Introduction
Ischemic heart disease is the most common cause of
mortality worldwide despite continued improvements in the
prevention and treatment of coronary artery disease. However, the
majority of patients are not qualified for the conventional
revascularization techniques of balloon angioplasty and stenting,
or coronary artery bypass grafting. Over the last decade, stem cell
transplantation has been considered as a promising approach for the
recovery of heart function in ischemic disease. Mesenchymal stem
cells (MSCs) can be easily isolated and expanded, and have a stable
genetic background and a low risk of immunorejection. Furthermore,
stem cells possess the capacity of multilineage differentiation and
bioenergetic modulation (1,2).
As a result, MSCs are used as seeding cells in tissue engineering
and stem cell therapy. They have been used in both experimental and
clinical settings to attenuate infarct size expansion, enhance
myocardial regeneration and cardiac function (1–5).
Previous studies have indicated that the functional benefits
observed after MSC transplantation in animal models of cardiac
injury may be related to the secretion of soluble factors that,
acting in a paracrine fashion, attenuate pathological ventricular
remodeling, induce angiogenesis and promote myocardium regeneration
(1,2,4,6).
Angiogenesis is essential for cardiac repair following myocardial
infarction (MI), when collateral vessels form at the site of the
infarct and maintain blood flow to ischemic tissue (7). MSCs may differentiate into vascular
endothelial-like cells and incorporate into growing new blood
vessels by the paracrine release of a series of angiogenic
cytokines, including vascular endothelial growth factor (VEGF) and
basic fibroblast growth factor (bFGF) (8). However, the low survival rate and
angiogenic potential of transplanted cells in the ischemic
myocardium influence the outcome of MSC transplantation for the
treatment of ischemic heart disease.
microRNAs (miRNAs or miRs) are naturally occurring
RNAs that are transcribed in the nucleus, processed by the RNases
Drosha/DGCR8 and Dicer into mature ∼22-nucleotide RNAs that bind to
complementary target mRNAs through Watson-Crick base pairing
between the miRNA ‘seed region’ and sequences commonly located in
the 3′-untranslated regions (3′-UTR) (9–12).
As they can regulate numerous genes, miRNAs are considered as
master regulators of cellular processes, such as cellular
differentiation and organogenesis. The endothelial cell-specific
miRNA, miR-126, has been shown to positively regulate the response
of endothelial cells to VEGF and to improve angiogenesis partly by
directly repressing negative regulators of the VEGF pathway,
including the Sprouty-related protein 1 (SPRED1), and
phosphoinositol-3 kinase regulatory subunit 2 (PIK3R2) (13). Thus, the overexpression of miR-126
relieves the repressive influence of SPRED1/PIK3R2 on the signaling
pathways activated by VEGF and bFGF, favoring angiogenesis. It
plays an essential role in neoangiogenesis following MI and in the
maintenance of vascular integrity. The targeted deletion of miR-126
causes a loss of vascular integrity in mice and zebrafish during
development and results in defective angiogenesis (14). A recent study showed that the
blood flow-induced upregulation of miR-126 by the mechanosensitive
transcription factor, Klf2, in endothelial cells activated the VEGF
signaling pathway and led to sprouting and remodeling of the aortic
arch in developing zebrafish (15). These findings establish the
involvement of miR-126 in potently promoting physiological
angiogenesis and enhancing blood flow to ischemic tissue.
In addition to VEGF, many Notch pathway components
are expressed and play a critical role during vessel development
(16–19). In mammals, the Notch pathway
involves 5 transmembrane ligands, Delta-like (Dll)-1, -3, -4,
Jagged (Jag)-1 and Jag-2, and 4 transmembrane receptors, Notch-1,
-2, -3 and -4. Notch receptor activation results in the cleavage of
the Notch intracellular domain (NICD) and translocation to the
nucleus, thereby activating downstream target genes, such as basic
helix-loop-helix (bHLH) protein (18,20). Functional studies have provided us
with convincing evidence that the Notch pathway is involved in a
variety of cell fate decisions and regulates many biological
processes, particularly angiogenesis.
Therapies combining genes and stem cells hold
promise for the treatment of ischemic heart disease. In particular,
MSCs are excellent carriers of therapeutic genes to the ischemic
zone. Accordingly, we hypothesized that the transplantation of MSCs
modified with miR-126 may be superior to MSCs, increasing the stem
cell paracrine activity and inducing the Notch pathway to promote
ischemic angiogenesis, thereby enhancing cardiac functional
recovery.
Materials and methods
Animal ethics
All procedures were performed in compliance with the
guidelines for the Care and Use of Laboratory Animals published by
the National Institutes of Health (NIH publication no. 85-23,
revised 1996) and approved by the Animal Care and Use Committee of
the Second Xiangya Hospital, Central South University. The
investigators responsible for molecular, histological and
functional studies were blinded to the treatment groups.
Isolation and primary cultivation of
MSCs
Six-week-old male C57BL/6 mice were sacrificed by
cervical dislocation. The hind legs and vertebrae were dissected
and carefully separated from the adherent tissues. After the tips
of each bone were removed, bone marrow was collected by flushing
out the content of the femurs and tibia with phosphate-buffered
saline (PBS). The collected cells were plated at 1×106
cells/ml in 100-mm plastic dishes and cultured in complete medium,
consisting of Dulbecco’s modified Eagle’s medium/Nutrient Mixture
F12 (DMEM/F12), 10% fetal bovine serum and 1%
penicillin/streptomycin (all from Gibco). The passaged cells were
analyzed by fluorescence-activated cell sorting as previously
described (21). After blocking
for non-specific binding with buffer containing 1% bovine serum
albumin, the cells were incubated for 20 min at 4°C with the
following antibodies (Santa Cruz Biotechnology, Inc.):
anti-CD29-fluorescein isothiocyanate (FITC), anti-CD34-FITC,
anti-c-kit-FITC, anti-CD45-FITC, anti-CD90-FITC and
anti-CD105-FITC. The differentiation of MSCs in vitro
towards the adipogenic and osteogenic lineage was carried out as
previously described (22,23).
Recombinant lentiviral vector
construction, cell infection and stable cell line generation
To construct recombinant lentiviral miRNA expression
vectors, mature miR-126, TRE promoter and enhanced green
fluorescent protein (eGFP) sequences were inserted into plasmids
for the generation of pUp-TRE, pDown-miR126 and pTail-IRES/eGFP.
pLV.EX3d.P/puro-TRE>miR126>IRES/eGFP was obtained with the
incubation of donors and accepter vectors catalyzed by LR clonase
(Gateway® LR Clonase™ Plus Enzyme Mix; Invitrogen). The
envelope helper plasmids, pLV/helper-SL3, pLV/helper-SL4,
pLV/helper-SL5, with pLV. EX3d.P/puro-TRE-miR126-IRES/eGFP or
pLVrtTA/neo, were co-transfected into 293T cells with Lipofectamine
2000 according to the manufacturer’s instructions (Invitrogen), to
produce Lenti-miR126-eGFP/puro or Lenti-rtTA/neo, respectively. In
order to perform lentiviral infections, MSCs were first treated by
Lenti-rtTA/neo; 48 h later, infected cells were selected in 0.5
mg/ml neomycin and fresh medium every 2 days. Selection was
terminated when the control cells were completely dead and
antibiotic-free medium was used for propagation. Neomycin-resistant
cells were infected by Lenti-miR126-eGFP/puro and grown with 2
μg/ml puromycin. Double-resistant cells were then ultimately
obtained, and 2 μg/ml doxycycline was added to medium. The
overexpression of miR-126 was achieved. MSCs infected with miR-126
recombinant lentiviral vectors encoding eGFP were termed
MSCmiR-126, and MSCs infected with mock vectors carrying
eGFP but without miR-126 were termed MSCnull, which was
used to determine whether the mock vectors influence the cells in a
paracrine manner and the signaling molecules involved compared to
the control MSCs.
To determine whether Dll-4 plays a role in
tubulogenesis, MSCmiR-126 colony was infected with
lentiviral constructs encoding short hairpin RNA (shRNA) to
knockdown Dll-4. Three types of plasmids,
pAJ-U6-shRNA-CMV-Puro/eGFP, psPAX2 (gag/pol element), and pMD2.G
(VSVG element), were transfected into 293T cells according to the
instructions for Lipofectamine 2000 (Invitrogen). After 48 h of
transduction, the stable shRNA line was selected.
Quantitative real-time PCR to identify
the efficiency of miR-126 transfection
Total RNA was extracted from each sample with TRIzol
reagent (Invitrogen) according to the manufacturer’s instructions.
Complimentary DNA was synthesized in a 20-μl reaction
mixture using the SuperScript III First-Strand Synthesis System for
RT-PCR (Invitrogen). The expression of miR-126 in the transduced
cells was detected by real-time PCR using the All-in-One™ miRNA
quantitative real-time PCR Detection kit and All-in-One™ miRNA qPCR
Primer (from GeneCopoeia). The primer sequence was 5′-CATT
ATTACTTTTGGTACGCGAAA-3′. miR-126 transcript levels were normalized
to the control, U6 mRNA, whose primer sequence was
5′-TCGTGAAGCGTTCCATATTTTTAA-3′.
VEGF and bFGF secretion from
miR-126-modified MSCs under hypoxic conditions
After the MSCs, mock-vector-infected MSCs and
miR-126-transduced MSCs completed adherence, they were incubated
for 24 h at 37°C in a humidified modular hypoxia chamber (Billups
Rothenberg, Inc.) containing 95% nitrogen and 5% carbon dioxide
(n=4 in each group). Subsequently, the conditioned medium was
collected for analysis. Commercial VEGF or bFGF enzyme-linked
immunosorbent assay (ELISA) kits (R&D Systems Inc.,
Minneapolis, MN, USA) were used to quantify the concentration of
VEGF and bFGF in each sample. The supernatant from MSCs cultured
under normal conditions was used as the control. Each experiment
was repeated 3 times.
Creation of MI model and cell
transplantation
Eight-week-old female C57BL/6 mice weighing 20±2 g
underwent ligation of the left coronary artery to produce MI. After
being anesthetized by an intraperitoneal injection of 150 μl
(1%) pentobarbital sodium (Merck, Darmstadt, Germany), the mice
were orally intubated with a 1.0-mm OD intubation cannula and
connected to a small animal volume-control ventilator (HES-HA
MiniVent 845; Harvard Apparatus, Holliston, MA). The left anterior
descending artery (LAD) was ligated 2–3 mm from its origin between
the pulmonary artery conus and the left atrium by using 8-0
sutures. Mice were subjected to electrocardiographs using the
RM6240BD system (Chengdu Instrument Company, Changdu, China) to
determine MI. After pumping out the gas in the chest with a 1-ml
syringe to create negative pressure, the intercostal space incision
was closed with 6-0 sutures, and skin incision was closed with 5-0
sutures.
One week after coronary ligation, the surviving mice
were randomly divided into 4 groups (PBS group, MSC group,
MSCnull group and MSCmiR-126 group; 20 in
each), and received re-thoracotomy and an intramyocardial injection
of PBS, MSC, MSCnull or MSCmiR-126. Each
received an injection of 5×106 cells/100 μl in
PBS or PBS alone with a total volume of 50 μl at 5 sites
(basal anterior, mid-anterior, mid-lateral, apical anterior and
apical lateral) in the peri-infarcted area, with 10 μl at
each site. The chest was then closed and the animals were allowed
to recover.
Assessment of cardiac function by
echocardiography
Transthoracic echocardiographic studies were
performed by an experienced blinded cardiologist 14 days after the
cell transplantation using an echocardiographic system (Sonos 5500;
Hewlett-Packard, Andover, MA) equipped with a 15.0 MHz transducer.
For analysis of left ventricular (LV) function, left ventricular
internal dimensions (LVID) were measured. The echo transducer was
placed on the left hemithorax and views were recorded.
Two-dimensional images were obtained at the midpapillary muscle
level. LV ejection fraction (EF; LVEF) (%) = (EDV − ESV)/EDV ×100%,
fractional shortening (FS) (%) = [(LVIDD − LVIDS)/LVIDD] ×100%,
where D stands for diastole, S for systole and EDV and ESV stand
for end-diastolic and end-systolic LVID, respectively. The images
were obtained in each view and each parameter was measured from 3
consecutive beat cycles in each image.
Western blot analysis
Cells of each group were harvested after being
cultured for 72 h. Cells of MSC, MSCnull and
MSCmiR-126 colonies were extracted to examine the
alteration of Notch signaling compare to MSCs. At 1 and 2 weeks
after cell transplantation, the infarcted areas in the PBS, MSC,
MSCnull and MSCmiR126 groups (10 in each
group) were excised for VEGF, bFGF and Notch pathway components
assay. Different samples were lysed in 0.2 ml lysis buffer (0.1%
SDS, 1% NP-40, 50 mM HEPES, pH 7.4, 2 mM EDTA, 100 mM NaCl, 5 mM
sodium orthovanadate, 40 μM p-nitrophenyl phosphate, and 1%
protease inhibitor mixture set I; Calbiochem). Lysates were
centrifuged at 12,000 rpm for 15 min. The supernatant was collected
and denatured. Protein concentrations were determined by the
Bradford method (Bio-Rad Laboratories, Inc., Hercules, CA). Protein
samples (20 μg) were heated to 95°C for 5 min, run on 10%
SDS-PAGE gels, and transferred onto PVDF membranes (Millipore)
using the semi-dry transfer method. The membranes were blocked for
1 h in Tris-buffered saline containing 0.01% Tween-20 with 10%
non-fat dried milk, and incubated overnight at 4°C with the
relevant antibodies (Santa Cruz Biotechnology, Inc.): anti-Notch-1,
-2, -3, -4, Dll-1, -3, -4, Jag-1, -2, VEGF and bFGF antibodies. The
membranes were rinsed and incubated for 1 h with the corresponding
peroxidase-conjugated secondary antibodies. Chemiluminescent
detection was performed using the ECL kit (Pierce). All bands from
western blot analysis were analyzed using ImageJ software (version
1.6 NIH) to verify the relative level of Notch molecules, VEGF and
bFGF markers compared to the internal control, β-actin.
Measurement of MSC survival in ischemic
myocardium
To measure MSC survival in the ischemic myocardium,
the animals were euthanized on day 7 after transplantation. The
survival of male donor MSCs that expressed the Sry gene in the
female recipient hearts was calculated by using real-time PCR.
Different numbers of male DNA (MSCs) were added to female DNA
(mouse heart tissue) as standards: 100, 50, 25, 10, 5, 1 and
0×104 MSCs/10 mg heart tissue. The expression levels of
Sry were quantified by normalizing the values relative to the mouse
housekeeping gene, β-actin. The number of stem cells was calculated
according to the calibration curve, which showed the threshold
cycles (Ct) of Sry expression against serially diluted MSCs
(24).
Myocardial blood flow
Two weeks after transplantation and following the
echocardiography study, measurements were taken to determine
whether MSCmiR-126 increased blood flow to the infarcted
area of mouse hearts. A total volume of 20 μl
(7.2×104) of 10 μm blue fluorescent microspheres
was injected into the left atrium. After 1 min, a reference blood
sample was withdrawn from the descending aorta at a rate of 1
ml/min. The heart was then excised and weighed. The border,
infarcted, and normal regions of the mouse hearts were dissected
and analyzed separately. Microspheres were extracted from the blood
and heart of each mouse by 0.2 ml potassium hydroxide digestion,
and fluorescence was measured with a 96-well plate reader.
Myocardial blood flow (Qs) was calculated as Qs (ml/min/g) =
(As/Ar)Qr (ml/min)/Wt (g), where Qr represents the withdrawal rate
of the reference blood, As and Ar represent the absorbance in
sample tissue and reference blood, respectively, and Wt represents
tissue weight (25). Blood flow
in border and infarcted areas was expressed as a percentage of the
normal myocardium.
Capillary density
To identify capillary density, the tissue sections
(5 μm) of the infarcted zone were stained with anti-VIII
factor antibody (Santa Cruz Biotechnology, Inc.).
Immunohistochemical staining was performed using the two-step
immunohistochemical technique with DAB (Maixin-Bio, Fuzhou, China)
as described in the manufacturer’s specifications. After being
restained with hematine, the samples were coverslipped and
photographed. The capillaries were counted with a ×200 microscopic
objective in 10 randomly selected fields in 6 sections/animal (10
animals in each group) and averaged. Criteria for being counted
consisted of lumen having diameters <50 μm and including
single or tiny vascular with integral endothelial cells. Capillary
density was expressed as the number of vessels per microscopic
surface area (0.95 mm2).
Statistical analysis
Data re presented as the means ± standard deviation.
analysis of variance (ANOVA) with Scheffe’s post hoc test was used
to identify differences among all groups. A P-value of <0.05 was
considered to indicate a statistically significant difference.
Results
Phenotypic characterization and
differentiation capacity of cells
Cells were scattered in a number of colony
distributions 3 days after planting. On days 8 and 9, the bottle
was covered with long, spindle-like cells. The passaged cells
(mostly spindle-shaped or fibroblast-like cells) were uniformly
distributed and covered the bottom every 4–5 days. They highly
expressed the MSC surface marker molecules, CD29, CD90 and CD105,
and negatively expressed the blood cell surface molecules, c-kit,
CD34 and CD45. Following 3 weeks of adipogenic induction, the
passaged cells stained positive for Oil Red O, showing a
lipid-laden adipocyte phenotype. Similarly, when induced with
osteogenic induction medium for 3 weeks, these cells showed
osteogenesis upon staining with Alizarin Red S for calcium
deposits. These results demonstrated that the stem cells posses
pluripotent differentiation ability.
Efficiency of miR-126 transfection and
resistance of infected cells to hypoxia
To determine the efficiency of gene transfection and
the influence of miR-126 on MSC differentiation, a 10-fold higher
expression level is required. After infection with miR-126
recombinant lentiviral vectors, MSCs overexpressed eGFP.
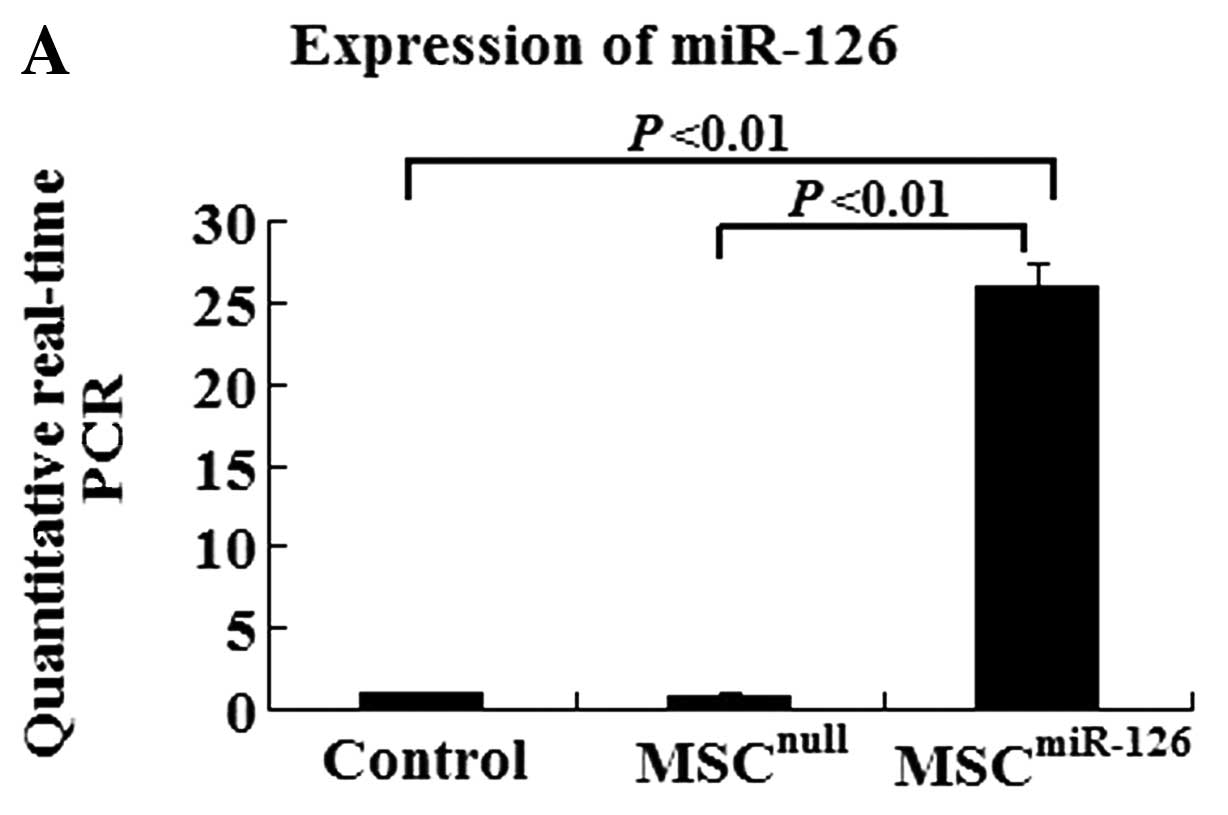
Quantitative real-time PCR data indicated that the efficiency of
gene transfection in the MSCmiR-126 group was similar to
that in the MSCnull group (90.2 vs. 91.7%). The
expression of miR-126 in the MSCmiR-126 group was
26-fold higher than that in the MSC group (P<0.01), and 30-fold
higher than the expression in the MSCnull group
(P<0.01) (Fig. 1A). The TRE
promoter induced by doxycycline was proved to be a strong
cis-element in miRNA expression.
To examine the resistance of MSCs under hypoxic
conditions, MSCs were exposed to hypoxia for 24–72 h and
(3,4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
intake was assessed. In our experiment, MTT intake was not
significantly different between the MSCs exposed to hypoxia for 24
h and those in normal culture. However, it was significantly
decreased when MSCs were exposed to hypoxia for 48 or 72 h. Similar
results were observed in the MSCnull group. Of note, the
overexpression of miR-126 partially prevented this reduction
(Fig. 1B).
Overexpression of miR-126 increases VEGF,
bFGF and Dll-4 expression in vitro and in vivo
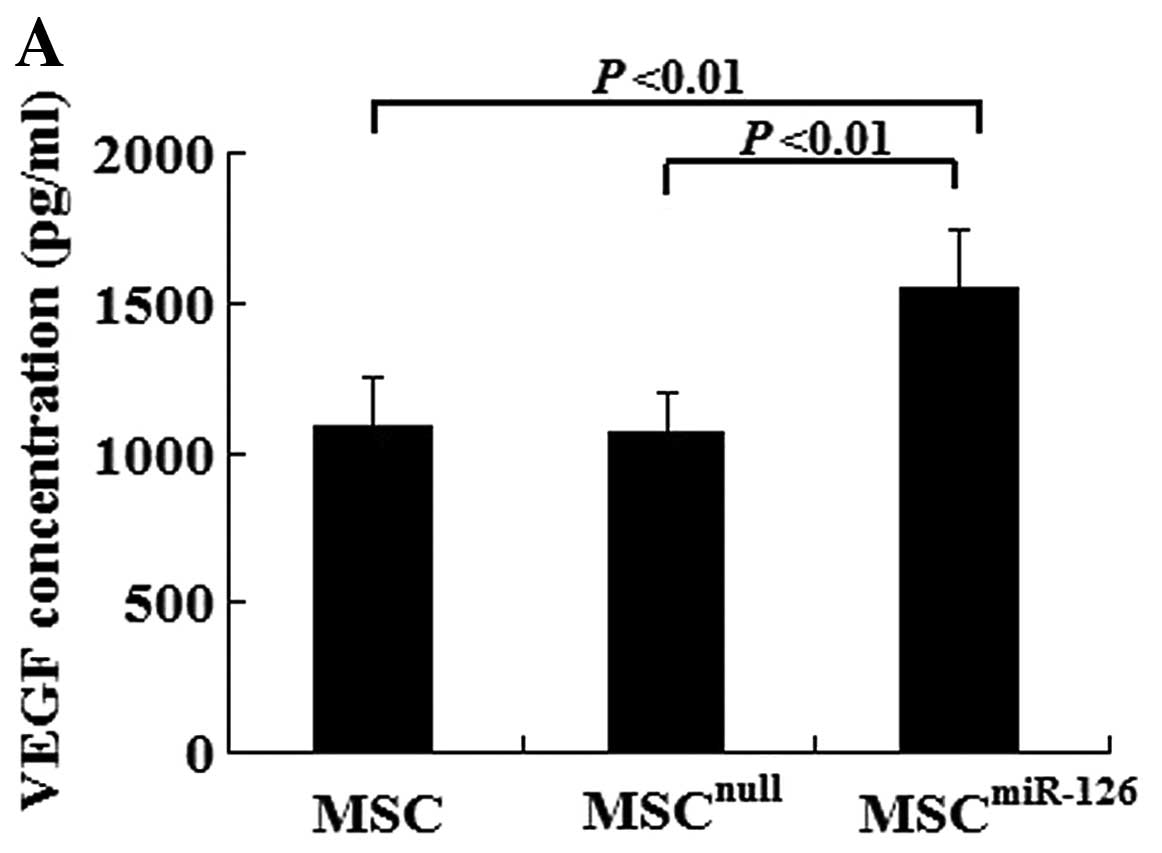
In vitro, there was no difference in the VEGF
and bFGF concentration between the MSCnull group and the
MSC group (VEGF, 1082.5±167.3 vs. 1063.2±138.1, P>0.05; bFGF,
475.6±73.6 vs. 482.4±67.3, P>0.05). However, MSCs overexpressing
miR-126 had an increased secretion of VEGF (1552.4±189.6) and bFGF
(608.9±97.4) compared with the MSCnull (P<0.01) or
the MSC group (P<0.01) under hypoxic conditions (Fig. 2A and B). We also examined Notch
signaling, which plays an important role in angiogenesis. The
expression of Notch-1, -2, -4, Dll-1, -4 and Jag-1 in the MSCs was
examined by western blot analysis. There was no significant
difference in the expression of Notch-1, -2, -4, Dll-1 or Jag-1 in
the MSCmiR-126 group compared to the MSCs and
MSCnull group. However, the Dll-4 protein level was
dramatically increased with the increased expression of miR-126 in
the MSCs (Fig. 2C).
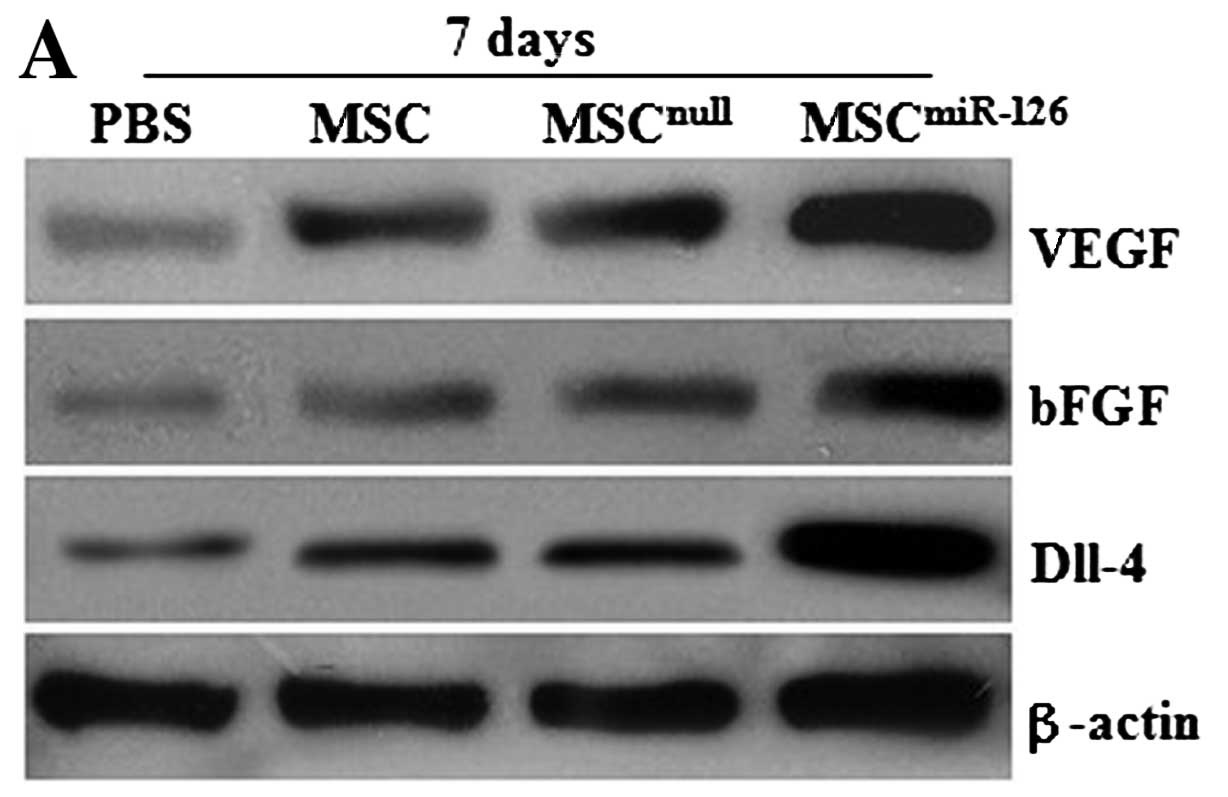
Similarly, no difference was observed in the
expression of VEGF and bFGF proteins between the MSCnull
and the MSC group on days 7 and 14 after transplantation (all
P>0.05) in vivo. However, the protein levels of VEGF and
bFGF in the MSC or the MSCnull group significantly
increased on day 7 compared with the PBS group (all P<0.05), and
obvious differences were also detected on day 14 (all P<0.05).
MSCs modified with miR-126 demonstrated an upregulated expression
of VEGF and bFGF proteins, which was significantly higher than that
in the MSC (P<0.05), the MSCnull (P<0.05), or the
PBS group (P<0.01) (Fig.
3A–D). In addition, 7 days after transplantation, the
expression level of Dll-4 protein in the infarcted tissue was also
significantly higher in the MSCsmiR-126 group than in
the PBS (P<0.001), the MSC (P<0.01), or the
MSCnull group (P<0.01) (Fig. 3A, B and E). Similar effects were
observed at 2 weeks post-transplantation. The level of Dll-4 in the
MSC and the MSCnull group increased compared to the PBS
group at the 2 time-points (both P<0.05); however, no
statistically significant difference was observed between the
MSCnull and the MSC group (P>0.05).
MSC survival in ischemic myocardium
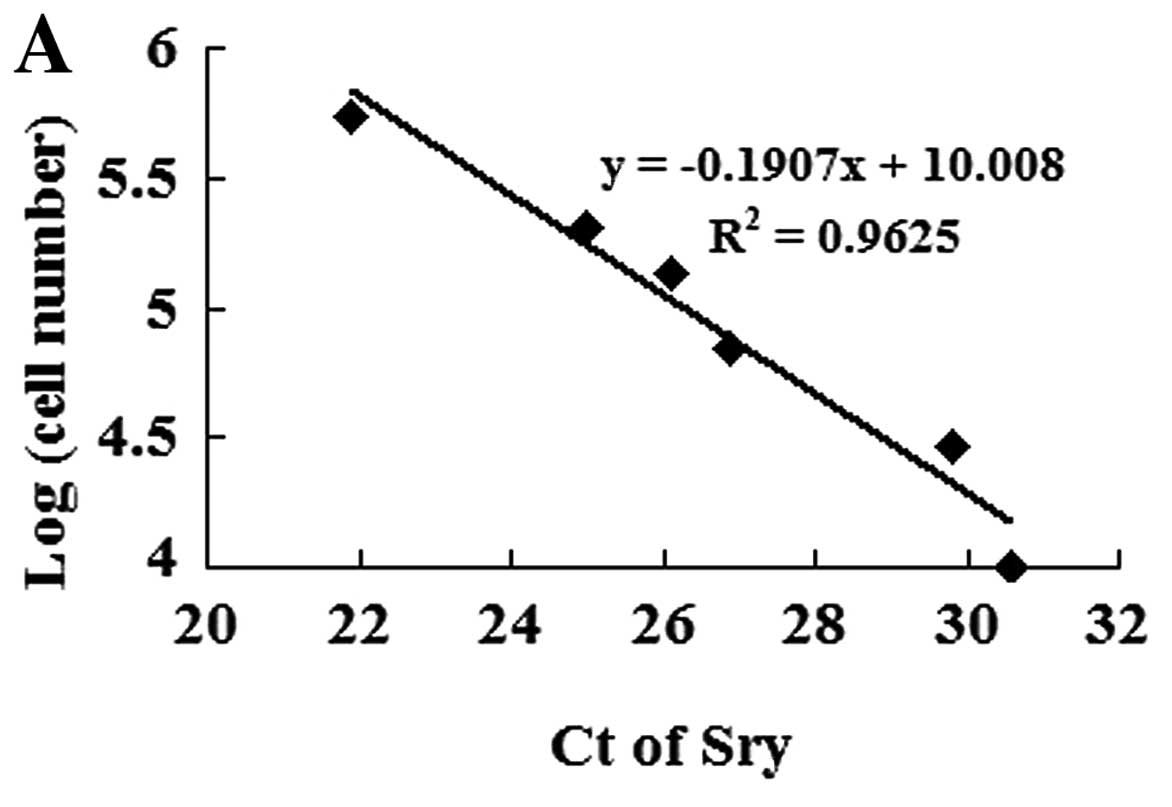
To identify transplanted MSC survival in the
ischemic myocardium, we established a calibration curve of the
number of MSCs in recipient hearts vs. Sry gene expression
(Fig. 4A). Ct values of the Sry
gene were plotted on a semilogarithmic scale of MSC number to
obtain a balanced contribution of all reference dilutions.
Subsequently, hearts (4 in each group) were harvested 7 days after
their respective treatment. In comparison with the
MSCnull or the MSC group, the expression of the Sry gene
in the peri-infarcted and infarcted myocardium was significantly
higher in the MSCmiR-126 group. However, no Sry
expression was detected in the medium-control animals. The number
of MSCs was calculated from the above calibration curve. The
expression of miR-126 significantly increased MSC survival in the
ischemic myocardium (Fig.
4B).
Transplantation of MCSs overexpressing
miR-126 enhances angiogenesis and tubulogenesis
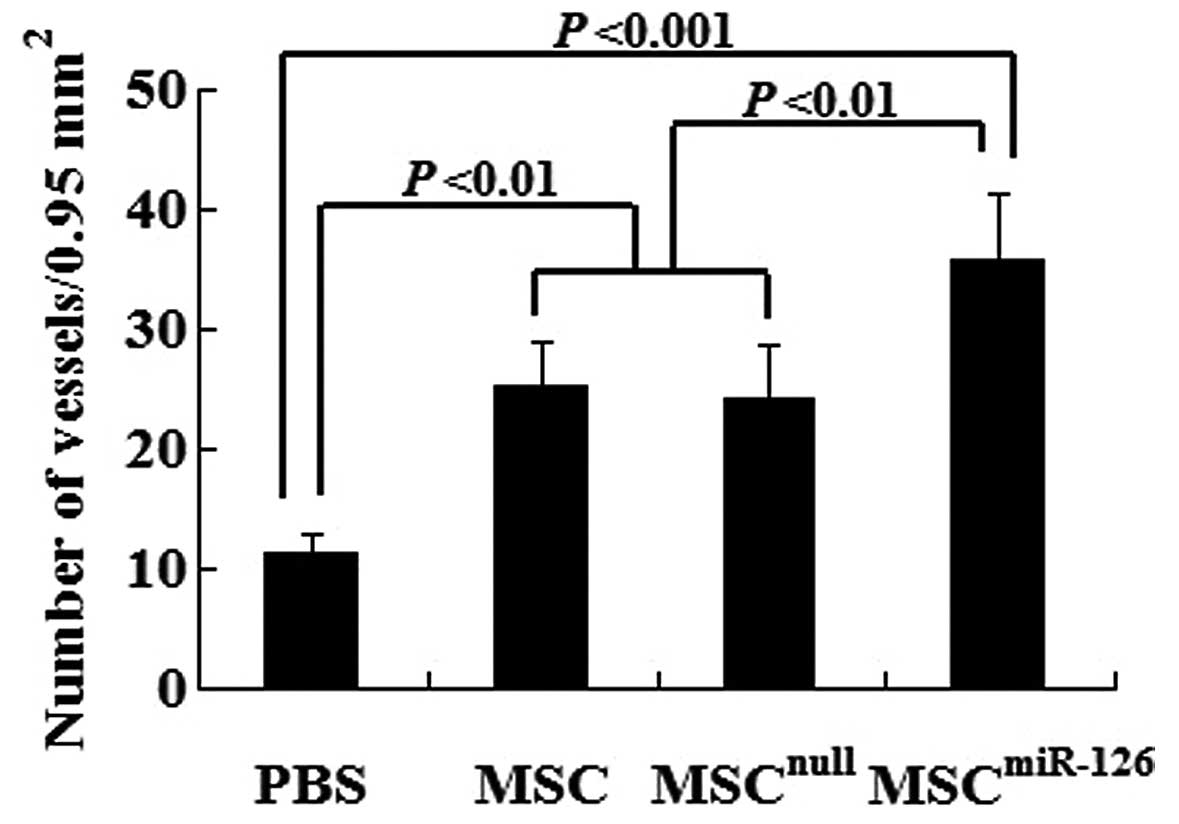
Semiquantitative analysis showed that 2 weeks after
transplantation, the number of mature vessels in the peri-infarcted
and infarcted tissue was significantly increased in the MSC
(25.3±3.6, P<0.01), the MSCnull (24.2±4.5, P<0.01)
and the MSCmiR-126 group (35.7±5.7, P<0.001) compared
to the PBS group (11.2±1.6). No statistically significant
difference was observed between the MSCnull and the MSC
group (P>0.05). However, the mature microvessel number was
significantly greater in the MSCmiR-126 than in the MSC
(P<0.01) or MSCnull group (P<0.01) (Fig. 5).
In addition, to determine whether Notch ligand Dll-4
play a key role in tubulogenesis, we used shRNA to repress the
expression of the Dll-4 gene in MSCmiR-126 cultures. Two
weeks after the transplantation of MSCmiR-126 cultures
in which the expression of Dll-4 had been knocked down, we found
increased immature vessel proliferation in the peri-infarcted area
of mouse hearts. However, mature blood vessels with lumen were
barely detected. This indicates that Dll-4 contributes to
functional angiogenesis.
Transplantation of miR-126-transfected
MCSs increases blood flow in ischemic myocardium
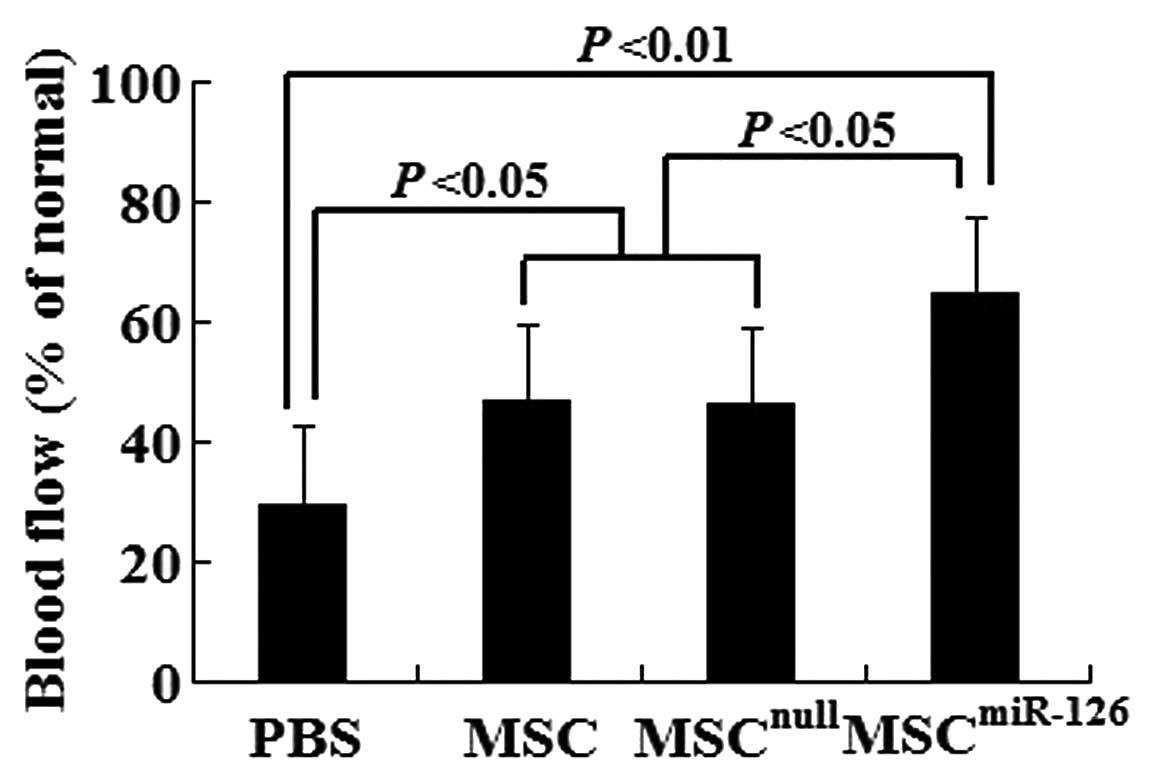
To determine whether new blood vessels translate to
increased coronary blood flow to the infarcted myocardium, we
identified functional microvessels in the infarcted hearts using
the the fluorescent microsphere method for regional blood flow
assessment 2 weeks after transplantation. There was a significant
decrease in blood flow to the infarcted and peri-infarcted zone.
However, MSC and MSCnull treatment increased it to
almost 47% of the normal levels. Of note, the transplantation of
MSCmiR-126 further increased the blood flow to almost
65% of the normal levels (Fig.
6).
Overexpression of miR-126 in MCSs for
transplantation stimulates the improvement of cardiac function
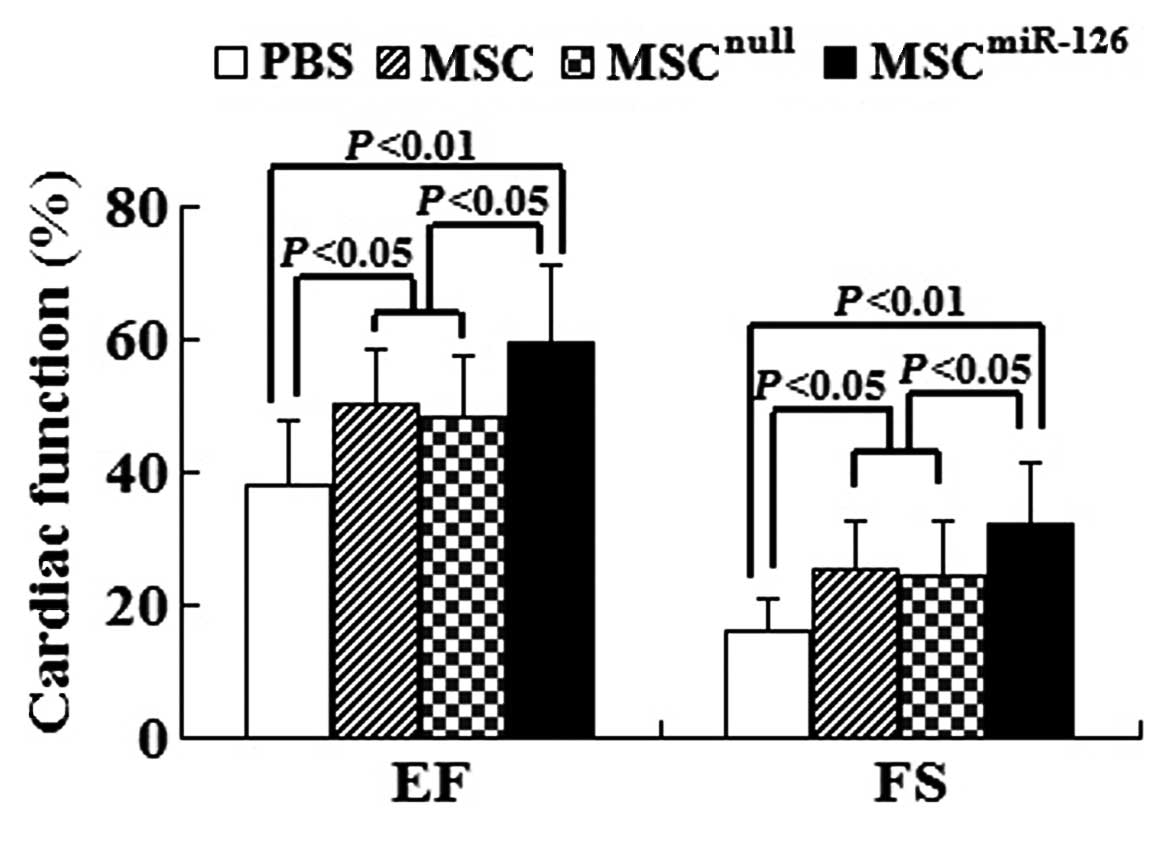
Global heart function was assessed by
echocardiography at 2 weeks post-transplantation. The results
showed that LV function in infarcted C57BL/6 mice in the PBS group
[EF (%), 38.25±9.36; FS (%), 16.31±4.55] was significantly reduced
compared to the MSC group [EF (%), 50.19±8.89; FS (%), 25.58±7.32],
the MSCnull group [EF (%), 48.32±9.37; FS (%),
24.29±8.26] and the MSCmiR-126 group [EF (%),
59.37±10.71; FS (%), 32.41±8.75]. EF and FS in the MSC group and
the MSCnull group were evidently higher than those in
the PBS group (all P<0.05). In comparison with the PBS group, EF
and FS in the MSCmiR-126 group were significantly
improved (both P<0.01). Furthermore, animals transplanted with
MSCmiR-126 showed a marked improvement in EF and FS
compared with transplantation of MSC or MSCnull (all
P<0.05) (Fig. 7).
Discussion
Cell-based therapy appears to be a useful modality
for myocardial repair and reverse remodeling in ischemic heart
disease (26). The direct
injection of isolated cardiomyocytes into injured tissue fails to
enhance heart function since these cells do not thrive or integrate
within the recipient heart (27).
Bone marrow-derived MSCs have been proposed as a novel therapeutic
approach for the improvement of infracted heart function through
the regeneration of the myocardium or neovascularization (28,29). However, low survival and
angiogenic potential of transplanted cells influences the outcome
of MSCs transplantation for the therapy of ischemic heart disease.
Cell sheet grafts with genetically engineered properties to prolong
cell survival and promote blood vessel networks integrated with
pre-existing coronary may offer a potential approach to repair the
dead or injured myocardium after MI.
We characterized the bone marrow-derived MSCs by
immunophenotyping and confirmed that MSCs can be obtained from
mouse bone marrow by serial passage of adherent cells. MSCs can be
transfected with lentiviral vectors ecoding miR-126 with high
efficiency and without any adverse effects on cell viability. MSCs
modified with miR-126 can survive for long periods of time under
hypoxic conditions and effectively express miR-126 for at least 2
weeks in the injected area.
Transplanted MSCs can be identified in the ischemic
area by eGFP or Y-chromosome FITC staining after transplantation.
However, it is not easy to compare the difference in survival rate
of various MSCs. A number of previous studies have evaluated stem
cell survival by observing Sry gene expression in the LV or the
whole heart (25,30,31). Since the Sry gene is located on
the Y chromosome, the gene-positive stem cells can be inferred to
be of male origin. However, Dresske et al (32) reported that the Sry
gene-containing part was mainly localized in the LV adjacent to the
infarcted area after MSCs were injected into the ischemic region.
In our study, the expression of the Sry gene in the entire LV was
extremely low. We assessed the survival of MSCs in the ischemic
myocardium at 7 days post-transplantation, based on the expression
of the Sry gene in the region. To ensure that all transplanted male
MSCs were harvested, we collected enough ischemic tissue including
the border and infarcted zone. To avoid uneven MSC distribution,
MSCs were multiply injected into the peri-infarcted area.
Angiogenesis is the important process by which new
vessels form through the growth of existing vessels, and includes
the proliferation, sprouting, migration and tubulogenesis of
endothelial cells, followed by pruning and remodeling of the
vascular network. It is well known that VEGF and bFGF are major
pro-angiogenic growth factors that play a pivotal role in
angiogenesis. They can also activate several downstream pathways,
including the mitogen-activated protein kinase (MAPK) and PI3K
pathways, to regulate cell motility, proliferation and survival
(33,34). miRNAs, first known as regulators
of development in worms and fruit flies, now act as important
modulators of mammalian cardiovascular development and disease.
miR-126 promotes angiogenesis in response to angiogenic growth
factors, such as VEGF, bFGF and epidermal growth factor (EGF), by
repressing negative regulators of signal transduction pathways.
Thus, it plays an essential role in neoangiogenesis after MI and in
the maintenance of vascular integrity (14). Of note, our data demonstrate that
compared with the MSC and MSCnull cultures,
MSCmiR-126 cultures significantly increased the
secretion of angiogenic factors, such as VEGF and bFGF in
vitro and in vivo. Paracrine effects include the humoral
stimulation of the preservation of pre-existing cells and play a
pivotal role in MSCmiR-126-mediated blood vessel
formation. Both of the factors released from the modified
transplanted stem cells promote angiogenesis in the ischemic area
and represent another therapeutic strategy for cardiac repair.
However, it remains unclear as to how the overexpression of miR-126
increases the paracrine effects of MSCs.
Our study shows that the intra-myocardial injection
of MSCs overexpressing miR-126 into infracted area significantly
enhances microvessel formation in ischemic tissue. Our results
demonstrated a significantly greater capacity to form functional
microvessels and increase blood flow to the infarcted and
peri-infarcted zone in the group receiving MSCs modified with
miR-126 than in the group transplanted with untreated MSCs. In
tandem with the continuous improvement in capillary denisty, the
transplantation of MSCs overexpressing miR-126 further enhanced the
cardiac function in MI animals; this effect was superior to that
exerted by the untransduced MSCs. At 2 weeks post-transplantation,
the cardiac function in the MSCmiR-126 group gradually
recovered, whereas the MSC group or the MSCnull group
did not exhibit such evident effects. This suggests that the
increase in adequately functional vessels and the enhancement of
tissue perfusion play a key role in the improvement of cardiac
function.
The VEGF pathway and Notch signaling are perhaps two
of the most important mechanisms in the regulation of vascular
development. As one of the Notch ligands, Dll-4 functions as an
essential regulator in angiogenesis and tubulogenesis. The
predominant site of Dll-4 expression is the vasculature,
particularly the arteries, arterioles and capillaries during
embryonic development, while in adults its expression is mostly
restricted to small arteries and capillary networks (16,35). Dll-4 plays a role in selecting tip
cells in sprouting and appears to function as an anti-angiogenic
branch factor, negatively regulating proangiogenic factors and
positively regulating vessel maturation factors (36,37). In our study, Dll-4 dramatically
increased with the increased expression of miR-126 in vitro.
The treatment of MSCs overexpressing miR-126 led to upregulation of
Dll-4 in the ischemic myocardium. However, the transplantation of
Dll-4-silenced MSCmiR-126 cultures to the infarcted
heart induced the proliferation of immature vessels with no lumen
and resulted in poor tissue perfusion, which was consistent with
previous reports (38–40). Vascular network formation is
coordinated by VEGF and Dll-4; Dll-4 may act downstream of VEGF as
a ‘brake’ on VEGF-mediated angiogenic sprouting (41). Our findings indicated that the
transplantation of MSCs overexpressing miR-126 led to the
upregulation of Dll-4, which inhibited the angiogenic branch and
enhanced tubulogenesis in the ischemic myocardium, forming
functional microvessels.
In brief, there are 3 main findings in the current
study: i) the genetic modification of MSCs to overexpress miR-126
led to their increased resistance under hypoxic conditions; ii)
miR-126-transduced MSCs increased the release of angiogenic
factors; iii) MSCs overexpressing miR-126 activated Dll-4, which
contributed to the enhanced tubulogenesis. Targeting the expression
of miR-126 in the transplanted MSCs may be a novel therapeutic
strategy for angiogenesis and to restore cardiac function following
MI.
In conclusion, our data demonstrate that the
overexpression of miR-126 results in the upregulation of VEGF, bFGF
and Notch ligand Dll-4 in the MSCs, as well as in the improved
survival of engrafted MSCs and enhanced functional angiogenesis in
the ischemic myocardium. MSCs genetically modified with miR-126
thus be represent a promising approach in neoangiogenesis and
thereby the treatment of ischemic heart disease.
Abbreviations:
|
MSCs
|
mesenchymal stem cells;
|
|
MI
|
myocardial infarction;
|
|
VEGF
|
vascular endothelial growth
factor;
|
|
bFGF
|
basic fibroblast growth factor;
|
|
miRNAs or miRs
|
microRNAs;
|
|
3′-UTR
|
3′-untranslated regions;
|
|
SPRED1
|
Sprouty-related protein 1;
|
|
PIK3R2
|
phosphoinositol-3 kinase regulatory
subunit 2;
|
|
Dll
|
Delta-like;
|
|
Jag
|
jagged;
|
|
NICD
|
Notch intracellular domain;
|
|
bHLH
|
basic helix-loop-helix;
|
|
PBS
|
phosphate-buffered saline;
|
|
shRNA
|
short hairpin RNA;
|
|
FITC
|
fluorescein isothi ocyanate;
|
|
eGFP
|
enhanced green fluorescent
protein;
|
|
LV
|
left ventricular;
|
|
LAD
|
left anterior descending artery;
|
|
LVID
|
left ventricular internal
dimensions;
|
|
EF
|
ejection fraction;
|
|
FS
|
fractional shortening;
|
|
EDV
|
end-diastolic LVID;
|
|
ESV
|
end-systolic LVID;
|
|
ANOVA
|
analysis of variance;
|
|
MTT
|
(3,4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide;
|
|
Ct
|
threshold cycles;
|
|
EGF
|
epidermal growth factor;
|
|
MAPK
|
mitogen-activated protein kinase
|
Acknowledgements
This study was supported by a grant
from the National Nature Scientific Funding of China, 2009 (no.
30871053).
References
|
1.
|
Feygin J, Mansoor A, Eckman P, Swingen C
and Zhang J: Functional and bioenergetic modulations in the infarct
border zone following autologous mesenchymal stem cell
transplantation. Am J Physiol Heart Circ Physiol. 293:H1772–H1780.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Tang YL, Zhao Q, Qin X, et al: Paracrine
action enhances the effects of autologous mesenchymal stem cell
transplantation on vascular regeneration in rat model of myocardial
infarction. Ann Thorac Surg. 80:229–237. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Stamm C, Westphal B, Kleine HD, et al:
Autologous bone-marrow stem-cell transplantation for myocardial
regeneration. Lancet. 361:45–46. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Kinnaird T, Stabile E, Burnett MS, et al:
Local delivery of marrow-derived stromal cells augments collateral
perfusion through paracrine mechanisms. Circulation. 109:1543–1549.
2004. View Article : Google Scholar
|
|
5.
|
Meyer GP, Wollert KC, Lotz J, et al:
Intracoronary bone marrow cell transfer after myocardial
infarction: eighteen months’ follow-up data from the randomized,
controlled BOOST (Bone marrow transfer to enhance ST-elevation
infarct regeneration) trial. Circulation. 113:1287–1294.
2006.PubMed/NCBI
|
|
6.
|
Uemura R, Xu M, Ahmad N and Ashraf M: Bone
marrow stem cells prevent left ventricular remodeling of ischemic
heart through paracrine signaling. Circ Res. 98:1414–1421. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Tang J, Xie Q, Pan G, Wang J and Wang M:
Mesenchymal stem cells participate in angiogenesis and improve
heart function in rat model of myocardial ischemia with
reperfusion. Eur J Cardiothorac Surg. 30:353–361. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Kinnaird T, Stabile E, Burnett MS, et al:
Marrow-derived stromal cells express genes encoding a broad
spectrum of arteriogenic cytokines and promote in vitro and in vivo
arteriogenesis through paracrine mechanisms. Circ Res. 94:678–685.
2004. View Article : Google Scholar
|
|
9.
|
Zhao Y, Samal E and Srivastava D: Serum
response factor regulates a muscle-specific microRNA that targets
Hand2 during cardiogenesis. Nature. 436:214–220. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Zhao Y and Srivastava D: A developmental
view of microRNA function. Trends Biochem Sci. 32:189–197. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Kertesz M, Iovino N, Unnerstall U, Gaul U
and Segal E: The role of site accessibility in microRNA target
recognition. Nat Genet. 39:1278–1284. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Wu L and Belasco JG: Let me count the
ways: mechanisms of gene regulation by miRNAs and siRNAs. Mol Cell.
29:1–7. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Wang S, Aurora AB, Johnson BA, et al: The
endothelial-specific microRNA miR-126 governs vascular integrity
and angiogenesis. Dev Cell. 15:261–271. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Fish JE, Santoro MM, Morton SU, et al:
miR-126 regulates angiogenic signaling and vascular integrity. Dev
Cell. 15:272–284. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Nicoli S, Standley C, Walker P, Hurlstone
A, Fogarty KE and Lawson ND: MicroRNA-mediated integration of
haemodynamics and Vegf signalling during angiogenesis. Nature.
464:1196–1200. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Duarte A, Hirashima M, Benedito R, et al:
Dosage-sensitive requirement for mouse Dll4 in artery development.
Genes Dev. 18:2474–2478. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Krebs LT, Xue Y, Norton CR, et al: Notch
signaling is essential for vascular morphogenesis in mice. Genes
Dev. 14:1343–1352. 2000.PubMed/NCBI
|
|
18.
|
Roca C and Adams RH: Regulation of
vascular morphogenesis by Notch signaling. Genes Dev. 21:2511–2524.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Phng LK and Gerhardt H: Angiogenesis: a
team effort coordinated by notch. Dev Cell. 16:196–208. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Artavanis-Tsakonas S, Rand MD and Lake RJ:
Notch signaling: cell fate control and signal integration in
development. Science. 284:770–776. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Grajales L, Garcia J, Banach K and Geenen
DL: Delayed enrichment of mesenchymal cells promotes cardiac
lineage and calcium transient development. J Mol Cell Cardiol.
48:735–745. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Krampera M, Pasini A, Rigo A, et al:
HB-EGF/HER-1 signaling in bone marrow mesenchymal stem cells:
inducing cell expansion and reversibly preventing multilineage
differentiation. Blood. 106:59–66. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Wang H, Cao F, De A, et al: Trafficking
mesenchymal stem cell engraftment and differentiation in
tumor-bearing mice by bioluminescence imaging. Stem Cells.
27:1548–1558. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Pons J, Huang Y, Takagawa J, et al:
Combining angiogenic gene and stem cell therapies for myocardial
infarction. J Gene Med. 11:743–753. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Li H, Zuo S, He Z, et al: Paracrine
factors released by GATA-4 overexpressed mesenchymal stem cells
increase angiogenesis and cell survival. Am J Physiol Heart Circ
Physiol. 299:H1772–H1781. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Macia E and Boyden PA: Stem cell therapy
is proarrhythmic. Circulation. 119:1814–1823. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Yuasa S and Fukuda K: Cardiac regenerative
medicine. Circ J. 72(Suppl A): A49–A55. 2008. View Article : Google Scholar
|
|
28.
|
Orlic D, Kajstura J, Chimenti S, et al:
Bone marrow cells regenerate infarcted myocardium. Nature.
410:701–705. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Kutryk MJ and Stewart DJ: Angiogenesis of
the heart. Microsc Res Tech. 60:138–158. 2003. View Article : Google Scholar
|
|
30.
|
Sheikh AY, Lin SA, Cao F, et al: Molecular
imaging of bone marrow mononuclear cell homing and engraftment in
ischemic myocardium. Stem Cells. 25:2677–2684. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Terrovitis J, Lautamaki R, Bonios M, et
al: Noninvasive quantification and optimization of acute cell
retention by in vivo positron emission tomography after
intramyocardial cardiac-derived stem cell delivery. J Am Coll
Cardiol. 54:1619–1626. 2009. View Article : Google Scholar
|
|
32.
|
Dresske B, El Mokhtari NE, Ungefroren H,
et al: Multipotent cells of monocytic origin improve damaged heart
function. Am J Transplant. 6:947–958. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Graupera M, Guillermet-Guibert J, Foukas
LC, et al: Angiogenesis selectively requires the p110alpha isoform
of PI3K to control endothelial cell migration. Nature. 453:662–666.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Kerbel RS: Tumor angiogenesis. New Engl J
Med. 358:2039–2049. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Benedito R and Duarte A: Expression of
Dll4 during mouse embryogenesis suggests multiple developmental
roles. Gene Expr Patterns. 5:750–755. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Trindade A, Kumar SR, Scehnet JS, et al:
Overexpression of delta-like 4 induces arterialization and
attenuates vessel formation in developing mouse embryos. Blood.
112:1720–1729. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Williams CK, Li JL, Murga M, Harris AL and
Tosato G: Up-regulation of the Notch ligand Delta-like 4 inhibits
VEGF-induced endothelial cell function. Blood. 107:931–939. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Scehnet JS, Jiang W, Kumar SR, et al:
Inhibition of Dll4-mediated signaling induces proliferation of
immature vessels and results in poor tissue perfusion. Blood.
109:4753–4760. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Noguera-Troise I, Daly C, Papadopoulos NJ,
et al: Blockade of Dll4 inhibits tumour growth by promoting
non-productive angiogenesis. Nature. 444:1032–1037. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Ridgway J, Zhang G, Wu Y, et al:
Inhibition ofDll4 signalling inhibits tumour growth by deregulating
angiogenesis. Nature. 444:1083–1087. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Suchting S, Freitas C, le Noble F, et al:
The Notch ligand Delta-like 4 negatively regulates endothelial tip
cell formation and vessel branching. Proc Natl Acad Sci USA.
104:3225–3230. 2007. View Article : Google Scholar : PubMed/NCBI
|