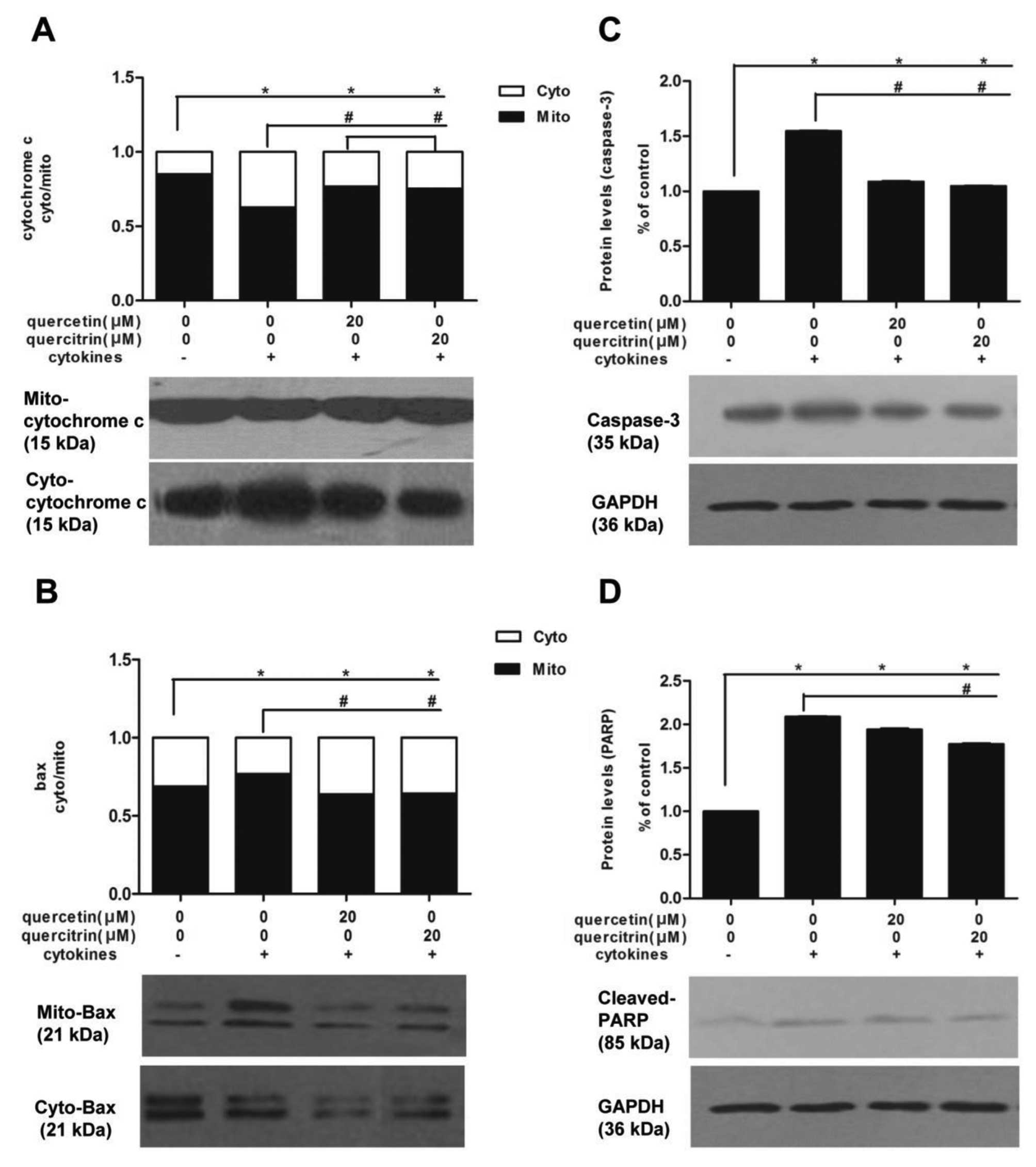
First, we investigated cytochrome c and Bax
protein expressions in subcellular fraction. Cytokines promoted
cytochrome c to translocate from mitochondria to cytoplasm
in RINm5F cells, and the fraction of band density (mitochondria to
cytoplasm) were significantly decreased compared to untreated
cells. However, we observed a moderate rise of fraction
(mitochondria to cytoplasm) in cells pretreated with quercetin or
quercitrin, compared to the cytokine alone group (Fig. 5A). Also, cytokines caused an
enrichment of Bax in mitochondira in RINm5F cells, however, this
enrichment was inhibited by a 2-h preincubation of quercetin or
quercitrin (20 μM) to a certain extent (Fig. 5B). We also examined cellular
levels of caspase-3 and cleaved PARP. Caspase-3 (Fig. 5C) and cleaved PARP (Fig. 5D) levels of the cytokine group
were higher than in the untreated control, yet the addition of
quercetin or quercitrin (20 μM) reduced their expression.
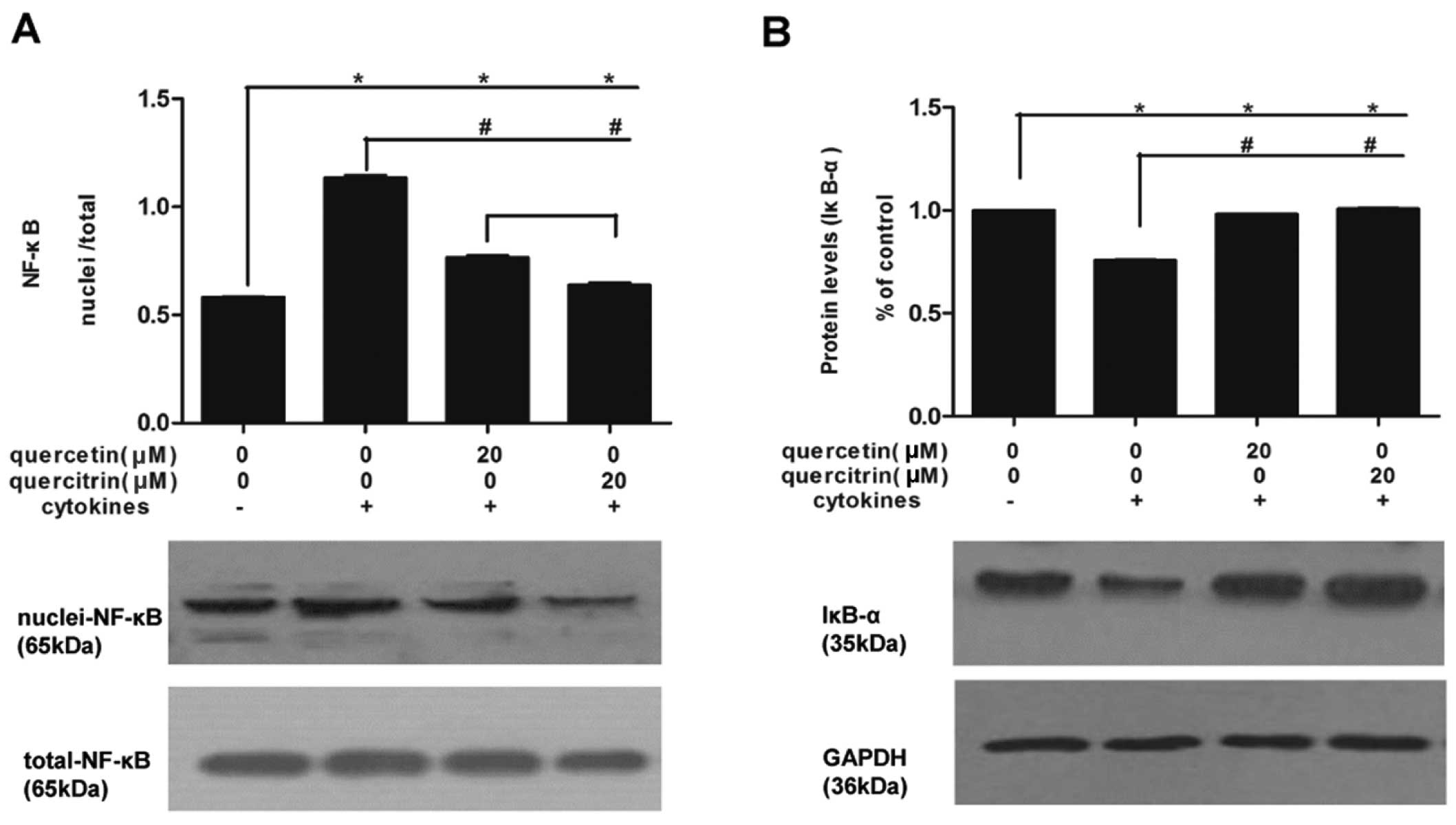
We also measured NF-κB (Fig. 6A) and IκBα (Fig. 6B) expression in nuclei and
cellular levels. Cytokines promoted NF-κB assembled in the nucleus
of RINm5F cells, which was relieved by quercetin or quercitrin, and
quercetin had a greater inhibitory effect compared to quercitrin.
At the same time, total protein expression of IκBα moderately
decreased in the cytokine group compared to that in the untreated
group, while this decline was limited in the group with either
quercetin or quercitrin pretreatment.
Discussion
In the present study, we found that
quercetin/quercitrin improved the cytokine-induced impairment of
viability and insulin secretion in RINm5F cells. We propose that
quercetin/quercitrin protect β-cells by mediating ROS accumulation,
iNOS expression, NF-κB pathway and mitochondria cytochrome c
signaling.
Tumor necrosis factor-α (TNF-α), interleukin-1β
(IL-1β), and interferon-γ (IFN-γ) have a direct influence on β-cell
destruction, possibly due to apoptosis, necrosis or other processes
(19,20). Here, we found that the combined
cytokines significantly decreased both the viability and
glucose-stimulated insulin secretion (GSIS) in RINm5F β-cells, and
this toxicity existed with an intensified oxidative stress.
Oxidative stress was considered a causal link between inflammation
and cell death (21–23), and it could activate caspases,
induce polyadenosine diphosphate-ribose polymerase (PARP) cleavage
and cell death (24). Thus,
exploring relevant nutritional substances benefitting cellular
redox maintenance such as quercetin may shed light on possible
treatment of β-cell failure. Quercetin is the most ubiquitous
antioxidative flavonoid in nature, and it is usually presented in
its glycosylated form, quercitrin (2). Quercetin and quercitrin have been
found to have protective effects on β-cells, although findings
remain limited and controversial. Our results demonstrated that
quercetin was able to maintain impaired β-cell viability and GSIS,
which had linkages with inhibited accumulation of ROS.
Accumulating evidence shows that the mitochondrial
intrinsic pathway of apoptosis plays a major role in pancreatic
β-cell death in diabetes (23,25). We found that a combination of
TNF-α, IL-1β and IFN-γ promoted mitochondrial apoptosis, which is
consistent with previous studies that these combined cytokines
induce mitochondrial membrane depolarization and cytochrome
c release in the RINm5F β-cell line (26,27). Notably, quercetin/quercitrin
suppressed cytokine-induced translocation of the pro-apoptotic
Bcl-2 family protein (Bax), cytochrome c release and
caspase-3 alteration, thereby indicating its role as an
anti-apoptotic agent in cytokine-induced β-cell suffering.
Cytochrome c promoted by the pro-apoptotic proteins releases
from mitochondrial, and these upstream alteration inspire the
downstream caspase-3 cleavage, which is an essential signal of
mitochondrial events of apoptosis (28). Our study also indicated that an
increased caspase-3 expression was concomitant with the altered
metabolism of PARP, which is consistent with a previous report on
caspase-3 involvement in PARP cleavage and cell death (29). In the damaged pancreatic β-cells,
PARP activates via cleavage and repairs the damaged DNA with
nicotinamide adenine dinucleotide + (NAD+) (30). When cleaved PARP is exhausted,
cells are destroyed by apoptosis (30). Quercetin/quercitrin protected
β-cells via inhibition of mitochondrial apoptosis and the
prevention of PARP activation. However, the direct cause-effect
relationship between apoptosis and cleavage was not verified in
this study, and requires further elucidation.
Cytokines were also able to induce nitric oxide (NO)
formation, which is generated by inducible nitric oxide synthase
(iNOS) (31). Our results
indicated that NO/iNOS production was involved in the protection of
the toxicity to RINm5F cells of quercetin/quercitrin. The iNOS gene
was also found to play a role in the neuroprotective effect of
quercetin (32), therefore we
hypothesized that the NO/iNOS system was an important part of the
mechanism of quercetin. The relationship between the iNOS pathway
and mitochondrial apoptosis was not fully confirmed, however, a
previous study reported that NO could lead to mitochondrial
membrane potential changes and the following release of cytochrome
c triggers apoptosis (33).
The transcription factor NF-κB is one of the key
mediators of cytokine-induced β-cell death, and it has been
reported to regulate expression of iNOS (8,34).
When stimulated by inducers, presumably via phosphorylation of
IκBα, NF-κB dimers release from initially inactive complex in the
cytoplasm and translocate into the nucleus and promote the
transcription of target genes (34,35). Thus, we assumed that NF-κB
activation, a crucial mediator of iNOS gene expression, could be an
upstream target for the inhibitory effects of quercetin/quercitrin.
Our results demonstrated that degradation of IκBα and the
translocation of NF-κB subunit p65 to nuclei induced by cytokines
were remarkably attenuated when RINm5F cells were pretreated by
quercetin/quercitrin. This is consistent with the effect of
quercetin on NF-κB signal transduction in osteoclast precursors
(36). This finding provides
insight into the mechanism underlying the protective effects of
quercetin/quercitrin against cytokine-impaired β-cells. However, we
did not verify that the NF-κB activation directly induced iNOS
expression.
In this study, we investigated the aglycone and
rhamnose glucoside form of quercetin. As a glycosylated form of
quercetin, quercitrin contains a rhamnose at C3 (Fig. 1A). Due to its additional radical
and high bioavailability in the digestive tract, quercitrin was
considered to be a more potent antioxidant and neuroprotective
agent compared to quercetin (15,16,37). However, the results of our study
were not in accordance with this. On the contrary, quercetin
possessed even stronger protective effects on RINm5F β-cells. This
was consistent with previous studies, such as the one by Wagner
et al (1), who found that
quercetin afforded more protective effects on MeHg-induced lipid
peroxidation and ROS generation in mitochondria and brain slices,
as well as the study of Chow et al (17), who reported that quercetin rather
than quercitrin protected RAW264.7 macrophages from oxidative
stress-induced apoptosis and mitochondrial dysfunction. We
hypothesized that the sugar moiety of the glycosides inhibited the
lipophilicity of quercetin or their effect relied on different
pathways or cell types; however, this requires further
investigations.
In conclusion, we evaluated the anti-diabetic and
protective effects of quercetin/quercitrin and identified the
underlying mechanisms of cytokine-induced RINm5F pancreatic
β-cells. The interaction of the underlying mechanisms remains to be
confirmed in future studies. In addition, quercetin may be more
efficacious than quercitrin as an anti-diabetic agent.
Acknowledgements
This study was part of a project sponsored by the
National Natural Science Foundation of China (NSFC) with code
H2603/81172652.
References
|
1
|
Wagner C, Vargas AP, Roos DH, et al:
Comparative study of quercetin and its two glycoside derivatives
quercitrin and rutin against methylmercury (MeHg)-induced ROS
production in rat brain slices. Arch Toxicol. 84:89–97. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hertog MG, Feskens EJ, Hollman PC, Katan
MB and Kromhout D: Dietary antioxidant flavonoids and risk of
coronary heart disease: the Zutphen Elderly Study. Lancet.
342:1007–1011. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Babujanarthanam R, Kavitha P, Mahadeva Rao
US and Pandian MR: Quercitrin a bioflavonoid improves the
antioxidant status in streptozotocin: induced diabetic rat tissues.
Mol Cell Biochem. 358:121–129. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Coskun O, Kanter M, Korkmaz A and Oter S:
Quercetin, a flavonoid antioxidant, prevents and protects
streptozotocin-induced oxidative stress and beta-cell damage in rat
pancreas. Pharmacol Res. 51:117–123. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mathis D, Vence L and Benoist C: beta-Cell
death during progression to diabetes. Nature. 414:792–798. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mandrup-Poulsen T: Apoptotic signal
transduction pathways in diabetes. Biochem Pharmacol. 66:1433–1440.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Saldeen J: Cytokines induce both necrosis
and apoptosis via a common Bcl-2-inhibitable pathway in rat
insulin-producing cells. Endocrinology. 141:2003–2010.
2000.PubMed/NCBI
|
|
8
|
Cnop M, Welsh N, Jonas JC, Jorns A, Lenzen
S and Eizirik DL: Mechanisms of pancreatic beta-cell death in type
1 and type 2 diabetes: many differences, few similarities.
Diabetes. 54(Suppl 2): S97–S107. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tsujimoto Y: Cell death regulation by the
Bcl-2 protein family in the mitochondria. J Cell Physiol.
195:158–167. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Menegazzi M, Novelli M, Beffy P, et al:
Protective effects of St. John’s wort extract and its component
hyperforin against cytokine-induced cytotoxicity in a pancreatic
beta-cell line. Int J Biochem Cell Biol. 40:1509–1521. 2008.
|
|
11
|
Adewole SO, Caxton-Martins EA and Ojewole
JA: Protective effect of quercetin on the morphology of pancreatic
beta-cells of streptozotocin-treated diabetic rats. Afr J Tradit
Complement Altern Med. 4:64–74. 2006.PubMed/NCBI
|
|
12
|
Youl E, Bardy G, Magous R, et al:
Quercetin potentiates insulin secretion and protects INS-1
pancreatic beta-cells against oxidative damage via the ERK1/2
pathway. Br J Pharmacol. 161:799–814. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cho JM, Chang SY, Kim DB, Needs PW, Jo YH
and Kim MJ: Effects of physiological quercetin metabolites on
interleukin-1beta-induced inducible NOS expression. J Nutr Biochem.
Jan 3–2012.(Epub ahead of print).
|
|
14
|
Babujanarthanam R, Kavitha P and Pandian
MR: Quercitrin, a bioflavonoid improves glucose homeostasis in
streptozotocin-induced diabetic tissues by altering glycolytic and
gluconeogenic enzymes. Fundam Clin Pharmacol. 24:357–364. 2010.
View Article : Google Scholar
|
|
15
|
Hollman PC, de Vries JH, van Leeuwen SD,
Mengelers MJ and Katan MB: Absorption of dietary quercetin
glycosides and quercetin in healthy ileostomy volunteers. Am J Clin
Nutr. 62:1276–1282. 1995.PubMed/NCBI
|
|
16
|
Rattanajarasroj S and Unchern S:
Comparable attenuation of Abeta(25–35)-induced neurotoxicity by
quercitrin and 17beta-estradiol in cultured rat hippocampal
neurons. Neurochem Res. 35:1196–1205. 2010.PubMed/NCBI
|
|
17
|
Chow JM, Shen SC, Huan SK, Lin HY and Chen
YC: Quercetin, but not rutin and quercitrin, prevention of
H2O2-induced apoptosis via anti-oxidant
activity and heme oxygenase 1 gene expression in macrophages.
Biochem Pharmacol. 69:1839–1851. 2005.PubMed/NCBI
|
|
18
|
Cai L, Li W, Wang G, Guo L, Jiang Y and
Kang YJ: Hyperglycemia-induced apoptosis in mouse myocardium:
mitochondrial cytochrome C-mediated caspase-3 activation pathway.
Diabetes. 51:1938–1948. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Veluthakal R, Jangati GR and Kowluru A:
IL-1beta-induced iNOS expression, NO release and loss in metabolic
cell viability are resistant to inhibitors of ceramide synthase and
sphingomyelinase in INS 832/13 cells. JOP. 7:593–601.
2006.PubMed/NCBI
|
|
20
|
Denis MC, Mahmood U, Benoist C, Mathis D
and Weissleder R: Imaging inflammation of the pancreatic islets in
type 1 diabetes. Proc Natl Acad Sci USA. 101:12634–12639. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Michalska M, Wolf G, Walther R and
Newsholme P: Effects of pharmacological inhibition of NADPH oxidase
or iNOS on pro-inflammatory cytokine, palmitic acid or
H2O2-induced mouse islet or clonal pancreatic
beta-cell dysfunction. Biosci Rep. 30:445–453. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pi J, Bai Y, Zhang Q, et al: Reactive
oxygen species as a signal in glucose-stimulated insulin secretion.
Diabetes. 56:1783–1791. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cumaoglu A, Ari N, Kartal M and Karasu C:
Polyphenolic extracts from Olea europea L. protect against
cytokine-induced beta-cell damage through maintenance of redox
homeostasis. Rejuvenation Res. 14:325–334. 2011.
|
|
24
|
Burkle A: Physiology and pathophysiology
of poly(ADP-ribosyl)ation. Bioessays. 23:795–806. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Allagnat F, Cunha D, Moore F, Vanderwinden
JM, Eizirik DL and Cardozo AK: Mcl-1 downregulation by
pro-inflammatory cytokines and palmitate is an early event
contributing to beta-cell apoptosis. Cell Death Differ. 18:328–337.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Barbu A, Welsh N and Saldeen J:
Cytokine-induced apoptosis and necrosis are preceded by disruption
of the mitochondrial membrane potential (Deltapsi(m)) in pancreatic
RINm5F cells: prevention by Bcl-2. Mol Cell Endocrinol. 190:75–82.
2002. View Article : Google Scholar
|
|
27
|
Mehmeti I, Lenzen S and Lortz S:
Modulation of Bcl-2-related protein expression in pancreatic beta
cells by pro-inflammatory cytokines and its dependence on the
antioxidative defense status. Mol Cell Endocrinol. 332:88–96. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lakhani SA, Masud A, Kuida K, et al:
Caspases 3 and 7: key mediators of mitochondrial events of
apoptosis. Science. 311:847–851. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Muranyi M, Fujioka M, He Q, et al:
Diabetes activates cell death pathway after transient focal
cerebral ischemia. Diabetes. 52:481–486. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Virag L, Marmer DJ and Szabo C: Crucial
role of apopain in the peroxynitrite-induced apoptotic DNA
fragmentation. Free Radic Biol Med. 25:1075–1082. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Roeske-Nielsen A, Dalgaard LT, Mansson JE
and Buschard K: The glycolipid sulfatide protects insulin-producing
cells against cytokine-induced apoptosis, a possible role in
diabetes. Diabetes Metab Res Rev. 26:631–638. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang ZJ, Cheang LC, Wang MW and Lee SM:
Quercetin exerts a neuroprotective effect through inhibition of the
iNOS/NO system and pro-inflammation gene expression in PC12 cells
and in zebrafish. Int J Mol Med. 27:195–203. 2011.PubMed/NCBI
|
|
33
|
Hirst DG and Robson T: Nitrosative stress
as a mediator of apoptosis: implications for cancer therapy. Curr
Pharm Des. 16:45–55. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Melloul D: Role of NF-kappaB in beta-cell
death. Biochem Soc Trans. 36:334–339. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ghosh S and Karin M: Missing pieces in the
NF-kappaB puzzle. Cell. 109(Suppl): S81–S96. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yamaguchi M and Weitzmann MN: Quercetin, a
potent suppressor of NF-κB and Smad activation in osteoblasts. Int
J Mol Med. 28:521–525. 2011.PubMed/NCBI
|
|
37
|
Morand C, Manach C, Crespy V and Remesy C:
Quercetin 3-O-beta-glucoside is better absorbed than other
quercetin forms and is not present in rat plasma. Free Radic Res.
33:667–676. 2000. View Article : Google Scholar : PubMed/NCBI
|