Introduction
In muscle fibers, interactions between the cell and
the extracellular matrix (ECM) are considered to be important for
muscular development during somitogenesis, cell migration from
somites, correct innervation, and muscle patterning (1,2).
Cell-ECM interactions play a key role in mechanotransduction
transmitting forces across the plasma membrane (3,4).
Experimental evidence for the importance of cell-ECM
interactions during muscle formation can be found in transgenic
mice lacking fibronectin, which have defective somites (5). Myoblast migration from the somite
can be inhibited by anti-β1-integrin antibodies (6), and during in vitro
differentiation in which myotube formation can be inhibited with
antibodies to integrins (7). In
the adult muscle, an intact basement membrane-cytoskeletal linkage
is important for skeletal muscle stability and integrity (8).
Integrins are a family of heterodimeric cell surface
membrane proteins that mediate the interaction of cells with each
other, with ECM proteins, and with additional molecules in their
environment (9,10). These proteins also link ECM to
cytoskeletal actin providing bidirectional signaling between the
ECM and the cytoplasm (11).
Thus, integrins also play a key role in cell adhesion including
cell-matrix and intercellular interactions and therefore, they are
involved in various biological phenomena, such as cell migration,
differentiation, tissues repair and programmed cell death (12).
Each integrin is composed of a noncovalently-linked
pair of α and β subunits. In particular, the α7β1-integrin is
concentrated at neuromuscular and myotendinous junctions and it is
located along the sarcolemma at costameres with an important role
in the functions of the skeletal muscle (13). It was demonstrated that congenital
myopathies may be caused by mutations in the human integrin α7 gene
(ITGA7) confirming the importance of the α7β1-integrin in
maintaining normal skeletal muscle physiology (14).
Regarding the α7 subunit, the α7B isoform is
detected in proliferating and adult myofibres and is exclusively
localized in the neuromuscular and myotendinous junctions (13,15). Instead, the α7A isoform, detected
in differentiating myofibres, plays a key role in muscle
regeneration during the dynamic adhesion stage, whereas it appears
to have a minor role in mature skeletal muscle (16).
The β1 chain cytoplasmic domain also undergoes
developmentally regulated alternative splicing (17). β1A is the most common isoform of
the β1 chain and its expression levels appear to be very low or
negligible in mature skeletal muscle, whereas the β1D isoform is
the predominant β1 isoform in adult striated muscle (18). Thus, β1D-integrin plays a crucial
role in linking the subsarcolemmal cytoskeleton to the surrounding
extracellular matrix in adult muscle tissues (12,19).
The role of integrins was also studied in Duchenne
muscular dystrophy evidencing the detection of high levels of
α7β1-integrin in order to compensate for the absence of dystrophin;
these results reinforced the role played by integrins in the
integrity and stability of skeletal muscle fibers (20).
In agreement with several reports (21–23), we offered support for the
hypothesis that integrins have a role in the function of human
adult skeletal muscle (24) and
demonstrated a bidirectional signaling between sarcoglycans and
integrins (25). This reciprocal
control may determine the prevalence of one system over another
with a consequent transmission of different messages to the
sarcolemma-associated cytoskeleton (24,25).
Thus, we studied α7B and β1D integrin during
muscular inactivity and we showed that these isoforms were
displaced by the relative isoforms α7A and β1A due to loss of
regulatory effects on gene expression of these proteins (26). Moreover, we studied
muscle-specific integrins and their relative isoforms, α7A and β1A
in chimpanzee’s masseter muscle, analyzing biopsies of alpha male
and non-alpha male subjects, since this particular muscle plays a
key role in many behavioral functions in respect to tasks of
subjects. This study demonstrated high levels of α7A and β1A
integrin in alpha males in respect to non-alpha male subjects in
which only α7B and β1D showed normal staining patterns (27).
The masseter muscle shows a very high ATPase
activity for contracting very quickly and forcefully participating
in a wide variety of functional activities of the stomatognathic
system including mastication, swallowing and speech (28,29). This diversity of functions
requires coordination of motor output elements of the neuromuscular
system with appropriate activation of tongue, facial and
oropharyngeal muscles (23).
About this, mastication is one of the most complex and co-ordinated
functional movements involving diverse and accurate mandibular
patterns to incise and grind food suitable for swallowing. The
pattern of mandibular movement during chewing is influenced by
factors such as the bolus type and the type of occlusion (30,31). The relative position of the upper
and lower teeth determines occlusal stability, which is related to
muscular performance (31).
Subjects with unilateral posterior crossbite exhibit
different kinematics of the mandible during mastication when
chewing on the affected side, resulting in an increased frequency
of reverse chewing cycles (32,33). The masseter of the crossbite side
is less active than the counterpart, and the co-ordination of the
masticatory muscles on the two sides is altered with respect to
controls (33,34).
Although the significance of masticatory muscle
function has been illustrated in previous experimental studies on
animals (35,36), there are insufficient data on the
influence of proteins about crossbite malocclusion. In our opinion,
integrins could play an important role during malocclusion diseases
in masticatory muscle and in particular in masseter, in which all
networks of proteins could be modified.
Thus, considering the important function of masseter
muscle, and its particular role in chewing cycles, we aim to study
this muscle in order to verify its composition in integrin network.
Then, by immunohistochemical and molecular technique, we analyzed
human masseter muscles of surgical patients affected by severe
class III malocclusion to comprehend the role of integrins in this
masticatory muscle.
Materials and methods
Patients and ethics
Five surgical patients, 3 men and 2 women, age
31.3±5.5 years (mean ± standard deviation), with unilateral
posterior crossbite, all on the right side, were selected for the
study. All the patients gave informed consent.
The inclusion criteria for the crossbite patient
group were: i) severe class III malocclusion with right posterior
crossbite of two or more posterior teeth, ii) complete permanent
dentition, iii) no erupting teeth, iv) no caries, and v) no
temporomandibular disorders. The exclusion criteria were: no
history of connective tissue disorders, myopathies, endocrine
disorders, autoimmune disease, bone disease, bleeding
disorders.
The investigation conformed with guidelines
established by the University Internal Review Board for use of
Human Subjects and with the principles outlined in the Helsinki
Declaration of 1975.
Muscle biopsies
Biopsies were obtained under general anesthesia from
the superficial and anterior portion of both masseter muscles of
patients undergoing orthognathic surgery to reposition one or both
jaws in conjunction with orthodontic treatment following the
protocol suggested by Boyd et al (37). The supero-inferior level of the
biopsy was determined by the mandibular occlusal plane and was
excised from the anterior, deep surface of the masseter adjacent to
the anterior aspect of the mandibular ramus (38). All biopsies were obtained by the
same surgeon via an intraoral incision through the mucosa and
buccinator muscle, approximately 3×3×3 mm. The biopsy specimens of
both masseter muscles were analyzed using immunohistochemical
analysis.
Immunohistochemical analysis
The biopsies were fixed in 3% paraformaldehyde in
0.2 M phosphate buffer, pH 7.4, for 2 h at room temperature. They
were then washed extensively with 0.2 M phosphate buffer, pH 7.4,
and then with phosphate-buffered saline (PBS), containing 12 and
18% sucrose. The samples were snap-frozen in liquid nitrogen and
20-μm sections were prepared in a cryostat for use in a protocol to
perform immunofluorescence. The sections were placed on glass
slides that were coated with 0.5% gelatin and 0.005% chromium
potassium sulphate.
To block non-specific binding sites and to
permeabilize the membranes, the sections were preincubated with 1%
bovine serum albumin (BSA), 0.3% Triton X-100 in PBS at room
temperature for 15 min. Finally, the sections were incubated with
primary antibodies. The following primary antibodies were used:
anti-α7B integrin diluted 1:50, anti-β1D integrin diluted 1:50,
anti-α7A integrin diluted 1:100, and anti-β1A integrin diluted 1:50
(synthetic peptides from the COOH terminal region; kindly provided
by the laboratory of Professor Tarone, University of Torino).
Primary antibodies were detected using Texas Red-conjugated IgG
(Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA).
Slides were finally washed in PBS and sealed with mounting
medium.
The sections were then analyzed and images acquired
using a Zeiss LSM 5 DUO (Carl Zeiss, Jena, Germany) confocal laser
scanning microscope. All images were digitalized at a resolution of
8 bits into an array of 2,048×2,048 pixels. Optical sections of
fluorescent specimens were obtained using a helium-neon (HeNe)
laser (wavelength, 543 nm) at a 62 sec scanning speed with up to
eight averages; 1.50 μm sections were obtained using a pinhole of
250. For each reaction, at least 100 individual fibers were
examined. Contrast and brightness were established by examining the
most brightly labeled pixels and choosing the settings that allowed
clear visualization of the structural details while keeping the
pixel intensity at its highest (∼200). Each image was acquired
within 62 sec, in order to minimize photodegradation.
The ‘display profile’ function of the laser scanning
microscope was used to show the intensity profile across an image;
the intensity curves are shown in graphs. Digital images were
cropped and figure montages prepared using Adobe Photoshop 7.0
(Adobe Systems; Palo Alto, CA, USA).
Statistical analysis
All our observations were analyzed by an internal
software for image analysis, included in the CLSM software and
named ‘Histo’, measuring the distribution of pixel intensity of all
areas corresponding to each fiber; pixel intensities were converted
in a data table indicating values of single pixel intensity. By
this software, we analyzed 100 fibers for each reaction, using for
all reactions the same confocal parameters, and a mean and standard
deviation for single fibers were obtained.
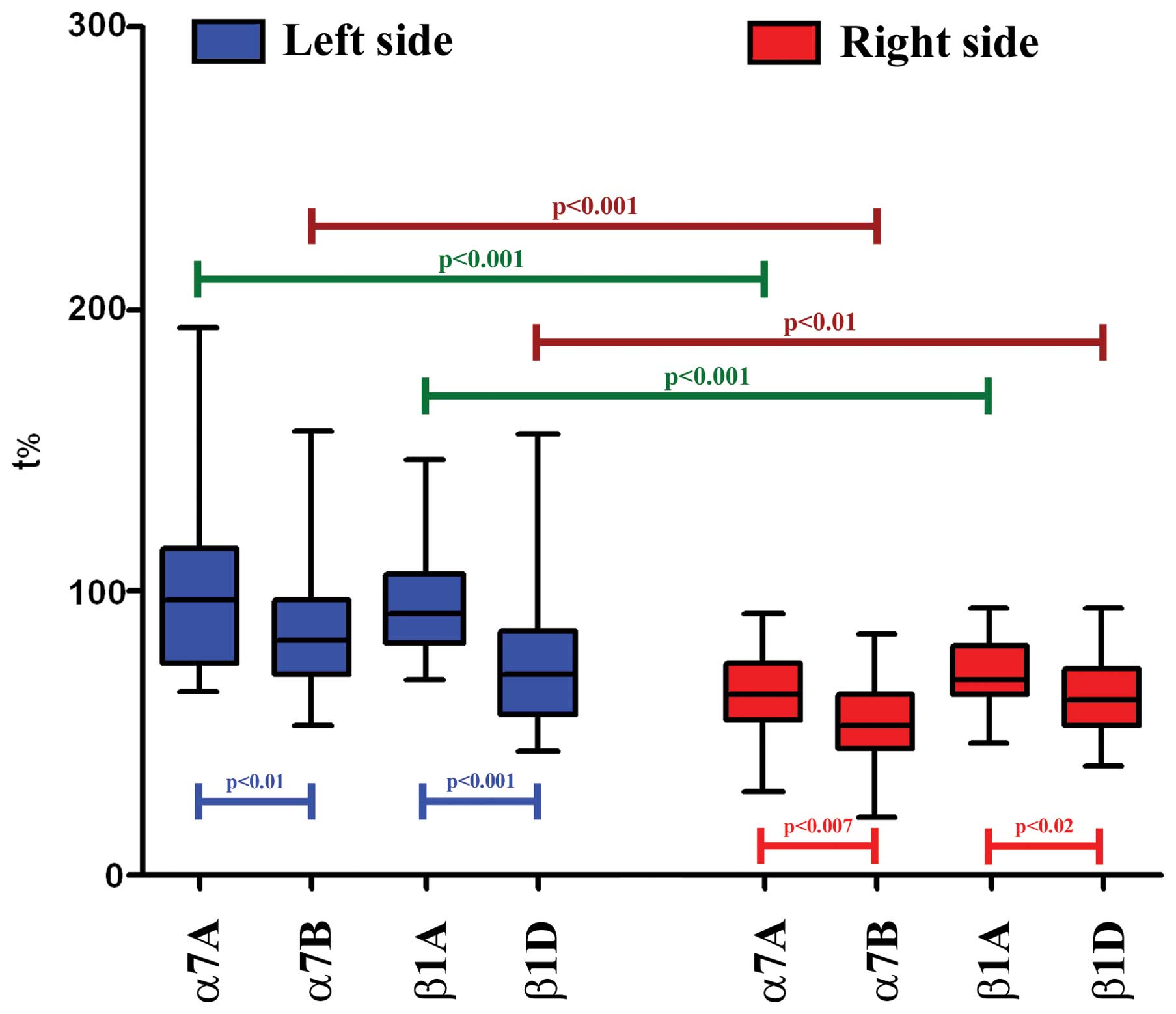
The box-and-whisker plots with the median values of
fluorescence intensity in both muscle fibers crossbite (right side)
and left side are shown in Fig.
4. Data analysis and graphs were plotted on GraphPad Prism v.5
(GraphPad Software, San Diego, CA, USA) and inter-integrin
differences among the isoforms in masseter muscle fibers, were
assessed by the Wilcoxon rank test (w); two tailed p-values
<0.05 were considered statistically significant.
Total-RNA isolation
Samples containing 50–100 mg of tissue were
homogenized using a power homogenizer (Ultra Turrax, IKA-Werke
GmbH, Staufen, Germany). Total-RNA was isolated by a single-step
RNA isolation procedure (TRIzol®, Invitrogen) (39) that uses a monophasic solution of
phenol and guanidine isothiocyanate.
RT-PCR analysis
In the present study, we collected muscle biopsies
of the human subjects all affected by right posterior crossbite
taking muscle tissue both from the right and left side (control),
in order to evaluate the expression of α7A, α7B, β1A and β1D
integrin by RT-PCR. RT-PCR was carried out using the GeneAmp Gold
RNA PCR Reagent kit (Applied Biosystems, Foster City, CA, USA) in a
GeneAmp PCR System 9600 thermal cycler (Applied Biosystems). In the
first step, an initial reverse transcription (RT) reaction was
carried out in a volume of 20 μl containing 3 μg of total-RNA, 10
units RNase inhibitor, 10 mM DTT, 15 units MultiScribe reverse
transcriptase and 1.25 μM oligo(dt)16 using the following thermal
cycler conditions: 10 min at 25°C, followed by 12 min at 42°C. In
the second step, a PCR was performed in a volume of 50 μl
containing 5 μl of cDNA from the first step (RT) as a template, 2.5
units AmpliTaq Gold DNA polymerase and each primer at a
concentration of 0.2 μM. The primer pairs used are shown in
Table I.
 | Table IOligonucleotide primer sequences of
the integrin isoforms and of the internal control GAPDH used for
RT-PCR. |
Table I
Oligonucleotide primer sequences of
the integrin isoforms and of the internal control GAPDH used for
RT-PCR.
| Primers | Forward | Reverse | Length (bp) | Exons | Nucleotides | Accession NCBI |
|---|
| α7A |
5′-CGGGCCAACATCACAGTGAA-3′ |
5′-TCCGATGGAAGAAGCCACACT-3′ | 208 | 24–26 | 3342–3553 |
ENST00000257880 |
| α7B |
5′-CGGGCCAACATCACAGTGAA-3′ |
5′-GTTTGAAGAATCCCATCTTCCACAG-3′ | 205 | 23–25 | 3206–3410 | NM_002206 |
| β1A |
5′-TGCCGTAACAACTGTGGTCA-3′ |
5′-TAACCATCCTGTCTCAAGTC-3′ | 255 | 16 | 2567–2821 | NM_002211 |
| β1D |
5′-TGGAGAATCCAGAGTGTCCC-3′ |
5′-AGAGACCAGCTTTACGTCCG-3′ | 252 | 13–15 | 2144–2395 | NM_033668 |
| GAPDH |
5′-AACCTGCCAAATATGATGAC-3′ |
5′-ACTGAGTGTGGCAGGGACTC-3′ | 340 | 8–9 | 854–1192 | NM_002046 |
PCR conditions were as follows: an initial 10 min
denaturation step at 95°C, followed by 35 cycles of denaturation at
94°C for 40 sec, annealing at 62°C for 40 sec and extension at 72°C
for 45 sec with a final extension at 72°C for 10 min. For each
protein, human GAPDH cDNA was used as an internal control and the
primers are shown in Table I.
Digital images were cropped and figure montages prepared using
Adobe Photoshop 7.0.
Results
Immunohistochemical analysis
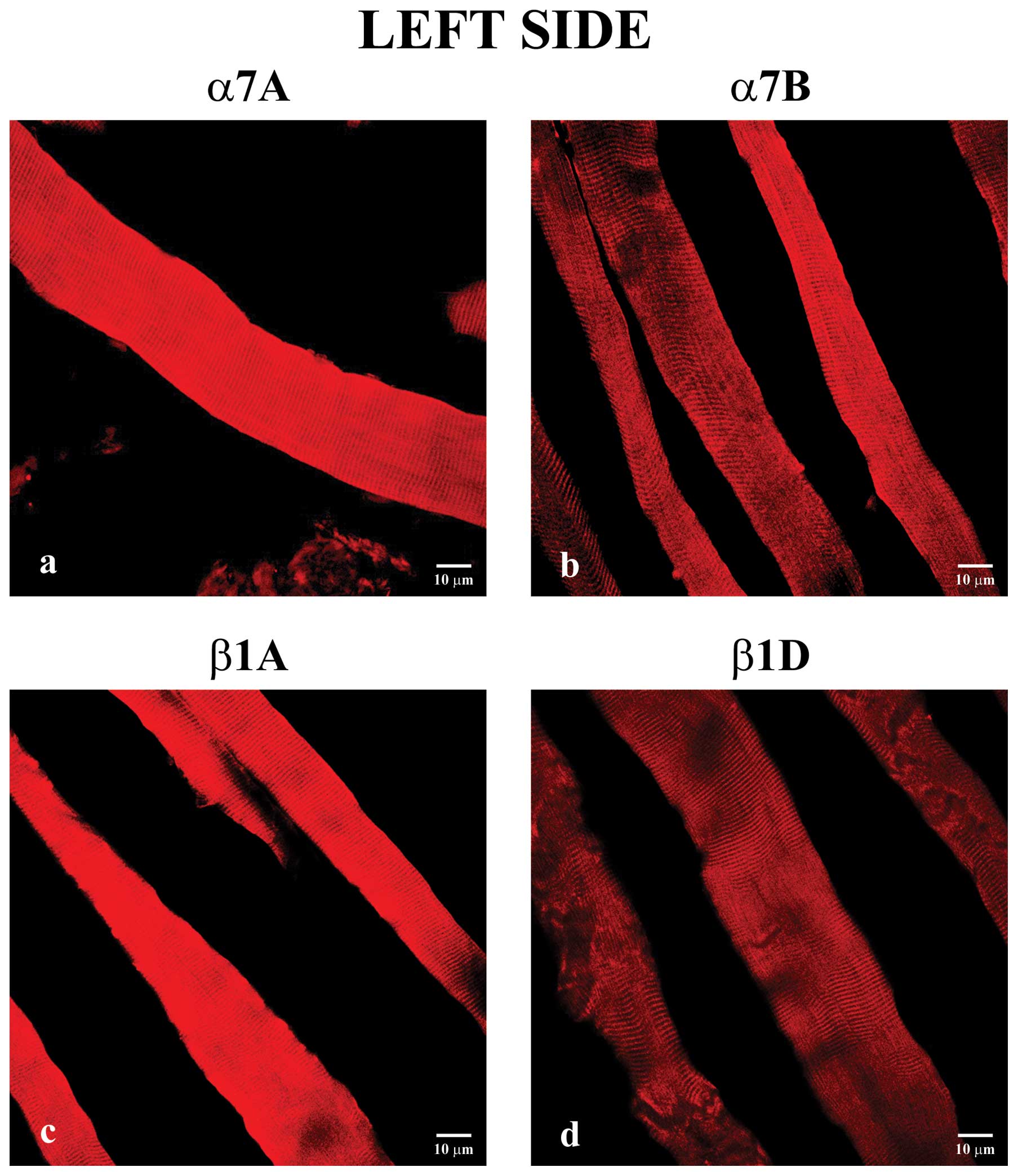
For immunohistochemical analysis, first, we analyzed
longitudinal sections of biopsy samples from left masseter muscle
without crossbite. These reactions showed that all of the integrins
were detected to varying degrees along the sarcolemma (Fig. 1). In particular, immunostaining of
α7A integrin (Fig. 1a) was
increased in respect to the corresponding α7B isoform (Fig. 1b). Moreover, β1A integrin
(Fig. 1c) immunofluorescence was
increased in respect to the β1D isoform (Fig. 1d).
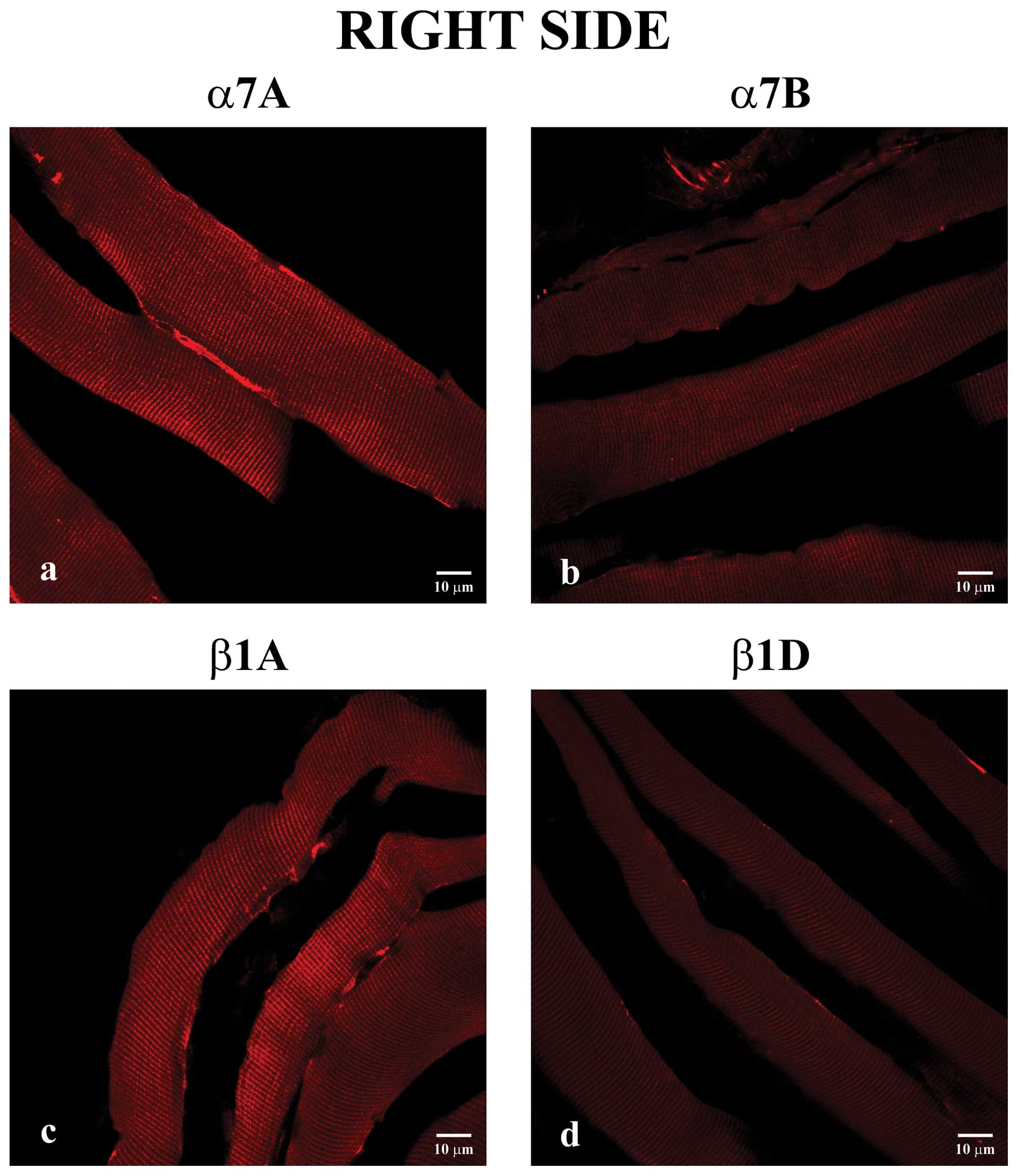
Secondly, we performed immunostaining on
longitudinal sections of masseter muscle fibers from the right
masseter. All of the integrins were detected to varying degrees
along the sarcolemma, and, generally, all showed decreased
immunofluorescence compared to integrins of the left side. In
particular, these reactions showed an increased staining pattern
for α7A (Fig. 2a) and β1A
integrin (Fig. 2c) compared to
the α7B (Fig. 2b) and β1D
(Fig. 2d) isoforms,
respectively.
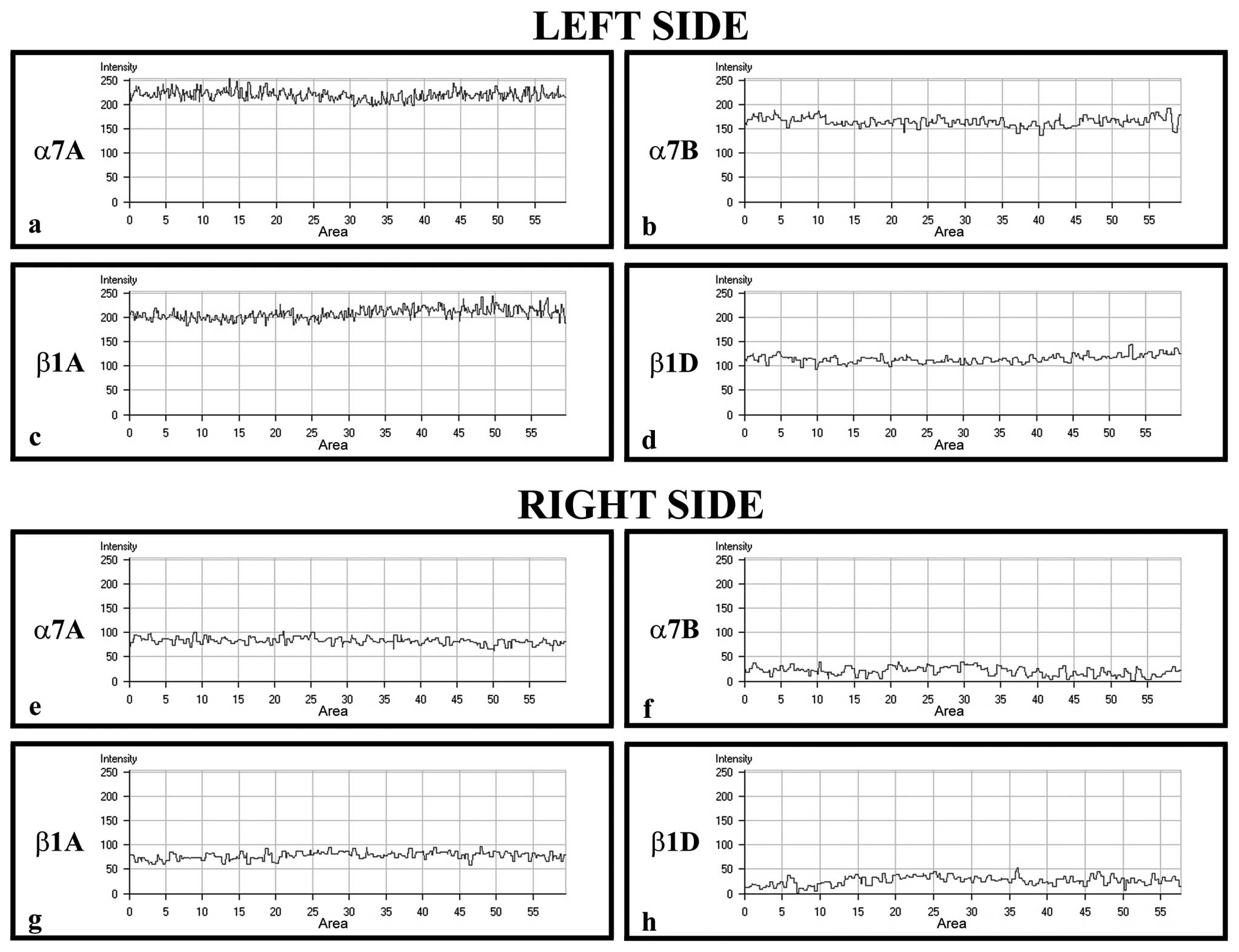
To confirm the protein staining patterns, we used
the ‘display profile’ software function of the laser scanning
microscope for selected samples. This additional analysis, which
reveals the fluorescence intensity profile across the image,
converted the immunofluorescence signal into a graph (Fig. 3) in order to quantitate the
differences between the antibodies. The display profile of the left
masseter showed increased fluorescence peaks for α7A (Fig. 3a) and β1A integrin (Fig. 3c), whereas peaks of the α7B
(Fig. 3b) and β1D isoform
(Fig. 3d) revealed decreased
intensity values. By applying this analysis to the samples of
muscle fibers taken from right masseter, it was possible to show
that the fluorescence peaks of all integrins showed a general
decrease compared with those of all integrins observed in the left
masseter, and in particular α7B (Fig.
3f) and β1D integrin (Fig.
3h) fluorescence intensity showed never reached 50, whereas the
fluorescence intensity of α7A (Fig.
3e) and β1A (Fig. 3g)
isoforms values were between 50 and 100.
Statistical analysis
Expression of α7A and β1A integrin in muscle fibers
on the right side (crossbite) was higher compared with that of α7B
and β1D isoforms of same side (w=2.655, p<0.007; w=2.258,
p<0.02, respectively). Compared to isoforms of controlateral
muscle fibers, the α7A, α7B and β1A isoforms of masseter fibers
crossbite were markedly decreased (w=5.403, p<0.001; w=5.303,
p<0.001; and w=4.886, p<0.001, respectively) whereas β1D
integrin, in crossbite, showed, a significant decrease of
distribution compared with muscle fibers β1D integrin, in left side
(w=2.353, p<0.01). In the left side the α7A and β1A integrin
showed a remarkable increase when compared to the α7B and β1D
isoforms, respectively, (w=2.574, p<0.01; w=4.180, p<0.001)
(Fig. 4).
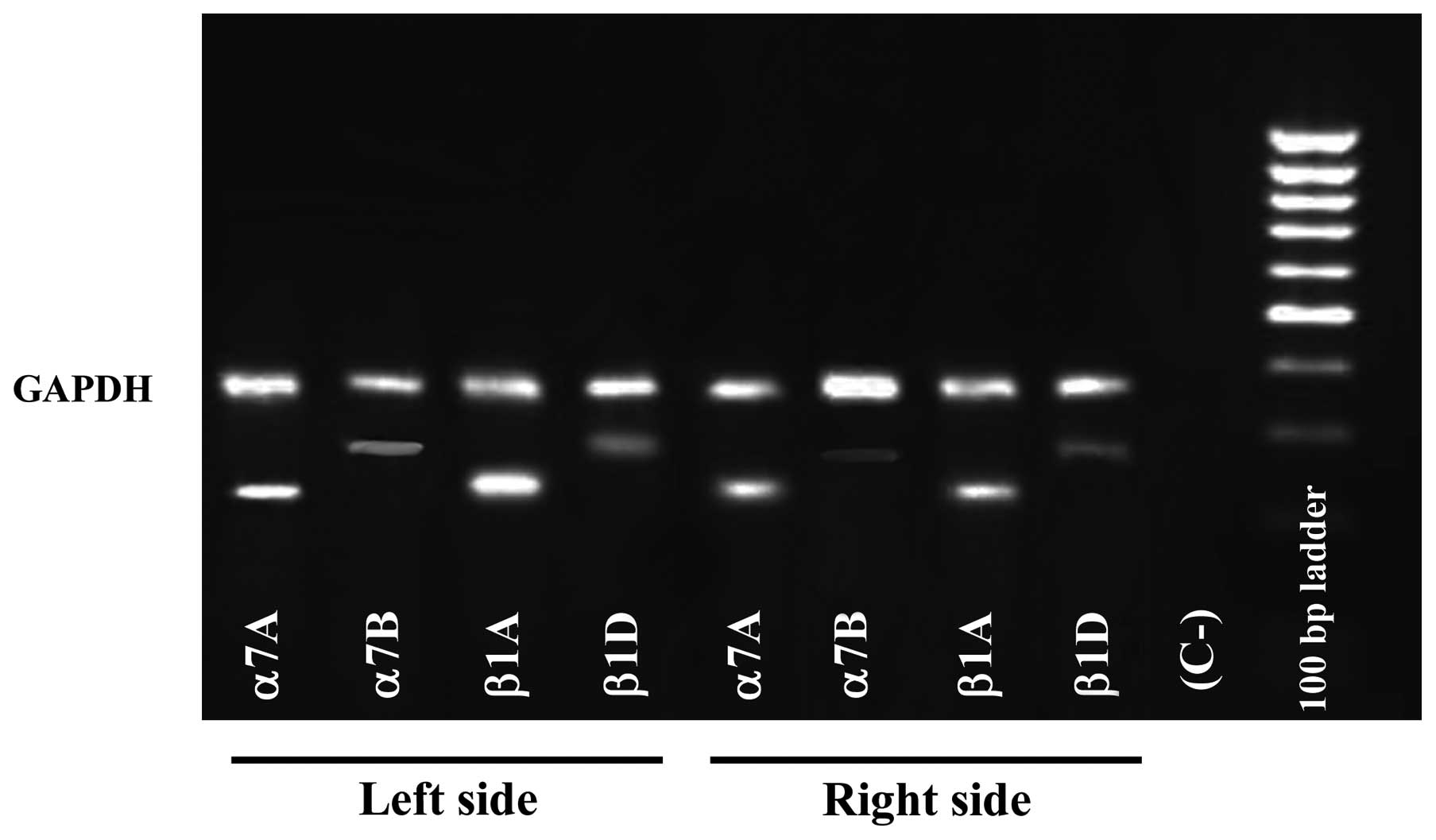
RT-PCR analysis
In order to determine whether differences in the
expression of integrins are due to the presence of different
integrin mRNA levels, we performed RT-PCR with primers specific for
muscle integrins. Using RNA samples isolated from masseter muscle
biopsies, we confirmed that levels of integrins appeared
significantly lower, in the right side, in comparison with those of
left side. Furthermore, α7A and β1A, compared to the α7B and β1D
isoform, respectively, were predominant in both masseter muscle
specimens (Fig. 5)
Discussion
In this report, for the first time, we analyzed
muscle-specific integrins, using human samples of muscle fibers on
both masseters obtained by patients affected by right posterior
crossbite.
Our results showed that the amount of integrins
appeared to be significantly lower, in the crossbite side, than
that detected in their left counterpart. Furthermore, the α7A and
β1A isoforms, compared to the α7B and β1D isoforms, respectively,
were predominant in both masseters.
Our previous report, evaluating the muscular
activation during chewing in unilateral posterior crossbite
patients, showed that the kinematics and electromyography
characteristics of the masseters of the non-affected side were
similar to those of controls, whereas the masseters of the
crossbite side were less active (34) meaning that patients with
unilateral posterior crossbite show a serious functional asymmetry
during chewing (33).
We thus hypothesized that the decreased functional
activity of the masseter of the crossbite side can be strongly
related to the behavior of integrins. It is well established that
integrins play a crucial role in cell adhesion, differentiation,
remodelling and programmed cell death (10,40). In particular, it was demonstrated
that the β1D isoform can be associated with α7A and α7B in adult
skeletal muscle, appearing immediately after myoblast fusion and
continuing to rise during myotube growth and maturation (12). In this way, β1D integrin plays a
key role in linking the sarcolemmal cytoskeleton to the surrounding
extracellular matrix in muscle tissue (12,19). The β1A isoform is involved in
signaling (41–44) and it not detected in adult
skeletal muscle fibers by immunofluorescence (45). Our preliminary results demonstrate
that the β1A isoform is clearly detectable in adult masseter muscle
and this could be due to the particular composition of
masseter.
Indeed, masseter muscle, compared with limb and
trunk muscles, is highly unusual; in fact, in addition to normal
slow and fast fibers, type I and type II respectively, this muscle
contains fiber types which are typical for developing or cardiac
muscle.
Moreover, many fibers of the masseter are hybrid;
these fiber types, in limb and trunk muscles, are thought to be
those that are in transition from one fiber type into another,
since they are predominantly found during disuse or during extreme
usage of the muscles (46) or in
regenerating fibers (47). Hybrid
fibers are abundantly present in normal jaw-closing muscle, both in
rabbit (48), and in humans
(49–51). The important presence of hybrid
fibers in considerable numbers, may be due to specific functional
demands of the masseter muscle; these fiber types probably increase
the capacity of the masseter muscle to generate a large variety of
motor tasks, since they have contractile features which lie between
those of pure fibers.
In this way, the presence of hybrid fibers could
reflect the adaptive ability of masseter muscle fibers, showing the
capacity to modify their contractile properties to optimize the
efficiency during contraction. Then, the masseter muscle could
continuously switch from one fiber type to another. Based on its
functional demand, in our opinion, these continuous changes in
phenotypic structures could also influence normal arrangement of
the entire muscle, and in masseter muscle this could provoke a
rearrangement of integrin network with consequential increase of
the β1A isoform.
Another possible explanation for the different
arrangement of the integrin network in masseter muscle, observed in
the present study, also could be due to evidence that, the
membranes of normal muscle fibers are ruptured by stretch and
relaxation (52), with
stimulation of satellite cells to repair the damage. Thus, since
masseter muscle is subject to continuous mechanical stress, as a
result of continuous control of the position and motion of the
mandible and creation of forces at the teeth and temporomandibular
joint (53), this muscle is
characterized by a particular high turnover, which produces a
regeneration process initiating by fibers typical for developing
muscle.
A further remarkable feature is the relationship
between fiber size and fiber type in masseter muscle; fast type
fibers of masseter muscle have a smaller cross-sectional area than
the slow type fibers whereas in the limb and trunk muscles, the
reverse is true (54). This
characteristic facilitates an increase in the exchange of
O2, improving the resistance to fatigue so that it is
most advantageous for mastication (55). The continued expression of these
fiber types in adult masseter muscle might indicate a longer time
to heal, and this could justify the loss of β1D and the
consequential increased amount of β1A integrin showed by the
present data.
Furthermore, interesting features of integrins allow
us to propose an intriguing hypothesis on masseter muscle in
patients with unilateral posterior crossbite. Previous reports have
found that α-actinin, a focal adhesion component that interacts
with β1A (56) reinforcing links
between actin filaments, binds β1D less strongly than β1A (12). This feature correlates with the
absence of α-actinin at the myotendinous junction, the major sites
of force transmission in muscle (57), suggesting that β1D may be less
effective than β1A with regard to integrin-mediated signaling
(45). Therefore, based on our
results, we could hypothesize that the lower activity of masseter
on the crossbite side may cause a loss of β1D integrin and that the
β1A isoform may have a role in reinforcing the arrangement of the
muscle fibers and in recovering signaling role of the entire
membrane in order to restore force transmission.
Previously, a similar behavior of the integrin
network was demonstrated analyzing human gastrocnemius muscle of
subjects affected by sensitive-motor polyneuropathy (26,58). During this muscular inactivity it
is possible to hypothesize that a reorganization of the
transmembrane occurred, maintaining the viability of the skeletal
muscle fibers (58).
The present data provide the first suggestion that
integrins in masseter muscle play a key role in regulating muscular
functional activity and allowing the optimization of the
contractile forces of this muscle. Therefore, these results reveal
a new venue of research, which will have the aim to understand the
differences of the protein composition and structural arrangement
of masseter muscle fibers in respect to other muscle fibers. Thus,
it is intriguing to examine whether other proteins, such as
sarcoglycans, are involved in this different protein arrangement of
masseter muscle fibers.
References
|
1.
|
JM ErvastiK OhlendieckSD KahlMG GaverKP
CampbellDeficiency of a glycoprotein component of the dystrophin
complex in dystrophic
muscleNature345315319199010.1038/345315a02188135
|
|
2.
|
M YoshidaA SuzukiH YamamotoS NoguchiY
MizunoE OzawaDissociation of the complex of dystrophin and its
associated proteins into several unique groups by n-octyl
β-D-glucosideEur J Biochem2221055106119948026484
|
|
3.
|
M YoshidaE OzawaGlycoprotein complex
anchoring dystrophin to sarcolemmaJ Biochem10874875219902081733
|
|
4.
|
JM ErvastiKP CampbellA role for the
dystrophin-glycoprotein complex as a transmembrane linker between
laminin and actinJ Cell
Biol122809823199310.1083/jcb.122.4.8098349731
|
|
5.
|
EL GeorgeEN Georges-LabouesseRS
Patel-KingH RayburnRO HynesDefects in mesoderm, neural tube and
vascular development in mouse embryos lacking
fibronectinDevelopment1191079109119938306876
|
|
6.
|
T JaffredoAF HorwitzCA BuckPM RongF
Dieterlen-LievreMyoblast migration specifically inhibited in the
chick embryo by grafted CSAT hybridoma cells secreting an
anti-integrin antibodyDevelopment10343144619883266744
|
|
7.
|
AS MenkoD BoettigerOccupation of the
extracellular matrix receptor, integrin, is a control point for
myogenic
differentiationCell515157198710.1016/0092-8674(87)90009-23115595
|
|
8.
|
JS CampbellMP WenderothSD HauschkaEG
KrebsDifferential activation of mitogen-activated protein kinase in
response to basic fibroblast growth factor in skeletal muscle
cellsProc Natl Acad Sci USA92870874199510.1073/pnas.92.3.870
|
|
9.
|
Q ChenMS KinchTH LinK BurridgeRL
JulianoIntegrin-mediated cell adhesion activates mitogen-activated
protein kinasesJ Biol Chem269266022660519947929388
|
|
10.
|
RO HynesIntegrins: versatility,
modulation, and signaling in cell
adhesionCell691125199210.1016/0092-8674(92)90115-S1555235
|
|
11.
|
K BurridgeM Chrzanowska-WodnickaFocal
adhesions, contractility and signalingAnnu Rev Cell Dev
Biol12463518199610.1146/annurev.cellbio.12.1.4638970735
|
|
12.
|
AM BelkinNI ZhidkovaF BalzacF AltrudaD
TomatisA MaierG TaroneVE KotelianskyK Burridgeβ1D integrin
displaces the β1A isoform in striated muscles: localization at
junctional structures and signaling potential in nonmuscle cellsJ
Cell Biol1322112261996
|
|
13.
|
PT MartinSJ KaufmanRH KramerJR
SanesSynaptic integrins in developing, adult, and mutant muscle:
selective association of β1, α7A, and α7B integrins with the
neuromuscular junctionDev Biol17412513919968626012
|
|
14.
|
YK HayashiFL ChouE EngvallM OgawaC
MatsudaS HirabayashiK YokochiBL ZioberRH KramerSJ KaufmanMutations
in the integrin α7 gene cause congenital myopathyNat
Genet1994971998
|
|
15.
|
ZZ BaoM LakonishokS KaufmanAF Horwitzα7β1
integrin is a component of the myotendinous junction on skeletal
muscleJ Cell Sci1065795901993
|
|
16.
|
DJ BurkinGQ WallaceKJ NicolDJ KaufmanSJ
KaufmanEnhanced expression of the α7β1 integrin reduces muscular
dystrophy and restores viability in dystrophic miceJ Cell
Biol152120712182001
|
|
17.
|
A Van der FlierI KuikmanC BaudoinR van der
NeutA SonnenbergA novel beta 1 integrin isoform produced by
alternative splicing: unique expression in cardiac and skeletal
muscleFEBS Lett36934034419957544298
|
|
18.
|
NI ZhidkovaAM BelkinR MayneNovel isoform
of beta 1 integrin expressed in skeletal and cardiac muscleBiochem
Biophys Res Commun214279285199510.1006/bbrc.1995.22857545396
|
|
19.
|
R FasslerJ RohmedelV MaltsenW BlochS
LentiniK GuanD GullbergJ HeschelerK AddicksAM WodusDifferentiation
and integrity of cardiac muscle cells are impaired in the presence
of β1 integrinJ Cell Sci1092989299919969004034
|
|
20.
|
PH VachonH XuL LiuF LoechelY HayashiK
ArahataJC ReedUM WewerE EngvallIntegrins (α7β1) in muscle function
and survival. Disrupted expression in merosin-deficient congenital
muscular dystrophyJ Clin Invest100187018811997
|
|
21.
|
WK SongW WangRF FosterDA BielserSJ
KaufmanH36-α7 is a novel integrin alpha chain that is
developmentally regulated during skeletal myogenesisJ Cell
Biol1176436571992
|
|
22.
|
M George-WeinsteinRF FosterJV GerhartSJ
KaufmanIn vitro and in vivo expression of α7 integrin and desmin
define the primary and secondary myogenic lineagesDev
Biol1562092291993
|
|
23.
|
M MonemiF KadiJX LiuLE ThornellPO
ErikssonAdverse changes in fibre type and myosin heavy chain
composition of human jawActa Physiol
Scand167339345199910.1046/j.1365-201x.1999.00625.x10632637
|
|
24.
|
G AnastasiG CutroneoG RizzoA ArcoG
SantoroP BramantiAG VitettaA PisaniF TrimarchiA FavaloroSarcoglycan
and integrin localization in normal human skeletal muscle: a
confocal laser scanning microscope studyEur J
Histochem482452522004
|
|
25.
|
G AnastasiA AmatoG TaroneG VitaMC MoniciL
MagauddaM BrancaccioA SidotiF TrimarchiA FavaloroG
CutroneoDistribution and localization of vinculin-talin-integrin
system and dystrophin-glycoprotein complex in human skeletal
muscleCells Tissues
Organs175151164200310.1159/00007463114663158
|
|
26.
|
G AnastasiG CutroneoG SantoroA ArcoG
RizzoC TromminoP BramantiL SosciaA FavaloroIntegrins, muscle agrin
and sarcoglycans during muscular inactivity conditions: an
immunohistochemical studyEur J Histochem50327336200617213042
|
|
27.
|
A FavaloroG SperanzaS RezzaV GattaG
VaccarinoL StuppiaF FestaG AnastasiMuscle-specific integrins in
masseter muscle fibers of chimpanzees: an immunohistochemical
studyFolia Histochem Cytobiol47551558200920430719
|
|
28.
|
AG HannamAS McMillanInternal organization
in the human jaw musclesCrit Rev Oral Biol Med5558919947999950
|
|
29.
|
TS MilesMA NordstromAfferent and cortical
control of human masticatory musclesAdv Exp Med
Biol508443449200210.1007/978-1-4615-0713-0_5012171141
|
|
30.
|
RJ WildingA LewinThe determination of
optimal human jaw movements based on their association with chewing
performanceArch Oral
Biol39333343199410.1016/0003-9969(94)90125-28024498
|
|
31.
|
MG PiancinoP BraccoT VallelongaA MerloD
FarinaEffect of bolus hardness on the chewing pattern and
activation of masticatory muscles in subjects with normal dental
occlusionJ Electromyogr
Kinesiol18931937200810.1016/j.jelekin.2007.05.00617616401
|
|
32.
|
GS ThrockmortonPH BuschangH HayasakiAS
PintoChanges in the masticatory cycle following treatment of
posterior unilateral crossbite in childrenAm J Orthod Dentofacial
Orthop120521529200110.1067/mod.2001.11862611709671
|
|
33.
|
MG PiancinoF TalponeP DalmassoC
DebernardiA LewinP BraccoReverse-sequencing chewing patterns before
and after treatment of children with unilateral posterior
crossbiteEur J Orthod28480484200610.1093/ejo/cjl01416772316
|
|
34.
|
MG PiancinoD FarinaF TalponeA MerloP
BraccoMuscular activation during reverse and non-reverse chewing
cycles in unilateral posterior crossbiteEur J Oral
Sci117122128200910.1111/j.1600-0722.2008.00601.x19320720
|
|
35.
|
A UsamiS AbeY IdeMyosin heavy chain
isoforms of the murine masseter muscle during pre- and post-natal
developmentAnat Histol
Embryol32244248200310.1046/j.1439-0264.2003.00481.x12919077
|
|
36.
|
N KawaiR SanoJA KorfageS NakamuraE TanakaT
van WesselGE LangenbachK TanneFunctional characteristics of the rat
jaw muscles: daily muscle activity and fiber type compositionJ
Anat215656662200910.1111/j.1469-7580.2009.01152.x19811563
|
|
37.
|
SB BoydWJ GonyeaHL LeganWH BellMasseter
muscle adaptation following surgical correction of vertical
maxillary excessJ Oral Maxillofac
Surg47953962198910.1016/0278-2391(89)90380-72760732
|
|
38.
|
SB BoydWJ GonyeaRA FinnCE WoodardWH
BellHistochemical study of the masseter muscle in patients with
vertical maxillary excessJ Oral Maxillofac
Surg427583198410.1016/0278-2391(84)90315-X6229616
|
|
39.
|
P ChomczynskiN SacchiSingle-step method of
RNA isolation by acid guanidinium thiocyanate-phenol-chloroform
extractionAnal
Biochem162156159198710.1016/0003-2697(87)90021-22440339
|
|
40.
|
S SastryA HorwitzIntegrin cytoplasmic
domains: mediators of cytoskeletal linkages and extra- and
intracellular initiated transmembrane signalingCurr Opin Cell
Biol5819831199310.1016/0955-0674(93)90031-K8240826
|
|
41.
|
SE La FlammeSK AkiyamaKM YamadaRegulation
of fibronectin receptor distributionJ Cell Biol1174374471992
|
|
42.
|
AA ReszkaY HayashiAF HorwitzIdentification
of aminoacid sequences in the integrin β1 cytoplasmic domain
implicated in cytoskeletal associationJ Cell
Biol117132113301992
|
|
43.
|
SE LaFlammeLA ThomasSS YamadaKM
YamadaSingle subunit chimeric integrins as mimics and inhibitors of
endogenous integrin functions in receptor localization, cell
spreading and migration, and matrix assemblyJ Cell
Biol12612871298199410.1083/jcb.126.5.12878063864
|
|
44.
|
JM LewisMA SchwartzMapping in vivo
associations of cytoplasmic proteins with integrin β1 cytoplasmic
domain mutantsMol Biol Cell61511601995
|
|
45.
|
AM BelkinSF RettaOY PletjushkinaF BalzacL
SilengoR FasslerVE KotelianskyK BurridgeG TaroneMuscle β1D integrin
reinforces the cytoskeleton-matrix link: modulation of integrin
adhesive function by alternative splicingJ Cell
Biol139158315951997
|
|
46.
|
H KlitgaardM ZhouS SchiaffinoR BettoG
SalviatiB SaltinAgeing alters the myosin heavy chain composition of
single fibres from human skeletal muscleActa Physiol
Scand1405562199010.1111/j.1748-1716.1990.tb08975.x2275405
|
|
47.
|
D PetteJ SketeljD SkorjancE LeisnerI
TraubF BajrovicPartial fast-to-slow conversion of regenerating rat
fast-twitch muscle by chronic low-frequency stimulationJ Muscle Res
Cell Motil23215221200210.1023/A:102097471038912500901
|
|
48.
|
SH KwaWA WeijsPJ JüchContraction
characteristics and myosin heavy chain composition of rabbit
masseter motor unitsJ Neurophysiol7353854919957539059
|
|
49.
|
JJ BredmanA WesselsWA WeijsJA KorfageCA
SoffersAF MoormanDemonstration of ‘cardiac-specific’ myosin heavy
chain in masticatory muscles of human and rabbitHistochem
J231601701991
|
|
50.
|
P StålPO ErikssonS SchiaffinoGS
Butler-BrowneLE ThornellDifferences in myosin composition between
human oro-facial, masticatory and limb muscles: enzyme-,
immunohistoand biochemical studiesJ Muscle Res Cell
Motil1551753419947860700
|
|
51.
|
M MonemiPO ErikssonA ErikssonLE
ThornellAdverse changes in fibre type composition of the human
masseter versus biceps brachii muscle during agingJ Neurol
Sci1543548199810.1016/S0022-510X(97)00208-69543320
|
|
52.
|
BJ PetrofHH StedmanJB ShragerJ EbyHL
SweeneyAM KellyAdaptations in myosin heavy chain expression and
contractile function in dystrophic mouse diaphragmAm J
Physiol65C834C84119938214039
|
|
53.
|
T GrünheidGEJ LangenbachJAM KorfageA
ZentnerTMGJ van EijdenThe adaptive response of jaw muscles to
varying functional demandsEur J Orthodont31596612200919656804
|
|
54.
|
JAM KorfageP BrugmanTMGJ van
EijdenIntermuscular and intramuscular differences in myosin heavy
chain composition of the human masticatory musclesJ Neurol
Sci17895106200010.1016/S0022-510X(00)00372-511018701
|
|
55.
|
L LarssonX LiWR FronteraEffects of aging
on shortening velocity and myosin isoform composition in single
human skeletal muscle cellsAm J Physiol272C638C64919979124308
|
|
56.
|
CA OteyFM PavalkoK BurridgeAn interaction
between α-actinin and the β1 integrin subunit in vitroJ Cell
Biol1117217291990
|
|
57.
|
JG Tidballα-actinin is absent from the
terminal segments of myofibrils and from subsarcolemmal densities
in frog skeletal muscleExp Cell Res1704694821987
|
|
58.
|
G AnastasiG CutroneoG SantoroA ArcoG
RizzoP BramantiC RinaldiA SidotiA AmatoA FavaloroCostameric
proteins in human skeletal muscle during muscular inactivityJ
Anat213284295200810.1111/j.1469-7580.2008.00921.x18537849
|