Introduction
Chronic low-grade inflammation plays an important
role in the development of metabolic disorders such as insulin
resistance and diabetes mellitus (1,2).
High levels of saturated fatty acids are among the main culprits in
these metabolic disorders (3).
Palmitate is a type of saturated free fatty acid that stimulates
macrophages to express high levels of pro-inflammatory cytokines
such as tumor necrosis factor (TNF)-α, interleukin (IL)-6 and
IL-1β. These unique properties are reported to be the cause of
insulin resistance (2,4,5).
Inhibition of pro-inflammatory cytokines is reported to recover the
anti-inflammatory phenotype, improve insulin resistance and
alleviate metabolic disorders (5,6).
These observations suggest that regulation of macrophage
inflammation induced by palmitate may be an important therapeutic
target for treating metabolic disorders.
The Astragalus polysaccharide (APS) is widely used
as an antimicrobial agent and an immune stimulator in developing
countries (7). Recent studies
have shown that APS has insulin-sensitizing, hypoglycemic effects
(8,9). Research demonstrated that APS
increases the expression of anti-inflammatory cytokines including
IL-10 and transforming growth factor (TGF)-β in diabetic mice. The
authors also demonstrated that APS ameliorates diabetes, which may
suggest that APS has anti-inflammatory effects (10,11). Furthermore, in our previous study
we found that APS increased AMPK activity in skeletal muscle
(12). A previous study
demonstrated that AMPK is involved in regulating inflammatory
responses (13). These results
suggest that APS may play anti-inflammatory roles in vivo
and in vitro. Furthermore, studies have demonstrated that
APS exerted anti-inflammatory effects against lipopolysaccharide
(LPS)-induced inflammation in several cell lines (14,15). However, APS was found to stimulate
the expression of TNF-α and iNOS to exert pro-inflammatory effects
in the murine macrophage RAW264.7 cell line (16,17). The RAW264.7 cell line is widely
used to study inflammatory responses associated with metabolic
disorders (4–6). However, the pro-inflammatory effects
of APS in RAW264.7 cells conflict with its beneficial effects in
reducing insulin resistance in metabolic disorders. Questions still
remain whether APS plays an anti-inflammatory or pro-inflammatory
role in RAW264.7 cells and how APS affects macrophage cells.
In the present study, we demonstrated that APS
affects inflammatory responses in murine macrophage RAW264.7 cells
with or without palmitate treatment. APS stimulated RAW264.7 cells
to produce IL-10 protein and express most of the anti-inflammatory
genes. Additionally, APS induced recovery of IL-10 protein, the
expression of a number of anti-inflammatory genes and inhibited
IL-1β protein production, as well as several pro-inflammatory
genes, in the presence of palmitate in RAW264.7 cells. These
anti-inflammatory effects of APS in palmitate-induced RAW264.7
cells were abrogated by inhibiting AMPK activity. Taken together,
our results revealed that Astragalus polysaccharide evoked
anti-inflammatory effects that were dependent on AMPK activity in
palmitate-induced RAW264.7 cells.
Materials and methods
Preparation of APS extracts
Astragalus membranaceus (Fisch.) Bunge var.
mongholicus (Bunge) Hsiao was purchased from Shanghai
Medicinal Materials Co. (Shanghai, China). We extracted the main
bioactive component, Astragalus polysaccharide (APS), with
optimized techniques using direct water decoction according to a
previously described procedure with some modifications (18). In brief, APS, a hazel-colored and
water-soluble powder, was diluted in DMEM (SH30022.1B; HyClone) to
a working concentration of 10%. This solution was filtered three
times with a 0.22-μm filter before use. All APS dilutions
were limited to a one-time use. The extract was identified by the
Department of Authentication of Chinese Medicine, Hubei College of
Chinese Traditional Medicine (Wuhan, China). The endotoxin
contaminants in the APS working solution was tested and limited to
<0.05 EU/ml.
Preparation of palmitate
BSA-bound palmitate was constructed according to a
previously described procedure with some modifications (19). In brief, palmitate was dissolved
in ethanol to a concentration of 0.75 M, and then diluted with 20%
FFA-free BSA to a working concentration of 7.5 mM (the molar ratio
of palmitate to BSA was ∼2.5). This stock solution was filtered and
stored at −20°C to be used within two weeks. The same concentration
of ethanol mixed with 20% of BSA was used as a control. The
endotoxin contaminants in the palmitate working solution was tested
and limited to <0.3 EU/ml. This amount of contaminating
endotoxin was previously proven not to be sufficient to activate
RAW264.7 cells (5).
Cell culture
The murine macrophage cell line RAW264.7 was
purchased from the Shanghai Cellular Research Institute (China) and
cultured in DMEM supplemented with 10% fetal bovine serum (SV30087;
HyClone), 100 U/ml penicillin and 100 μg/ml streptomycin at
37°C in a humidified atmosphere of 5% CO2. The cells
were trypsinized at 80% confluence with 0.25% trypsin/0.02% EDTA in
Hank’s solution for 1–3 min and resuspended in a complete culture
medium. These culture materials were all endotoxin-free.
Western blotting and antibody
reagent
The cells in 6-well plates (1×106
cells/plate) were lysed in cold buffer containing 50 mM HEPES, pH
7.4, 150 mM NaCl, 200 mM NaF, 20 mM sodium pyrophosphate, 10%
glycerol, 1% Triton X-100, 4 mM sodium orthovanadate, 2 mM
phenylmethylsulfonyl fluoride and 1 mM EDTA. Whole cell lysates (20
μg) were resolved by SDS-PAGE and transferred onto
polyvinylidene difluoride membranes (Immobilon-P; Millipore,
Bedford, MA, USA). Following blocking with 5% milk in TBST, the
membranes were probed with primary antibodies as indicated and
subsequently incubated with horseradish peroxidase-conjugated
secondary antibodies for visualization with enhanced
chemiluminescence (Thermo Scientific, USA). The blots were stripped
in western blotting stripping buffer containing 2% SDS, 62.5 mM
Tris-HCl (pH 6.8) and 100 mM β-mercaptoethanol. Phosphorylated-AMPK
(Thr172) and AMPK antibodies were purchased from Cell Signaling
Technology (Beverly, MA, USA). Phospho-AMPK and the total AMPK
levels were assayed by densitometry with Quantity One 4.4
software.
Reverse transcription and real-time
PCR
Total RNA obtained from murine RAW264.7 macrophages
in 6-well plates (1×106 cells/plate) was prepared with
RNeasy® Mini kit columns (Qiagen) using the
manufacturer’s protocol. cDNA synthesis was performed with 1
μg of total RNA using hexamers for priming and the
QuantiTect® Reverse Transcription kit (Qiagen) according
to the manufacturer’s recommendations. Quantitative real-time PCR
was performed on a LightCycler system (Bio-Rad iQ-2; Bio-Rad) using
QuantiFast™ SYBR®-Green PCR (Genocopy). Ten microliters
of reaction mixture was incubated, and amplification was performed
for 50 cycles (30 sec at 95°C and 30 sec at 60°C). The primers (at
a final concentration of 10 mM) were designed with the Primer 5
software (Table I). The β-actin
mRNA level was used as the internal control. All samples were run
in triplicates. The qRT-PCR results were analyzed as previously
described (20).
 | Table IPrimer sequences used in this
study. |
Table I
Primer sequences used in this
study.
| Gene | Primer sequences
(5′-3′) |
|---|
| β-actin | |
| Sense | TCA CCC ACA CTG TGC
CCA TCT ACG A |
| Antisense | GGA TGC CAC AGG ATT
CCA TAC CCA |
| IL-10 | |
| Sense | AGA AGC ATG GCC CAG
AAA TC |
| Antisense | CCA AGG AGT TGT TTC
CGT TAGC |
| Mannose
receptor | |
| Sense | ATG CCA AGT GGG AAA
ATC TG |
| Antisense | TGT AGC AGT GGC CTG
CAT AG |
| Dectin-1 | |
| Sense | CAT CGT CTC ACC GTA
TTA ATG CAT |
| Antisense | CCC AGA ACC ATG GCC
CTT |
| YM-1 | |
| Sense | AAT GAT TCC TGC TCC
TGT GG |
| Antisense | ACT TTG ATG GCC TCA
ACC TG |
| YM-2 | |
| Sense | CAC GGC ACC TCC TAA
ATT GT |
| Antisense | GCT GGA CCA CCA GGA
AAG TA |
| TNF-α | |
| Sense | AAA ATT CGA GTG ACA
AGC CTG TAG |
| Antisense | CCC TTG AAG AGA ACC
TGG GAG TAG |
| IL-1β | |
| Sense | GAT CCA CAC TCT CCA
GCT GCA |
| Antisense | CAA CCA ACA AGT GAT
ATT CTC CAT G |
| IL-6 | |
| Sense | AAG TGC ATC ATC GTT
GTT CAT ACA |
| Antisense | GAG GAT ACC ACT CCC
AAC AGA CC |
| CD11c | |
| Sense | ACA CAG TGT GCT CCA
GTA TGA |
| Antisense | GCC CAG GGA TAT GTT
CAC AGC |
| iNOS | |
| Sense | CAG CTG GGC TGT ACA
AAC CTT |
| Antisense | CAT TGG AAG TGA AGC
GTT TCG |
| Arginase | |
| Sense | AGA GCT GAC AGC AAC
CCT GT |
| Antisense | GGA TCC AGA AGG TGA
TGG AA |
| MCP-1 | |
| Sense | TCT GGG CCT GCT GTT
CAC A |
| Antisense | CCT ACT CAT TGG GAT
CAT CTT GCT |
IL-10 and IL-1β ELISA assays
The RAW264.7 cells in 6-well plates
(1×106 cells/plate) were incubated with APS.
Supernatants were recovered and frozen at −80°C before analysis.
The production of IL-10 and IL-1β in cell supernatants was
determined with an ELISA kit (R&D Systems, USA) according to
the manufacturer’s instructions.
DN-A MPK and pcDNA-Zeo plasmid
transfection
DN-AMPKα1 (dominant negative) and the control
vector, pcDNA-Zeo, were gifts from Professor Carling Dave (Imperial
College, London, UK). The plasmids were transfected into RAW264.7
cells using Lipofectamine™ 2000 (Invitrogen) according to the
manufacturer’s protocol. All plasmids used here were removed from
endotoxin, and the amount of contaminating lipopolysaccharide was
tested to be <0.01 EU/ml.
Statistical analysis
Data are expressed as the means ± standard deviation
(SD). Data were analyzed with SPSS 17.0 software and tested by
one-way analysis of variance (ANOVA). P<0.05 was defined as
indicating a statistically significant result.
Results
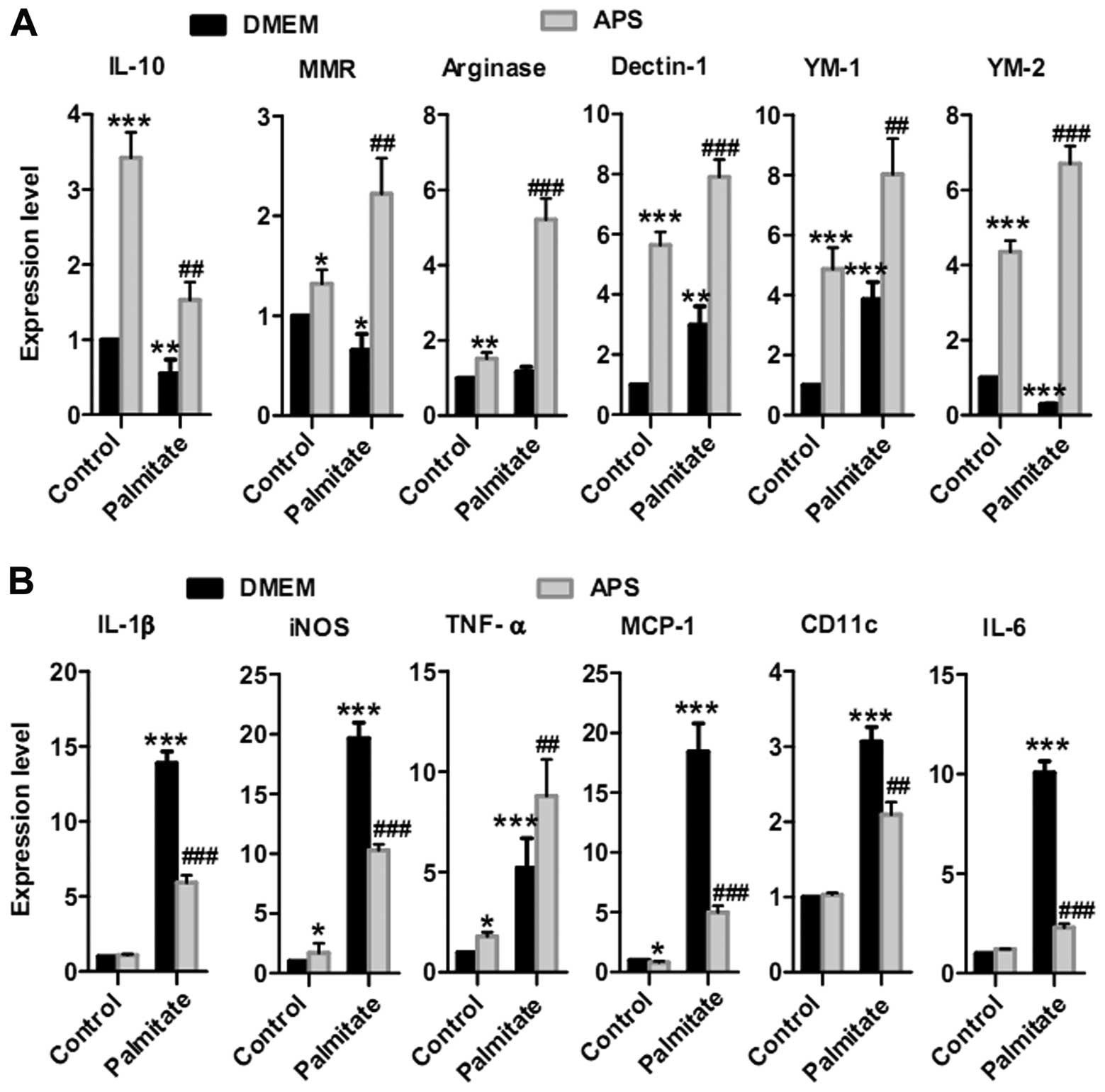
APS modulates the expression of
inflammatory genes in RAW264.7 cells
To ascertain whether APS modulates macrophage
inflammatory responses with or without palmitate, IL-10, macrophage
mannose receptor (MMR), arginase, Dectin-1, YM-1 and YM-2 were
analyzed as anti-inflammatory genes. Conversely, IL-1β, iNOS,
TNF-α, monocyte chemoattractant protein-1 (MCP-1), CD11c and IL-6
were analyzed as pro-inflammatory genes (20,21). APS stimulated anti-inflammatory
gene expression in the RAW264.7 cells: 3.4±0.3-fold increase in
IL-10, 1.3±0.1-fold increase in MMR, 1.5±0.2-fold increase in
arginase, 5.6±0.4-fold increase in Dectin-1, 4.9±0.6-fold increase
in YM-1 and 4.4±0.3-fold increase in YM-2 (Fig. 1A). APS also recovered the
expression of most of the anti-inflammatory genes in the
palmitate-treated groups: 2.8±0.4-fold increase in IL-10,
3.4±0.6-fold increase in MMR, 4.5±0.5-fold increase in arginase,
2.6±0.2-fold increase in Dectin-1, 2.0±0.3 fold increase in YM-1
and 23.1±1.6-fold increase in YM-2 (Fig. 1A).
There was a small increase in pro-inflammatory gene
expression when RAW264.7 cells were treated with 400 μg/ml
APS (Fig. 1B). For example, APS
increased iNOS expression by 1.7±0.8-fold and TNF-α by
1.8±0.2-fold. No statistically significant increase in IL-6, IL-1β
and CD11c expression was achieved. There was also a small
inhibition in MCP-1 mRNA expression. Additionally, APS inhibited
palmitate-stimulated expression of most pro-inflammatory genes in
RAW264.7 cells, including IL-1β by 57±4%, iNOS by 48±2%, MCP-1 by
73±3%, CD11c by 32±5% and IL-6 by 77±2% (Fig. 1B). Taken together, these results
revealed that APS modulates the expression of most of the
pro-inflammatory genes and induces the expression of
anti-inflammatory genes in RAW264.7 cells following palmitate
treatment.
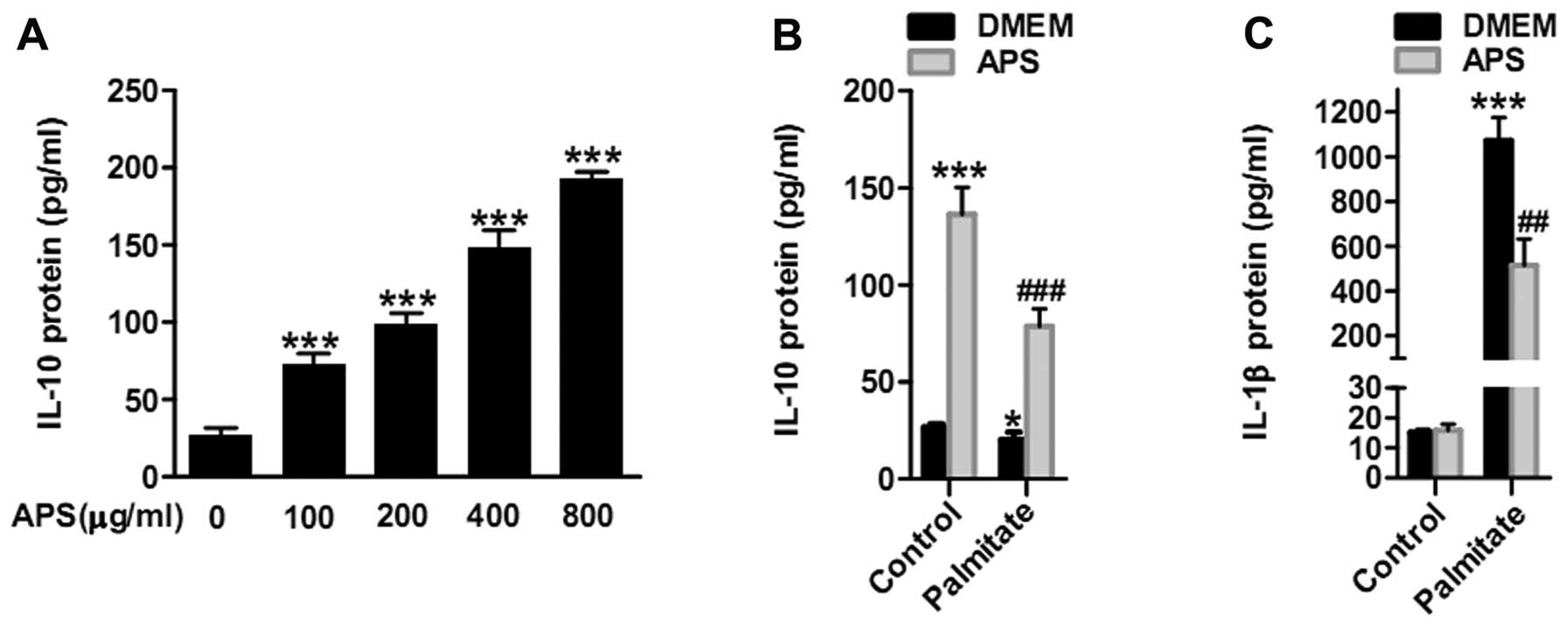
APS enhances the expression of IL-10
protein and reduces IL-1β secretion in RAW264.7 cells
To evaluate the anti-inflammatory effects of APS in
RAW264.7 cells, IL-10 protein was analyzed following APS treatment
for 24 h. IL-10 protein gradually increased with escalating APS
concentrations of 100, 200, 400 and 800 μg/ml (Fig. 2A). To further ascertain whether
APS regulates protein secretion in the presence of palmitate, IL-10
and IL-1β protein was analyzed to evaluate the inflammatory effects
of APS in RAW264.7 cells. RAW264.7 cells were pretreated with 400
μg/ml APS for 24 h. APS enhanced IL-10 protein secretion
(3.9±0.4 fold in the palmitate groups) and reduced IL-1β protein
production (by 51.9±10% in the palmitate groups) in the
palmitate-induced macrophage RAW264.7 cells (Fig. 2B and C). These results
demonstrated that APS enhanced the expression of IL-10 protein and
suppressed IL-1β secretion in palmitate-treated RAW264.7 cells.
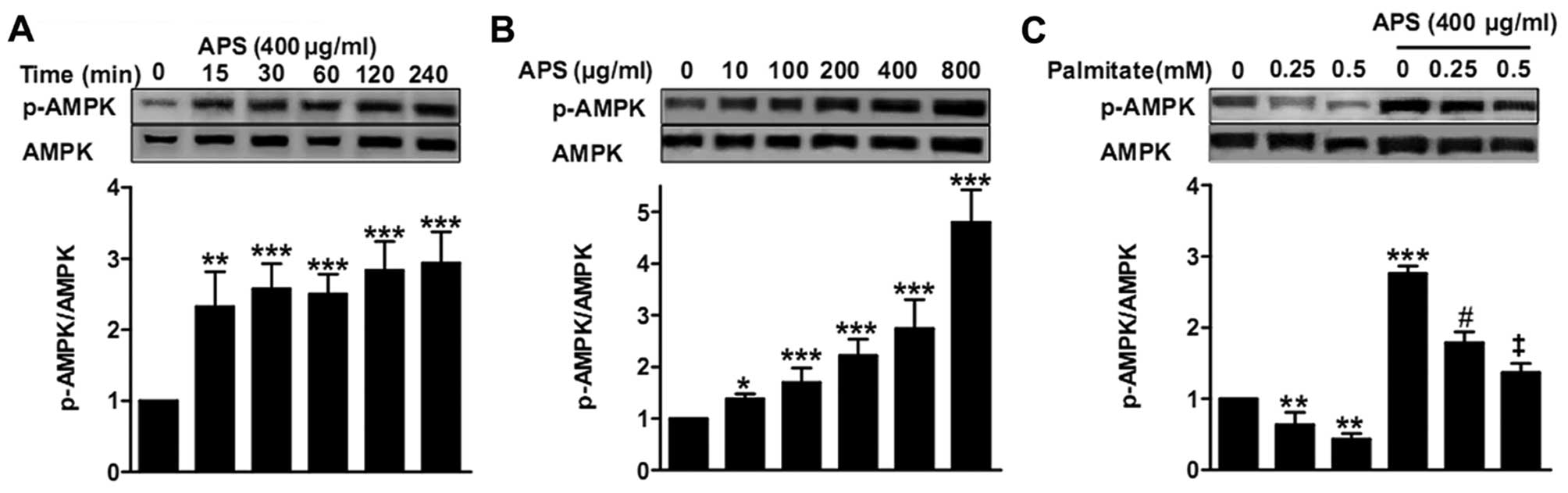
APS recovers the impaired AMPK activity
induced by palmitate
APS rapidly stimulated AMPK activity in the RAW264.7
cells in a concentration-dependent manner after 15 min (Fig. 3A and B). APS recovered the
impaired AMPK activity induced by palmitate (1.8±0.2-fold with 0.25
mM palmitate, and 2.1±0.3-fold with 0.5 mM palmitate, respectively)
(Fig. 3C).
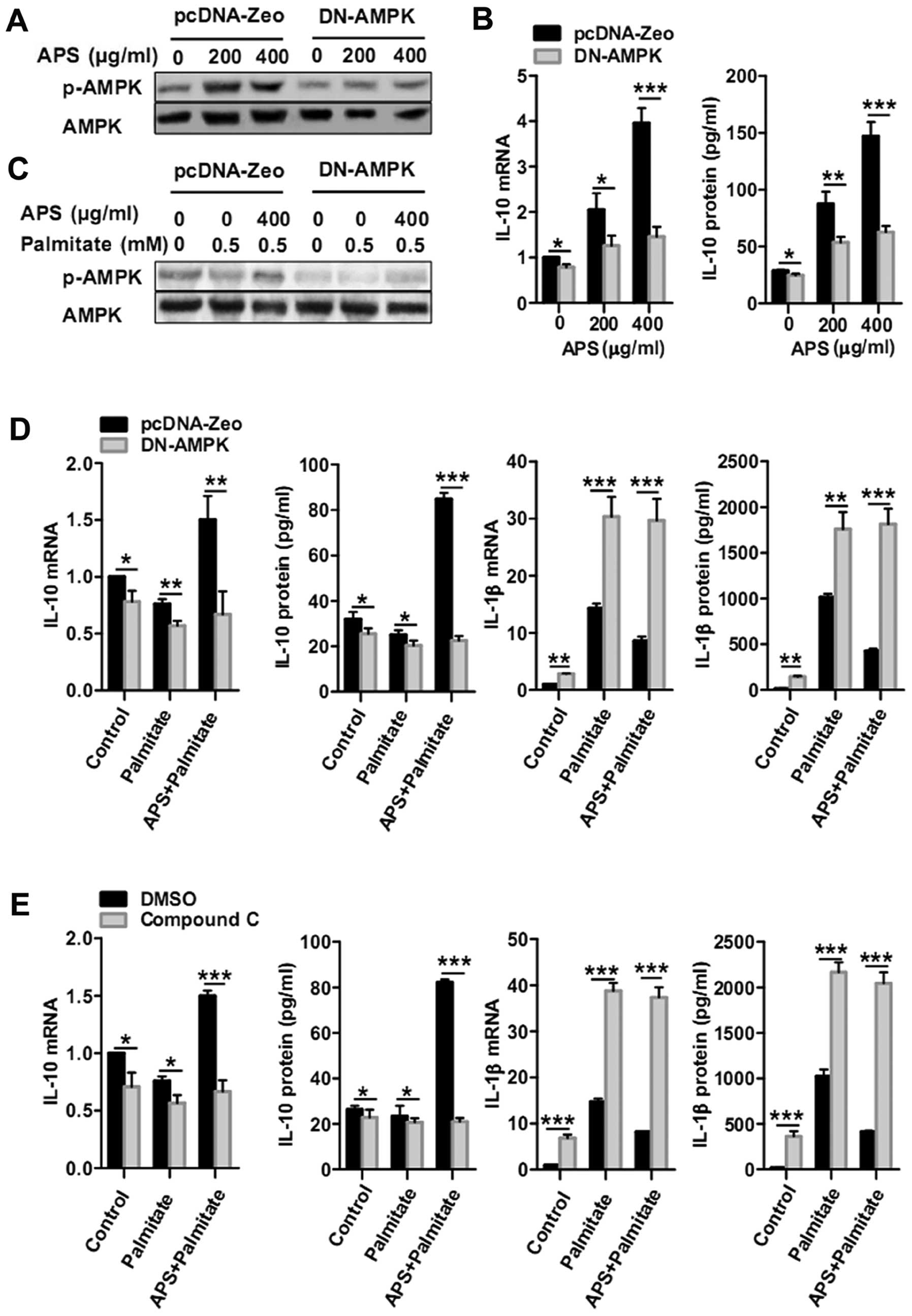
APS modulates IL-10 and IL-1β gene and
protein expression, dependent on AMPK activity
To further ascertain whether APS-regulated IL-10 and
IL-1β expression is dependent on AMPK activity, RAW264.7 cells were
transfected with DN-AMPKα to inhibit AMPK activity (Fig. 4A and C). Compared with the
pcDNA-Zeo control group, DN-AMPK groups treated for 24 h with 200
and 400 μg/ml of APS showed significant decreases in IL-10
mRNA by 39±10 and 63±6%, respectively. IL-10 protein secretion in
the DN-AMPK groups was abrogated by 39±5% with 200 μg/ml APS
treatment and by 57±3% with 400 μg/ml APS treatment
(Fig. 4B). Furthermore, DN-AMPK
macrophages were pretreated with 400 μg/ml APS for 24 h and
treated again with palmitate for 12 h. APS-dependent recovery of
IL-10 mRNA in the presence of palmitate (0.5 mM) was inhibited by
AMPK impairment by 55±13%. AMPK impairment also inhibited the
recovery of IL-10 protein production by 73±2% (Fig. 4D). Meanwhile, APS-dependent
inhibition of IL-1β mRNA and protein in the presence of palmitate
(0.5 mM) was abrogated by AMPK impairment (Fig. 4D). Consistently, compound C, an
AMPK inhibitor, also abrogated the APS-dependent IL-10 recovery and
IL-1β inhibition in the presence of palmitate (0.5 mM) (Fig. 4E). These results demonstrate that
the enhancement of IL-10 and suppression of IL-1β in RAW264.7 cells
following palmitate treatment was caused by APS and was dependent
on AMPK activity.
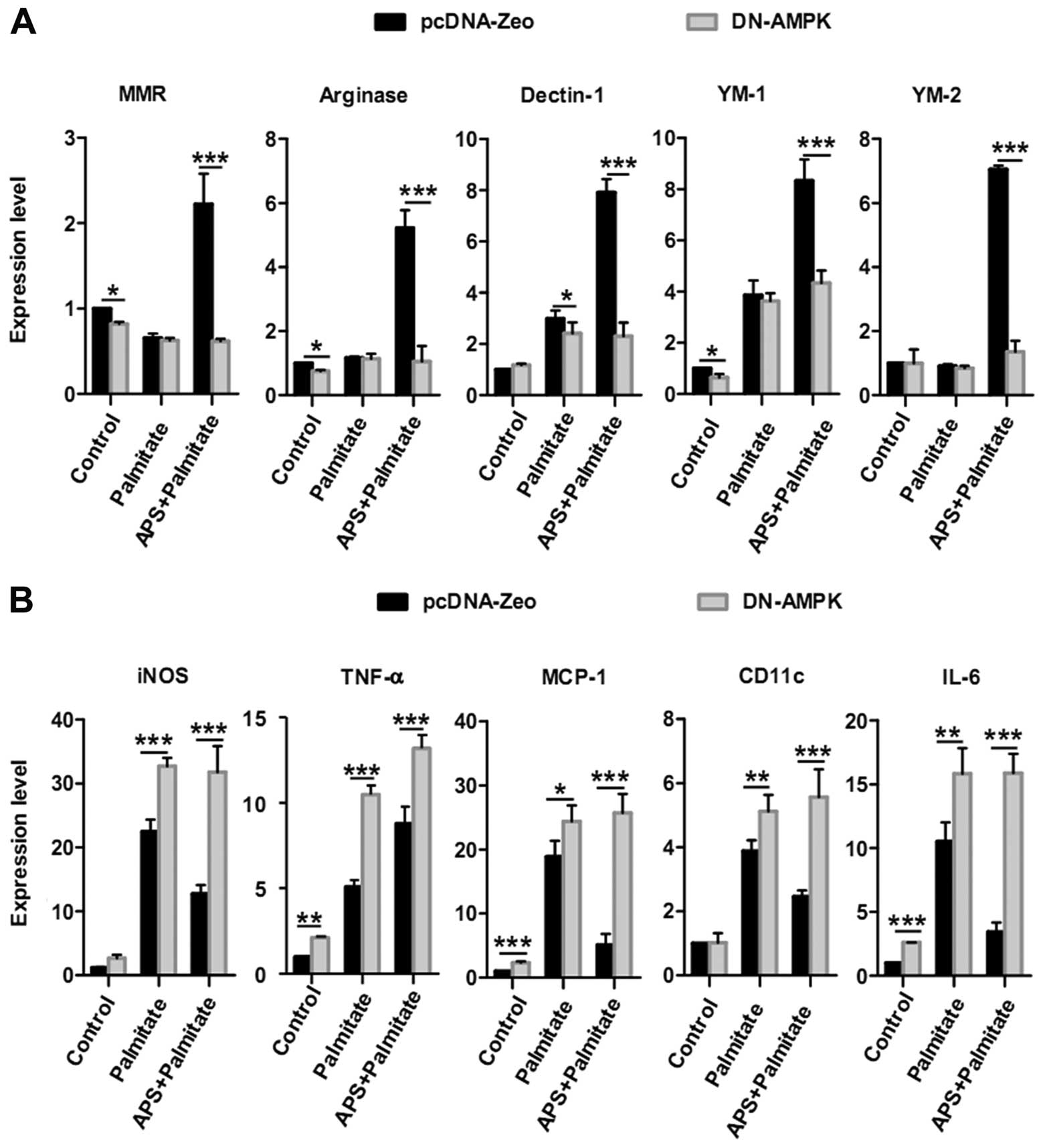
APS modulates the expression of
inflammatory genes in a manner dependent on AMPK activity
To further identify whether APS modulates the
expression of inflammatory genes through AMPK activity, DN-AMPK and
pcDNA-Zeo plasmids were transfected into RAW264.7 cells to inhibit
AMPK activity, respectively (13). When comparing the DN-AMPK and
pcDNA-Zeo groups, the APS-dependent recovery of anti-inflammatory
genes in the present of palmitate (0.5 mM) had a decrease of 72±1%
for MMR, 80±9% for arginase, 71±7% for Dectin-1, 48±6% for YM-1 and
81±5% for YM-2 (Fig. 5A).
Conversely, when comparing the DN-AMPK and pcDNA-Zeo groups,
APS-dependent suppression of pro-inflammatory genes in the present
of palmitate (0.5 mM) showed an increase of 1.5±0.3-fold for iNOS,
4.0±0.6-fold for MCP-1, 1.3±0.6-fold for CD11c and 3.6±0.4-fold for
IL-6 (Fig. 5B). Unexpectedly,
AMPK impairment also increased the expression of TNF-α mRNA in the
palmitate or APS-pretreated groups, but this increase did not
depend on AMPK activity. These results imply that the loss of AMPK
activity inhibits the expression of most anti-inflammatory genes
and abrogates APS-induced inhibition of pro-inflammatory genes
except for TNF-α.
Discussion
Many studies have established the relationship
between chronic low-grade inflammation and metabolic disorders such
as insulin resistance and diabetes mellitus (1,2).
Inflammatory cells and inflammation are key players in obesity and
insulin resistance (2,4,21).
APS has hypoglycemic activity and increases insulin sensitivity
(8,9,12,18). Recent studies have demonstrated
that APS ameliorates diabetes and may have anti-inflammatory
effects (10,11). However, the effect of APS on
inflammatory cells is still uncertain. RAW264.7 is a murine
macrophage cell line that is widely used to study inflammatory
responses associated with metabolic disorders (4–6).
Palmitate is one of the main culprits involved in macrophage
inflammatory responses in metabolic disorders (4,5).
Here, we demonstrated that APS evokes anti-inflammatory responses
in murine macrophage RAW264.7 cells with or without palmitate
treatment. This suggests that APS plays an essential role in
regulating inflammatory responses. Thus, APS may potentially be
used for the prevention and treatment of metabolic disorders.
Recent research has demonstrated that APS increases
pro-inflammatory cytokine expression levels, including TNF-α and NO
production, in RAW264.7 cells (16,17). However, this observation conflicts
with the roles of APS in improving insulin resistance and
hypoglycemic activity (8,9,12,18). In the present study, we evaluated
the expression of several important anti-inflammatory and
pro-inflammatory genes activated by APS to establish this
regulation in RAW264.7 cells. As a whole, APS exhibited
anti-inflammatory effects. Firstly, an important anti-inflammatory
gene, IL-10, was analyzed and increased gene levels were found.
Furthermore, expression of anti-inflammatory genes, MMR, arginase,
Dectin-1, YM-1 and YM-2, increased after APS treatment in RAW264.7
cells (Fig. 1A). However, only
two genes, iNOS and TNF-α, showed increased expression among the
pro-inflammatory genes. Other pro-inflammatory genes, including
IL-6, IL-1β and CD11c, had no statistically significant change and
MCP-1 exhibited a slight inhibition (Fig. 1B). IL-10 protein expression
increased gradually with escalating APS concentrations (Fig. 2A). APS enhanced IL-10 protein
production but IL-1β protein secretion had no statistically
significant change after APS treatment in RAW264.7 cells (Fig. 2B and C). IL-10 plays important
beneficial roles in improving metabolic disorders (22), while IL-1β plays important roles
in accelerating metabolic disorders (23,24). Our results better explain the
roles of APS in metabolic disorders (8,9,12,18) and these results also showed that
APS stimulated the expression of most anti-inflammatory genes and
only two pro-inflammatory genes. Therefore, we demonstrated that
APS stimulates RAW264.7 cells to an anti-inflammatory polarization
(20,21).
AMPK was demonstrated to be a good cellular
regulator for insulin resistance and diabetes in metabolic
disorders (25). Recent evidence
revealed that AMPK is also involved in regulating inflammatory
responses (6,13,26). We found that APS stimulates
RAW264.7 cells to an anti-inflammatory polarization. However,
whether APS stimulates AMPK activity in RAW264.7 cells is still
unknown. Phosphorylation of the α subunit at the site of Thr172
residue is crucial for AMPK activity (25). In the present study, RAW264.7
cells were treated with APS for the indicated time periods or
treated with various APS concentrations for the same time. Results
showed that APS rapidly increased phosphorylation of AMPK (Thr172)
in a dose-dependent manner (Fig. 3A
and B). Furthermore, APS recovered the impaired AMPK
phosphorylation induced by palmitate (Fig. 3C). Therefore, we demonstrated that
APS stimulates AMPK activity in RAW264.7 cells with or without
palmitate treatment.
APS has shown anti-inflammatory effects on
LPS-induced inflammation in several cell lines (14,15). One published study demonstrated
that the characterization of inflammation induced by LPS was
different from that induced by palmitate (4). However, whether APS has
anti-inflammatory effects on palmitate-induced inflammation is
still unknown. In this study, we provide a model for APS regulation
of inflammation in metabolic disorders by showing that APS enhanced
anti-inflammatory gene expression in palmitate-treated groups
(Fig. 1A). Meanwhile, APS
decreased the expression of most of the palmitate-induced
pro-inflammatory genes, except for TNF-α (Fig. 1B). Additionally, APS reduced IL-1β
protein and recovery of IL-10 protein production caused by
palmitate in RAW264.7 cells (Fig. 2B
and C). These results demonstrated that APS plays
anti-inflammatory roles in RAW264.7 cells following palmitate
treatment.
Although studies have found that APS has an
anti-inflammatory effect, involvement of AMPK activity has not been
demonstrated to be correlated with the roles of APS (14,15,27,28). In the present study, to identify
whether the anti-inflammatory effects of APS in RAW264.7 cells
following the treatment of palmitate are associated with AMPK
activity, inhibitors of AMPK, such as the DN-AMPK plasmid or
compound C, were used to investigate the anti-inflammatory effect
of APS while AMPK activity was blocked. Inhibition of AMPK activity
abrogated these effects besides expression of anti-inflammatory
genes, including IL-10, MMR, arginase, Dectin-1, YM-1 and YM-2
(Figs. 4 and 5A). Additionally, APS decreased the
expression of most of the palmitate-induced pro-inflammatory genes,
except for TNF-α (Fig. 1B). These
effects were abrogated by the loss of AMPK activity (Fig. 5B). Moreover, other unknown
mechanisms may be involved in the promotion of TNF-α expression in
APS-treated RAW264.7 cells in the presence of palmitate,
independent of AMPK activity. Thus, APS recovers the expression of
most of the anti-inflammatory genes and inhibits the expression of
several pro-inflammatory genes in the presence of palmitate, in a
manner dependent on AMPK activity.
In summary, we demonstrated that APS stimulates
RAW264.7 cells to an anti-inflammatory polarity and has
anti-inflammatory effects in palmitate-induced RAW264.7 cells.
These anti-inflammatory effects are dependent on AMPK activity.
Thus, APS can potentially be a useful therapeutic candidate for the
prevention and treatment of inflammatory disorders.
Acknowledgments
This study was supported by grants from the National
Basic Research Program of China (2010CB529800), the National
Science and Technology Major Projects of New Drugs
(2012ZX09103301-028), the National Natural Sciences Foundation of
China to H.X.H. and H.S. (nos. 81171127 and 31000344), and the
Natural Science Foundation of Hubei Province of China
(2010CDA045).
References
|
1
|
Greenfield JR and Campbell LV:
Relationship between inflammation, insulin resistance and type 2
diabetes: ‘cause or effect’? Curr Diabetes Rev. 2:195–211.
2006.
|
|
2
|
Permana PA, Menge C and Reaven PD:
Macrophage-secreted factors induce adipocyte inflammation and
insulin resistance. Biochem Biophys Res Commun. 341:507–514. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bray GA, Lovejoy JC, Smith SR, et al: The
influence of different fats and fatty acids on obesity, insulin
resistance and inflammation. J Nutr. 132:2488–2491. 2002.PubMed/NCBI
|
|
4
|
Samokhvalov V, Bilan PJ, Schertzer JD,
Antonescu CN and Klip A: Palmitate- and
lipopolysaccharide-activated macrophages evoke contrasting insulin
responses in muscle cells. Am J Physiol Endocrinol Metab.
296:E37–E46. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nguyen MT, Favelyukis S, Nguyen AK, et al:
A subpopulation of macrophages infiltrates hypertrophic adipose
tissue and is activated by free fatty acids via Toll-like receptors
2 and 4 and JNK-dependent pathways. J Biol Chem. 282:35279–35292.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jeong HW, Hsu KC, Lee JW, et al: Berberine
suppresses proinflammatory responses through AMPK activation in
macrophages. Am J Physiol Endocrinol Metab. 296:E955–E964. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Denzler KL, Waters R, Jacobs BL, Rochon Y
and Langland JO: Regulation of inflammatory gene expression in
PBMCs by immunostimulatory botanicals. PLoS One. 5:e125612010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen W, Xia YP, Chen WJ, Yu MH, Li YM and
Ye HY: Improvement of myocardial glycolipid metabolic disorder in
diabetic hamster with Astragalus polysaccharide treatment. Mol Biol
Rep. 39:7609–7615. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu M, Wu K, Mao X, Wu Y and Ouyang J:
Astragalus polysaccharide improves insulin sensitivity in KKAy
mice: regulation of PKB/GLUT4 signaling in skeletal muscle. J
Ethnopharmacol. 127:32–37. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen W, Li Y and Yu M: Astragalus
polysaccharides: an effective treatment for diabetes prevention in
NOD mice. Exp Clin Endocrinol Diabetes. 116:468–474. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li RJ, Qiu SD, Chen HX, Tian H and Wang
HX: The immunotherapeutic effects of Astragalus polysaccharide in
type 1 diabetic mice. Biol Pharm Bull. 30:470–476. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zou F, Mao XQ, Wang N, Liu J and Ou-Yang
JP: Astragalus polysaccharides alleviate glucose toxicity and
restores glucose homeostasis in diabetic states via activation of
AMPK. Acta Pharmacol Sin. 30:1607–1615. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sag D, Carling D, Stout RD and Suttles J:
Adenosine 5′-monophosphate-activated protein kinase promotes
macrophage polarization to an anti-inflammatory functional
phenotype. J Immunol. 181:8633–8641. 2008.
|
|
14
|
Shon YH, Kim JH and Nam KS: Effect of
Astragali radix extract on lipopolysaccharide-induced inflammation
in human amnion. Biol Pharm Bull. 25:77–80. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
He X, Shu J, Xu L, Lu C and Lu A:
Inhibitory Effect of Astragalus polysaccharides on
lipopolysaccharide-induced TNF-α and IL-1β production in THP-1
cells. Molecules. 17:3155–3164. 2012.PubMed/NCBI
|
|
16
|
Zhao LH, Ma ZX, Zhu J, Yu XH and Weng DP:
Characterization of polysaccharide from Astragalus radix as the
macrophage stimulator. Cell Immunol. 271:329–334. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee KY and Jeon YJ: Macrophage activation
by polysaccharide isolated from Astragalus membranaceus. Int
Immunopharmacol. 5:1225–1233. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang N, Zhang D, Mao X, Zou F, Jin H and
Ouyang J: Astragalus polysaccharides decreased the expression of
PTP1B through relieving ER stress induced activation of ATF6 in a
rat model of type 2 diabetes. Mol Cell Endocrinol. 307:89–98. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schmitz-Peiffer C, Craig DL and Biden TJ:
Ceramide generation is sufficient to account for the inhibition of
the insulin-stimulated PKB pathway in C2C12 skeletal muscle cells
pretreated with palmitate. J Biol Chem. 274:24202–24210. 1999.
View Article : Google Scholar
|
|
20
|
Lefevre L, Gales A, Olagnier D, et al:
PPARgamma ligands switched high fat diet-induced macrophage M2b
polarization toward M2a thereby improving intestinal Candida
elimination. PLoS One. 5:e128282010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Oh DY, Talukdar S, Bae EJ, et al: GPR120
is an omega-3 fatty acid receptor mediating potent
anti-inflammatory and insulin-sensitizing effects. Cell.
142:687–698. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hong EG, Ko HJ, Cho YR, et al:
Interleukin-10 prevents diet-induced insulin resistance by
attenuating macrophage and cytokine response in skeletal muscle.
Diabetes. 58:2525–2535. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gabay C, Lamacchia C and Palmer G: IL-1
pathways in inflammation and human diseases. Nat Rev Rheumatol.
6:232–241. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Speaker KJ and Fleshner M: Interleukin-1
beta: a potential link between stress and the development of
visceral obesity. BMC Physiol. 12:82012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schimmack G, Defronzo RA and Musi N:
AMP-activated protein kinase: role in metabolism and therapeutic
implications. Diabetes Obes Metab. 8:591–602. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hattori Y, Suzuki K, Hattori S and Kasai
K: Metformin inhibits cytokine-induced nuclear factor kappaB
activation via AMP-activated protein kinase activation in vascular
endothelial cells. Hypertension. 47:1183–1188. 2006. View Article : Google Scholar
|
|
27
|
Yuan Y, Sun M and Li KS: Astragalus
mongholicus polysaccharide inhibits lipopolysaccharide-induced
production of TNF-alpha and interleukin-8. World J Gastroenterol.
15:3676–3680. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jiang JB, Qiu JD, Yang LH, He JP, Smith GW
and Li HQ: Therapeutic effects of astragalus polysaccharides on
inflammation and synovial apoptosis in rats with adjuvant-induced
arthritis. Int J Rheum Dis. 13:396–405. 2010. View Article : Google Scholar : PubMed/NCBI
|