Introduction
The generation of neurons in the mammalian central
nervous system (CNS) was traditionally believed to end by early
postnatal life. However, it has been shown that the adult mammalian
brain contains self-renewable, multipotent neural stem cells (NSCs)
that are responsible for neurogenesis and plasticity in specific
regions of the adult brain (1,2).
In the hippocampus, newly generated neurons originate from stem
cells in the subgranular zone of the dentate gyrus (DG), and
neurogenesis continues throughout adult life (3,4).
Although the mechanisms by which neurons are generated from stem
cells are not yet well known, studies have indicated that the
number of newly generated hippocampal neurons in adults is
influenced specifically by enriched environments, learning, and
voluntary exercise (5,6).
Mastication is a behavior that is closely related
with CNS activities. Chewing causes regional increases in cerebral
blood flow and neuronal activity in the human brain (7,8).
By contrast, it has been shown that reduced mastication and
occlusal disharmony impair spatial memory and promote the
degeneration of hippocampal neurons (9,10).
We, as well as others, have also previously demonstrated that
reducing mastication by feeding mice a soft diet inhibits the
survival of newly generated neurons in the DG (11,12). However, it remains unknown whether
the induction of mastication affects the differentiation,
proliferation, or survival of newly generated cells in the adult
DG. In this study, we aimed to investigate the effects of forced
mastication by hard diet feeding on the differentiation and
survival of NSCs in the adult DG.
Materials and methods
Animals
Six-week-old C57BL/6 mice were housed in standard
cages. Room conditions were kept at a 12:12-h light/dark cycle and
an ambient temperature of 22±2°C. Animals were allowed free access
to food and water. All animal procedures were performed in
accordance with the guidelines for the Care and Use of Laboratory
Animals of Tokushima University Graduate School of Dentistry.
Diet
Mice were fed a standard laboratory diet, MF
(Oriental Yeast, Tokyo, Japan). To create a hard diet, MF chow was
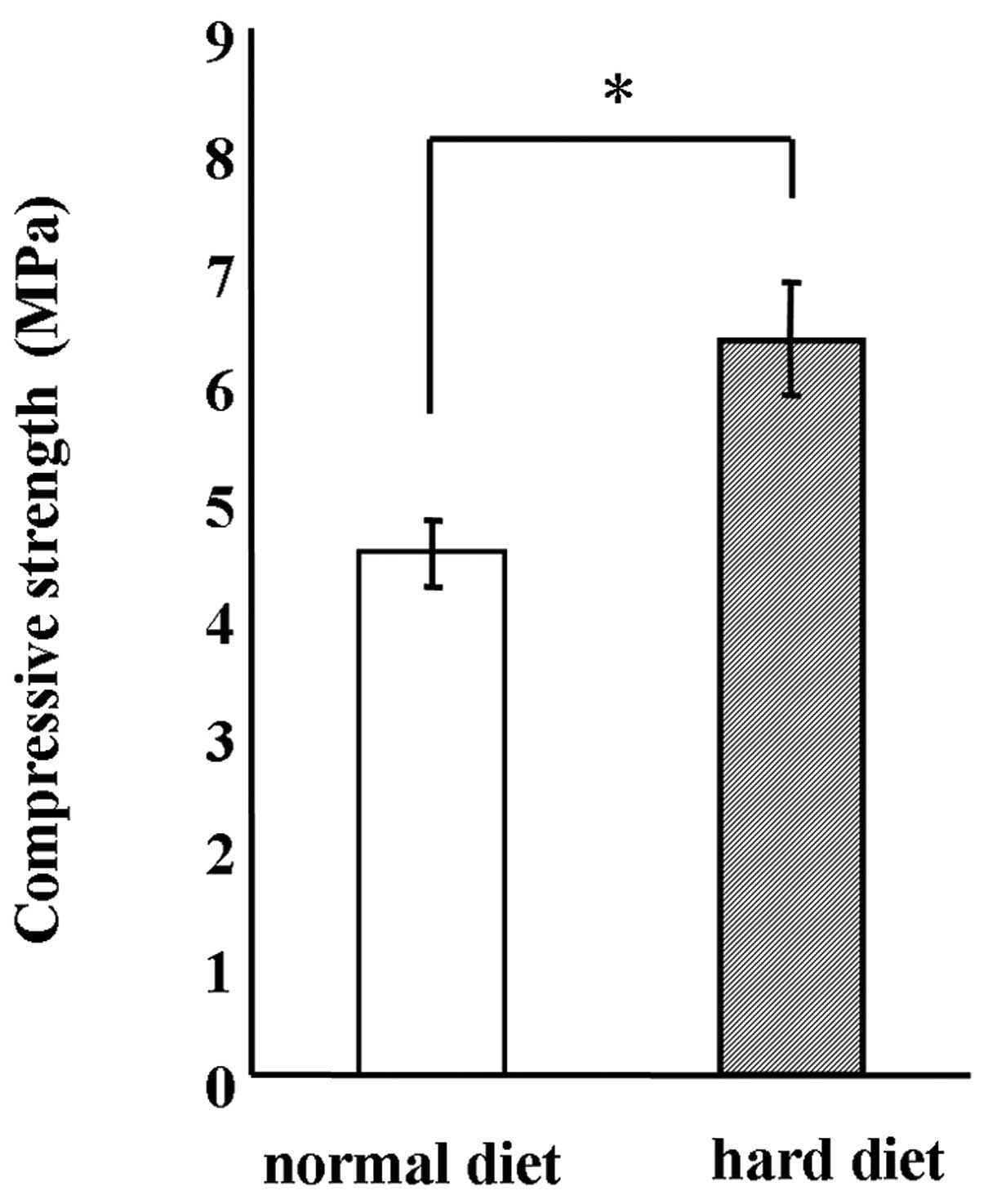
autoclaved. The hardness of the MF chow was significantly increased
by 1.5-fold by autoclave treatment (Fig. 1). Therefore, autoclaved normal
diet chow was used as the hard diet to enforce mastication in this
study.
Experimental conditions
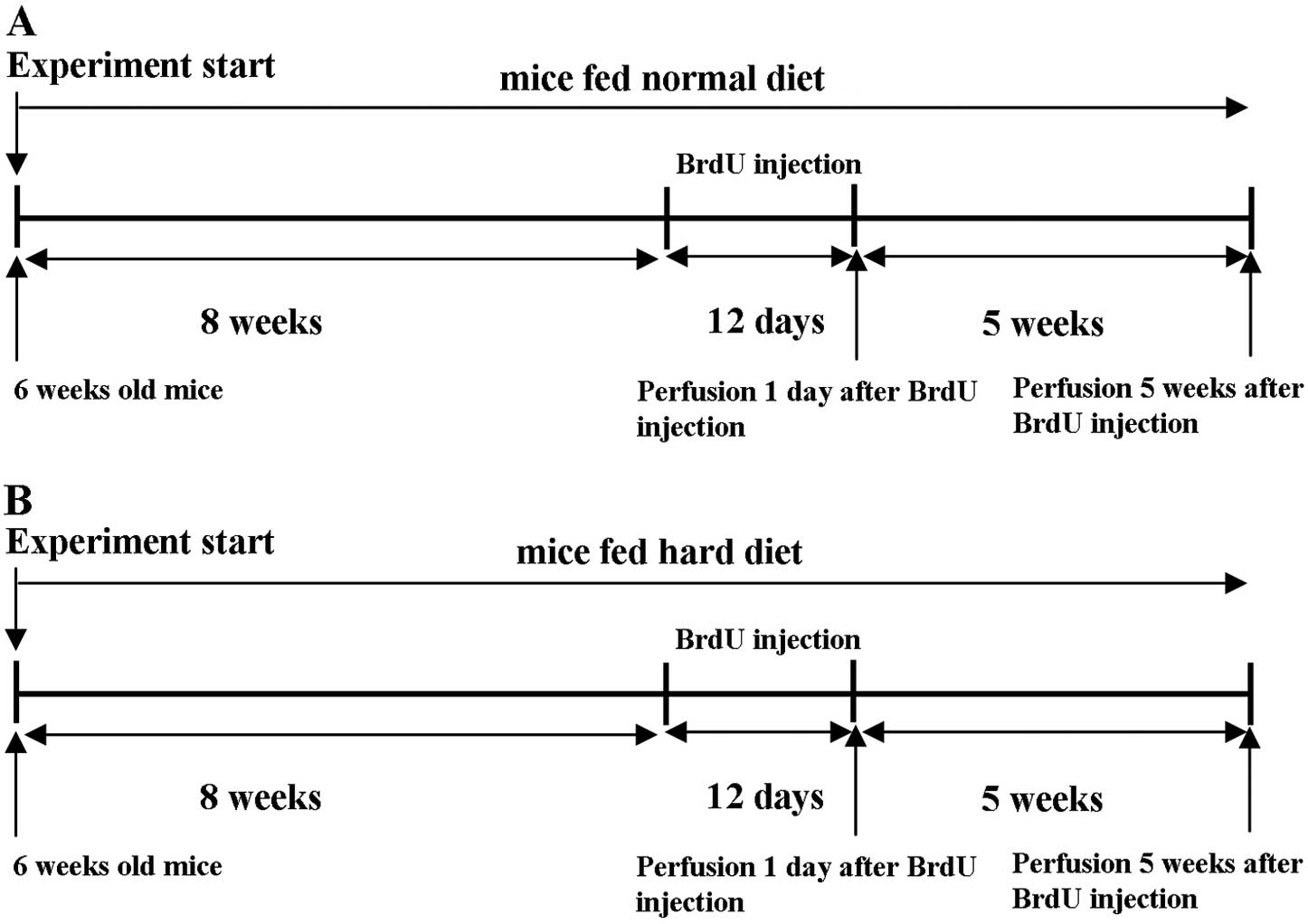
The time schedule of this experiment is shown in
Fig. 2. At 6 post-natal weeks,
the animals were placed in standard cages (4 or 5 animals/cage).
Control mice were allowed free access to the standard pellet
(Fig. 2A). Mice in the
experimental group were given free access to a hard diet
(autoclaved normal diet) (Fig.
2B). The animals were maintained in each experimental condition
for 8 weeks and then received bromodeoxyuridine (BrdU;
Sigma-Aldrich, St. Louis, MO, USA) injections (50 mg/g body weight)
intraperitoneally once a day for 12 consecutive days. One day after
the last BrdU injection, half the mice in each group were given an
overdose of anesthetic and perfused transcardially with cold 4%
paraformaldehyde in 0.1 M phosphate-buffered saline (PBS) (11). The remaining animals in each group
were kept in their respective experimental conditions for 5
additional weeks and were perfused in a similar manner.
Body weight measurement
Throughout the experiment, the nutritional status of
the mice was monitored by weighing the mice regularly once a week
until the day of sacrifice.
Immunohistochemistry
Mice were sacrificed and perfused transcardially
with cold 4% paraformaldehyde in 0.1 M PBS as previously described
(11). The brains were removed
from the skulls and immersed in the same fixative for 5 h at 4°C
with constant rotation followed by washing with PBS overnight at
4°C. Coronal 50-μm hippocampal sections were made using a
vibratome and stored at −10°C in cryoprotectant reagent (30%
sucrose, 30% ethylene glycol and 0.25 mM polyvinylpyrrolidone in
PBS) (11). Vibratome sections
were rinsed in 1X Tris-buffered saline (TBS), blocked with 10%
normal goat serum (NGS), 1X Triton X-100 in TBS for 1 h, followed
by incubation with primary antibodies overnight. The following
primary antibodies were used: mouse monoclonal antibodies against
neuronal nuclei (NeuN; 1:1,000; Millipore) and neuronal nitric
oxide synthase (nNOS; 1:1,000; Santa Cruz Biotechnology, Inc.),
rat-monoclonal antibodies against BrdU (1:20; Funakoshi),
rabbit-polyclonal antibodies for c-Fos (1:10,000; Santa Cruz
Biotechnology, Inc.). For evaluation of the changes in neural
proliferation and survival in the DG, the BrdU in vivo
labeling protocol was used. BrdU is incorporated only in the cells
that are dividing and is integrated in the synthesized DNA enabling
them to be detected immunohistochemically. The free-floating brain
slices were initially treated with denatured solution (12 N HCl +
1X TBS) and incubated for 30 min at 37°C. After washing in TBS, the
sections were incubated for 1 h at room temperature with the
appropriate secondary antibodies: anti-mouse Alexa 594 (1:1,000)
and anti-rabbit Alexa 488 (1:1,000). Finally, nuclei were stained
with 4′,6-diamidino-2-phenylindole (DAPI). Slices were mounted,
coverslipped in ProLong® antifade reagent (Invitrogen
Life Technologies, Carlsbad, CA, USA) and examined under an
epifluorescence microscope.
Volume measurement of the granular cell
layer of the DG
Brain slices stained with anti-NeuN antibody were
examined and images of the 12 sections were acquired using an
epifluorescence microscope. To calculate the DG volume, the area of
the granular cell layer was measured using ImageJ software and
multiplied by the thickness of the sections (50 μm)
according to Cavalieri’s principle (13).
Cell count
The DG was examined under a conventional
epifluorescence microscope after the slices were
immunohistochemically treated with specific antibodies. A total of
60 slices of 50-μm coronal sections were originally stored
in 5 separated tubes containing 12 slices in each tube. Sections
from every 200 μm across the whole hippocampus were selected
(12 sections from anterior to posterior). BrdU, c-Fos and nNOS
labeled cells were manually counted in each section by a blinded
observer. A ×20 objective lens was used for all counting (11). The labeled cells within the DG
were examined for both the group fed the normal diet and the group
fed the hard diet.
Morris water maze
The Morris water maze task was preformed to test
spatial learning. The task used a circular pool filled with water
containing white ink. A platform was placed in the pool so that it
was invisible at water level. The experiment was carried out after
the 5 weeks following BrdU injection. Tasks were conducted between
09:00 and 15:00 during the light cycle. After training, mice
underwent 3 trials/day for 10 consecutive days. During testing,
mice were required to locate the platform, and escape latency time
was recorded. Once mice located the platform, they were permitted
to remain on it for 10 sec. If mice did not locate the platform
within 90 sec, they were placed on the platform for 10 sec and then
removed from the pool, as previously described (14,15).
Probe test
Mice were trained in the water maze as described
above and then were subjected to a probe test. Each mouse was
subjected to a probe test immediately after the final training
trial on the 10th day. Before beginning the probe test, the
platform was removed from the pool. Mice were released from the
quadrant opposite to the previous platform quadrant, and the
swimming was tracked for 90 sec, as previously described (16,17).
Real-time PCR
Total RNA was isolated from 21-week-old mice. Mice
were sacrificed by an overdose of anesthetic and the hippocampus
was dissected in RNAlater (Sigma). Total RNA of hippocampal cells
was isolated using the TRIzol reagent (Invitrogen Life
Technologies) according to the manufacturer’s instructions. RNA (1
μg) was reverse- transcribed to first-strand cDNA using a
PrimeScript™ RT reagent kit (Takara Bio, Inc., Shiga, Japan)
according to the manufacturer’s instructions. A Thermal Cycler Dice
real-time system (Takara Bio, Inc.) was used for real-time PCR
(18–20). The cDNAs were amplified with
SYBR® Premix Ex Taq and specific primer pairs for
fibroblast growth factor (FGF)-1 (21), FGF-2 (22), epidermal growth factor (EGF)
(23), vascular endothelial
growth factor (VEGF) (24,25),
insulin-like growth factor-1 (IGF-1) (26), nerve growth factor (NGF),
neurotrophin-3 (NT-3), brain-derived neurotrophic factor (BDNF),
glial cell line-derived neurotrophic factor (GDNF), neuropeptide Y,
β-tubulin III, 2′,3′-cyclic-nucleotide phosphodiesterase (CNPase),
microtubule-associated protein 2 (Map2), glial fibrillary acidic
protein (GFAP), nestin, glutamate receptor 1 (GluR1) (27), glutamate receptor 2 (GluR2)
(27), glutamate receptor 3
(GluR3) (27), glutamate receptor
4 (GluR4) (27), N-methyl
D-aspartate receptor subtype 2B (NR2B) (28) and glyceraldehyde 3-phosphate
dehydrogenase (GAPDH). Primers for NGF, NT-3, GDNF, β-tubulin III,
CNPase, Map2, GFAP, nestin and GAPDH were designed with Perfect
Real-Time Primer Design software (Takara Bio, Inc.). The primers
used in this study are listed in Table I. The PCR conditions were as
follows: 10 sec at 95°C, followed by 40 cycles of 95°C for 5 sec
and 60°C for 30 sec, and finally, 15 sec at 95°C and 30 sec at
60°C.
 | Table I.Primer list for real-time PCR
(5′→3′). |
Table I.
Primer list for real-time PCR
(5′→3′).
| Genes | Primer
sequences |
|---|
| GAPDH | F:
AACTTTGGCATTGTGGAAGG |
| R:
ACACATTGGGGGTAGGAACA |
| FGF-1 | F:
AGAGTACCGAGACTGGCCAGTAC |
| R:
TCTGGCCATAGTGAGTCCGAG |
| FGF-2 | F:
CCAACCGGTACCTTGCTATG |
| R:
TATGGCCTTCTGTCCAGGTC |
| EGF | F:
ACGGTTTGCCTCTTTTCCTT |
| R:
GTTCCAAGCGTTCCTGAGAG |
| VEGF | F:
GGAGATCCTTCGAGGAGCACTT |
| R:
GGCGATTTAGCAGCAGATATAAGAA |
| IGF-1 | F:
GTGTGGACCGAGGGGCTTTTACTTC |
| R:
GCTTCAGTGGGGCACAGTACATCTC |
| NGF | F:
CAGACCCGCAACATCACTGTA |
| R:
CCATGGGCCTGGAAGTCTAG |
| NT-3 | F:
CATTCGGGGACACCAGGTC |
| R:
TTTGCACTGAGAGTTCCAGTGTTT |
| BDNF | F:
GGTATCCAAAGGCCAACTGA |
| R:
CTTATGAATCGCCAGCCAAT |
| GDNF | F:
ACCCGCTTCCATAAGGCTTTA |
| R:
CAGCCTTGTGCCGAAAGAC |
| Neuropeptide Y | F:
GCAGAGGACATGGCCAGATAC |
| R:
TGGATCTCTTGCCATATCTCTGTCT |
| β-tubulin III | F:
GGGCATTCCAACCTT |
| R:
AGCTCGGCGCCCTCTGTGTAGT |
| CNPase | F:
CCTTAGCAACAGCCGAGTGGATA |
| R:
GTTCCCAGATCACAAGCCAACA |
| Map2 | F:
AATAGACCTAAGCCATGTGACATCC |
| R:
AGAACCAACTTTAGCTTGGGCC |
| GFAP | F:
GTACCAGGACCTGCTCAATG |
| R:
TGTGCTCCTGCTTGGACTCCTT |
| Nestin | F:
CTCCAAGAATGGAGGCTGTAGGAA |
| R:
CCTATGAGATGGAGCAGGCAAGA |
| GluR1 | F:
ATGCTGGTTGCCTTAATCGAG |
| R:
ATTGATGGATTGCTGTGGGAT |
| GluR2 | F:
TTGAGTTCTGTTACAAGTCAAGGGC |
| R:
AGGAAGATGGGTTAATATTCTGTGGA |
| GluR3 | F:
AACGCCTGTAAACCTTGCAGT |
| R:
AGTCCTTGGCTCCACATTCC |
| GluR4 | F:
CCAGGGCAGAGGCGAAG |
| R:
CGTTTTCTCCCACACTCCCA |
| NR2B | F:
TCCGCCGTGAGTCTTCTGTCTATG |
| R:
CTGGGTGGTAAAGGGTGGGTTGTC |
Statistical analysis
The results are presented as the means ± SEM. For
statistical analysis of the results, the Student’s t-test and ANOVA
(Scheffe’s post hoc test) were used to compare the group fed the
normal diet with the group fed the hard diet.
Results
Effect of different food textures on
mouse growth and dietary intake
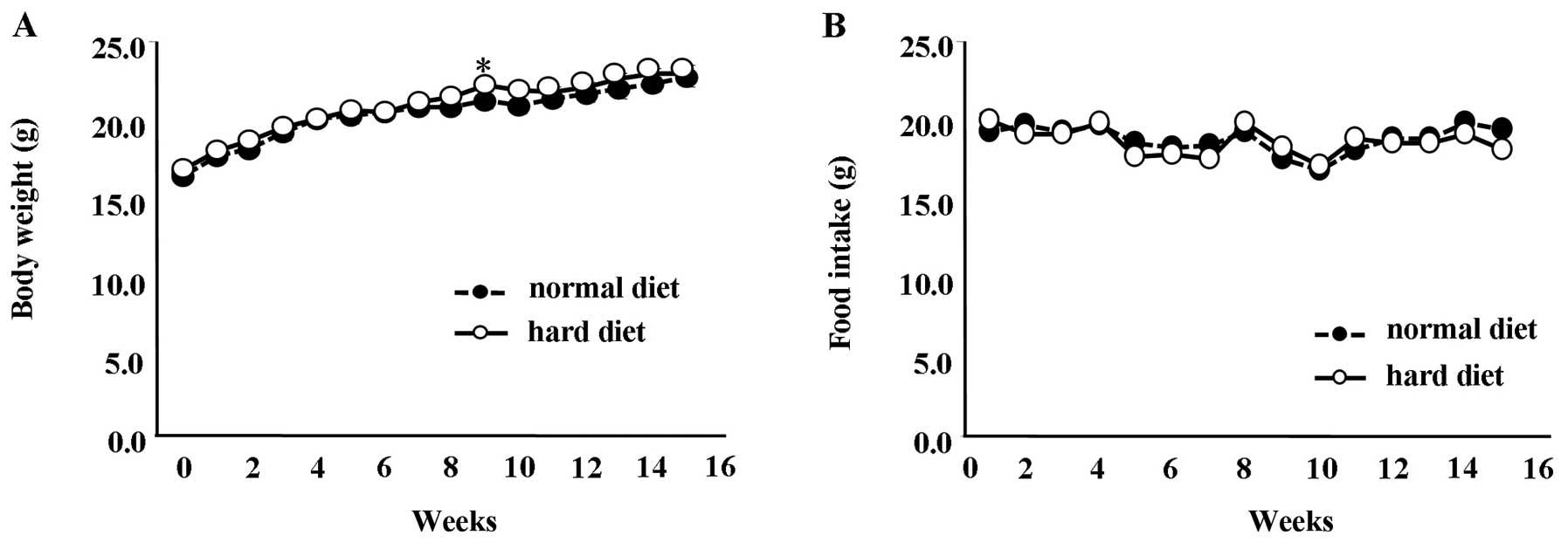
Mice fed a normal diet between the ages of 6 and 20
weeks showed a significant difference in body weight compared to
the mice fed a hard diet at 11 weeks (Fig. 3A) (P<0.01). However, at all
other time-points, the difference in body weight between the groups
was not statistically significant. Similarly, the amount of food
intake between the group fed the normal diet and the group fed the
hard diet showed no significant differences (Fig. 3B).
Proliferation of newly generated cells in
the DG
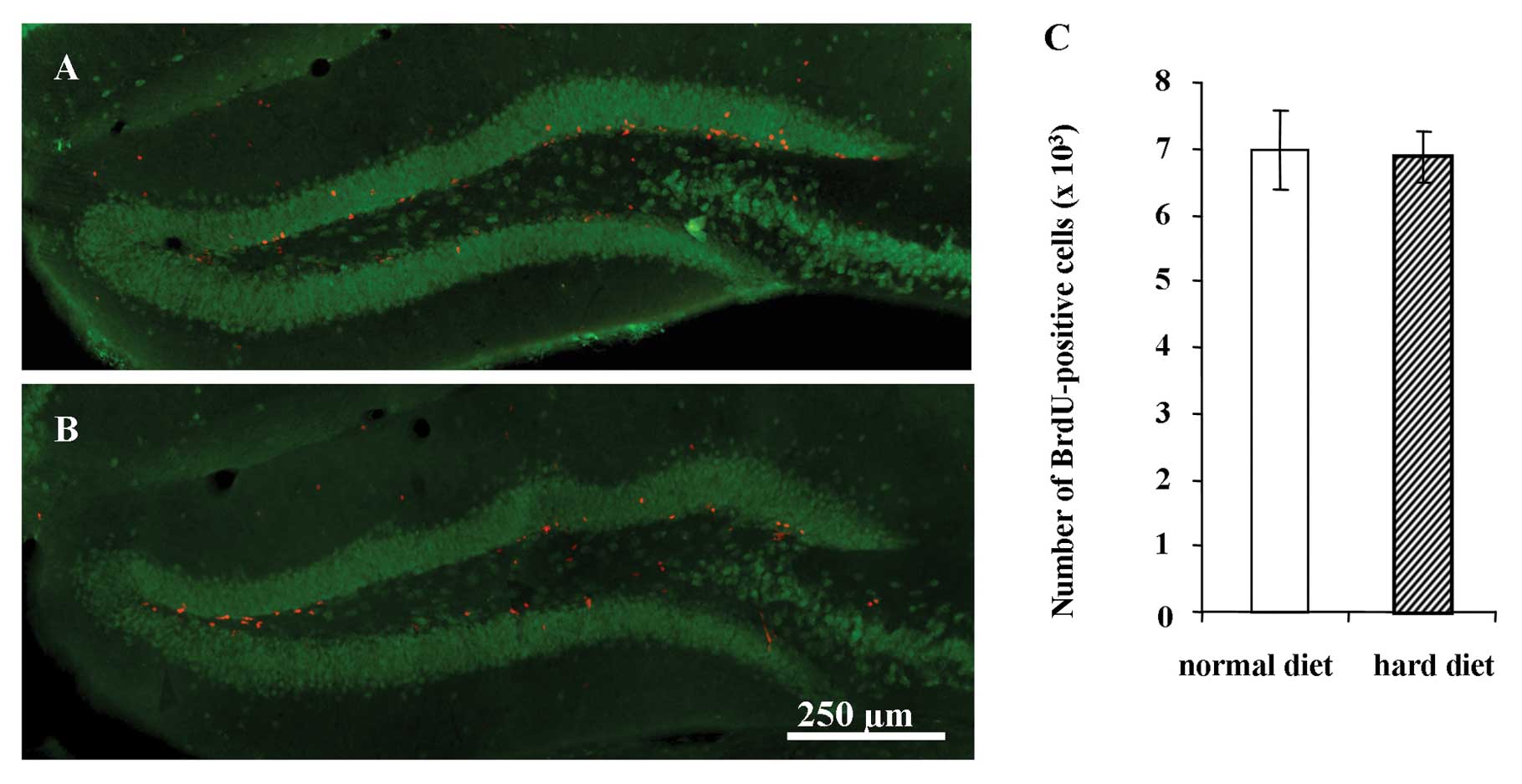
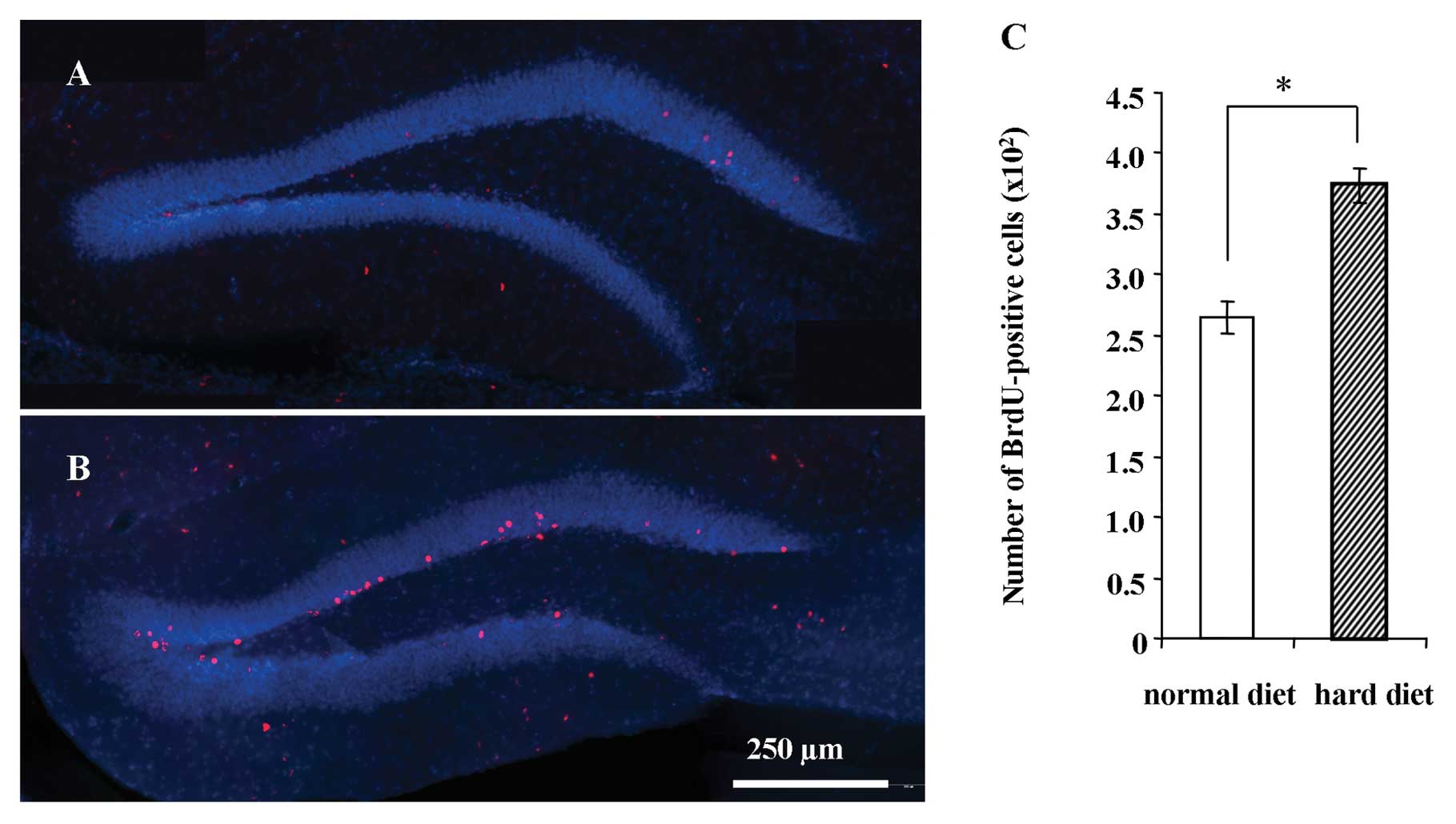
Representative images of BrdU-positive cells in the
hippocampus 1 day after the BrdU injection are shown in Fig. 4 for the group fed the normal diet
(Fig. 4A) and group fed the hard
diet (Fig. 4B). The number of
BrdU-positive cells in the DG 1 day after the BrdU injection was
not significantly different between the groups (Fig. 4C).
Survival of newly generated cells in the
DG
Representative images of BrdU-positive cells in the
hippocampus 5 weeks after BrdU injections are shown for the group
fed the normal diet (Fig. 5A) and
the group fed the hard diet (Fig.
5B). There was a significantly larger number of BrdU-positive
cells in the group fed the hard diet compared to the group fed the
normal diet 5 weeks after BrdU injection (P<0.001) (Fig. 5C).
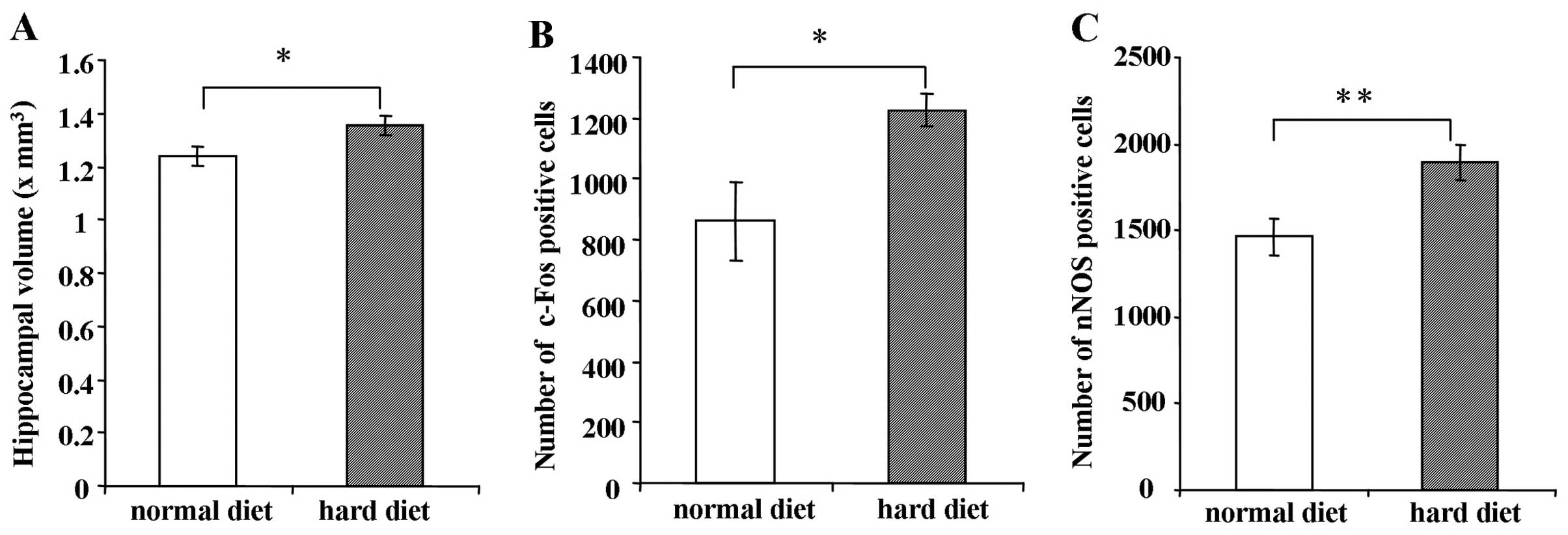
Hippocampal volume in mice fed the hard
or normal diet
As shown in Fig.
6A, the hippocampal volume of the mice fed the hard diet was
significantly larger than the hippocampal volume of the mice fed
the normal diet when measured 5 weeks after BrdU injection
(P<0.05).
Immunostaining of c-Fos and nNOS in the
DG
The number of c-Fos-positive cells in the group fed
the hard diet was greater than that in the group fed the normal
diet 5 weeks after BrdU injection (Fig. 6B) (P<0.05). Similarly, the
number of nNOS-positive cells in the DG was significantly increased
in the group fed the hard diet compared to the group fed the normal
diet 5 weeks after BrdU injection (Fig. 6C) (P<0.01).
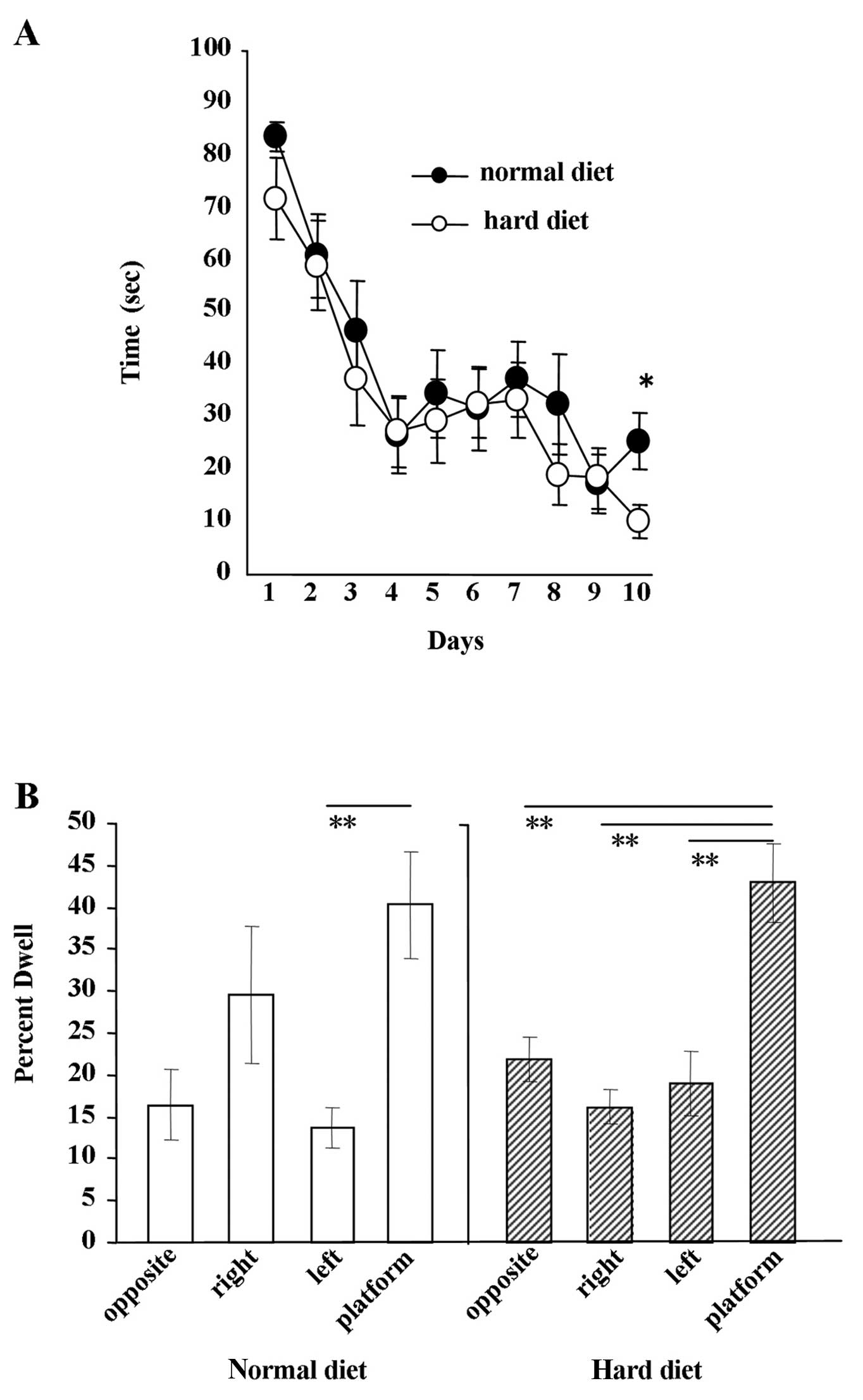
Water maze experiment
The results of the Morris water maze experiment are
shown in Fig. 7. The escape
latency in each group decreased as the number of trials progressed.
No significant difference in the escape latency was found between
the group fed the hard diet and the group fed the normal diet from
day 1 to 9 (Fig. 7A). Ten days
after the beginning of the experiment, we observed a significant
difference between the groups (Fig.
7A) (P<0.005). The results of the water maze probe test are
shown in Fig. 7B. After 10 days
of water maze experiments, the probe test was performed. When mice
fed the hard diet were allowed to search for the platform, the
period during which the mice focally searched around the former
platform location was significantly longer than searches in other
locations (P<0.01). By contrast, when mice fed the normal diet
were subjected to the probe test, the only difference observed in
their searches was between the time spent around the former
platform location and the left side of the arena (P<0.05).
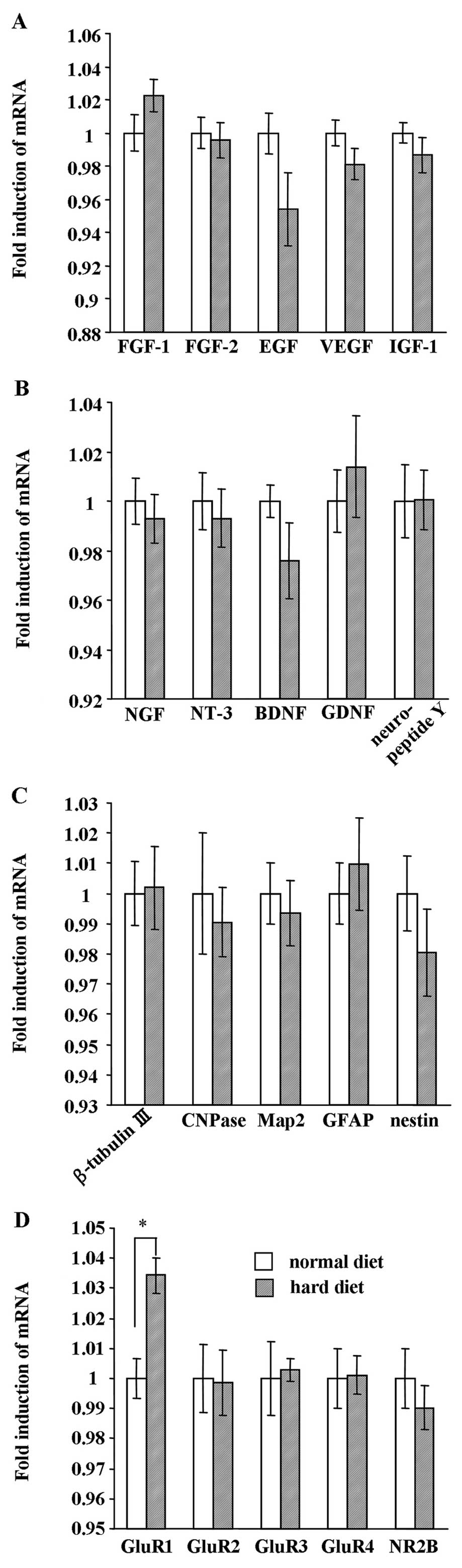
Effect of hard diet feeding on mRNA
expression of growth factors, neurotrophic factors, NSC markers and
receptors in the hippocampus
As is shown in Fig.
8, we analyzed the mRNA expression of growth factors,
neurotrophic factors, NSC markers and receptors in the hippocampus
between the group fed the hard diet and the group fed the normal
diet at the end of the experiment. Real-time PCR analysis revealed
that the mRNA expression of the growth factors, FGF-1, FGF-2, EGF,
VEGF and IGF-1, was not affected by food texture (Fig. 8A). In addition, the expression of
neurotrophic factors (NGF, NT-3, BDNF, GDNF and neuropeptide Y)
(Fig. 8B) and NSC markers
(β-tubulin III, CNPase, Map2, GFAP and nestin) (Fig. 8C) did not differ between the group
fed the hard diet and the group fed the normal diet. While the
expression of the receptors, GluR2, GluR3, GluR4 and NR2B (Fig. 8D), was not affected by food
texture, GluR1 mRNA expression was significantly increased in the
group fed the hard diet (P<0.01).
Discussion
To date, information as to how enforced mastication
regulates NSC proliferation and survival in the hippocampus is
lacking. To the best of our knowledge, this is the first report to
demonstrate that the NSC survival in the hippocampus is increased
and spatial memory is improved by forced mastication.
In this study, the normal diet was autoclaved to
increase its hardness and thus force the mice to chew. With respect
to body weight and food intake (Fig.
3), the growth of the mice was not affected by the autoclaved
diet. Therefore, we inferred that the major nutritional components
were not markedly different between the normal and the autoclaved
normal diet.
We assessed the proliferation of subgranular
progenitor cells in the DG of adult mice immediately after
consecutive injections of BrdU. We observed that neither the hard
diet nor the normal diet caused a change in the number of
BrdU-positive cells (Fig. 4).
These results indicated that the enforced mastication had no effect
on the proliferation of progenitor cells as evaluated by BrdU
labeling. Five weeks after the BrdU injections, a decrease in the
number of BrdU-positive cells was observed not only in the group
fed the normal diet but also in the group fed the hard diet
(Fig. 5). However, we found that
the number of surviving BrdU-positive cells was significantly
increased in the group fed the hard diet compared to the group fed
the normal diet (Fig. 5C).
Therefore, these data suggest that forced mastication in the group
fed the hard diet increased the survival rate of the newly
generated cells in the DG.
Hippocampal volume in mice fed the hard diet was
significantly increased compared to the mice fed the normal diet
(Fig. 6A). Since a number of
studies have shown that hippocampal volume affects memory functions
(29), forced mastication due to
a hard diet may be able to indirectly improve memory. For an
assessment of neural activity after forced mastication, the
expression of c-Fos in the DG of the group fed the hard diet and
the group fed the normal diet was evaluated. It has been shown that
the immunostaining of c-Fos is expressed in an activity-dependent
manner and has been used as a marker for activated neurons in the
DG (30,31). In this study, since c-Fos levels
in the DG of the mice fed the hard diet significantly increased
compared with the mice fed the normal diet (Fig. 6B), mastication does indeed appear
to influence neural activity in the DG.
Nitric oxide (NO) is an intercellular signaling
molecule that is involved in several physiological processes
(32,33). At least 3 isoforms of NO synthase
(NOS) have been identified in the CNS, including endothelial
(eNOS), neuronal (nNOS) and inducible (iNOS) forms (32,33). It has been reported that
nNOS-knockout increases the survival rate of neuronal cells in the
hippocampus (34). However, our
results showed that the number of nNOS-positive cells in the mice
fed the hard diet was significantly increased (Fig. 6C), and that the number of
BrdU-positive cells in the DG of the group fed the hard diet was
greater than that in the group fed the normal diet (Fig. 5C). Therefore, the survival of
newborn cells during adult neurogenesis in the DG may depend upon
other mechanisms in addition to nNOS signaling. For example,
sensory-motor information taken from the oral cavity may reach the
hippocampus, consequently affecting the neurogenesis rate. Another
possibility is that the muscular contractions involved in
mastication may increase cerebral blood flow, allowing an increase
in the entry of growth factors into the DG that could affect the
rate of neurogenesis.
The Morris water maze test was used to examine the
effect of induced mastication on spatial learning ability, as
previously described (35).
Although the group fed the hard diet showed a significant decrease
in escape latency only at 10 days compared with the group fed the
normal diet (Fig. 7A)
(P<0.005), the latency on the probe test after 10 days differed
between the group fed the hard diet and the group fed the normal
diet (Fig. 7B). This finding
indicated that the group fed the hard diet spent significantly more
time in the former location of the platform compared to all other
locations. For this reason, we suggest that forced mastication can
improve spatial learning ability.
We also assessed the effect of a hard diet on the
mRNA expression of various growth factors, neurotrophic factors,
neuronal stem cell markers, and receptors in the hippocampus by
real-time PCR. Although several factors were examined, we observed
a small but significant increase in the expression of GluR1 mRNA in
the group fed the hard diet compared with the group fed the normal
diet (P<0.01). GluRs are synaptic receptors located primarily on
the membranes of neuronal cells (36). One of the major functions of GluRs
appears to be the modulation of synaptic plasticity, memory and
learning (36). Therefore, these
changes in the group fed the hard diet may be attributed to the
slight increase in GluR1 mRNA expression in the hippocampus
(Fig. 8D), as well as in
hippocampal volume (Fig. 6A)
compared with the group fed the normal diet. However, since these
results may be sensitive to the timing of experimentation, further
experiments may be required to detect the critical periods of mRNA
expression in the hippocampus.
In conclusion, forced mastication alters the
survival rate and NSC activity in the DG. In addition, spatial
memory was improved in the group fed the hard diet compared to the
group fed the normal diet. These results suggest that mastication
caused by daily food texture may improve or accelerate the recovery
of spatial memory functions in the hippocampus, preventing senile
dementia.
Acknowledgements
This study was supported in part by
the Grants-in-Aid for Scientific Research (no. 21390548 to M.M. and
no. 22592296 to T.H.) from the Ministry of Education, Culture,
Sports, Science and Technology of Japan (2010–2014).
References
|
1.
|
Gage FH: Mammalian neural stem cells.
Science. 287:1433–1438. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Taupin P and Gage FH: Adult neurogenesis
and neural stem cells of the central nervous system in mammals. J
Neurosci Res. 69:745–749. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Altman J and Das GD: Autoradiographic and
histological evidence of postnatal hippocampal neurogenesis in
rats. J Comp Neurol. 124:319–335. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Kuhn HG, Dickinson-Anson H and Gage FH:
Neurogenesis in the dentate gyrus of the adult rat: age-related
decrease of neuronal progenitor proliferation. J Neurosci.
16:2027–2033. 1996.PubMed/NCBI
|
|
5.
|
van Praag H, Kempermann G and Gage FH:
Running increases cell proliferation and neurogenesis in the adult
mouse dentate gyrus. Nat Neurosci. 2:266–270. 1999.
|
|
6.
|
Gould E, Beylin A, Tanapat P, Reeves A and
Shors TJ: Learning enhances adult neurogenesis in the hippocampal
formation. Nat Neurosci. 2:260–265. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Momose T, Nishikawa J, Watanabe T, Sasaki
Y, Senda M, Kubota K, Sato Y, Funakoshi M and Minakuchi S: Effect
of mastication on regional cerebral blood flow in humans examined
by positron-emission tomography with 15O-labelled water
and magnetic resonance imaging. Arch Oral Biol. 42:57–61. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Onozuka M, Fujita M, Watanabe K, Hirano Y,
Niwa M, Nishiyama K and Saito S: Mapping brain region activity
during chewing: a functional magnetic resonance imaging study. J
Dent Res. 81:743–746. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Kato T, Usami T, Noda Y, Hasegawa M, Ueda
M and Nabeshima T: The effect of the loss of molar teeth on spatial
memory and acetylcholine release from the parietal cortex in aged
rats. Behav Brain Res. 83:239–242. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Terasawa H, Hirai T, Ninomiya T, Ikeda Y,
Ishijima T, Yajima T, Hamaue N, Nagase Y, Kang Y and Minami M:
Influence of tooth-loss and concomitant masticatory alterations on
cholinergic neurons in rats: immunohistochemical and biochemical
studies. Neurosci Res. 43:373–379. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Mitome M, Hasegawa T and Shirakawa T:
Mastication influences the survival of newly generated cells in
mouse dentate gyrus. Neuroreport. 16:249–252. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Tsutsui K, Kaku M, Motokawa M, Tohma Y,
Kawata T, Fujita T, Kohno S, Ohtani J, Tenjoh K, Nakano M, Kamada H
and Tanne K: Influences of reduced masticatory sensory input from
soft-diet feeding upon spatial memory/learning ability in mice.
Biomed Res. 28:1–7. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Gundersen HJ, Bagger P, Bendtsen TF, et
al: The new stereological tools: disector fractionator, nucleator
and point sampled intercepts and their use in pathological research
and diagnosis. APMIS. 96:857–881. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Fujioka A, Fujioka T, Tsuruta R, Izumi T,
Kasaoka S and Maekawa T: Effects of a constant light environment on
hippocampal neurogenesis and memory in mice. Neurosci Lett.
488:41–44. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Tauber SC, Bunkowski S, Ebert S, Schulz D,
Kellert B, Nau R and Gerber J: Enriched environment fails to
increase meningitis-induced neurogenesis and spatial memory in a
mouse model of pneumococcal meningitis. J Neurosci Res.
87:1877–1883. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Moser E, Moser MB and Andersen P: Spatial
learning impairment parallels the magnitude of dorsal hippocampal
lesions, but is hardly present following ventral lesions. J
Neurosci. 13:3916–3925. 1993.
|
|
17.
|
Stackman RW Jr, Lora JC and Williams SB:
Directional responding of C57BL/6J mice in the Morris water maze is
influenced by visual and vestibular cues and is dependent on the
anterior thalamic nuclei. J Neurosci. 32:10211–10225. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Hasegawa T, Chosa N, Asakawa T, Yoshimura
Y, Ishisaki A and Tanaka M: Establishment of immortalized human
periodontal ligament cells derived from deciduous teeth. Int J Mol
Med. 26:701–705. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Asakawa T, Chosa N, Yoshimura Y, Asakawa
A, Tanaka M, Ishisaki A, Mitome M and Hasegawa T: Fibroblast growth
factor 2 inhibits the expression of stromal cell-derived factor 1α
in periodontal ligament cells derived from human permanent teeth
in vitro. Int J Mol Med. 29:569–573. 2012.
|
|
20.
|
Hasegawa T, Chosa N, Asakawa T, Yoshimura
Y, Fujihara Y, Kitamura T, Tanaka M, Ishisaki A and Mitome M:
Differential effects of TGF-β1 and FGF-2 on SDF-1α expression in
human periodontal ligament cells derived from deciduous tooth in
vitro. Int J Mol Med. 30:35–40. 2012.
|
|
21.
|
Arnold RS, Sun CQ, Richards JC, Grigoriev
G, Coleman IM, Nelson PS, Hsieh CL, Lee JK, Xu Z, Rogatko A,
Osunkoya AO, Zayzafoon M, Chung L and Petros JA: Mitochondrial DNA
mutation stimulates prostate cancer growth in bone stromal
environment. Prostate. 69:1–11. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Huang CC, Yeh CM, Wu MY, Chang AY, Chan
JY, Chan SH and Hsu KS: Cocaine withdrawal impairs metabotropic
glutamate receptor-dependent long-term depression in the nucleus
accumbens. J Neurosci. 31:4194–4203. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Qu R, Li Y, Gao Q, Shen L, Zhang J, Liu Z,
Chen X and Chopp M: Neurotrophic and growth factor gene expression
profiling of mouse bone marrow stromal cells induced by ischemic
brain extracts. Neuropathology. 27:355–363. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Fabel K, Fabel K, Tam B, Kaufer D, Baiker
A, Simmons N, Kuo CJ and Palmer TD: VEGF is necessary for
exercise-induced adult hippocampal neurogenesis. Eur J Neurosci.
18:2803–2812. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Cao L, Jiao X, Zuzga DS, Liu Y, Fong DM,
Young D and During MJ: VEGF links hippocampal activity with
neurogenesis, learning and memory. Nat Genet. 36:827–835. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Iida K, Del Rincon JP, Kim DS, Itoh E,
Nass R, Coschigano KT, Kopchick JJ and Thorner MO: Tissue-specific
regulation of growth hormone (GH) receptor and insulin-like growth
factor-I gene expression in the pituitary and liver of GH-deficient
(lit/lit) mice and transgenic mice that overexpress bovine GH (bGH)
or a bGH antagonist. Endocrinology. 145:1564–1570. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Destot-Wong KD, Liang K, Gupta SK, Favrais
G, Schwendimann L, Pansiot J, Baud O, Spedding M, Lelièvre V, Mani
S and Gressens P: The AMPA receptor positive allosteric modulator,
S18986, is neuroprotective against neonatal excitotoxic and
inflammatory brain damage through BDNF synthesis.
Neuropharmacology. 57:277–286. 2009. View Article : Google Scholar
|
|
28.
|
Boon WC, Diepstraten J, van der Burg J,
Jones ME, Simpson ER and van den Buuse M: Hippocampal NMDA receptor
subunit expression and water maze learning in estrogen deficient
female mice. Brain Res Mol Brain Res. 140:127–132. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Ystad MA, Lundervold AJ, Wehling E,
Espeseth T, Rootwelt H, Westlye LT, Andersson M, Adolfsdottir S,
Geitung JT, Fjell AM, Reinvang I and Lundervold A: Hippocampal
volumes are important predictors for memory function in elderly
women. BMC Med Imaging. 9:172009.PubMed/NCBI
|
|
30.
|
Kee N, Teixeira CM, Wang AH and Frankland
PW: Preferential incorporation of adult-generated granule cells
into spatial memory networks in the dentate gyrus. Nat Neurosci.
10:355–362. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Ahn SN, Guu JJ, Tobin AJ, Edgerton VR and
Tillakaratne NJ: Use of c-fos to identify activity-dependent spinal
neurons after stepping in intact adult rats. Spinal Cord.
44:547–559. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Bredt DS, Hwang PM and Snyder SH:
Localization of nitric oxide synthase indicating a neural role for
nitric oxide. Nature. 347:768–770. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Brenman JE and Bredt DS: Synaptic
signaling by nitric oxide. Curr Opin Neurobiol. 7:374–378. 1997.
View Article : Google Scholar
|
|
34.
|
Fritzen S, Schmitt A, Köth K, Sommer C,
Lesch KP and Reif A: Neuronal nitric oxide synthase (NOS-I)
knockout increases the survival rate of neural cells in the
hippocampus independently of BDNF. Mol Cell Neurosci. 35:261–271.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Sharma S, Rakoczy S and Brown-Borg H:
Assessment of spatial memory in mice. Life Sci. 87:521–536. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Peng S, Zhang Y, Zhang J, Wang H and Ren
B: Glutamate receptors and signal transduction in learning and
memory. Mol Biol Rep. 38:453–460. 2011. View Article : Google Scholar : PubMed/NCBI
|