Introduction
Breast cancer is the most common cancer and the
second leading cause of cancer-related mortalities among women
after lung cancer. Since 1896, clinical and experimental studies
have supported the idea that breast cancer is a classical model of
a hormone-dependent malignancy and that estrogens are mammary
carcinogens. Estrogen plays an important role in the growth and
differentiation of the normal mammary gland. The effects of
estrogens are mediated by the intracellular estrogen receptors
(ERs) and ERα is mostly found in the uterus and mammary gland
(1). ER is a transcription
activator and binds to the estrogen response element of the target
gene activating gene transcription (2). It has been noted that estrogen
administration enhances tumor growth while estrogen deprivation
reduces tumors (3); therefore,
elevated levels of estrogen in the body are a risk factor for
breast cancer.
Breast cancer treatments include chemotherapy,
hormone therapy as well as surgery and radiotherapy depending on
the stage. Doxorubicin and tamoxifen are widely used drugs in
chemotherapy and hormonal therapy, respectively. The exact
mechanism of how doxorubicin works is complex, but it is thought
that it interacts with DNA, prevents the DNA double helix from
being resealed and stops the process of replication (4). Previous studies including ours have
shown that doxorubicin induces autophagy (5). Tamoxifen is an estrogen receptor
antagonist which competes with estrogen to bind to its receptor.
The estrogen receptor/tamoxifen complex stops the genes being
switched on by estrogen (6,7).
Studies have shown that tamoxifen induces autophagy and apoptotic
cell death in estrogen-positive breast cancer cells (8,9).
Cancer stem cells (CSCs) are cancer cells that
possess the characteristics of normal stem cells (10). The cancer stem cell hypothesis
proposes that tumors are initiated and maintained by a small
fraction of cells, but the origin of these tumorigenic cells are
actually not known. The stem cell theory of cancer proposes two
major concepts. One theory claims that CSCs arise from mutated stem
cells and then these mutated cells expand in such a manner that the
mutation is shared by many of the descendants (11). An alternative theory proposes that
transformed and differentiated cells acquire stem cell-like
characteristics (12). This
hypothesis is also known as epithelial to mesenchymal transition
(EMT).
The development of the mammary gland suggests that
stem cells play an important role in the biology of the breast.
This fact suggests that the mammary gland is also particularly
prone to carcinogenesis, and that breast stem cells play a very
important role in breast cancer. CSCs also have been described in
breast cancers. The CSC hypothesis in breast cancer assumes that
CSCs can generate cells with a certain type of aberrant and limited
differentiation (13).
Conventional therapies used at present such as chemotherapy and
radiation kill the growing differentiated cells but fail to kill
these cancer-initiating cells (14). The reason is assumed that CSCs
share the properties of normal stem cells which have mechanisms
that make them relatively resistant to chemotherapy. These
properties include resistance to drugs and toxins by expression of
several ABC transporters, increased expression of BcL-2 family
proteins, increased expression of pumps such as breast cancer
resistance protein (BCRP) (15)
and P-glycoprotein (PGP) (16)
and active DNA repair capacity (13). In addition, stem cells divide
infrequently in contrast to differentiated cells, and antimitotic
chemotherapies are less effective against stem cells than mature
cancer cells (13). Moreover,
stem cells survive, grow and form colonies as tumorospheres in
serum-free suspension, while more differentiated cells die under
these conditions. In solid tumors, it has been shown that only a
small proportion of the tumor cells are able to form colonies as
revealed by in vitro clonogenic assays (17).
It has been proposed that CSCs are able to be
isolated from breast cancer cell lines using specific cell markers,
CD44+/CD24−/low (18). CD44 is a stem cell marker that is
common to different organs and pathologies. However, the
CD44+/CD24−/low phenotype is probably tissue
restricted. Al-Hajj et al (19) used these cell-surface markers to
isolate a subpopulation of highly tumorigenic breast cancer cells
from human breast tumor. In breast cancer, a population of
CD44+/CD24−/low cells is considered to be
highly enriched in cancer-initiating cells which are 1,000 times
more tumorigenic than other cell populations, and injection of as
few as 200 cells was found to lead to tumor formation in SCID mice
.
Autophagy is a tightly regulated process involving
the degradation of a cell’s own components through the lysosomal
machinery. Autophagy plays a normal part in cell growth,
development, and homeostasis, helping to maintain a balance between
the synthesis, degradation, and the subsequent recycling of
cellular products. During nutrient starvation, autophagy leads to
the breakdown of non-vital components and the release of nutrients
to maintain metabolism and ATP levels to ensure cell survival
(20). In addition, the
autophagylysosomal pathway is an important mechanism for regulating
the homeostasis of intracellular long-lived proteins and organelles
(21). Moreover, autophagy plays
a role in innate and adaptive immune responses (22). During apoptosis, induced autophagy
can be either a protective mechanism or a process that causes cell
death. Recent studies have shown that autophagy delays apoptotic
death in noninvasive breast cancer cells following DNA damage
(23). On the other hand, in the
absence of apoptosis, autophagy can trigger a form of cell death
(24). A previous study also
demonstrated that prolonged autophagy in the absence apoptotic
machinery is a cell survival mechanism that delays cell death in
hematopoietic cells when growth factors and nutrients are short in
supply (25). Malfunctioning of
autophagy is observed in many human diseases such as cancer,
neurodegenerative, infectious and inflammatory diseases, heart
diseases and diabetes (26,27).
Understanding the role of CSCs during carcinogenesis
has become a major focus in stem cell biology and cancer research.
Therefore, the aim of this study was to isolate breast CSCs and
test whether these cells show resistance to hormonal therapy
(tamoxifen) similar to their resistance shown to chemotherapy. For
that purpose, CSCs were tested initially for their chemotherapy
resistance using doxorubicin. The possible autophagic cell death
mechanism was then assessed in breast CSCs after they were treated
with tamoxifen.
Materials and methods
Culture of MCF-7 cells
MCF-7 cells were kindly provided by Osmangazi
University Medical School and cultured in Dulbecco’s modified
Eagle’s medium (DMEM)/F12 GlutaMAX containing 10% fetal bovine
serum (FBS) and 1% penicillin-streptomycin (PS) solution (100 U/ml
penicillin and 100 μg/ml streptomycin). Cells were grown in
T-25 flasks with 4 ml medium and incubated in a CO2
incubator at 37°C in 5% CO2. The medium of the cells was
replaced every other day, and cells were passaged when they reached
confluence.
Isolation of
CD44+/CD24−/low breast CSCs
MCF-7 cells were detached from the flask by
treatment with trypsin-EDTA solution, and the cells were
centrifuged. After cells were washed with PBS (pH 7.4), the cell
pellet was resuspended in FITC-conjugated CD44 antibody (BD
Pharmingen, USA) and PE-conjugated CD24 antibody (BioLegend, USA).
The antibody concentration was 10 μl of antibody solution
per 1×106 cells. Cells were incubated with the
antibodies for 40 min at room temperature (RT) in the dark. Unbound
antibodies were washed off with PBS, and the cell pellet was
resuspended in culture medium after centrifugation to be sorted.
The cells were sorted by a fluorescence-activated cell sorting
device (FACSAria II; BD Pharmingen).
Comparison of the growth rate of
CD44+/CD24−/low and MCF-7 cells
CD44+/CD24−/low and MCF-7
cells were seeded into 24-well plates. At 24, 48, 72 and 96 h of
incubation times, the values of the growth rates were determined by
a cell proliferation assay. Culture medium was mixed with MTS One
Solution (Promega, USA) at a ratio of 5:1, and 240 μl of
this mix was added into each well. Cells were incubated for 2.5 h
at 37°C in a CO2 incubator. Then, 200 μl of
solution from each well was transferred into a 96-well plate.
Absorbances were measured at 490 nm.
Determination of the inhibitory
concentrations of tamoxifen and doxorubicin
CD44+/CD24−/low and MCF-7
cells (1,250 cells/well) were seeded into each well of a 96-well
plate, and different concentrations of tamoxifen and doxorubicin
were applied to the cells one day later by diluting the drugs with
culture medium. Following 72 h of drug treatment, the MTS
experiment was performed. Culture medium was mixed with MTS One
Solution at a ratio of 5:1, and 120 μl of MTS/culture medium
was added to each well. The cells were incubated with the solution
for 2 h at 37°C. Then, absorbances were measured at 490 nm.
Analysis of apoptosis by Annexin V
staining and flow cytometry
The FITC Annexin V Apoptosis Detection Kit II (BD
Pharmingen) was used to detect apoptotic cell death in the
tamoxifen- and doxorubicin-treated cells incubated for 48 h.
Untreated and drug-treated CD44+/CD24−/low
and MCF-7 cells were detached from the flasks. Cells were
transferred to FACS tubes, washed with PBS and centrifuged. The
cell pellets were dissolved in 5 μl Annexin V and 5
μl propidium iodide (PI) solution and incubated at RT for 20
min in the dark. Meanwhile, 1 ml binding buffer was diluted with 9
ml PBS. Then, 400 μl diluted binding buffer was added into
each FACS tube and the cells were analyzed by flow cytometry.
Analysis of autophagy by flow
cytometry
Tamoxifen- and doxorubicin-treated
CD44+/CD24−/low and MCF-7 cells and untreated
cells were detached from flasks following 48 h of treatment and
transferred to FACS tubes. Acridine orange (1 μl;
Sigma-Aldrich Co., Germany) was mixed with 10 ml culture medium.
The mixture (1 ml) was added into each tube after they were
centrifuged. The cell pellets were resuspended in the acridine
orange staining solution and incubated at 37°C for 15 min. The cell
pellets were then washed with PBS, and finally the cell pellets
were resuspended in 300 μl PBS to be analyzed by flow
cytometry.
Western blot analysis of the LC3
protein
Both MCF-7 and CD44+/CD24−/low
cells were seeded, and one day later they were treated with
tamoxifen and doxorubicin, respectively. At 48 h, treated and
control cells were collected by trypsinization, washed with PBS,
and the pellets were lysed with 100–150 μl lysis buffer. The
protein concentration of the lysates was determined with Coomassie
protein assay reagent (Thermo Scientific, USA) following the
manufacturer’s instructions. Subsequently, 20 μg protein of
each sample was loaded onto a 12% separating acrylamide gel. After
blotting, the membrane was incubated with LC3 (Axxora Nanotools,
Germany) and β-actin (Cell Signaling, Technology, Inc., USA)
primary antibodies at a concentration of 1 μg/ml at 4°C. On
the next day, the membrane was then incubated with the secondary
antibody at a dilution of 1:10,000 for 1 h at RT with gentle
agitation. Finally, the membrane was washed with TBST and then
incubated with SuperSignal West Pico Chemiluminescent Substrate
(Thermo Scientific) for 5 min. Images of the proteins were captured
with a molecular imager (ChemiDoc XRS+; Bio-Rad, USA) at a suitable
time (change with respect to the primary antibody).
Results
Determination of the half maximal
inhibitory concentrations of tamoxifen and doxorubicin
The initial step for evaluating the effect of
tamoxifen and doxorubicin on isolated breast CSCs was to determine
the half maximal inhibitory concentration (IC50) of the
drugs. Cell proliferation assay (MTS) was used to determine the
IC50 of each drug by treating the MCF-7 cells with
different concentations. Following 72 h of treatment, the results
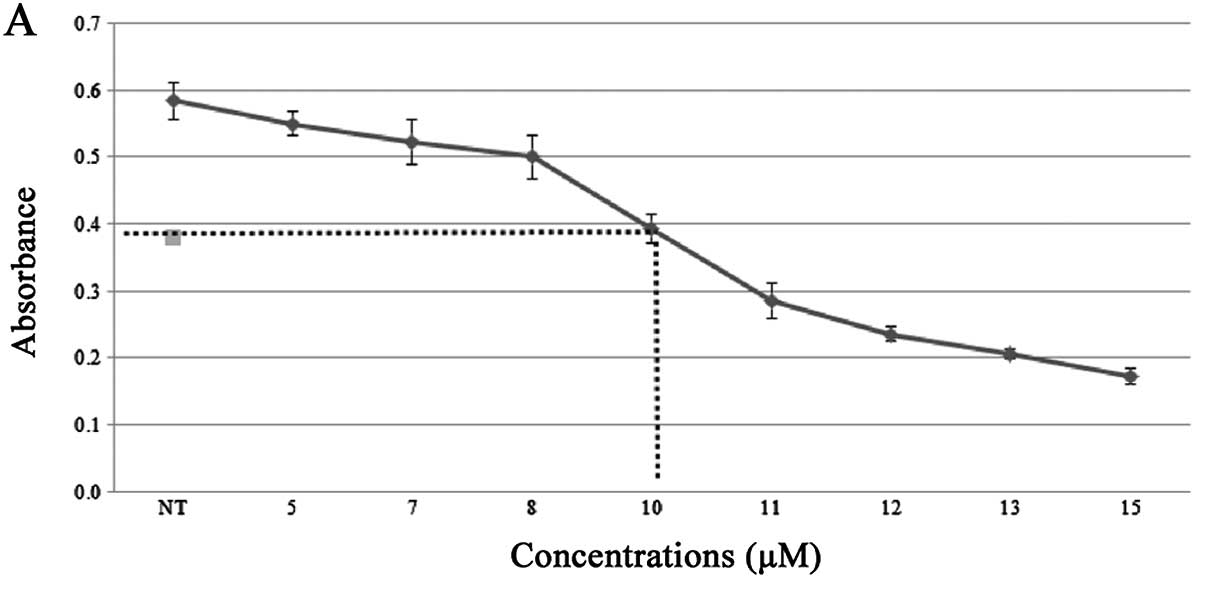
showed that the IC50 of tamoxifen and doxorubicin in
MCF-7 cells was ∼10 and 0.65 μM, respectively (Fig. 1). Therefore, 10 μM
tamoxifen and 0.7 μM doxorubicin were used for the
subsequent experiments. Moreover, we found that tamoxifen-induced
effects on apoptosis and autophagy were significant at 48 h in the
MCF-7 cells. For this reason, both tamoxifen and doxorubicin were
incubated with the cells for 48 h.
Isolation of
CD44+/CD24−/low cells
Different techniques for the isolation of the breast
CSC population have been used. In this study, breast CSCs were
isolated from the breast cancer cell line (MCF-7) using specific
cell markers. These isolated cells were positive for CD44 and
negative/low for CD24 markers.
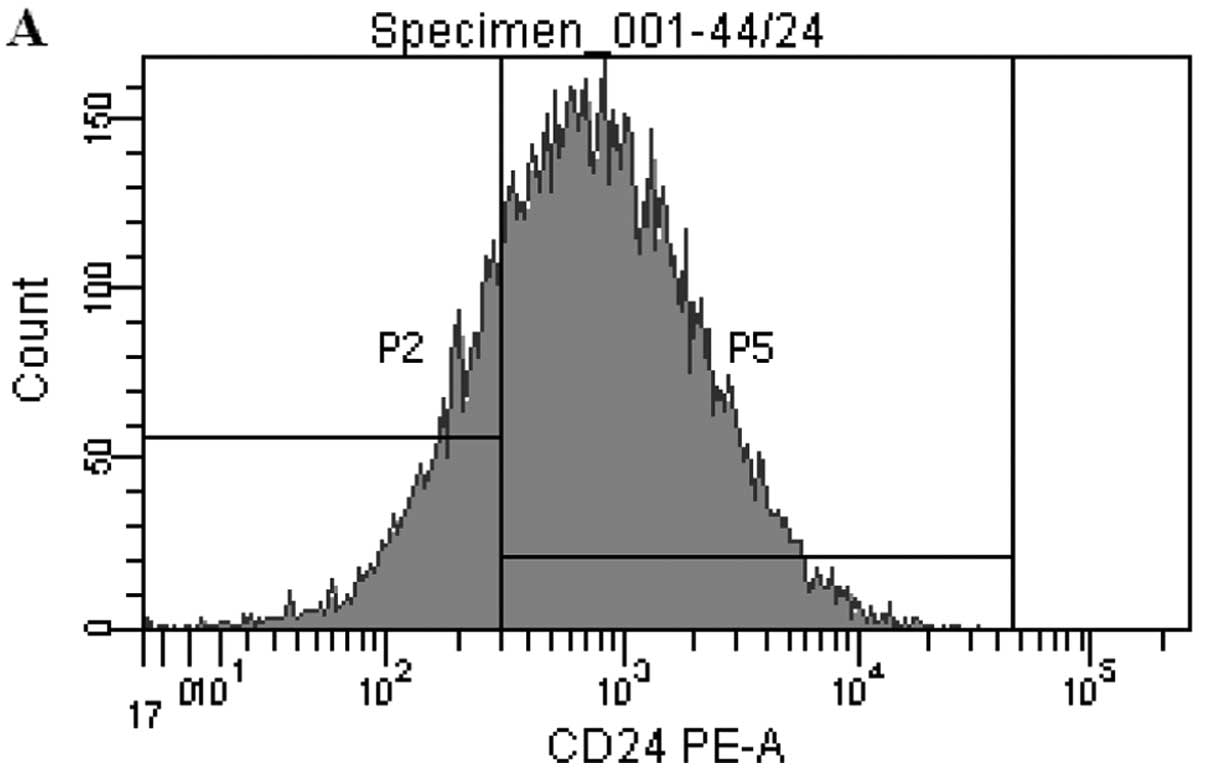
CD44+/CD24−/low cells were sorted from the
MCF-7 cells using CD44 and CD24 antibodies at the same time. At the
end, it was observed that there were ∼32% CD44+ cells
and 23% CD24−/low cells in the MCF-7 cells. Overall,
there was ∼1% CD44+/CD24−/low cells present
in the MCF-7 cell line (Fig. 2).
CD44+/CD24− cells were used immediately
following sorting since they start to lose their properties by half
in 5 days.
Comparison of the growth rate of
CD44+/CD24−/low cells and MCF-7 cells
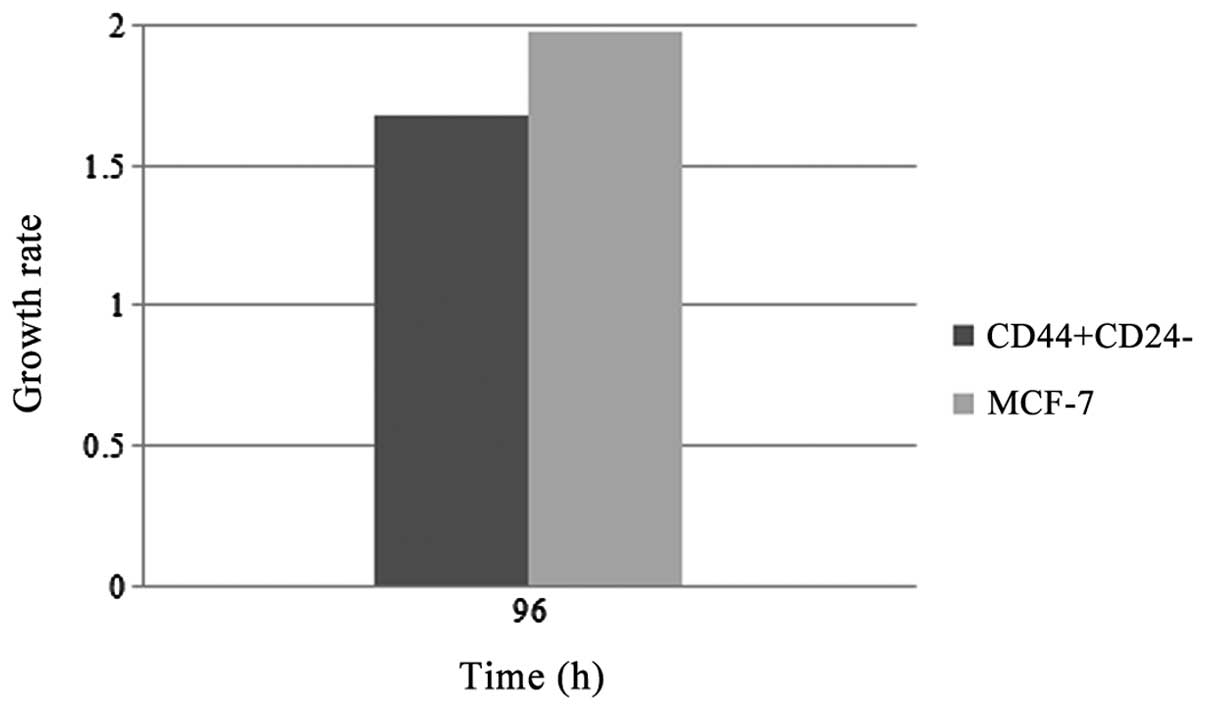
CSCs display particular features; one of which is
slow cell growth. To test this parameter, cell cycle rates of
sorted cells and parental cells were compared. The proliferation
assay results demonstrated that
CD44+/CD24−/low cells proliferated slower
than the MCF-7 cells when they were allowed to grow for 96 h
(Fig. 3).
Effect of tamoxifen and doxorubicin on
the cell proliferation of MCF-7 and
CD44+/CD24−/low cells
Previous studies have shown that
CD44+/CD24−/low cells show resistance to
chemotherapeutic drugs. Based on these studies, we tested the
sorted cells for resistance to chemotherapy using doxorubicin and
evaluated their viability by MTS cell proliferation assay. We
compared CD44+/CD24−/low cells to MCF-7 cells
after treatment using the same drug. Moreover, the same procedures
were also performed to evaluate the effects of tamoxifen in this
CSC population.
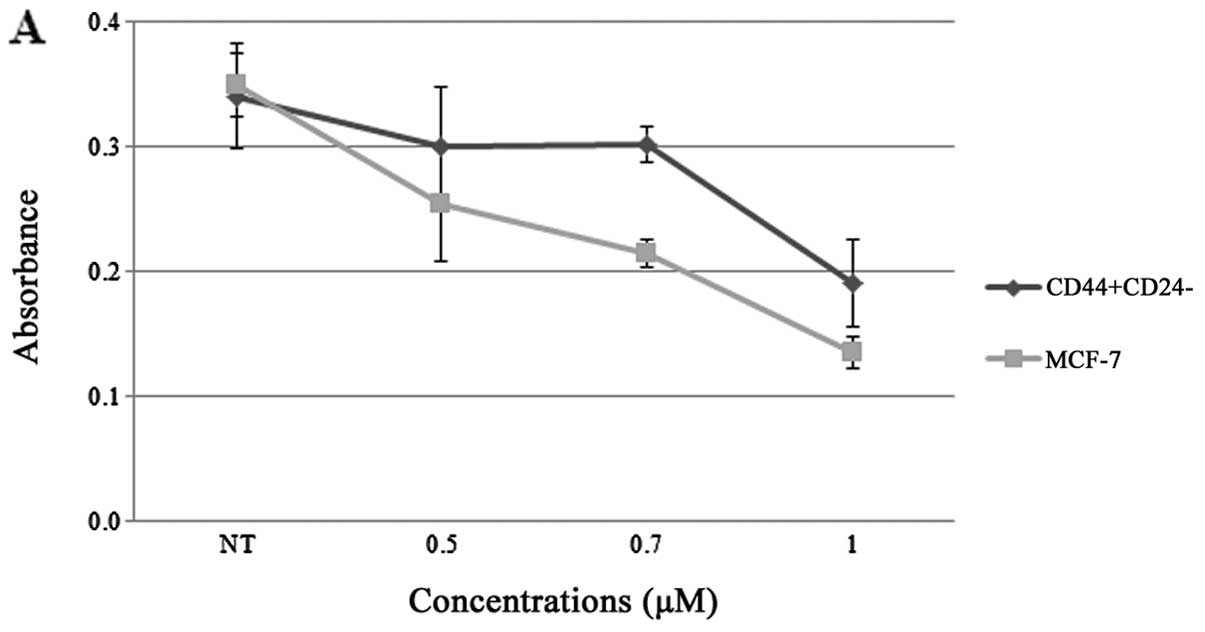
Both isolated breast CSCs and parental cells were
treated with doxorubicin for 48 h. Proliferation assay results
demonstrated that the CD44+/CD24−/low cells
were less sensitive to doxorubicin when compared to MCF-7 cells
when these cells were treated with concentrations at 0.5, 0.7 and 1
μM (Fig. 4A). In contrast,
the proliferation assay results of tamoxifen experiments revealed
that CD44+/CD24−/low cells did show a slight
resistance to tamoxifen when compared to the MCF-7 cells (Fig. 4B).
Induction of apoptosis and autophagy in
CD44+/CD24−/low cells by doxorubicin and
tamoxifen
In addition to the proliferation assay, we carried
out experiments to ascertain whether
CD44+/CD24−/low breast CSCs exhibit
resistance to apoptosis or autophagy in response to chemotherapy
and hormonal therapy. Both sorted and parental cells were treated
with doxorubicin and tamoxifen, and then stained by Annexin V and
PI for detection of apoptosis and acridine orange for the autophagy
studies. The cells were then analyzed by flow cytometry. Flow
cytometric results demonstrated that
CD44+/CD24−/low cells underwent ∼10% less
apoptotic cell death comparing to the MCF-7 parental cells
following treatment with doxorubicin (Fig. 5A), supporting the results of the
cell proliferation assay. Therefore, the results of the doxorubicin
studies revealed that breast CSCs exhibit slight resistance to
apoptosis following treatment with chemotherapy drugs. With respect
to autophagy, on the other hand,
CD44+/CD24−/low cells did not show any
resistance to doxorubicin as indicated by acridine orange stain and
flow cytometric analysis when compared to the MCF-7 cells (Fig. 5B).
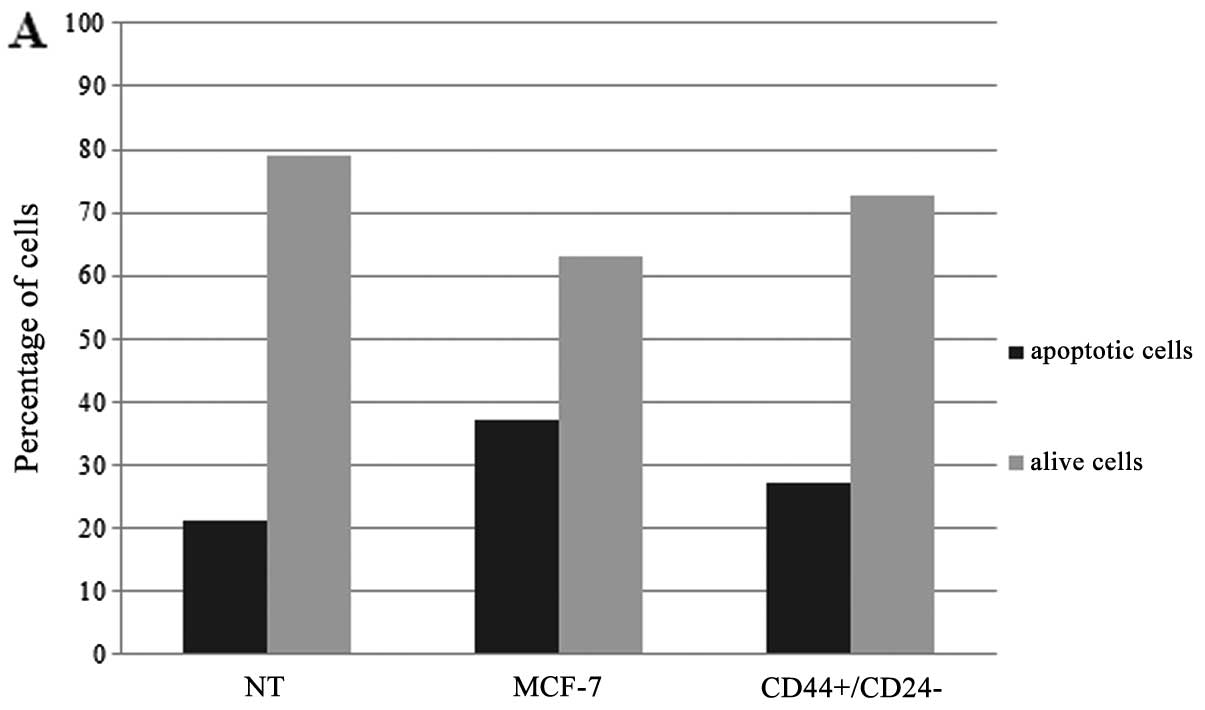
In the case of tamoxifen,
CD44+/CD24−/low cells did not show any
difference in terms of resistance to tamoxifen when compared to the
parental MCF-7 cells (Fig. 5C and
D). The apoptosis and autophagy results of the
CD44+/CD24−/low cells and MCF-7 cells were
similar.
Effect of tamoxifen in
CD44+/CD24−/low and MCF-7 cells in respect to
the autophagy marker LC3
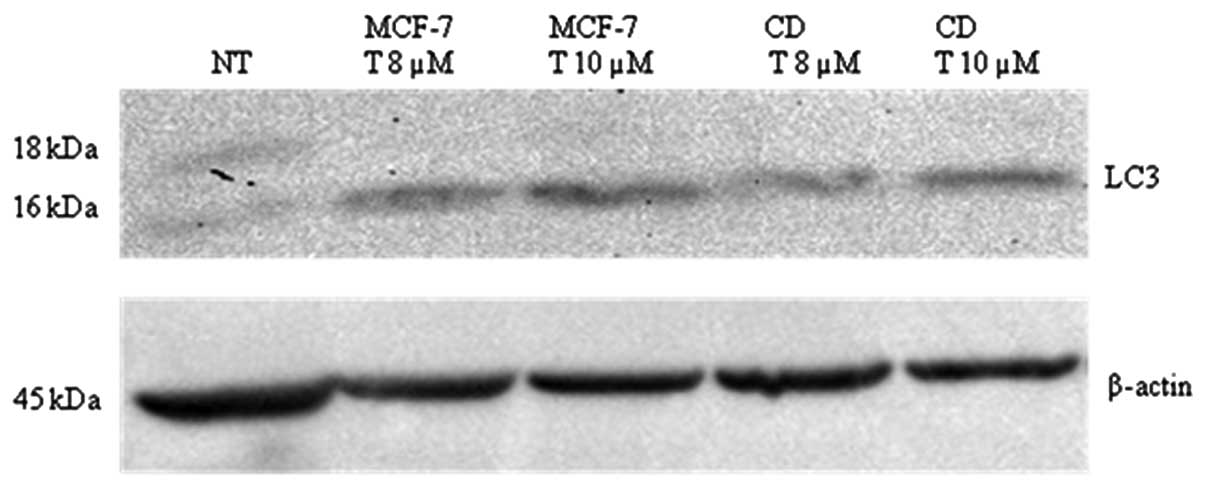
In order to observe whether tamoxifen has a
differential effect on autophagy in sorted
CD44+/CD24−/low breast CSCs compared to the
parental MCF-7 cells, we analyzed LC3-I and II protein by western
blotting. Tamoxifen induced autophagy in both
CD44+/CD24−/low breast CSCs and MCF-7 cells
at 48 h (Fig. 6). LC3-I (18 kDa)
to LC3-II (16 kDa) conversion was clearly visualized in the treated
cells. Densitometric analysis of the LC3-II bands of the samples
was carried out by ImageJ program (Table I). According to these results, 10
μM tamoxifen induced more extensive autophagic cell death in
both the sorted and parental cells when compared to the untreated
and 8 μM drug-treated cells. However, the breast CSCs did
not show a significant difference in the extent of autophagic cell
death when compared to the MCF-7 cells.
 | Table IRelative density of the LC3-II bands
of the drug-treated cells compared to the untreated cells. |
Table I
Relative density of the LC3-II bands
of the drug-treated cells compared to the untreated cells.
| Samples | Relative
density |
|---|
| NT | 1 |
| MCF-7 (8 μM
tamoxifen) | 2.70 |
| MCF-7 (10 μM
tamoxifen) | 3.30 |
|
CD44+/CD24−/low (8
μM tamoxifen) | 2.80 |
|
CD44+/CD24−/low (10
μM tamoxifen) | 3.19 |
Discussion
CSCs were first described by the evidence that the
growth and propagation of leukemia were driven by a small
population of leukemia cells that have the ability for continual
self-renewal, and these cells were termed as CSCs (13). Later, it was proposed that
inhibition of tumor stem cells could prevent the recurrence of
leukemia (28). Since that time,
CSCs have been recognized as important components in carcinogenesis
and have been isolated from many types of cancers including breast,
brain, skin, head and neck and thyroid (13).
The objective of this study was to isolate breast
CSCs from the MCF-7 breast cancer cell line and ascertain whether
these isolated cells exhibit a difference in the autophagic
response to tamoxifen-based hormonal therapy, which is used to
treat ∼50–60% of breast cancer patients (8). For that purpose, breast CSCs were
isolated from MCF-7 cells by using CD44 and CD24 markers. Sorting
results showed that only 1% of MCF-7 cells had
CD44+/CD24−/low breast cancer stem cell
markers. This finding corroborated the results of Al-Hajj et
al (19) who also reported
the presence of ∼1% of CD44+/CD24−/low cells
in the MCF-7 cell line. Moreover, we observed that the sorted cells
started to lose their CD44+/CD24−/low
properties by half in ∼5 days. Wright et al (29) also demonstrated that these sorted
cells did not exhibit the CD44+ and CD24−
stem cell markers and lost chemotherapy drug resistance compared to
parental cells when they were passaged four times as a
monolayer.
Slow growth rate is a well-known parameter of breast
CSCs (13). To ensure that our
isolated cells carry stem cell features, we tested and demonstrated
that sorted breast CSCs proliferated slower than the parental
cells, as supported by Fillmore and Kuperwasser (30). Moreover, Kim et al
(31) sorted CD24−/low
cells from MCF-7 cells and compared the proliferation rate of these
cells with the parental and the growth of CD24+ cells by
growing them for 72 h in DMEM. Their result revealed that the
numbers of cultured CD24+ and parental cells were higher
than that in the CD24−/low cells. These results reveal
that CD24+ and MCF-7 cells have a higher proliferative
capacity than CD24−/low cells and suggest that the
expression of CD24 may enhance the growth and proliferation of
MCF-7 cells.
It is known that both chemotherapy and radiation
kill growing differentiated cells. Conventional chemotherapies are
initially effective in controlling tumor growth. However, many
patients relapse over time. One explanation for relapse is that
cells which have a high tumorigenic potential are resistant to
therapy (32). Resistance of
CSCs, including breast CSCs toward chemotherapy drugs and radiation
has been shown (14).
Specifically, a previous study showed that
CD44+/CD24−/low breast CSCs were more
resistant to chemotherapy drugs than the parental cells when the
cells were treated for 48 h (29). Therefore, we tested the effect of
doxorubicin on our sorted CD44+/CD24−/low
cells which were described as a highly tumorigenic subpopulation of
breast cancer cells. Proliferation results demonstrated that sorted
cells consisted of more viable cells comparing to the MCF-7 cells
when these cells were treated with 0.5, 0.7 and 1 μM
doxorubicin. In addition, flow cytometric results revealed that
apoptotic cell death was ∼10% less in the
CD44+/CD24−/low cells than that in the MCF7
cells when treated with 0.7 μM doxorubicin for 48 h, while
the autophagic cell death ratio remained the same.
Tamoxifen is an FDA approved drug for the prevention
and the treatment of breast cancer. For many years, it has been
used as endocrine therapy for the treatment of both early and
advanced breast cancer for patients with hormone receptor-positive
breast cancer. Studies have shown that tamoxifen induces autophagy
and apoptotic cell death in estrogen-positive breast cancer cells
(8,9). In addition, we desired to ascertain
how tamoxifen affects breast CSCs and whether or not these sorted
cells display any resistance to hormonal therapy drugs. Therefore,
we initially optimized and identified the effective dose and
treatment time of tamoxifen. We then set out to study the effects
of tamoxifen on isolated breast CSCs and compared the results with
the MCF-7 parental cells. In contrast to doxorubicin, the tamoxifen
experimentation results obtained by flow cytometric assays, western
blot analysis and cell proliferation assay showed that both
apoptotic and autophagic cell death ratios were similar in the
CD44+/CD24−/low cells and MCF7 cells.
Overall, our studies demonstrated that isolated
CD44+/CD24−/low breast CSCs do not show
significant resistance to tamoxifen which is the front-line therapy
in most hormonal therapies for breast cancer patients. In
conclusion, previous studies have demonstrated that CSCs show
resistance to chemotherapy and radiation. This resistance is also
thought to be the reason for the reoccurrence of tumor formation
after chemotherapy or radiation therapy. In the present study,
breast CSCs were isolated and studied to observe whether they were
resistant to hormonal therapy. This study supported the results of
previous studies showing that isolated breast CSCs from the MCF-7
breast cancer cell line show slight resistance to undergo apoptosis
in response to doxorubicin. In the case of the hormonal therapy
drug, tamoxifen, our studies demonstrated that tamoxifen-induced
apoptotic and or autophagic cell death in these isolated cells were
similar to that noted in MCF-7 cells and did not show a significant
difference between the isolated breast CSCs and the parental
cells.
References
|
1
|
Gustafsson JA: Novel aspects of estrogen
action. J Soc Gynecol Investig. 7(Suppl 1): S8–S9. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Moggs JG and Orphanides G: Estrogen
receptors: orchestrators of pleiotropic cellular responses. EMBO
Rep. 2:775–781. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cos S, González A, Martínez-Campa C,
Mediavilla MD, Alonso-González C and Sánchez-Barceló EJ:
Estrogen-signaling pathway: a link between breast cancer and
melatonin oncostatic actions. Cancer Detect Prev. 30:118–128. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fornari FA, Randolph JK, Yalowich JC,
Ritke MK and Gewirtz DA: Interference by doxorubicin with DNA
unwinding in MCF-7 breast tumor cells. Mol Pharmacol. 45:649–656.
1994.PubMed/NCBI
|
|
5
|
Akar U, Chaves-Reyez A, Barria M, Tari A,
Sanguino A, Kondo Y, Kondo S, Arun B, Lopez-Berestein G and Ozpolat
B: Silencing of Bcl-2 expression by small interfering RNA induces
autophagic cell death in MCF-7 breast cancer cells. Autophagy.
4:669–679. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Deroo BJ and Korach KS: Estrogen receptors
and human disease. J Clin Invest. 116:561–570. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shang Y, Hu X, DiRenzo J, Lazar MA and
Brown M: Cofactor dynamics and sufficiency in estrogen
receptor-regulated transcription. Cell. 103:843–852. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bursch W, Ellinger A, Kienzl H, Török L,
Pandey S, Sikorska M, Walker R and Hermann RS: Active cell death
induced by the anti-estrogens tamoxifen and ICI 164 384 in human
mammary carcinoma cells (MCF-7) in culture: the role of autophagy.
Carcinogenesis. 17:1595–1607. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Paglin S, Hollister T, Delohery T, Hackett
N, McMahill M, Sphicas E, Domingo D and Yahalom J: A novel response
of cancer cells to radiation involves autophagy and formation of
acidic vesicles. Cancer Res. 61:439–444. 2001.PubMed/NCBI
|
|
10
|
Charafe-Jauffret E, Ginestier C and
Birnbaum D: Breast cancer stem cells: tools and models to rely on.
BMC Cancer. 9:2022009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang Y, Yang J, Zheng H, Tomasek GJ, Zhang
P, McKeever PE, Lee EY and Zhu Y: Expression of mutant p53 proteins
implicates a lineage relationship between neural stem cells and
malignant astrocytic glioma in a murine model. Cancer Cell.
15:514–526. 2009. View Article : Google Scholar
|
|
12
|
Santisteban M, Reiman JM, Asiedu MK,
Behrens MD, Nassar A, Kalli KR, Haluska P, Ingle JN, Hartmann LC,
Manjili MH, Radisky DC, Ferrone S and Knutson KL: Immune-induced
epithelial to mesenchymal transition in vivo generates breast
cancer stem cells. Cancer Res. 69:2887–2895. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Charafe-Jauffret E, Monville F, Ginestier
C, Dontu G, Birnbaum D and Wicha MS: Cancer stem cells in breast:
current opinion and future challenges. Pathobiology. 75:75–84.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dean M, Fojo T and Bates S: Tumour stem
cells and drug resistance. Nat Rev Cancer. 5:275–284. 2005.
View Article : Google Scholar
|
|
15
|
Zhou S, Schuetz JD, Bunting KD, Colapietro
AM, Sampath J, Morris JJ, Lagutina I, Grosveld GC, Osawa M,
Nakauchi H and Sorrentino BP: The ABC transporter Bcrp1/ABCG2 is
expressed in a wide variety of stem cells and is a molecular
determinant of the side-population phenotype. Nat Med. 7:1028–1034.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hait WN and Yang JM: Clinical management
of recurrent breast cancer: development of multidrug resistance
(MDR) and strategies to circumvent it. Semin Oncol. 32(Suppl 7):
S16–S21. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wolman SR, Heppner GH and Wolman E: New
directions in breast cancer research. FASEB J. 11:535–543.
1997.PubMed/NCBI
|
|
18
|
Ponti D, Costa A, Zaffaroni N, Pratesi G,
Petrangolini G, Coradini D, Pilotti S, Pierotti MA and Daidone MG:
Isolation and in vitro propagation of tumorigenic breast cancer
cells with stem/progenitor cell properties. Cancer Res.
65:5506–5511. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yorimitsu T and Klionsky DJ: Autophagy:
molecular machinery for self-eating. Cell Death Differ. 12(Supp 2):
S1542–S1552. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shintani T and Klionsky DJ: Autophagy in
health and disease: a double-edged sword. Science. 306:990–995.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ravikumar B, Moreau K, Jahreiss L, Puri C
and Rubinsztein DC: Plasma membrane contributes to the formation of
pre-autophagosomal structures. Nat Cell Biol. 12:747–757. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Abedin MJ, Wang D, McDonnell MA, Lehmann U
and Kelekar A: Autophagy delays apoptotic death in breast cancer
cells following DNA damage. Cell Death Differ. 14:500–510. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Codogno P and Meijer AJ: Autophagy and
signaling: their role in cell survival and cell death. Cell Death
Differ. 12:1509–1518. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lum JJ, Bauer DE, Kong M, Harris MH, Li C,
Lindsten T and Thompson CB: Growth factor regulation of autophagy
and cell survival in the absence of apoptosis. Cell. 120:237–248.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Beau I, Mehrpour M and Codogno P:
Autophagosomes and human diseases. Int J Biochem Cell Biol.
43:460–464. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schneider L and Zhang J: Lysosomal
function in macromolecular homeostasis and bioenergetics in
Parkinson’s disease. Mol Neurodegener. Apr 13–2010.(Epub ahead of
print). View Article : Google Scholar
|
|
28
|
Weissman IL, Anderson DJ and Gage F: Stem
and progenitor cells: origins, phenotypes, lineage commitments, and
transdifferentiations. Annu Rev Cell Dev Biol. 17:387–403. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wright MH, Calcagno AM, Salcido CD,
Carlson MD, Ambudkar SV and Varticovski L: Brca1 breast tumors
contain distinct CD44+/CD24− and
CD133+ cells with cancer stem cell characteristics.
Breast Cancer Res. 10:R102008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fillmore CM and Kuperwasser C: Human
breast cancer cell lines contain stem-like cells that self-renew,
give rise to phenotypically diverse progeny and survive
chemotherapy. Breast Cancer Res. 10:R252008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim HJ, Kim JB, Lee KM, Shin I, Han W, Ko
E, Bae JY and Noh DY: Isolation of CD24high and
CD24low/− cells from MCF-7: CD24 expression is
positively related with proliferation, adhesion and invasion in
MCF-7. Cancer Lett. 258:98–108. 2007.
|
|
32
|
Li X, Lewis MT, Huang J, Gutierrez C,
Osborne CK, Wu MF, Hilsenbeck SG, Pavlick A, Zhang X, Chamness GC,
Wong H, Rosen J and Chang JC: Intrinsic resistance of tumorigenic
breast cancer cells to chemotherapy. J Natl Cancer Inst.
100:672–679. 2008. View Article : Google Scholar : PubMed/NCBI
|