Introduction
Colorectal cancer (CRC) is the fourth most
frequently diagnosed cancer and the second leading cause of
cancer-related mortality in the United States. According to cancer
statistics in 2012, 143,460 new cases of CRC are diagnosed with
colon or rectal cancer, and 51,690 succumb to CRC, most with
metastatic tumors (1).
Approximately 50% of patients diagnosed with CRC will develop CRC
liver metastases, and 70–80% of these have unresectable metastatic
liver disease (2). The lung is
the second most frequent site of metastasis of CRC. Approximately
8–10% of patients develop pulmonary metastases after resection of
the primary CRC (3). The majority
of patients with metastatic CRC cannot be cured, although a subset
of patients with liver and/or lung-isolated metastatic disease,
local recurrence, or limited intra-abdominal disease is potentially
curable with surgery. For other patients with metastatic CRC,
treatment is palliative and generally consists of systemic
chemotherapy. Significant progress in the systemic treatment of CRC
has been achieved over the past twelve years, with various active
drugs, either in combination or as single agents: 5-FU/LV,
capecitabine, irinotecan, oxaliplatin, bevacizumab, cetuximab and
panitumumab (4). Median overall
survival for patients with metastatic CRC has increased from less
than 9 months with no treatment to approximately 24 months.
However, the majority of patients can not be cured of CRC, and
succumb to the disease in less than 2 years. Novel therapeutic
agents that can provide significant clinical benefit for patients
with advanced CRC are urgently required.
Novel molecular therapies in pre-clinical animal
models and early clinical trials to treat hepatic metastasis from
CRC have been reported (5–7).
Vesicular stomatitis virus (VSV) is a negative-strand RNA virus of
the family Rhabdoviridae with potent oncolytic properties
that is exquisitely sensitive to the antiviral actions of type 1
interferons (IFN-α/β) in normal but not in cancer cells (8,9).
It is considered to be due to the fact that IFN-responsive
anti-viral pathways are defective in many types of tumors (8–11).
We previously demonstrated that repeated administrations (every
other day for 4 days, 3 injections total) of VSV-NDV/F(L289A),
which is an engineered VSV expressing a fusogenic membrane
glycoprotein from a heterologous virus, through a permanent cannula
surgically implanted into the hepatic artery, led to sustained
tumor-selective virus replication and substantially enhanced its
oncolytic potential in the treatment of advanced multifocal
hepatocellular carcinoma in the liver of rats (12).
In this study, we generated an orthotropic model of
CRC in the livers or the lungs of syngeneic and immune competent
rats and investigated the feasibility of repeated intravenous
infusions of rVSV-NDV/F(L289A) for multiple CRC lung metastases,
compared with the feasibility of repeated hepatic arterial
infusions for multifocal CRC liver metastases in immune competent
rats.
Materials and methods
Cell lines and culture conditions
The rat CRC cell line RCN-H4 was obtained from RIKEN
BioResource Center Cell Bank (Ibaraki, Japan). RCN-H4 is a subclone
established by Inoue et al (13) according to Fidler’s method; it has
a high potency for forming experimental liver metastatic tumors.
RCN-H4 was maintained in RPMI-1640 medium (Sigma-Aldrich, St.
Louis, MO, USA) containing 10% heat-inactivated fetal bovine serum
(FBS) and 0.05% penicillin-streptomycin solution (were from
Sigma-Aldrich).
Recombinant VSV-NDV/F(L289A) vectors
Recombinant VSV vector expressing mutant (L289A)
Newcastle disease virus fusion protein [rVSV-NDV/F(L289A)] has
previously been described (14).
Viral titers of working stocks were determined on BHK-21 cells by
using standard plaque assays. A resulting titer for
rVSV-NDV/F(L289A) was 1.4×109 plaque-forming units
(pfu)/ml.
In vitro cytotoxicity assay
RCN-H4 cells were seeded in 24-well plates at
5×104 cells/well with RPMI-1640 medium containing 10%
FBS. After two days, they were infected with rVSV-NDV/F(L289A) at a
multiplicity of infection (MOI) of 0.0001, 0.001, 0.01 or 0.1. Cell
viability was measured at the indicated time points after infection
by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
(MTT) assay (Cell Proliferation kit I; Roche Diagnostics,
Indianapolis, IN, USA). All cell viability results are expressed as
percentage of viable cells compared to mock-infected control at
each time point. To test the effect of fresh serum on rVSV tumor
cell killing, RCN-H4 was cultured in RPMI-1640 medium containing
10% heat-inactivated rat serum or fresh serum. Rat serum was
collected from F344/DuCrj rats. Fresh or heat-inactivated serum was
diluted with RPMI-1640 medium (1:9 dilution).
Orthotopically multifocal CRC metastasis
model in syngeneic rats
Inbred male F344/DuCrj rats (7–8 weeks old; 150–180
g) were purchased from Charles River Japan, Inc. (Chiba, Japan) and
housed in a specific pathogen-free environment under standard
conditions. All procedures involving animals were approved by the
Hiroshima University Animal Ethics Committee and were performed
according to their guidelines. Rats were anesthetized with 100
mg/kg ketamine intraperitoneally and isoflurane using an inhalation
anesthesia system (Bio Machinery, Chiba, Japan). Subsequently, rats
were implanted with 5×106 syngeneic RCN-H4 cells in 20
μl of RPMI-1640 in the left lateral and right central lobe
of the liver to establish multiple liver metastases of CRC. In
order to establish multiple lung metastases of CRC, rats were
infused with 1×106 syngeneic RCN-H4 cells in 1 ml of
RPMI-1640 via the penial vein.
Surgical placement of an indwelling
intrahepatic artery cannula for repeated vector administration
Rats were anesthetized and underwent laparotomy 21
days after tumor cell implantation. The hepatic vessels (common
hepatic artery, proper hepatic artery, and gastroduodenal artery)
were dissected with the aid of an operating microscope. The
Preclinical Mini-Port implantable access device (Deltec, Inc., St.
Paul, MN, USA) was used to administer the vector repeatedly via the
hepatic artery. After ligation of the gastroduodenal artery with
7-0 Prolene (Ethicon, Somerville, NJ, USA), a 2-French clear
Polyurethane catheter (outer diameter 0.63 mm, inner diameter 0.30
mm) was inserted into the gastroduodenal artery. The common hepatic
artery was ligated to prevent the rVSV vector from flowing
backward. The Preclinical Mini-Port device was then implanted into
a subcutaneous pocket in the umbilical area.
rVSV-NDV/F(L289A) virotherapy
rVSV-NDV/F(L289A) vector (4.0×106 pfu) in
1 ml of phosphate-buffered saline (PBS) was administered 3 times
for 3 consecutive days via the port system. CRC lung metastasis
models were infused intravenously via the penial vein at 14 days
after tumor cell infusion.
Assessment of serum chemistries
Blood samples were collected from the inferior vena
cava of each rat on Days 1 and 3. The levels of alanine transferase
(ALT), aspartate transferase (AST), creatinine, and blood urea
nitrogen (BUN) were determined at the Chemistry Laboratory at
Fukuyama Medical Laboratories (Fukuyama, Japan).
Histology and immunohistochemical
stainings
At indicated time points after rVSV-NDV/F(L289A)
vector infusion into the hepatic artery, animals were sacrificed.
Livers were fixed in 4% paraformaldehyde overnight, and were then
paraffin-embedded. Sections (5 μm) were subjected to either
hematoxylin and eosin (H&E) staining for histological analysis
or immunohistochemistry using rabbit polyclonal antibodies against
cleaved caspase-3 (Cell Signaling Technology, Danvers, MA, USA) for
assessment of rVSV-mediated apoptosis. Immunohistochemistry
sections were counterstained with hematoxylin.
Statistical analysis
For comparison of the means of two groups, the
two-sided Student’s t-test for independent groups was applied to
determine statistical significance. Survival curves of animals were
plotted according to the Kaplan-Meier method. Statistical
significance in different treatment groups was compared using the
log-rank test. All statistical analyses were performed using
StatView software version 5.0 (Abacus Concepts, Berkeley, CA).
Results
Oncolytic activities of rVSV-NDV/F(L289A)
in rat CRC cells in vitro
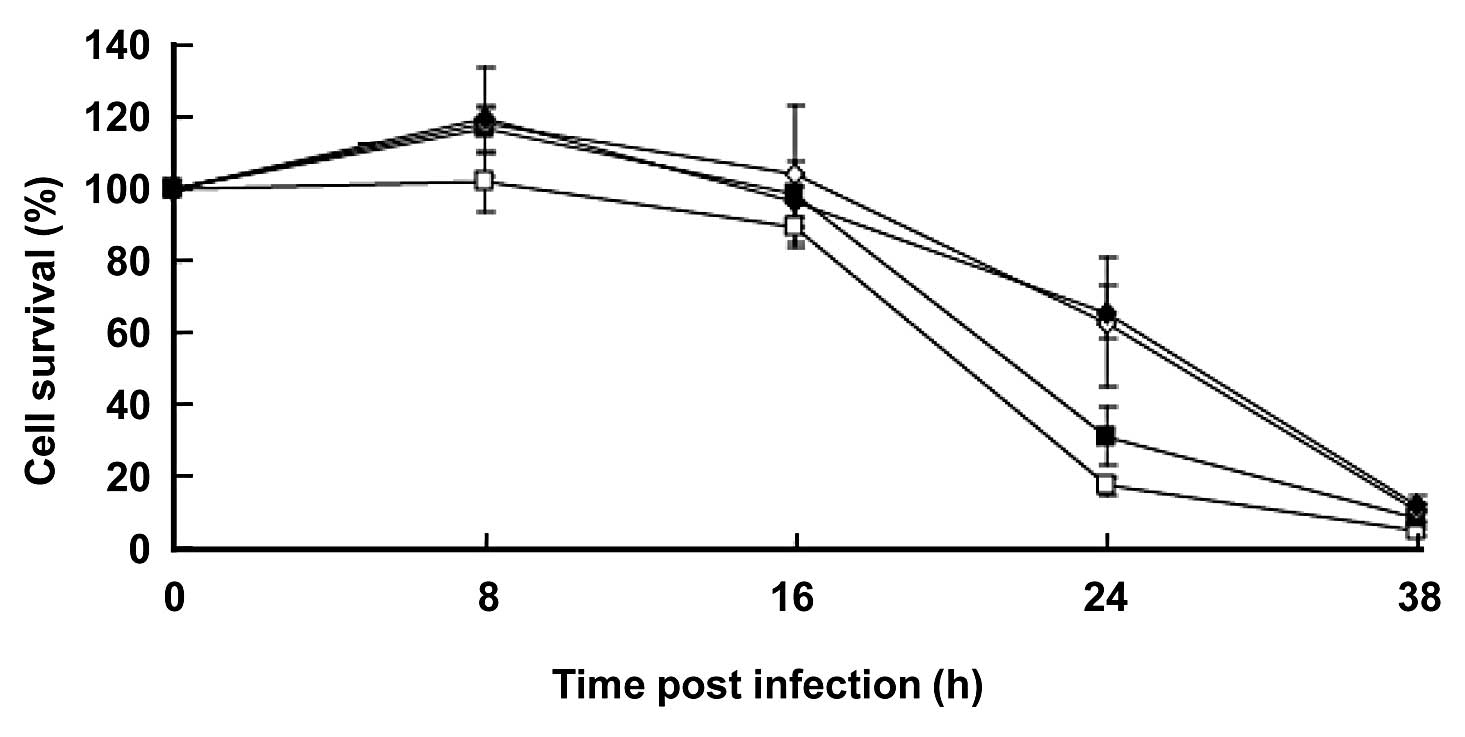
To assess the cytotoxic effect of rVSV-NDV/F(L289A)
on the rat CRC cell line, we infected RCN-H4 cells with the virus
at various MOIs. Percentage of cell survival was quantified by MTT
assays as a fraction of mock-infected cells at each time point
(Fig. 1). The rVSV-NDV/F(L289A)
killed tumor cells in a dose-dependent manner up to 24 h. At 32 h,
almost 100% of the cells were killed even at 0.0001 MOI of
rVSV-NDV/F(L289A). This suggested that extremely low doses of
rVSV-NDV/F(L289F) can kill RCN-H4 cells efficiently in
vitro.
Animal models of multifocal CRC liver or
multiple CRC lung metastasis
At 21 days after implantation of RCN-H4 cells in the
left lateral and right central lobe of the liver, 100% of animals
developed two sites of CRC lesions of up to 20 mm in diameter in
their livers. Peritoneal dissemination was not detected. At 14 days
following venous infusion of RCN-H4 cells, 100% of animals
developed multiple CRC metastatic lesions of up to 2 mm in diameter
in their lungs (data not shown).
Absence of organ toxicity after repeated
hepatic arterial infusions or intravenous infusions of
rVSV-NDV/F(L289A)
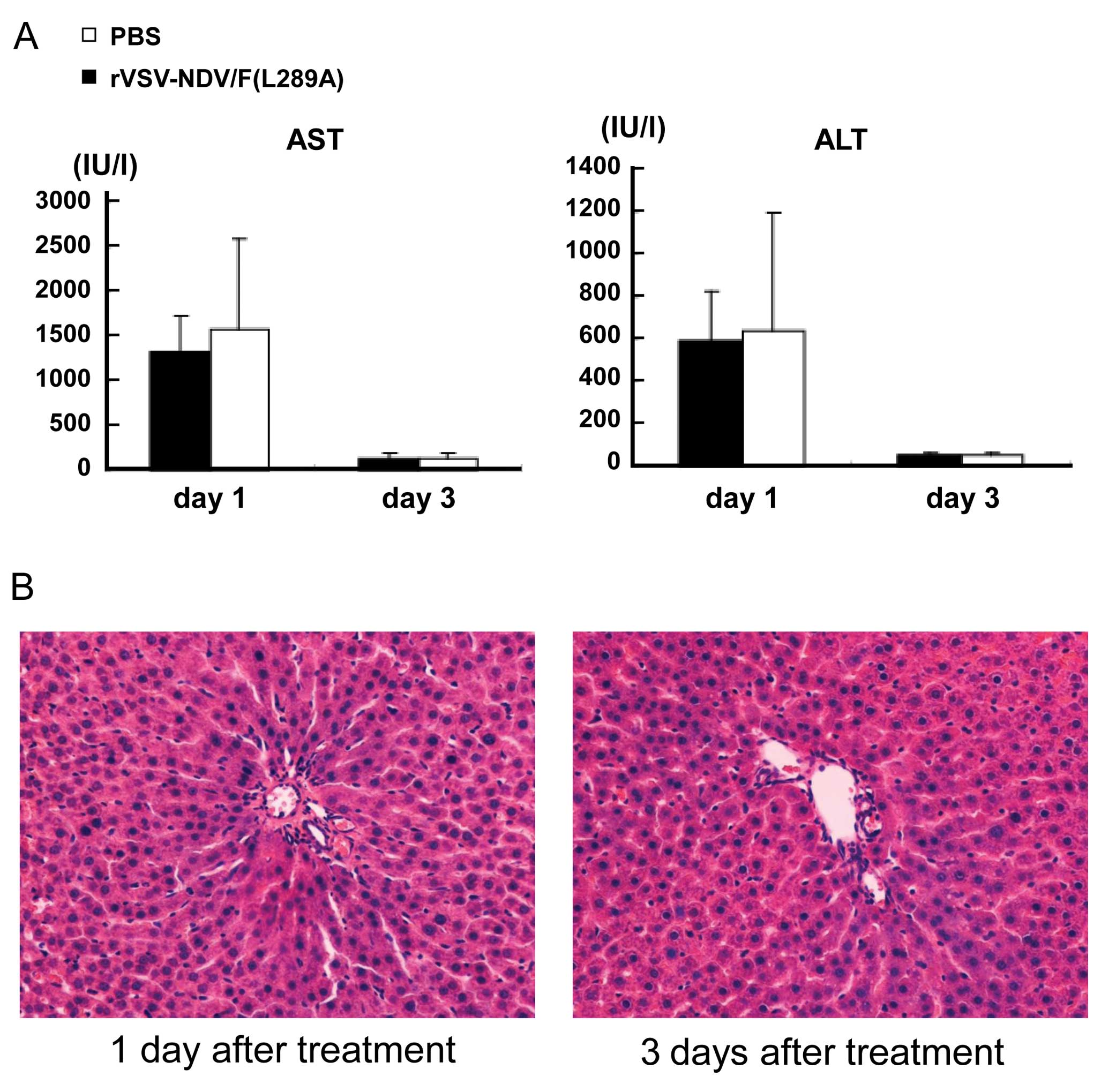
The maximum tolerated dose of rVSV-NDV/F(L289A) of
immune competent rats was 1.3×107 pfu (15). We evaluated the potential
hepatotoxicity after three injections of 4.0×106 pfu of
rVSV-NDV/F(L289A) through the indwelling intrahepatic artery
catheter. Rats with liver metastases were infused with
rVSV-NDV/F(L289A) (n=3) on Days 0–2, or sham operation was
performed (n=3). Blood samples were obtained from each rat on Days
1 and 3. The kinetic profiles of serum transaminase levels
(aspartate aminotransferase and alanine aminotransferase) were
determined (Fig. 2A). When
rVSV-NDV/F(L289A) was administered via the hepatic artery, the
common hepatic artery was ligated to prevent rVSV-NDV/F(L289A) from
flowing backward. Transient elevations of serum transaminases (AST
and ALT) were seen at Day 1 after treatment in both groups, but the
levels rapidly returned to baseline at Day 3. There were no
significant differences in both groups. Additionally, the histology
of the neighboring hepatic parenchyma was completely normal
(Fig. 2B). Therefore, transient
elevation of serum transaminase was possibly due to ligation of the
common hepatic artery, and there was no remarkable hepatotoxicity
associated with three injections of rVSV-NDV/F(L289A) via the
hepatic artery.
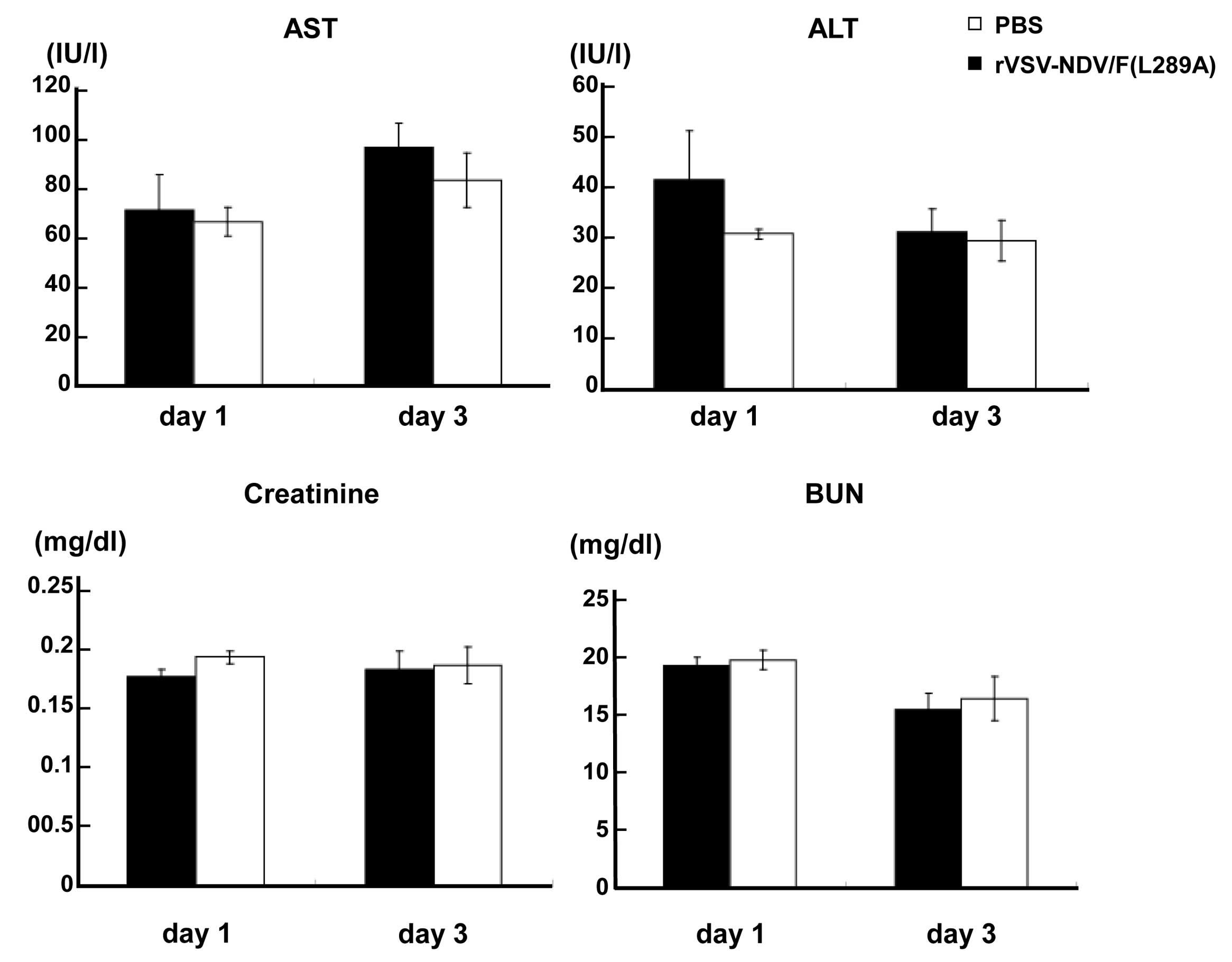
In addition, we evaluated potential organ toxicity
after 3 injections of intravenous infusions of 4.0×106
pfu of rVSV-NDV/F(L289A) in the multiple lung metastases model.
Blood samples were obtained from each rat on Days 1 and 3. The
kinetic profiles of serum transaminases (AST and ALT), creatinine
and BUN as indications of liver and kidney damage, respectively,
were determined (Fig. 3). There
were no significant differences of AST, ALT, creatinine and BUN in
both groups, indicating a lack of hepato- and nephrotoxicity
associated with repeated intravenous infusions of
rVSV-NDV/F(L289A).
In vivo antitumor efficacy of repeated
hepatic arterial infusions in multifocal CRC liver metastases
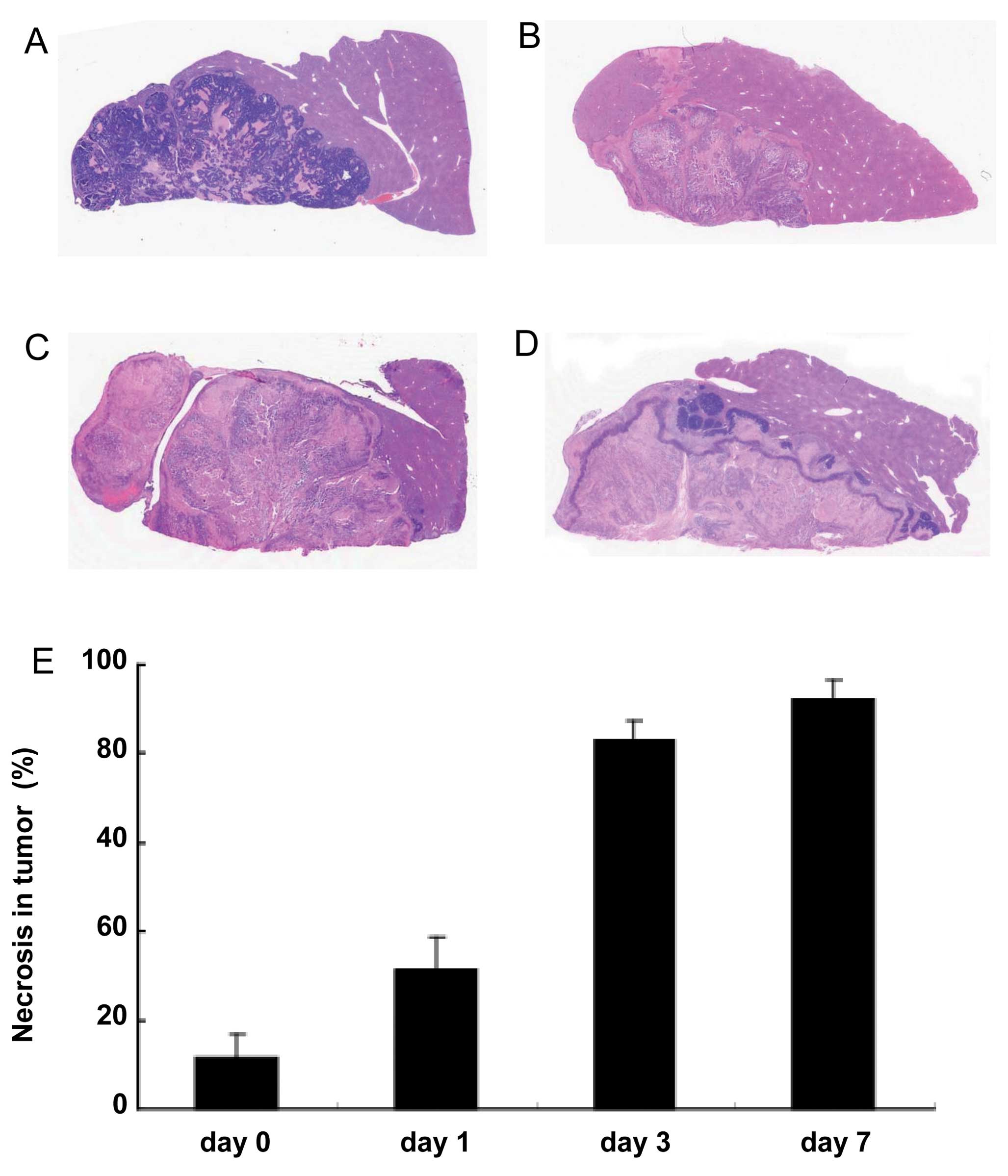
To demonstrate the antitumor efficacy of repeated
hepatic arterial infusions of rVSV-NDV/F(L289A), rats were
sacrificed at indicated time points (Days 0, 1, 3 and 7) and the
tumor-bearing livers were excised for histological analysis. In the
control liver sections, most areas within the tumor were viable
tumor cells (Fig. 4A). By
contrast, we detected large necrotic areas within the tumor in the
rVSV-NDV/F(L289A)-treated liver at Day 7 (Fig. 4D). We measured the percentage of
necrotic areas to the total area in representative tumor sections
by using a BZ-H1M3 program and image analyzer (Keyence, Osaka)
(Fig. 4E). The percentage of
necrotic areas before treatment was 11.5%. At Day 1, after a single
infusion of rVSV-NDV/F(L289A), the percentage was 31.4% (Fig. 4B). At Day 3, after repeated
infusions of rVSV-NDV/F(L289A), the percentage was 83.2% (Fig. 4C). Finally, the percentage of
necrotic areas further increased to 92.3% at Day 7 (Fig. 4D). Liver parenchyma neighboring
tumors showed no signs of pathology. These results suggested that 3
injections of rVSV-NDV/F(L289A) through the hepatic artery resulted
in tumor-selective necrosis and no remarkable hepatotoxicity.
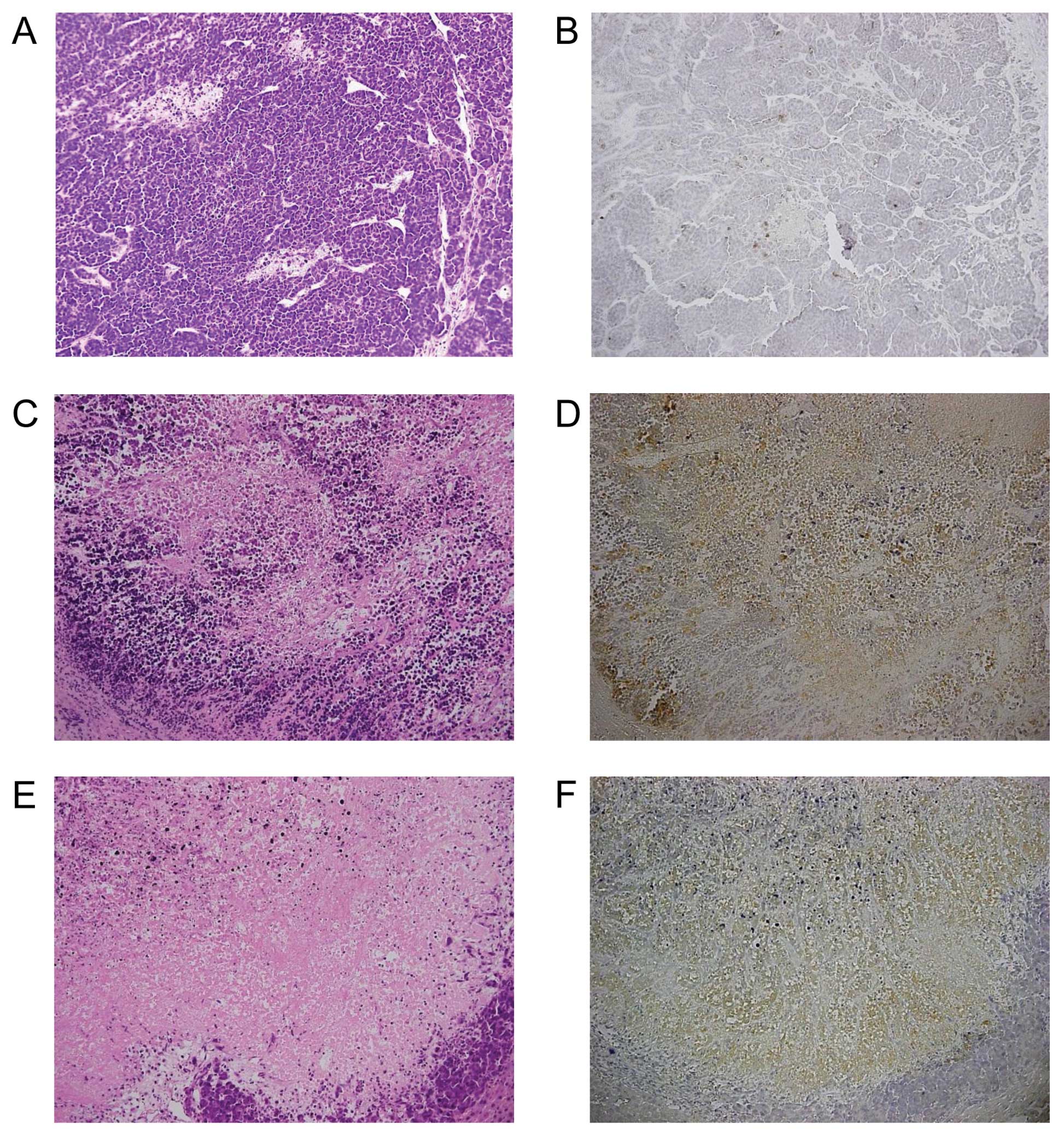
In order to elucidate the mechanism of the antitumor
effect by rVSV, we analyzed tumor samples obtained at Day 0 (before
treatment), Days 1 and 3 by immunohistochemistry using rabbit
polyclonal antibodies against activated caspase-3. Caspase-3 is one
of the key executioners of apoptosis, as it is either partially or
totally responsible for the proteolytic cleavage of many key
proteins such as the nuclear enzyme polymerase (16). rVSV causes lysis of infected tumor
cells through activation of apoptotic pathways (17). At Day 0, minimal spontaneous
necrotic areas within the tumor lesions were found (Fig. 5A), which were stained indistinctly
by cleaved caspase-3 antibody (Fig.
5B). By contrast, at Day 1, we detected more necrotic areas in
the tumor lesions (Fig. 5C),
which were stained diffusely positive by cleaved caspase-3 antibody
(Fig. 5D). At Day 3, most areas
within the tumors were necrotic (Fig.
5E). A few viable tumor cells were detected at the rim of the
tumor, which were stained weakly (Fig. 5F). It was suggested that
rVSV-NDV/F(L289A) induced the apoptosis of tumor cells.
Prolonged survival of CRC liver
metastasis-bearing rats treated with hepatic arterial infusions of
rVSV-NDV/F(L289A)
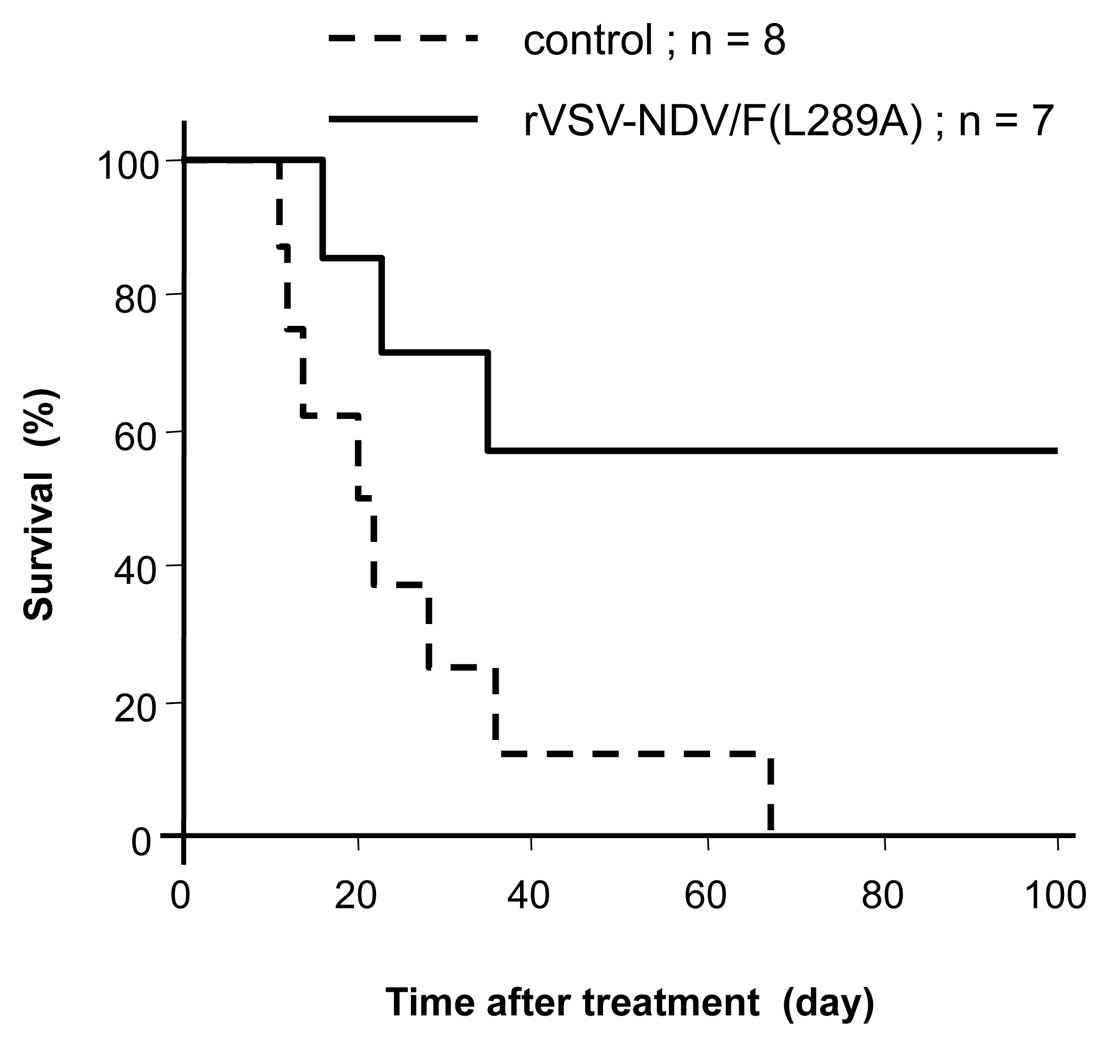
To assess the potential of rVSV-NDV/F(L289A) as a
therapeutic agent for multifocal CRC liver metastasis, rats bearing
two sites of CRC tumors in their livers with sizes ≤20 mm in
diameter were randomly assigned to 3 injections of hepatic arterial
infusions with 4.0×106 pfu of rVSV-NDV/F(L289A) (n=7) or
subjected to sham operation (n=8) and survival was followed
(Fig. 6). Sham-operated rats
started to die of tumor progression in 11 more days and all of them
expired at 68 days (median survival, 25 days). Autopsy revealed
massive liver metastases. By contrast, rats treated with
intrahepatic arterial infusion of rVSV showed significantly
prolonged survival (P= 0.0196). By 100 days post-treatment, the
median survival had not been reached. Autopsy of the VSV-treated
rats that died demonstrated multiple lung metastases and solitary
liver metastases. However, we did not detect massive hepatomegaly
with disseminated liver metastases nor peritoneal dissemination.
The long-term surviving rats were sacrificed at 190 days after
treatment and evaluated for residual malignancy. No metastatic
liver tumors were detected, although some rats had metastatic lung
tumors.
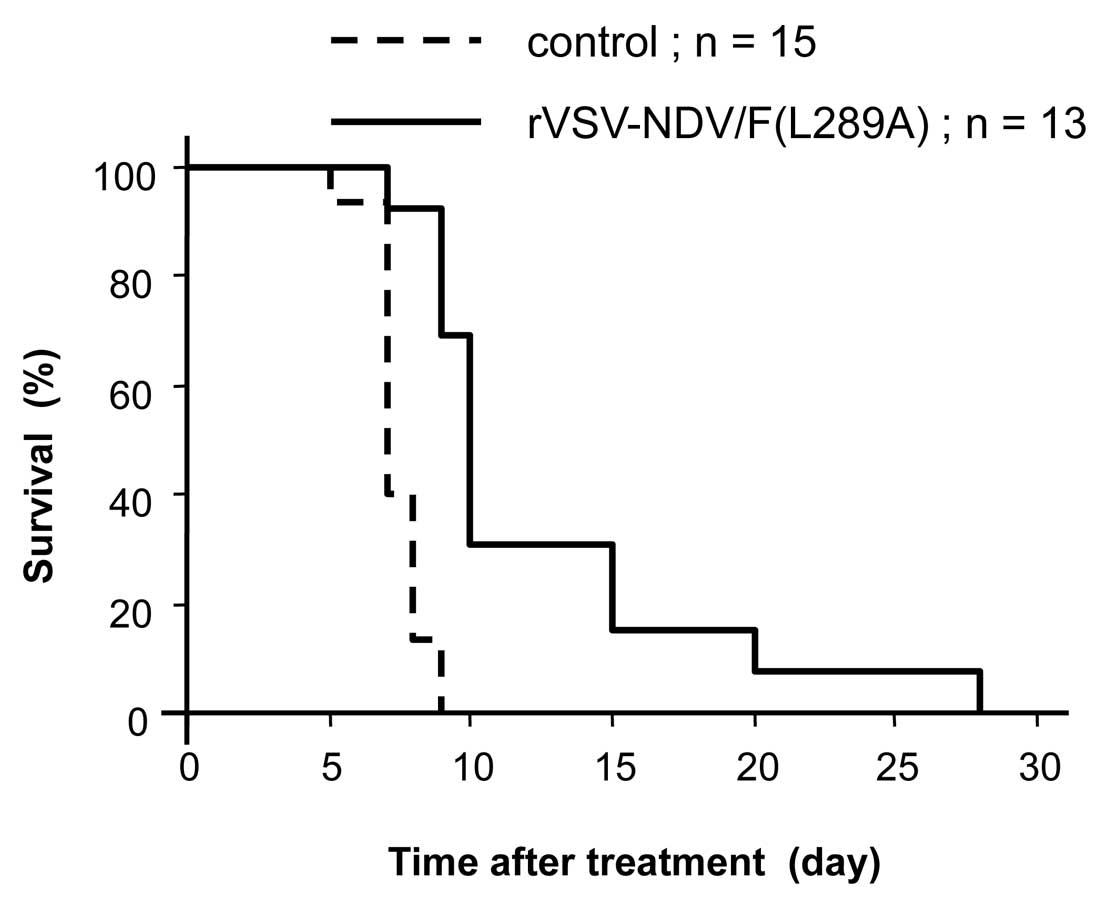
Prolonged survival of CRC lung
metastasis-bearing rats treated with intravenous infusions of
rVSV-NDV/F(L289A)
To assess the potential of rVSV-NDV/F(L289A) as a
therapeutic agent for multiple CRC lung metastases, rats bearing
multiple lung metastases were randomly assigned to 3 injections of
intravenous infusions with 4.0×106 pfu of
rVSV-NDV/F(L289A) (n=13) or PBS (n=15) via the penial vein, and
survival was followed (Fig. 7).
PBS-treated rats started to die of tumor progression in 5 more days
and all of them expired at 9 days (median survival, 7 days). The
rVSV-NDV/F(L289A)-treated rats survived until 28 days post vector
injection (median survival, 10 days). Although the differential
survival rates were statistically significant by log-rank test
analysis (P<0.001), no rats treated with rVSV-NDV/F(L289A)
achieved long-term survival.
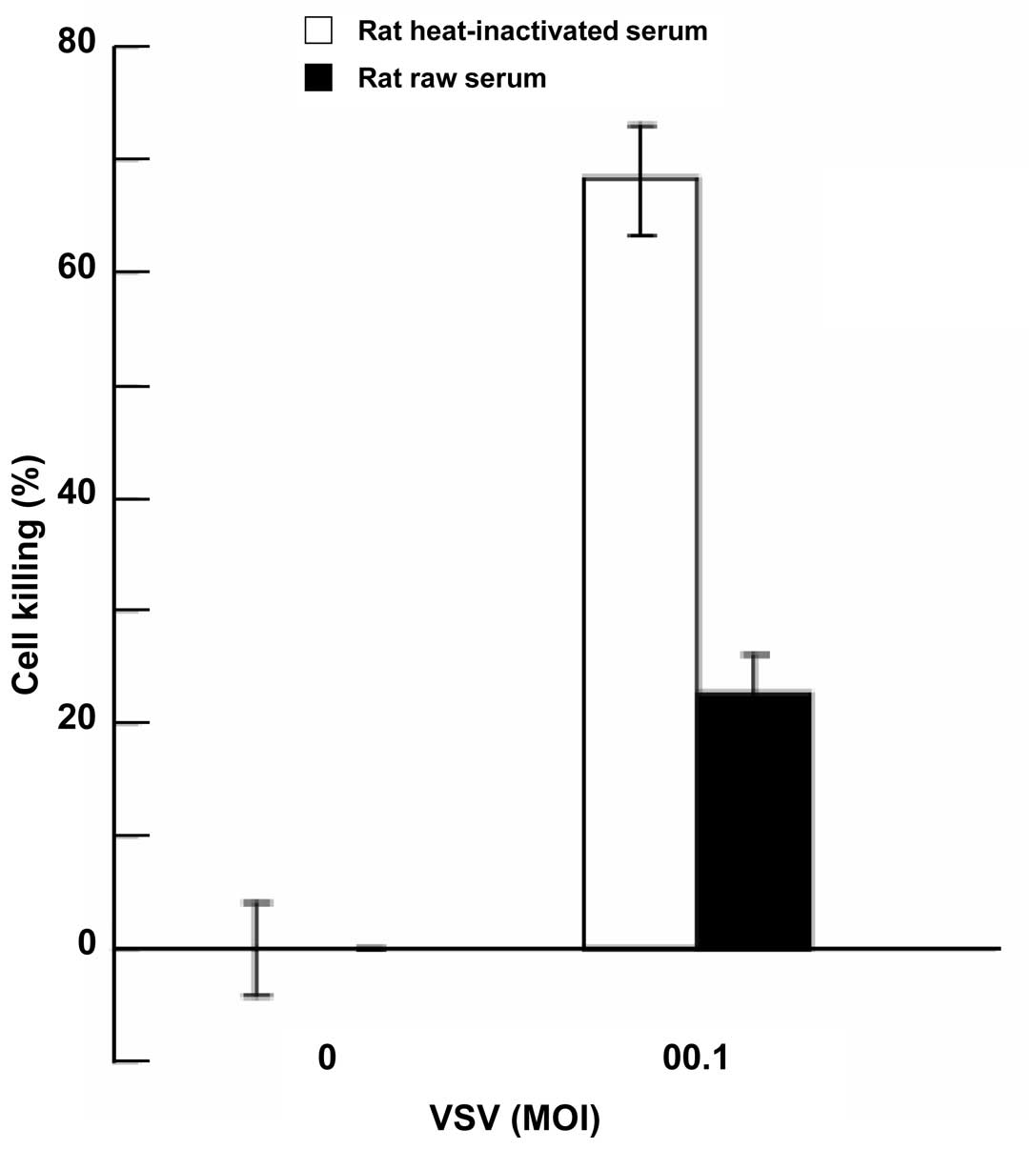
Effect of fresh rat serum on
rVSV-NDV/F(L289A)-mediated tumor killing
This study suggested that antitumor efficacy of
intravenous infusion of rVSV-NDV/F(L289A) against CRC lung
metastasis was strongly reduced, compared with locoregional
delivery such as hepatic arterial infusion against CRC liver
metastasis. Locoregional delivery is generally applied with the
intention of obtaining a high drug concentration within the tumor
tissue and low systemic drug levels avoiding systemic toxicity.
Intravenous infusion might reduce the local concentration of rVSV
within the tumors. To assess the effect of rat serum on
rVSV-NDV/F(L289A)-mediated tumor cell killing, tumor cells were
infected with rVSV-NDV/F(L289A) at an MOI of 0.01 in RPMI-1640
medium containing 10% rat fresh serum or 10% rat heat-inactivated
serum. rVSV-mediated oncolysis at 24 h after infection was
significantly suppressed by rat fresh serum (P=0.0015) (Fig. 8).
Discussion
Oncolytic virotherapy is expected to prove valuable
for treating cancer patients. VSV as well as other viruses
including adenovirus, herpes simplex virus, reovirus, autonomous
parvovirus, Newcastle disease virus, measles virus and several more
have been developed as oncolytic agents for cancer treatment
(18). Compared with other
replication-competent oncolytic vectors, VSV is very attractive as
an oncolytic agent. The occurrence of antibodies against VSV in the
general human population is extremely low, except in those regions
where it is endemic, such as Georgia, USA, and Central America
(19,20). In addition, VSV can infect
numerous types of tumor cells due to the widely tropic nature of
the VSV G protein (21).
Oncolytic virus therapy has the potential to destroy a tumor mass
of unlimited size, if indeed productive virus infection spreads
from one infected cell to another (22). However, the use of oncolytic
viruses in destroying tumors in clinical trials has not yet been
very successful in most cases (23). This indicates that the infectious
virus dose can not spread efficiently through a tumor mass. In the
present study, we demonstrated that repeated hepatic arterial
infusions of rVSV-NDV/F(L289A) in multifocal liver metastases from
CRC dramatically increased antitumor efficacy and caused long-term
survival (more than 100 days) in the majority of the treated
animals. We previously reported that a single hepatic arterial
infusion of rVSV led to prolongation of rats bearing multiple CRC
liver metastases without any long-term survivor (24). This difference in anti-tumor
efficacy in vivo suggests that repeated administrations
might cause rVSV to spread more efficiently within the tumor
lesion. Repeated virus administration might increase tumor vessel
leakage.
The lung is the second most frequent site of
metastasis of CRC. To assess the potential of repeated infusions of
rVSV in pulmonary CRC metastases, we established the multiple
pulmonary metastases models with the same tumor cell line in
syngeneic rats. To treat pulmonary CRC metastases with
rVSV-NDV/F(L289A), the same dose of virus was used. The only
difference was the injection route, which was via the penial vein.
Although the differential survival rates were statistically
significant by log-rank test analysis (P<0.001), no rats treated
with rVSV-NDV/F(L289A) achieved long-term survival. When an
oncolytic virus is given systemically to animals (for example, by
intravenous injection), there are many barriers that prevent it
from reaching the tumor and infecting cancer cells. The virus
enters the circulation, where it can be quickly neutralized through
absorption by blood cells, through the complement cascade or by
neutralizing antibodies (25). We
examined the effect of rat serum on rVSV-NDV/F(L289A)-mediated
tumor cell killing. We confirmed that rVSV-mediated oncolysis could
be impeded by an antiviral activity present in rat fresh serum. The
mechanism of inactivation of rVSV by rat fresh serum might involve
the components of the classical C pathway through C3b and a
nonimmunoglobulin serum factor (26). To treat metastatic CRC more
effectively with intravenous infusion of rVSV, we will need to
overcome these barriers. Depending on a virus’s clearance, a marked
increase in the local concentration of the virus could be achieved
by injection into the feeding artery.
In the present study, we demonstrated that repeated
infusions of rVSV-NDV/F(L289A) were both effective and safe in the
treatment of multifocal liver metastases as well as lung metastases
of CRC in immune competent rat models, although systemic venous
delivery is less effective than locoregional delivery such as
hepatic arterial infusion. Locoregional deliver of rVSV could avoid
its inactivation by fresh serum to maximize its antitumor
efficacy.
Acknowledgements
The study was supported by
Grants-in-Aid for Scientific Research (C) 16591322 from the
Ministry of Education, Science, Sports, Culture and Technology of
Japan.
References
|
1.
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar
|
|
2.
|
Wieser M, Sauerland S, Arnold D, Schmiegel
W and Reinacher-Schick A: Peri-operative chemotherapy for the
treatment of resectable liver metastases from colorectal cancer: A
systematic review and meta-analysis of randomized trials. BMC
Cancer. 10:3092010. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Murata S, Moriya Y, Akasu T, Fujita S and
Sugihara K: Resection of both hepatic and pulmonary metastases in
patients with colorectal carcinoma. Cancer. 83:1086–1093. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Wolpin BM and Mayer RJ: Systemic treatment
of colorectal cancer. Gastroenterology. 134:1296–1310. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Havlik R, Jiao LR, Nicholls J, Jensen SL
and Habib NA: Gene therapy for liver metastases. Semin Oncol.
29:202–208. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Mayer-Kuckuk P, Banerjee D, Kemeny N, Fong
Y and Bertino JR: Molecular therapies for colorectal cancer
metastatic to the liver. Mol Ther. 5:492–500. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Ruan DT and Warren RS: Liver-directed
therapies in colorectal cancer. Semin Oncol. 32:85–94. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Stojdl DF, Lichty B, Knowles S, et al:
Exploiting tumor-specific defects in the interferon pathway with a
previously unknown oncolytic virus. Nat Med. 6:821–825. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Balachandran S and Barber GN: Vesicular
stomatitis virus (VSV) therapy of tumors. IUBMB Life. 50:135–138.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Balachandran S, Porosnicu M and Barber GN:
Oncolytic activity of vesicular stomatitis virus is effective
against tumors exhibiting aberrant p53, Ras, or myc function and
involves the induction of apoptosis. J Virol. 75:3474–3479. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Balachandran S and Barber GN: Defective
translational control facilitates vesicular stomatitis virus
oncolysis. Cancer Cell. 5:51–65. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Shinozaki K, Ebert O and Woo SL:
Eradication of advanced hepatocellular carcinoma in rats via
repeated hepatic arterial infusions of recombinant VSV. Hepatology.
41:196–203. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Inoue Y, Kashima Y, Aizawa K and
Hatakeyama K: A new rat colon cancer cell line metastasizes
spontaneously: biologic characteristics and chemotherapeutic
response. Jpn J Cancer Res. 82:90–97. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Ebert O, Shinozaki K, Kournioti C, Park
MS, Garcia-Sastre A and Woo SL: Syncytia induction enhances the
oncolytic potential of vesicular stomatitis virus in virotherapy
for cancer. Cancer Res. 64:3265–3270. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Shinozaki K, Ebert O, Kournioti C, Tai YS
and Woo SL: Oncolysis of multifocal hepatocellular carcinoma in the
rat liver by hepatic artery infusion of vesicular stomatitis virus.
Mol Ther. 9:368–376. 2004. View Article : Google Scholar
|
|
16.
|
Fernandes-Alnemri T, Litwack G and Alnemri
ES: CPP32, a novel human apoptotic protein with homology to
Caenorhabditis elegans cell death protein Ced-3 and
mammalian interleukin-1 beta-converting enzyme. J Biol Chem.
269:30761–30764. 1994.PubMed/NCBI
|
|
17.
|
Kopecky SA, Willingham MC and Lyles DS:
Matrix protein and another viral component contribute to induction
of apoptosis in cells infected with vesicular stomatitis virus. J
Virol. 75:12169–12181. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Bell JC, Lichty B and Stojdl D: Getting
oncolytic virus therapies off the ground. Cancer Cell. 4:7–11.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Giedlin MA, Cook DN and Dubensky TW Jr:
Vesicular stomatitis virus: an exciting new therapeutic oncolytic
virus candidate for cancer or just another chapter from Field’s
Virology? Cancer Cell. 4:241–243. 2003.
|
|
20.
|
Lichty BD, Power AT, Stojdl DF and Bell
JC: Vesicular stomatitis virus: re-inventing the bullet. Trends Mol
Med. 10:210–216. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Barber GN: Vesicular stomatitis virus as
an oncolytic vector. Viral Immunol. 17:516–527. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
McCormick F: Future prospects for
oncolytic therapy. Oncogene. 24:7817–7819. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Everts B and van der Poel HG:
Replication-selective oncolytic viruses in the treatment of cancer.
Cancer Gene Ther. 12:141–161. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Shinozaki K, Ebert O and Woo SL: Treatment
of multi-focal colorectal carcinoma metastatic to the liver of
immune-competent and syngeneic rats by hepatic artery infusion of
oncolytic vesicular stomatitis virus. Int J Cancer. 114:659–664.
2005. View Article : Google Scholar
|
|
25.
|
Parato KA, Senger D, Forsyth PA and Bell
JC: Recent progress in the battle between oncolytic viruses and
tumours. Nat Rev Cancer. 5:965–976. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Mills BJ and Cooper NR:
Antibody-independent neutralization of vesicular stomatitis virus
by human complement. I. Complement requirements. J Immunol.
121:1549–1557. 1978.PubMed/NCBI
|