Introduction
Atrial fibrillation (AF) is the most common form of
cardiac arrhythmia encountered in clinical practice and the main
cause of arrhythmia-related hospitalizations, accounting for
approximately 1/3 of hospitalizations for heart rhythm disorders
(1). The prevalence of AF is
estimated to be 1% in the general population, and it increases
strikingly as the population ages, with a prevalence of
approximately 0.1% in individuals younger than 55 years of age,
roughly 4% among those over 60 years and nearly 10% in those aged
80 years and older (2). According
to the Framingham Heart Study, the lifetime risk of developing AF
is at least 25% for subjects who have reached the age of 40
(3). AF is associated with
substantially increased cardiovascular morbidity and mortality; it
increases the risk of stroke by 3 to 5-fold, imposing a large
economic burden on national healthcare systems around the world and
a deleterious impact on the quality of life of patients (4). The risk of cerebrovascular
thromboembolism ascribed to AF also increases abruptly with
advancing age, rising from 1.5% at age 50–59 years up to 23.5% at
age 80–89 years (4). AF also
independently increases the risk of congestive heart failure and
the risk of mortality by 1.5 to 2-fold compared with cases in sinus
rhythm (5). Additionally, AF is
responsible for complications such as adverse hemodynamics, reduced
exercise capacity, impaired cognitive function or dementia and
tachycardia-induced cardiomyopathy (6). AF has traditionally been regarded as
an acquired disease secondary to miscellaneous cardiac or systemic
conditions, including hypertension, coronary artery disease,
congenital heart disease, rheumatic heart disease, chronic
pulmonary heart disease, cardiomyopathy, cardiac surgery,
obstructive sleep apnea, diabetes mellitus, hyperthyroidism and
electrolyte imbalance (1).
However, in 30–45% of AF patients, no established risk factors are
identified by routine procedures, and such AF is defined as
‘idiopathic’ or ‘lone’ (1), of
which at least 15% have a positive family history, so termed
familial AF (7). Growing evidence
has documented the familial aggregation of AF and an enhanced
susceptibility to AF in the close relatives of patients with AF,
indicating that hereditary defects may play an important role in
the pathogenesis of AF in a subset of patients (8–14).
Genome-wide linkage analysis with polymorphic genetic markers
mapped multiple susceptibility loci for AF on human chromosomes
10q22, 6q14–16, 11p15.5, 5p13, 10p11-q21 and 5p15, of which
AF-causing mutations in 2 genes, KCNQ1 on chromosome 11p15.5
and NUP155 on chromosome 5p13, were identified and
functionally characterized (15–21). Additionally, a genetic scan of
candidate genes revealed a long list of AF associated genes,
including KCNE2, KCNE3, KCNE5, KCNH2, KCNJ2, KCNA5, SCN5A,
SCN1B, SCN2B, SCN3B, NPPA, GJA1 and GJA5 (22–37). Nevertheless, AF is a genetically
heterogeneous disease and the genetic determinants for AF in a
large proportion of patients remain unclear.
Emerging evidence underscores the crucial role for
several transcription factors, including NKX2-5, GATA4 and GATA6,
in the proper cardiogenesis (38–40) and mutations in these genes have
been causally linked to congenital cardiovascular anomalies and AF
(41–56). GATA5 is another member of the GATA
family and its expression and function overlap with those of GATA4
and GATA6 during cardiac development, particularly in the
regulation of target gene expression synergistically with NKX2-5
(57,58), suggesting the potential
association of functionally compromised GATA5 with AF.
To assess the prevalence of GATA5 mutations
in patients with lone AF and to explore the mechanism by which
mutated GATA5 causes or confers susceptibility to AF, the
coding exons and exon/intron boundaries of GATA5 were
sequenced in patients with lone AF in contrast to control
individuals and the functional effect of the mutant GATA5 was
characterized in comparison with its wild-type counterpart using a
luciferase reporter assay system.
Materials and methods
Study population
A cohort of 118 unrelated patients with lone AF was
identified among the Han Chinese population in China. The available
relatives of the index patients were enrolled and a total of 200
ethnically-matched unrelated healthy individuals were recruited as
controls. Peripheral venous blood specimens were prepared and
clinical data including medical records, electrocardiogram and
echocardiography reports were collected. The study subjects were
clinically classified using a consistently applied set of
definitions (7,53). Briefly, diagnosis of AF was made
by a standard 12-lead electrocardiogram demonstrating no P waves
and irregular R-R intervals regardless of clinical symptoms. Lone
AF was defined as AF occurring in individuals <60 years of age
without other cardiac or systemic diseases by physical examination,
electrocardiogram, transthoracic echocardiogram and extensive
laboratory tests. Familial AF was defined as the presence of
documented lone AF in 2 or more first- or second-degree relatives.
Relatives with AF occurring at any age in the setting of structural
heart disease (hypertensive, ischemic, myocardial or valvular) were
classified as ‘undetermined’ for having an inherited form of AF.
The ‘undetermined’ classification was also used if documentation of
AF on an electrocardiogram tracing was lacking in relatives with
symptoms consistent with AF (palpitations, dyspnea and
light-headedness), or if a screening electrocardiogram and
echocardiogram were not performed, irrespective of the symptoms.
Relatives were classified as ‘unaffected’ if they were asymptomatic
and had a normal electrocardiogram. Paroxysmal AF was defined as AF
lasting >30 sec that terminated spontaneously. Persistent AF was
defined as AF lasting >7 days and requiring either
pharmacological therapy or electrical cardioversion for
termination. AF that was refractory to cardioversion or that was
allowed to continue was classified as permanent. The study protocol
was reviewed and approved by the local institutional ethics
committee and written informed consent was obtained from all
research participants prior to conducting investigation.
Genotyping
Genomic DNA from all participants was extracted from
blood lymphocytes with the Wizard® Genomic DNA
Purification kit (Promega Corporation, Madison, WI, USA).
Initially, the whole coding sequence and splice junctions of the
GATA5 gene were screened in 118 unrelated patients with lone
AF. Subsequently, genotyping GATA5 in the available
relatives of the index patient carrying an identified mutation and
in the 200 ethnically-matched unrelated healthy individuals used as
controls was performed. The referential genomic DNA sequence of
GATA5 was derived from GenBank (accession no. HM015595).
With the assistance of online Primer3 software (http://frodo.wi.mit.edu), the primer pairs used to
amplify the coding exons (exons 2–7) and intron-exon boundaries of
GATA5 by polymerase chain reaction (PCR) were designed as
shown in Table I. PCR was carried
out using HotStar Taq DNA Polymerase (Qiagen, Hilden, Germany) on a
PE 9700 Thermal Cycler (Applied Biosystems, Foster City, CA, USA)
with standard conditions and concentrations of reagents. Amplified
products were purified with the QIAquick Gel Extraction kit
(Qiagen). Both strands of each PCR product were sequenced with a
BigDye® Terminator v3.1 Cycle Sequencing kit (Applied
Biosystems) under an ABI PRISM 3130XL DNA analyzer (Applied
Biosystems). The sequencing primers were those designed previously
for specific region amplifications. DNA sequences were viewed and
analyzed with the DNA Sequencing Analysis Software v5.1 (Applied
Biosystems). The variant was validated by resequencing of an
independent PCR-generated amplicon from the subject and met our
quality control threshold with a call rate exceeding 99%.
 | Table IThe intronic primers used to amplify
the coding exons and exon-intron boundaries of GATA5. |
Table I
The intronic primers used to amplify
the coding exons and exon-intron boundaries of GATA5.
| Exon | Forward primer
(5′→3′′) | Reverse primer
(5′→3′) | Amplicon (bp) |
|---|
| 2 | GGC ATA AGC TCG GGC
GCT GG | TGG GCC CCG AGA CTG
TGG AG | 648 |
| 3 | TGA CGA AAG CCG CCA
GGC TC | CCC CAG GGG CTC TGG
TGT CA | 375 |
| 4 | CCG CAA GGC CGA CCT
GAG TC | CCG CTC CTC CCC AGC
CTC TT | 312 |
| 5 | GGG AAT CCA GCT CCA
CGG GC | CTG GAG GCA CCG AAG
GCC AC | 331 |
| 6 | GCC TGC GGT GTG ACC
GTG AG | GGT GTG TCC AGC CCA
CCT GC | 370 |
| 7 | CCC CCA TGC CAT TCC
AGG GC | GGG GCC TGC TGG TCT
CTG CT | 402 |
Alignment of multiple GATA5 protein
sequences across species
The multiple GATA5 protein sequences across various
species were aligned using an online program MUSCLE, version 3.6
(http://www.ncbi.nlm.nih.gov/).
Construction of recombinant
pcDNA3.1-hGATA5 expression plasmid
Human fetal cardiac tissue specimens were previously
collected and preserved in RNAlater RNA Stabilization Reagent
(Qiagen). Total-RNA was prepared using an RNeasy Protect Mini kit
(Qiagen). Reverse transcription was performed with
Oligo(dT)20 primer using SuperScript III reverse
transcriptase (Invitrogen Life Technologies, Carlsbad, CA, USA).
The full-length wild-type cDNA of the human GATA5 gene,
including partial 5′- and 3′-untranslated regions, was PCR
amplified using pfuUltra high-fidelity DNA polymerase (Stratagene,
La Jolla, CA, USA). The primer pairs used for the specific
amplification of the GATA5 transcript were: forward, 5′-GTA
GCT AGC CAC CGC CGT GCC CTG CCG-3′ and reverse, 5′-GAT GCG GCC GCT
GTT CCC CTG ACA TGG GC-3′. The PCR fragment with a length of 1,296
base pairs was doubly digested by endonuclease NheI and
NotI. The digested product was fractionated by 1.5% agarose
gel electrophoresis, purified with the QIAquick Gel Extraction kit
(Qiagen) and then subcloned into pcDNA3.1 (Promega Corporation) to
form a eukaryotic expression vector, pcDNA3.1-hGATA5.
Site-directed mutagenesis
The identified mutation was introduced into the
wild-type GATA5 using a QuikChange II XL Site-Directed
Mutagenesis kit (Stratagene) with a complementary pair of primers.
The mutant was sequenced to confirm the desired mutation and to
exclude any other sequence variations. The 4 pairs of primers used
to confirm the mutant clone were: primer 1, forward, 5′-TAA TAC GAC
TCA CTA TAG GG-3′ and reverse, 5′-TGG TAG GCA CTG CCG TCT, CG-3′
(product size, 443 bp); primer 2, forward, 5′-CCT TCC CTT TCG CGC
ACA GC-3′ and reverse, 5′-CGA GGA CAG GCG CTT CTG AG-3′ (product
size, 431 bp); primer 3, forward, 5′-GCA ATG CCT GCG GCC TCT AC-3′
and reverse, 5′-GAG CTG TCA GTG CTG GCG AC-3′ (product size, 340
bp); primer 4, forward, 5′-CCA GAC ACG GAA GCG GAA GC-3′ and
reverse, 5′-CCT CGA CTG TGC CTT CTA-3′ (product size, 426 bp).
Reporter gene assays
The atrial natriuretic factor (ANF)-luciferase
reporter gene, which contains the 2600-bp 5′-flanking region of the
ANF gene, namely ANF(-2600)-Luc, was kindly provided by Dr
Ichiro Shiojima, from the Department of Cardiovascular Science and
Medicine, Chiba University Graduate School of Medicine, Chiba,
Japan. HEK-293 cells were cultured in Dulbecco’s modified Eagle’s
medium supplemented with 10% fetal calf serum. The ANF(-2600)-Luc
reporter construct and an internal control reporter plasmid pGL4.75
(hRluc/CMV; Promega Corporation) were used in transient
transfection assays to examine the transcriptional activation
function of the GATA5 mutant. HEK-293 cells were transfected
with 0.4 μg of wild-type or mutant pcDNA3.1-hGATA5 expression
vector, 0.4 μg of ANF(-2600)-Luc reporter construct and 0.04 μg of
pGL4.75 control reporter vector using PolyFect Transfection Reagent
(Qiagen). For co-transfection experiments, 0.2 μg of wild-type
pcDNA3.1-hGATA5, 0.2 μg of mutant pcDNA3.1-hGATA5, 0.4 μg of
ANF(-2600)-Luc and 0.04 μg of pGL4.75 were used. Firefly luciferase
and Renilla luciferase activities were measured with the
Dual-Glo® luciferase assay system (Promega Corporation)
48 h after transfection. A minimum of 3 independent experiments
were performed for wild-type and mutant GATA5.
Statistical analysis
Data are expressed as the means ± SD. Continuous
variables were tested for normality of distribution and Student’s
unpaired t-test was used for comparison of numeric variables
between 2 groups. Comparison of the categorical variables between 2
groups was performed using Pearson’s χ2 test or Fisher’s
exact test when appropriate. A two-tailed P-value <0.05 was
considered to indicate statistically significant differences.
Results
Characteristics of the study
subjects
A total of 118 unrelated patients with lone AF and a
cohort of 200 ethnically-matched unrelated healthy individuals used
as controls were enrolled and clinically evaluated. None of them
had overt traditional risk factors for AF. There were no
significant differences between patient and control groups in
baseline characteristics including age, gender, body mass index,
blood pressure, fasting blood glucose, serum lipid, left atrial
dimension, left ventricular ejection fraction, heart rate at rest,
as well as life style (data not shown). At the time of the present
study, 8 patients were also diagnosed with hypertension in
accordance with the criterion that the average systolic or
diastolic blood pressure (2 readings made after 5 min of rest in
the sitting position) was ≥140 or ≥90 mm Hg, respectively, but at
the time of initial diagnosis of AF, their blood pressures were
normal. The baseline clinical characteristics of the 118 patients
with lone AF are summarized in Table
II.
 | Table IIBaseline clinical characteristics of
the 118 patients with lone atrial fibrillation. |
Table II
Baseline clinical characteristics of
the 118 patients with lone atrial fibrillation.
| Parameter | No. or
quantity | Percentage or
range |
|---|
| Male | 65 | 55 |
| Age at first
diagnosis of atrial fibrillation (years) | 52.84 | 32–59 |
| Type of atrial
fibrillation at presentation |
| Paroxysmal | 82 | 69 |
| Persistent | 22 | 19 |
| Permanent | 14 | 12 |
| Positive family
history of atrial fibrillation | 35 | 30 |
| History of
cardioversion | 38 | 32 |
| History of cardiac
pacemaker | 6 | 5 |
| Resting heart rate
(bpm) | 78.31 | 52–176 |
| Systolic blood
pressure (mmHg) | 125.73 | 95–138 |
| Diastolic blood
pressure (mmHg) | 85.02 | 70–88 |
| Body mass index
(kg/m2) | 22.38 | 20–24 |
| Left atrial
diameter (mm) | 38.17 | 28–42 |
| Left ventricular
ejection fraction (%) | 64 | 50–78 |
| Fasting blood
glucose (mmol/l) | 4.40 | 4–6 |
| Total cholesterol
(mmol/l) | 3.52 | 3–5 |
| Triglycerides
(mmol/l) | 1.33 | 1–2 |
| Medications |
| Amiodarone | 89 | 75 |
| Aspirin | 24 | 20 |
| Warfarin | 72 | 61 |
| Beta-blocker | 15 | 13 |
| Calcium channel
blocker | 12 | 10 |
| Digoxin | 48 | 41 |
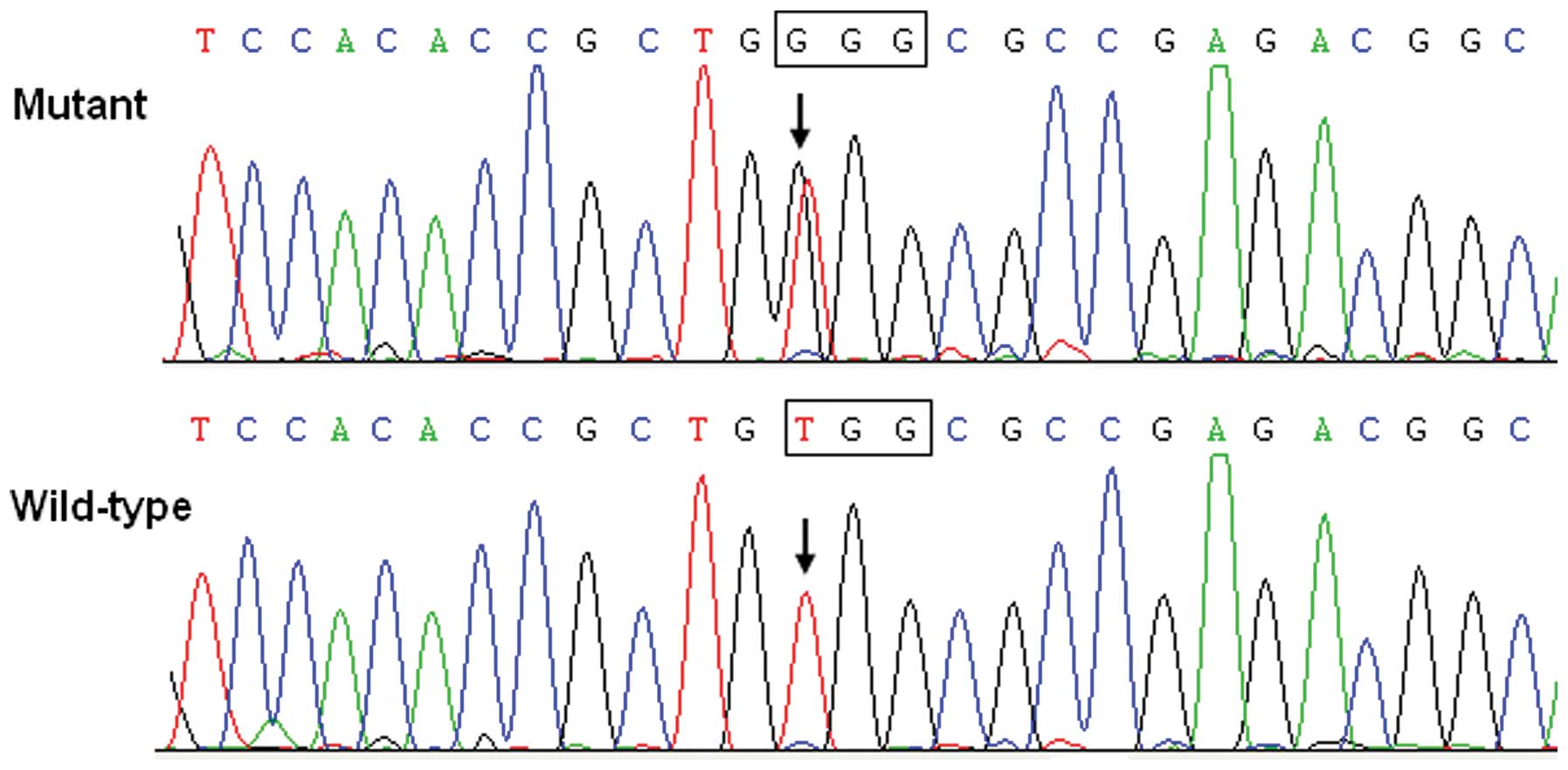
GATA5 mutation
Direct sequencing of the coding exons and
exon-intron boundaries of the GATA5 gene was conducted after
PCR amplification of genomic DNA from each of the 118 patients with
lone AF. A heterozygous GATA5 mutation was identified in 1
out of 118 unrelated patients, with a prevalence of ~0.85% for
GATA5 mutation. In particular, a substitution of guanine (G)
for thymine (T) in the first nucleotide of codon 200 (c.598T>G),
predicting the transition of tryptophane (W) into glycine (G) at
amino acid 200 (p.W200G) was identified in a patient with positive
family history. The sequence chromatograms showing the detected
heterozygous GATA5 mutation of c.598T>G compared with the
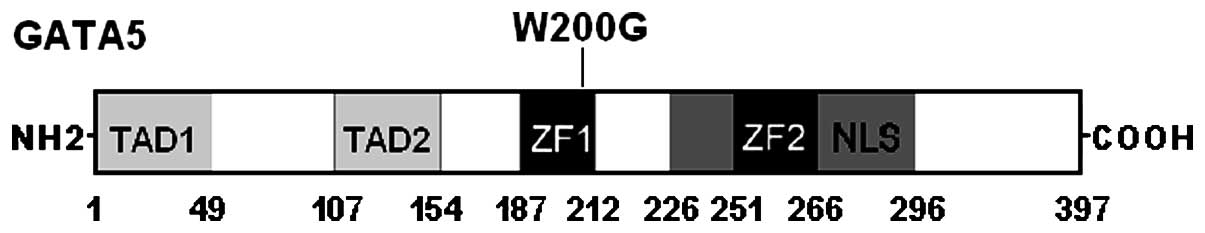
corresponding control sequence are shown in Fig. 1. A schematic diagram of GATA5
depicting the putative structural domains and location of the
mutation identified in AF patients is presented in Fig. 2. The missense mutation was not
found in the control population nor was it reported in the NCBI’s
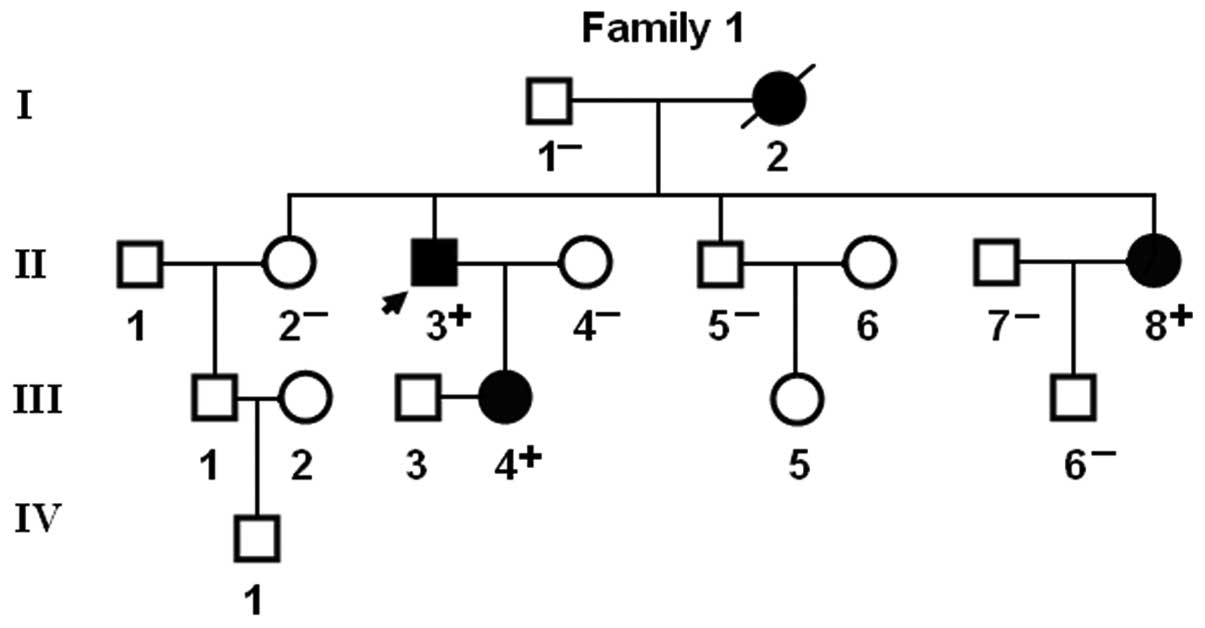
SNP database (http://www.ncbi.nlm.nih.gov/SNP). Genetic screening of
the available family members demonstrated that the mutation was
present in all affected living family members, but absent in
unaffected family members examined. Analysis of the pedigree showed
that the mutation cosegregated with AF transmitted as an autosomal
dominant trait in the family with complete penetrance. The pedigree
structure of the family is illustrated in Fig. 3. The phenotypic characteristics
and results of genetic screening of the affected family members are
listed in Table III. Congenital
atrial septal defect was confirmed by medical records of previous
catheter-based repairs in 2 AF patients (I-2 and II-8).
 | Table IIIPhenotypic characteristics and status
of the GATA5 mutation of the affected pedigree members. |
Table III
Phenotypic characteristics and status
of the GATA5 mutation of the affected pedigree members.
| Subject
information | Phenotype |
Electrocardiogram | Echocardiogram | Genotype |
|---|
|
|
|
|
|
|---|
| Identity | Gender | Age at time of
study (years) | Age at diagnosis of
AF (years) | AF
(classification) | Heart rate
(beats/min) | QRS interval
(ms) | QTc | LAD (mm) | LVEF (%) | GATA5 mutation
(W200G) |
|---|
| I-2 | F | 64a | 42 | Permanent | 90 | 118 | 458 | 53 | 52 | NA |
| II-3 | M | 58 | 30 | Permanent | 82 | 98 | 440 | 38 | 65 | +/− |
| II-8 | F | 52 | 43 | Persistent | 93 | 104 | 465 | 56 | 60 | +/− |
| III-4 | F | 32 | 32 | Paroxysmal | 78 | 90 | 420 | 32 | 62 | +/− |
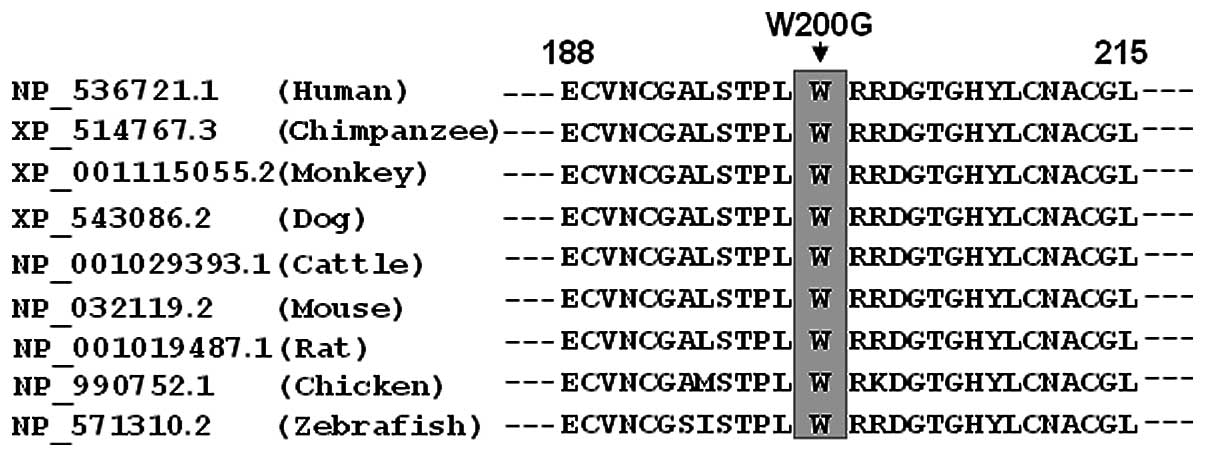
Alignment of multiple GATA5 protein
sequences
A cross-species alignment of GATA5 protein sequences
showed that the altered amino acid was completely conserved
evolutionarily, as presented in Fig.
4, suggesting that the amino acid is functionally
important.
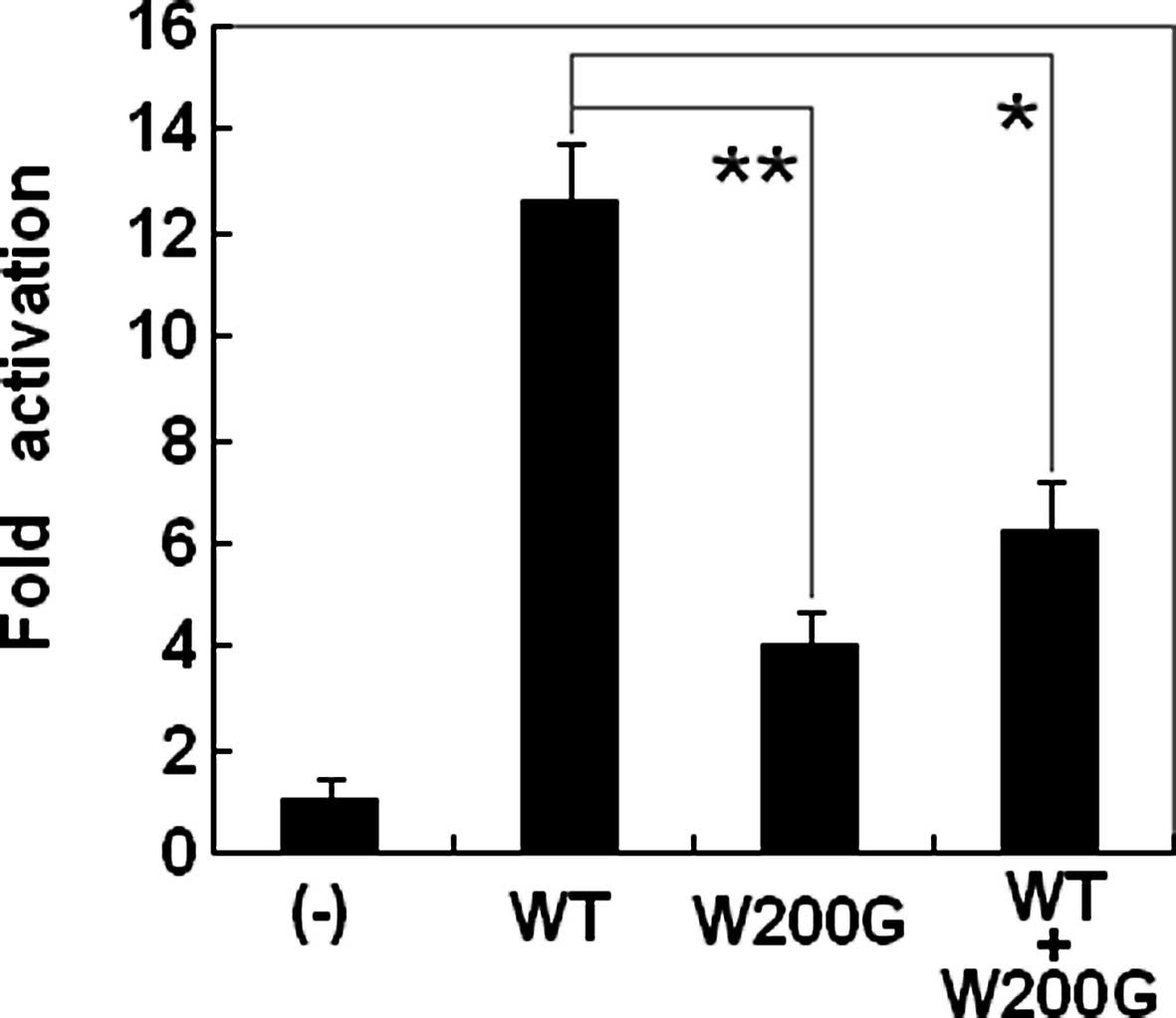
Transcriptional activity of the GATA5
mutant
The transcriptional activation characterization of
the mutated GATA5 in HEK-293 cells was examined using one of its
direct cardiac downstream target genes, ANP, as a luciferase
reporter and the activity of the ANP promoter was presented
as fold activation of Firefly luciferase relative to Renilla
luciferase. The same amounts of wild-type (0.4 μg) and W200G-mutant
GATA5 (0.4 μg) activated the ANP promoter by ~13- and
4-fold, respectively. When the same amount of wild-type
GATA5 (0.2 μg) was cotransfected with W200G-mutant
GATA5 (0.2 μg), the induced activation of the ANP promoter
was ~6-fold. These results suggest that the GATA5 mutation has a
significantly reduced transcriptional activation compared with its
wild-type counterpart (Fig.
5).
Discussion
In the present study, a novel heterozygous GATA5
mutation of p.W200G identified in a family with lone AF is
reported. This missense mutation of GATA5 was present in all
the affected family members examined but was absent in the
unaffected family members available and in the 400 normal
chromosomes from a matched control population. A cross-species
alignment of multiple GATA5 protein sequences showed that the
altered amino acid was completely conserved evolutionarily.
Functional analysis demonstrated that the p.W200G mutation of GATA5
was associated with a significantly decreased transcriptional
activity. Therefore, it is highly likely that functionally impaired
GATA5 is involved in the pathogenesis of AF in this family. To the
best of our knowledge, this is the first report on the relationship
between GATA5 loss-of-function mutation and susceptibility to AF.
These results expand the spectrum of mutations in GATA5 linked to
AF and provide significant insight into the molecular basis
underlying AF.
GATA transcription factors are a group of DNA
binding proteins characteristic of preferential binding to the
consensus DNA sequence GATA of target gene promoters. The GATA
family comprises 6 members (GATA1 to GATA6), of which GATA4, GATA5
and GATA6 are expressed in various mesoderm and endoderm-derived
tissues, particularly in the embryonic and adult heart (39). GATA5 maps to human chromosome
20q13.33 by fluorescence in situ hybridization, which
encodes a predicted 397-amino-acid protein (58). Compared with the functional
domains of GATA4, GATA5 is predicted to consist of 2
transcriptional activation domains (TADs), 2 adjacent zinc fingers
(ZFs) and 1 nuclear localization signal (NLS). The 2 TADs are both
essential for the transcriptional activity of GATA5. The C-terminal
ZF is required for DNA sequence recognition and binding to the
consensus motif, while the N-terminal ZF is responsible for
stability and sequence specificity of protein-DNA binding as well
as transcriptional activation by GATA factors. Most of the
protein-protein interactions of GATA factors are mediated by its
C-terminal ZF. The NLS sequence is associated with the sub-cellular
trafficking and distribution of GATA5. The GATA5 mutation of
p.W200G identified in this study is located in the N-terminal ZF,
thus it may be expected to exert influence on the transcriptional
activity of GATA5.
It has been corroborated that GATA5 is an upstream
regulator of multiple genes transcribed during embryogenesis and
cardiac morphogenesis including the genes that encode atrial
natriuretic peptide (ANP), brain natriuretic peptide, α-myosin
heavy chain, β-myosin heavy chain and cardiac troponin C and I
(39). Hence, the functional
effects of the GATA5 mutation may be ascertained by analysis
of the transcriptional activity of the ANP promoter in cells
transfected with the GATA5 mutant in contrast to its
wild-type counterpart. In this study, the functional role of the
novel p.G200W mutation of GATA5 identified in our familial AF
patients was characterized by transcriptional activity assays and
the results demonstrated a significantly decreased transcriptional
activity on a downstream gene. These findings indicate that
haploinsufficiency resulting from GATA5 mutations is potentially an
alternative pathophysiological mechanism involved in AF, although
the functional roles of the recently reported AF related GATA5
mutations remain to be explored (59).
The findings that functionally impaired GATA5
predisposes to AF can be partially attributed to the abnormally
developed pulmonary vein myocardium. The pulmonary venous vessel is
ensheathed by a layer of myocardium termed pulmonary myocardial
sleeve, which has been substantiated to be responsible for the
initiation and perpetuation of AF by several potential
arrhythmogenic mechanisms including intrinsic pacemaker activity
and properties that facilitate re-entrance (60–62). Genetic-labeling lineage tracing
studies have revealed that NKX2-5 is expressed in the atria and
pulmonary myocardium and is crucial for the localized formation of
the sinoatrial node during embryogenesis. NKX2-5 may function as a
suppressor of the sinoatrial node lineage gene program, which
limits pacemaker activity to the sinoatrial and atrioventricular
nodes. When the NKX2-5 protein decreased in a hypomorphic model,
the pulmonary cardiomyocytes switched to connexin40-negative,
HCN4-positive cells, a nodal-like phenotype with pacemaker activity
(61). In NKX2-5-null
mouse embryos, HCN4 was activated in the entire embryonic
heart tube, whereas connexin40 expression was inhibited, and
ectopic pacemaker cells were observed throughout the heart tube
(63). In humans, AF was observed
as an isolated phenotype or as a part of compound phenotypes in
patients carrying NKX2-5 mutations (45,64,65). Therefore, as a transcriptionally
cooperative partner of NKX2-5, GATA5, when a dominant
negative mutation occurs, may contribute to the formation of the
pulmonary myocardium sleeve and the shift of the pulmonary
myocardium to a sinoatrial node-like phenotype by reducing NKX2-5,
hence creating an atrial electrophysiological substrate liable to
AF.
There are some downstream genes transactivated by
GATA5, and mutations in several target genes have been implicated
in AF, including the genes that encode β-myosin heavy chain, atrial
natriuretic peptide and gap junction protein connexin40 (32,33,35–37,66). Therefore, it is highly likely that
mutated GATA5 confers susceptibility to AF by decreasing expression
of target genes.
Markedly, congenital atrial septal defect has been
documented in 2 AF patients harboring the p.G200W mutation of
GATA5. Similar to our findings, congenital cardiovascular
malformations were previously confirmed in AF patients carrying
NKX2-5, GATA4 or GATA6 mutations (45,51–53,56,59). Considering some congenital cardiac
structural defects may close spontaneously, we cannot rule out the
possibility that some mutation carriers had minor cardiac septal
defects that closed shortly after birth on their own. These
observations indicate that AF may share a common genetic origin
with congenital heart disease.
In conclusion, our findings provide novel insight
into the molecular mechanism associated with AF, suggesting
potential implications for early prophylaxis and gene-specific
therapy of this common tachycardia.
Acknowledgements
We are indebted to the participants for their
dedication to the study. This study was supported by grants from
the National Natural Science Fund of China (81000082, 81070153 and
30570768), the National Basic Research Program of China
(2010CB912604), the Personnel Development Foundation of Shanghai,
China (2010019) and the Key Program of Basic Research of Shanghai,
China (10JC1414000, 10JC1414001 and 10JC1414002).
References
|
1
|
Fuster V, Rydén LE, Cannom DS, Crijns HJ,
Curtis AB, Ellenbogen KA, Halperin JL, Kay GN, Le Huezey JY, Lowe
JE, et al: American College of Cardiology Foundation/American Heart
Association Task Force: 2011 ACCF/AHA/HRS focused updates
incorporated into the ACC/AHA/ESC 2006 guidelines for the
management of patients with atrial fibrillation: a report of the
American College of Cardiology Foundation/American Heart
Association Task Force on practice guidelines. Circulation.
123:e269–e367. 2011.
|
|
2
|
Go AS, Hylek EM, Phillips KA, Chang Y,
Henault LE, Selby JV and Singer DE: Prevalence of diagnosed atrial
fibrillation in adults: national implications for rhythm management
and stroke prevention: the AnTicoagulation and Risk Factors in
Atrial Fibrillation (ATRIA) Study. JAMA. 285:2370–2375. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lloyd-Jones DM, Wang TJ, Leip EP, Larson
MG, Levy D, Vasan RS, D’Agostino RB, Massaro JM, Beiser A, Wolf PA
and Benjamin EJ: Lifetime risk for development of atrial
fibrillation: the Framingham Heart Study. Circulation.
110:1042–1046. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wolf PA, Abbott RD and Kannel WB: Atrial
fibrillation as an independent risk factor for stroke: the
Framingham Study. Stroke. 22:983–988. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Benjamin EJ, Wolf PA, D’Agostino RB,
Silbershatz H, Kannel WB and Levy D: Impact of atrial fibrillation
on the risk of death: the Framingham Heart Study. Circulation.
98:946–952. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Magnani JW, Rienstra M, Lin H, Sinner MF,
Lubitz SA, McManus DD, Dupuis J, Ellinor PT and Benjamin EJ: Atrial
fibrillation: current knowledge and future directions in
epidemiology and genomics. Circulation. 124:1982–1993. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Darbar D, Herron KJ, Ballew JD, Jahangir
A, Gersh BJ, Shen WK, Hammill SC, Packer DL and Olson TM: Familial
atrial fibrillation is a genetically heterogeneous disorder. J Am
Coll Cardiol. 41:2185–2192. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ellinor PT, Yoerger DM, Ruskin JN and
MacRae CA: Familial aggregation in lone atrial fibrillation. Hum
Genet. 118:179–184. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Arnar DO, Thorvaldsson S, Manolio TA,
Thorgeirsson G, Kristjansson K, Hakonarson H and Stefansson K:
Familial aggregation of atrial fibrillation in Iceland. Eur Heart
J. 27:708–712. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Junttila MJ, Raatikainen MJ, Perkiömäki
JS, Hong K, Brugada R and Huikuri HV: Familial clustering of lone
atrial fibrillation in patients with saddleback-type ST-segment
elevation in right precordial leads. Eur Heart J. 28:463–468. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Christophersen IE, Ravn LS,
Budtz-Joergensen E, Skytthe A, Haunsoe S, Svendsen JH and
Christensen K: Familial aggregation of atrial fibrillation: a study
in Danish twins. Circ Arrhythm Electrophysiol. 2:378–383. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang YQ, Zhang XL, Wang XH, Tan HW, Shi
HF, Fang WY and Liu X: Familial aggregation of lone atrial
fibrillation in the Chinese population. Intern Med. 49:2385–2391.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lubitz SA, Yin X, Fontes JD, Magnani JW,
Rienstra M, Pai M, Villalon ML, Vasan RS, Pencina MJ, Levy D, et
al: Association between familial atrial fibrillation and risk of
new-onset atrial fibrillation. JAMA. 304:2263–2269. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fox CS, Parise H, D’Agostino RB Sr,
Lloyd-Jones DM, Vasan RS, Wang TJ, Levy D, Wolf PA and Benjamin EJ:
Parental atrial fibrillation as a risk factor for atrial
fibrillation in offspring. JAMA. 291:2851–2855. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Brugada R, Tapscott T, Czernuszewicz GZ,
Marian AJ, Iglesias A, Mont L, Brugada J, Girona J, Domingo A,
Bachinski LL and Roberts R: Identification of a genetic locus for
familial atrial fibrillation. N Engl J Med. 336:905–911. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ellinor PT, Shin JT, Moore RK, Yoerger DM
and MacRae CA: Locus for atrial fibrillation maps to chromosome
6q14–16. Circulation. 107:2880–2883. 2003.PubMed/NCBI
|
|
17
|
Chen YH, Xu SJ, Bendahhou S, Wang XL, Wang
Y, Xu WY, Jin HW, Sun H, Su XY, Zhuang QN, et al: KCNQ1
gain-of-function mutation in familial atrial fibrillation. Science.
299:251–254. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Oberti C, Wang L, Li L, Dong J, Rao S, Du
W and Wang Q: Genome-wide linkage scan identifies a novel genetic
locus on chromosome 5p13 for neonatal atrial fibrillation
associated with sudden death and variable cardiomyopathy.
Circulation. 110:3753–3759. 2004. View Article : Google Scholar
|
|
19
|
Zhang X, Chen S, Yoo S, Chakrabarti S,
Zhang T, Ke T, Oberti C, Yong SL, Fang F, Li L, et al: Mutation in
nuclear pore component NUP155 leads to atrial fibrillation and
early sudden cardiac death. Cell. 135:1017–1027. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Volders PG, Zhu Q, Timmermans C, Eurlings
PM, Su X, Arens YH, Li L, Jongbloed RJ, Xia M, Rodriguez LM and
Chen YH: Mapping a novel locus for familial atrial fibrillation on
chromosome 10p11–q21. Heart Rhythm. 4:469–475. 2007.PubMed/NCBI
|
|
21
|
Darbar D, Hardy A, Haines JL and Roden DM:
Prolonged signal-averaged P-wave duration as an intermediate
phenotype for familial atrial fibrillation. J Am Coll Cardiol.
51:1083–1089. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang Y, Xia M, Jin Q, Bendahhou S, Shi J,
Chen Y, Liang B, Lin J, Liu Y, Liu B, et al: Identification of a
KCNE2 gain-of-function mutation in patients with familial atrial
fibrillation. Am J Hum Genet. 75:899–905. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lundby A, Ravn LS, Svendsen JH, Hauns S,
Olesen SP and Schmitt N: KCNE3 mutation V17M identified in a
patient with lone atrial fibrillation. Cell Physiol Biochem.
21:47–54. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ravn LS, Aizawa Y, Pollevick GD,
Hofman-Bang J, Cordeiro JM, Dixen U, Jensen G, Wu Y, Burashnikov E,
Haunso S, et al: Gain of function in IKs secondary to a mutation in
KCNE5 associated with atrial fibrillation. Heart Rhythm. 5:427–435.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hong K, Bjerregaard P, Gussak I and
Brugada R: Short QT syndrome and atrial fibrillation caused by
mutation in KCNH2. J Cardiovasc Electrophysiol. 16:394–396. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xia M, Jin Q, Bendahhou S, He Y, Larroque
MM, Chen Y, Zhou Q, Yang Y, Liu Y, Liu B, et al: A Kir2.1
gain-of-function mutation underlies familial atrial fibrillation.
Biochem Biophys Res Commun. 332:1012–1019. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Olson TM, Alekseev AE, Liu XK, Park S,
Zingman LV, Bienengraeber M, Sattiraju S, Ballew JD, Jahangir A and
Terzic A: Kv1.5 channelopathy due to KCNA5 loss-of-function
mutation causes human atrial fibrillation. Hum Mol Genet.
15:2185–2191. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang Y, Li J, Lin X, Yang Y, Hong K, Wang
L, Liu J, Li L, Yan D, Liang D, et al: Novel KCNA5 loss-of-function
mutations responsible for atrial fibrillation. J Hum Genet.
54:277–283. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Olson TM, Michels VV, Ballew JD, Reyna SP,
Karst ML, Herron KJ, Horton SC, Rodeheffer RJ and Anderson JL:
Sodium channel mutations and susceptibility to heart failure and
atrial fibrillation. JAMA. 293:447–454. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Watanabe H, Darbar D, Kaiser DW,
Jiramongkolchai K, Chopra S, Donahue BS, Kannankeril PJ and Roden
DM: Mutations in sodium channel beta1- and beta2-subunits
associated with atrial fibrillation. Circ Arrhythm Electrophysiol.
2:268–275. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang P, Yang Q, Wu X, Yang Y, Shi L, Wang
C, Wu G, Xia Y, Yang B, Zhang R, et al: Functional
dominant-negative mutation of sodium channel subunit gene SCN3B
associated with atrial fibrillation in a Chinese GeneID population.
Biochem Biophys Res Commun. 398:98–104. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hodgson-Zingman DM, Karst ML, Zingman LV,
Heublein DM, Darbar D, Herron KJ, Ballew JD, de Andrade M, Burnett
JC Jr and Olson TM: Atrial natriuretic peptide frameshift mutation
in familial atrial fibrillation. N Engl J Med. 359:158–165. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ren X, Xu C, Zhan C, Yang Y, Shi L, Wang
F, Wang C, Xia Y, Yang B, Wu G, et al: Identification of NPPA
variants associated with atrial fibrillation in a Chinese GeneID
population. Clin Chim Acta. 411:481–485. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Thibodeau IL, Xu J, Li Q, Liu G, Lam K,
Veinot JP, Birnie DH, Jones DL, Krahn AD, Lemery R, et al: Paradigm
of genetic mosaicism and lone atrial fibrillation: physiological
characterization of a connexin 43-deletion mutant identified from
atrial tissue. Circulation. 122:236–244. 2010. View Article : Google Scholar
|
|
35
|
Gollob MH, Jones DL, Krahn AD, Danis L,
Gong XQ, Shao Q, Liu X, Veinot JP, Tang AS, Stewart AF, et al:
Somatic mutations in the connexin 40 gene (GJA5) in atrial
fibrillation. N Engl J Med. 354:2677–2688. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yang YQ, Zhang XL, Wang XH, Tan HW, Shi
HF, Jiang WF, Fang WY and Liu X: Connexin40 nonsense mutation in
familial atrial fibrillation. Int J Mol Med. 26:605–610.
2010.PubMed/NCBI
|
|
37
|
Yang YQ, Liu X, Zhang XL, Wang XH, Tan HW,
Shi HF, Jiang WF and Fang WY: Novel connexin40 missense mutations
in patients with familial atrial fibrillation. Europace.
12:1421–1427. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bruneau BG: The developmental genetics of
congenital heart disease. Nature. 451:943–948. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pikkarainen S, Tokola H, Kerkelä R and
Ruskoaho H: GATA transcription factors in the developing and adult
heart. Cardiovasc Res. 63:196–207. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hu DL, Chen FK, Liu YQ, Sheng YH, Yang R,
Kong XQ, Cao KJ, Gu HT and Qian LM: GATA-4 promotes the
differentiation of P19 cells into cardiac myocytes. Int J Mol Med.
26:365–372. 2010.PubMed/NCBI
|
|
41
|
Schott JJ, Benson DW, Basson CT, Pease W,
Silberbach GM, Moak JP, Maron BJ, Seidman CE and Seidman JG:
Congenital heart disease caused by mutations in the transcription
factor NKX2-5. Science. 281:108–111. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang J, Xin YF, Liu XY, Liu ZM, Wang XZ
and Yang YQ: A novel NKX2-5 mutation in familial ventricular septal
defect. Int J Mol Med. 27:369–375. 2011.PubMed/NCBI
|
|
43
|
Liu XY, Wang J, Yang YQ, Zhang YY, Chen
XZ, Zhang W, Wang XZ, Zheng JH and Chen YH: Novel NKX2-5 mutations
in patients with familial atrial septal defects. Pediatr Cardiol.
32:193–201. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang J, Liu XY and Yang YQ: Novel NKX2-5
mutations responsible for congenital heart disease. Genet Mol Res.
10:2905–2915. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gutierrez-Roelens I, De Roy L, Ovaert C,
Sluysmans T, Devriendt K, Brunner HG and Vikkula M: A novel
CSX/NKX2-5 mutation causes autosomal-dominant AV block: are atrial
fibrillation and syncopes part of the phenotype? Eur J Hum Genet.
14:1313–1316. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Boldt LH, Posch MG, Perrot A, Polotzki M,
Rolf S, Parwani AS, Huemer M, Wutzler A, Ozcelik C and Haverkamp W:
Mutational analysis of the PITX2 and NKX2-5 genes in patients with
idiopathic atrial fibrillation. Int J Cardiol. 145:316–317. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Garg V, Kathiriya IS, Barnes R,
Schluterman MK, King IN, Butler CA, Rothrock CR, Eapen RS,
Hirayama-Yamada K, Joo K, Matsuoka R, et al: GATA4 mutations cause
human congenital heart defects and reveal an interaction with TBX5.
Nature. 424:443–447. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wang J, Fang M, Liu XY, Xin YF, Liu ZM,
Chen XZ, Wang XZ, Fang WY, Liu X and Yang YQ: A novel GATA4
mutation responsible for congenital ventricular septal defects. Int
J Mol Med. 28:557–564. 2011.PubMed/NCBI
|
|
49
|
Liu XY, Wang J, Zheng JH, Bai K, Liu ZM,
Wang XZ, Liu X, Fang WY and Yang YQ: Involvement of a novel
GATA4 mutation in atrial septal defects. Int J Mol Med.
28:17–23. 2011.
|
|
50
|
Yang YQ, Li L, Wang J, Liu XY, Chen XZ,
Zhang W, Wang XZ, Jiang JQ, Liu X and Fang WY: A novel GATA4
loss-of-function mutation associated with congenital ventricular
septal defect. Pediatr Cardiol. 33:539–546. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Posch MG, Boldt LH, Polotzki M, Richter S,
Rolf S, Perrot A, Dietz R, Ozcelik C and Haverkamp W: Mutations in
the cardiac transcription factor GATA4 in patients with lone atrial
fibrillation. Eur J Med Genet. 53:201–203. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yang YQ, Wang MY, Zhang XL, Tan HW, Shi
HF, Jiang WF, Wang XH, Fang WY and Liu X: GATA4 loss-of-function
mutations in familial atrial fibrillation. Clin Chim Acta.
412:1825–1830. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Jiang JQ, Shen FF, Fang WY, Liu X and Yang
YQ: Novel GATA4 mutations in lone atrial fibrillation. Int J Mol
Med. 28:1025–1032. 2011.PubMed/NCBI
|
|
54
|
Lin X, Huo Z, Liu X, Zhang Y, Li L, Zhao
H, Yan B, Liu Y, Yang Y and Chen YH: A novel GATA6 mutation in
patients with tetralogy of Fallot or atrial septal defect. J Hum
Genet. 55:662–667. 2010. View Article : Google Scholar
|
|
55
|
Zheng GF, Wei D, Zhao H, Zhou N, Yang YQ
and Liu XY: A novel GATA6 mutation associated with
congenital ventricular septal defect. Int J Mol Med. 29:1065–1071.
2012.
|
|
56
|
Yang YQ, Wang XH, Tan HW, Jiang WF, Fang
WY and Liu X: Prevalence and spectrum of GATA6 mutations associated
with familial atrial fibrillation. Int J Cardiol. 155:494–496.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zhang Y, Rath N, Hannenhalli S, Wang Z,
Cappola T, Kimura S, Atochina-Vasserman E, Lu MM, Beers MF and
Morrisey EE: GATA and Nkx factors synergistically regulate
tissue-specific gene expression and development in vivo.
Development. 134:189–198. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Nemer G, Qureshi ST, Malo D and Nemer M:
Functional analysis and chromosomal mapping of Gata5, a gene
encoding a zinc finger DNA-binding protein. Mamm Genome.
10:993–999. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Yang YQ, Wang J, Wang XH, Wang Q, Tan HW,
Zhang M, Shen FF, Jiang JQ, Fang WY and Liu X: Mutational spectrum
of the GATA5 gene associated with familial atrial fibrillation. Int
J Cardiol. 157:305–307. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Haïssaguerre M, Jaïs P, Shah DC, Takahashi
A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Métayer P and
Clémenty J: Spontaneous initiation of atrial fibrillation by
ectopic beats originating in the pulmonary veins. N Engl J Med.
339:659–666. 1998.PubMed/NCBI
|
|
61
|
Mommersteeg MT, Brown NA, Prall OW, de
Gier-de Vries C, Harvey RP, Moorman AF and Christoffels VM: Pitx2c
and Nkx2-5 are required for the formation and identity of the
pulmonary myocardium. Circ Res. 101:902–909. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Mommersteeg MT, Christoffels VM, Anderson
RH and Moorman AF: Atrial fibrillation: a developmental point of
view. Heart Rhythm. 6:1818–1824. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Mommersteeg MT, Hoogaars WM, Prall OW, de
Gier-de Vries C, Wiese C, Clout DE, Papaioannou VE, Brown NA,
Harvey RP, Moorman AF and Christoffels VM: Molecular pathway for
the localized formation of the sinoatrial node. Circ Res.
100:354–362. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Watanabe Y, Benson DW, Yano S, Akagi T,
Yoshino M and Murray JC: Two novel frameshift mutations in NKX2.5
result in novel features including visceral inversus and sinus
venosus type ASD. J Med Genet. 39:807–811. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Pabst S, Wollnik B, Rohmann E, Hintz Y,
Glänzer K, Vetter H, Nickenig G and Grohé C: A novel stop mutation
truncating critical regions of the cardiac transcription factor
NKX2-5 in a large family with autosomal-dominant inherited
congenital heart disease. Clin Res Cardiol. 97:39–42. 2008.
View Article : Google Scholar
|
|
66
|
Gruver EJ, Fatkin D, Dodds GA, Kisslo J,
Maron BJ, Seidman JG and Seidman CE: Familial hypertrophic
cardiomyopathy and atrial fibrillation caused by Arg663His
beta-cardiac myosin heavy chain mutation. Am J Cardiol. 83:H13–H18.
1999. View Article : Google Scholar : PubMed/NCBI
|