Introduction
Asthma is a chronic condition of the respiratory
system in which the airways occasionally constrict, become
inflamed, and are congested with excessive amounts of mucus
(1). Similar to other complex
diseases, environmental and genetic factors have been identified as
potential causes of asthma although the precise mechanisms are not
fully understood (2). Most asthma
medications work by preventing bronchospasms and/or reducing
inflammation. Improvements in available therapies, such as the
development of fast-onset once-a-day combination drugs with better
safety profiles are highly desirable. Immune signal transduction
inhibitors and antioxidants that target specific pathways or
mediators could be useful for treating asthma (3). Biological compounds directed against
the IL-4 or IL-13 pathway, and new immunoregulatory agents that
modulate the functions of T-regulatory and T helper-17 cells are
also potential anti-asthmatic treatments (4). Although a cure is unlikely to be
developed in the near future, a greater understanding of the
mechanisms underlying asthma pathogenesis could make this a
reality.
The lung is composed of a unique tissue subjected
more frequently to oxidant stress compared to most organs as it is
directly exposed to higher oxygen tensions (5). Thus, partial pressure of oxygen in
the alveoli is much higher than that in other vital organs such as
the heart, liver and brain (6).
Because the lung is directly exposed to ambient air, lung cells
experience enhanced oxidant stress caused by environmental
irritants, oxidants and pollutants such as cigarette smoke, ozone
and environmental carcinogens that generate free radicals (7). A typical symptom of most lung
disorders and infections is inflammation and activation of
inflammatory cells (8).
While screening for anti-allergenic compounds using
high throughput-compatible assays with combinatorial chemical
libraries in a previous study, we obtained a unique hit that was
identified as a derivative (LX519290) of L-allo threonine (9). L-allo threonine is a diastereoisomer
of L-threonine that does not naturally exist in the human body
(10). This amino acid is a
component of globomycin, a new peptide antibiotic with
spheroplast-forming activity. In E. coli, the amino acid can
be produced by acetaldehyde and glycine with a serine hydroxymethyl
transferase-catalyzed aldol reaction in the presence of pyridoxal
phosphate (11). Interestingly,
L-allo threonine cannot be metabolized into L-threonine in chickens
although the ability of humans to metabolize this compound remains
unknown (12,13).
Recently, we published a study in which LX519290
exhibited anti-atopic activity in a 2,4-dinitrofluorobenzene
(DNFB)-induced animal model (14). In our murine asthmatic model, a
number of disease symptoms including infiltration of inflammatory
cells (particularly eosinophils), loss of airway structural
integrity and airway obstruction were markedly attenuate in
ovalbumin (OVA)-challenged mice compared to control animals. In the
present study, we therefore assessed the anti-asthmatic activity of
LX519290 administered after a single post-OVA-challenge in a mouse
model.
Materials and methods
Chemicals, reagents and cells
OVA, alum, hematoxylin and eosin (H&E) and
periodic acid-Schiff (PAS) were purchased from Sigma-Aldrich (St.
Louis, MO, USA). Phospho-specific as well as non-phospho-specific
p38, JNK and ERK antibodies were purchased from Cell Signaling
Technology, Inc. (Beverly, MA, USA). CD4+,
CD8+, IL-17E and HRP antibodies were obtained from Santa
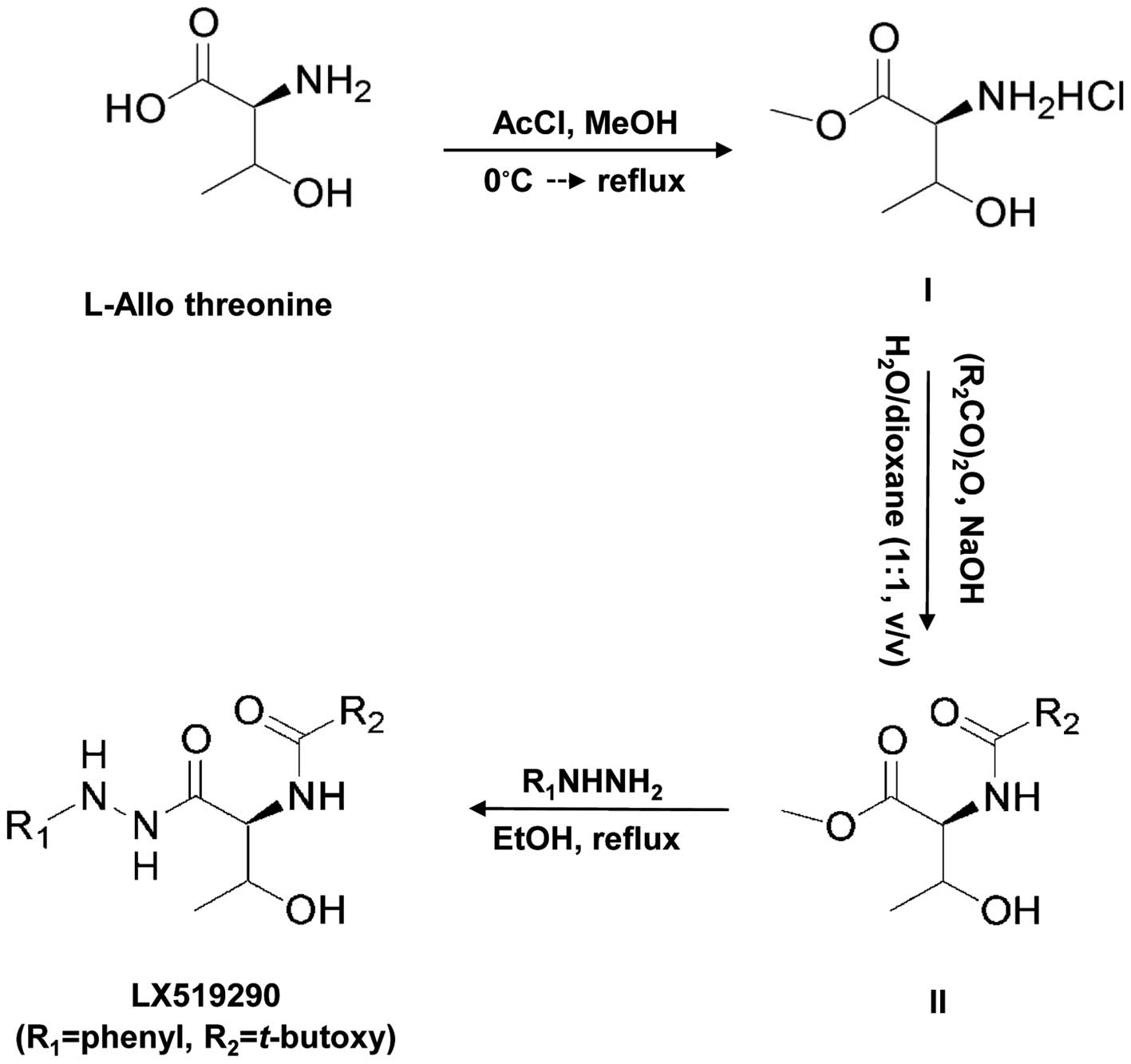
Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). LX519290 was
prepared as described in Fig. 1.
The detailed protocol used for preparing the compound is described
elsewhere (14). Other reagents
used were commercially available. CCRF-CEM cells (T lymphoblastic
cells) were purchased from American Type Culture Collection (ATCC,
Manassas, VA, USA; no. CCL-119).
Cell viability
Cell viability was assayed using a CCK-8 cell
proliferation assay kit (Dojindo, Kumamoto, Japan), as follows:
cells (5×105/ml) were plated in 96-well plates, and
incubated for 24 h in 100 μl of RPMI-1640 medium. Various
concentrations (1, 3, 10, 30, 100 and 300 μg/ml) of LX519290 were
added to the cells, and incubated for an additional 48 h. Next, 10
μl of MTT solution (5 mg/ml MTT in PBS) was added to each well,
followed by incubation at 37°C for 4 h. To stop the reaction, 100
μl of 0.04 M HCl was added to isopropanol with vigorous mixing.
Absorbance was measured with a Victor multilabel counter (Wallac,
Turku, Finland) at 564 nm.
Animal care
Male C57BL/6 mice (5–6 weeks of age, ~20–23 g) were
supplied by Samtaco (Osan, Korea). All mice had free access to tap
water and chow food (Purina Korea, Inc., Seoul, Korea). The animals
were kept in an air-conditioned room at 22±1°C and 55±5% humidity.
All procedures complied with the guidelines of the Committee of the
International Association for the Study of Pain Research and
Ethical Issues (15), and all
regulations of the Committee of Laboratory Animal Ethics, Kyungpook
National University (Daegu, Korea). All animals were acclimated to
the laboratory environment for at least 7 days prior to initiating
the experiments. The mice were divided into groups of 5
animals.
Immunization and OVA-challenge exposure
to mice
Immunization and subsequent challenge of the mice
were carried out as previously described by Heo et al
(16) with slight modification.
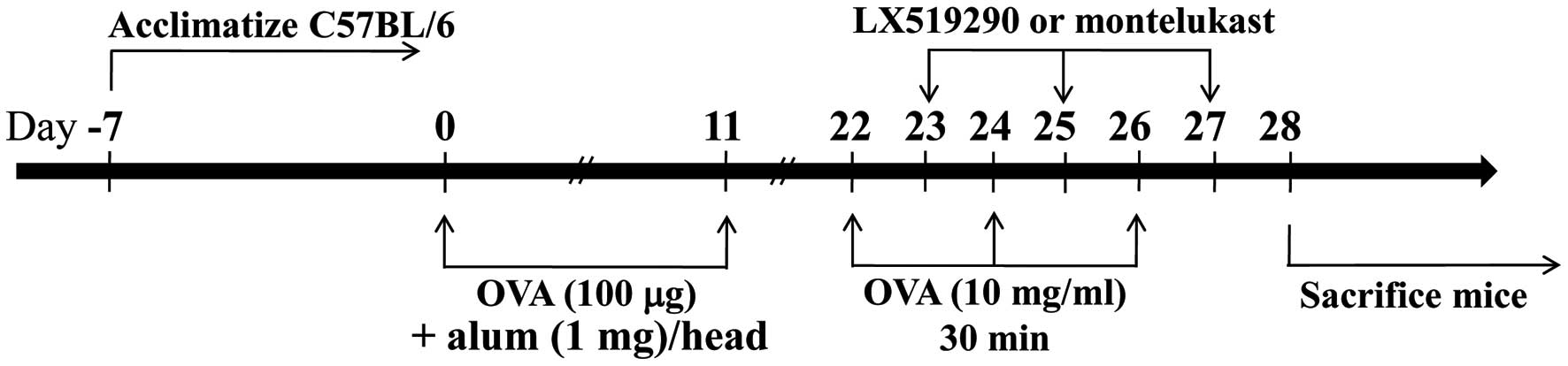
In brief, C57BL/6 mice were intraperitoneally (i.p.) injected with
50 μg of OVA absorbed on 1 mg of alum on days 0 and 11. On day 22,
mice were exposed 3 times to aerosolized OVA (10 mg/ml) in 0.9%
saline for 30 min, and then every other day for 6 more days (on
days 22, 24 and 26). Control mice were injected with 0.5 ml of
sterile saline using similar equipment and schedules (Fig. 2).
LX519290 treatment
The OVA-sensitized C57BL/6 mice were divided into 2
groups: one was challenged with OVA and the other was treated with
saline (as control). The OVA-challenged group was further divided
and treated with or without LX519290. One day after the first
challenge with OVA (day 22), the animals received 3 i.p. injections
of LX519290 (1 mg/kg in 0.2 ml of saline). This treatment was
repeated every second day for 6 days (days 23, 25 and 27). After 24
h of the last treatment (day 27), blood was collected to measure
serum IL-4, IL-13 and IgE. The lungs were then removed for
histological and cytokine analyses.
Lung histological evaluation
The lungs were fixed with 10% paraformaldehyde in
0.1 M PBS (pH 7.4) and embedded in paraffin as previously described
(16,17). Tissue sections were subjected to
staining with H&E to observe general morphology,
immunohistochemistry, and PAS reaction to assess mucus production
in the airway epithelium. Paraffin blocks were cut into section (6
μm), and mounted on glass slides. Paraffin in the lung sections was
removed by treatment with xylene and serial dilutions of ethanol,
and then stained with H&E. Cells positive for CD4+,
CD8+ and IL-17E that had migrated into the lung were
detected with immunostaining. All slides were incubated in 0.3%
H2O2 in methanol overnight at room
temperature to quench endogenous peroxidase activity.
Immunostaining was performed overnight at 4°C with anti-mouse
CD4+, CD8+ or IL-17E antibodies diluted 1:200
with 1% bovine serum albumin (BSA) in PBS. The sections were then
incubated with horseradish peroxidase (HRP)-conjugated secondary
antibody diluted 1:500 in 5% BSA in PBS at 37°C for 1 h. The
sections were also stained with 1% Schiff’s reagent, and finally
stained with Mayer’s hematoxylin for 5 min at room temperature.
Measurement of serum IgE levels
Serum IgE levels were measured by an ELISA as
previously described (18). Sera
were obtained from mice 24 h after challenge with either saline or
OVA. ELISAs specific for IL-4 and IL-13 were conducted using a
matching antibody pair (R&D Systems, Minneapolis, MN, USA)
according to the manufacturer’s instructions. The secondary
antibodies were conjugated to HRP. Residual substrate readings
taken at 450 nm were converted into pg/ml according to standard
curves generated with recombinant IL-4 and IL-13.
RT-PCR analysis of gene expression
Total RNA were extracted using TRI reagent
(Molecular Research Center, Cambridge, UK) according to the
manufacturer’s protocols. The RNA (1–10 μg) from mouse spleen cells
or CCRF-CEM cells (T lymphoblastic cells) was transcribed into
first-strand cDNA with random primers [or oligo(dT)] in a reaction
volume of 20 μl using an RT kit (Intron, Seoul, Korea), and 4 μl of
product was used as the PCR template. Sequences of the primers used
for PCR amplification are shown as follows: 5′-TGAGGC
CCAGCTTAAAGA-3′ and 5′-CTGTGGACCCTGCCATA GAT-3′ for Wiskott-Aldrich
syndrome gene-like protein (N-WASP); 5′-CACTGCTATGCTGCCTGCTC-3′ and
5′-TCT TCACCTGCTCCACTGCC-3′ for IL-10; 5′-ATGTTCCAG TATGACTCCAC-3′
and 5′-GCCAAAGTTGTCATGGA TGA-3′ for glyceraldehyde-3-phosphate
dehydrogenase (GAPDH). Genes for IL-10, N-WASP and GAPDH were
amplified with denaturing at 94°C for 30 sec, annealing at 60°C for
30 sec and extension at 72°C for 30 sec.
Western blot analysis
Cell lysates were prepared using an M-PER Mammalian
Protein Extraction Reagent, and the protein concentration was
determined using a BCA assay kit (both were from Pierce
Biotechnology, Rockford, IL, USA). The proteins were separated with
SDS-PAGE (8%), and immunoblotting was conducted with antibodies
against phospho-p38, p38, phospho-JNK, JNK, phospho-ERK and ERK as
described elsewhere (19).
Antibody binding was detected with an enhanced chemiluminescence
western blotting detection reagent (Animal Genetics, Inc., Suwon,
Korea), and viewed with an LAS3000 imaging system (Fujifilm Co.,
Tokyo, Japan).
Statistical analysis
Data are expressed as the mean ± standard deviation.
The results were analyzed by a multiple range test using SPSS 9.0
(SPSS, Chicago, IL, USA). P-values <0.1 or <0.05 were
considered to indicate statistically significant results.
Results
In vitro cytotoxic effects of
LX519290
The effects of LX519290 on the cell viability in
RAW264.7 cells was extensively investigated. Cell viability with
various concentrations of LX519290 was measured with a CCK-8 cell
proliferation assay kit. The results showed cell viability of
100±7.8, 93±2.2, 97±5.2, 94±6.6, 87±7.3 and 86±5.2%, respectively,
indicating that the present concentrations (1, 3, 10, 30, 100 and
300 μg/ml) did not adversely affect cell viability (data not
shown).
Histological analysis of lungs from
LX519290-treated mice
Forty-eight hours after the final OVA or saline
treatment in each group, lung tissues were excised to assess
inflammatory cell infiltration, mucus release and cytokine
expression. The effect of LX519290 on airway inflammation and
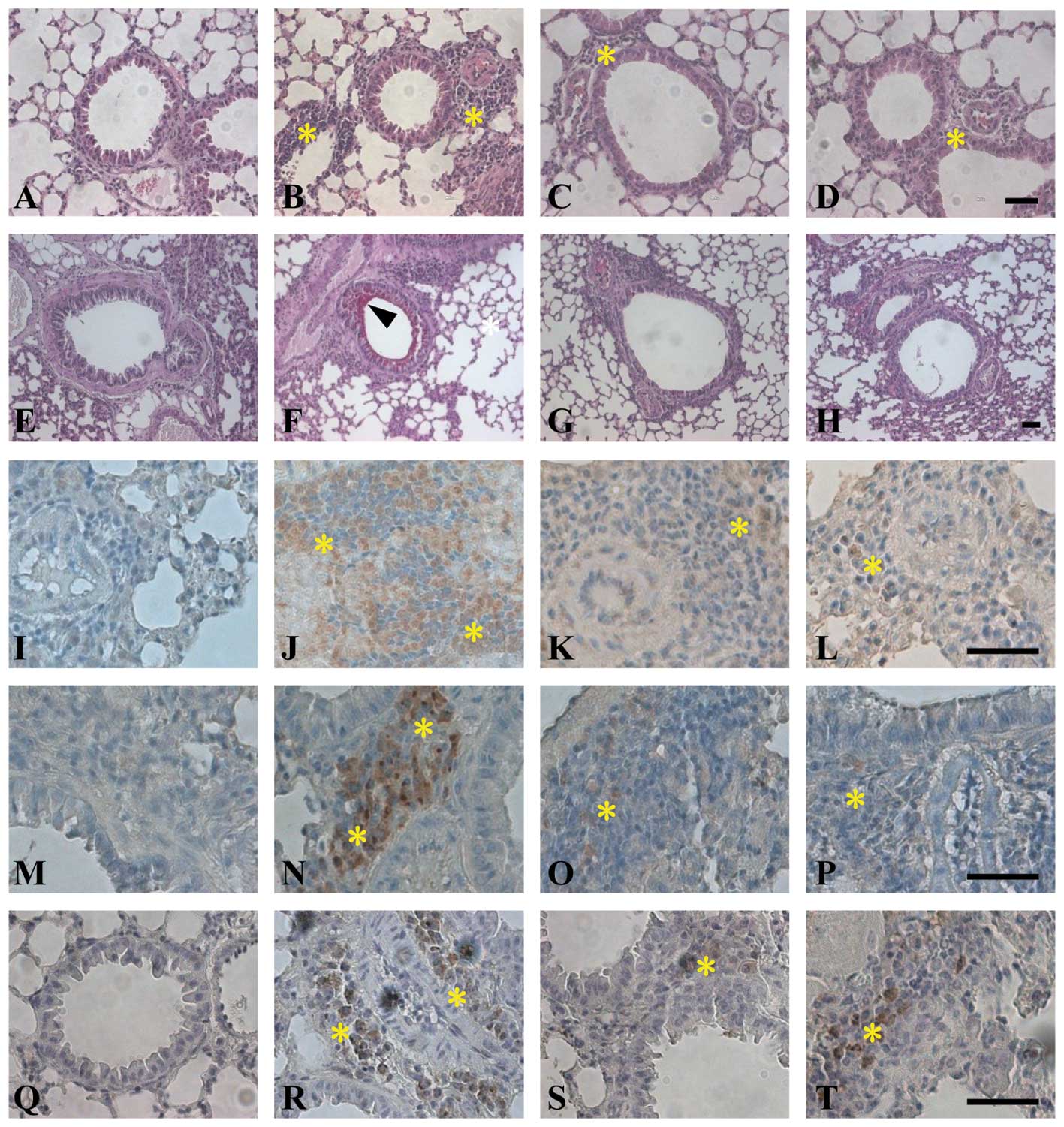
remodeling was evaluated according to lung histology. Histological
analysis of lungs taken from both OVA-sensitized mice and control
animals exposed to saline aerosol showed that the lungs of all mice
were normal (Fig. 3). In
contrast, lungs from mice challenged with aerosolized OVA showed
severe inflammation, showing that the lungs showed clear and
widespread inflammatory cell infiltration (Fig. 3B, F, J, N and R). OVA-challenged
animals had increased smooth muscle thickness in the airways
compared with the control mice (Fig.
3F, arrowhead). On the other hand, LX519290 prevented airway
smooth muscle hypertrophy observed in the OVA-challenged animals
(Fig. 3C, G, K, O and S). Lung
sections from LX519290-treated mice also showed a decrease in the
number of inflammatory cells. The OVA-challenged group showed
remarked increases in CD4+ (Fig. 3I-L) and CD8+ (Fig. 3M-P) T cells. PAS staining of
neutral mucus revealed that the epithelial cells surrounding airway
tissues increased in number (Fig.
3F) compared to the control group (Fig. 3E). Mucus production in airway
tissues was decreased in the LX519290-treated group (Fig. 3C) compared to the OVA-challenged
group (Fig. 3B). These results
demonstrated that LX519290 strongly inhibits asthmatic symptoms in
the lung.
Cytokine expression evaluated by
immunohistochemistry
Immunohistochemistry revealed that only a few cells
positive for IL-17E staining were present in the lungs of the
control group (Fig. 3Q). In the
OVA-challenged group, IL-17E immunoreactivity was observed in some
mononuclear cells infiltrating around the airways and vessels
(Fig. 3R). The number of
mononuclear cells expressing IL-17E in lung tissues was
significantly decreased in the LX519290-treated group (Fig. 3R and S). To further study the
changes in cytokine expression, we measured IL-4 and IL-13 levels
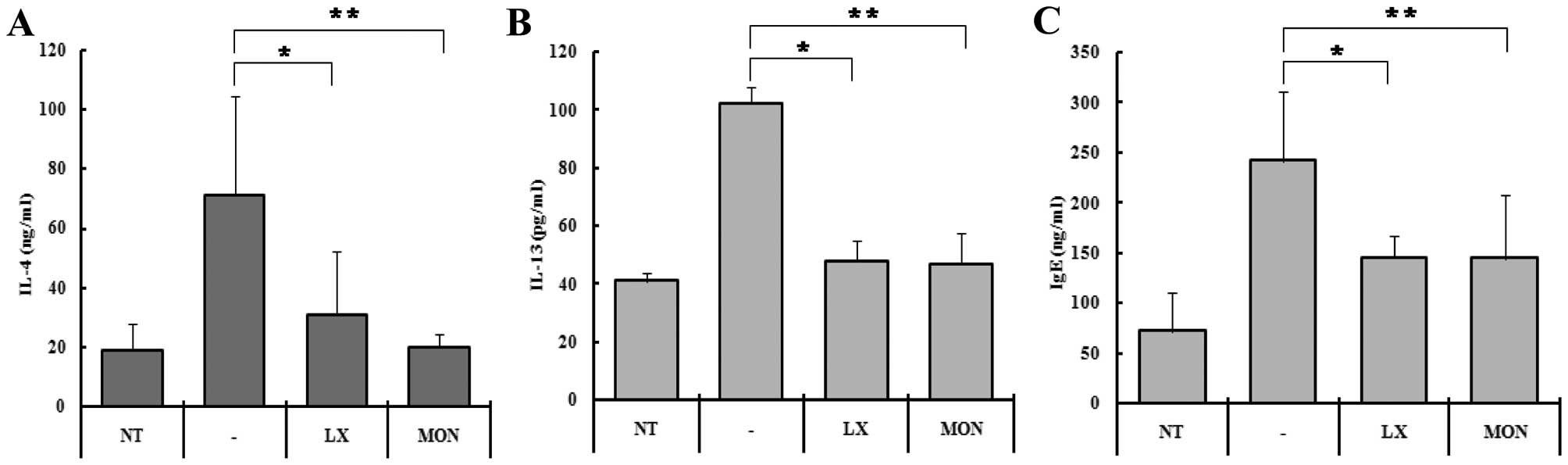
in serum (Fig. 4). IL-4
concentration in the serum of the control mice increased from
375±184 to 1420±665 ng/ml following OVA-challenge (Fig. 4A). In the mice treated with
LX519290 and Montelukast, the IL-4 production rate was reduced ~60%
when compared to the OVA-challenged mice (Fig. 4A). IL-13 levels in the serum of
the control mice increased from 41±2.9 to 102±5.9 ng/ml following
OVA-challenge (Fig. 4B). In the
mice treated with LX519290 and Montelukast, the IL-13 levels were
reduced to ~53% compared to the OVA-challenged mice.
In order to determine whether the expression of
cytokines related to asthma was decreased by LX519290, we measured
the serum levels of IL-4 and IL-13 with an ELISA. In the control
group, the IL-4 concentration was ~375±184 pg/ml, whereas it was
1420±665 in the OVA-challenged group (Fig. 4A). This increase in IL-4 explains
why the OVA-induced immune responses in the leukocytes increased
the cell numbers 5.5-fold (Fig.
4A, second column). Treatment with LX519290, however, reduced
the level of IL-4 to ~10% (Fig.
4A, third column) which was a dramatic decrease compared to
that achieved with Montelukast as a positive control. We also
hypothesized that the level of IL-13 would also decrease in the
LX519290-treated group. We were able to demonstrate that IL-13
levels were reduced by 91.6% in the LX519290-treated mice
challenged with OVA (Fig. 4B).
This effect was also observed in lung tissues from LX519290-treated
mice (data not shown). To further confirm whether IgE levels in the
serum were affected by LX519290 treatment, we performed an ELISA.
IgE concentrations in the serum increased in the OVA-treated mice
(242±68 ng/ml), but in the mice treated with LX519290, serum IL-4
levels were sharply reduced to 145±21 ng/ml (Fig. 4C).
Analysis of gene expression by
RT-PCR
We examined whether mRNA levels of CD4+,
CD8+, the Th1-type cytokines IFN-γ and TNF-β, and
Th2-type cytokine IL-10 are altered in mouse primary spleen cells
by treatment with LX519290 for 24 h. For this, we isolated mouse
spleen cells, treated them with LX519290, and compared the gene
expression profiles to those of the control. At a concentration of
30 μg/ml, LX519290 did not significantly change CD4+ and
CD8+ mRNA expression levels (data not shown).
Western blot analysis of signaling
molecules
We next examined whether LX519290 affects the
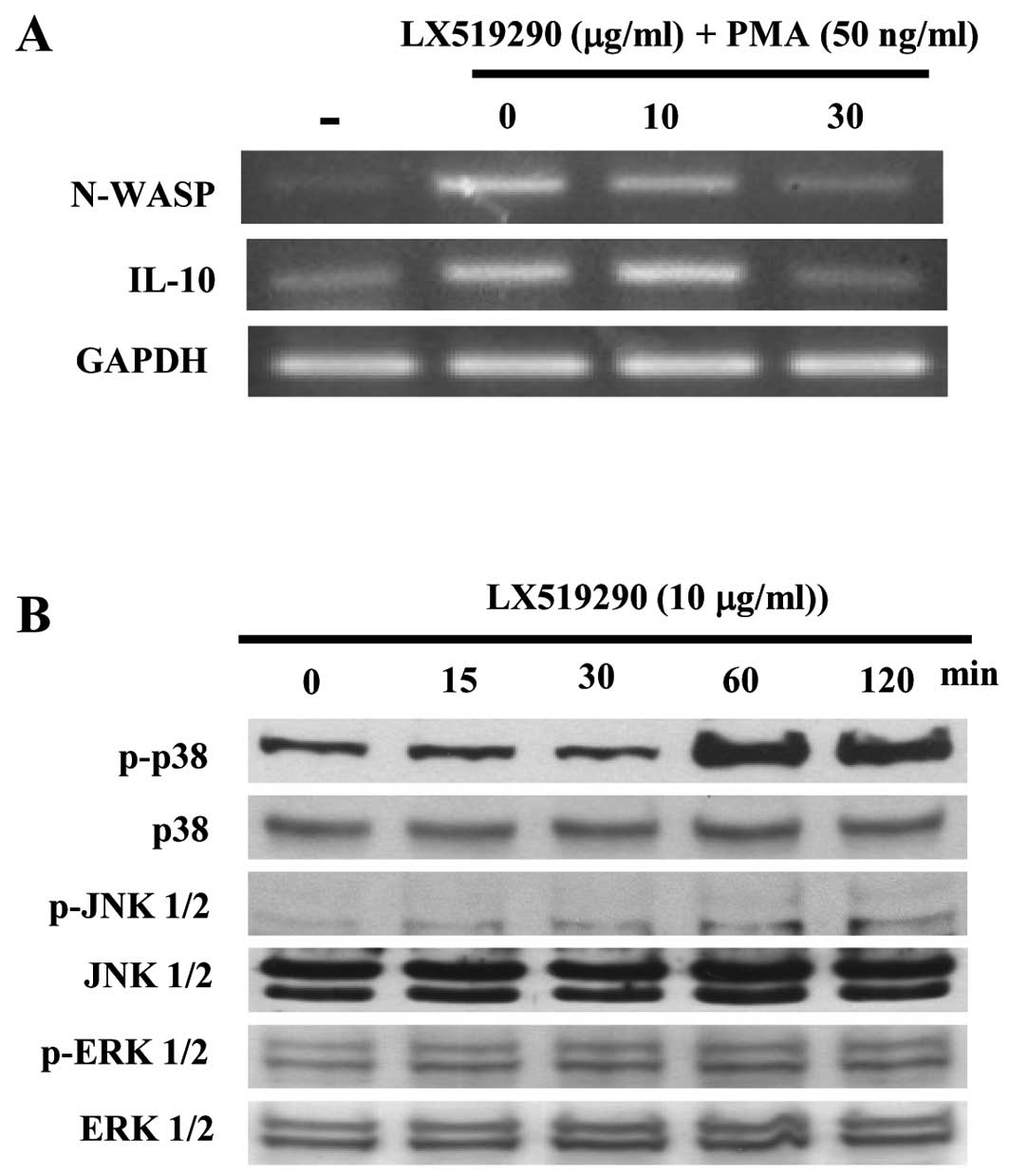
expression of cytokines and signaling molecules. As shown in
Fig. 5A, IFN-γ expression sharply
increased in a concentration-dependent manner while IL-10
expression dramatically decreased following treatment with up to 30
μg/ml of LX519290 (Fig. 5A).
Notably, N-WASP expression in mouse spleen cells was inhibited by
LX519290 treatment (Fig. 5A). The
level of p38 phosphorylation in CCRF-CEM cells significantly
increased after 60 and 120 min of LX519290 treatment whereas the
levels of phospho- as well as non-phospho-JNK and -ERK were
unchanged (Fig. 5B).
Discussion
Research has shown that various antioxidants help
prevent DNA damage, mutagenesis, carcinogenesis and the growth of
pathogenic bacteria (20).
Antioxidants mitigate many inflammatory events to permit the
healing of tissues damaged by free radicals, and are often
associated with the prevention of free radical production in
biological systems (21). It has
been estimated that all cells in our body produce many radicals.
Ubiquitous scavenging enzymes including catalase, superoxide
dismutase and xanthine oxidase may successfully clear surplus
radicals in tissues (22).
Dietary supplements including vitamins C and E can prevent damage
caused by free radicals (23).
Thus, the antioxidant capacity is widely used as a parameter to
characterized beneficial foods or medicinal compounds that possess
bioactive properties which help regulate body homeostasis (23). It is therefore important to
evaluate the molecular mechanisms underlying the inflammatory
process and oxidative stress. This is needed to develop novel
anti-asthmatic therapies (24).
Moreover, the demand for novel agent(s) that can prevent or treat
allergic asthma is increasing. Allergen-induced airway diseases are
caused by allergen-specific Th2-mediated cytokines (24). Production of these factors can be
induced by airway inflammation, Th2 cell activation, inflammatory
cells, and Ig isotype switching involving T and B cells. Certain
agent(s) can reduce asthmatic symptoms by affecting the production
and secretion of various Th2-type cytokines such as IL-4, IL-5,
IL-13 and TNF-α (25,26). In our study, we examined whether
LX519290 administration affects cytokine expression in an in
vivo model. It was evident that LX519290 decreased IL-10 mRNA
expression whereas IFN-γ mRNA expression levels were increased
(Figs. 4 and 5).
It has been suggested that onset of the asthmatic
response is controlled by CD4+ T cells which produce
Th2-type cytokines, such as IL-3, IL-4, IL-5, IL-10 and granulocyte
macrophage-colony stimulatory factor (GM-CSF). IL-4 and IL-13 are
thought to be most closely associated with allergic asthma although
IL-17E, IL-23, IL-31, IL-33 and IL-35 are potential initiators of
asthmatic events (27). IL-13 is
involved in the growth, differentiation, and activation of
eosinophils, which are effector cells of the asthmatic response.
IL-4 stimulates the switching of B cell isotypes to promote the
production of IgE (28). IL-4
also plays a crucial role in the differentiation of Th1 into Th2
lymphocytes in vitro and in vivo. A mutant mouse
model of asthma indicated that IL-4 and IL-13 are essential for
activating allergen-induced pulmonary eosinophils (29).
As expected, LX519290 reduced IL-4 and IL-13
cytokine expression in mice, but these changes in saline-challenged
mice were not significant compared to control animals. After
exposure to OVA, serum obtained from vehicle-treated mice contained
significantly increased amounts of IL-4 and IL-13 in conjunction
with eosinophil infiltration in the lung. Compared to the
corresponding results in saline-challenged mice, the number of
total leukocytes from the OVA-challenged animals treated with
LX519290 (1 mg/kg) significantly decreased compared with that of
the OVA-challenged lung tissues (Fig.
3). When we observed cells positive for CD4+,
CD8+ and IL-17E by immunostaining, we found that there
were a significant decrease in these parameters compared to lung
tissues from OVA-challenged mice (Fig. 3).
The Wiskott-Aldrich syndrome protein (WASP) family
of proteins activates the ARF2/3 complex in response to signals
that induce cell migration (30).
In the present study, LX519290 induced changes in cell morphology,
and inhibited N-WASP and ARF3 mRNA expression when spleen cells
were treated with PMA (Fig. 5A
and data not shown). Elevated numbers of activated CD4+
T cells, mast cells, and eosinophils, in both bronchial mucosa and
bronchoalveolar lavage (BAL) fluid are classic features of asthma
and related symptoms (31). These
inflammatory cells may release cytokines that have the potential to
augment cell-mediated immune reactions, and induce tissue damage
and dysfunction. In the present study, we evaluated a unique
approach for treating asthma by obtaining a derivative of L-allo
threonine. To do this, we performed a high throughput
screening-compatible assay to measure antioxidant activity along
with a T-bet promoter assay. The results showed that LX519290
possessed strong antioxidant activity. There is evidence of ERK1/2
and p38 phosphorylation with the development of asthmatic symptoms
in human patients (19). In our
study, we did not observe a similar phenomenon in mice following
LX519290 treatment. Instead, we found that p38 phosphorylation was
dramatically increased in CCRF-CEM cells, which are derived from T
cells originating from lymphoblasts. It is assumed that CCRF-CEM
cells act similar to cells of Th1 lineage since the cells showed a
strong IL-2 production following PMA treatment (32). This finding suggests that LX519290
affects the activation of Th1 cell populations. It is possible that
the number of Th2 cells in the general cell population is normally
decreased whereas that of Th1 cells is increased during the onset
of asthmatic inflammation.
In conclusion, this is the first report
demonstrating that LX519290 has anti-asthmatic effects in
vitro and in vivo by modulating IL-4 and IL-13
expression. These activities were confirmed by H&E staining,
ELISA, immunohistochemistry, and IgE activity assays. If the
anti-asthmatic activity of LX519290 could be manipulated by
controlling asthma-related molecular expression, this may be useful
for approaches in preventive and curative medicine. Further
investigations should be conducted to study the homeostasis between
Th1 and Th2 cell balance in lung tissue; in that there is no drug
that can effectively cure asthma and/or related disorders.
Acknowledgements
The present study was supported by a grant from the
Regional Innovation Project, Ministry of Industry and Commerce,
Republic of Korea (S.H.L.).
References
|
1
|
Holgate ST: The sentinel role of the
airway epithelium in asthma pathogenesis. Immunol Rev. 242:205–219.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
von Mutius E: Gene-environment
interactions in asthma. J Allergy Clin Immunol. 123:3–11.
2009.PubMed/NCBI
|
|
3
|
Walsh GM: Emerging drugs for asthma.
Expert Opin Emerg Drugs. 13:643–653. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chiaramonte MG, Mentink-Kane M, Jacobson
BA, Cheever AW, Whitters MJ, Goad ME, Wong A, Collins M, Donaldson
DD, Grusby MJ and Wynn TA: Regulation and function of the
interleukin 13 receptor alpha 2 during a T helper cell type
2-dominant immune response. J Exp Med. 197:687–701. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
López-Torres M, Gil P and Barja de Quiroga
G: Effect of hyperoxia acclimation on catalase and glutathione
peroxidase activities and in vivo peroxidation products in various
tissues of the frog Rana ridibunda perezi. J Exp Zool.
248:7–18. 1998.PubMed/NCBI
|
|
6
|
Møller S, Iversen JS, Krag A, Bie P, Kjaer
A and Bendtsen F: Reduced baroreflex sensitivity and pulmonary
dysfunction in alcoholic cirrhosis: effect of hyperoxia. Am J
Physiol Gastrointest Liver Physiol. 299:G784–G790. 2010.PubMed/NCBI
|
|
7
|
Olin AC, Ljungkvist G, Bake B, Hagberg S,
Henriksson L and Torén K: Exhaled nitric oxide among pulpmill
workers reporting gassing incidents involving ozone and chlorine
dioxide. Eur Respir J. 14:828–831. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Moraes TJ, Zurawska JH and Downey GP:
Neutrophil granule contents in the pathogenesis of lung injury.
Curr Opin Hematol. 13:21–27. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim SW, Lee SH, Heo JC, Lee EJ and Kim DM:
Pharmaceutical and functional food composition for treating,
preventing or improving respiratory diseases comprising amino
acid-based derivatives. Korea Patent Registration # 10-1076769.
1–17. November 19–2011.
|
|
10
|
Baker DH, Webel DM and Fernandez SR:
D-allo threonine has no growth promoting efficacy for chicks. Poult
Sci. 77:1397–1399. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao H, Hamase K, Morikawa A, Qiu Z and
Zaitsu K: Determination of D- and L-enantiomers of threonine and
allo-threonine in mammals using two-step high-performance liquid
chromatography. J Chromatogr B Analyt Technol Biomed Life Sci.
810:245–250. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nakajima M, Inukai M, Haneishi T, Terahara
A, Arai M, Kinoshita T and Tamura C: Globomycin, a new peptide
antibiotic with spheroplast-forming activity. III Structural
determination of globomycin. J Antibiot. 31:426–432. 1978.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Makart S, Bechtold M and Panke S:
Model-based characterization of an amino acid racemase from
Pseudomonas putida DSM 3263 for application in
medium-constrained continuous processes. J Biotechnol. 130:402–410.
2007.
|
|
14
|
Heo JC, Son HU, Kim SL and Lee SH: A
derivative of L-allo threonine alleviates
2,4-dinitrofluorobenzene-induced atopic dermatitis indications.
Biosci Biotechnol Biochem. 76:2012–2015. 2012.PubMed/NCBI
|
|
15
|
Zimmermann M: Ethical guidelines for
investigations of experimental pain in conscious animals. Pain.
16:109–110. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Heo JC, Rho JR, Kim TH, Kim SY and Lee SH:
An aqueous extract of green tea Camellia sinensis increase
expression of Th-1 cell-specific anti-asthmatic markers. Int J Mol
Med. 22:763–768. 2008.PubMed/NCBI
|
|
17
|
Heo JC, Woo SU, Kweon MA, Park JY, Lee HK,
Son M, Rho JR and Lee SH: Aqueous extract of the Helianthus
annuus seed alleviates asthmatic symptoms in vivo. Int J
Mol Med. 21:57–61. 2008.
|
|
18
|
Heo JC, Park CH, Lee HJ, Kim SO, Kim TH
and Lee SH: Amelioration of asthmatic inflammation by an aqueous
extract of Spinacia oleracea Linn. Int J Mol Med.
25:409–415. 2010.PubMed/NCBI
|
|
19
|
Liu W, Liang Q, Balzar S, Wenzel S, Gorska
M and Alam R: Cell-specific activation profile of extracellular
signal regulated kinase 1/2, Jun N-terminal kinase, and p38
mitogen-activated protein kinases in asthmatic airways. J Allergy
Clin Immunol. 121:893–902. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Valko M, Izakovic M, Mazur M, Rhodes CJ
and Telser J: Role of oxygen radicals in DNA damage and cancer
incidence. Mol Cell Biochem. 266:37–56. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Korkina LG, Mikhalśhik E, Suprun MV,
Pastore S and Dal Toso R: Molecular mechanisms underlying wound
healing and anti-inflammatory properties of naturally occurring
biotechnologically produced phenylpropanoid glycosides. Cell Mol
Biol. 53:84–91. 2007.
|
|
22
|
Fang YZ, Yang S and Wu G: Free radicals,
antioxidants, and nutrition. Nutrition. 18:872–879. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dut R, Dizdar EA, Birben E, Sackesen C,
Soyer OU, Besler T and Kalayci O: Oxidative stress and its
determinants in the airways of children with asthma. Allergy.
63:1605–1609. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Russo C, Arcidiacono G and Polosa R:
Adenosine receptors: promising targets for the development of novel
therapeutics and diagnostics for asthma. Fundam Clin Pharmacol.
20:9–19. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li L, Xia Y, Nguyen A, Lai YH, Feng L,
Mosmann TR and Lo D: Effects of Th2 cytokines on chemokine
expression in the lung: IL-13 potently induces eotaxin expression
by airway epithelial cells. J Immunol. 162:2477–2487.
1999.PubMed/NCBI
|
|
26
|
Huang CH, Loo EX, Kuo IC, Soh GH, Goh DL,
Lee BW and Chua KY: Airway inflammation and IgE production induced
by dust mite allergen-specific memory/effector Th2 cell line can be
effectively attenuated by IL-35. J Immunol. 187:462–471. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Whitehead GS, Wilson RH, Nakano K, Burch
LH, Nakano H and Cook DN: IL-35 production by inducible
costimulator (ICOS)-positive regulatory T cells reverses
established IL-17-dependent allergic airways disease. J Allergy
Clin Immunol. 129:207–215. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu Z, MacPhee IA and Oliveira DB: Reactive
oxygen species in the initiation of IL-4 driven autoimmunity as a
potential therapeutic target. Curr Pharm Des. 10:899–913. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Munitz A, Brandt EB, Mingler M, Finkelman
FD and Rothenberg ME: Distinct roles for IL-13 and IL-4 via IL-13
receptor alpha1 and the type II IL-4 receptor in asthma
pathogenesis. Proc Natl Acad Sci USA. 105:7240–7245. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen JL, Lacomis L, Erdjument-Bromage H,
Tempst P and Stamnes M: Cytosol-derived proteins are sufficient for
Arp2/3 recruitment and ARF/coatomer-dependent actin polymerization
on Golgi membranes. FEBS Lett. 566:281–286. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Doherty TA, Soroosh P, Broide DH and Croft
M: CD4+ cells are required for chronic eosinophilic lung
inflammation but not airway remodeling. Am J Physiol Lung Cell Mol
Physiol. 296:L229–L235. 2009.PubMed/NCBI
|
|
32
|
Asai K, Moriwaki S and Maeda-Yamamoto M:
Enhancement of interleukin-2 production in CCRF-CEM, human T-cell
leukemia, by tea flavonols. Jpn Agric Res Q. 39:51–55. 2005.
View Article : Google Scholar
|