Introduction
Due to the necessary clamping of the hepatic pedicle
during resection of liver tumor or liver transplantation, hepatic
ischemia/reperfusion (IR) injury occurs with the resumption of
oxygen delivery to the liver, aggravating ischemia injury, and is
considered the major cause for surgical failure (1). Reactive oxygen species (ROS)
production is increased in response to IR. Ischemia is
characterized by ATP depletion and oxygen free radical production
(2,3). Also, dysregulated electron transport
chain in mitochondria may contribute to increased oxidative stress
(4). However, the major source of
ROS following reperfusion has been shown to be the resident
macrophages in the liver, or Kupffer cells (5,6).
In an early phase (up to 6 h after reperfusion), Kupffer cells are
activated, leading to increased ROS production and secretion of
proinflammatory cytokines (7). In
the late phase (6–24 h after reperfusion), recruitment of
neutrophils and T-lymphocytes further increases the synthesis of
ROS, signaling molecules and complement factors (8). Apoptosis is also increased in
response to oxidative stress and inflammation (9). Therefore, ROS play a crucial role in
the pathology of hepatic IR injury.
Mesenchymal stem cells (MSCs) are adult stem cells.
Similar to other stem cells, they can renew themselves and are
capable of multipotent differentiation. MSCs are considered
suitable for repairing damaged organs as they are non-immunogenic
and immunosuppressive cells able to differentiate into different
lineages, and as they secrete a number of cytokines (10). Preventing IR damage using MSCs has
been shown in the brain (11),
heart (12), and kidney (13). Previous studies showed that both
MSCs and MSC-conditioned medium have the potential to improve the
hepatic condition in rat models of liver fibrosis or acute hepatic
failure (14). These studies
indicate that the hepatoprotective effects of MSCs are mainly due
to their paracrine function (15,16).
However, it remains unclear whether bone
marrow-derived mesenchymal stem cells (BM-MSCs) could also
ameliorate hepatic damage induced by IR injury. Therefore, in the
present study, we generated a rat model of hepatic IR injury that
closely mimics clinical conditions. Using this model, we
investigated the protective role of BM-MSCs and the underlying
molecular mechanisms during the first 24 h after reperfusion.
Materials and methods
Animals and experimental design
Seventy-two male Wistar rats (weighing 230–250 g and
aged 8–10 weeks) were used for this study. Prior to the
experiments, the rats were housed under standard conditions at the
Animal Center of the Second Affiliated Hospital, Harbin Medical
University. Experimental procedures used in this study were
approved by the Administrative Panel on Laboratory Animal Care of
Harbin Medical University.
Rats were randomly divided into three groups of 24
rats in each group. Rats in the sham-operated group were treated by
laparotomy only; rats in the other two groups were subjected to IR.
Upon reperfusion, the IR-transplanted group was immediately
injected with BM-MSCs via the hepatic portal vein, while the
IR-control group was injected with phosphate-buffered saline (PBS)
in the same manner. At 2, 6, 12 or 24 h after IR, 2 ml of blood was
collected from the inferior vena cava of 6 rats from each group
before the animals were euthanized to harvest their livers.
Isolation of BM-MSCs
BM-MSCs were isolated using the density
centrifugation method as previously described (17). Briefly, whole BM cells were
flushed from the femurs and tibias of male 4-week old Wistar rats,
and then fractionated in Lymphoprep™ density solution (density
1.077; Nycomed Pharma, Oslo, Norway). Following centrifugation at
800 × g for 20 min, the cells at the interface were collected and
suspended in Dulbecco’s modified Eagle’s medium and Ham’s F-12
nutrient mixture (DMEM/F12; Gibco-Invitrogen Inc., Carlsbad, CA,
USA) containing 10% fetal bovine serum (HyClone-Thermo Fisher
Scientific, Waltham, MA, USA) and 1% penicillin/streptomycin. Cells
were incubated at 37°C with 95% humidity and 5% CO2.
Forty-eight hours later, the culture medium was changed to remove
non-adherent cells.
Labeling of BM-MSCs
To trace BM-MSCs following transplantation, we
labeled them with the fluorescent dye PKH26 (Sigma, St. Louis, MO,
USA), according to the manufacturer’s instructions. Labeled cells
were then cultured in growth medium for at least 24 h before
transplantation.
Induction of hepatic IR injury and cell
transplantation
Rats were anesthetized with pentobarbital sodium (60
mg/kg). A midline laparotomy was performed under aseptic
conditions, the portal circulation to the left lateral and median
lobes of the liver was carefully dissected, and a microaneurysm
clamp was placed on the hepatic artery and portal vein to block the
blood supply to these lobes. This treatment caused ischemia of 70%
of the segmental liver and prevented mesenteric venous (18). The clamp was removed after 60 min
and, immediately, 1×106 PKH26-labeled MSCs resuspended
in 200 μl PBS or PBS alone were injected into the portal
vein with a 30-gauge needle. Sham-operated rats received only the
laparotomy. Surgery was closed with 4/0 silk suture. When fully
awake, rats had free access to food and water. During the entire
procedure, the core body temperature of each rat was continuously
monitored with a rectal probe and maintained at 37.0±0.4°C with a
heating lamp.
Measurement of aspartate aminotransferase
(AST) and alanine aminotransferase (ALT)
To estimate the degree of hepatic IR injury, we
measured the levels of serum AST and ALT. Rats were anesthetized as
described above, and 2 ml of blood was collected from the inferior
vena cava with a 20-gauge needle, placed in a microtainer tube with
serum separator (Eppendorf, Hamburg, Germany), and centrifuged at
4,000 × g for 12 min. AST and ALT levels in the serum were measured
using an automatic analyzer (Hitachi, Tokyo, Japan) and expressed
as U/L.
Histological analysis
Livers were fixed in 10% buffered formalin, embedded
in paraffin, and cut into 5 μm sections. Sections were
stained in hematoxylin and eosin (H&E) and observed with a
Nikon Eclipse 80i microscope (Nikon, Tokyo, Japan) connected to a
DXM1200F digital camera. The severity of the liver injury was
assessed in accordance with the modified Suzuki classification
(19), modified, by a pathologist
who was blinded to the experimental design. Scores for severity
were: none, 0; minimal, 1; moderate, 2; and severe, 3. For each
rat, three liver sections were examined and three randomly selected
high-power fields (×100) were analyzed in each section. The mean
score for each animal was then determined by summation of all
scores, divided by 9.
Assays for superoxide dismutase (SOD),
glutathione peroxidase (GSH-Px), and malondialdehyde (MDA) in
livers
A portion of the injured rat liver was harvested and
homogenized in ice-cold 0.9% saline. Following centrifugation at
1,500 × g for 15 min, the supernatant was collected and used to
measure the activity of SOD, GSH-Px and MDA using SOD, GSH-Px, or
MDA detection kits (Nanjing Jiancheng Biotech, Nanjing, China),
respectively, in accordance with the manufacturer’s instructions
(20).
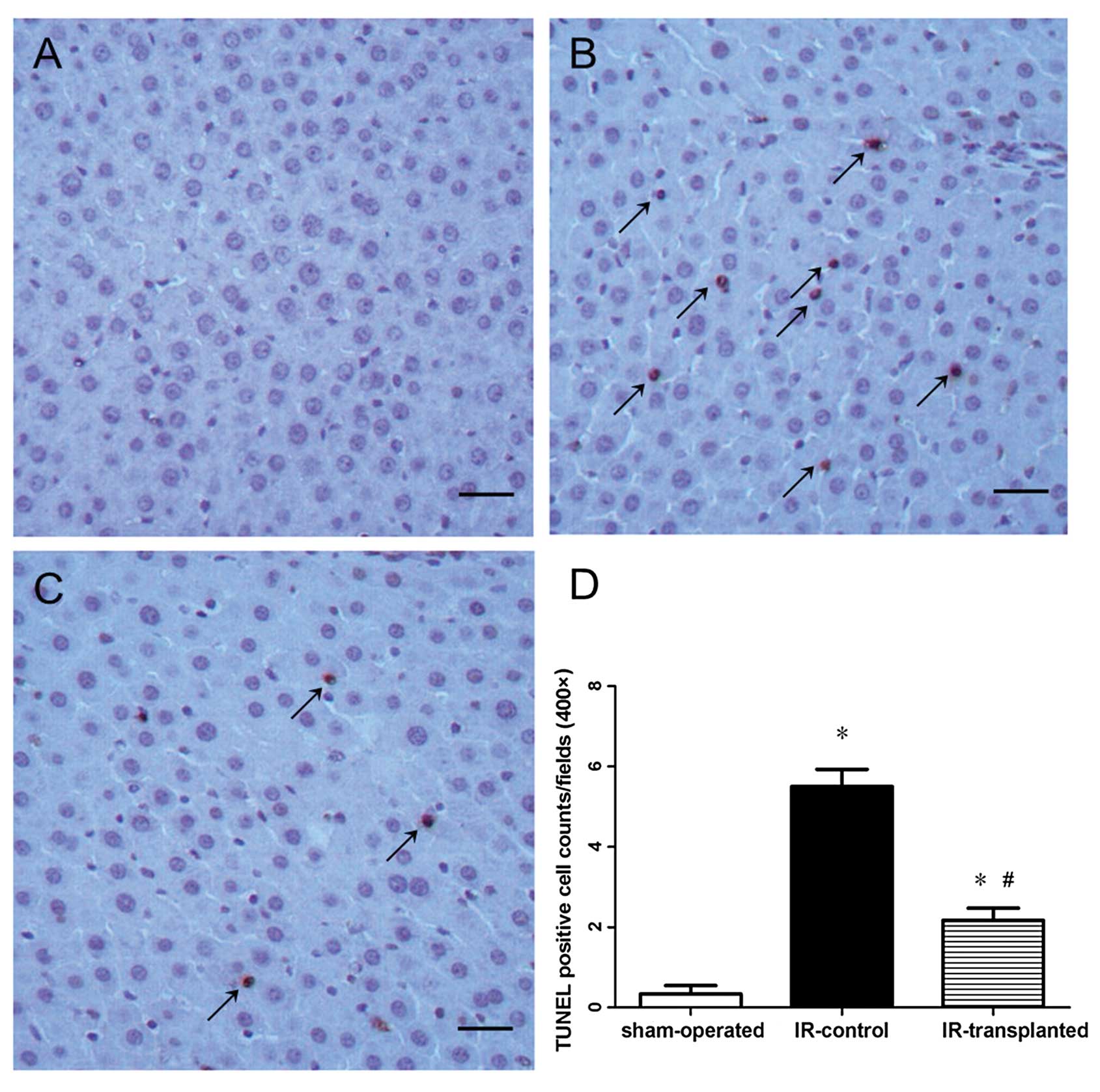
Detection of apoptotic cells in liver
tissues
We used a terminal deoxynucleotidyl transferase
(TdT)-mediated dUTP nick-end labeling (TUNEL) kit (Roche Applied
Science, Penzberg, Germany) to detect the apoptotic hepatocytes
(21). Liver sections (5
μm) were stained and six sections were analyzed for each
rat. Numbers of apoptotic cells and total hepatic cells in each
section were counted in three randomly selected fields (×400). An
apoptosis index (AI) was expressed as the mean percentage of
apoptotic cells within the total number of hepatic cells for each
animal.
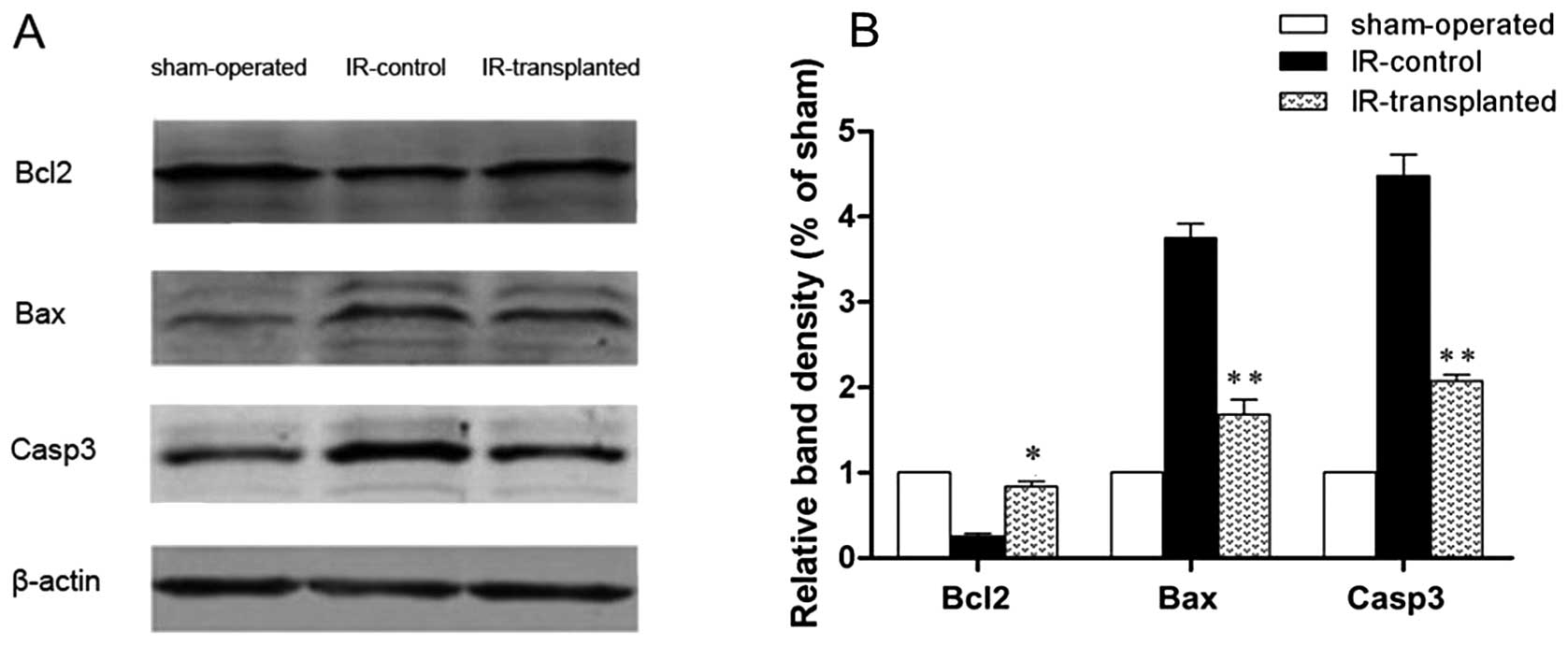
Western blot analysis
Whole protein extracts were prepared from liver
tissues. Freshly harvested liver tissues were homogenized in a
radioimmunoprecipitation assay (RIPA) lysis buffer (Solarbio
Shanghai, China) and centrifuged at 12,000 × g for 15 min. The
protein concentration in the supernatant of each sample was
measured with a DCTM Protein Assay Kit (Bio-Rad, Hercules, CA, USA)
according to the manufacturer’s instructions. Proteins were
separated using a 12% polyacrylamide gel, and then transferred to
immunoblot polyvinylidene difluoride (PVDF) membranes. After
blocking in 5% milk in Tris-buffered saline containing 0.05%
Tween-20 at room temperature, membranes were serially incubated
with the following primary antibodies: mouse anti-Actb, rabbit
anti-B cell lymphoma 2 (Bcl-2), rabbit anti-Bcl-2-associated X
protein (Bax), and rabbit anti-caspase-3 (Casp3) (1:200 dilution;
Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) at 4°C
overnight. Membranes were then washed and incubated with
fluorescence-conjugated anti-mouse or anti-rabbit IgG (1:2,000
dilution; Invitrogen). The bound secondary antibodies were analyzed
with an Odyssey Infrared Imaging System (LI-COR Biosciences,
Lincoln, NE, USA), normalized to β-actin.
Statistical analysis
SPSS 13.0 (SPSS Inc., Chicago, IL, USA) was used for
statistical analyses. For multiple comparisons, data were analyzed
using analysis of variance (ANOVA). Analysis between two groups was
performed using the unpaired Student’s t-test (two-tailed) where
ANOVA indicated significance for the multiple comparisons. Data are
reported as mean ± standard deviation. A P-value <0.05 was
considered to indicate a statistically significant difference.
Results
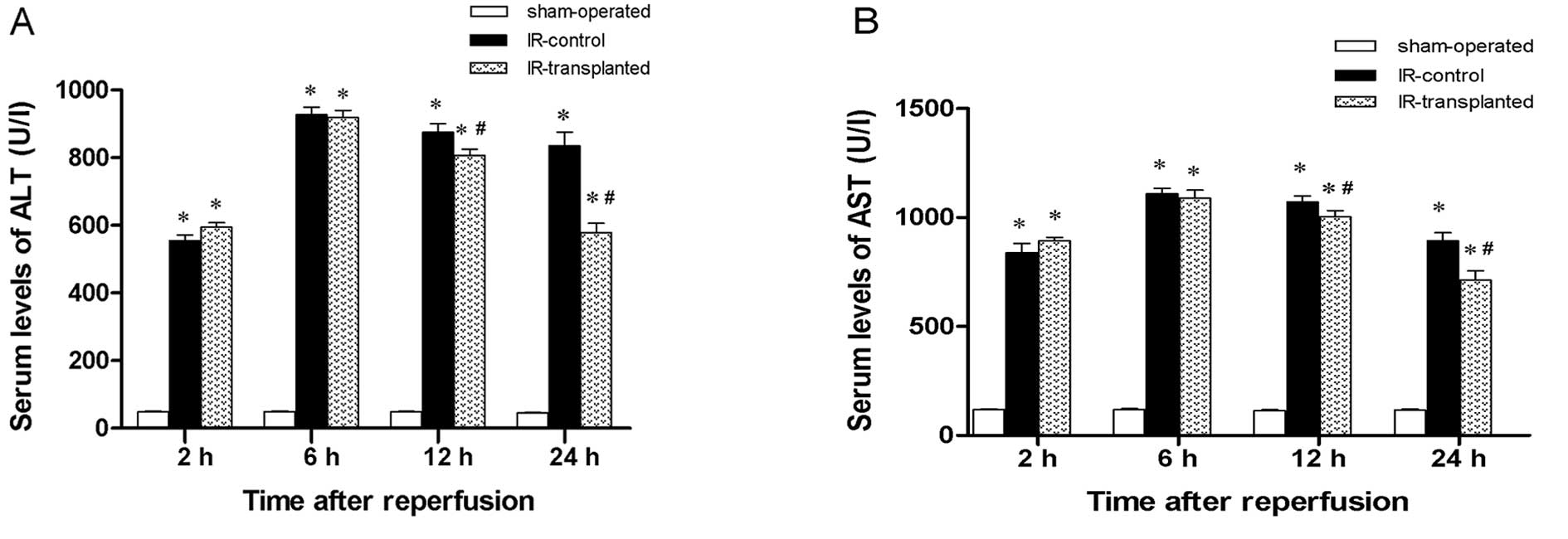
Effect of transplanted BM-MSCs on ALT and
AST serum levels
To determine the degree of IR-induced hepatic injury
in the rat livers receiving BM-MSCs or PBS, we measured ALT and AST
levels in sera collected at 2, 6, 12 and 24 h after IR induction
and cell transplantation. Compared to the sham-operated rats, ALT
and AST levels in IR model rats were higher at every time-point,
with the greatest difference 6 h after induction (Fig. 1). This suggests that IR injury was
successfully induced in the IR model rats. At 12 and 24 h after
reperfusion, ALT and AST levels in the IR-transplanted group
significantly decreased compared to the IR-control group. This
suggests that the transplantation of BM-MSCs had a protective
effect against hepatic injury induced by the IR injury.
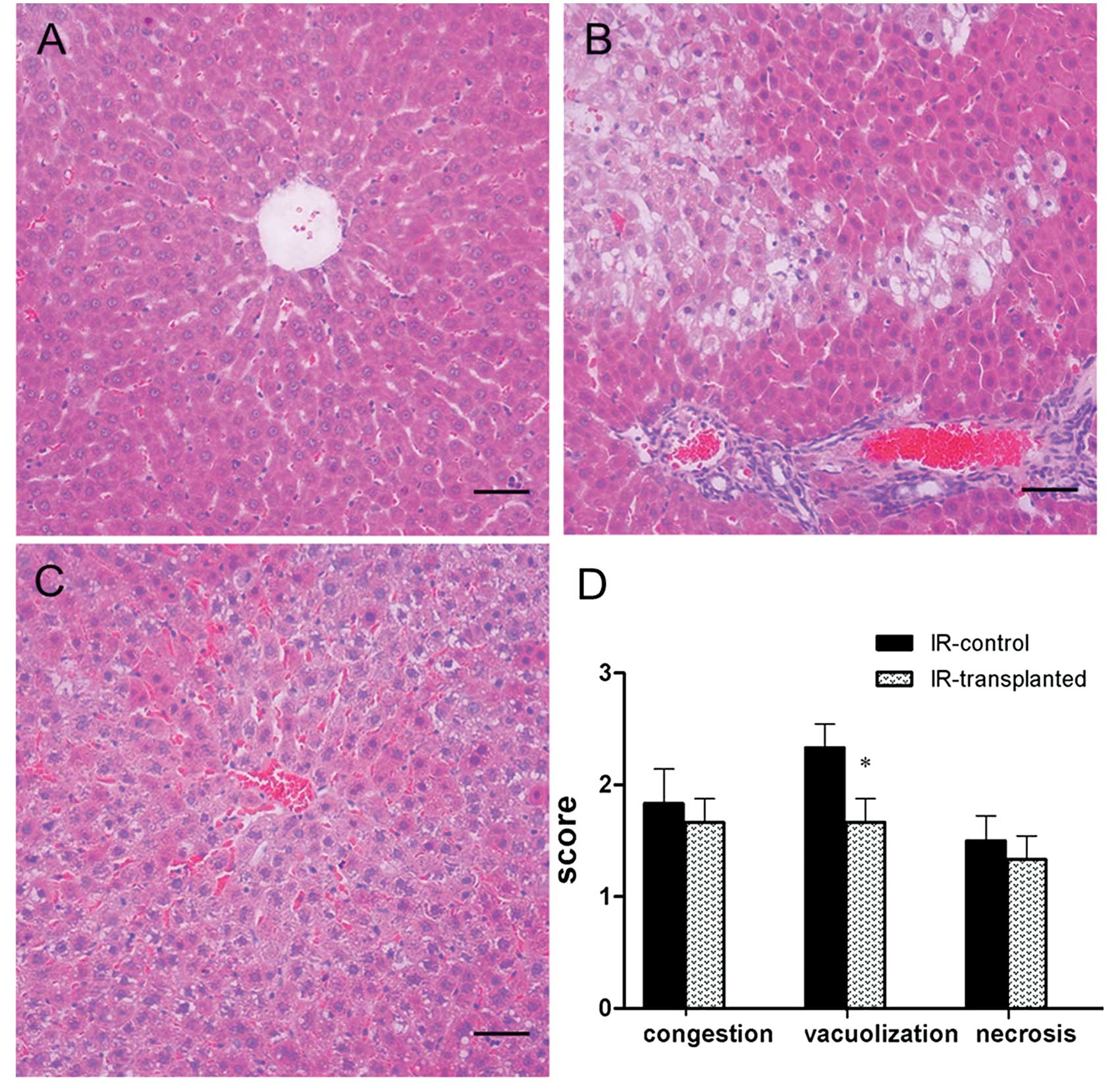
Transplanted BM-MSCs improve the
histopathology of IR-induced livers
To further confirm the protective role of BM-MSCs in
IR-induced hepatic injuries in rats, we examined the histopathology
of livers harvested 2, 6, 12 or 24 h after IR induction and cell
transplantation. At every time-point, all IR-induced livers showed
sinusoidal congestion, cytoplasmic vacuolization, and focal
necrosis, which are indicative of severe damage. When comparing the
Suzuki scores between the livers of the IR-transplanted group and
the IR-control group, improved histopathology and significantly
lower Suzuki scores were found in the IR-transplanted group only at
the 24 h time-point (Fig. 2).
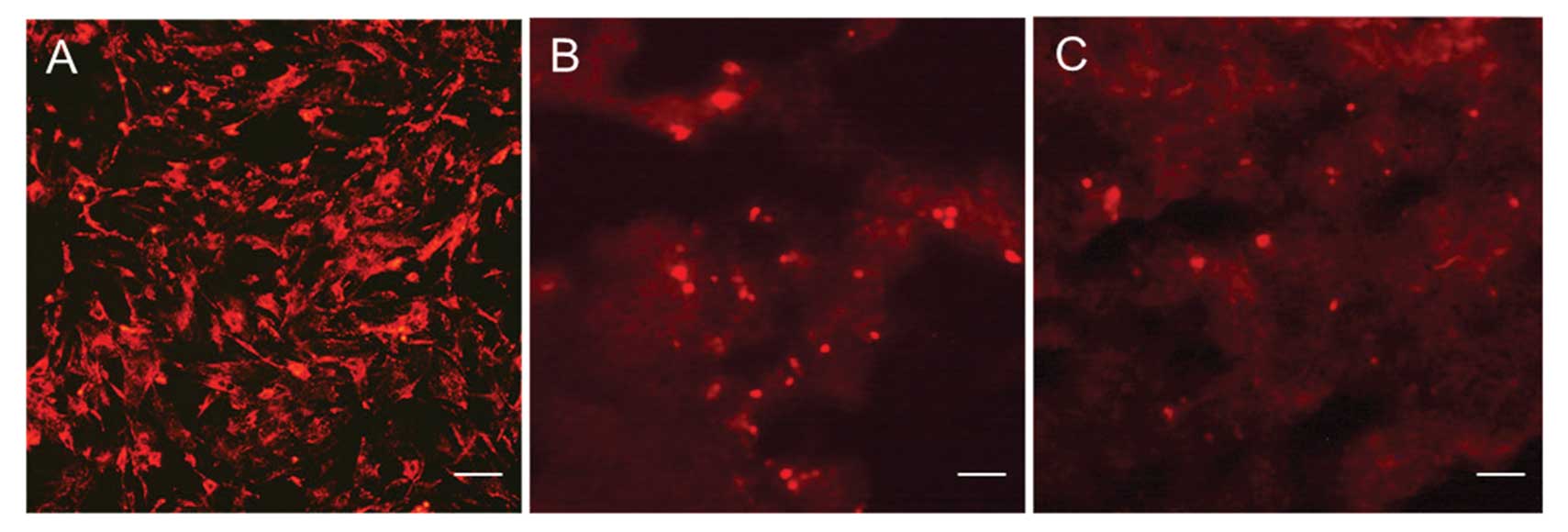
Dynamic distribution of transplanted
BM-MSCs in IR-induced livers
We therefore investigated the distribution pattern
of BM-MSCs transplanted via the portal vein as a function of time.
BM-MSCs were stained by PKH26 fluorescent dye in vitro. We
observed that at 2 h, transplanted PKH26-labeled cells were
clustered around the main branches of the portal triad; at 6 h,
these cells had moved to the periportal area, and at 12 and 24 h
they were scattered within the portal tract areas (Fig. 3).
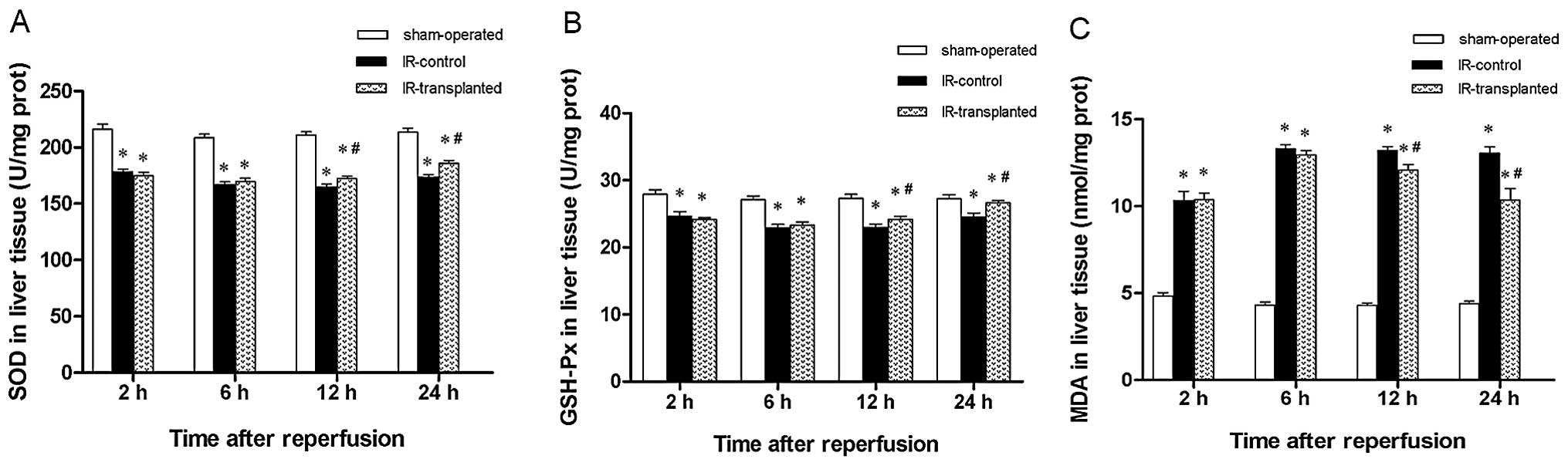
Transplanted BM-MSCs attenuate the
oxidative stress response in IR-induced livers
Previous studies have shown that the oxidative
stress response is involved in IR-induced liver injury. We
therefore assessed if transplanted BM-MSCs would attenuate the
oxidative stress response in liver IR injury, by comparing SOD and
GSH-Px activity levels, and MDA levels. We observed that SOD and
GSH-Px levels were markedly decreased and that MDA levels markedly
increased at 2, 6, 12 and 24 h in IR model rats compared with the
sham-operated rats. This suggests that oxidative stress was indeed
involved in IR-induced liver injury. Furthermore, we observed that
transplantation of BM-MSCs significantly increased SOD and GSH-Px
levels and significantly decreased MDA levels at 12 and 24 h,
compared to rats in the IR-control group (Fig. 4). These results indicate that
transplanted BM-MSCs attenuated the oxidative stress response in
IR-induced liver injury.
Transplanted BM-MSCs inhibit apoptosis in
IR-induced livers
Increased oxidative stress in a tissue often results
in apoptosis. We therefore examined apoptotic activity in the
IR-model rat livers and if the transplantation of BM-MSCs protected
cells from apoptosis. We first assessed apoptotic cells in the
livers using TUNEL staining, and then analyzed the expression
levels of the anti-apoptotic protein, Bcl-2, and the pro-apoptotic
proteins, Bax and Casp3. Twenty-four hours after reperfusion and
cell transplantation, the livers from the IR-model rats had a
markedly higher apoptotic index than those from the sham-operated
rats, suggesting that IR induction caused hepatic apoptosis
(Fig. 5). This was further
confirmed by a significant decrease in Bcl-2 levels, and by
significant increases in Bax and Casp3 levels in IR-injured livers
(Fig. 6). The apoptotic index was
lower in livers from the IR-transplanted rats (Fig. 5), Bax and Casp3 levels were lower,
and Bcl-2 levels were higher. Collectively, these results indicate
that the transplanted BM-MSCs had a protective effect against
apoptosis in the IR-induced livers.
Discussion
In the present study, we investigated the damage and
molecular mechanisms of liver IR injury in a rat model as well as
the protective role of BM-MSCs during the crucial first 24-h
period. We found evidence of IR injury during the first 24 h after
reperfusion. From 2 h after reperfusion, AST and ALT serum levels
were higher than in the sham-operated rats and severe damage was
observed in the histopathology. In addition, evidence of increased
oxidative response was observed from 2 h after reperfusion, while
apparent apoptosis was observed at 24 h. We also observed that
BM-MSCs transplanted via the portal vein exerted a protective
effect against hepatic IR damage during the late phase following
reperfusion. This observation was further supported by the dynamic
distribution of BM-MSCs; 24 h after reperfusion, the majority of
the cells were scattered in the portal tract area of the
livers.
For a therapeutic use of stem cells to be efficient,
cells must be easy to isolate, available in large amounts, able to
expand in vitro, and able to survive in vivo in
sometimes harsh conditions. Based on these criteria, BM-MSCs are
suitable candidates (22). Once
injected, they are recruited at the site of injury via homing
mechanisms involving the CXCR4 receptor and the stromal derived
factor-1 (SDF-1) (23). Once
implanted, stem cells improve the injured tissue by secreting
growth factors [including vascular endothelial growth factor
(VEGF), hepatocyte growth factor (HGF), and insulin-like growth
factor 1 (IGF-1)] (24,25), by differentiating into organ
cells, by transdifferentiating the organ cells and by inducing
neovascularization (11–13). Results from the present study are
in agreement with results obtained from studies of other organs,
such as the heart (12), brain
(11) and kidney (13). Our results show that BM-MSCs homed
to IR-injured liver tissue and improved liver conditions, as
assessed by apoptosis measurement. Also, the protective role of
BM-MSCs was observed during the late phase of the crucial period
(from 12 to 24 h after reperfusion) of IR damage induction,
indicating that BM-MSCs were viable and able to function in a short
time after transplantation. Differentiating into hepatocytes or
hepatocyte-like cells requires time; therefore, it is more likely
that the short-term effects of transplanted BM-MSCs on IR injury
were achieved via a paracrine mechanism, rather than via
repopulation.
The oxidative stress response has long been
recognized as central to the pathogenesis of hepatic IR injury
(26). In the present study, we
observed that at 2, 6, 12 and 24 h after reperfusion, the levels of
the antioxidative enzymes SOD and GSH-Px were significantly
decreased, while MDA levels, a marker for oxidative activity, was
significantly increased, compared with the sham-operated control
rats. Therefore, our observations are consistent with other studies
showing that the oxidative stress response is involved in the
formation of hepatic IR injury in the first 24 h.
We demonstrated in the present study that BM-MSCs
transplanted via the portal vein attenuated the oxidative stress
response in IR-injured livers. The suppressive role of BM-MSCs in
the oxidative stress response was also observed in other studies of
drug-induced animal models of liver disease. In a mouse model of
liver cirrhosis induced by carbon tetrachloride (CCl4),
transplantation of human BM-MSCs suppressed the oxidative stress
response and improved liver conditions (27). In a rat model of hepatic injury
induced by CCl4, administration of BM-MSCs induced a
cytoprotective response by an antioxidative process (28). MSC suppression of the oxidative
stress response was also shown in rats with IR-induced lung and
kidney injury (29). Our study
showed that at 12 and 24 h after reperfusion, the BM-MSC
transplants attenuated the decrease in SOD and GSH-Px, and the
increase in MDA, that otherwise occurred in the IR-control rats
that received PBS only. These results indicate that BM-MSCs are
capable of reducing the oxidative stress response in livers
subjected to IR injury.
Apoptosis plays an important role in IR-injured
liver and is triggered either by the mitochondria or by tumor
necrosis factor (TNF) signaling. Indeed, hypoxia leads to reduced
ATP production and ATP-dependent cellular events are stopped
(30). Mitochondria also regulate
apoptosis by caspase activation (31). Hypoxia-induced injury also induces
an immune response resulting in the secretion of cytokines
(including TNF, SDF-1 and interleukins) and the attraction of a
number of cells, including MSCs. TNF signaling activates caspases,
resulting in apoptosis (32).
During reperfusion, immune and endothelial cells are activated and
produce ROS, which directly damage nearby cells and activate Ras,
increasing apoptosis (33). In a
mouse model of IR-induced hepatic injury, administrating the
antioxidant mangafodipir reestablished oxidative balance and
suppressed apoptosis (34). Using
the same mouse model, Yu et al (35) demonstrated that the Notch-Janus
kinase 2 (JAK2)/signal transducer and activator of transcription 3
(STAT3) signaling pathway protected hepatocytes from IR injury by
attenuating oxidative stress and inhibiting apoptosis in
vitro and in vivo. In the present study, we observed
apoptosis in liver tissues subjected to IR 24 h after reperfusion,
as shown by an increased apoptotic index, decreased expression of
Bcl-2, and increased expression of Bax and Casp3. Furthermore, we
showed that transplantation of BM-MSCs via the portal vein
decreased apoptosis. Therefore, our results are consistent with
previous studies suggesting that apoptosis is involved in
IR-induced hepatic injury and that BM-MSCs inhibit apoptosis.
BM-MSCs inhibit hepatocyte apoptosis by secreting
cytokines, thus regulating cellular signal transduction pathways.
In rats, BM-MSCs secrete VEGF, which attenuates myocardial IR
injury by activating the PI3K signaling pathway (36), and the PI3K pathway can regulate
the expression of Bcl-2, an anti-apoptotic protein (37). In rat neurons, MSCs secrete
cytokines that reduce chronic ethanol-induced injury by modulating
the extracellular-signal-regulated kinase (ERK)1/2 pathway
(38). The ERK1/2 pathway
regulates apoptosis by increasing the Bax/Bcl-2 ratio, Casp3 levels
and TNF levels (39). In the
present study, we observed, at 24 h after reperfusion and BM-MSC
transplantation, that Bcl-2 levels, an anti-apoptotic protein, was
increased, and that the expression levels of pro-apoptotic
proteins, Bax and Casp3, were decreased. We thus inferred that
BM-MSCs inhibit apoptosis in IR-induced hepatic injury via their
paracrine activity.
In conclusion, we demonstrated that in a rat model
of IR injury, hepatic injury occurred within 24 h of reperfusion
and that the oxidative stress response and subsequent apoptosis
were involved in the process of hepatic IR damage. We also observed
that BM-MSCs transplanted via the portal vein could attenuate IR
injury by, at least in part, suppressing the oxidative stress
response and inhibiting apoptosis.
Acknowledgements
The authors thank Dr Liang Hongsheng
and Dr Du Wenzhong for their technical assistance.
References
|
1
|
Carden DL and Granger DN: Pathophysiology
of ischaemia-reperfusion injury. J Pathol. 190:255–266. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nieuwenhuijs VB, De Bruijn MT, Padbury RT
and Barritt GJ: Hepatic ischemia-reperfusion injury: roles of
Ca2+ and other intracellular mediators of impaired bile
flow and hepatocyte damage. Dig Dis Sci. 51:1087–1102. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jaeschke H, Smith CV and Mitchell JR:
Hypoxic damage generates reactive oxygen species in isolated
perfused rat liver. Biochem Biophys Res Commun. 150:568–574. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jaeschke H and Mitchell JR: Mitochondria
and xanthine oxidase both generate reactive oxygen species in
isolated perfused rat liver after hypoxic injury. Biochem Biophys
Res Commun. 160:140–147. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jaeschke H, Bautista AP, Spolarics Z and
Spitzer JJ: Superoxide generation by Kupffer cells and priming of
neutrophils during reperfusion after hepatic ischemia. Free Radic
Res Commun. 15:277–284. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shibuya H, Ohkohchi N, Seya K and Satomi
S: Kupffer cells generate superoxide anions and modulate
reperfusion injury in rat livers after cold preservation.
Hepatology. 25:356–360. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Walsh KB, Toledo AH, Rivera-Chavez FA,
Lopez-Neblina F and Toledo-Pereyra LH: Inflammatory mediators of
liver ischemia-reperfusion injury. Exp Clin Transplant. 7:78–93.
2009.PubMed/NCBI
|
|
8
|
Arumugam TV, Magnus T, Woodruff TM,
Proctor LM, Shiels IA and Taylor SM: Complement mediators in
ischemia-reperfusion injury. Clin Chim Acta. 374:33–45. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chang WJ and Toledo-Pereyra LH: Toll-like
receptor signaling in liver ischemia and reperfusion. J Invest
Surg. 25:271–277. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Caplan AI and Dennis JE: Mesenchymal stem
cells as trophic mediators. J Cell Biochem. 98:1076–1084. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Borlongan CV, Glover LE, Tajiri N, Kaneko
Y and Freeman TB: The great migration of bone marrow-derived stem
cells toward the ischemic brain: therapeutic implications for
stroke and other neurological disorders. Prog Neurobiol.
95:213–228. 2011. View Article : Google Scholar
|
|
12
|
Li SC, Acevedo J, Wang L, et al:
Mechanisms for progenitor cell-mediated repair for ischemic heart
injury. Curr Stem Cell Res Ther. 7:2–14. 2011.PubMed/NCBI
|
|
13
|
Wise AF and Ricardo SD: Mesenchymal stem
cells in kidney inflammation and repair. Nephrology. 17:1–10. 2011.
View Article : Google Scholar
|
|
14
|
Pan RL, Wang P, Xiang LX and Shao JZ:
Delta-like 1 serves as a new target and contributor to liver
fibrosis down-regulated by mesenchymal stem cell transplantation. J
Biol Chem. 286:12340–12348. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kuo TK, Hung SP, Chuang CH, et al: Stem
cell therapy for liver disease: parameters governing the success of
using bone marrow mesenchymal stem cells. Gastroenterology.
134:2111–2121. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Banas A, Teratani T, Yamamoto Y, et al:
IFATS collection: in vivo therapeutic potential of human adipose
tissue mesenchymal stem cells after transplantation into mice with
liver injury. Stem Cells. 26:2705–2712. 2008. View Article : Google Scholar
|
|
17
|
Zhang XM, Du F, Yang D, et al:
Transplanted bone marrow stem cells relocate to infarct penumbra
and co-express endogenous proliferative and immature neuronal
markers in a mouse model of ischemic cerebral stroke. BMC Neurosci.
11:1382010. View Article : Google Scholar
|
|
18
|
Nauta RJ, Tsimoyiannis E, Uribe M, Walsh
DB, Miller D and Butterfield A: Oxygen-derived free radicals in
hepatic ischemia and reperfusion injury in the rat. Surg Gynecol
Obstet. 171:120–125. 1990.PubMed/NCBI
|
|
19
|
Suzuki S, Nakamura S, Koizumi T, et al:
The beneficial effect of a prostaglandin I2 analog on ischemic rat
liver. Transplantation. 52:979–983. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xue T, Luo P, Zhu H, et al: Oxidative
stress is involved in Dasatinib-induced apoptosis in rat primary
hepatocytes. Toxicol Appl Pharmacol. 261:280–291. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gavrieli Y, Sherman Y and Ben-Sasson SA:
Identification of programmed cell death in situ via specific
labeling of nuclear DNA fragmentation. J Cell Biol. 119:493–501.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li SC, Wang L, Jiang H, Acevedo J, Chang
AC and Loudon WG: Stem cell engineering for treatment of heart
diseases: potentials and challenges. Cell Biol Int. 33:255–267.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Petit I, Jin D and Rafii S: The
SDF-1-CXCR4 signaling pathway: a molecular hub modulating
neo-angiogenesis. Trends Immunol. 28:299–307. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen L, Tredget EE, Wu PY and Wu Y:
Paracrine factors of mesenchymal stem cells recruit macrophages and
endothelial lineage cells and enhance wound healing. PLoS One.
3:e18862008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jin SZ, Meng XW, Sun X, et al: Hepatocyte
growth factor promotes liver regeneration induced by transfusion of
bone marrow mononuclear cells in a murine acute liver failure
model. J Hepatobiliary Pancreat Sci. 18:397–405. 2011. View Article : Google Scholar
|
|
26
|
McCord JM: Oxygen-derived free radicals in
postischemic tissue injury. N Engl J Med. 312:159–163. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang D, Jiang M and Miao D: Transplanted
human amniotic membrane-derived mesenchymal stem cells ameliorate
carbon tetrachloride-induced liver cirrhosis in mouse. PLoS One.
6:e167892011. View Article : Google Scholar
|
|
28
|
Cho KA, Woo SY, Seoh JY, Han HS and Ryu
KH: Mesenchymal stem cells restore CCl4-induced liver
injury by an antioxidative process. Cell Biol Int. 36:1267–1274.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen YT, Sun CK, Lin YC, et al:
Adipose-derived mesenchymal stem cell protects kidneys against
ischemia-reperfusion injury through suppressing oxidative stress
and inflammatory reaction. J Transl Med. 9:512011. View Article : Google Scholar
|
|
30
|
Guzun R, Timohhina N, Tepp K, et al:
Regulation of respiration controlled by mitochondrial creatine
kinase in permeabilized cardiac cells in situ. Importance of system
level properties. Biochim Biophys Acta. 1787:1089–1105. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang GW, Klein JB and Kang YJ:
Metallothionein inhibits doxorubicin-induced mitochondrial
cytochrome c release and caspase-3 activation in cardiomyocytes. J
Pharmacol Exp Ther. 298:461–468. 2001.PubMed/NCBI
|
|
32
|
Waetzig GH, Rosenstiel P, Arlt A, et al:
Soluble tumor necrosis factor (TNF) receptor-1 induces apoptosis
via reverse TNF signaling and autocrine transforming growth
factor-beta1. FASEB J. 19:91–93. 2005.PubMed/NCBI
|
|
33
|
Bueno OF, De Windt LJ, Tymitz KM, et al:
The MEK1-ERK1/2 signaling pathway promotes compensated cardiac
hypertrophy in transgenic mice. EMBO J. 19:6341–6350. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Coriat R, Leconte M, Kavian N, et al:
Mangafodipir protects against hepatic ischemia-reperfusion injury
in mice. PLoS One. 6:e270052011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yu HC, Qin HY, He F, et al: Canonical
notch pathway protects hepatocytes from ischemia/reperfusion injury
in mice by repressing reactive oxygen species production through
JAK2/STAT3 signaling. Hepatology. 54:979–988. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Angoulvant D, Ivanes F, Ferrera R,
Matthews PG, Nataf S and Ovize M: Mesenchymal stem cell conditioned
media attenuates in vitro and ex vivo myocardial reperfusion
injury. J Heart Lung Transplant. 30:95–102. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shroff EH, Snyder CM, Budinger GR, et al:
BH3 peptides induce mitochondrial fission and cell death
independent of BAX/BAK. PLoS One. 4:e56462009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu L, Cao JX, Sun B, et al: Mesenchymal
stem cells inhibition of chronic ethanol-induced oxidative damage
via upregulation of phosphatidylinositol-3-kinase/Akt and
modulation of extracellular signal-regulated kinase 1/2 activation
in PC12 cells and neurons. Neuroscience. 167:1115–1124. 2010.
View Article : Google Scholar
|
|
39
|
Zhuang S and Schnellmann RG: A
death-promoting role for extracellular signal-regulated kinase. J
Pharmacol Exp Ther. 319:991–997. 2006. View Article : Google Scholar : PubMed/NCBI
|