Introduction
Human parathyroid hormone (PTH), an 84-amino acid
peptide (PTH 1-84), is a principal hormone that regulates bone
remodeling via its actions on both bone formation and resorption.
Osteoblast lineage cells are the target cells for the effects of
PTH on bone tissue. When administered intermittently by daily
subcutaneous injection, recombinant human PTH1-34 (teriparatide)
increases bone mineral density and reduces the incidence of
skeletal fractures in osteoporosis (‘anabolic’ effects of PTH)
(1). In contrast, the ‘catabolic’
effects result from pathological conditions in which the
parathyroid glands continuously secrete an excess of hormone. Such
continuous secretion of PTH induces bone resorption by stimulation
of receptor activator of nuclear factor (NF)-κB ligand (RANKL) and
inhibition of osteoprotegerin (OPG) in osteoblasts (2). The effects of PTH on osteoblasts are
known to be mediated via binding of PTH to the seven
membrane-spanning G protein-coupled receptor, PTH receptor 1
(PTHR1), and activation of both the cyclic AMP (cAMP)-dependent
protein kinase A (PKA) pathway and the phosphoinositide-dependent
protein kinase C (PKC) pathway (3). Intermittent PTH administration in
mice has been shown to increase osteoblast numbers by stimulating
survival signaling, preventing their apoptosis, and thus increasing
bone formation (4). Intermittent
PTH also affects the synthesis of many osteogenic growth factors
and cytokines, as well as that of their antagonists (1). Insulin-like growth factor (IGF)-1
and fibroblast growth factor (FGF)-2 may contribute to the anabolic
effect of intermittent PTH in increasing osteoblast numbers
(1). Intermittent PTH also
promotes osteoblast differentiation, activating Wnt signaling in
osteoblasts, and inhibiting the Wnt antagonist, sclerostin, in
osteocytes (5). However, the
molecular and cellular mechanisms underlying the anabolic action of
PTH are not completely understood and remain controversial.
The Wiskott-Aldrich syndrome protein (WASP) is a
cytoplasmic protein implicated in regulating the actin cytoskeleton
and cytoskeletal reorganization involved in cellular functions such
as migration, phagocytosis and immune synapse formation (6). WASP family protein member 2 (Wasf2;
also known as WASP family verprolin-homologous protein 2; WAVE2) is
one of the WASP family proteins which is ubiquitously expressed in
mammals (7). Yamazaki et
al (8) demonstrated that
Wasf2 is crucial for Rac-induced membrane ruffling, which is
important in cell motility. Wasf2-deficient mice survived only
until embryonic day 12.5 and displayed growth retardation and
certain morphological defects (9). Since remodeling of the cytoskeleton
is involved in mechanotransduction, Wasf2 is implicated in this
process. To date, however, little is known concerning the
regulation and molecular basis of action of Wasf2 in
osteoblasts.
In this study, we investigated the regulation of
gene expression following intermittent PTH administration in
osteoblastic MC3T3-E1 cells. Here, we showed that intermittent PTH
regulated Wasf2 expression and that the Wnt inhibitor, IWP-2, or
the protein kinase C inhibitor, Go6976, inhibited this
downregulation, indicating that Wasf2 is a novel target of
intermittent PTH administration via the Wnt and PKC signaling
pathways.
Materials and methods
Cell cultures
Cells of the mouse cell line MC3T3-E1 were cultured
in α-MEM containing 100 μg/ml kanamycin (Meiji, Tokyo, Japan) and
supplemented with 10% fetal bovine serum (FBS; SAFC Bioscience,
Inc., Lenexa, KS, USA) at 37°C in 100-mm cell culture dishes
(Corning Inc., Corning, NY, USA) in a humidified atmosphere of 5%
CO2 in air.
Compounds and reagents
PTH1-34, PTH3-34, Go6976, IWR-1 and dorsomorphin
were purchased from Sigma-Aldrich (St. Louis, MO, USA). IWP-2 was
obtained from Stemgent (San Diego, CA, USA). The Wasf2 siRNA and
silencer negative control scramble siRNA (no. 4611; Ambion) were
purchased from Applied Biosystems (Foster City, CA, USA).
Recombinant human bone morphogenetic protein (BMP) 2 was kindly
supplied by Astellas Pharma Inc. (Tokyo, Japan).
PTH administration
MC3T3-E1 cells were plated at 1×105
cells/cm2. After 24 h, the cells were cultured in the
presence of 10−8 M PTH or in control medium for 1, 6
(intermittent) or 48 h (continuous) within each 48-h incubation
cycle. These cycles were repeated three times. The cells were
harvested after 6 days.
Microarray analysis
Total RNA was extracted from the cells using the
ReliaPrep™ RNA Cell Miniprep System (Promega), and quality was
evaluated by Bioanalyzer (Agilent Technologies, Palo Alto, CA,
USA). Total RNA was labeled with Cy3. Samples were hybridized using
a SurePrint G3 Mouse GE microarray kit (Agilent Technologies)
according to the manufacturer’s protocol. Arrays were scanned with
a G2539A Microarray Scanner System. Data were analyzed using
GeneSpringer GX software (both from Agilent Technologies).
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was extracted from the cells at the
indicated time points using Isogen (Nippongene, Toyama, Japan), and
RT-PCR was performed as previously described (10). The primer sequences for each gene
were as follows: Wasf2, 5-TTGCCAAAGCCCTCATAAAC-3 (forward) and
5-AGCCAGGGTACCATCAACAG-3 (reverse); OPG,
5-TCCTGGCACCTACCTAAAACAGCA-3 (forward) and
5-CTACACTCTCGGCATTCACTTTGG-3 (reverse); RANKL,
5-GTCACTCTGTCCTCTTGGTAC-3 (forward) and 5-TGAAACCCCAAAGTACGTCG-3
(reverse); osterix, 5-GGGTTAAGGGGAGCAAAGTCAGAT-3 (forward) and
5-CTGGGGAAAGGAGGCACAAAGAAG-3 (reverse); glyceraldehyde-3-phosphate
dehydrogenase (GAPDH), 5′-TCCACCACCCTGTTGCTGTA-3′ (forward) and
5′-ACCACAGTCCATGCCATCAC-3′ (reverse).
To account for any difference in the amount of RNA,
GAPDH was chosen as our endogenous control and amplified using the
primers described above. The amplification products were
electrophoresed on 2% agarose gels.
Quantification of gene expression by
quantitative reverse transcription-polymerase chain reaction
(qRT-PCR)
qRT-PCR was performed using Assay-on-Demand™ TaqMan
probes (Applied Biosystems) and the StepOne® real-time
PCR system. The relative level of gene expression was quantified
using the comparative CT method with GAPDH as the
endogenous control.
Transfection of small interfering RNA
(siRNA)
The Wasf2 siRNA sequences for each gene were as
follows: 5′-CGUAAAAUCAAGACACGCAtt-3′ (sense) and
5′-UGCGUGUCUUGAUUUUACGtg-3′ (antisense). MC3T3-E1 cells were plated
at 1×105 cells/cm2. After 24 h, the cells
were transfected with 10 nM Wasf2 siRNA or scramble siRNA (no.
4611; Ambion) complexed with Lipofectamine RNAiMAX (Invitrogen,
Carlsbad, CA, USA). After the cells were cultured for a further 5
days, total RNA was extracted from the cells.
Statistical analysis
All experiments were repeated three to four times
and representative results are shown. The data are presented as the
mean ± standard deviation, and were analyzed by the Student’s
t-test. Values of P<0.05 were considered to indicate
statistically significant results.
Results and Discussion
Regulation of gene expression by
intermittent PTH administration
PTH has both anabolic and catabolic effects on bone
depending on the mode of administration (11). Several molecules have previously
been identified as mediators of the effects of intermittent PTH. In
this study, we used the mouse osteoblastic cell line MC3T3-E1 to
evaluate the anabolic effect of intermittent PTH administration
using an in vitro model. The cells were cultured in the
presence of PTH or in control medium for 1, 6 (intermittent PTH
administration) or 48 h (continuous PTH administration) within each
48-h incubation cycle, and these cycles were carried out three
times. To identify candidate intermittent PTH-responsive genes, we
performed DNA microarray analyses to study the effect of
intermittent PTH on gene expression. We used Agilent mouse DNA
arrays that contain oligonucleotide probe sets representing 55,684
genetic elements, all the characterized mouse genes. Comparison of
the intermittent (6 h) PTH1-34 treatment to the continuous PTH1-34
treatment revealed that 1% or less of the genes changed >2-fold
(data not shown). Among these genes, the Wasf2 gene was identified
as a candidate intermittent PTH-responsive gene. To confirm this
observation, RT-PCR and qRT-PCR analyses were performed to examine
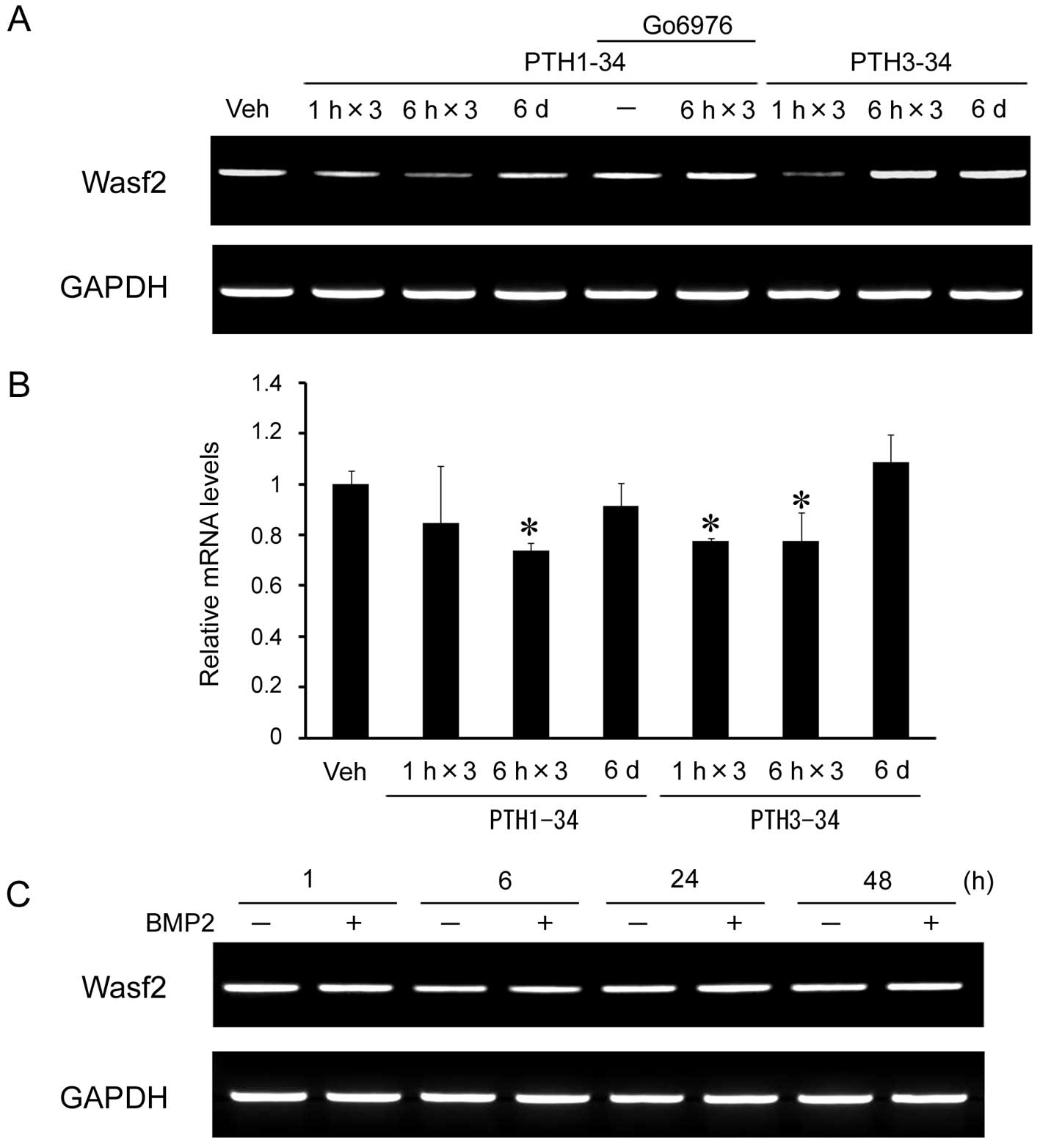
Wasf2 mRNA expression levels in these cells. The expression level
of Wasf2 mRNA declined to ~80% following intermittent PTH1-34 (6 h)
as compared with continuous PTH1-34 (48 h) (Fig. 1A and B).
PTH activates both the adenylate cyclase/PKA and the
plasma membrane phospholipase C/PKC pathways and multiple
mechanisms are involved in the anabolic effect induced by
intermittent PTH treatment (12).
Using PTH3-34 which lacks the PKA-activating domain, Wasf2
expression was also downregulated by intermittent treatment (1 and
6 h) (Fig. 1A and B). Treatment
with intermittent PTH1-34 in combination with the PKC inhibitor,
Go6976, which was added to the culture before treatment, inhibited
the downregulation of Wasf2 mRNA expression. These results suggest
that the response of Wasf2 expression to intermittent PTH
administration is mediated by the PKC signaling pathway. Since BMPs
are known to regulate the differentiation and function of
osteoblasts, we analyzed the level of Wasf2 expression following
administration of BMP2. However, BMP2 had no effect on Wasf2 mRNA
expression (Fig. 1C).
Effects of the Wnt and BMP/Smad
inhibitors on downregulation of Wasf2 mRNA expression by
intermittent PTH treatment
Previously, in vivo and in vitro
studies have indicated that PTH-regulated anti-apoptotic signaling
promotes osteoblastic differentiation and increases production
and/or activation of osteogenic growth factors such as IGF-1 or BMP
(1,13). Recent studies in vitro have
shown that Wnt signaling can mediate the actions of G-protein
coupling receptors in many tissues (14–16) and have explored links between PTH
and Wnt signaling pathways. Continuous PTH administration to
MC3T3-E1 cells was found to increase the stabilized β-catenin level
in those cells (17). PTH
receptor signaling has also been shown to result in binding of the
receptor to Lrp6, phosphorylation of Lrp6 and stabilization of
β-catenin in osteoblasts (18).
Most importantly, new data show that intermittent PTH
administration in Lrp5−/− and Lrp5+/+ mice
show equal enhancement of skeletal mass (19), indicating that the anabolic
effects due to PTH are independent of Lrp5 and thus of canonical
Wnt signaling. Bergenstock and Partridge (20) provide evidence for a link between
intermittent PTH actions and non-canonical Wnt signaling in bone.
Despite all these observations, little is known about the
contribution of Wnt signaling to the actions of intermittent
PTH.
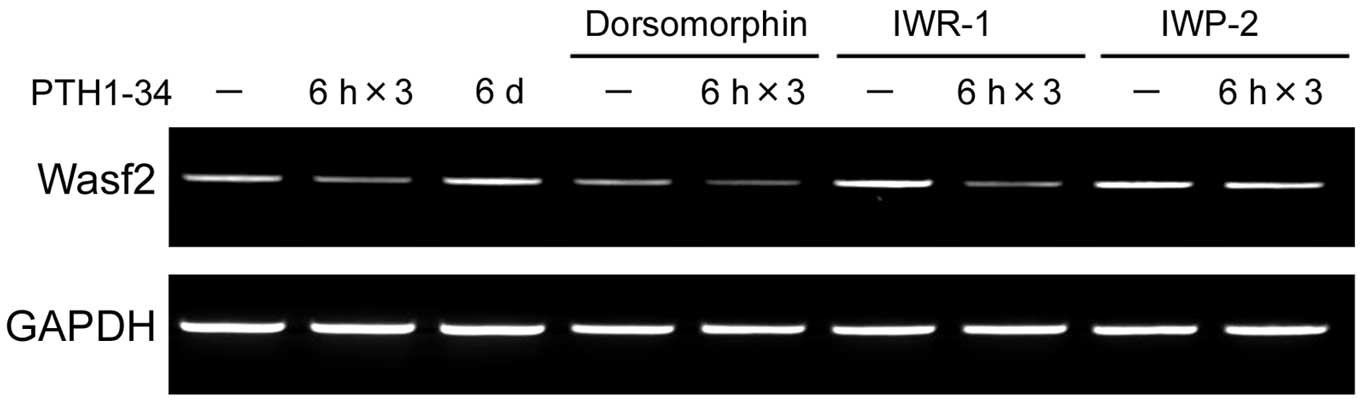
Recently, a novel class of small-molecule inhibitors
that blocks Wnt signaling was identified (21). IWR-1 promotes β-catenin
phosphorylation by stabilizing Axin-scaffolded destruction
complexes; IWP-2 prevents palmitylation of Wnt proteins by
porcupine, thereby blocking Wnt secretion and activity. Therefore,
we examined the effects of these inhibitors on PTH administration.
Although IWP-2 inhibited downregulation of Wasf2 mRNA expression by
intermittent PTH1-34 in MC3T3-E1 cells, IWR-1 did not inhibit its
regulation (Fig. 2). The
non-canonical Wnt pathway functions in a β-catenin-independent
manner and signals are transduced through Fz family receptors and a
co-receptor, such as Ror2 or RYK, but not Lrp-5/6 (22). Our data suggest that the Wasf2
response following intermittent PTH administration may mediate the
non-canonical Wnt signaling pathway. It is also consistent with
recent studies of PTH1R showing that Lrp6 is not required for
intermittent PTH action. In contrast, the BMP/Smad inhibitor
dorsomorphin was unable to regulate the Wasf2 response to PTH
(Fig. 2), indicating that the
BMP/Smad signaling pathway could not mediate regulation of this
gene by intermittent PTH, consistent with the results shown in
Fig. 1C.
There are three types of pathways involved in
non-canonical Wnt signaling. Among them, the Wnt/Ca2+
pathway, in which non-canonical Wnts such as Wnt-5a bind to the Fz
receptor and to a co-receptor such as Knypek or Ror2 and stimulate
heterotrimeric G proteins, increases intracellular calcium levels,
decreases cyclin GMP (cGMP) levels, and then activates PKC or
calcium/calmodulin-dependent protein kinase II (CamKII) to induce
nuclear factor of activated T cells (NFAT) and other transcription
factors (23). Takada et
al (24) reported that the
non-canonical Wnt pathway, through CamKII, transcriptionally
induces Runx2 and represses peroxisome proliferator-activated
receptor-γ (PPAR-γ) transactivation. Taken together, these findings
suggest that this pathway may be involved in the bone formation
induced by intermittent PTH administration. Thus, the
Wnt/Ca2+ pathway induced through Wasf2 expression may
regulate cell adhesion and cell movement in bone tissue.
Effects of Wasf2 knockdown on gene
expression in MC3T3-E1 cells
Wasf2 is ubiquitously expressed in mammalian cells,
plays roles in lamellipodia formation and cell-cell contact
organization under a Rho family small GTPase Rac1 signal and is the
most mechanosensitive cytoskeleton-related gene (25). Based on the results of the present
study, intermittent PTH administration regulates Wasf2 expression.
Until now, little was known of the molecular basis of action of
Wasf2 on osteoblasts. To investigate the function of Wasf2 in
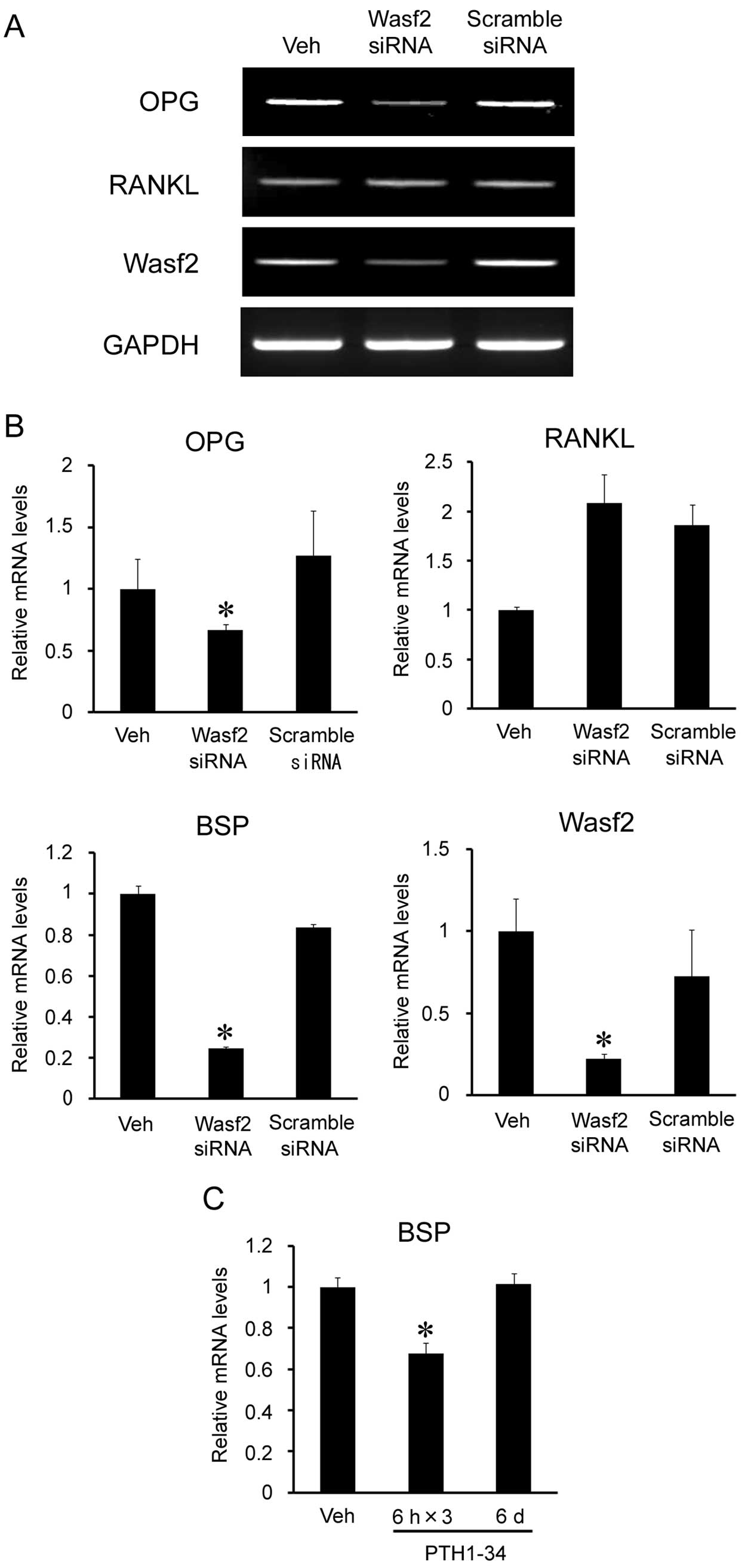
osteoblasts, we transfected MC3T3-E1 cells with Wasf2 siRNA.
Transfection of Wasf2 siRNA reduced Wasf2 mRNA expression (Fig. 3A). In these cells, the expression
levels of bone sialoprotein (BSP) and OPG mRNA were reduced by 20
and 60%, respectively (Fig. 3B).
Intermittent PTH1-34 administration also reduced BSP expression
(Fig. 3C), suggesting that
downregulation of BSP expression by intermittent PTH may be
mediated by Wasf2.
BSP is a phosphorylated bone matrix glycoprotein and
is an Arg-Gly-Asp (RGD)-containing protein which is mainly produced
by mature osteoblasts and osteocytes. BSP is thought to function as
an adaptor molecule between cells and bone mineral and may also be
important in the differentiation, tissue organization and
remodeling of bone (26). BSP is
necessary for promoting cell adhesions through interaction with
integrins (27,28). In addition, the RGD motif in BSP
can mediate both cell attachment and signaling activities (26). According to our experiments, cell
adhesion and the cytoskeleton-related gene Wasf2 could together
regulate expression of BSP which may be involved in the regulation
of cell attachment. It was previously reported that BSP also
modulates the activity of osteoclasts through the vitronectin
receptor which is present on the surfaces of osteoclasts (29), and it is also reported that the
Wnt/Ca2+ pathway can affect cell adhesion and cell
movement during gastrulation (30,31). The present study supports the
hypothesis that intermittent PTH administration decreases Wasf2
mRNA expression, and then regulates BSP expression by altering cell
attachment and/or signaling activities, and thus regulates the
osteoclastic activities in bone tissue.
References
|
1
|
Jilka RL: Molecular and cellular
mechanisms of the anabolic effect of intermittent PTH. Bone.
40:1434–1446. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ma YL, Cain RL, Halladay DL, et al:
Catabolic effects of continuous human PTH (1-38) in vivo is
associated with sustained stimulation of RANKL and inhibition of
osteoprotegerin and gene-associated bone formation. Endocrinology.
142:4047–4054. 2001.PubMed/NCBI
|
|
3
|
Kronenberg HM, Lanske B, Kovacs CS, et al:
Functional analysis of the PTH/PTHrP network of ligands and
receptors. Recent Prog Horm Res. 53:283–303. 1998.PubMed/NCBI
|
|
4
|
Jilka RL, Weinstein RS, Bellido T,
Roberson P, Parfitt AM and Manolagas SC: Increased bone formation
by prevention of osteoblast apoptosis with parathyroid hormone. J
Clin Invest. 104:439–446. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Keller H and Kneissel M: SOST is a target
gene for PTH in bone. Bone. 37:148–158. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thrasher AJ and Burns SO: WASP: a key
immunological multitasker. Nat Rev Immunol. 10:182–192. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yamashita H, Ueda K and Kioka N: WAVE2
forms a complex with PKA and is involved in PKA enhancement of
membrane protrusions. J Biol Chem. 286:3907–3914. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yamazaki D, Suetsugu S, Miki H, et al:
WAVE2 is required for directed cell migration and cardiovascular
development. Nature. 424:452–456. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yan C, Martinez-Quiles N, Eden S, et al:
WAVE2 deficiency reveals distinct roles in embryogenesis and
Rac-mediated actin-based motility. EMBO J. 22:3602–3612. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nakashima A, Katagiri T and Tamura M:
Cross-talk between Wnt and bone morphogenetic protein 2 (BMP-2)
signaling in differentiation pathway of C2C12 myoblasts. J Biol
Chem. 280:37660–37668. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Poole KE and Reeve J: Parathyroid hormone
- a bone anabolic and catabolic agent. Curr Opin Pharmacol.
5:612–617. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ishizuya T, Yokose S, Hori M, et al:
Parathyroid hormone exerts disparate effects on osteoblast
differentiation depending on exposure time in rat osteoblastic
cells. J Clin Invest. 99:2961–2970. 1997. View Article : Google Scholar
|
|
13
|
Zhang R, Edwards JR, Ko SY, et al:
Transcriptional regulation of BMP2 expression by the PTH-CREB
signaling pathway in osteoblasts. PLoS One. 6:e207802011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Castellone MD, Teramoto H, Williams BO,
Druey KM and Gutkind JS: Prostaglandin E2 promotes colon cancer
cell growth through a Gs-axin-beta-catenin signaling axis. Science.
310:1504–1510. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Goessling W, North TE, Loewer S, et al:
Genetic interaction of PGE2 and Wnt signaling regulates
developmental specification of stem cells and regeneration. Cell.
136:1136–1147. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu Z and Habener JF: Glucagon-like
peptide-1 activation of TCF7L2-dependent Wnt signaling enhances
pancreatic beta cell proliferation. J Biol Chem. 283:8723–8735.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tobimatsu T, Kaji H, Sowa H, et al:
Parathyroid hormone increases beta-catenin levels through Smad3 in
mouse osteoblastic cells. Endocrinology. 147:2583–2590. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wan M, Yang C, Li J, et al: Parathyroid
hormone signaling through low-density lipoprotein-related protein
6. Genes Dev. 22:2968–2979. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sawakami K, Robling AG, Ai M, et al: The
Wnt co-receptor LRP5 is essential for skeletal mechanotransduction
but not for the anabolic bone response to parathyroid hormone
treatment. J Biol Chem. 281:23698–23711. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bergenstock MK and Partridge NC:
Parathyroid hormone stimulation of noncanonical Wnt signaling in
bone. Ann NY Acad Sci. 1116:354–359. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen B, Dodge ME, Tang W, et al: Small
molecule-mediated disruption of Wnt-dependent signaling in tissue
regeneration and cancer. Nat Chem Biol. 5:100–107. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gordon MD and Nusse R: Wnt signaling:
multiple pathways, multiple receptors, and multiple transcription
factors. J Biol Chem. 281:22429–22433. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang HY and Malbon CC: Wnt-frizzled
signaling to G-protein-coupled effectors. Cell Mol Life Sci.
61:69–75. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Takada I, Suzawa M, Matsumoto K and Kato
S: Suppression of PPAR transactivation switches cell fate of bone
marrow stem cells from adipocytes into osteoblasts. Ann NY Acad
Sci. 1116:182–195. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Qian A, Di S, Gao X, et al: cDNA
microarray reveals the alterations of cytoskeleton-related genes in
osteoblast under high magneto-gravitational environment. Acta
Biochim Biophys Sin. 41:561–577. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ganss B, Kim RH and Sodek J: Bone
sialoprotein. Crit Rev Oral Biol Med. 10:79–98. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Somerman MJ, Fisher LW, Foster RA and Sauk
JJ: Human bone sialoprotein I and II enhance fibroblast attachment
in vitro. Calcif Tissue Int. 43:50–53. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Helfrich MH, Nesbitt SA, Dorey EL and
Horton MA: Rat osteoclasts adhere to a wide range of RGD
(Arg-Gly-Asp) peptide-containing proteins, including the bone
sialoproteins and fibronectin, via a beta 3 integrin. J Bone Miner
Res. 7:335–343. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lakkakorpi PT, Helfrich MH, Horton MA and
Väänänen HK: Spatial organization of microfilaments and vitronectin
receptor, alpha v beta 3, in osteoclasts. A study using confocal
laser scanning microscopy. J Cell Sci. 104:663–670. 1993.
|
|
30
|
Veeman MT, Axelrod JD and Moon RT: A
second canon. Functions and mechanisms of beta-catenin-independent
Wnt signaling. Dev Cell. 5:367–377. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chisholm AD: Gastrulation: Wnts signal
constriction. Curr Biol. 16:R874–R876. 2006. View Article : Google Scholar : PubMed/NCBI
|