Introduction
Acute lung injury (ALI) is a common complication
following sepsis. Despite advances in supportive care and
pharmacologic treatment, mortality from ALI remains unacceptably
high (1). Previous studies have
demonstrated that mesenchymal stem cells (MSCs) have therapeutic
effects on ALI. MSCs are able to attenuate pulmonary edema and
inflammatory factors, but enhance anti-inflammatory cytokine
(2). MSCs can be engrafted in
injured lung tissue to differentiate into epithelial cells types,
which are closed to alveolar fluid transport (3–5).
However, the mechanisms of the effects of MSCs on pulmonary
vascular function have yet to be fully elucidated.
MSCs can be obtained through different methods as
compared with other tissues, the standard adipose tissue can easily
be sampled as the source of multipotent cells, known as
adipose-derived stem cells (ADSCs). A larger number of MSCs in
adipose tissue can easily be harvested and rapidly proliferated.
Increasing evidence suggests that adipose tissue may be a suitable
source of MSCs (6).
Nitric oxide (NO) has been reported to be an
essential signaling molecule with several roles that affect a
number of physiological and pathophysiological functions, including
angiogenesis, vascular tone, and it may also lead to endothelial
tube formation (7). NO is
synthesized by a family of enzymes known as NO synthases. Among
these enzymes is endothelial nitric oxide synthase (eNOS). eNOS is
essential to endothelial cells in keeping their functions and
homeostasis. eNOS is able to modulate pulmonary arterial
hypertension (8). Moreover, the
reduced eNOS expression causes endothelial cells to be vulnerable
to apoptosis (9). Therefore, eNOS
and eNOS-derived NO are considered to play a role in the regulation
of vascular function.
We hypothesized that the roles of eNOS and
eNOS-derived NO were important targets as contributors to pulmonary
microvascular endothelial cells (PMVECs) and lung protective
effects of ADSCs during ALI.
Materials and methods
Experimental animals
In the present study, adult Sprague-Dawley (SD) rats
weighing ∼220 g were obtained from the Animal Center of Dalian
Medical University, China.
Isolation and culture of ADSCs
ADSCs were isolated from human adipose tissue as
previously reported (6). Human
adipose tissue of 15–20 g was isolated from a grossly
normal-appearing region farthest away from the cancer following
surgery for localized breast cancer. Adipose tissue was washed
extensively with sterile phosphate-buffered saline (PBS) to remove
contaminating debris, extracted blood vessels, and was cut into
small fragments of tissue (1 mm3). The small fragments
of tissue were treated with 1% collagenase I in PBS for 1 h at 37°C
with gas bath and gentle agitation. The cellular pellet was
resuspended in high glucose DMEM (Gibco, USA) supplemented with 15%
FBS (Gibco) and 1% penicillin-streptomycin. ADSCs were used for all
experiments at passages 6–12.
Isolation and culture of PMVECs
Rat PMVECs were isolated from rat lung as previously
reported (10). PMVECs were
isolated from an ∼80 g SD rat which was anesthetized and sacrificed
by bloodletting. Rat lungs were perfused with D-Hank’s solution to
whiten them from the right ventricles. Peripheral lung tissue was
isolated and cut into small fragments of tissue (1 mm3).
The small fragments of tissue were seeded in a 25-cm2
flask. Subsequently, the tissue fragments were incubated in low
glucose DMEM (Gibco) supplemented with 20% FBS and 1%
penicillin-streptomycin. PMVECs were used for experiments at
passages 5–8. The PMVECs were identified by von Willebrand factor
(vWF) and KDR (11).
Experimental design
In vitro, PMVECs were treated with 5
μg/ml lipopolysaccharide (LPS) (Sigma, USA) for 4 h at 37°C,
and then washed with sterile PBS. In the co-culture group, PMVECs
were co-cultured with ADSCs in either a standard single well or in
a Transwell (0.4 μm pore size; Costar) for 72 h. In the
control group, PMVECs were cultured without ADSCs. In vivo,
rats were randomly assigned to three groups: the sham group (n=5),
the ALI group (n=10) and the ADSC group (n=10). Sham group rats
received normal saline instead of LPS or ADSCs in the same manner
and served as control. The ALI group rats received an
intraperitoneal injection of 6 mg/kg LPS to induce ALI. The ADSC
group rats were injected with ∼5×105 ADSCs through the
caudal vein. ALI group rats received normal saline instead of
ADSCs. All animals were used to test at 7 days.
Flow cytometry
Flow cytometry for expression of a panel of surface
markers was performed using a BD Biosciences FACS machine using
standard techniques. ADSCs were harvested and washed with PBS, and
stained by PE-CD34, FITC-CD90 and PE-106 antibodies (BD
Biosciences, USA).
ADSC differentiation into vascular
endothelial cells
Human ADSCs were plated on 6-well plates at a
density of ∼1.2×104 cells/plate. Before plating, the
6-well plates were coated with human fibronectin (Millipore, USA).
Human ADSCs were supplemented with low glucose DMEM, 5% FBS, 40
ng/ml vascular endothelial growth factor (VEGF) (PeproTech, Inc.,
USA) and 1% penicillin-streptomycin for 7 days.
Immunofluorescence staining
The cells were fixed in 4% paraformaldehyde at 4°C
overnight, and permeabilized with 0.1% Triton X-100 prior to the
addition of antibodies for 15 min. Primary antibodies included vWF
(1:50 dilution; Wuhan Sanying Biotechnology, Inc., China), KDR
(1:50 dilution), eNOS (1:50 dilution) (both from Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) and were incubated for 2
h. FITC-labeled secondary antibodies were incubated for 1.5 h.
Hoechst 33258 was used to trace the nucleus and were incubated for
15 min. The immune complex was carried out using fluorescent
microscopy.
NO measurement
Culture medium was collected at 24, 48 and 72 h; the
plasma of rats was collected at 7 days. All samples were analyzed
for NO production using an NO assay kit. These analyses were
carried out in accordance with the manufacturer’s instructions
(Nanjing Jiancheng Bioengineering Institute, China).
Lung wet-to-dry ratio
A lung wet-to-dry weight ratio (W/D ratio) was
valued as the degree of pulmonary edema. Lung wet weight was
determined immediately after removal of the left lung. Lung dry
weight was determined after the lung had been dried at 60°C for 48
h, and the W/D ratio was calculated by division.
Western blot analysis
Total proteins were extracted from the rat inferior
lobe of the right lung. The proteins were loaded onto a linear
gradient polyacrylamide gel of 10% with a stacking gel of 4%
polyacrylamide, and subjected to electrically transfer onto a PVDF
membrane following electrophoresis. Membranes were blocked by
incubation with blocking buffer containing 5% skimmed powdered milk
for 1 h, followed by incubation with primary antibodies at 4°C
overnight. Primary antibodies including eNOS (1:250 dilution),
inducible nitric oxide synthase (iNOS) (1:200 dilution), β-actin
(1:400 dilution) (all from Santa Cruz Biotechnology, Inc.) were
used. After washing, the membranes were incubated with anti-IgG
horseradish peroxidase-linked secondary antibodies (1:6,000
dilution). After detecting a standard ECL, they were subjected to
image analysis using ImageJ software program.
Statistical analysis
Results are presented as the means ± SEM and
analyzed using SPSS 17.0 software. Experimental data were analyzed
using one-way analysis of variance (ANOVA) except for the
quantitative analysis of eNOS expression in PMVECs which was
performed using Student’s t-test. P<0.05 was considered to
indicate statistically significant differences.
Results
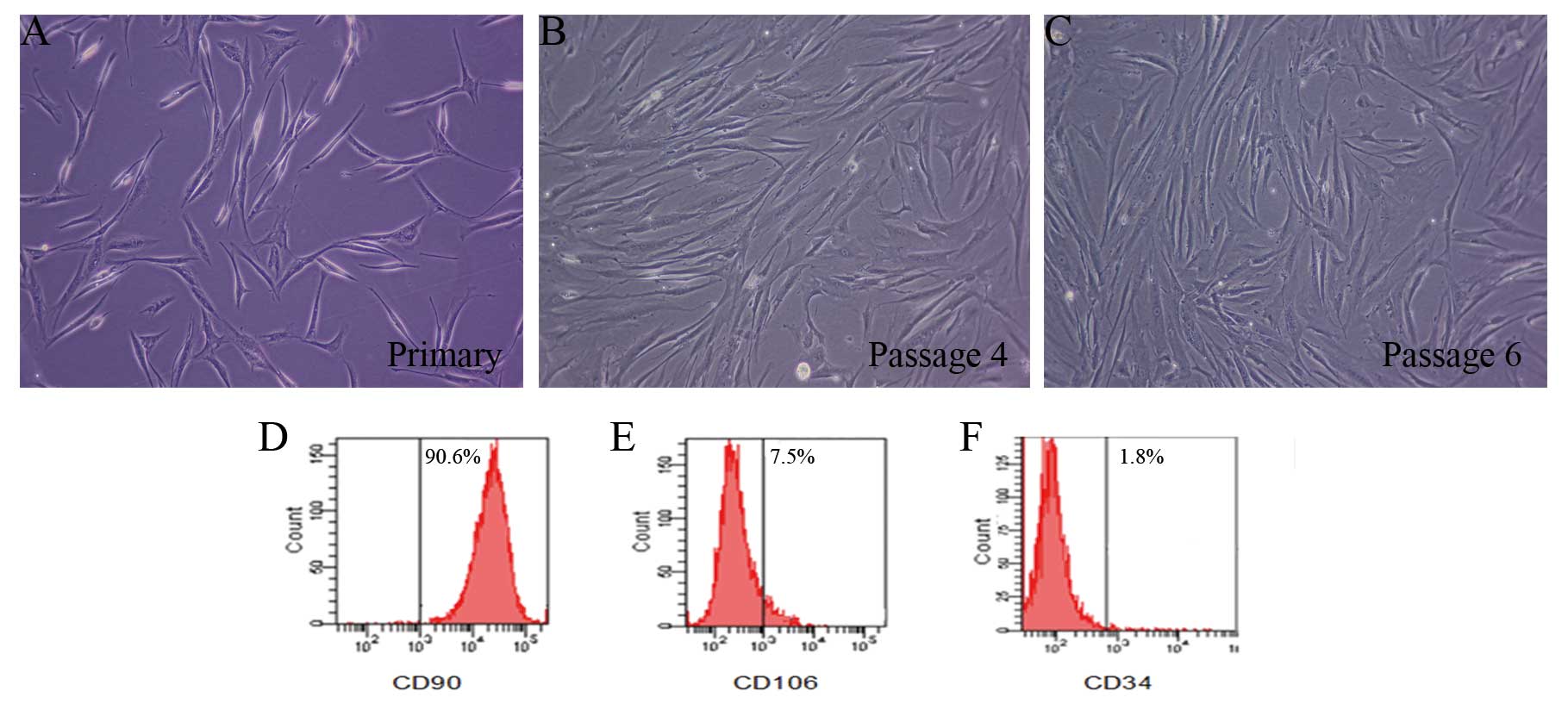
Characteristics of ADSCs in culture
The majority of cells displayed a spindle-like shape
or fibroblast-like shape (Fig.
1A–C). Flow cytometry analysis demonstrated expression of ADSC
surface markers. ADSCs were positive for expression of CD90 that
expressed ∼90.6%, but showed low expression for CD106 and CD34,
which expressed ∼7.5 and 1.8%, respectively (Fig. 1D–F).
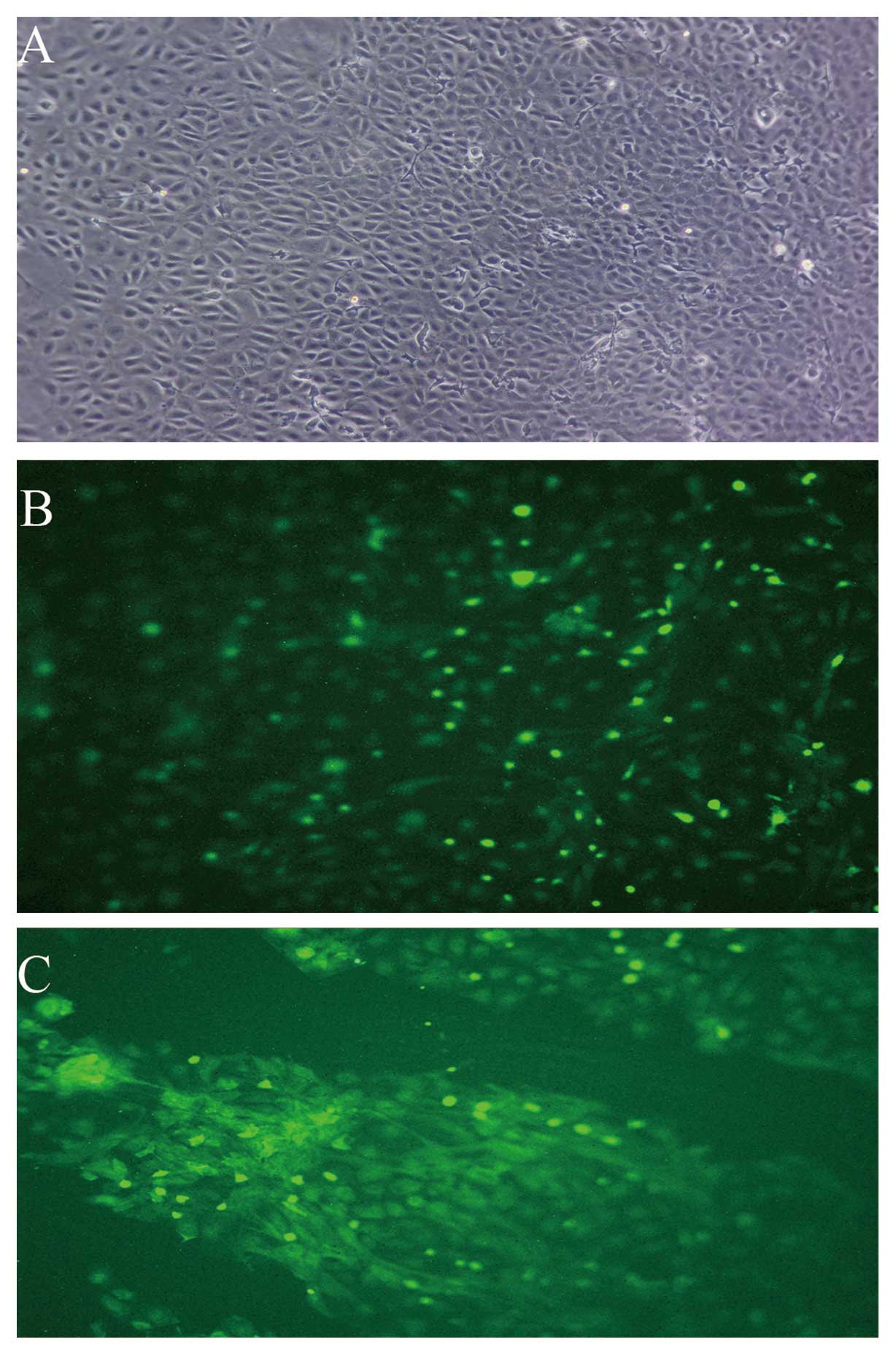
Characteristics of PMVECs in culture
The majority of cells displayed a cobblestone-like
shape (Fig. 2A).
Immunofluorescence analysis demonstrated that >95% PMVECs were
positive for expression of vWF and KDR (Fig. 2B and C).
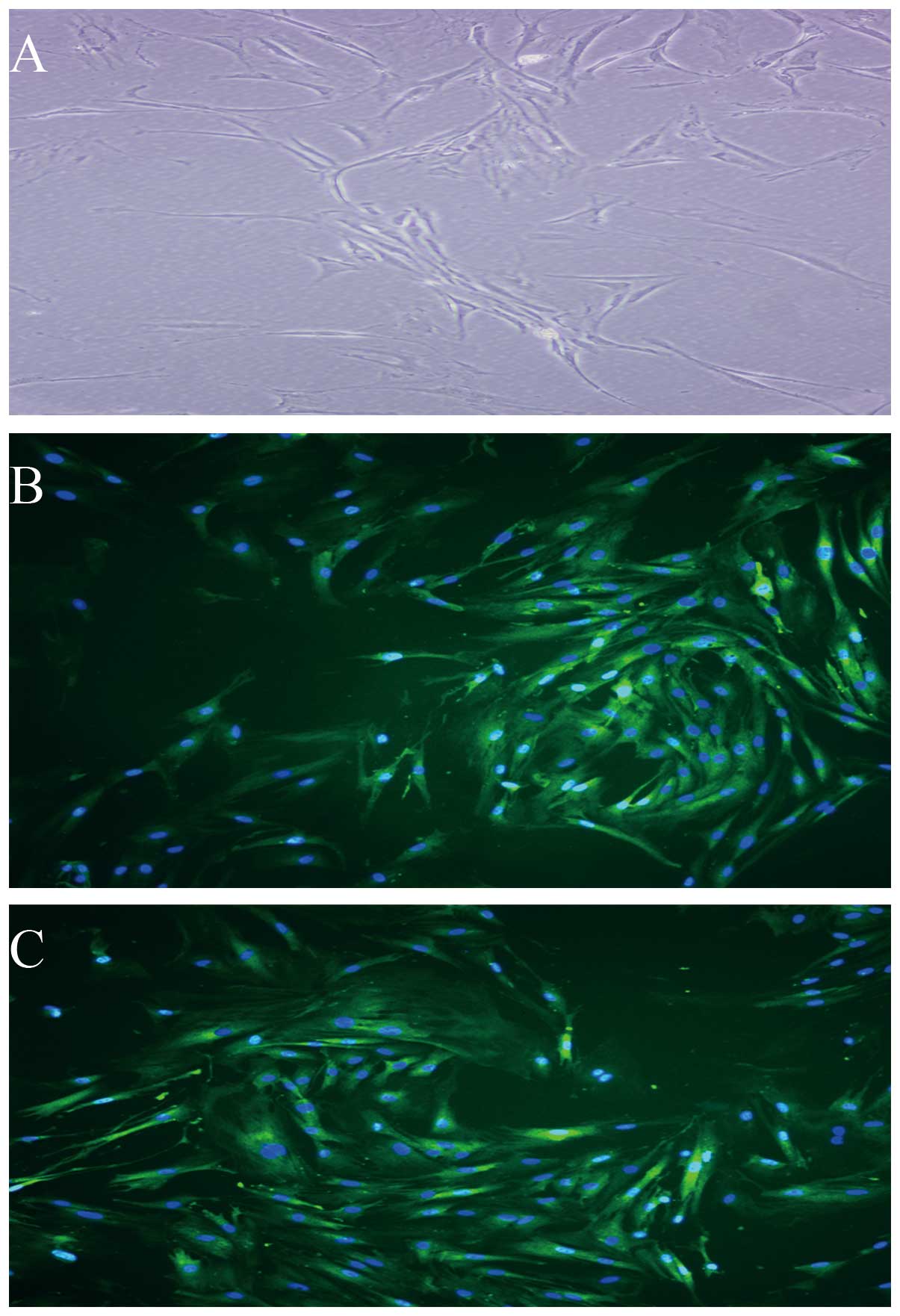
ADSCs differentiate into vascular
endothelial cells
Under the inductive conditions, the ADSCs showed
morphology of vascular endothelial cells and expressed vWF and KDR
(Fig. 3A–C), which are vascular
endothelial lineage-specific markers. By contrast, ADSCs under
control conditions (non-induction) did not express these
markers.
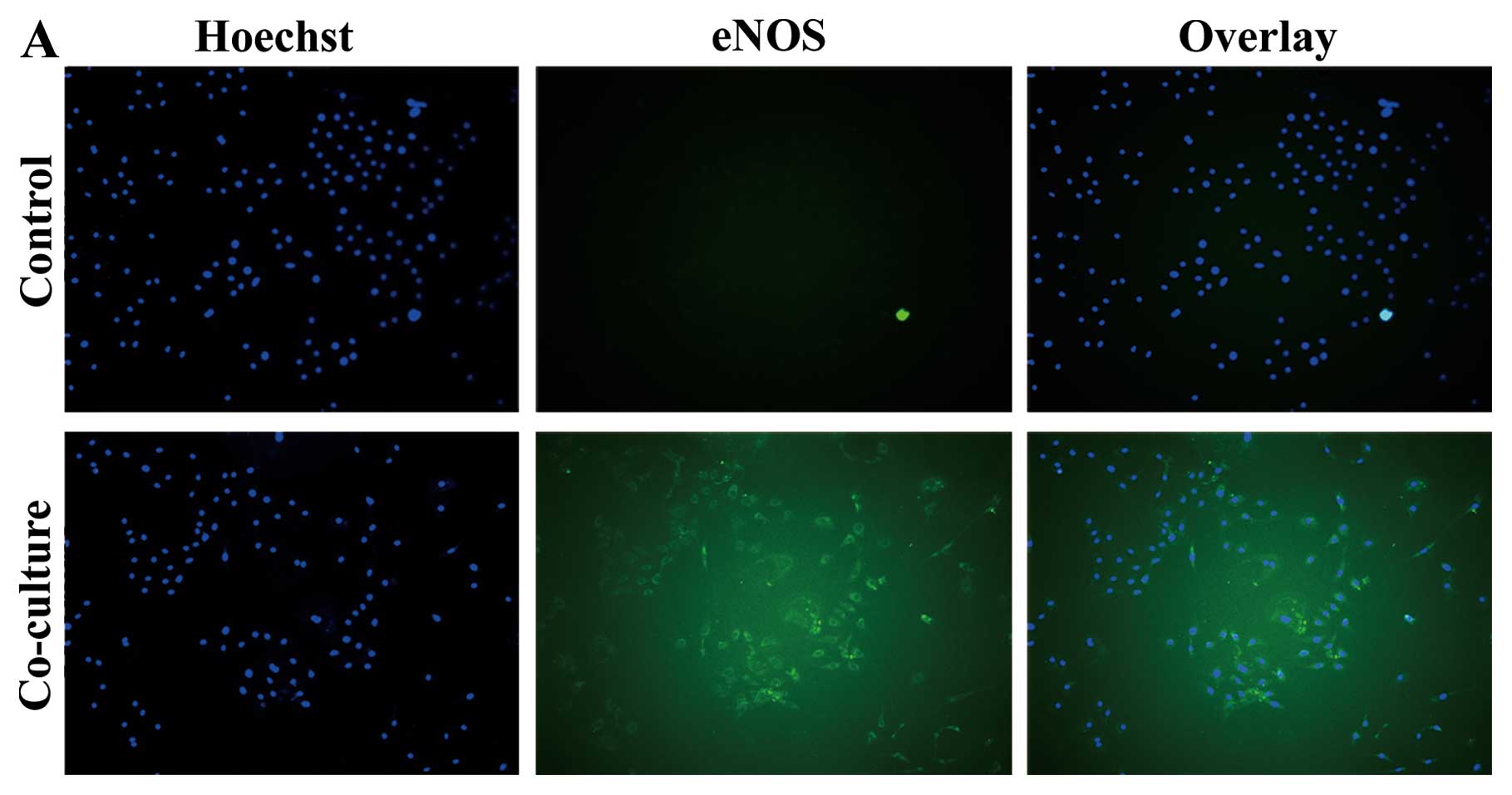
ADSCs enhance eNOS expression in PMVECs
following co-culture
PMVECs were co-cultured with ADSCs in Transwell for
72 h. In the co-culture group, eNOS expression increased
significantly compared to the control group at 72 h (Fig. 4A and B). Meanwhile, ADSCs showed
morphology of vascular endothelial lineage cells and expressed vWF
following co-culture with PMVECs (Fig. 4C). ADSCs differentiated into
vascular endothelial lineage cells.
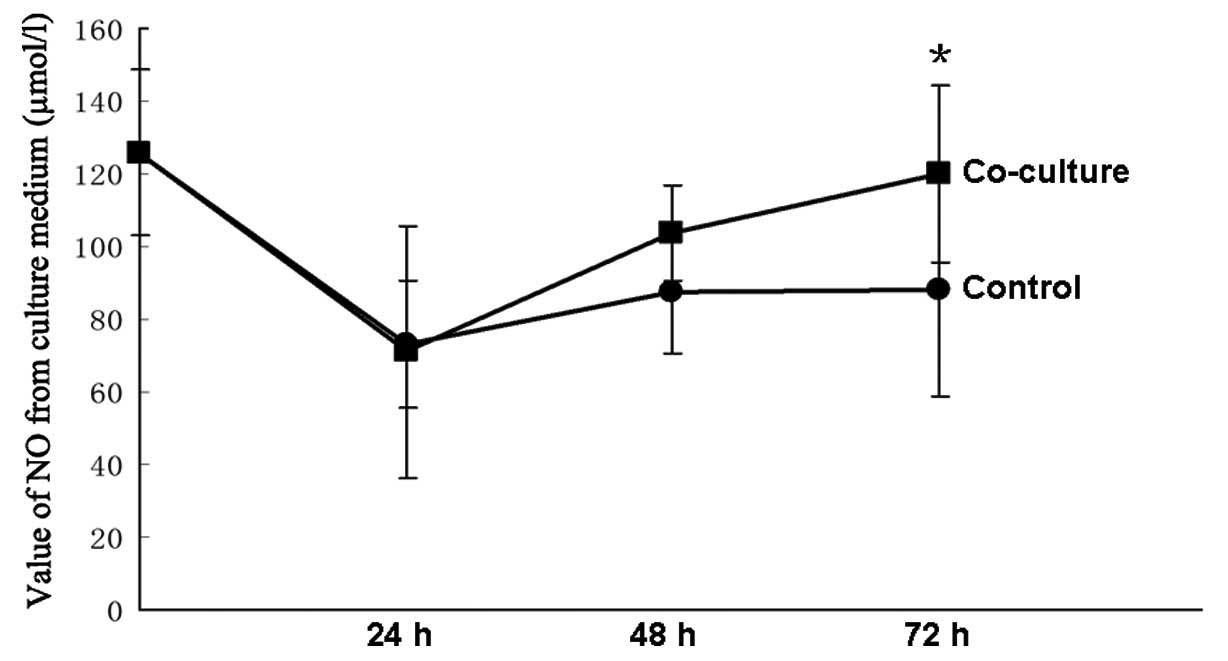
NO concentration is higher in the
co-culture medium
The concentrations of NO were measured at 24, 48 and
72 h. There was no difference between the control group and the
co-culture group at the initial 24 and 48 h. The concentration of
NO was higher in the co-culture group than in the control group at
72 h (P<0.05), and the co-culture group was similar to their
baseline (Fig. 5).
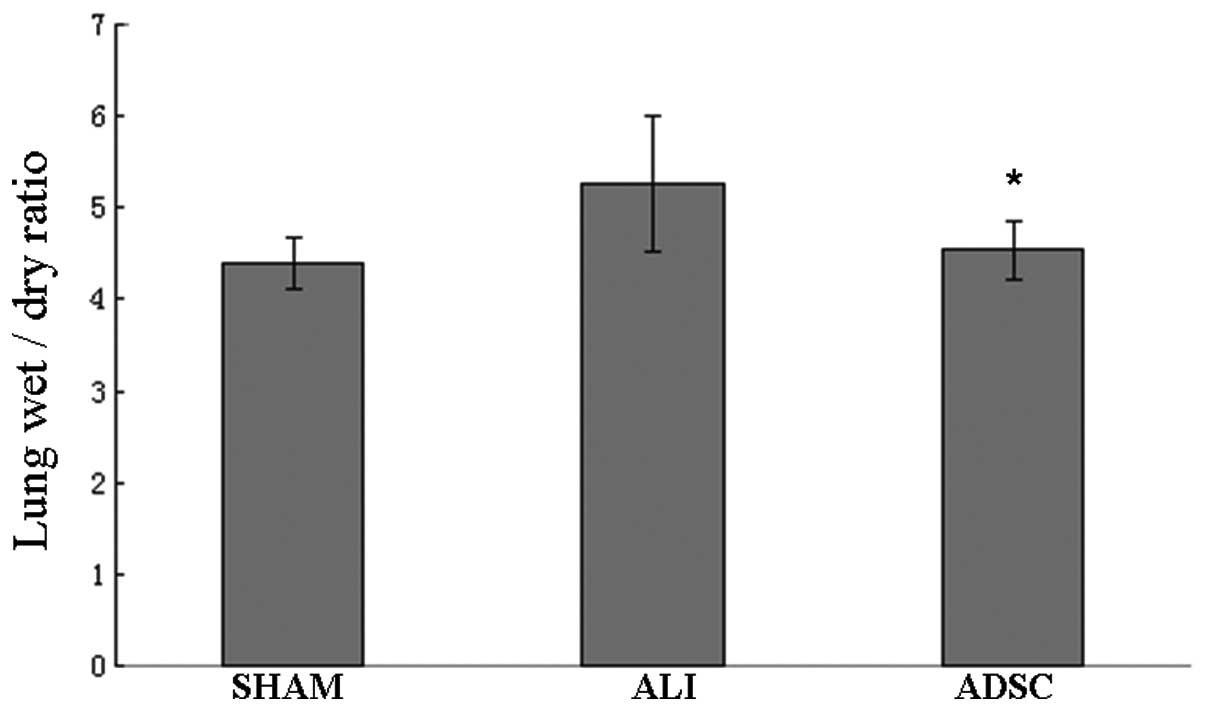
ADSCs reduce lung W/D ratio
Results showed that the lung W/D ratio of rats
treated with ADSCs decreased significantly compared with the ALI
group at 7 days (P<0.05). The lung W/D ratio of the ADSC group
was similar to the sham group (Fig.
6). Our results indicated that ADSCs could reduce the severity
of lung injury and pulmonary edema.
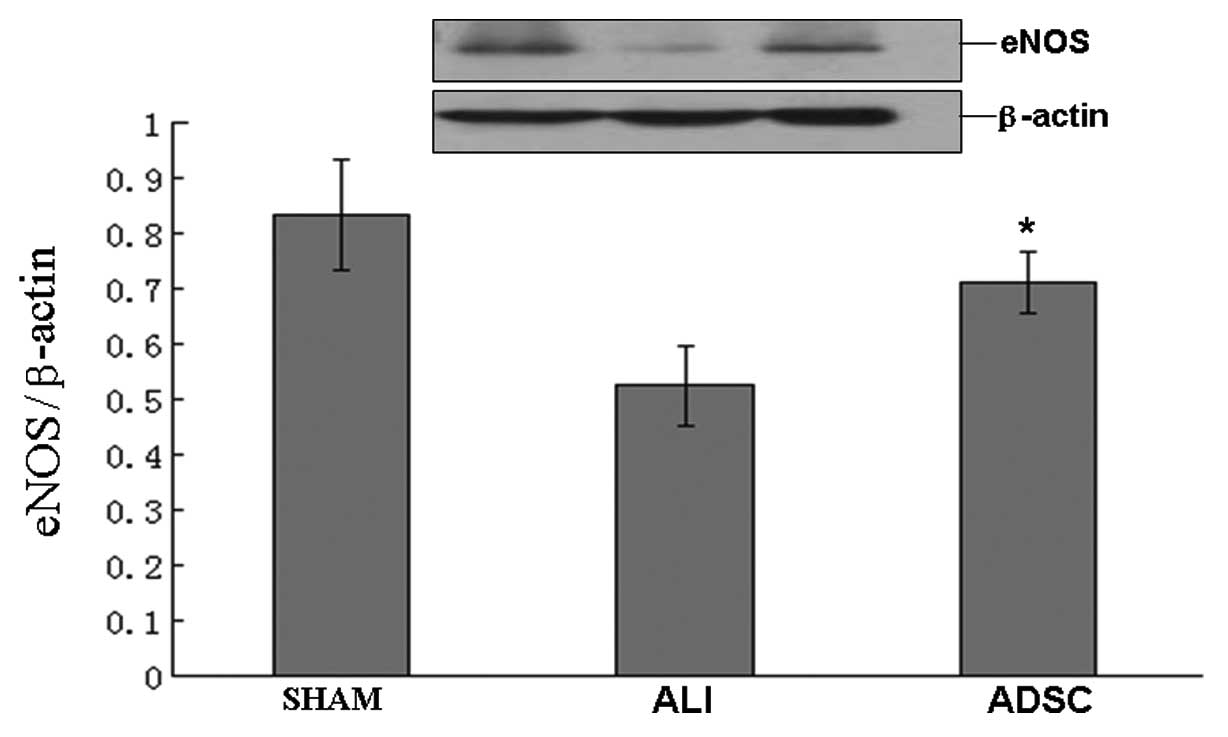
ADSCs enhance eNOS protein expression in
the lung
By transplantation of ADSCs, eNOS protein expression
increased significantly in the ADSC group compared with the ALI
group at 7 days (P<0.05), but the ADSC group was similar to the
sham group (Fig. 7). Our results
indicated that ADSCs restored and enhanced eNOS, which contributed
to protect and remodel ALI.
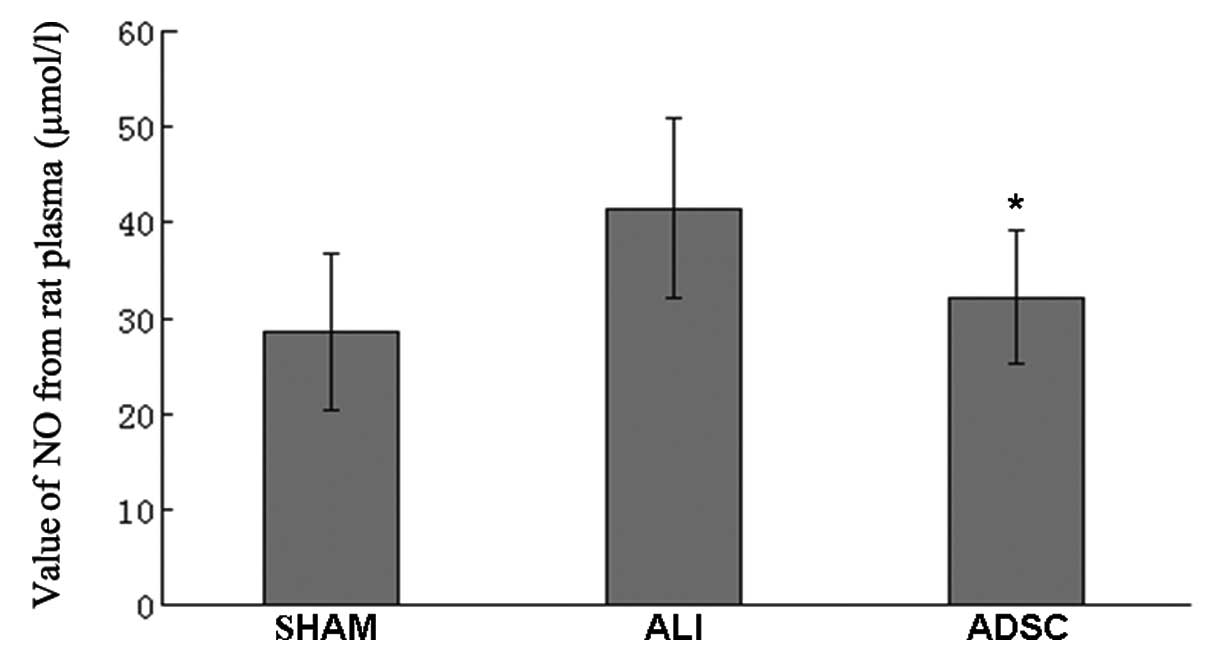
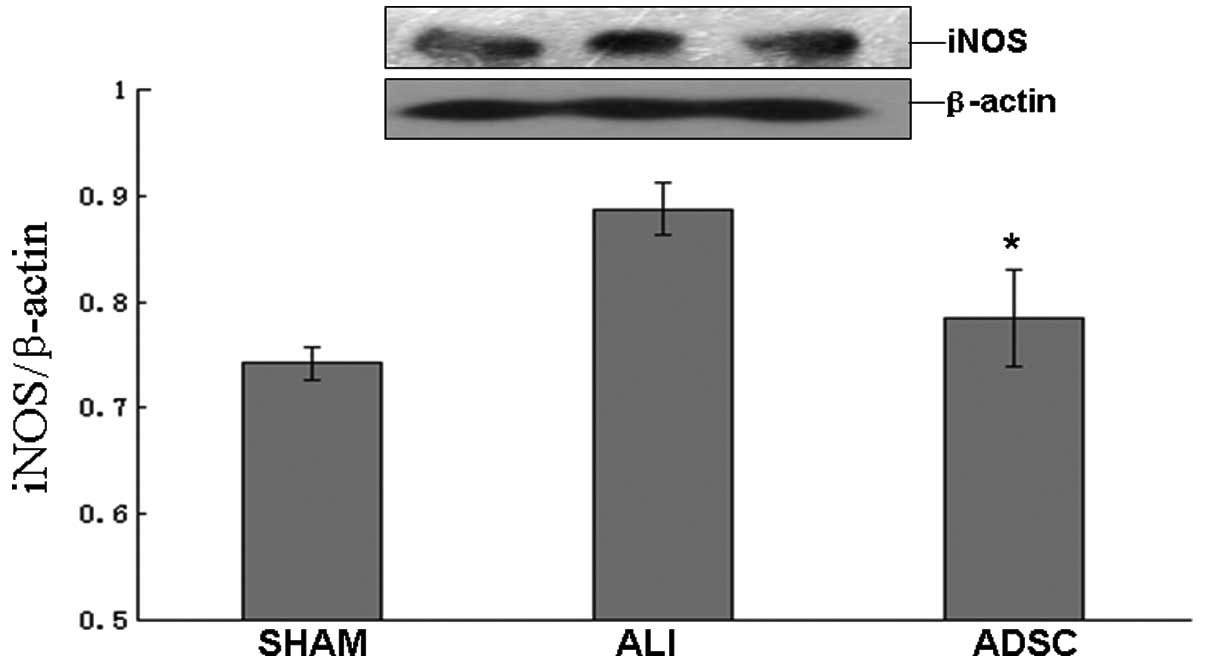
ADSCs attenuate the concentration of NO,
possibly due to the attenuation of iNOS protein expression in
vivo
In the ADSC group, the concentration of NO decreased
significantly compared with the ALI group at 7 days (P<0.05),
but the ADSC group was similar to the sham group (Fig. 8). Moreover, the iNOS protein
expression decreased significantly in the ADSC group compared with
the ALI group at 7 days (P<0.05), but the ADSC group was similar
to the sham group (Fig. 9).
Discussion
The present study successfully harvested ADSCs from
adipose tissue and identified their surface markers by the FACS
machine. It was demonstrated that most of the ADSCs were positive
to surface antigen CD90, but negative for CD34 and CD106, which
confirmed previous studies (12–14). It has been reported that
multi-lineage capacity of a population of stem cells are
successfully isolated from human adipose tissue (6). The adipose tissues are shown to be
an abundant source for isolating multipotent cells (15). In order to induce stem cell
differentiation into vascular endothelial cells in vitro,
the ADSCs require angiogenic factors such as VEGF, transforming
growth factor-β (TGF-β) and basic growth fibroblast growth factor
(bFGF) (16). However, we
successfully used the VEGF to induce ADSC differentiation into
endothelial cells. This result demonstrated that ADSCs efficiently
differentiated into vascular endothelial cells in vitro.
Hyperpermeability response and pulmonary arterial
hypertension are key among most pathophysiological characteristics
of ALI. The integrity of the PMVECs is essential to prevent the
influx of protein-rich fluid from the plasma. When the PMVECs are
destroyed by inflammation, it may further lead to high-permeability
and pulmonary edema. Therefore, the injured PMVECs are a principal
event to ALI. Our observations showed that the ADSCs enhanced eNOS
expression in PMVECs and NO was highly produced in the co-culture
medium. When PMVECs were exposed to LPS and did not co-culture with
ADSCs, eNOS expression was only slight at 72 h. Moreover, ADSCs
differentiated into vascular endothelial lineage cells in Transwell
co-culture experiments. Previous studies have shown that ADSCs are
capable of regenerative angiogenesis due to their differentiation
into vascular endothelial cells. ADSCs secrete angiogenic factors,
such as VEGF, TGF-β, bFGF, which are inductive factors that have
the role in differentiating into vascular endothelial cells
(16,17). Furthermore, the injured PMVECs
also had a limited endothelial cell capability and characteristic
in Transwell co-culture experiments. ADSCs were affected by PMVECs
and differentiated into vascular endothelial lineage cells to
activate the secretion of eNOS-derived NO accompanied by secreted
PMVECs.
Several studies have reported that MSCs are not
labeled and traced in the in vivo model of ALI, but the MSCs
have therapeutic effects on ALI (2,18).
We studied the effects of transplantation of ADSCs in rats
suffering from ALI. When ADSCs were administered to ALI, ADSCs
reduced the lung W/D ratio of ALI, which is a marker of the
severity of lung injury and pulmonary edema. The eNOS and
eNOS-derived NO signaling played an essential role in resisting
injuries in vascular endothelial cells during ALI. eNOS and
eNOS-derived NO are fundamental factors in eliciting the
hyperpermeability response in experiments (19,20). The NO-soluble guanylate cyclase
(sGC)-cyclic guanosine monophosphate (cGMP) signaling pathway plays
a critical role in the regulation of pulmonary vascular tone and
resistance in pulmonary arterial hypertension. The effects of sGC
stimulators and activators depend on ongoing eNOS-derived NO
generation (21). LPS abolishes
acetylcholine-induced relaxation mediated by endothelium-derived
hyperpolarizing factor, although partially inhibited the
eNOS-derived NO mediate relaxation component in the rat pulmonary
artery (22). Our data showed
that eNOS protein expression increased in lung tissue after
administering ADSCs. Therefore, it was considered that relieving
pulmonary vascular injuries depended on eNOS expression by
administering ADSCs. We found that eNOS-derived NO signaling was
activated, but the concentration of NO was decreased significantly
in vivo. To explain this phenomenon, we consider that
several cell types are involved in ALI, such as alveolar epithelial
cell types I and II, vascular endothelial cells, smooth muscle
cells, fibroblasts, neutrophils and macrophages. Macrophages, in
particular, play a significant role in the pathophysiology of
LPS-induced ALI. eNOS produces low amounts of NO and activities in
endothelial cells. However, iNOS activates in macrophages and
produces large amounts of NO following stimulation by LPS. It is
believed that iNOS and iNOS-derived NO mediate the
pathophysiological processes of ALI, including pulmonary neutrophil
infiltration, oxidant stress and pulmonary arterial hypertension.
PMVEC barrier dysfunction is caused by iNOS came from alveolar
macrophage (23). We observed
that iNOS protein and iNOS-derived NO concentration decreased
significantly following delivery of ADSCs. Therefore, our evidence
demonstrated that decreased iNOS was a part of the protection from
ALI provided by ADSCs.
We suggest that ADSCs restored and enhanced eNOS and
eNOS-derived NO, which contributed to protect and remodel ALI. The
ADSCs decreased iNOS, which was a part of protection during ALI.
Collectively, ADSCs may be a promising therapeutic strategy for
ALI.
Acknowledgements
This study was supported by the
National Natural Science Fund of China (no. 81273923).
References
|
1
|
Rubenfeld GD, Caldwell E, Peabody E, et
al: Incidence and outcomes of acute lung injury. N Engl J Med.
353:1685–1693. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gupta N, Su X, Popov B, et al:
Intrapulmonary delivery of bone marrow-derived mesenchymal stem
cells improves survival and attenuates endotoxin-induced acute lung
injury in mice. J Immunol. 179:1855–1863. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ortiz LF, Gambelli C, McBride D, et al:
Mesenchymal stem cell engraftment in lung is enhanced in response
to bleomycin exposure and ameliorates its fibrotic effects. Proc
Natl Acad Sci USA. 100:8407–8411. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rojas M, Xu J, Woods CR, et al: Bone
marrow derived mesenchymal stem cells in repair of the injured
lung. Am J Respir Cell Mol Biol. 33:145–152. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kotton DN, Ma BY, Cardoso WV, et al: Bone
marrow-derived cells as progenitors of lung alveolar epithelium.
Development. 128:5181–5188. 2001.PubMed/NCBI
|
|
6
|
Zuk PA, Zhu M, Mizuno H, et al:
Multilineage cells from human adipose tissue: implications for
cell-based therapies. Tissue Eng. 7:211–228. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ito H, Matsushita S, Ishikawa S, et al:
Significant correlation between endothelial nitric oxide synthase
(eNOS) expression and alveolar repair in elastase-induced rat
pulmonary emphysema. Surg Today. 43:293–299. 2013. View Article : Google Scholar
|
|
8
|
Maron BA, Zhang YY, White K, et al:
Aldosterone inactivates the endothelin-B receptor via a cysteinyl
thiol redox switch to decrease pulmonary endothelial nitric oxide
levels and modulate pulmonary arterial hypertension. Circulation.
126:963–974. 2012. View Article : Google Scholar
|
|
9
|
Hoffmann J, Haendeler J, Aicher A, et al:
Aging enhances the sensitivity of endothelial cells toward
apoptotic stimuli: important role of nitric oxide. Circ Res.
89:709–715. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ryan US, White LA, Lopez M and Ryan JW:
Use of microcarriers to isolate and culture pulmonary microvascular
endothelium. Tissue Cell. 14:597–606. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Solodushko V, Parker JC and Fouty B:
Pulmonary microvascular endothelial cells form a tighter monolayer
when grown in chronic hypoxia. Am J Respir Cell Mol Biol.
38:491–497. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chieregato K, Castegnaro S, Madeo D, et
al: Epidermal growth factor, basic fibroblast growth factor and
platelet-derived growth factor-bb can substitute for fetal bovine
serum and compete with human platelet-rich plasma in the ex vivo
expansion of mesenchymal stromal cells derived from adipose tissue.
Cytotherapy. 13:933–943. 2011.
|
|
13
|
Katz AJ, Tholpady A, Tholpady SS, Shang H
and Oqle RC: Cell surface and transcriptional characterization of
human adipose-derived adherent stromal (hADAS) cells. Stem Cells.
23:412–423. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
De Ugarte DA, Alfonso Z, Zuk PA, et al:
Differential expression of stem cell mobilization-associated
molecules on multi-lineage cells from adipose tissue and bone
marrow. Immunol Lett. 89:267–270. 2003.PubMed/NCBI
|
|
15
|
Lee RH, Kim B, Choi I, et al:
Characterization and expression analysis of mesenchymal stem cells
from human bone marrow and adipose tissue. Cell Physiol Biochem.
14:311–324. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rehman J, Traktuev D, Li J, et al:
Secretion of angiogenic and antiapoptotic factors by human adipose
stromal cells. Circulation. 109:1292–1298. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Miranville A, Heeschen C, Sengenes C, et
al: Improvement of postnatal neovascularization by human adipose
tissue-derived stem cells. Circulation. 110:349–355. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Burnham EL, Taylor WR, Quyyumi AA, et al:
Increased circulating endothelial progenitor cells are associated
with survival in acute lung injury. Am J Respir Crit Care Med.
172:854–860. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hatakeyama T, Pappas PJ, Hobson RW, et al:
Endothelial nitric oxide synthase regulates microvascular
hyperpermeability in vivo. J Physiol. 574:275–281. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sanchez FA, Savalia NB, Duran RG, et al:
Functional significance of differential eNOS translocation. Am J
Physiol Heart Circ Physiol. 291:H1058–H1064. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lang M, Kojonazarov B, Tian X, et al: The
soluble guanylate cyclase stimulator riociguat ameliorates
pulmonary hypertension induced by hypoxia and SU5416 in rats. PLoS
One. 7:e434332012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Subramani J, Leo MD, Kathirvel K, et al:
Essential role of nitric oxide in sepsis-induced impairment of
endothelium-derived hyperpolarizing factor mediated relaxation in
rat pulmonary artery. Eur J Pharmacol. 630:84–91. 2010. View Article : Google Scholar
|
|
23
|
Farley KS, Wang LF, Law C and Mehta S:
Alveolar macrophage inducible nitric oxide synthase-dependent
pulmonary microvascular endothelial cell septic barrier
dysfunction. Microvasc Res. 76:208–216. 2008. View Article : Google Scholar
|