Introduction
Myocardial edema is closely related to myocardial
stunning, and may lead to death or complications following surgery.
Heart dysfunction caused by myocardial edema can dramatically
affect both systolic and diastolic function, and may endure over a
long period of time (1,2). It has been previously demonstrated
that a water content increase of 3.5% can lead to a cardiac output
decrease of 30 to 50% (3,4). Myocardial edema is caused by
ischemia-reperfusion injury, hemodilution during cardiopulmonary
bypass (CPB), and an inflammatory response to CPB (5,6).
Aquaporin water channels are comprised of highly
conserved proteins that exist in both bacteria and humans. These
water channels are involved in many physiological processes, such
as the osmotic environment and overall body fluid balance,
including osmotically driven transepithelial fluid transport that
occurs in the kidney, eye and secretory glands, and facilitation of
water movement into and out of the brain in various pathologies
such as stroke, tumor and infection (7–10).
There are 13 members of AQPs (AQP0-AQP12) in animals and their
expression is organ-specific. Aquaporin 1 (AQP1) is the most
predominant and least tissue expression-specific subtype. The
monomeric structure consists of two triple transmembrane helices
connected by a long flexible extracellular loop. Studies in rodents
have demonstrated that AQP1 is expressed in cardiac muscle cells
and that it can be reversibly internalized from the cell membrane
in response to the osmotic environment (10–12).
Cardiac myocytes are tightly interconnected by means
of highly specialized regions of the plasma membrane called gap
junctions. Gap junctions are composed of clusters of transmembrane
channels connecting the cytosol of adjacent cells. Studies have
demonstrated that Connexin 43 (Cx43) plays an important role in
myocardial protection in ischemic preconditioning (13–15), but whether it plays the same role
in myocardial edema remains unknown.
In this study, we demonstrated the variations in
mRNA expression and protein levels of AQP1 following CPB surgery in
a goat animal model. To maintain constant extracellular volume,
water is cleared from the cytoplasm via gap junctions. We also
tested whether a functional relationship exists between water
channels and gap junctions. We found that AQP1 plays an important
role in myocardial edema via regulation of Cx43 expression.
Materials and methods
Animal model
The study protocol was approved by the Medical
Ethics Committee of Shanghai Xinhua Hospital, conforms to the
Principles of Laboratory Animal Care (National Society for Medical
Research), and was conducted according to National Institutes of
Health guidelines.
Twenty-four adult goats weighing 50–60 kg (Slac
Laboratory Animal) were used for this study. Myocardium tissues
from the left and right cardiac ventricles were obtained from six
adult goats anesthetized and bled from the dorsal aorta until
expiration. Tissues were stored in liquid nitrogen for further
use.
Animal studies and extracorporeal
circulation model
The goats underwent general anesthesia with
isoflurane and nitrous oxide, and were endotracheally intubated. A
left anterolateral thoracotomy was performed in the fifth
intercostal space, followed by dissection. After systemic
anticoagulation with sodium heparin (300 U/kg), the aorta was
cannulated for arterial perfusion. A venous cannula was placed in
the right atrium. Upon initiation of CPB surgery, the body
temperature was cooled to 28°C and the aorta was cross-clamped. The
heart was arrested with crystalloid cardioplegic solution.
The pericardial cavity was filled with cold
physiological saline after cardiac arrest and perfused with
crystalloid cardioplegic solution every 20 min. Following 60 min of
cardiac arrest, the aorta was declamped. All animals were weaned
from CPB without inotropic support 60 min after the release of the
aortic cross-clamp. Protamine was used to neutralize the heparin.
Following placement of the drainage tube, the thoracic cavity was
closed.
Myocardium tissue samples from the left and right
cardiac ventricles were obtained 0, 2, 6, 12, 24, 48 and 72 h
following CPB, and stored in liquid nitrogen until further use.
Real-time PCR analysis of AQP1
expression
Real-time PCR was performed to assess AQP1
expression in the left ventricle (LV) and right ventricle (RV).
Heart tissue was homogenized and RNA was isolated using RNeasy
Fibrous Tissue Mini kit (Qiagen) according to the manufacturer’s
protocol, and any contaminating DNA was degraded by a 15-min
incubation with RNase-free DNase. Real-time PCR was performed using
the High Capacity cDNA Archive kit (Applied Biosystems). We created
two standard curves, one for mouse AQP1 and the other using the
internal control GAPDH with real-time PCR using appropriate primers
and probes (Applied Biosystems). Each sample was loaded in
triplicate for both AQP1 and GAPDH. The primers for AQP1 were:
forward, 5′-GCC AGC GAG TTC AAG AAG-3′ and reverse, 5′-CCC CAC CCA
GAA AAT CC-3′. The primers for GAPDH were: forward, 5′-GCC AGC GAG
TTC AAG AAG-3′ and reverse, 5′-CCC CAC CCA GAA AAT CC-3′. Real-time
PCR was conducted using the ABI Prism 7000 sequence detection
system, and data were analyzed with ABI Prism 7300 SDS
software.
Western blot analysis
Western blot analysis was performed as described
previously (16), with minor
modifications. In brief, each goat myocardium tissue sample was
crushed and ground using a mortar, pestle and liquid nitrogen. The
resulting powder was immediately suspended in lysis buffer (pH 7.4,
4% SDS, 100 mM DTT, 125 mM Tris, 40% glycerol and trace cocktail
protease inhibitor), and ultrasonically homogenized. Next, the
solution was heated to 94°C for 4 min, and the cell debris and
insoluble substances were removed by centrifugation at 15,000 × g
for 3 min. The supernatant was separated by SDS-PAGE with 20
μg protein/lane and transferred onto a PVDF membrane
(Millipore) using transfer buffer [pH 11.0, 25 mM Tris, 0.2 M
glycine, 20% (v/v) methanol] on a semi-dry electroblotter
(Bio-Rad). After blocking with 5% skim milk and 0.1% Tween-20 for 1
h at room temperature (RT), AQP1 was detected by incubating the
samples with rabbit polyclonal anti-AQP1 and Cx43 antibodies at a
1:1,000 dilution (Abcam Ltd.) overnight at 4°C. Alkaline
phosphatase-goat anti-rabbit IgG at a 1:3,000 dilution (GeneTex,
Inc., USA) was used as the secondary antibody. The immunoblots were
developed using ECL detection reagent (Pierce Chemical).
Lentivirus production and
transfection
We obtained the lentivirus packaging system for AQP1
overexpression from Tronolab. The lentivirus packaging system for
AQP1 RNAi was obtained from Addgene. The RNAi target sequences were
5′-ATC ATC AGC ATC CAA GGT CAT ACT CC-3′ and 5′-AAG AGC TTC TTC TTG
ATT TCG CTG G-3′. Recombinant lentivirus was produced by
co-transfecting 293T cells with the expression plasmid and
packaging plasmids, and RNAi plasmids and packaging plasmids
separately. Infectious cells were harvested at 48 and 72 h post
transfection. Cell debris was eliminated by centrifugation.
Recombinant lentiviral suspension (200 μg/kg body weight)
was injected into the ventricular myocardium of the ventricular
wall using 30-gauge syringe. The injection did not cause any
hemodynamic changes, allergies, or other side effects during the
entire experiment. The empty vector virus was injected in an
identical manner and served as the negative control.
Immunofluorescence
Cryosections (8-μm) were fixed with 3%
paraformaldehyde in PBS, washed, and incubated in blocking buffer
(PBS containing 2% BSA). Primary antibodies diluted in blocking
buffer were applied for 1 h at RT, or overnight at 4°C (1:1,000
dilutions for the monoclonal AQP1 antibody; Abcam). Samples were
washed with PBS and incubated for 2 h with appropriate secondary
antibodies diluted in blocking buffer (Alexa Fluor 488-conjugated
donkey anti-rabbit IgG; Molecular Probes-Invitrogen). Samples were
washed three times in PBS and mounted using Shandon Immu-mount™.
Sections were analyzed with a Leica SP2 confocal laser-scanning
microscope.
Analysis of myocardial edema
Myocardial edema was determined according to the
water content of the myocardial muscle tissue. The wet weight (WW)
of myocardial tissue was weighed using an analytical balance. The
samples were then dried using a microwave oven, and the dry weight
(DW) was recorded. Water content was calculated using the equation:
Water content = (WW - DW)/WW × 100%.
Cardiac function measurement
A micromanometer tipped catheter was positioned in
the LV. Hemodynamic parameters were recorded using a data recording
unit. Echocardiography was performed using a Sonos 5500 (Hewlett
Packard, USA).
Statistical analysis
All values are expressed as the mean ± standard
deviation (SD). Statistical analysis was performed by one-way
analysis of variance (ANOVA), followed by the Student’s and
Newman-Keuls test using SPSS 11.0. A P-value <0.05 was
considered to indicate a statistically significant result.
Results
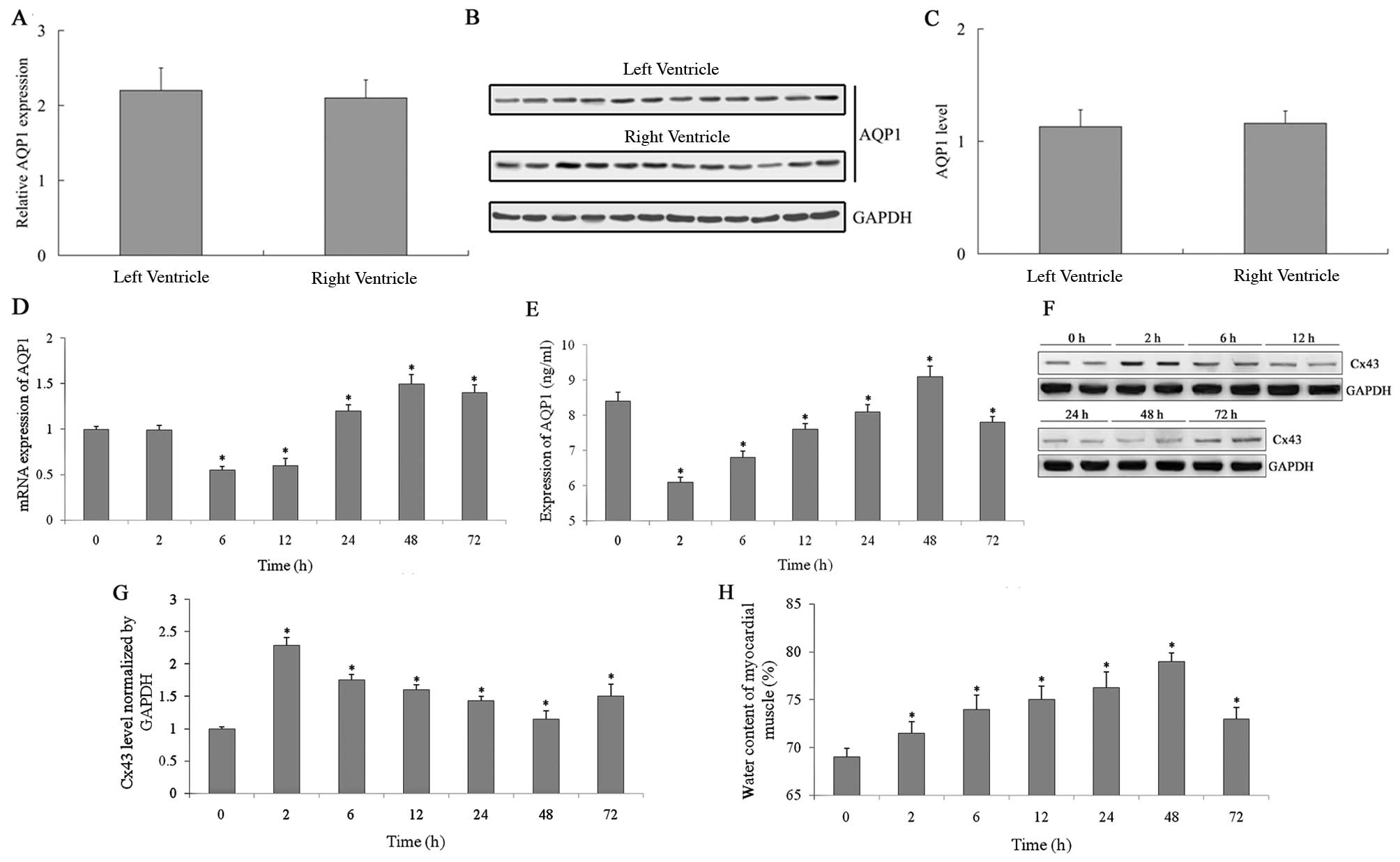
Right and left ventricular expression of
AQP1 in goats
The expression of AQP1 in human cardiac muscle is
rather high. In our study, goat right and left ventricular mRNA
expression levels of AQP1 were detected by real-time PCR, and the
results indicated that there was little difference between the
right and the left ventricles (P>0.05) (Fig. 1A). The protein levels of AQP1 were
also detected by western blot analysis (Fig. 1B). A histogram was created based
on the gray values of the western blot analysis (Fig. 1C). The protein level of AQP1 in
the left and right ventricles was also nearly identical (P>0.05)
(Fig. 1B and C).
Left ventricular expression of AQP1 and
Cx43 after CPB surgery
The mRNA expression of AQP1 was measured by
real-time-PCR 0, 2, 6, 12, 24, 48 and 72 h following CPB surgery
(Fig. 1D). Initially, mRNA
expression decreased after aortic occlusion. Six hours later, the
expression began to increase and reached a maximum level after 48
h. Next, the mRNA expression level of AQP1 slowly decreased. The
protein expression of AQP1 was measured using ELISA (Fig. 1E), and the expression pattern was
similar to mRNA expression. Initially the protein expression
decreased, 6 h later the expression level began to increase, 48 h
later it reached the peak value, and then the expression level
began to slowly decrease. The protein expression of Cx43 (Fig. 1F) detected by western blot
analysis, was inversely correlated with AQP1.
Myocardial edema was assessed according to the water
content of the myocardial muscle. The water content of the
myocardial muscle was measured 2, 6, 12, 24, 48 and 72 h following
CPB surgery (Fig. 1H). The degree
of myocardial edema increased following the cardiopulmonary
surgery, and 48 h later peaked at the same time that the mRNA and
protein expression levels of AQP1 reached maximum values. Finally,
the water content of the myocardial muscle slowly decreased.
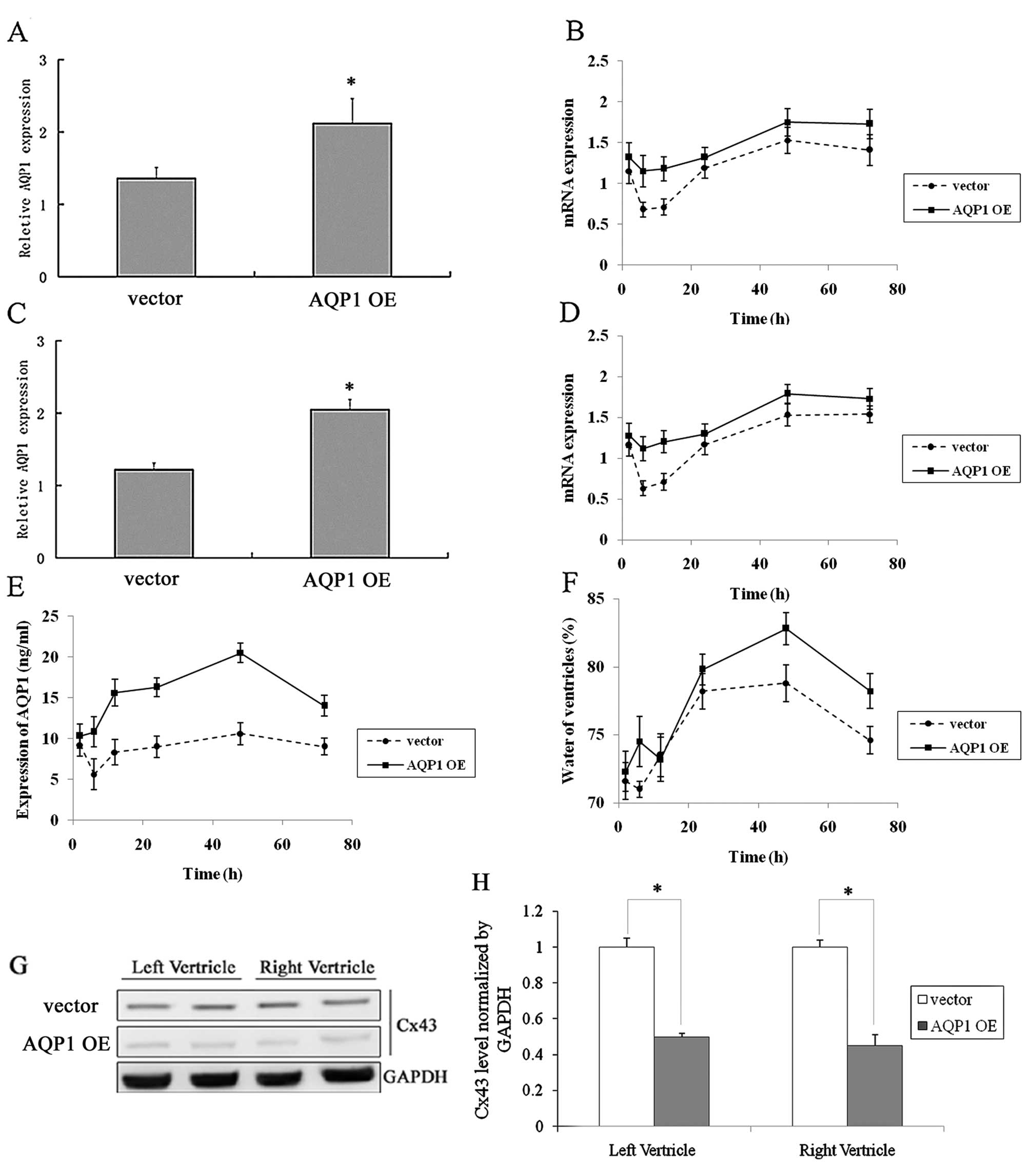
Lentiviral-mediated overexpression of
AQP1 causes myocardial edema
Left and right ventricular cells were infected by a
lentivirus expressing AQP1 during CPB surgery. The expression of
AQP1, as detected by real-time PCR (Fig. 2A–D), and the protein expression of
Cx43, detected by western blot analysis (Fig. 2G–H), decreased after infection
with the lentivirus expressing AQP1. A similar trend was observed
between AQP1 expression levels and the control; however, the
quantity of AQP1 protein expression was greater compared to the
control (Fig. 2B and D).
Myocardial edema was determined by measuring the water content of
the myocardial muscle tissue (Fig.
2F). The myocardial edema of the infection group was much
greater compared to the control group. The changes of AQP1 protein
over time were also measured (Fig.
2E). The trend of the curve correlated with the degree of
myocardial edema.
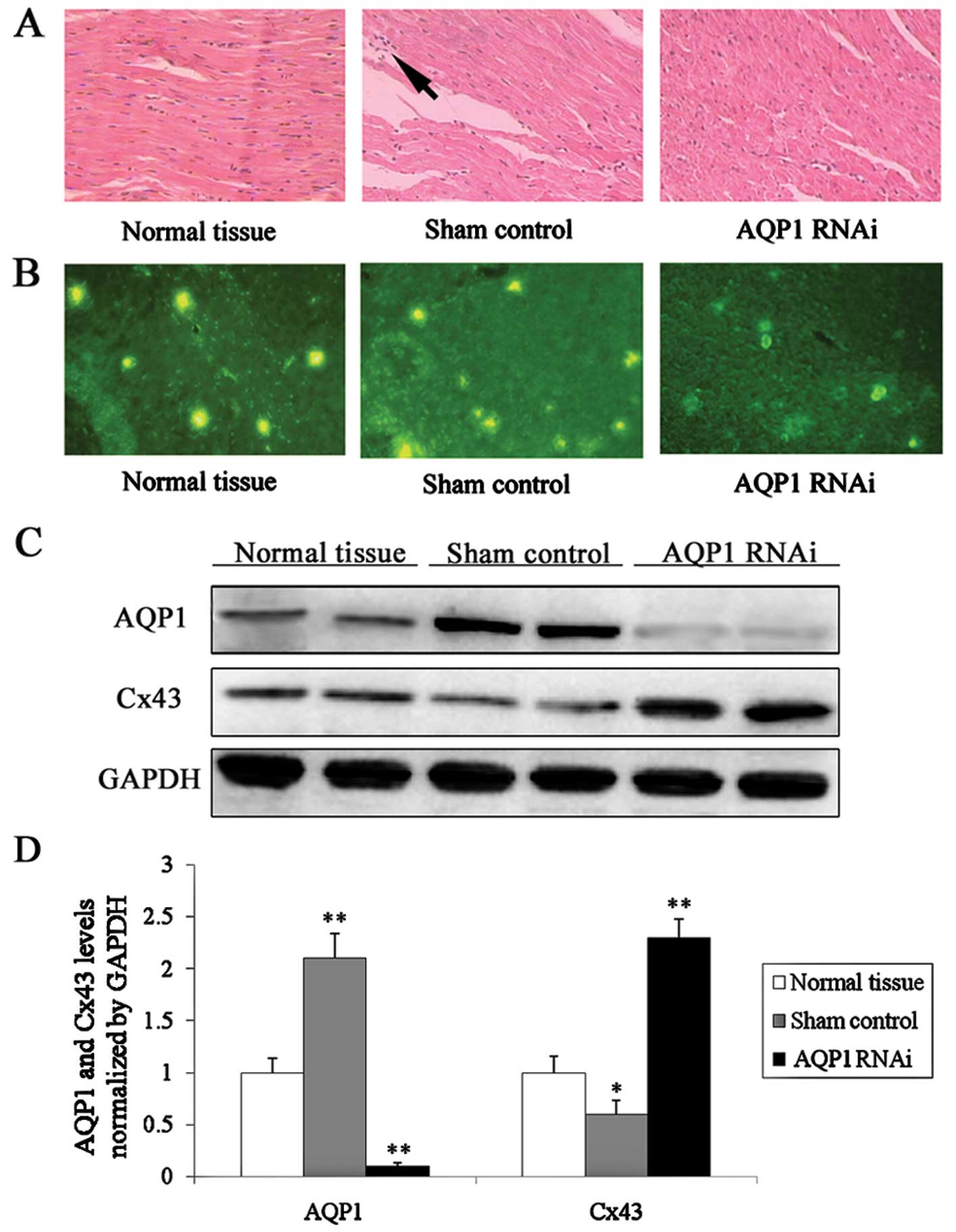
Inflammation was observed in conjunction
with elevated AQP1 expression following CPB surgery
AQP1 was knocked down following injection of an RNAi
lentivirus during CPB surgery. Tissue samples were obtained from
the left ventricle. Sections were cut (5 μm) and stained
using the standard hematoxylin and eosin staining. The efficiency
of knockdown was detected by immunofluorescence staining (Fig. 3B). In the control group, a greater
number of nuclei was released from the myocardial cells (Fig. 3A). This finding revealed that
inflammation had occurred along with myocardial edema following CPB
surgery. Inflammation did not occur in the normal tissue group or
in the surgery with lentivirus-infection group. The expression of
Cx43 (Fig. 3C) increased in the
surgery with lentivirus-infection group compared to the control
group; this demonstrated that the inflammation caused by myocardial
edema correlated with AQP1 and Cx43 expression.
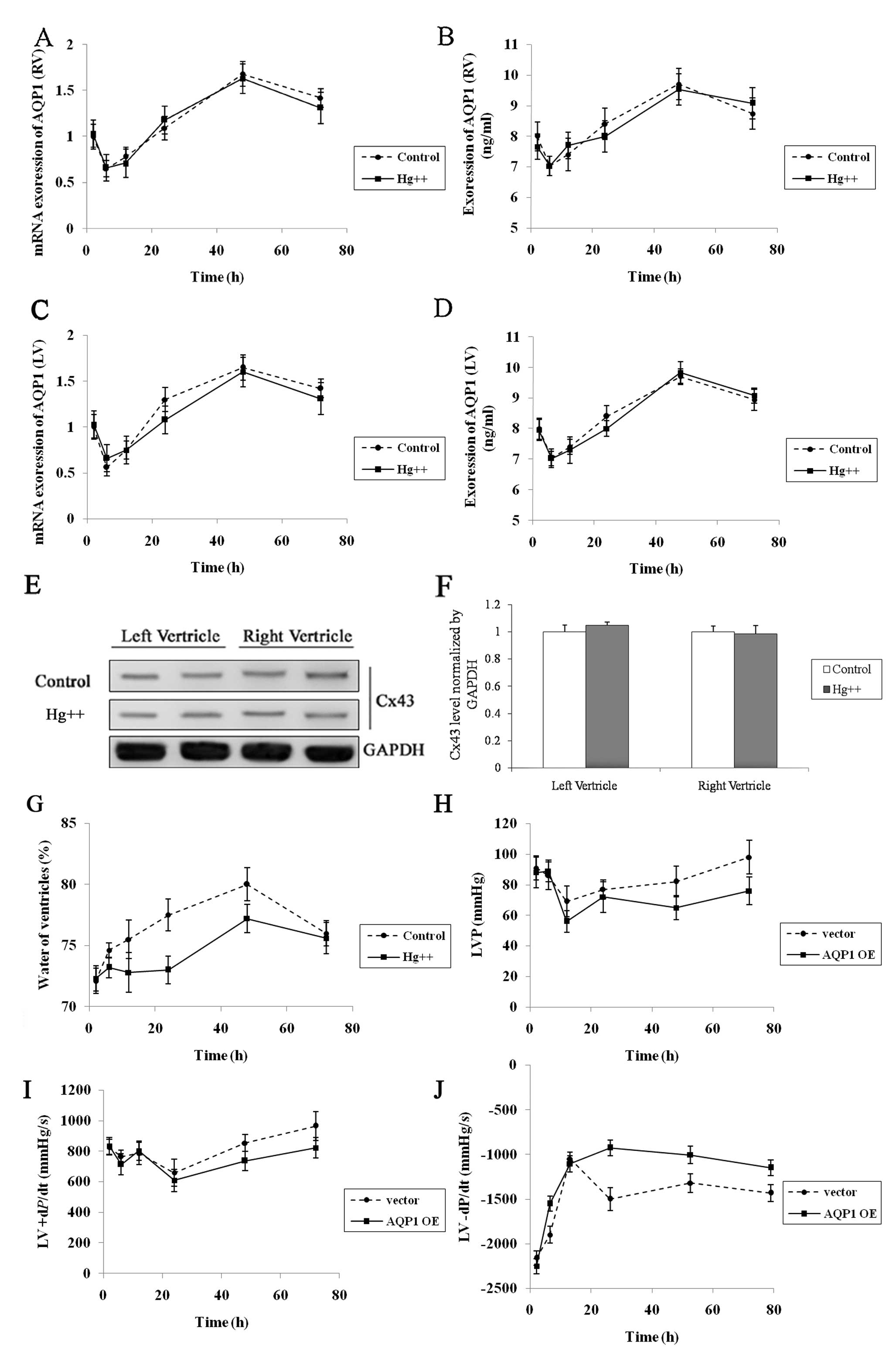
Effect of water channel protein inhibitor
Hg2+ on myocardial edema caused by CPB
Hg2+ is a water channel protein
inhibitor. During CPB surgery, 3 μM Hg2+ was
added to cardioplegia. The mRNA and protein expression levels of
AQP1 were measured 2, 6, 12, 24, 48 and 72 h following CPB surgery
(Fig. 4A–D). The protein
expression of Cx43 was measured 48 h after CPB surgery (Fig. 4E and F). Our results revealed that
Hg2+ exhibited no effect on both the left and right
ventricular expression of AQP1 (P>0.05). However, the degree of
myocardial edema was low compared with the control group.
The effect of lentiviral-mediated
overexpression of AQP1 on cardiac function following CPB
surgery
To confirm the effects of lentiviral-mediated
overexpression of AQP1 in myocardial edema, lentiviral vector of
AQP1 or empty vector was injected intramuscularly into goats during
CPB surgery; cardiac function was then assessed using hemodynamic
parameters and catheterization analysis 2, 6, 12, 24, 48 and 72 h
following CPB surgery. Catheterization analysis revealed the LV
pressure (LVP) of AQP1 lentiviral vector- and empty vector-treated
goats (Fig. 4H). In both groups,
the LVP gradually recovered following CPB surgery; however, at the
72 h time-point, the LVP of the AQP1 lentiviral vector-treated
goats was significantly lower compared to the empty vector-treated
goats (AQP1 lentiviral vector, 76.12±7 mmHg; empty vector, 98.03±10
mmHg; P<0.05). Positive and negative dP/dt was used to measure
the overall cardiac contractility and relaxation, respectively
(17). Positive dP/dt decreased
following CPB surgery in the empty vector- and AQP1 lentiviral
vector-treated goats (Fig. 4I).
The positive dP/dt of the AQP1 lentiviral vector-treated goats was
significantly lower compared to empty vector-treated goats 72 h
after CPB surgery (AQP1 lentiviral vector, 822±59 mmHg; empty
vector, 965±72 mmHg; P<0.05). Moreover, recovery of negative
dP/dt after CPB surgery was observed in the empty vector- and AQP1
lentiviral vector-treated goats (Fig.
4J). The negative dP/dt of AQP1 lentiviral vector-treated goats
was significantly worse compared to empty vector goats 24 h after
CPB surgery (AQP1 lentiviral vector, −1150±126 mmHg; empty vector,
−1432±97 mmHg; P<0.05).
Discussion
This study was designed to examine the role of AQP1
in myocardial edema caused by CPB surgery. Additionally, we
investigated whether there is a functional relationship between
water channels and gap junctions. Using a goat extracorporeal
circulation model, we examined APQ1 and Cx43 expression and the
degree of myocardial edema during CPB surgery. We found a
correlation between increased expression of AQP1 and myocardial
edema following CPB surgery. Additionally, overexpression of AQP1
by a lentivirus enhanced myocardial edema and downregulated Cx43
expression.
AQP1 is the ubiquitous water channel protein found
in endothelial cell membranes of vascular tissues throughout the
body. It is found in the plasma membranes of red blood cells as
well as the kidney, lung, brain, and eye (18). Gap junctions are composed
primarily of Cx43, which has been previously demonstrated to be an
essential element in the protective response of the myocardium to
ischemic preconditioning (19).
To date, there are fewer reports on AQPs and their
relationship with connexins in the heart when compared to the
kidney, brain, eye, and other tissues. The function of AQPs and
connexins in the heart is poorly understood, and there is
considerable controversy among different studies. AQP1 has been
identified in rodent heart and human heart (20), and it has been confirmed that AQP1
is the dominant AQP in the human heart and that it is co-localized
with t-tubular and caveolar proteins, in particular.
It has been hypothesized that the transmembrane ion
current of myocardial cells, mediated by AQP1, requires rapid
equilibrium of internal and external osmotic pressure (21,22). In a previous report, it was
demonstrated that AQP1 expression was increased in anemic fetuses
compared to age-matched controls, suggesting that AQP1 plays an
important role in physiological accommodation to fetal anemia
(23). In this study, left
ventricular mRNA and protein expression levels of AQP1 were
measured following CPB surgery and the water content of myocardial
muscle was determined. Our results indicated that cardiac AQP1
plays a role during the osmotic stress of CPB and the osmotic
stress that occurs following cardiac edema.
It was previous demonstrated that AQP1 is present in
the pulmonary microvascular endothelium, but on the expression of
AQP1 decreases in alveolar microvessels upon pulmonary edema in
chronic heart failure. It has been hypothesized that downregulation
of AQP1 in alveolar microvessels potentially acts as a compensatory
mechanism to protect against the formation of excessive pulmonary
edema (24). The expression of
AQP1 in alveolar microvessels following edema was not observed in
this study. This was most likely because AQP proteins in myocardial
cells are the major water transport channels; moreover,
Hg2+ (AQP protein non-specific inhibitor) limits the
extent of myocardial edema. We found no statistically significant
differences between mRNA and protein expression levels of AQP1
following Hg2+ treatment during CPB surgery. However,
the water content of myocardial muscle tissue significantly
decreased after Hg2+ treatment.
In this study, we analyzed the expression level of
the main gap junction protein, Cx43; we hypothesized that there is
a potential relationship between connexins and aquaporins. Goats
with a silenced AQP1 gene exhibit increased Cx43 levels following
CPB surgery, suggesting a close relationship between AQP1 and
Cx43.
In summary, we found that AQP1 plays a role in
myocardial edema following CPB surgery; moreover, a close
relationship between AQP1 and Cx43 was demonstrated. Further
studies are warranted to investigate potential methods of
regulating AQP1 expression in order to control the degree of
myocardial edema during surgery.
Acknowledgements
This study was partly supported by
grants from the National Natural Science Foundation of China
(30872558) and the Science and Technology Foundation of School of
Medicine, Shanghai Jiaotong University (2008XJ019).
References
|
1
|
Albers J, Schroeder A, de Simone R, Mockel
R, Vahl CF and Hagl S: 3D evaluation of myocardial edema:
experimental study on 22 pigs using magnetic resonance and tissue
analysis. Thorac Cardiovasc Surg. 49:199–203. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jia CX, Rabkin DG, Hart JP, Dean DA,
Cabreriza SA, Weinberg AD and Spotnitz HM: Regional variation in
myocardial water content in the edematous pig heart. J Surg Res.
106:70–75. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Foglia RP, Lazar HL, Steed DL, Follette
DM, Manganaro AJ, Deland E and Buckberg GD: Iatrogenic myocardial
edema with crystalloid primes: effects on left ventricular
compliance, performance, and perfusion. Surg Forum. 29:312–315.
1978.
|
|
4
|
Laine GA and Allen SJ: Left ventricular
myocardial edema. Lymph flow, interstitial fibrosis, and cardiac
function. Circ Res. 68:1713–1721. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Spotnitz HM and Hsu DT: Myocardial edema:
importance in the study of left ventricular function. Adv Card
Surg. 5:1–25. 1994.PubMed/NCBI
|
|
6
|
Palmer BS, Hadziahmetovic M, Veci T and
Angelos MG: Global ischemic duration and reperfusion function in
the isolated perfused rat heart. Resuscitation. 62:97–106. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Verkman AS: Physiological importance of
aquaporins: lessons from knockout mice. Curr Opin Nephrol
Hypertens. 9:517–522. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Verkman AS: Aquaporin water channels and
endothelial cell function. J Anat. 200:617–627. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
King LS, Kozono D and Agre P: From
structure to disease: the evolving tale of aquaporin biology. Nat
Rev Mol Cell Biol. 5:687–698. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Page E, Winterfield J, Goings G,
Bastawrous A and Upshaw-Earley J: Water channel proteins in rat
cardiac myocyte caveolae: osmolarity-dependent reversible
internalization. Am J Physiol. 274:H1988–H2000. 1998.
|
|
11
|
Umenishi F, Verkman AS and Gropper MA:
Quantitative analysis of aquaporin mRNA expression in rat tissues
by RNase protection assay. DNA Cell Biol. 15:475–480. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nielsen S, Smith BL, Christensen EI and
Agre P: Distribution of the aquaporin CHIP in secretory and
resorptive epithelia and capillary endothelia. Proc Natl Acad Sci
USA. 90:7275–7279. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Boengler K, Dodoni G, Rodriguez-Sinovas A,
Cabestrero A, Ruiz-Meana M, Gres P, Konietzka I, Lopez-Iglesias C,
Garcia-Dorado D, Di Lisa F, et al: Connexin 43 in cardiomyocyte
mitochondria and its increase by ischemic preconditioning.
Cardiovasc Res. 67:234–244. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gorbe A, Varga ZV, Kupai K, Bencsik P,
Kocsis GF, Csont T, Boengler K, Schulz R and Ferdinandy P:
Cholesterol diet leads to attenuation of ischemic
preconditioning-induced cardiac protection: the role of connexin
43. Am J Physiol Heart Circ Physiol. 300:H1907–H1913. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ruiz-Meana M, Rodriguez-Sinovas A,
Cabestrero A, Boengler K, Heusch G and Garcia-Dorado D:
Mitochondrial connexin43 as a new player in the pathophysiology of
myocardial ischaemiareperfusion injury. Cardiovasc Res. 77:325–333.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gross C, Nakamoto M, Yao X, Chan CB, Yim
SY, Ye K, Warren ST and Bassell GJ: Excess phosphoinositide
3-kinase subunit synthesis and activity as a novel therapeutic
target in fragile X syndrome. J Neurosci. 30:10624–10638. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Parsa CJ, Matsumoto A, Kim J, Riel RU,
Pascal LS, Walton GB, Thompson RB, Petrofski JA, Annex BH, Stamler
JS, et al: A novel protective effect of erythropoietin in the
infarcted heart. J Clin Invest. 112:999–1007. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Egan JR, Butler TL, Au CG, Tan YM, North
KN and Winlaw DS: Myocardial water handling and the role of
aquaporins. Biochim Biophys Acta. 1758:1043–1052. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Garcia-Dorado D, Ruiz-Meana M, Padilla F,
Rodriguez-Sinovas A and Mirabet M: Gap junction-mediated
intercellular communication in ischemic preconditioning. Cardiovasc
Res. 55:456–465. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Butler TL, Au CG, Yang B, Egan JR, Tan YM,
Hardeman EC, North KN, Verkman AS and Winlaw DS: Cardiac aquaporin
expression in humans, rats, and mice. Am J Physiol Heart Circ
Physiol. 291:H705–H713. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Au CG, Cooper ST, Lo HP, Compton AG, Yang
N, Wintour EM, North KN and Winlaw DS: Expression of aquaporin 1 in
human cardiac and skeletal muscle. J Mol Cell Cardiol. 36:655–662.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Suleymanian MA and Baumgarten CM: Osmotic
gradient-induced water permeation across the sarcolemma of rabbit
ventricular myocytes. J Gen Physiol. 107:503–514. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jonker S, Davis LE, van der Bilt JD,
Hadder B, Hohimer AR, Giraud GD and Thornburg KL: Anaemia
stimulates aquaporin 1 expression in the fetal sheep heart. Exp
Physiol. 88:691–698. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mullertz KM, Strom C, Trautner S, Amtorp
O, Nielsen S, Christensen S, Haunso S and Jonassen TE:
Downregulation of aquaporin-1 in alveolar microvessels in lungs
adapted to chronic heart failure. Lung. 189:157–166. 2011.
View Article : Google Scholar : PubMed/NCBI
|