Introduction
Irritable bowel syndrome (IBS) is a chronic,
functional gastrointestinal disorder affecting approximately 10–15%
of the world’s population. It is characterized by abdominal pain
and discomfort and altered bowel habits (1–3).
According to bowel habits, IBS is classified into four subgroups:
IBS with diarrhea (IBS-D), IBS with constipation (IBS-C), mixed IBS
(IBS-M), and untyped IBS (IBS-U) (4,5).
Alterations in gut motility and visceral hypersensitivity are two
main features of IBS. IBS-associated abdominal pain is believed to
result from smooth muscle activity disorder and visceral
hypersensitivity (6).
The glucagon-like peptide-1 (GLP-1) analogue
ROSE-010 has been found to relieve abdominal pain in patients with
IBS (7). GLP-1 is an incretin
hormone secreted from L-cells in response to ingested nutrients
(8). GLP-1 decreases
gastrointestinal motility and gastric emptying in healthy subjects
and rodents (9–13). GLP-1 also decreases motility in
the antro-duodeno-jejunal region and inhibits the migrating motor
complex (MMC) in IBS patients (9). Moreover, GLP-1 exerts inhibitory
effects on gastrointestinal motility, not only through vagal
afferents and central nervous mechanisms, but also through a
peripheral motor action mechanism (14).
The expression of GLP-1 receptors (GLP-1R) has been
detected in human small and large intestines (15). In the present study, we aimed to
investigate the role of GLP-1 and its receptor in the pathogenesis
of experimental IBS rat models by detecting the serum level of
GLP-1 and the expression of GLP-1R in the gut. The effect of GLP-1
on circular smooth muscle and longitudinal muscle responses of
intestinal segments was also assessed.
Materials and methods
Animals
Fifty adult male Sprague-Dawley rats (200–220 g)
were obtained from Beijing Vital River Laboratories Animal
Technology Co., Ltd., and were divided into five groups: IBS-C
group (n=10); IBS-C control group (n=10); IBS-D group (n=10); IBS-D
control group (n=10); and a blank control group (n=10). IBS-C rats
were pretreated with 0–4°C cool water (2 ml) to irrigate the
stomach daily for 14 days (16).
The IBS-C control rats were treated with normal drinking water (2
ml). IBS-D rats were established by wrap restraint stress as
described by Williams et al (17) with slight modifications. The IBS-D
control rats were given sham restraint stress. Rats were given free
access to standard rat chow (GB14924.3-2001, China) and water, and
they were acclimated for at least 7 days before experiments. The
protocol was approved by the Animal Use and Care Committee of
Nanjing Medical University.
Water content of feces
The rats of each group were placed in separate cages
for 3 h, and the amount and weight of the feces expelled by each
rat were collected and recorded during this time period. The feces
dried in an oven were weighed again. Water content of the feces was
calculated by the following formula: Water content % = [wet weight
of the feces (g) − dried weight of the feces (g)]/wet weight of the
feces (g) × 100% (18).
Small intestine transit rate
After fasting for 12 h, each rat received 2 ml of 2%
India ink by gavage. Rats were rapidly euthanized and the small
intestine samples from the pylorus to the cecum were removed 30 min
after receiving India ink. The percentage of small intestine
transit was defined as: small intestine transit rate % = the
promoting length of the ink (cm)/ the total length of intestine
(cm) ×100% (19).
Visceromotor response
Behavioral responses to colorectal distention (CRD)
were assessed by a measurement of the electromyogram (EMG) activity
of the external oblique (20).
Seven days prior to the experiment, stainless steel EMG electrodes
were implanted into the lateral abdominal wall of the rats, and the
electrode leads were exteriorized at the back of the neck. On the
day of the experiment, rats were sedated with isofluorane, and a
flexible balloon (5 cm) constructed from a surgical glove finger
attached to a catheter was inserted into the descending colon and
rectum via the anus; the catheter was fixed to the base of the
tail. Rats were placed in small Lucite cubicles (20×8×8 cm)
(Medical Instrument Co., Ltd., Zhejiang, China). After the rats
were fully awake and acclimatized, CRD was performed. Each trial
consisted of graded intensity stimulation trials (0, 20, 40 and 60
mmHg) for a 20-sec stimulation period followed by a 2-min rest, and
then the procedure was repeated one more time (21). The EMG was recorded continuously
during the experiment on an EMG measurement system (Power Labs,
Australia). The maximum amplitude of the curve for the EMG signal
was calculated.
Sample preparation
Blood samples and the colon tissue samples were
collected for RNA extraction, western blotting. Colonic muscle
strips were made for the smooth muscle contraction experiment. The
colon tissue sections were used for hematoxylin and eosin (H&E)
staining, immunohistochemistry. H&E-stained sections were
scored for colonic inflammation in model rats as previously
described (22).
GLP-1R immunohistochemistry
The sections were incubated with anti-GLP-1R
antibody (AB-39072; Abcam) at a final dilution of 1:100 for 24 h at
4°C. The immunoreaction was revealed by using horseradish
peroxidase (HRP)-labeled secondary mouse anti-rabbit antibody
(1:500; Jingmei Biological Engineering Co., Ltd., Shenzhen, China)
for 2 h at room temperature. The slides were mounted and scanned
using a microscope (Olympus, Tokyo, Japan). Five high power fields
(×400) from each slide were randomly selected for analysis using
Image-Pro Plus image analysis software. The average optical density
value represents the relative expression of proteins.
Quantitative real-time PCR
GLP-1R gene expression in the colon was analyzed by
quantitative real-time polymerase chain reaction (qRT-PCR). Total
RNA was extracted using TRIzol (Invitrogen, Carlsbad, CA, USA). One
microgram of total RNA was reverse-transcribed in a final volume of
50 μl using the ABI PRISM cDNA Archive kit. β-actin and
GLP-1R were amplified using 5 μl of cDNA per reaction. The
primers and TaqMan fluorogenic probes were purchased from
Invitrogen. β-actin was used as an internal gene. The primers for
rat GLP-1R and β-actin were as follows: GLP-1R, forward, 5′-CA
TCGTGGTATCCAAACTGA-3′ and reverse, 5′-GCTCGTCC ATCACAAAGG-3′;
β-actin, forward, 5′-TATGACTTAGTTG CGTTACACC-3′ and reverse,
5′-CCTTCACCGTTCCAGT TT-3′. The amplification reaction was performed
in an ABI PRISM 7500 fluorescence quantitative PCR instrument using
the following conditions: 50°C for 2 min, 95°C for 10 min and 95°C
for 15 sec, and a final extension step of 1 min at 66°C, 40 cycles,
stored at 4°C. The data were analyzed by the relative gene
expression, which was determined using the 2−ΔΔ cycle
threshold (Ct) method (23).
Western blotting
A colon tissue sample (100 mg) was homogenized in a
buffer containing 25 mM Tris-HCl (pH 7.5), 5 mM EDTA, 5 mM EGTA,
0.5 mM PMSF, 25 μg/ml leupeptin, 10 μg/ml aprotinin,
and 1 mM sodium vanadate. The crude protein homogenates with sample
buffer were boiled for 5 min. Samples were subjected to 10% sodium
dodecyl sulfate polyacrylamide gel electrophoresis (10 mg/lane) and
then transferred to a polyvinylidene difluoride membrane for
immunoblotting. The membranes were incubated in blocking buffer (5%
non-fat milk in Tween/Tris-buffered salt solution, TTBS) containing
anti-GLP-1R antibody (1:400; Abcam Ltd.). Following multiple
washes, the membranes were incubated for 1.5 h at room temperature
with secondary antibody (1:1,000) linked to horseradish peroxidase
(HRP). The immunopositive proteins on the membrane were detected by
an enhanced chemiluminescence detection kit (ECL; Millipore,
Bedford, MA, USA). The target band and the band for β-actin were
quantified by the Tianneng GIS gel image processing system for
optical density analysis.
Detection of serum GLP-1 levels by
ELISA
The blood samples were collected and DPP-4 inhibitor
(Millipore) was added to the blood samples. Following
centrifugation at 4,000 × g for 10 min at 4°C, the supernatants
were stored at −20°C until detection. The serum level of GLP-1
(7–36) was measured by ELISA (Linco Research Inc.). An aliquot of
100 μl of each sample was added to each assay well. ELISA
has a working range of 2–100 pM.
Validation of the effects of GLP-1 and
its receptor agonists on rat colon circular and longitudinal muscle
strips in rat models of IBS-C and IBS-D
The muscle strips obtained by cutting along the
longitudinal axis of the colon were the longitudinal muscle strips
(2×10 mm), and along the circular axis were the circular muscle
strips (2×10 mm), as previously reported (24). The muscle strips were suspended in
a designed horizontal organ bath, which contained 10 ml of Krebs
solution, continuously perfused with a 95% O2 and 5%
CO2 mixture, and heated (37°C) in Krebs solution with
the following composition: 119 mM NaCl, 4.5 mM KCl, 2.5 mM
MgSO4, 25 mM NaHCO3, 1.2 mM
KH2PO4, 2.5 mM CaCl2, and 11.1 mM
glucose, pH 7.4. One end of each strip was fixed to organ holders,
and the other end was fastened by a silk tie to an isometric
tension transducer (National Institute for Physiology, Japan). Each
muscle strip was slowly stretched at an initial tension of 0.5 g
and allowed to equilibrate for 60 min to reach a steady baseline
before experiments. Then, saline, GLP-1, and exendin-4 were added,
respectively, and the effects of these peptides on the IBS subtypes
and matched controls were evaluated. After the addition of GLP-1
and exendin-4, respectively, the contraction curve was recorded
within 5 min. The peptides were then eluted in the bath, and the
next trial was not performed until the smooth muscle strips were
restored to a steady state. We took the area under the curve (AUC)
of saline as 100%: data were expressed as the percentage of the AUC
obtained in the presence of saline on the ACh pretreated muscle
strips. Mechanical signals from each strip were amplified with an
amplifier, recorded, and analyzed using Med Lab 6.0 software
(MedEase, Nanjing, China).
Statistical analysis
Data are presented as the means ± SD. Analyses were
performed using SPSS 13.0 software (Jandel Scientific-SPSS Science,
Chicago, IL, USA). The IBS subtypes and control groups were
compared, and data were analyzed using one-way ANOVA followed by
Bonferroni post-test comparisons. P<0.05 was considered to
indicate statistically significant differences.
Results
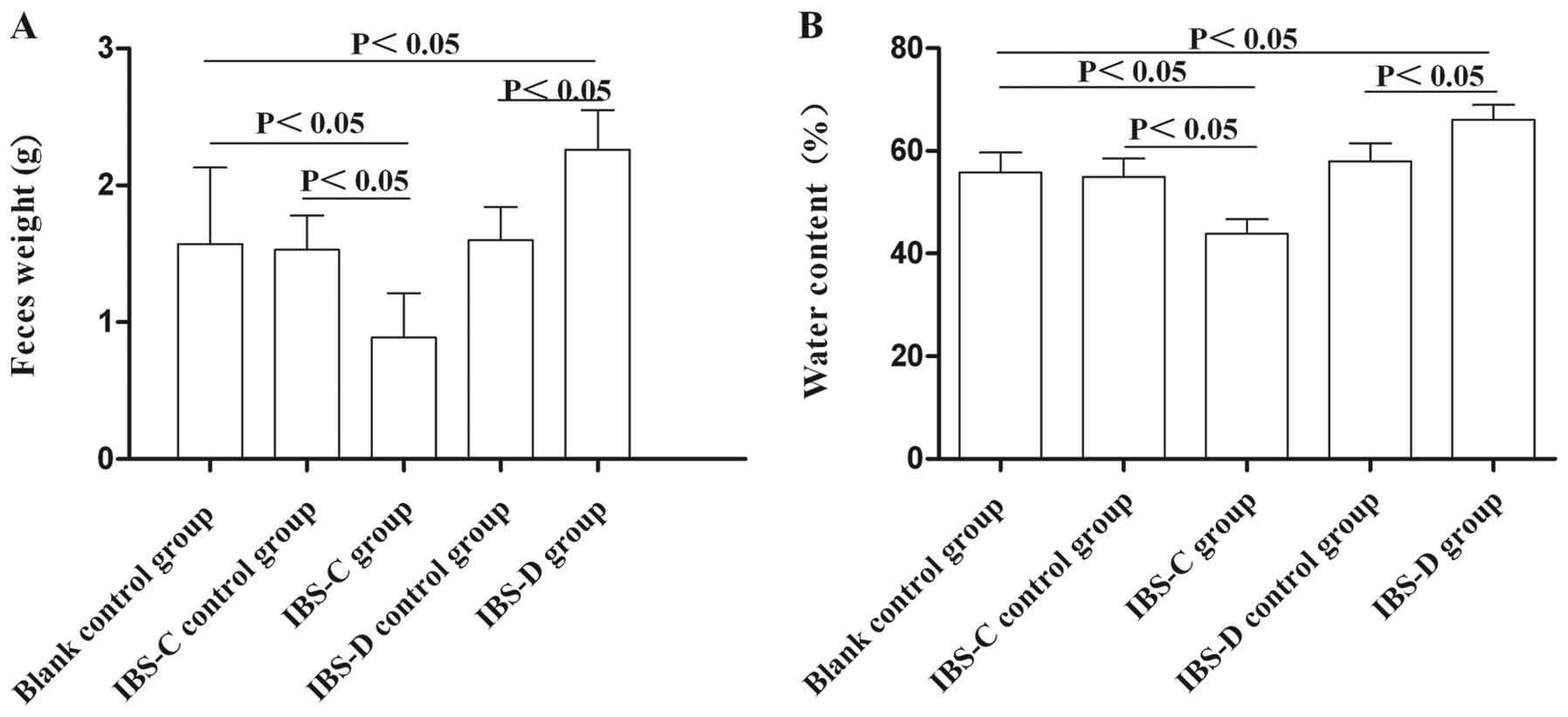
Feces changes in experimental IBS-C and
IBS-D rat models
To validate the successful establishment of rat
models of IBS-C and IBS-D, water content of the feces and feces
weight in the experimental IBS models were measured. The fecal
pellets in the IBS-C group weighed less than those in the control
group (P<0.05); the fecal pellets in the IBS-D group weighed
more than those in the control group (P<0.05) (Fig. 1). In addition, the water content
of the feces in the IBS-C rats was significantly less, and water
content in the feces of IBS-D rats was significantly more compared
with the control rats (P<0.05) (Fig. 1). These results suggested that the
models of IBS-C and IBS-D were established successfully.
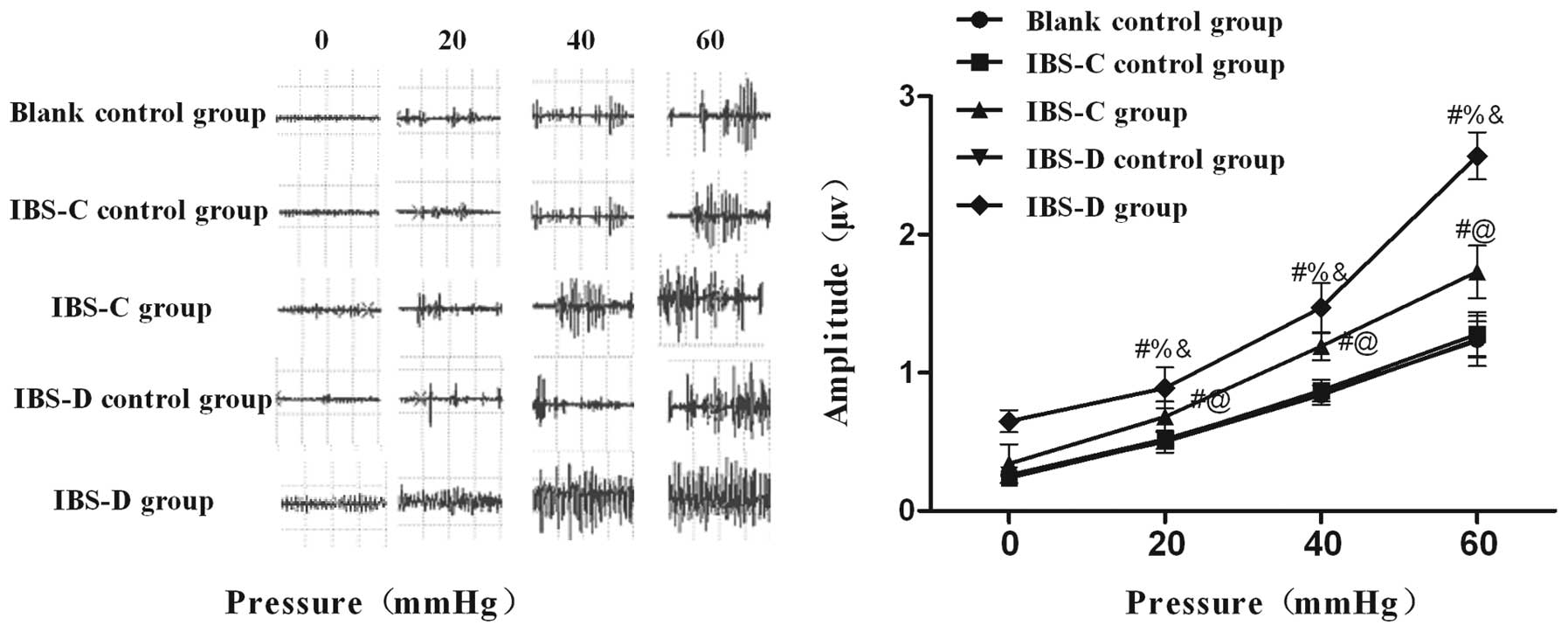
Visceromotor responses in experimental
IBS rat models
A significant increase in the maximum amplitude
curve of the EMG in response to increased CRD pressure in both
experimental IBS rats was detected (Fig. 2). The mean amplitudes in both
IBS-C and IBS-D rats exhibited higher responses to different
distention pressures compared with their matched controls,
respectively. These data indicated that the IBS model groups were
more sensitive to CRD compared with controls, suggesting that
gastric instillation of cool saline and wrap restraint stress
produced a persistent visceral hypersensitivity in IBS-C and IBS-D
rats.
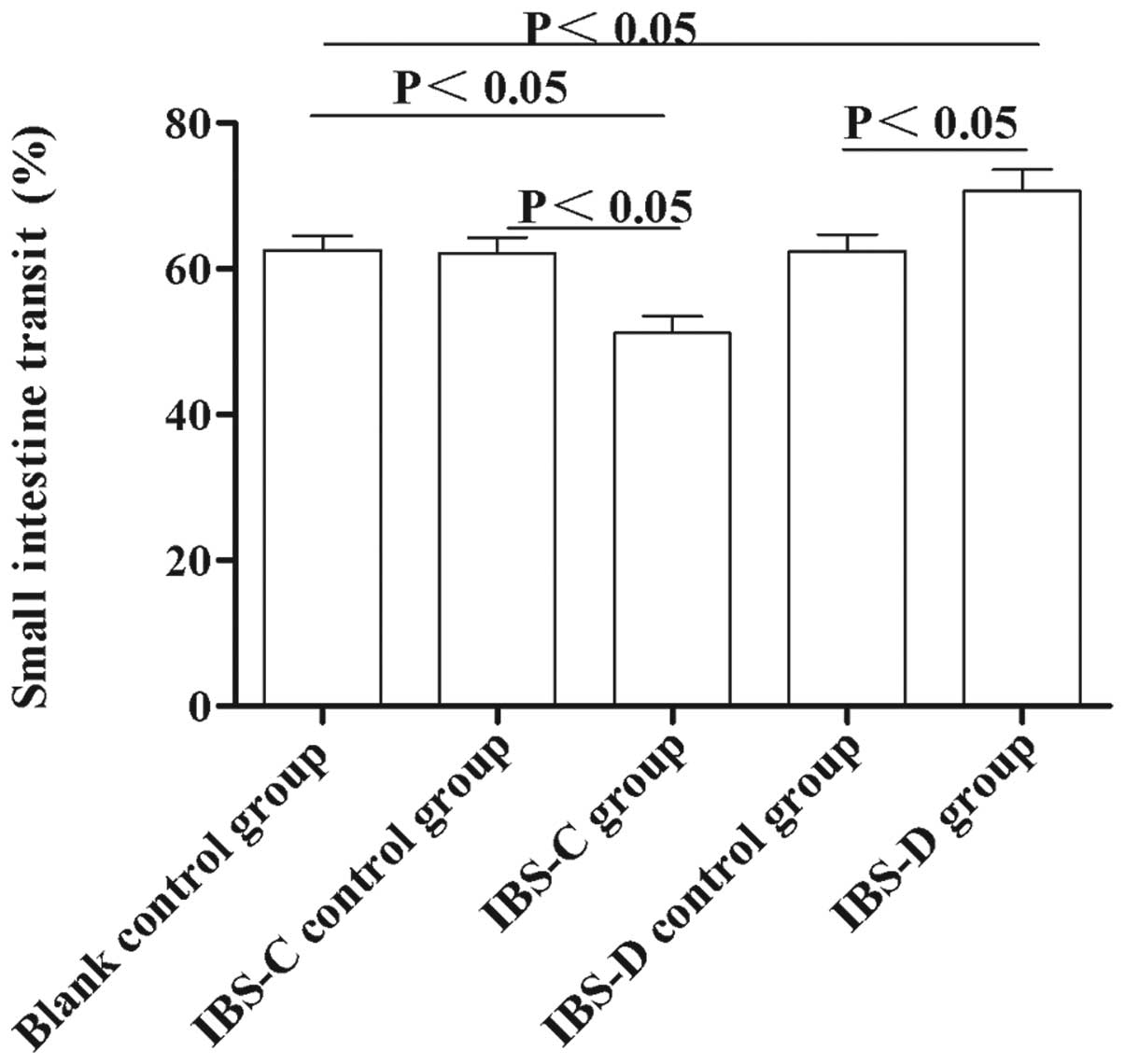
Small intestine transit rate in
experimental IBS rat models
To observe the motility changes in experimental
IBS-C and IBS-D in vivo, we measured the small intestine
transit rate. As shown in Fig. 3,
the small intestine transit rate in IBS-C rats was lower than that
in IBS controls and blank controls (51.17±2.32 vs. 62.09±2.22,
P<0.05 and 62.60±1.89, P<0.05, respectively). In IBS-D rats,
the small intestine transit rate was higher compared with that in
IBS-D controls and blank controls (70.69±2.91 vs. 62.39±2.31,
P<0.05 and 62.60±1.89, P<0.05, respectively). These results
suggested that small intestine transit in IBS-D rats was
accelerated, while the small intestine transit in IBS-C was
delayed, compared with their controls, respectively.
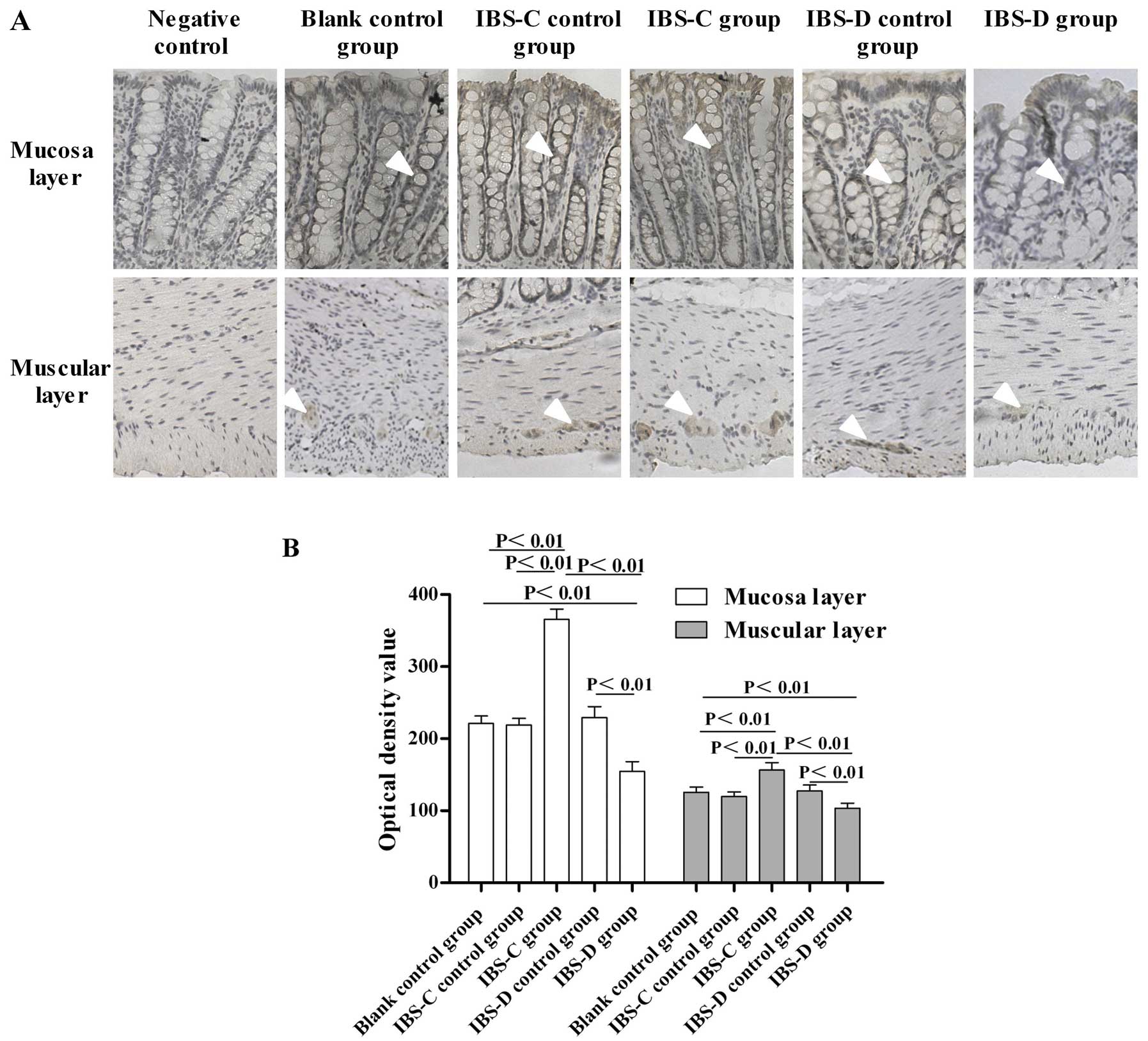
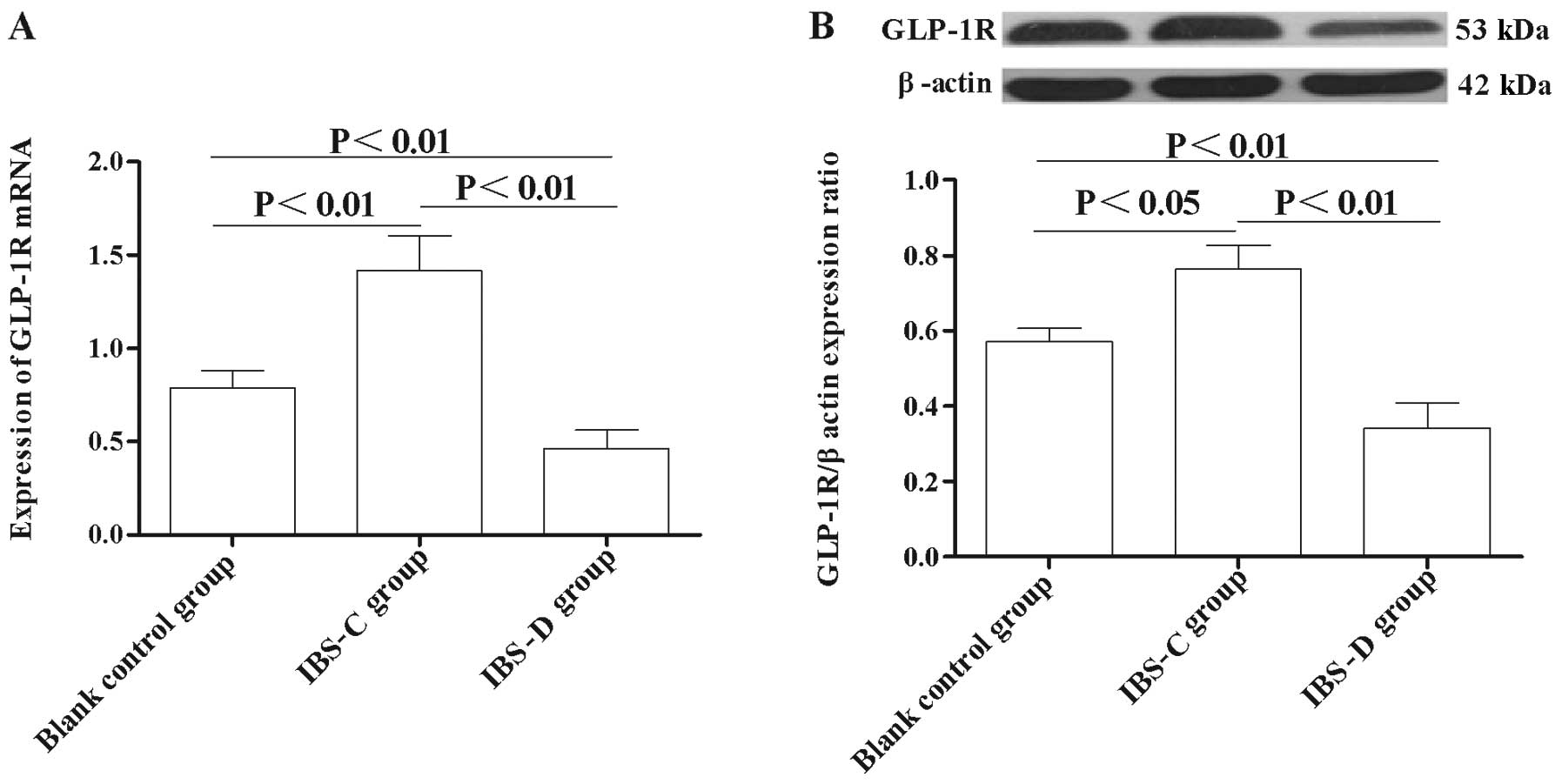
Expression of GLP-1R in the colon
To study the GLP-1 signaling implication in IBS, we
investigated the expression changes of GLP-1R in experimental IBS-C
and IBS-D. Immunohistochemistry results showed that GLP-1R
immunoreactivity was mainly located in the colonic mucosa layer and
in the circular muscle and myenteric plexus in both experimental
IBS rats (Fig. 4A). Markedly, the
expression of GLP-1R in the IBS-C group was higher than that in its
control group (P<0.01) (Fig.
4B). By contrast, the expression of GLP-1R in the IBS-D group
was significantly lower than that in its matched control
(P<0.01) (Fig. 4B).
Furthermore, the GLP-1R expression in the IBS-C group was much
higher than that in the IBS-D group (P<0.01) (Fig. 4B).
qRT-PCR analysis showed that the expression of
GLP-1R mRNA level in the IBS-C group was significantly higher
compared with the control group (P<0.01) (Fig. 5A), while in the IBS-D group, the
GLP-1R mRNA level was significantly lower compared with the matched
control (P<0.01) (Fig. 5A).
Moreover, the GLP-1R protein level was also significantly higher in
the IBS-C group compared with the control group (P<0.01)
(Fig. 5B), while the GLP-1R level
the IBS-D group was significantly lower than that in the control
group (P<0.01) (Fig. 5B).
These results indicated that abnormal GLP-1R expression is involved
in the pathogenesis of IBS.
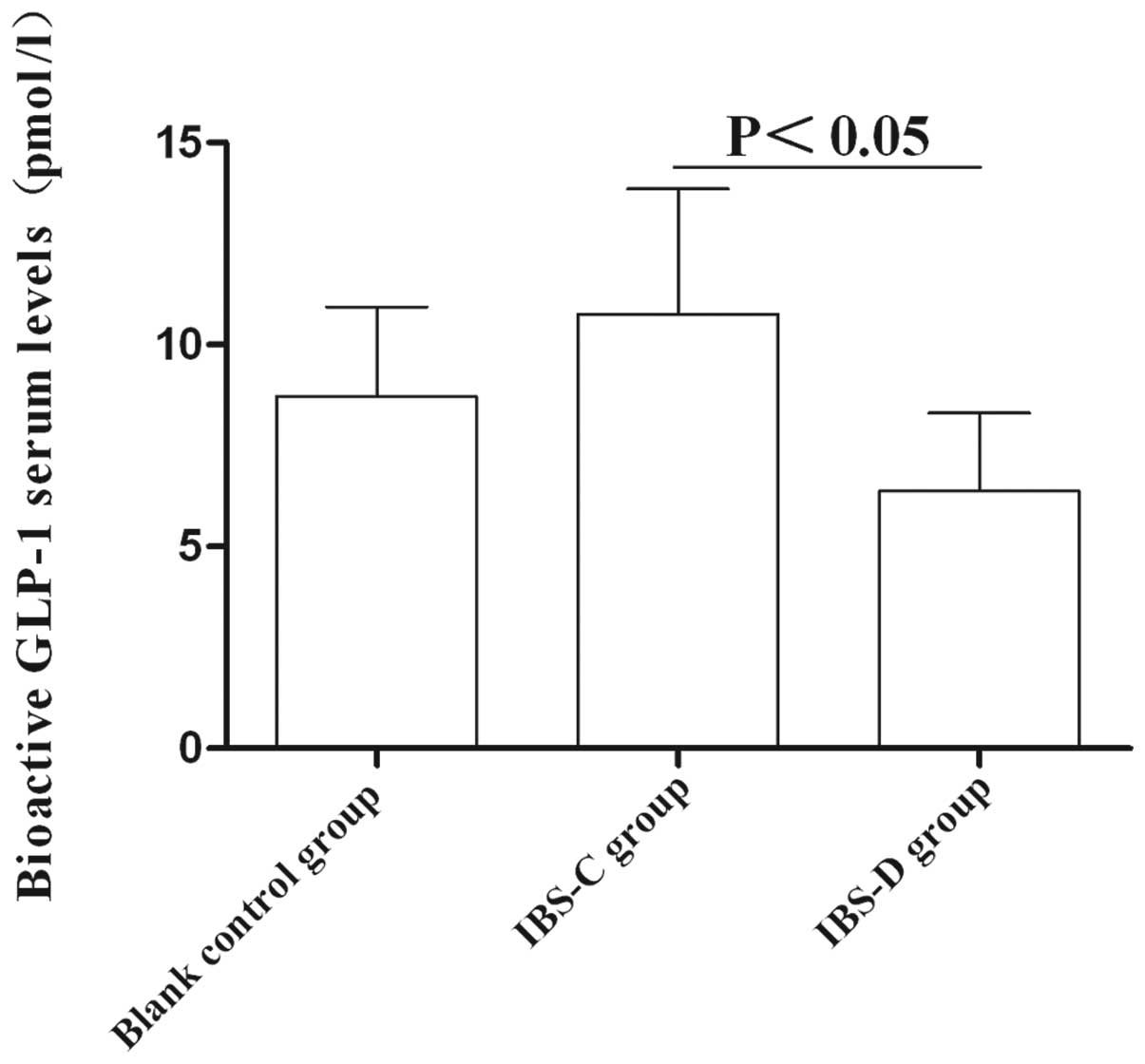
Circulating GLP-1 levels
The average serum level of GLP-1 in the IBS-C group
was significantly higher than that in the IBS-D group (10.76±3.1
vs. 6.37±1.93 pM, P<0.05). However, no significant difference of
serum GLP-1 level was detected between these two IBS subtype models
and control group (P>0.05) (Fig.
6). These results suggested that GLP-1 may be involved in IBS
subtype formation.
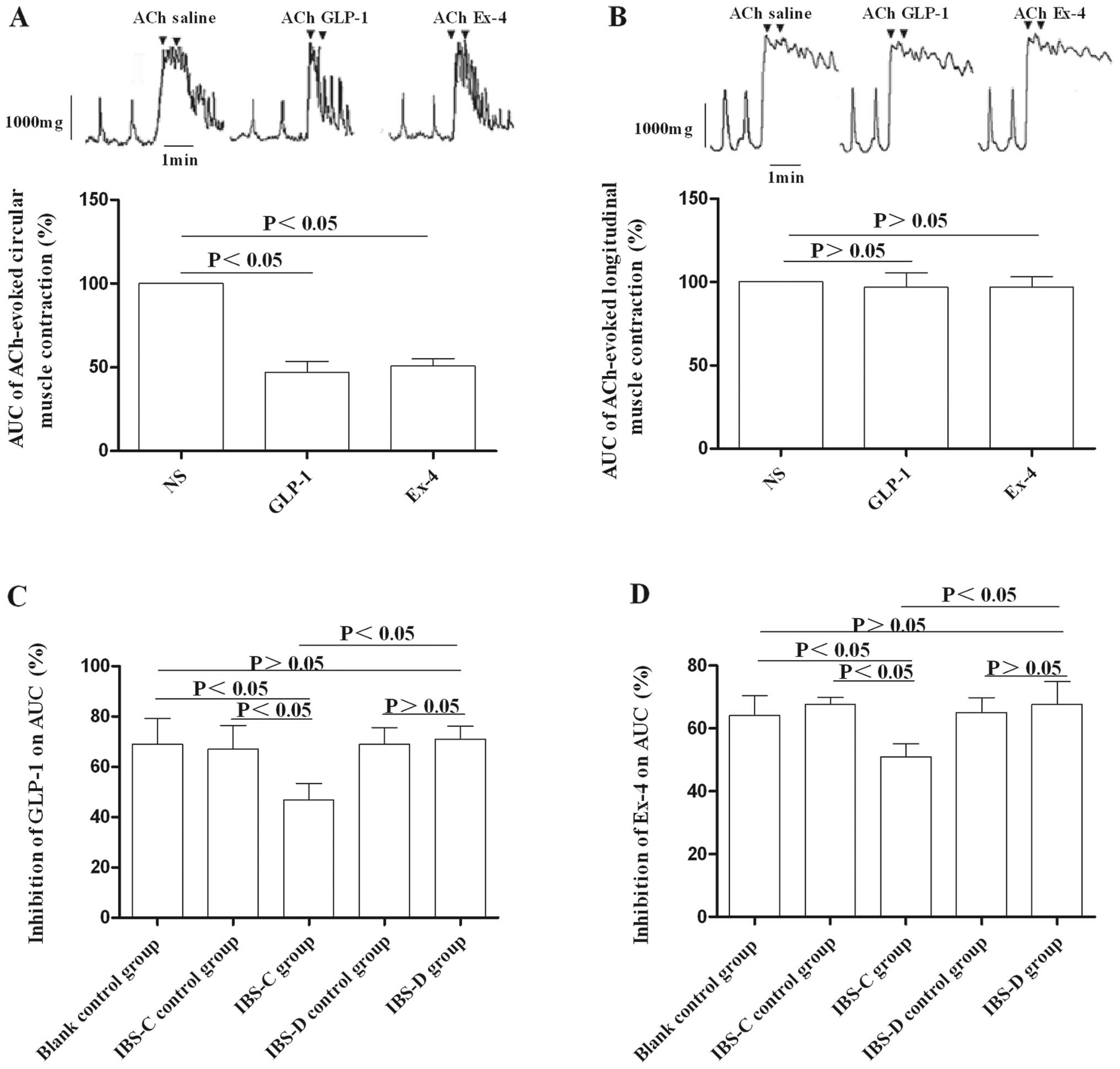
Effects of GLP-1 and its receptor
agonists on colon muscle strips in experimental IBS rat models
To determine the involvement of GLP-1 in the
pathogenesis of IBS, we tested the responses of colon muscle strips
to GLP-1 and exendin-4. Following pretreatment of muscle strips
with ACh, the optimal concentration of GLP-1 (1.0×10−7
M) or exendin-4 (8.0×10−8 M) was added. We found that
GLP-1 and exendin-4 had no effect on Ach-pretreated longitudinal
muscle strips (Fig. 7B), while
they exerted inhibitory effects on Ach-pretreated circular muscle
strips in both experimental IBS models and control groups (Fig. 7A). Furthermore, the inhibition by
GLP-1 or exendin-4 on Ach-pretreated circular muscle strips in the
IBS-C group was more obvious compared to that in the IBS-D group
(P<0.05) (Fig. 7C and D).
Discussion
In recent years, several different animal models of
IBS have been established to investigate the pathogenesis and
treatment of IBS. However, so far there has not been a perfect
model imitating the clinical manifestations of IBS patients. In our
study, the IBS-C group was established by gastric instillation of
0–4°C saline daily for 14 days (16), and the IBS-D group was established
by wrap restraint stress (17).
The IBS-D rats showed visceral hypersensitivity and defecated more
stool compared with the control rats, which was in accordance with
the clinical observations in IBS-D patients. Regarding the IBS-C
model, no marked inflammation was found in the colon after gastric
instillation with 0–4°C cool water daily for 14 days. Compared with
the blank control, the amount, weight, and water content of the
feces expelled by the IBS-C rats were significantly less, and the
time before the first black stool occurred in the IBS-C group was
significantly longer than that in the control groups.
Previous studies found that GLP-1 can slow gastric
emptying and inhibit intestinal movement (25–27). A clinical case report (28) indicated that a patient with a
neuroendocrine tumor who had high serum levels of GLP-1 (300 to
400-fold) had markedly delayed gastrointestinal transit and severe
intractable constipation. Normal bowel function was restored after
tumor resection. This indicates that GLP-1 is involved in the
formation of constipation, but the underlying mechanism remains
unclear. Our observations showed that the serum bioactive GLP-1
level in the IBS-C group was higher compared with the control
group, which indicates that an increased bioactive GLP-1 level may
be related to the formation of constipation in experimental rats.
In addition, our results showed that exogenous GLP-1 and its
receptor agonist, exendin-4, can effectively inhibit colon circular
muscle contraction in a rat experimental IBS model, while having no
effect on longitudinal muscle contraction. These results can be
explained by the fact that GLP-1R was located in the circular
muscle and myenteric neurons, while no GLP-1R-positive staining was
detected in the longitudinal muscle. These results are consistent
with the report of Amato et al (14), who showed that GLP-1R was detected
in some myenteric neurons in rats, and GLP-1 regulated the release
of NO to decrease the excitatory cholinergic neurotransmission by
acting on the presynaptic GLP-1Rs, and then inhibited the
intestinal movement.
As circular muscle contraction is dominant in
peristalsis, the action of GLP-1 could contribute to the reduction
of intestinal transit (14). In
our study, GLP-1 and its receptor agonist inhibited rat colonic
circular muscle, particularly in the IBS-C group, suggesting that
the high serum levels of GLP-1 and high expression of GLP-1R in the
IBS-C group inhibited colonic movement to cause the dry stool and
sluggish rat small intestine transit, leading to constipation. In
summary, GLP-1 and its receptors may be involved in altered
gastrointestinal motility in the pathogenesis of IBS.
The gastrointestinal mucosa may receive a variety of
stimuli to impart various types of signals to the central nervous
system and lead to sensory dysregulation. Our results showed that
GLP-1R is mainly expressed in the colon mucous layer, and its
expression varied among the experimental IBS subtypes, indicating
that GLP-1 and GLP-1R might be related to the formation of visceral
hypersensitivity in IBS models; however, this requires further
investigation.
In conclusion, our results showed that GLP-1 and
GLP-1R altered gastrointestinal motility of experimental IBS-C and
IBS-D, suggesting a potentially important role for GLP-1 and GLP-1R
in IBS-C and IBS-D and motility dysfunction.
Acknowledgements
This study was supported by grants
from the National Natural Science Foundation of China (no.
30771039) and the Key Medical Personnel of Jiangsu Province (no.
RC2011063)
References
|
1
|
Drossman DA: The functional
gastrointestinal disorders and the Rome III process.
Gastroenterology. 130:1377–1390. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Clarke G, Quigley EM, Cryan JF and Dinan
TG: Irritable bowel syndrome: towards biomarker identification.
Trends Mol Med. 15:478–489. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cremonini F and Talley NJ: Irritable bowel
syndrome: epidemiology, natural history, health care seeking and
emerging risk factors. Gastroenterol Clin North Am. 34:189–204.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Spiller RC and Thompson WG: Bowel
disorders. Am J Gastroenterol. 105:775–785. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Longstreth GF, Thompson WG, Chey WD,
Houghton LA, Mearin F and Spiller RC: Functional bowel disorders.
Gastroenterology. 130:1480–1491. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Camilleri M, McKinzie S, Busciglio I, et
al: Prospective study of motor, sensory, psychologic, and autonomic
functions in patients with irritable bowel syndrome. Clin
Gastroenterol Hepatol. 6:772–781. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hellstrom PM, Hein J, Bytzer P, Bjornsson
E, Kristensen J and Schamby H: Clinical trial: the glucagon-like
peptide-1 receptor agonist ROSE-010 for management of acute pain in
patients with irritable bowel syndrome: a randomized,
placebo-controlled, double-blind study. Aliment Pharmacol Ther.
29:198–206. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Elliott RM, Morgan LM, Tredger JA, Deacon
S, Wright J and Marks V: Glucagon-like peptide-1 (7–36)amide and
glucose-dependent insulinotropic polypeptide secretion in response
to nutrient ingestion in man: acute post-prandial and 24-h
secretion patterns. J Endocrinol. 138:159–166. 1993.
|
|
9
|
Hellstrom PM, Naslund E, Edholm T, et al:
GLP-1 suppresses gastrointestinal motility and inhibits the
migrating motor complex in healthy subjects and patients with
irritable bowel syndrome. Neurogastroenterol Motil. 20:649–659.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
McDonagh SC, Lee J, Izzo A and Brubaker
PL: Role of glial cell-line derived neurotropic factor family
receptor alpha2 in the actions of the glucagon-like peptides on the
murine intestine. Am J Physiol Gastrointest Liver Physiol.
293:G461–G468. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Miki T, Minami K, Shinozaki H, et al:
Distinct effects of glucose-dependent insulinotropic polypeptide
and glucagon-like peptide-1 on insulin secretion and gut motility.
Diabetes. 54:1056–1063. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schirra J, Houck P, Wank U, Arnold R, Goke
B and Katschinski M: Effects of glucagon-like peptide-1(7–36)amide
on antro-pyloroduodenal motility in the interdigestive state and
with duodenal lipid perfusion in humans. Gut. 46:622–631. 2000.
|
|
13
|
Schirra J, Nicolaus M, Roggel R, et al:
Endogenous glucagon-like peptide 1 controls endocrine pancreatic
secretion and antropyloro-duodenal motility in humans. Gut.
55:243–251. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Amato A, Cinci L, Rotondo A, Serio R,
Faussone-Pellegrini MS, Vannucchi MG and Mulè F: Peripheral motor
action of glucagon-like peptide-1 through enteric neuronal
receptors. Neurogastroenterol Motil. 22:e664–e203. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Körner M, Stöckli M, Waser B and Reubi JC:
GLP-1 receptor expression in human tumors and human normal tissues:
potential for in vivo targeting. J Nucl Med. 48:736–743.
2007.PubMed/NCBI
|
|
16
|
Peng LH, Yang YS, Sun G and Wang WF: A new
model of Constipation-predominant irritable bowel syndrome in rats.
Shijie Huaren Xiaohua Zazhi. 12:112–116. 2004.(In Chinese).
|
|
17
|
Williams CL, Villar RG, Peterson JM and
Burks TF: Stress-induced changes in intestinal transit in the rat:
a model for irritable bowel syndrome. Gastroenterology. 94:611–621.
1988.PubMed/NCBI
|
|
18
|
Gui XY, Pan GZ and Ke MY: Residual effect
of cold-restraint stress on colonic motility in rats. Chin J Dig
Dis. 17:94–96. 1997.(In Chinese).
|
|
19
|
Habold C, Reichardt F, Le Maho Y, et al:
Clay ingestion enhances intestinal triacylglycerol hydrolysis and
non-esterified fatty acid absorption. Br J Nutr. 102:249–257. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Winston J, Shenoy M, Medley D, Naniwadekar
A and Pasricha PJ: The vanilloid receptor initiates and maintains
colonic hypersensitivity induced by neonatal colon irritation in
rats. Gastroenterology. 132:615–627. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Al-Chaer ED, Kawasaki M and Pasricha PJ: A
new model of chronic visceral hypersensitivity in adult rats
induced by colon irritation during postnatal development.
Gastroenterology. 119:1276–1285. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Appleyard CB and Wallace JL: Reactivation
of hapten-induced colitis and its prevention by anti-inflammatory
drugs. Am J Physiol. 269:G119–G125. 1995.PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2[−Delta Delta C(T)] method. Methods. 25:402–408. 2001.
|
|
24
|
Tolessa T, Gutniak M, Holst JJ, Efendic S
and Hellstrom PM: Glucagon-like peptide-1 retards gastric emptying
and small bowel transit in the rat: effect mediated through central
or enteric nervous mechanisms. Dig Dis Sci. 43:2284–2290. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Näslund E, Bogefors J, Skogar S, et al:
GLP-1 slows solid gastric emptying and inhibits insulin, glucagon,
and PYY release in humans. Am J Physiol. 277:R910–R916.
1999.PubMed/NCBI
|
|
26
|
Nagell CF, Wettergren A, Ørskov C and
Holst JJ: Inhibitory effect of GLP-1 on gastric motility persists
after vagal deafferentation in pigs. Scand J Gastroenterol.
41:667–672. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bozkurt A, Näslund E, Holst JJ and
Hellström PM: GLP-1 and GLP-2 act in concert to inhibit fasted, but
not fed, small bowel motility in the rat. Regul Pept. 107:129–135.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Brubaker PL, Drucker DJ, Asa SL, Swallow
C, Redston M and Greenberg GR: Prolonged gastrointestinal transit
in a patient with a glucagon-like peptide (GLP)-1- and -2-producing
neuroendocrine tumor. J Clin Endocrinol Metab. 87:3078–3083. 2002.
View Article : Google Scholar : PubMed/NCBI
|