Introduction
Stroke is the second most common cause of mortality
and the leading cause of severe long-term disability in adults
worldwide, affecting approximately 15 million people each year
(1,2). Ischemic stroke accounts for
approximately 80% of all strokes, and occurs when a thrombus or
embolism blocks a major cerebral blood vessel or its branches
(3,4). This blockage eventually leads to
serious pathological changes and, possibly, to permanent impairment
of brain functions (4). To date,
thrombolysis with intravenous tissue plasminogen activator (t-PA)
is the only approved therapy for patients with acute ischemic
stroke, but only 1–2% of patients are eligible for this
thrombolytic therapy primarily due to a narrow time window for
administration (5,6). Thus, there is an urgent need to
develop novel therapeutic options for patients with ischemic
stroke.
Endothelial progenitor cells (EPCs) are a specific
subpopulation of hematopoietic stem cells capable of
differentiating into mature endothelial cells (7). EPCs are produced in the bone marrow
and mobilized into the peripheral circulation where they repair the
damaged endothelium and promote the formation of new blood vessels
(8). On the basis of their
endothelial regenerative potential, there has been a growing
interest in EPCs as novel biomarkers and potential therapeutic
agents for a variety of pathological conditions, particularly
cardiovascular diseases (9–11).
Ohta et al (12) reported
that administration of ex vivo-expanded bone marrow-derived
EPCs reduces infarct volume and neurological deficits in acute
focal brain ischemia and reperfusion (I/R) injury, possible by
attenuation of endothelial dysfunction. However, EPC studies in
stroke have been limited (13),
and the molecular mechanisms underlying the neuroprotective effects
of bone marrow-derived EPCs have not been fully elucidated.
Apoptosis is a major cellular mechanism responsible
for cell death in the pathological processes after cerebral I/R
injury (14). Oxidative stress
has been reported to occur in response to cerebral I/R injury and
is thought to be one of the major contributors to neuronal death
(15). Nuclear factor-κB (NF-κB),
an important transcriptional factor, plays a critical role in
regulating cellular responses to oxidative stress (16). In addition, previous studies have
demonstrated that NF-κB is activated during cerebral I/R injury
(17), and the inhibition of
NF-κB exhibits potent neuroprotective effects during cerebral
ischemia (18). Based on these
findings, we hypothesized that transplantation of bone
marrow-derived EPCs may attenuate cerebral I/R injury through the
inhibition of neuronal apoptosis and oxidative stress.
In the present study, we used a rat model of middle
cerebral artery occlusion (MCAO) to further investigate the
neuroprotective effects of bone marrow-derived EPCs as well as the
underlying mechanisms by focusing on neuronal apoptosis, oxidative
stress, and NF-κB expression.
Materials and methods
Animals
Male Sprague-Dawley rats, weighing 200–250 g, were
obtained from the Experimental Animal Centre of China Medical
University, Shenyang, China. The experimental protocol was reviewed
and approved by the Institutional Animal Care and Use Committee of
China Medical University. All animals were housed under diurnal
lighting conditions (22±3°C and a 12 h light/dark cycle) with free
access to standard laboratory chow and water.
Isolation and in vitro culture EPCs
Isolation and ex vivo expansion of EPCs were
performed as previously described (19,20). Briefly, femur and tibias were
excised from the donor rats after they were anesthetized with
chloral hydrate (300 mg/kg) and sacrificed by cervical dislocation.
Total bone marrow cells were harvested aseptically by flushing
femurs and tibias with phosphate buffered saline (PBS), and
mononuclear cells were collected by density gradient centrifugation
with Histopaque-1083 (Sigma-Aldrich, St. Louis, MO, USA). After
washing twice in PBS, the obtained mononuclear cells were seeded on
rat plasma fibronectin-coated dishes and maintained in EC basal
medium-2 (EBM-2) supplemented with 5% fetal bovine serum (FBS;
Invitrogen, Carlsbad, CA, USA), antibiotics, and growth factors.
Cells were cultured at 37°C in a humidified atmosphere with 5%
CO2. After 4 days of culture, the non-adherent cells
were removed by washing with PBS, and fresh culture medium was
applied. On the tenth day, the adherent cells were harvested by
trypsinization, washed, and resuspended for transplantation or
immunophenotypic characterization by flow cytometry.
Flow cytometry
To characterize the EPC population, flow cytometry
was performed on freshly isolated cells (at Day 0) and on cells
after 10 days of culture by using the antibodies against a panel of
surface markers, including CD31, CD34, CD133 and fetal liver
kinase-1 (Flk-1). Briefly, the cells were harvested, washed twice
with PBS, and then incubated for 30 min at 4°C with mouse anti-rat
antibodies for CD31 and CD34 (Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA) and rabbit anti-rat antibodies for CD133 and Flk-1
(Abnova, Taipei, Taiwan), followed by their corresponding
fluorescein isothiocyanate (FITC)-labeled secondary antibodies.
Finally, the samples were analyzed with a FACSCalibur cytometer (BD
Biosciences, San Jose, CA, USA) and data were processed using the
CellQuest software program.
EPC labeling for in vivo tracking
In order to track the fate of transplanted cells,
EPCs after 10 days of culture were labeled with a lipophilic red
fluorescent dye, CM-Dil (Invitrogen), according to the
manufacturer’s instructions. Thirty minutes before transplantation,
EPCs were incubated with 2 μl CM-Dil (1 mg/ml) at 37°C and then
resuspended in 500 μl culture medium for administration.
Experimental groups, rat model of
cerebral I/R injury and EPC administration
Fifty-four rats were randomly divided into three
groups as follows (18 rats in each group): the sham group, without
MCAO and culture medium-treated; the I/R group, 2 h occlusion
followed by 24 h reperfusion and culture medium-treated; the EPC
group, with MCAO and EPC administration.
A rat model of cerebral I/R injury was established
by MCAO as previously reported (21). In brief, after the rats were
anesthetized with 10% chloral hydrate (350 mg/kg, i.p.), the right
common carotid artery (CCA), internal carotid artery (ICA), and
external carotid artery (ECA) were surgically exposed through a
ventral middle line incision. A 4-0 nylon monofilament nylon suture
with a heat-rounded tip was introduced into the ECA lumen and
gently advanced into the ICA lumen until a slight resistance was
encountered, thus to occlude the origin of the middle cerebral
artery. Two hours later, the filament was carefully withdrawn to
restore cerebral blood flow and the neck incision was sutured. The
rectal temperature was maintained between 36.5 and 37.5°C by using
a heat pad and a heat lamp throughout the surgical procedure.
Sham-operated animals underwent the same surgical procedures but
without inserting a filament.
Labeled EPCs (106 cells in 500 μl culture
medium) were infused into the rats in the EPC group at the onset of
reperfusion and 12 h after reperfusion via the tail vein. Rats in
the sham and I/R groups were injected in the same manner but only
with an equal volume of fresh culture medium. After 24 h of
reperfusion, all animals were deeply anesthetized with chloral
hydrate (300 mg/kg) and sacrificed by decapitation.
Measurement of cerebral infarct
volume
After decapitation, the brains (n=6) were quickly
collected, chilled in ice-cold saline for 5 min, and sectioned
coronally into eight, 1.5 mm slices. The slices were stained with
2% 2,3,5-triphenyltetrazolium chloride (TTC; Sigma-Aldrich) at 37°C
for 20 min, followed by fixation in 4% paraformaldehyde overnight.
The normal tissues were stained dark red, while the infarct areas
remain unstained (white). The stained slices were photographed with
a digital camera, and the infarct area was quantified with an image
analysis system (Image-Pro Plus). The infarct volumes were
presented as percentages of the total brain volume (22).
Measurement of superoxide dismutase
(SOD), glutathione (GSH), glutathione peroxidase (GSH-PX),
malondialdehyde (MDA) and caspase-3 activities
Antioxidative enzyme activities including SOD,
GSH-PX, GSH and MDA in the hippocampus homogenate were measured by
using commercially available kits (Jiancheng Institute of
Bioengineering, Nanjing, China), according to the recommended
protocols. SOD activity was determined by monitoring its ability to
inhibit nucleotide oxidation. Results were reported as U/mg
protein. A GSH-PX unit activity was defined as the amount of enzyme
required to convert 1 μmol of reduced GSH to the oxidized form of
glutathione GSH in the presence of H2O2/min.
MDA content was determined based on its activity to react with
thiobarbituric acid and is expressed as nmol/mg protein. The
activity of caspase-3 in the hippocampus homogenate was assayed by
using a caspase-3 activity kit (Beyotime Institute of
Biotechnology, Haimen, P.R. China) following the manufacturer’s
instructions. Caspase-3 activity was estimated by measuring
Ac-DEVD-pNA reacting substances at the wavelength of 405 nm.
Immunohistochemistry
The brains were immersed in 10% buffered formalin
overnight, embedded in paraffin, and sectioned with a thickness of
5 μm. After dewaxing with xylene and rehydrating through descending
ethanol, the sections were subjected to heat-mediated antigen
retrieval in citrate buffer solution (pH 6.0). Subsequently,
endogenous peroxidase activity was inactivated using 3% (v/v)
hydrogen peroxide at room temperature for 15 min, and nonspecific
binding of antibodies was blocked with 10% normal goat serum. The
sections were then incubated overnight at 4°C in a humidifying
chamber with a rabbit anti-rat NF-κB polyclonal antibody (1:200
diluted; Santa Cruz Biotechnology, Inc.). After rinsing in PBS, the
corresponding secondary antibody [biotinylated goat anti-rabbit
immunoglobulin G (IgG), 1:200 diluted; Zhongshan Golden Bridge
Biotechnology, Beijing, China] was applied, followed by incubation
for 30 min with streptavidin-horseradish peroxidase conjugate. The
final immunoreactivity was visualized by using
3,3′-diaminobenzidine (DAB), and all sections were then
counterstained with hematoxylin, dehydrated, and mounted. For
negative controls, an isotype matched IgG was used instead of the
primary antibody.
Western blot analysis
Total proteins were extracted from the ipsilateral
ischemic cortex, and protein concentrations were quantified using
bicinchoninic acid (BCA) protein assay kit (Beyotime Institute of
Biotechnology). An equivalent amount of proteins was resolved on
sodium dodecyl sulfate polyacrylamide gels (SDS-PAGE), and then
electrotransferred onto polyvinylidene fluoride (PVDF) membranes
(Millipore, Bedford, MA, USA) under 70 V constant voltage
conditions for 90 min. After blocking with 5% fat-free milk at room
temperature for 2 h, the membranes were immunoblotted overnight at
4°C with rabbit anti-Bcl-2, anti-Bax, (1:1,000 diluted; Abcam,
Cambridge, MA, USA), or anti-NF-κB antibody (1:1,000 diluted; Santa
Cruz Biotechnology, Inc.), followed by horseradish
peroxidase-conjugated secondary antibodies. Following extensive
washing, immunoblots were visualized with an enhanced
chemiluminescence (ECL) detection kit (Millipore). The intensity of
the bands was quantified by densitometric analysis and normalized
to the loading control β-actin.
Statistical analysis
All data are expressed as the means ± standard
deviation (SD). Statistical analysis was performed using one-way
ANOVA followed by the Bonferroni post-hoc test for individual
comparisons between group means. Graphs were plotted and
statistical calculations were carried out using SigmaPlot 12.0
(Systat Software, Inc., San Jose, CA, USA). A P-value <0.05 was
considered to indicate statistically significant differences.
Results
In vitro characteristics of bone marrow
EPCs after 10-day culture
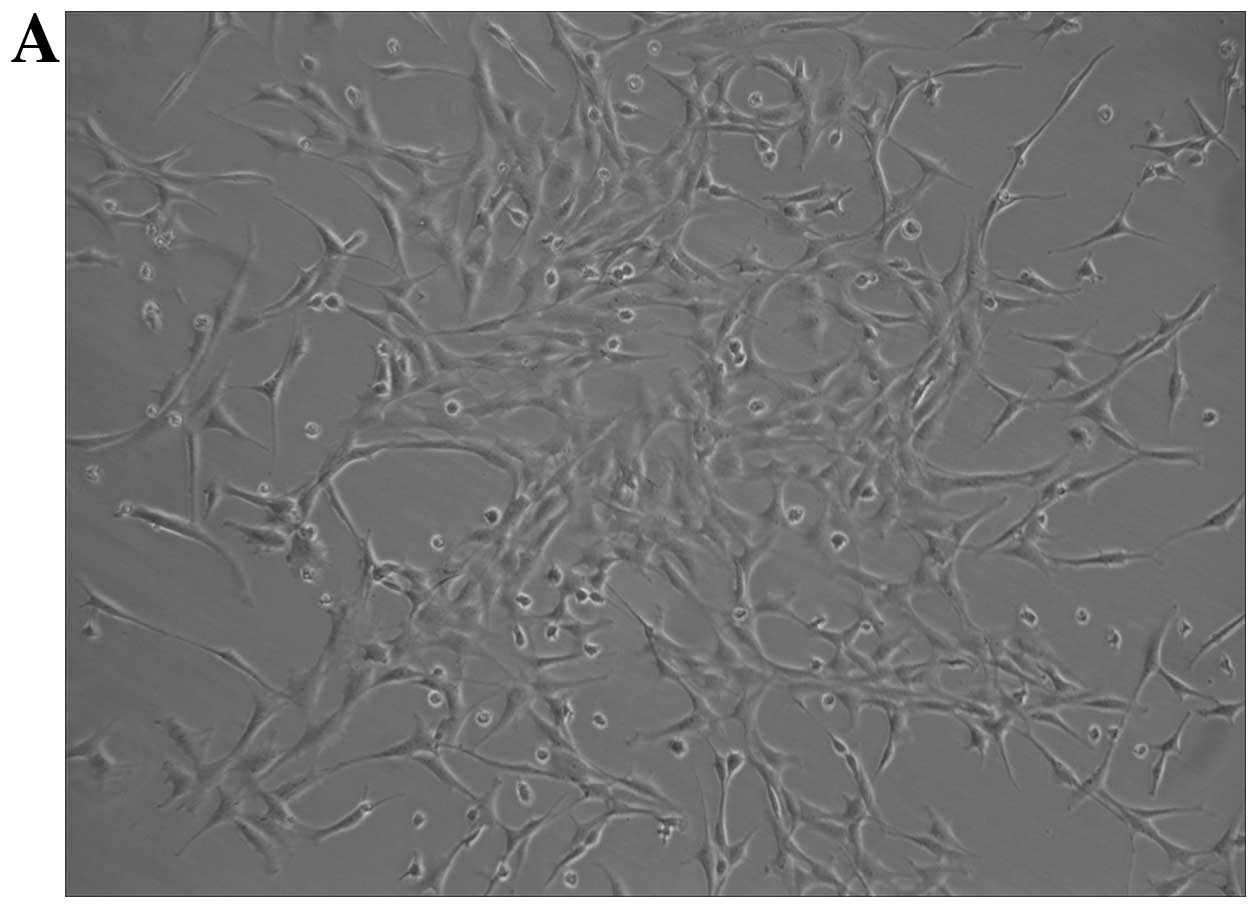
After 10 days of culture in EC basal medium, the
bone marrow-derived mononuclear cells exhibited spindle-shaped
morphology or cobblestone-like appearance typical of endothelial
cells (Fig. 1A). To further
characterize these cells, the stem cell markers (CD34 and CD133)
and endothelial cell markers (CD31 and Flk-1) were analyzed by flow
cytometry according to previous studies (23,24). Compared with freshly isolated
mononuclear cells, the percentages of cell population positive for
CD34, CD133, CD31 and Flk-1 were increased from 22.30±2.56 to
70.81±4.89%, 19.5±2.95 to 81.66±5.33%, 10.83±1.12 to 46.81±3.54%
and 12.94±1.87 to 60.05±3.91%, respectively (Fig. 1B). These cells were therefore
confirmed as bone marrow-derived EPCs.
Effects of EPC transplantation on brain
infarct volume
To investigate the neuroprotective effects of EPC
transplantation on cerebral I/R injury, EPCs (106 cells)
were administered intravenously at the onset of reperfusion and 12
h after reperfusion, and infarct volumes were assessed at 24 h
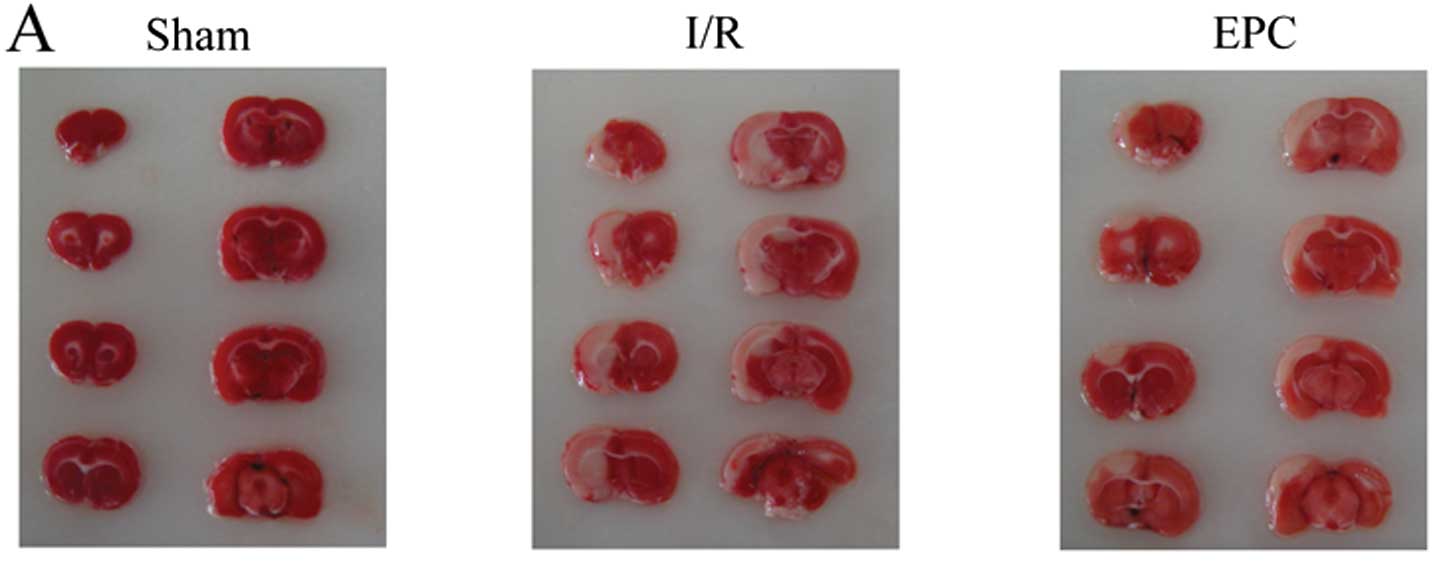
after reperfusion by TTC staining. As shown in Fig. 2A and B, no infarction was observed
in sham-operated group animals. By contrast, the infarct volume was
28.9±1.8% after 2 h MCAO and 24 h reperfusion in the I/R group.
However, EPC transplantation significantly reduced cerebral infarct
volumes, indicating neuroprotective effects of EPC transplantation
against cerebral I/R injury. Moreover, under immunofluorescence
microscopy, a significant number of CM-Dil-labeled EPCs showing red
fluorescence adoptively transferred into the infarct area,
suggesting that EPCs can migrate into the ischemic rat brain within
24 h after intravenous injection.
Effects of EPC transplantation on the
expression of Bcl-2 and Bax proteins and caspase-3 activity in
hippocampus after I/R injury
To test if EPC transplantation exerts its
neuroprotective effects against cerebral I/R injury via the
inhibition of neuronal apoptosis, we detected the expression of
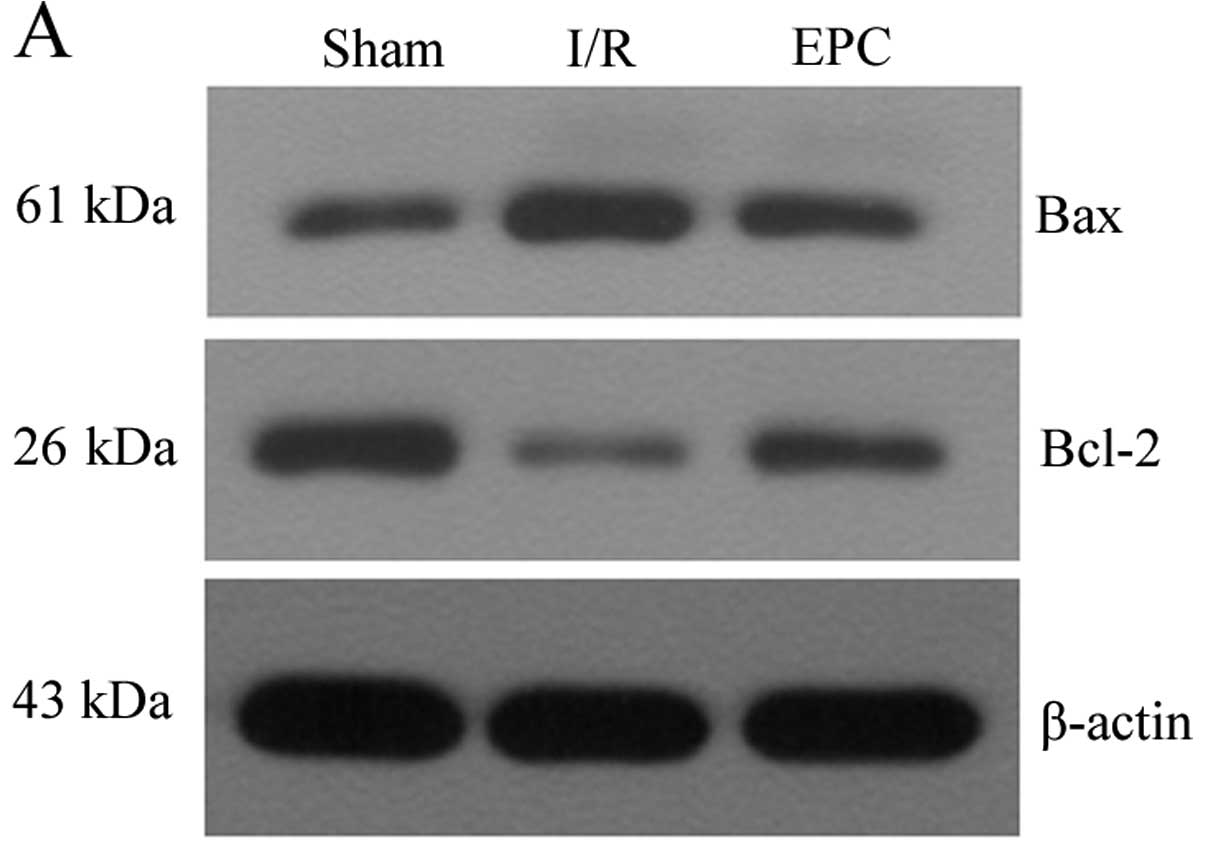
Bcl-2 and Bax protein in the ischemic penumbra of rats with 2 h
MCAO and 24 h reperfusion by western blot analysis. The results
showed that cerebral I/R injury induced by MCAO caused significant
upregulation of Bax and downregulation of Bcl-2 protein levels;
however, these changes were remarkably attenuated by EPC
transplantation (P<0.05, n=6) (Fig. 3A and B). We also measured the
enzymatic activity of caspase-3, a reliable indicator for
assessment of apoptosis. The activity of caspase-3 in the ischemic
penumbra of rats with 2 h MCAO and 24 h reperfusion was
significantly increased compared with the sham group, while the
caspase-3 activity was markedly reduced in the EPC group
(P<0.05, n=6) (Fig. 3C). These
findings suggest that EPC transplantation has the potential to
protect hippocampal neurons against cerebral I/R injury-induced
apoptosis.
Effects of EPC transplantation on the
content of MDA and the activities of antioxidant enzymes in
hippocampus after I/R injury
To further determine whether the neuroprotective
effects of EPC transplantation are associated with the inhibition
of oxidative stress, we evaluated the content of MDA and the
activities of antioxidant enzymes in the ischemic penumbra of rats
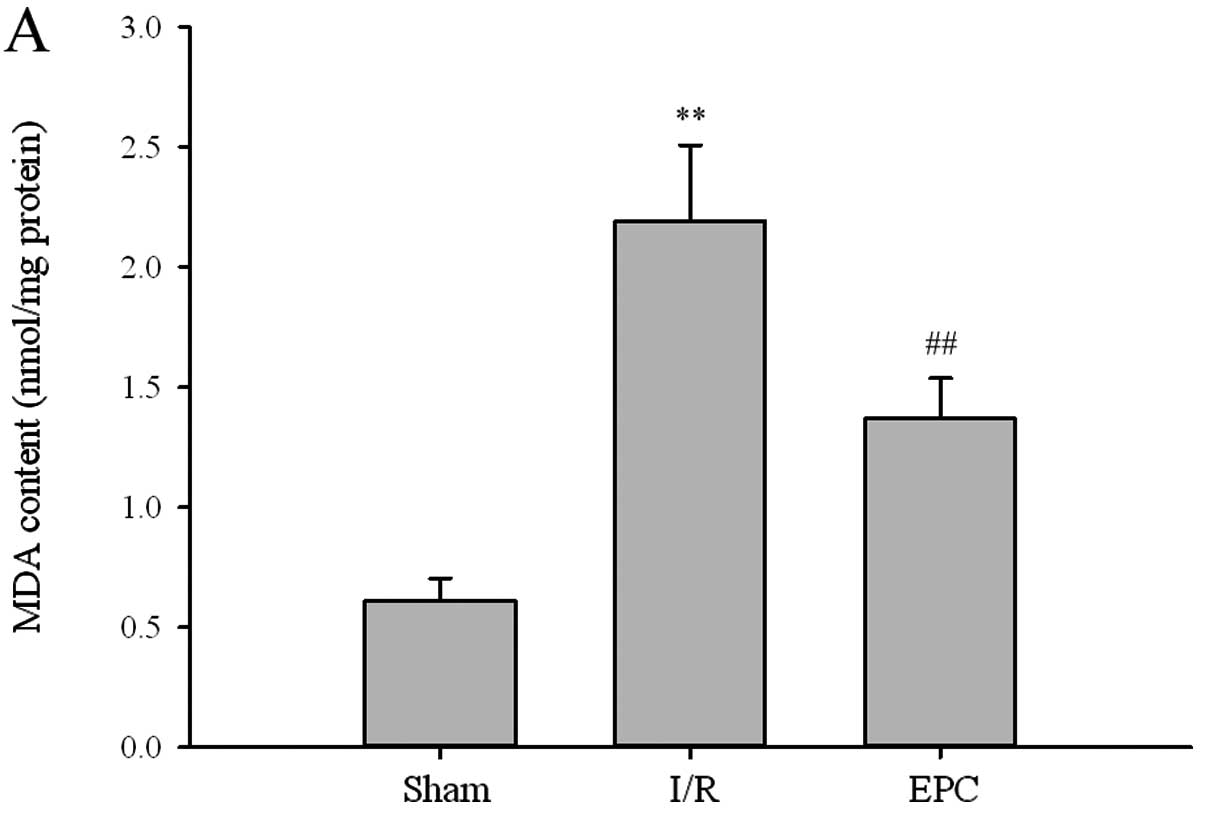
with 2 h MCAO and 24 h reperfusion. As illustrated in Fig. 4A, the level of MDA, an index of
lipid peroxidation, was significantly increased in culture
medium-treated ischemic hippocampus from 0.61±0.09 to 2.19±0.32
nmol/mg protein (P<0.01, n=6). After administration of EPCs, the
I/R-induced lipid peroxidation was evidently decreased to 1.37±0.17
nmol/mg protein (P<0.01, n=6) compared to the I/R group.
Additionally, the activities of antioxidant enzymes including GSH,
GSH-PX and SOD in the I/R group were remarkably diminished compared
to the sham group (P<0.01, n=6). However, EPC transplantation
resulted in enhanced activities compared to the I/R group
(P<0.05, n=6). Collectively, our data indicate that
administration of EPCs exerts neuroprotective effects via its
ability to inhibit oxidative stress.
Effects of EPC transplantation on NF-κB
expression in hippocampus after I/R injury
Next, we analyzed the effects of EPC transplantation
on NF-κB expression in hippocampus after I/R injury by
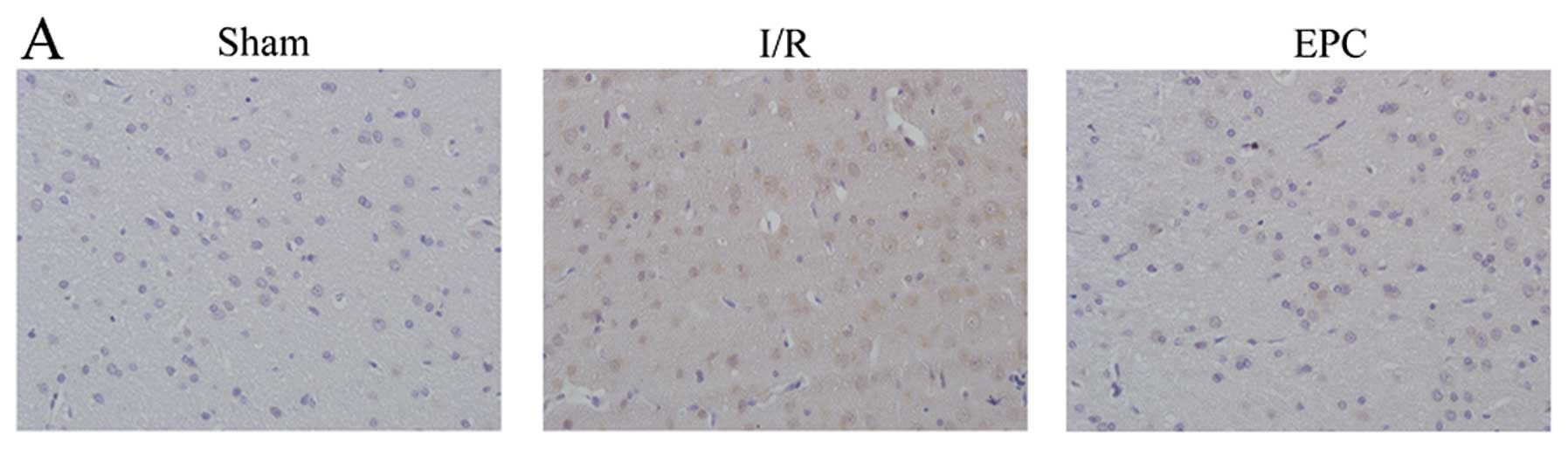
immunohistochemistry and western blot analysis. Few NF-κB positive
cells were observed in the hippocampus of sham-operated animals
(Fig. 5A). At 24 h after
reperfusion, an intense staining of NF-κB was found in the nucleus
in the ischemic hippocampus. However, EPC implantation
significantly attenuated the rise of brain I/R-induced NF-κB
expression. Meanwhile, changes observed by western blot analysis
were in accordance with the observations in the immunohistochemical
staining (Fig. 5B and C). These
findings demonstrate that EPC transplantation is capable of
inhibiting brain I/R injury-induced NF-κB activation.
Discussion
The intraluminal suture method of MCAO in rodents is
a well-characterized and classical animal model to mimic the
pathological features of human cerebral I/R injury and has been
widely used in pharmacological and molecular studies of brain I/R
injury (4). In the present study,
we used the MCAO animal model and demonstrated that EPC
transplantation protected against cerebral I/R injury by reducing
infarct volume, which is associated with the inhibition of neuronal
apoptosis, oxidative stress and NF-κB expression.
Despite extensive research efforts to standardize
the definitions of EPCs, the exact definition and molecular
characterization of EPCs is an ongoing debate (11). EPCs were initially identified by
Asahara et al (7) as
CD34-positive mononuclear cells from adult peripheral blood. It is
now known that EPCs migrate from the bone marrow to the peripheral
blood as circulating EPCs, where they express CD133, CD34 and Flk-1
(25). Currently, the most
commonly accepted definition of EPCs includes co-expression of
CD31, CD133, CD34 and Flk-1 (9).
In this study, the molecular characteristics of EPCs isolated and
expanded from bone marrow were similar to those described in
previous reports (10). EPC
administration significantly attenuated cerebral I/R injury, and
fluorescent-labeled EPCs were observed in the hippocampus of the
ischemia-affected hemisphere. These results are in agreement with
the findings from Ohta et al (12). Notably, the discovery rate of EPCs
was relatively low in the parenchyma of the ischemic hemisphere.
Our data suggest that EPCs may exert neuroprotective benefits via
their paracrine effective in addition to neovascularization.
A large body of evidence suggests that neuronal
apoptosis represents an important mechanism in the
pathophysiological mode of cell death in ischemic brain injury
(14). Caspase-3 plays a critical
role in apoptotic cell death, and the inhibition of caspase-3 has
been shown to ameliorate cerebral ischemic injury (26). In the current study, we
consistently found a marked increase in caspase-3 activity in the
ischemic rat penumbra, which could be effectively dampened by
administration of EPCs. Bcl-2 is an anti-apoptotic and Bax is a
pro-apoptotic protein belonging to the Bcl-2 family. They serve as
key regulators at the early stage of apoptosis (27). Our results demonstrated that I/R
injury increased Bax expression and decreased Bcl-2 expression in
the penumbra cortex after cerebral I/R injury. However, our
findings further revealed that EPC administration significantly
reversed these expression changes, indicating that EPCs may protect
neurons from apoptosis. These results are consistent with previous
findings showing that peripheral blood or bone marrow-derived EPCs
can release various potent inhibitors of apoptosis (28,29). In addition, several reports have
also shown that transplantation of EPCs can inhibit cardiac
apoptosis in rat models of myocardial infarction (30) and diabetic cardiomyopathy
(31). Collectively, our
observations demonstrated that EPC implantation is able to prevent
neuronal apoptosis induced by I/R injury.
It is well known that a cascade of pathological
events, such as oxidative stress, inflammatory responses, neuronal
apoptosis and brain damage, occurs within minutes after the onset
of cerebral ischemia (4).
Enhanced generation of reactive oxygen species (ROS) is considered
an important contributor to neuronal damage during ischemic insult.
Under normal physiological conditions, the basal amounts of ROS can
be rapidly scavenged by a system of endogenous antioxidant
defenses, such as SOD, GSH-PX and GSH. However, the relative
reduced capability of the antioxidant during cerebral ischemia
leads to an excess of ROS production and subsequent neuronal damage
(32). The level of MDA, an
indicator of oxidative stress, is also increased during cerebral
I/R injury. NF-κB plays a critical role in regulating cellular
responses to oxidative stress and has been shown to be activated
during cerebral I/R injury (17).
The present study showed that the marked reduction of MDA and NF-κB
expression and the significant elevation of SOD, GSH as well as
GSH-PX were observed after transplantation of EPCs to
ischemia-induced rats. Collectively, the neuroprotective effects of
EPC transplantation against brain I/R injury is, at least in part,
related to its antioxidant property.
In conclusion, the present study demonstrated that
transplantation of bone marrow EPCs exerts potent neuroprotective
functions against cerebral I/R injury in rats, and the protective
effects may be partly due to its antioxidative and anti-apoptotic
properties. Therefore, transplantation of bone marrow EPCs may
provide a novel therapeutic strategy for the treatment of ischemic
stroke.
Abbreviations:
|
EPC
|
endothelial progenitor cell
|
|
I/R
|
ischemia/reperfusion
|
|
MCAO
|
middle cerebral artery occlusion
|
|
TTC
|
triphenyltetrazolium chloride
|
|
SOD
|
superoxide dismutase
|
|
GSH-PX
|
glutathione peroxidase
|
|
GSH
|
glutathione
|
|
MDA
|
malondialdehyde
|
|
NF-κB
|
nuclear factor-κB
|
References
|
1
|
Feigin VL: Stroke epidemiology in the
developing world. Lancet. 365:2160–2161. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Donnan GA, Fisher M, Macleod M and Davis
SM: Stroke. Lancet. 371:1612–1623. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Feigin VL, Lawes CM, Bennett DA and
Anderson CS: Stroke epidemiology: a review of population-based
studies of incidence, prevalence, and case-fatality in the late
20th century. Lancet Neurol. 2:43–53. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Durukan A and Tatlisumak T: Acute ischemic
stroke: overview of major experimental rodent models,
pathophysiology, and therapy of focal cerebral ischemia. Pharmacol
Biochem Behav. 87:179–197. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kwiatkowski TG, Libman RB, Frankel M, et
al: Effects of tissue plasminogen activator for acute ischemic
stroke at one year. National Institute of Neurological Disorders
and Stroke Recombinant Tissue Plasminogen Activator Stroke Study
Group. N Engl J Med. 340:1781–1787. 1999.
|
|
6
|
Ginsberg MD: Neuroprotection for ischemic
stroke: past, present and future. Neuropharmacology. 55:363–389.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Asahara T, Murohara T, Sullivan A, et al:
Isolation of putative progenitor endothelial cells for
angiogenesis. Science. 275:964–967. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rafii S and Lyden D: Therapeutic stem and
progenitor cell transplantation for organ vascularization and
regeneration. Nat Med. 9:702–712. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Allegra A, Coppolino G, Bolignano D, et
al: Endothelial progenitor cells: pathogenetic role and therapeutic
perspectives. J Nephrol. 22:463–475. 2009.PubMed/NCBI
|
|
10
|
Sen S, McDonald SP, Coates PT and Bonder
CS: Endothelial progenitor cells: novel biomarker and promising
cell therapy for cardiovascular disease. Clin Sci (Lond).
120:263–283. 2011.PubMed/NCBI
|
|
11
|
Grisar JC, Haddad F, Gomari FA and Wu JC:
Endothelial progenitor cells in cardiovascular disease and chronic
inflammation: from biomarker to therapeutic agent. Biomark Med.
5:731–744. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ohta T, Kikuta K, Imamura H, et al:
Administration of ex vivo-expanded bone marrow-derived endothelial
progenitor cells attenuates focal cerebral ischemia-reperfusion
injury in rats. Neurosurgery. 59:679–686. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rouhl RP, van Oostenbrugge RJ, Damoiseaux
J, Tervaert JW and Lodder J: Endothelial progenitor cell research
in stroke: a potential shift in pathophysiological and
therapeutical concepts. Stroke. 39:2158–2165. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Broughton BR, Reutens DC and Sobey CG:
Apoptotic mechanisms after cerebral ischemia. Stroke. 40:e331–e339.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Song YS, Narasimhan P, Kim GS, Jung JE,
Park EH and Chan PH: The role of Akt signaling in oxidative stress
mediates NF-kappaB activation in mild transient focal cerebral
ischemia. J Cereb Blood Flow Metab. 28:1917–1926. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Denk A, Wirth T and Baumann B: NF-kappaB
transcription factors: critical regulators of hematopoiesis and
neuronal survival. Cytokine Growth Factor Rev. 11:303–320. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schneider A, Martin-Villalba A, Weih F,
Vogel J, Wirth T and Schwaninger M: NF-kappaB is activated and
promotes cell death in focal cerebral ischemia. Nat Med. 5:554–559.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Desai A, Singh N and Raghubir R:
Neuroprotective potential of the NF-kappaB inhibitor peptide
IKK-NBD in cerebral ischemia-reperfusion injury. Neurochem Int.
57:876–883. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang SJ, Zhang H, Hou M, et al: Is it
possible to obtain ‘true endothelial progenitor cells’ by in vitro
culture of bone marrow mononuclear cells? Stem Cells Dev.
16:683–690. 2007.
|
|
20
|
Timmermans F, Plum J, Yoder MC, Ingram DA,
Vandekerckhove B and Case J: Endothelial progenitor cells: identity
defined? J Cell Mol Med. 13:87–102. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Longa EZ, Weinstein PR, Carlson S and
Cummins R: Reversible middle cerebral artery occlusion without
craniectomy in rats. Stroke. 20:84–91. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Belayev L, Busto R, Zhao W, Fernandez G
and Ginsberg MD: Middle cerebral artery occlusion in the mouse by
intraluminal suture coated with poly-L-lysine: neurological and
histological validation. Brain Res. 833:181–190. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hirschi KK, Ingram DA and Yoder MC:
Assessing identity, phenotype, and fate of endothelial progenitor
cells. Arterioscler Thromb Vasc Biol. 28:1584–1595. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Devanesan AJ, Laughlan KA, Girn HR and
Homer-Vanniasinkam S: Endothelial progenitor cells as a therapeutic
option in peripheral arterial disease. Eur J Vasc Endovasc Surg.
38:475–481. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu Q: Progenitor cells in vascular repair.
Curr Opin Lipidol. 18:534–539. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li J, Han B, Ma X and Qi S: The effects of
propofol on hippocampal caspase-3 and Bcl-2 expression following
forebrain ischemia-reperfusion in rats. Brain Res. 1356:11–23.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jeong HS, Choi HY, Choi TW, et al:
Differential regulation of the antiapoptotic action of B-cell
lymphoma 2 (Bcl-2) and B-cell lymphoma extra long (Bcl-xL) by c-Jun
N-terminal protein kinase (JNK) 1-involved pathway in neuroglioma
cells. Biol Pharm Bull. 31:1686–1690. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kinnaird T, Stabile E, Burnett MS, et al:
Marrow-derived stromal cells express genes encoding a broad
spectrum of arteriogenic cytokines and promote in vitro and in vivo
arteriogenesis through paracrine mechanisms. Circ Res. 94:678–685.
2004. View Article : Google Scholar
|
|
29
|
Urbich C, Aicher A, Heeschen C, et al:
Soluble factors released by endothelial progenitor cells promote
migration of endothelial cells and cardiac resident progenitor
cells. J Mol Cell Cardiol. 39:733–742. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sen S, Merchan J, Dean J, et al:
Autologous transplantation of endothelial progenitor cells
genetically modified by adeno-associated viral vector delivering
insulin-like growth factor-1 gene after myocardial infarction. Hum
Gene Ther. 21:1327–1334. 2010. View Article : Google Scholar
|
|
31
|
Cheng Y, Guo S, Liu G, et al:
Transplantation of bone marrow-derived endothelial progenitor cells
attenuates myocardial interstitial fibrosis and cardiac dysfunction
in streptozotocin-induced diabetic rats. Int J Mol Med. 30:870–876.
2012.
|
|
32
|
Allen CL and Bayraktutan U: Oxidative
stress and its role in the pathogenesis of ischaemic stroke. Int J
Stroke. 4:461–470. 2009. View Article : Google Scholar : PubMed/NCBI
|