Introduction
Alcohol consumption is associated with the
development of various medical disorder such as alcoholic liver
disease (ALD) and pancreatitis. Several studies have found that
short-term excessive drinking is more common than alcohol addiction
and a dose-effect relationship between liver injury and alcohol is
not always present; the environment and genetics also play a
crucial role in ALD pathogenesis (1,2).
Intestinal barrier appears to be significantly involved in the
initiation of alcohol-induced tissue damage and its role is more
evident in liver injury. Disruption of the intestinal barrier
allows endotoxin and other bacterial products in the gut lumen to
pass into the portal circulation and cause hepatic inflammation and
development of alcoholic steatohepatitis (ASH), which could lead to
alcoholic cirrhosis and liver failure, which is a causal factor in
the development of alcoholic endotoxemia and hepatitis.
Keshavarzian et al (3)
found that only alcoholics with liver disease have intestinal
barrier dysfunction. The present study was undertaken to determine
the role of FoxO4 in the relationship between alcohol in the
intestinal barrier and liver injury.
The intestinal barrier is formed by the epithelial
cells and tight junctions (4,5).
The intestinal epithelium provides barrier functions between the
luminal triggers and the host. Intestinal barrier dysfunction could
lead to increased uptake of luminal antigens that promote mucosal
inflammation.
Increasing evidence suggests that Forkhead box
‘Other’ (FoxO) proteins, a subgroup of the Forkhead transcription
factor family, have an important role in mediating the effects of
insulin and growth factors on diverse physiological functions,
including cell proliferation, apoptosis and metabolism, as well as
on regulation of the immune response (6–13).
Prevention of FoxO phosphorylation by AKT in response to growth
factors such as platelet-derived growth factors (PDGF) and
insulin-like growth factor 1 (IGF-1) has been reported. FoxO4 is a
member of the FoxO subfamily that also includes FoxO1, FoxO3 and
FoxO6 (14). FoxO4 can also be
phosphorylated by phosphatidylinositol-3-kinase/AKT signaling
resulting in its inactivation and nuclear exclusion (15,16). The individual members of the FoxO
family have unique cell type-specific functions and their
regulation of target genes is context-dependent (12,13). However, it remains to be
determined whether FoxO4 has a similar immunoregulatory activity
and whether FoxO4 has a role in the relationship between
alcohol-induced intestinal barrier dysfunction and liver
injury.
In this study, we investigated the role of FoxO4 in
ALD using an animal model. We found that FoxO4 is an interacting
factor which forms a complex that represses tumor necrosis factor α
(TNFα) transcriptional activation of nuclear factor-κB (NF-κB) in
the ALD animals. Furthermore, it protects the intestinal barrier
from the alcohol by increasing the expression of tight junction
proteins.
Materials and methods
Rats, alcohol treatment
Six to eight-week-old littermates or age-matched
male WT rats (200±10 g at intake) were obtained from the Experiment
Animal Center, China Medical University. All rats in the study were
used strictly in accordance with the National Institution of Health
Guide for the Care and Use of Laboratory Animals. Our study
received the approval of the China Medical University Animal
Committee (no. 2011–1538).
Rats were deprived of food for 12 h prior to
induction of acute alcoholic liver injury. During the experiments,
the rats were divided into eight groups.
In the control group, eight rats were treated twice
daily with corn starch alone dissolved in 200 μl of PBS and
administered via gastric tube.
In the alcohol group, eight rats were given 40%
alcohol (5 g/kg body weight) through stomach feeding every 12
h/time, three times in total.
In the TNFα, the wortmannin (an AKT signaling
inhibitor) and the IGF-1 group (an AKT signaling agonist) rats were
separately injected intraperitoneally with TNFα (10 μg/kg
body weight), wortmannin (1.4 mg/kg body weight) or IGF-1 (0.2
mg/kg body weight) 30 min before alcohol administration.
In the anti-TNFα group, anti-TNFα (5 mg/kg body
weight) was injected intravenously into alcohol-treated rats 30 min
before alcohol administration.
In the two placebo groups, rats were treated with
PBS injected either intraperitoneally or intravenously prior to
induction of alcohol liver injury. The small intestine and liver of
all rats were swiss-rolled, formalin-fixed and
paraffin-embedded.
Assessment of tight junction proteins of
small intestine by electron microscopy
A standard fixation procedure was used for
conventional thin section electron microscopy. The procedure
involved incubation with OsO4 alone (1 or 2% in
phosphate buffer) at 0°C for 30 min. After fixation, the small
intestine was washed extensively in Veronal acetate buffer (90 mm,
pH 6.0), stained by incubation at 0°C for 60 min in uranyl
magnesium acetate (0.5%) in the same buffer, washed again,
dehydrated and embedded. Thin sections were cut at 60 nm with a
diamond knife and stained with uranyl acetate and lead citrate for
viewing on a 200 CX transmission electron microscope at 80 kV.
High-magnification images (×10,000) were captured to evaluate the
ultrastructure of tight junctions in small intestine tissue.
Immunohistochemistry
Paraffin slides were deparaffinized and rehydrated,
deparaffinized in xylene I, II and III for 10 min, dehydrated in
95, 90 and 70% ethanol for 2 min, and then tissue sections were
rinsed three times for 5 min each in 0.01 mol/l phosphate-buffered
saline (PBS, pH 7.4), pre-incubated for 15 min with 0.3%
H2O2, and then pre-incubated for 15 min with
5% normal goat serum and incubated overnight at 4°C with rabbit
anti-FoxO4 polyclonal antibody (rabbit anti-rat, 1:400). After
rinsing with 0.01 mol/l PBS, sections were incubated for 15 min
with secondary goat anti-rabbit immunoglobulin G at 37°C, rinsed
three times for 5 min each in 0.01 mol/l PBS, incubated for 15 min
with tertiary antibody at 37°C, and rinsed 3×5 min in 0.01 mol/l
PBS. A peroxidase reaction was performed to visualize FoxO4
immunolabeling by incubating with 0.05% 3,3-diaminobenzidine
tetrahydrochloride for 3 min and stopping the reaction with 0.01
mol/l PBS. To assess antibody specificity, incubation with the
primary antibody was omitted for some sections and no significant
staining was observed in this case. Positive results showed brown
and dark brown. Similarly, tissue sections were incubated overnight
at 4°C with rabbit anti-p-FoxO4 polyclonal antibody (rabbit
anti-rat, 1:400), and were then stained by the same
immunohistochemical method.
Measurement of TNFα, endotoxin, ALT and
AST in plasma
TNFα levels in the media were assessed using a rat
TNFα ELISA set according to the manufacturer’s instructions. TNFα
concentrations were determined using a standard and values were
normalized to total DNA present in the well using a DNA
quantification kit. Endotoxin was measured by Tachypleus Amebocye
Lysate. ALT and AST were measured by biochemical methods.
Western blot analysis
Using snap-frozen small intestine specimens with
histologically intact epithelium, we stripped the mucosa from the
underlying submucosal tissue, homogenized and sonicated it, and
transferred it into ice-cold lysis buffer with a protease inhibitor
cocktail for 60 min. Lysates were centrifuged and the protein
content of the supernatant was determined by using the BCA protein
assay kit. Depending on the antibody used, equivalent protein
concentrations of 10–75 lg were loaded onto each lane of
SDS-polyacrylamide gels. Electrophoretically separated samples were
transferred to an immobilon transfer membrane. Membranes were
incubated with the respective primary antibodies and a
corresponding peroxidase-conjugated secondary antibody. Blots were
visualized by chemiluminescence using immobilon Western
Chemiluminescent HRP substrate. After detection of specific tight
junctions, FoxO4 and p-FoxO4, all membranes were stripped with
Restore Western Blot Stripping Buffer, and an immunoblot for
β-actin was performed to ensure equal protein loading in each lane.
Densitometry was performed for each detected protein in each
group.
RNA isolation and reverse
transcription-polymerase chain reaction
The plasmids were cloned using PCR. RNA extraction,
reverse transcription-polymerase chain reaction (RT-PCR), and
microarray. The following primer pairs were used for amplification:
occludin, sense, 5′-GCTATGAAACCGACTACACGACA-3′; and antisense,
5′-ACTCTCCAGCAACCAGCATCT-3′; ZO-1, sense,
5′-AGGCTATTTCCAGCGTTTTGA-3′ and antisense, 5′-AA
TCCTGGTGGTGGTACTTGC-3′; NF-κB, sense, 5′-CGCAT TCTGACCTTGCCTATC-3′
and antisense, 5′-AGTCCAGTC TCCGAGTGAAGC-3′.
Statistical analysis
All data are expressed as the means ± SD and were
analyzed using one-way analysis of variance. P<0.05 was
considered to indicate statistically significant differences.
Results
The expression of tight junctions under
electron microscopy
We studied tight junctions in small intestine using
electron microscopy as an index of loss of colonic barrier
integrity. Acute alcohol administration significantly disrupted the
architecture of the tight junctions of the small intestine.
Wortmannin and anti-TNFα supplementation significantly protected
the cytoarchitecture of the intestinal barrier. However, in the
TNFα and IGF-1 groups, different degrees of tight junctions injured
were observed (Fig. 1).
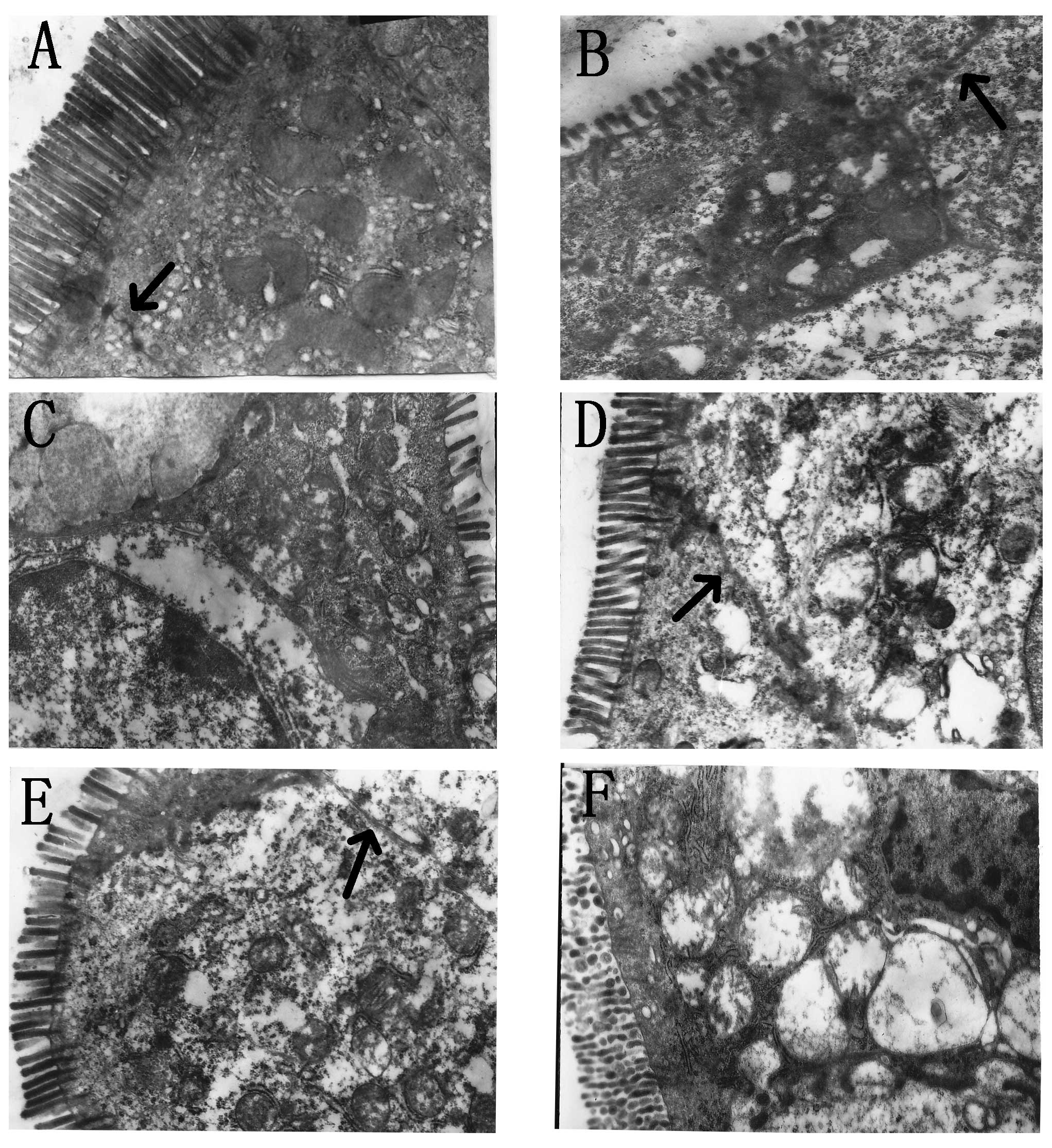 | Figure 1(A) Control group (×10,000); tight
junctions in the small intestine were visualized (arrow), and in
free surface, microvilli were arranged neatly and intensively. (B)
Alcohol group (×10,000); alcohol administration significantly
disrupted the tight junctions of the small intestine (arrow), and
microvilli became short and partly missing. (C) TNFα group
(×10,000); tight junctions were seriously disrupted and could not
be found under electron microscopy, microvilli became shorter and
fewer than in the alcohol group. (D) Anti-TNFα group (×10,000);
tight junctions could be found under microscopy (arrow) and
microvilli were normal. (E) Wortmannin group (×10,000); tight
junctions could be found under microscopy (arrow) and microvilli
were normal. (F) IGF-1 group (×10,000); tight junctions were
seriously disrupted and could not be found under electron
microscopy, microvilli became shorter and fewer than in the alcohol
group. |
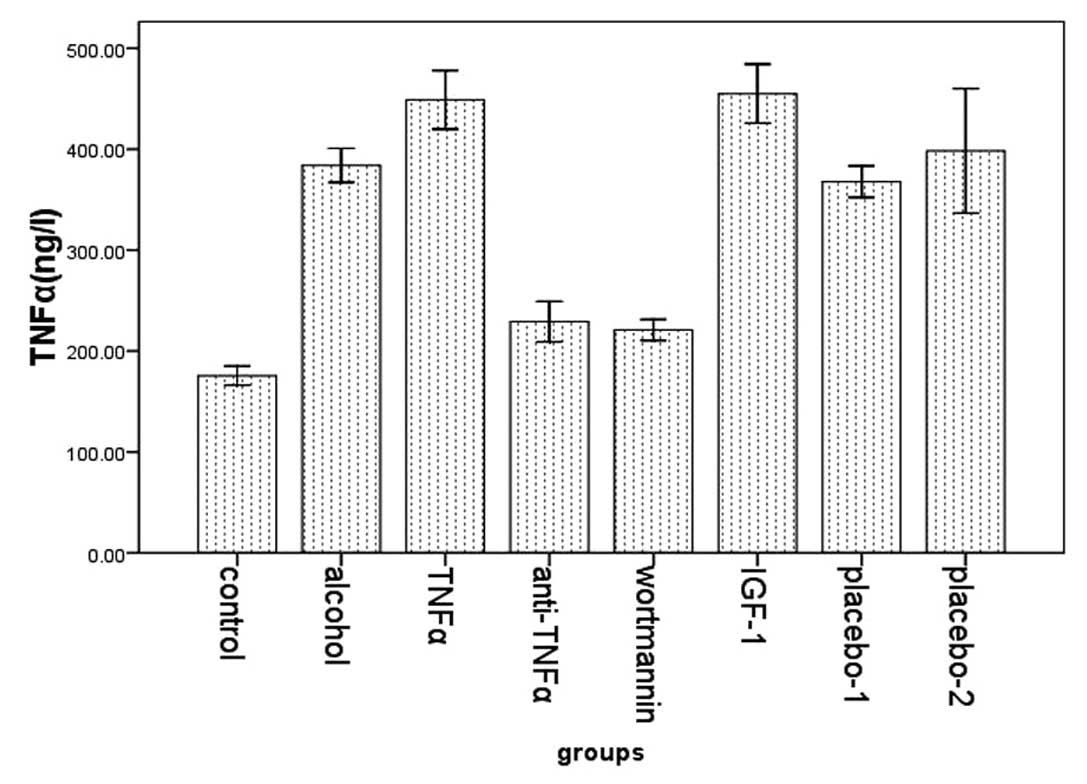
The expression of TNFα and endotoxin
We examined the expression of TNFα in plasma in the
eight groups using ELISA. Compared with the control group
(175.51±11.205), the expression of TNFα was higher in the alcohol
group (383.95±18.231). In the TNFα (448.91±31.447) and the IGF-1
group (455.00±27.917) the expression was significantly higher than
in the alcohol group. However, its expression in the wortmannin
(220.08±13.794) and the anti-TNFα group (229.10±24.027) was
significantly lower than that in the alcohol group (Fig. 2).
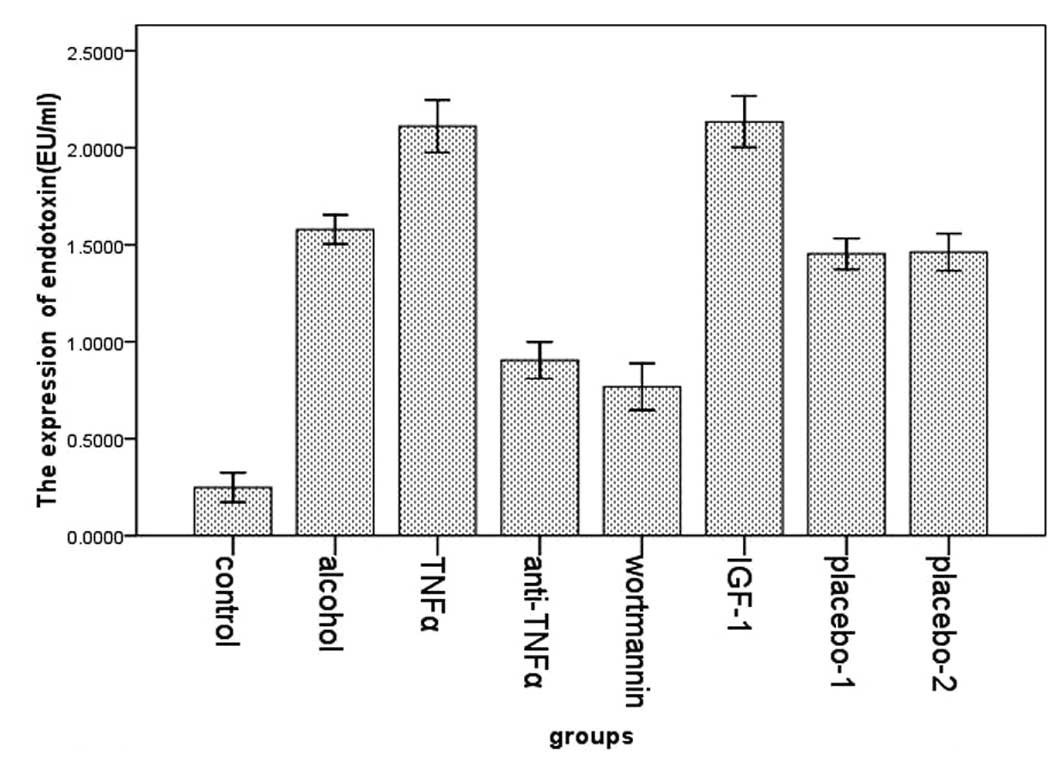
We examined the expression of endotoxin in the eight
groups by Tachypleus Amebocye Lysate. We found that the expression
of endotoxin was higher in the alcohol group (1.59±0.081) compared
with the control group (0.25±0.091). In the TNFα (2.11±0.147) and
the IGF-1 group (2.13±0.126) the levels were significantly higher
in the alcohol group. However, its expression in the wortmannin
(0.77±0.144) and the anti-TNFα group (0.90±0.113) was significantly
lower than that in the alcohol group (Fig. 3).
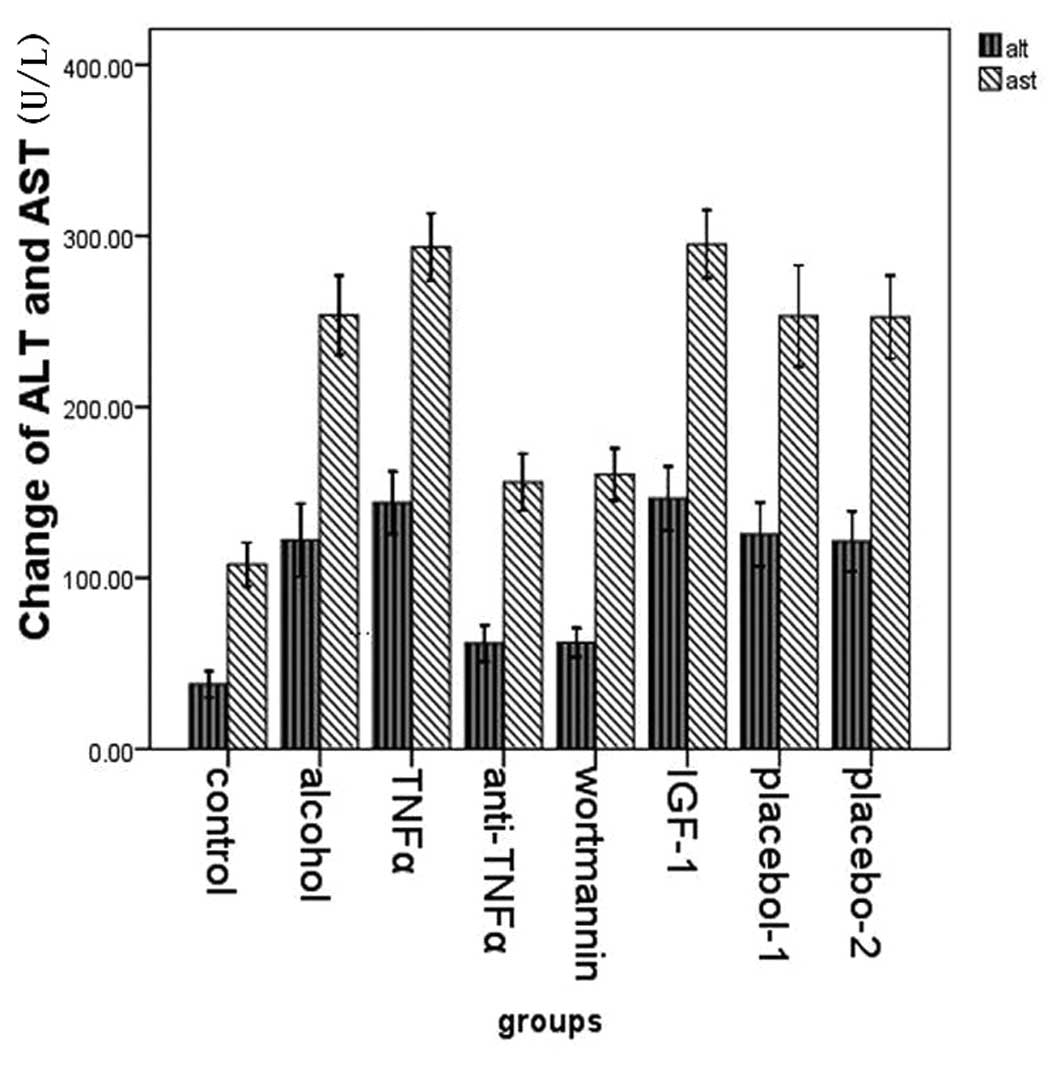
The expression of ALT and AST
We examined the expression of ALT and AST in plasma
in the eight groups. Compared with the control group (37.875±9.188;
107.875±15.292), the expression of ALT and AST was higher in the
alcohol group (122.143±23.061; 253.714±25.031). In the TNFα
(144.000±19.732; 293.571±21.259) and the IGF-1 group
(146.500±17.852; 295.167±18.957) the expression levels were
significantly higher than in the alcohol group. However, its
expression in the wortmannin (62.250±10.068; 160.625±18.213) and
the anti-TNFα group (61.750±12.669; 156.000±19.893) was
significantly lower than that in the alcohol group (Fig. 4).
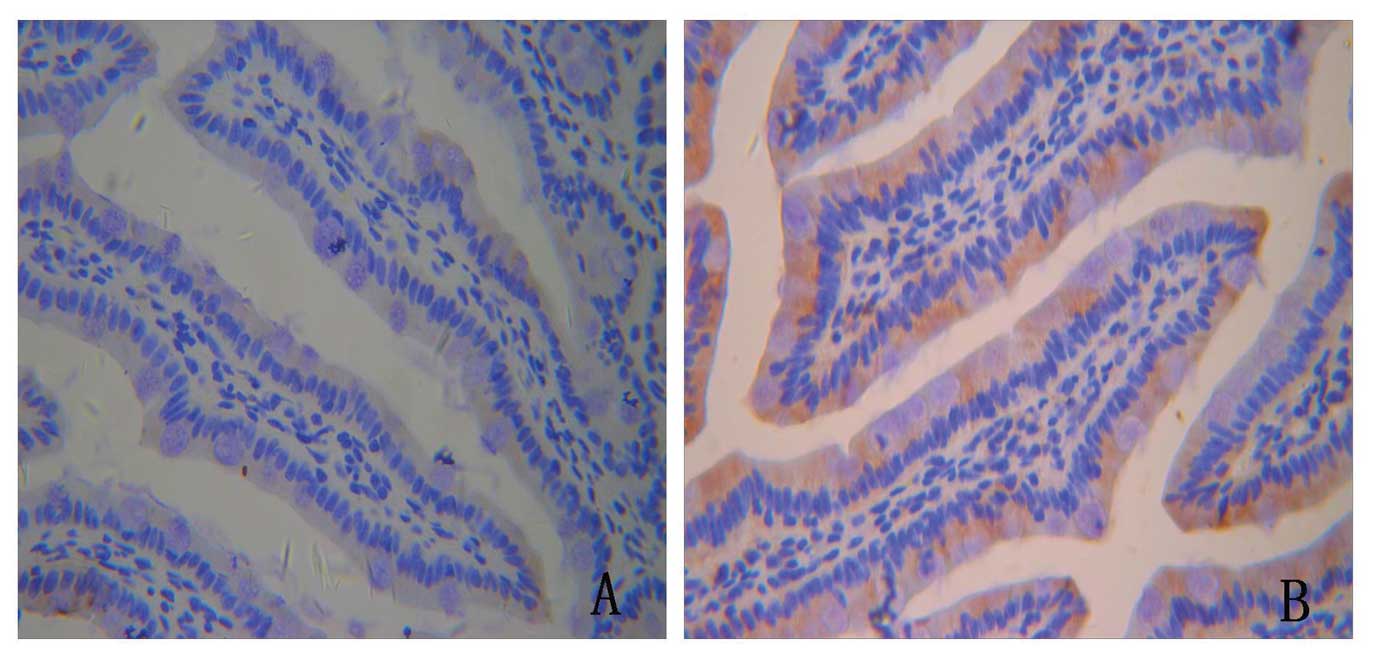
The expression and distribution of FoxO4
and p-FoxO4
In order to examine the role of FoxO4 in protein
expression and distribution of tight junction proteins, we first
used immunohistochemistry. During PKB/AKT activation (in the TNFα
and IGF-1 groups), FoxO4 became phosphorylated, upregulated and was
excluded into the cytoplasm (Fig.
5).
To extend our observations of changes of FoxO4 and
p-FoxO4 expression, the two proteins were analyzed by western
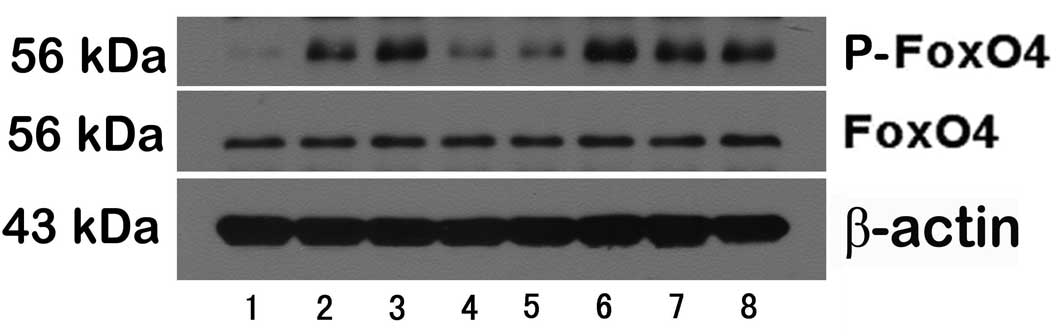
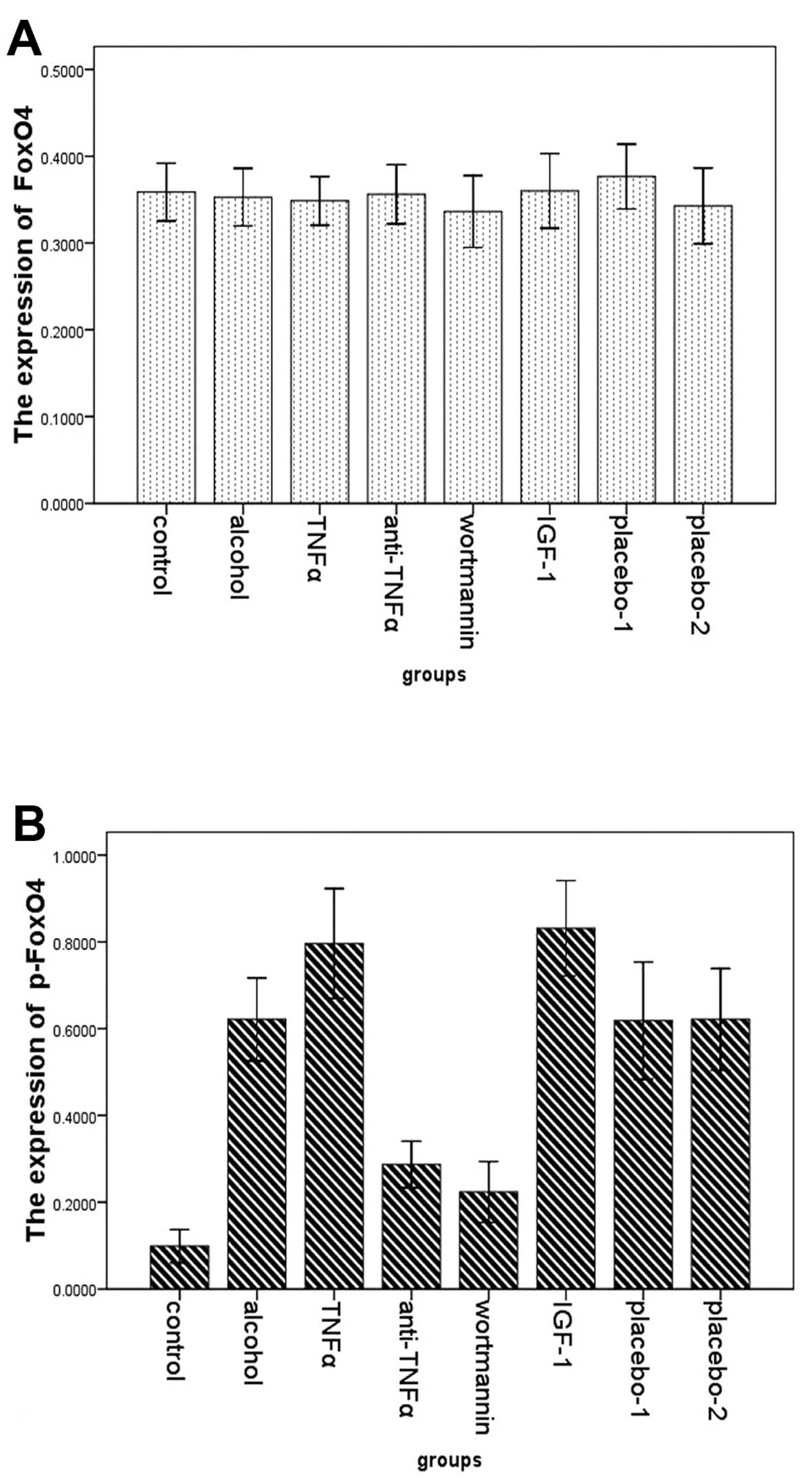
blotting in the small intestine of the eight groups. There were no
differences in the expression of FoxO4 among the eight groups. By
contrast, the expression of p-FoxO4 was markedly different among
the eight groups. Compared with rats in the alcohol group
(0.621±0.104), significant reductions in total protein for p-FoxO4
were observed in rats in the control (0.099±0.046), the wortmannin
(0.224±0.084) and the anti-TNFα group (0.287±0.064). The p-FoxO4
expression in rats in the TNFα (0.796±0.137) and the IGF-1 group
(0.831±0.104) were significantly higher than in the alcohol group
(Figs. 6 and 7).
The expression of NF-κB and tight
junction proteins
To observe the striking changes of NF-κB and tight
junction protein expression, the expression levels were analyzed by
western blotting and RT-PCR in the small intestine of the eight
groups.
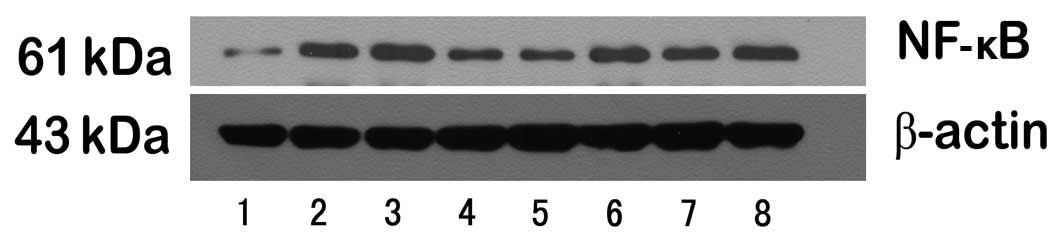
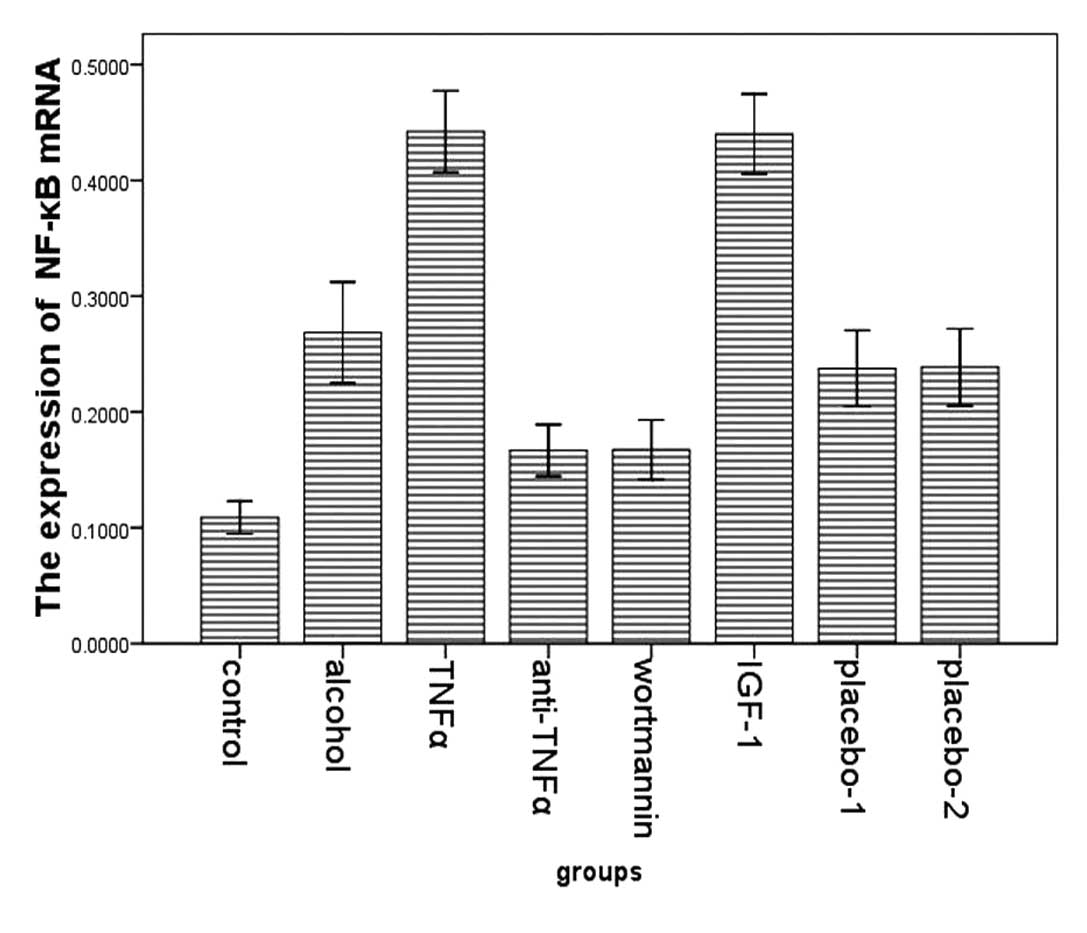
First, the trend of NF-κB was consistent with the
changes of p-FoxO4. Compared with rats in the alcohol group
(0.467±0.057, 0.269±0.047), significant reductions in total protein
and mRNA for NF-κB were observed in rats in the control
(0.174±0.033, 0.109±0.017), the wortmannin (0.244±0.045,
0.168±0.031) and in the anti-TNFα group (0.283±0.039, 0.167±0.027).
NF-κB expression in rats in the TNFα (0.578±0.071, 0.442±0.038) and
the IGF-1 group (0.559±0.080, 0.440±0.033) were significantly
higher than in the alcohol group (Figs. 8–11).
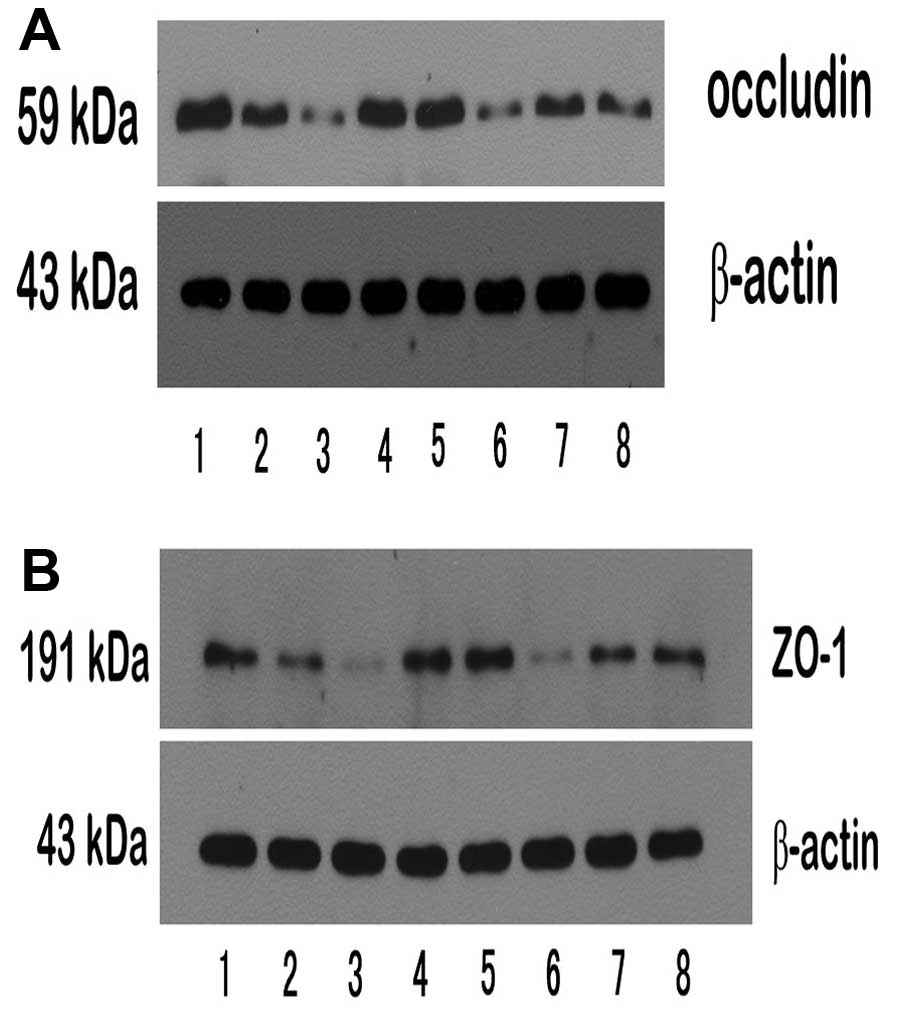
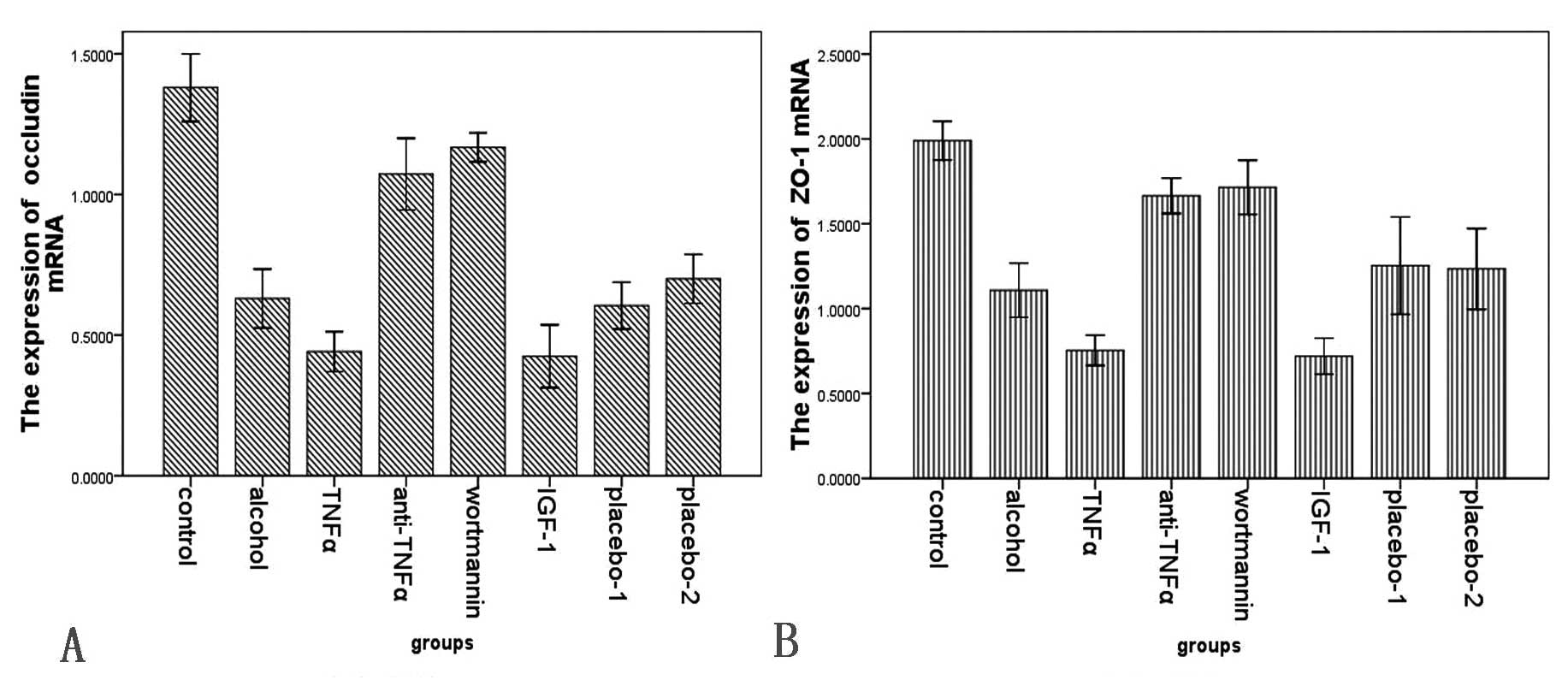
Second, the trend of tight junction proteins was
reversed with the change of NF-κB. Thus, in the alcohol group
(occludin, 0.305±0.949, ZO-1, 0.379±0.097; occludin mRNA,
0.630±0.114; ZO-1 mRNA, 1.109±0.172), the expression of protein and
mRNA for occludin and ZO-1 were markedly lower than in the control
group (occludin, 0.838±0.166, ZO-1, 1.029±0.145; occludin mRNA,
1.38±0.140, ZO-1 mRNA, 1.990±0.136), in the wortmannin group
(occludin, 0.634±0.156, ZO-1, 0.841±0.109; occludin mRNA,
1.168±0.061, ZO-1 mRNA, 1.715±0.191) and in the anti-TNFα group
(occludin, 0.536±0.100, ZO-1, 0.748±0.163; occludin mRNA,
1.073±0.153, ZO-1 mRNA, 1.665±0.125). However, in the TNFα group
(occludin, 0.176±0.078, ZO-1, 0.200±0.069; occludin mRNA,
0.441±0.077, ZO-1 mRNA, 0.754±0.096) and the IGF-1 group (occludin,
0.177±0.036, ZO-1, 0.201±0.082; occludin mRNA, 0.425±0.106, ZO-1
mRNA, 0.720±0.101) they were significantly lower than those in the
alcohol group (Figs. 12–15).
Discussion
In the present study, we investigated the mechanisms
by which FoxO4 regulates epithelial permeability, tight junction
protein expression and liver damage in a rat model of acute alcohol
liver disease.
Our results showed that the impairment of intestinal
barrier function in acute alcohol liver disease is associated with
loss of the tight junction proteins, including occludin and ZO-1.
Tight junctions are the major determinants of paracellular
permeability. Although changes in epithelial tight junction protein
expression have been studied extensively in monolayers stimulated
by cytokines, challenged by bacteria, or exposed to aspirin
(17–23), much remains to be understood about
barrier disruptive changes under acute alcohol consumption.
FoxO4 may regulate intestinal permeability through
tight junction proteins. Expression levels and distribution of
these tight junction proteins may influence the epithelial
permeability (4,5,24,25). Previous studies have shown that
downregulation of ZO-1 and claudin-1 in both FoxO4-deficient
epithelial cells in vivo and FoxO4-knocked down epithelial
cells in vitro, could provide a structural basis for the
increased intestinal epithelial permeability in FoxO4-null mice
(26).
The effects of FoxO4 on epithelial permeability are
likely through NF-κB (26–30).
Both loss and gain-of function of NF-κB have been shown to increase
epithelial permeability through different mechanisms. Nenci et
al (30) showed that NF-κB
inactivated epithelial cells have increased permeability due to an
increase in TNFα-mediated epithelial cell death. Upregulation of
epithelial permeability in FoxO4-null mice is likely due to the
downregulation of tight junction proteins as a result of increased
NF-κB activity in the epithelial cells. FoxO4 could inactivate
NF-κB through direct physical interaction; however, when FoxO4
becomes phosphorylated, it loses the ability to restrain NF-κB,
resulting in the downregulation of tight junction proteins and an
increase in epithelial permeability (26).
Our results showed that, first, when TNFα increased,
FoxO4 became phosphorylated and was excluded into cytoplasm. The
expression of p-FoxO4 increased and at the same time NF-κB became
active and tight junction proteins decreased. Then, when the rats
were given anti-TNFα, the expression of TNFα decreased, FoxO4
became dephosphorylated, active and located in nucleolus, and at
the same time NF-κB became inactive and tight junction proteins
increased. Subsequently, when the rats were given wortmannin, which
is an AKT signaling inhibitor, FoxO4 became dephosphorylated,
active and located in nucleolus, and at the same time NF-κB became
inactive and tight junction proteins increased. Finally, when the
rats were given IGF-1, an AKT signaling agonist, FoxO4 became
phosphorylated and was excluded into the cytoplasm, the expression
of p-FoxO4 increased, at the same time NF-κB became active and
tight junction proteins decreased. As a result, the intestinal
bacteria grew excessively, endotoxin was released into portal
circulation and liver injury deteriorated.
In summary, our findings suggest a complex network
of mechanisms involved in the beneficial effects of FoxO4 in the
epithelial barrier dysfunction. We demonstrated that TNFα can
upregulate phosphorylation of FoxO4. FoxO4, which is located in the
nucleus, is then excluded into the cytoplasm and inactivated, it
loses the ability to restrain NF-κB activity, and then
downregulates the expression of tight junction proteins and
increases epithelial permeability. At the same time, the endotoxin
was released into portal circulation, and liver injury
deteriorated.
Acknowledgements
The authors thank all the participants
in the present study and they also thank Miss. Cheng for her
editorial assistance.
References
|
1
|
Stewart S, Jones D and Day CP: Alcoholic
liver disease: new insights into mechanisms and preventative
strategies. Trends Mol Med. 7:408–413. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rouault TA: Hepatic iron overload in
alcoholic liver disease: why does it occur and what is its role in
pathogenesis? Alcohol. 30:103–106. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Keshavarzian A, Holmes EW, Patel M, et al:
Leaky gut in alcoholic cirrhosis: a possible mechanism for
alcohol-induced liver damage. Am J Gastroenterol. 94:200–207. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ma TY, Iwamoto GK, Hoa NT, et al:
TNF-alpha-induced increase in intestinal epithelial tight junction
permeability requires NF-kappa B activation. Am J Physiol
Gastrointest Liver Physiol. 286:G367–G376. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ma TY and Anderson JM: Tight junctions and
intestinal barrier. Physiology of the Gastrointestinal Tract. LR
Johnson: Elsevier Academic Press; New York, NY: pp. 1559–1594.
2006
|
|
6
|
Marchetti V, Menghini R, Rizza S, et al:
Benfotiamine counteracts glucose toxicity effects on endothelial
progenitor cell differentiation via Akt/FoxO signaling. Diabetes.
55:2231–2237. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Essaghir A, Dif N, Marbehant CY, et al:
The transcription of FOXO genes is stimulated by FOXO3 and
repressed by growth factors. J Biol Chem. 284:10334–10342. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu ZP, Wang Z, Yanagisawa H and Olson EN:
Phenotypic modulation of smooth muscle cells through interaction of
FoxO4 and myocardin. Dev Cell. 9:261–270. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Burgering BM: A brief introduction to
FOXOlogy. Oncogene. 27:2258–2262. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lin L, Hron JD and Peng SL: Regulation of
NF-kappaB, Th activation and autoinflammation by the forkhead
transcription factor Foxo3a. Immunity. 21:203–213. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dengler HS, Baracho GV, Omori SA, et al:
Distinct functions for the transcription factor Foxo1 at various
stages of B cell differentiation. Nat Immunol. 9:1388–1398. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Paik JH, Kollipara R, Chu G, et al: FoxOs
are lineage-restricted redundant tumor suppressors and regulate
endothelial cell homeostasis. Cell. 128:309–323. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tothova Z, Kollipara R, Huntly BJ, et al:
FoxOs are critical mediators of hematopoietic stem cell resistance
to physiologic oxidative stress. Cell. 128:325–339. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Birkenkamp KU and Coffer PJ: Regulation of
cell survival and proliferation by the FOXO (Forkhead box, class O)
subfamily of Forkhead transcription factors. Biochem Soc Trans.
31:292–297. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lüpertz R, Chovolou Y, Unfried K, et al:
The forkhead transcription factor FOXO4 sensitizes cancer cells to
doxorubicin-mediated cytotoxicity. Carcinogenesis. 29:2045–2052.
2008.PubMed/NCBI
|
|
16
|
Matsuzaki H, Ichino A, Hayashi T, et al:
Regulation of intracellular localization and transcriptional
activity of FOXO4 by protein kinase B through phosphorylation at
the motif sites conserved among the FOXO family. J Biochem.
138:485–491. 2005. View Article : Google Scholar
|
|
17
|
Anderson RC, Cookson AL, McNabb WC, et al:
Lactobacillus plantarum MB452 enhances the function of the
intestinal barrier by increasing the expression levels of genes
involved in tight junction formation. BMC Microbiol. 10:3162010.
View Article : Google Scholar
|
|
18
|
Donato KA, Gareau MG, Wang YJ and Sherman
PM: Lactobacillus rhamnosus GG attenuates interferon-(gamma)
and tumour necrosis factor-alpha-induced barrier dysfunction and
pro-inflammatory signalling. Microbiology. 156:3288–3297. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ewaschuk JB, Diaz H, Meddings L, et al:
Secreted bioactive factors from Bifidobacterium infantis
enhance epithelial cell barrier function. Am J Physiol Gastrointest
Liver Physiol. 295:G1025–G1034. 2008.PubMed/NCBI
|
|
20
|
Karczewski J, Troost FJ, Konings I, et al:
Regulation of human epithelial tight junction proteins by
Lactobacillus plantarum in vivo and protective effects on
the epithelial barrier. Am J Physiol Gastrointest Liver Physiol.
298:G851–G859. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mennigen R, Nolte K, Rijcken E, et al:
Probiotic mixture VSL#3 protects the epithelial barrier by
maintaining tight junction protein expression and preventing
apoptosis in a murine model of colitis. Am J Physiol Gastrointest
Liver Physiol. 296:G1140–G1149. 2009.
|
|
22
|
Resta-Lenert S and Barrett KE: Live
probiotics protect intestinal epithelial cells from the effects of
infection with enteroinvasive Escherichia coli (EIEC). Gut.
52:988–997. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zyrek AA, Cichon C, Helms S, et al:
Molecular mechanisms underlying the probiotic effects of
Escherichia coli Nissle 1917 involve ZO-2 and PKCzeta
redistribution resulting in tight junction and epithelial barrier
repair. Cell Microbiol. 9:804–816. 2007.PubMed/NCBI
|
|
24
|
Ye D, Ma I and Ma TY: Molecular mechanism
of tumor necrosis factor-alpha modulation of intestinal epithelial
tight junction barrier. Am J Physiol Gastrointest Liver Physiol.
290:G496–G504. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Atreya I, Atreya R and Neurath MF:
NF-kappaB in inflammatory bowel disease. J Intern Med. 263:591–596.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou W, Cao Q, Peng Y, Zhang QJ, et al:
FoxO4 inhibits NF-kappaB and protects mice against colonic injury
and inflammation. Gastroenterology. 137:1403–1414. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Andresen L, Jørgensen VL, Perner A, et al:
Activation of nuclear factor kappaB in colonic mucosa from patients
with collagenous and ulcerative colitis. Gut. 54:503–509. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Montufar-Solis D, Garza T and Klein JR:
T-cell activation in the intestinal mucosa. Immunol Rev.
215:189–201. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gadjeva M, Wang Y and Horwitz BH:
NF-kappaB p50 and p65 subunits control intestinal homeostasis. Eur
J Immunol. 37:2509–2517. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nenci A, Becker C, Wullaert A, et al:
Epithelial NEMO links innate immunity to chronic intestinal
inflammation. Nature. 446:557–561. 2007. View Article : Google Scholar : PubMed/NCBI
|