Introduction
Glucocorticoids, due to their anti-inflammatory and
immunosuppressive activity, are widely used as therapeutic tools
for the treatment of many inflammatory and/or immune diseases,
including rheumatoid arthritis, organ transplantation, leukemia,
multiple sclerosis or neurological disorders. However, there is
increasing evidence that glucocorticoid therapy is associated with
numerous toxic and/or side effects, including steroid-induced
diabetes (1,2). It has been demonstrated that
long-term and/or high-dose glucocorticoid therapy induces
hyperglycemia, in part, through the inhibition of insulin synthesis
and secretion, insulin resistance of peripheral tissues, and
increased hepatic glucose production, which may contribute to the
incidence and/or development of steroid diabetes (3,4).
Considering that steroid diabetes is also linked to steroid-induced
β-cell toxicity, research towards the better understanding of the
mechanisms underlying steroid-induced β-cell toxicity and
identification of new drugs or substances that prevent and/or
protect against steroid-induced β-cell toxicity is critical.
Previous investigations have shown that
dexamethasone (Dex), a synthetic glucocorticoid, reduces the
survival and induces the apoptosis of thymocytes, leukemia and
insulin-secreting cells (5-9),
and the effect is mediated through the release of mitochondrial
cytochrome c (5,6), the activation of caspases and
generation of reactive oxygen species (8), the inhibition of nuclear factor-κB
(9,10), and/or the inhibition of
phosphorylation of insulin receptor substrate-2 and protein kinase
B (PKB) (11). A large body of
evidence also demonstrates that Dex treatment leads to alteration
of the expression and/or activities of many intracellular signaling
proteins (12-14), including mitogen-activated protein
kinase (MAPK) phosphatase-1 (MKP-1) and the family of MAPKs, which
are involved in gene expression, cell survival and/or apoptosis.
Notably, a recent study revealed that Dex treatment inhibited the
phosphorylation of extracellular signal-regulated protein
kinase-1/2 (ERK-1/2), one member of the MAPK family, and stimulated
expression of MKP-1, contributing to a reduction in pancreatic
β-cell proliferation in islets from early lactating mothers
(15). These results suggest that
Dex-induced β-cell toxicity may be attributed, to a small extent,
to the ability of Dex to modulate the expression and/or activities
of these signaling proteins.
White-rot fungi, including Ganoderma, have
been used for medicinal purposes for centuries in China, Japan and
Korea (16). Ceriporia (C.)
lacerata is one of the white-rot fungi and has been used in
bioremediations in nature, such as lignocellulose degradation
(17). The pharmacological effect
of C. lacerata on steroid-induced β-cell toxicity is not
known. In this study, we evaluated the effect of a crude extract
from a submerged cultivation of C. lacerata on the survival
and apoptosis of INS-1 rat insulin-secreting cells treated with
Dex.
Materials and methods
Materials
RPMI-1640 medium, heat-inactivated fetal bovine
serum (FBS) and penicillin-streptomycin were purchased from
WelGENE, Inc. (Daegu, Korea). Antibody against procaspase-9 was
purchased from Enzo Life Science, Inc. (Farmingdale, NY, USA).
Antibody against phosphoeukaryotic initiation factor-2α (p-eIF-2α)
was purchased from Epitomics (Burlingame, CA, USA). Antibodies
against X-linked apoptosis of protein (XIAP) and interleukin-1β
(IL-1β) were purchased from R&D Systems (Minneapolis, MN, USA).
Antibodies against p-extracellular signal-regulated protein
kinase-1/2 (p-ERK-1/2), p-PKB, T-ERK-1/2 (total ERK-1/2), T-PKB
(total PKB) and T-eIF-2α (total eIF-2α) were purchased from Cell
Signaling Technology (Danvers, MA, USA). Antibody against
poly(ADP-ribose) polymerase (PARP) was purchased from Roche
Diagnostics (Mannheim, Germany). LY294002 and streptozotocin (STZ)
were purchased from Biomol (Plymouth Meeting, PA, USA). Antibodies
against p-pancreatic endoplasmic reticulum kinase (p-PERK), T-PERK
(total PERK), activation of transcription factor-6 (ATF-6), MKP-1,
glucose-regulated protein 78 (GRP78), goat anti-rabbit
immunoglobulin G-horseradish peroxidase (IgG-HRP), and goat
anti-mouse IgG-HRP were purchased from Santa Cruz Biotechnology,
Inc. (Santa Cruz, CA, USA). Enzyme-linked chemiluminescence (ECL)
Western detection reagents were purchased from Thermo Fisher
Scientific (Waltham, MA, USA). Proteinase inhibitor cocktail (100X)
was purchased from Calbiochem (Madison, WI, USA). Bradford reagent
was purchased from Bio-Rad (Hercules, CA, USA). Other reagents,
including Dex, and the actin antibody were purchased from Sigma
(St. Louis, MO, USA).
Preparation of a crude extract of C.
lacerata (CLCE)
C. lacerata was initially grown on a potato
dextrose agar medium and was transferred to 50 ml of a seed culture
(potato/ dextrose broth) medium (both were from Difco Laboratories,
Inc., Detroit, MI, USA) by punching out a portion (20 mm diameter).
Culture broth (~50 ml) was aseptically homogenized at 10,000 × g
for 3 min and inoculated 2% (v/v) into a culture medium with the
following composition (g/l): glucose, 20; peptone, 2; yeast
extract, 2; K2HPO4, 1;
KH2PO4, 0.5; MgSO4, 0.5. The pH
value was adjusted to 5.0 before sterilization. The submerged
culture was then carried out in a 5-liter jar fermentor (Fermentec
Co., Ltd., Cheongwon-gun, Korea) containing 4 liters of the medium
(at 300 × g, 25°C) for 10 days. The total culture broth cultivated
for 10 days was lyophilized and ground into powder. The powder (5
g), including mycelium, was added to distilled water (100 ml) and
then shaken at 150 × g at 25°C for 6 h. Samples were centrifuged at
10,000 × g for 20 min, and the resulting supernatant was filtered
through a Whatman filter paper no. 2 (Whatman International Ltd.,
Maidstone, UK). The culture filtrate was mixed with 4 volumes of
isopropyl alcohol, strirred vigorously and left overnight at 4°C,
following centrifugation at 10,000 × g for 20 min. The precipitate
of the crude extract was lyophilized and the weight was
estimated.
Cell culture
INS-1 rat insulin-secreting cells were grown in
RPMI-1640 supplemented with 10% heat-inactivated FBS, 100 U/ml
penicillin and 100 μg/ml streptomycin at 37°C under a
humidified condition in 95% air and 5% CO2.
Cell count analysis
INS-1 cells were seeded in 24-well plates
(1×105/500 μl/well) overnight. INS-1 cells were
then treated without or with Dex in the presence or absence of CLCE
plus LY294002 for 24 h. Alternatively, INS-1 cells seeded in
24-well plates (1×105/500 μl/well) were treated
for 24 h without or with Dex in the absence or presence of CLCE
(with no heating) or heated CLCE (95°C, 1 h). The number of
surviving cells, which were not stained by trypan blue dye, was
counted under a microscope. Approximately, <100 cells were
counted for the analysis.
Measurement of DNA fragmentation
INS-1 cells were seeded in 6-well plates at a
density of 0.5×106 cells/well in a 2-ml volume the day
before drug treatment. INS-1 cells were treated without or with Dex
in the presence or absence of CLCE for 24 h. The conditioned cells
were then harvested, washed, and lysed in buffer [50 mM Tris (pH
8.0), 0.5% sarkosyl, 0.5 mg/ml proteinase K and 1 mM EDTA] at 55°C
for 3 h, followed by addition of RNase A (0.5 μg/ml) and
further incubation at 55°C for 18 h. The lysates were centrifuged
at 10,000 × g for 20 min. The genomic DNA in the supernatant was
extracted with an equal volume of neutral phenol-chloroform-isoamyl
alcohol mixture (25:24:1), and analyzed by electrophoresis on 1.7%
agarose gel. The DNA was visualized and photographed under UV
illumination after staining with ethidium bromide (0.1
μg/ml).
Preparation of whole cell lysates
To measure the effects of Dex and/or CLCE on the
expression and/or activity of cell growth-, stress- or
apoptosis-related proteins, including procaspase-9, PARP, MKP-1,
PKB, ERK-1/2, PERK, eIF-2α, GRP78 and ATF-6, INS-1 cells were
seeded in 6-well plates (0.5×106/2 ml/well) the day
before drug treatment. INS-1 cells were then treated without or
with Dex in the presence or absence of CLCE for 2, 4 or 8 h. Each
time, the conditioned cells were washed twice with PBS and exposed
to cell lysis buffer [50 mM Tris-Cl (pH 7.4), 150 mM NaCl, 0.1%
sodium dodecyl sulfate, 0.25% sodium deoxycholate, 1% Triton X-100,
1% Nonidet P-40, 1 mM EDTA, 1 mM EGTA and proteinase inhibitor
cocktail (1X)]. The cell lysates were collected in a 1.5-ml tube
and centrifuged for 20 min at 4°C at 12,000 × g. The supernatant
was saved and protein concentrations were determined using Bradford
reagent.
Western blot analysis
Proteins (50 μg) were separated by SDS-PAGE
(10%) and transferred onto nitrocellulose membranes (Millipore).
The membranes were washed with TBS (10 mM Tris, 150 mM NaCl)
supplemented with 0.05% (vol/vol) Tween-20 (TBST) followed by
blocking with TBST containing 5% (wt/vol) non-fat dried milk. The
membranes were incubated overnight with antibodies specific for
MKP-1 (1:2,000), p-PKB (1:2,000), T-PKB (1:2,000), p-ERK-1/2
(1:2,000), T-ERK-1/2 (1:2,000), p-PERK (1:2,000), T-PERK (1:2,000),
p-eIF-2α (1:2,000), T-eIF-2α (1:2,000), GRP78 (1:2,000),
procaspase-9 (1:2,000), PARP (1:2,000) or actin (1:5,000) at 4°C.
The membranes were then exposed to secondary antibodies coupled to
horseradish peroxidase for 2 h at room temperature. The membranes
were washed three times with TBST at room temperature.
Immunoreactivities were detected by ECL reagents. Equal protein
loading was assessed by the expression level of the actin
protein.
Statistical analysis
Cell count analysis was carried out in triplicates
and repeated three times. Data are expressed as the means ±
standard error (SE). The significance of difference was determined
by one-way ANOVA. All significance testing was based on a P-value
of <0.05.
Results
Treatment with C. lacerata crude extract
suppresses the Dex-induced reduction in the survival and apoptosis
of INS-1 cells
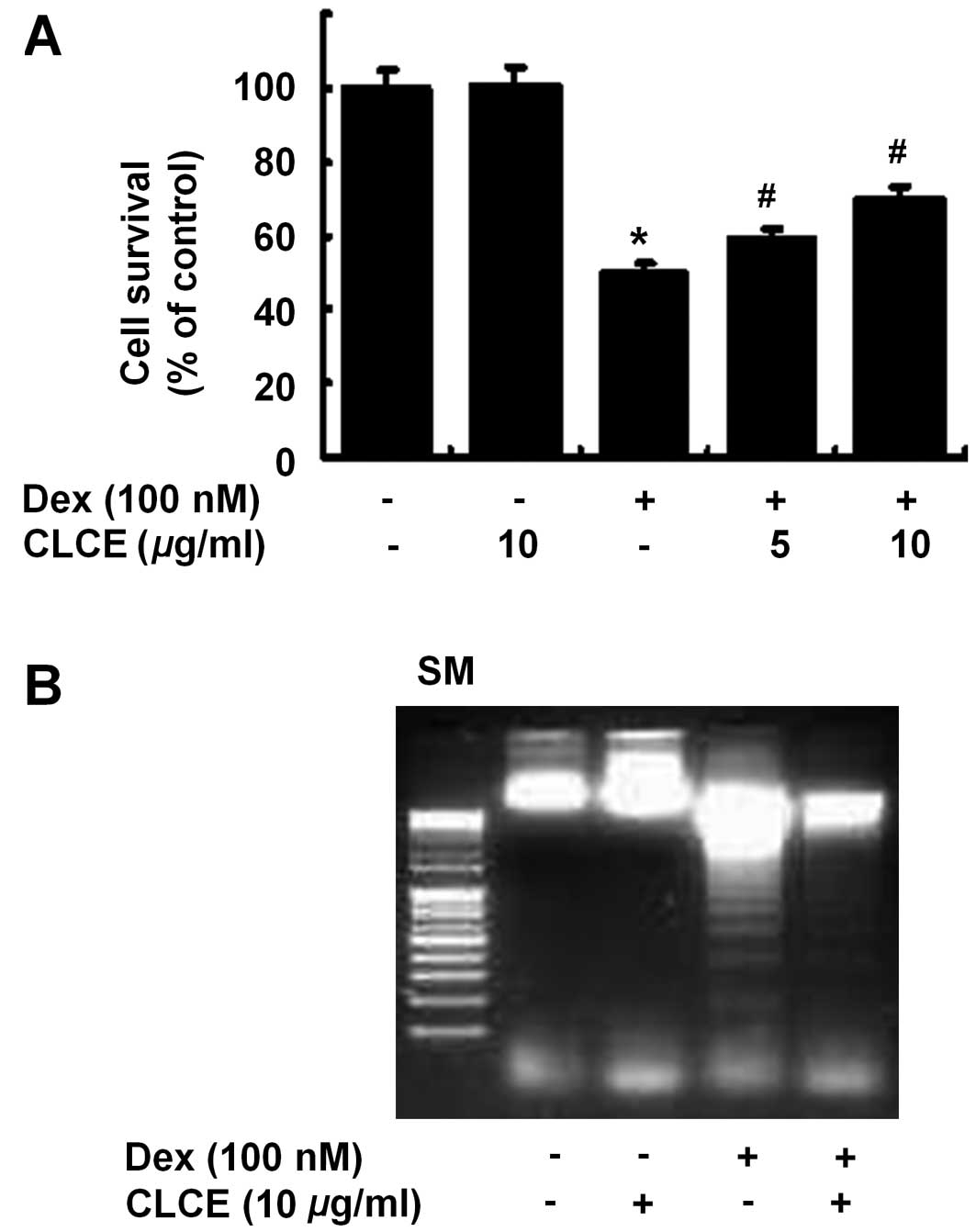
Initially, the effect of CLCE on the survival of
Dex-treated INS-1 cells was determined by cell count analysis.
Compared with the control (column 1), treatment with Dex (100 nM,
24 h) largely reduced the survival of INS-1 cells (column 3)
(Fig. 1A). However, treatment
with CLCE at 5 or 10 μg/ml substantially blocked the
reduction in INS-1 cell survival by Dex. Using a 10 μg/ml
concentration, we next investigated whether CLCE modulates the
Dex-induced apoptosis of INS-1 cells by measuring nuclear DNA
fragmentation, an apoptotic marker. Compared with the control (lane
1), Dex treatment induced nuclear DNA fragmentation in INS-1 cells
(lane 3) (Fig. 1B). However, CLCE
treatment largely suppressed the Dex-induced nuclear DNA
fragmentation in INS-1 cells.
Dex treatment leads to MKP-1 upregulation
and PKB and ERK-1/2 dephosphorylation, but does not alter the
expression and/or activity of GRP78, ATF-6, PERK, eIF-2α,
caspase-9, and PARP in INS-1 cells
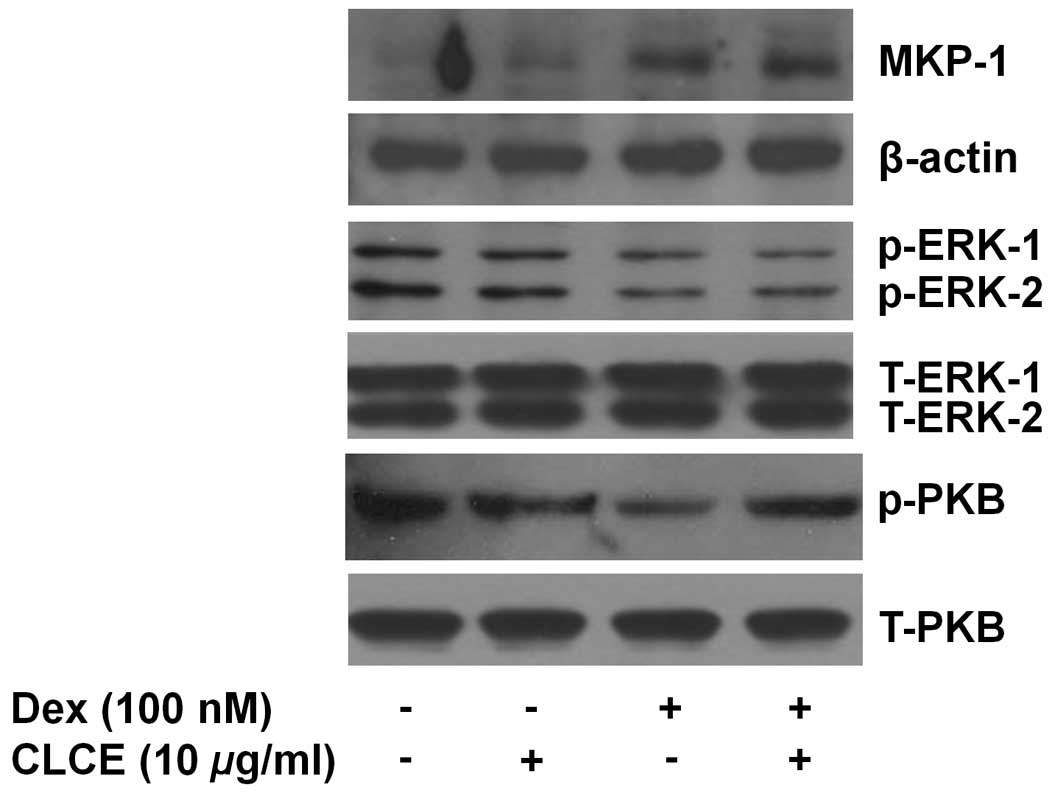
To understand the molecular and cellular mechanisms
underlying Dex-induced INS-1 cytotoxicity, the effect of Dex
treatment at early time points (2, 4 or 8 h) on the expression
and/or activity of growth-, stress- and apoptosis-related proteins,
including MKP-1, PKB, MKP-1, ERK-1/2, caspases, GRP78, PERK, eIF-2α
and ATF-6, in INS-1 cells was investigated by western blot
analysis. In INS-1 cells, Dex treatment at 2 h led to a strong
upregulation of MKP-1 protein and the MKP-1 upregulation was
maintained until 8 h (Fig. 2).
Dex treatment at 2 h, however, did not affect PKB phosphorylation,
but there was a strong and sustained repression of PKB
phosphorylation thereafter. Strong and sustained ERK-1/2
dephosphorylation by Dex treatment at the times tested was also
noted. However, expression of total PKB and ERK-1/2 remained
unchanged by Dex treatment at the times applied. The expression of
not only GRP78 and ATF-6 but also the phosphorylation of PERK and
eIF-2α, four well-known ER stress markers, were not largely altered
by Dex treatment at the times applied. Dex treatment also did not
affect the expression of the inactive proform of caspase-9
(procaspase-9) and PARP. Expression of control actin remained
constant during Dex treatment at the times applied.
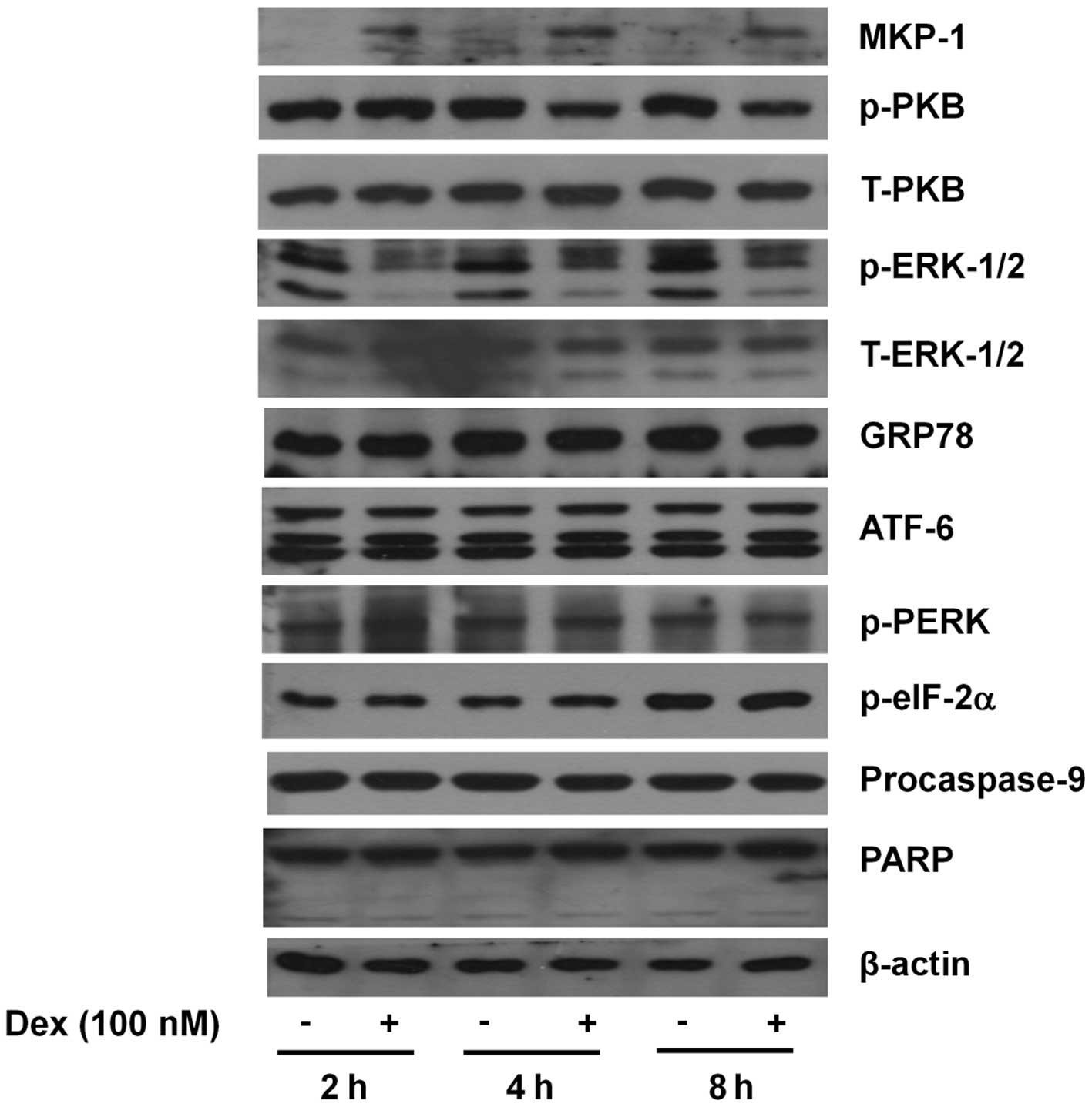 | Figure 2.Effects of Dex on the expression
and/or activities of MKP-1, PKB, ERK-1/2, GRP78, ATF-6, PERK,
eIF-2α, procaspase-9 and PARP in INS-1 cells. INS-1 cells were
treated without or with Dex (100 nM) for the indicated times. At
each time, whole cell lysates were prepared and analyzed by western
blot analysis using the respective antibody. Alternatively, the
immunoblotting used for measuring phospho-protein was further
stripped and reprobed with the antibody which recognizes total
expression levels of the protein. The image is a representative of
three independent experiments. p-PKB, phosphorylated PKB;
p-ERK-1/2, phosphorylated ERK-1/2; T-ERK-1/2, total ERK-1/2;
p-PERK, phosphorylated PERK; p-eIF-2α, phosphorylated eIF-2α. |
CLCE specifically blocks the Dex-induced
PKB dephosphorylation in INS-1 cells
We next determined the effect of CLCE on the
Dex-mediated induction of MKP-1 expression and dephosphorylation of
PKB and ERK-1/2 in INS-1 cells. For this, INS-1 cells were
pretreated without or with CLCE for 1 h and then treated without or
with Dex in the absence or presence of CLCE for an additional 4 h,
followed by measurement of the expression and/or phosphorylation of
MKP-1, PKB and ERK-1/2 in the conditioned cells. CLCE treatment did
not interfere with the Dex-induced MKP-1 upregulation and ERK-1/2
dephosphorylation, but attenuated the effect of Dex on PKB
dephosphorylation (Fig. 3).
Expression of actin, total ERK-1/2 and PKB remained constant
following treatment with Dex and/or CLCE.
Protective effect of CLCE on the
Dex-induced INS-1 cytotoxicity is attenuated by LY294002
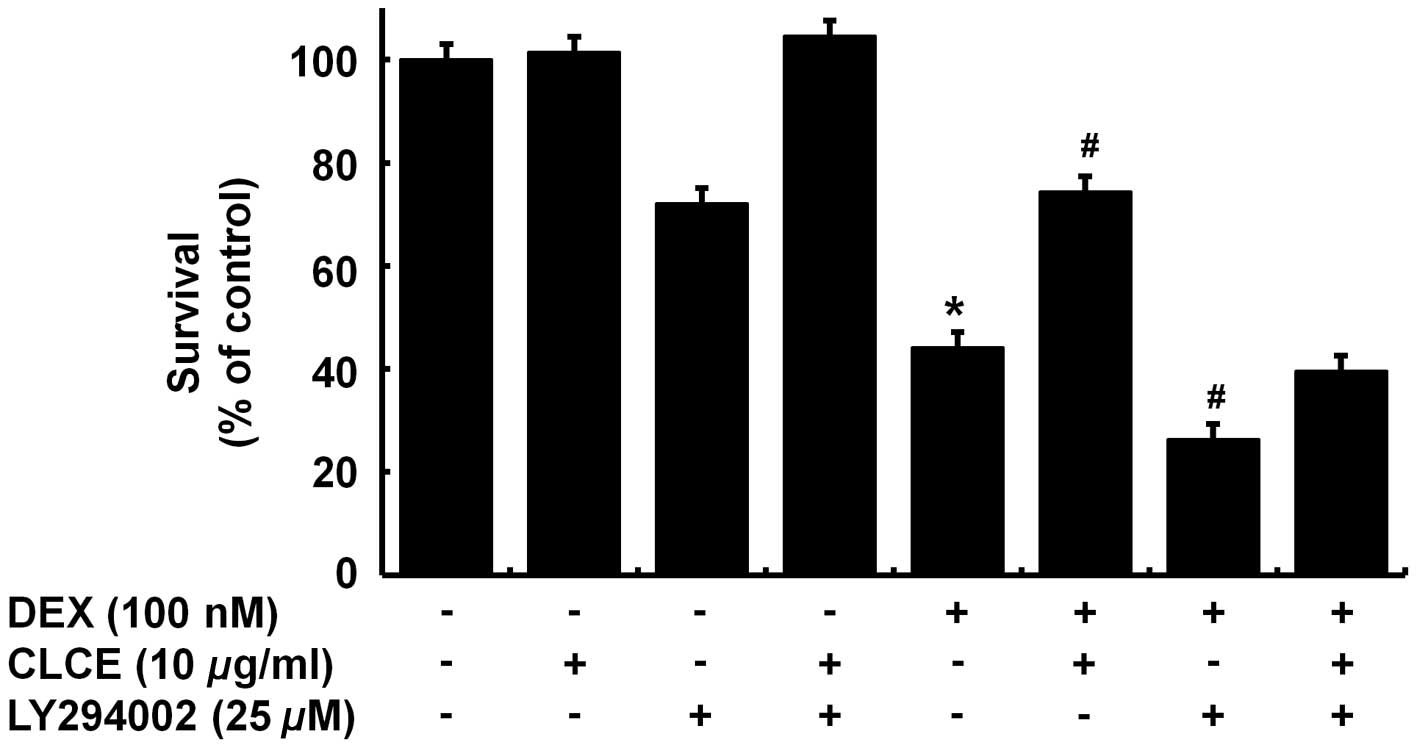
We next investigated the enhanced PKB
phosphorylation regarding the protective effect of CLCE on the
Dex-induced cytotoxicity to INS-1 cells using LY294002, a
pharmacological inhibitor of PI3K/PKB. INS-1 cells were pretreated
without or with LY294002 for 1 h and treated without or with Dex in
the absence or presence of CLCE for an additional 24 h, following
measurement of the number of surviving cells by cell count
analysis. As anticipated, single treatment with Dex largely reduced
the survival of INS-1 cells (column 5) compared with the control
(column 1) (Fig. 4). Of note,
treatment with LY294002 alone slightly decreased the survival of
INS-1 cells (column 3) and co-treatment with Dex and LY294002
resulted in a more repressive effect on the INS-1 cell survival
(column 7) than Dex treatment alone (column 5), suggesting that the
necessity of endogenous PI3K/PKB expression and/or activity for the
survival of INS-1 cells. Notably, the protective effect of CLCE on
the Dex-induced cytotoxicity of INS-1 cells was not evident in the
presence of LY294002.
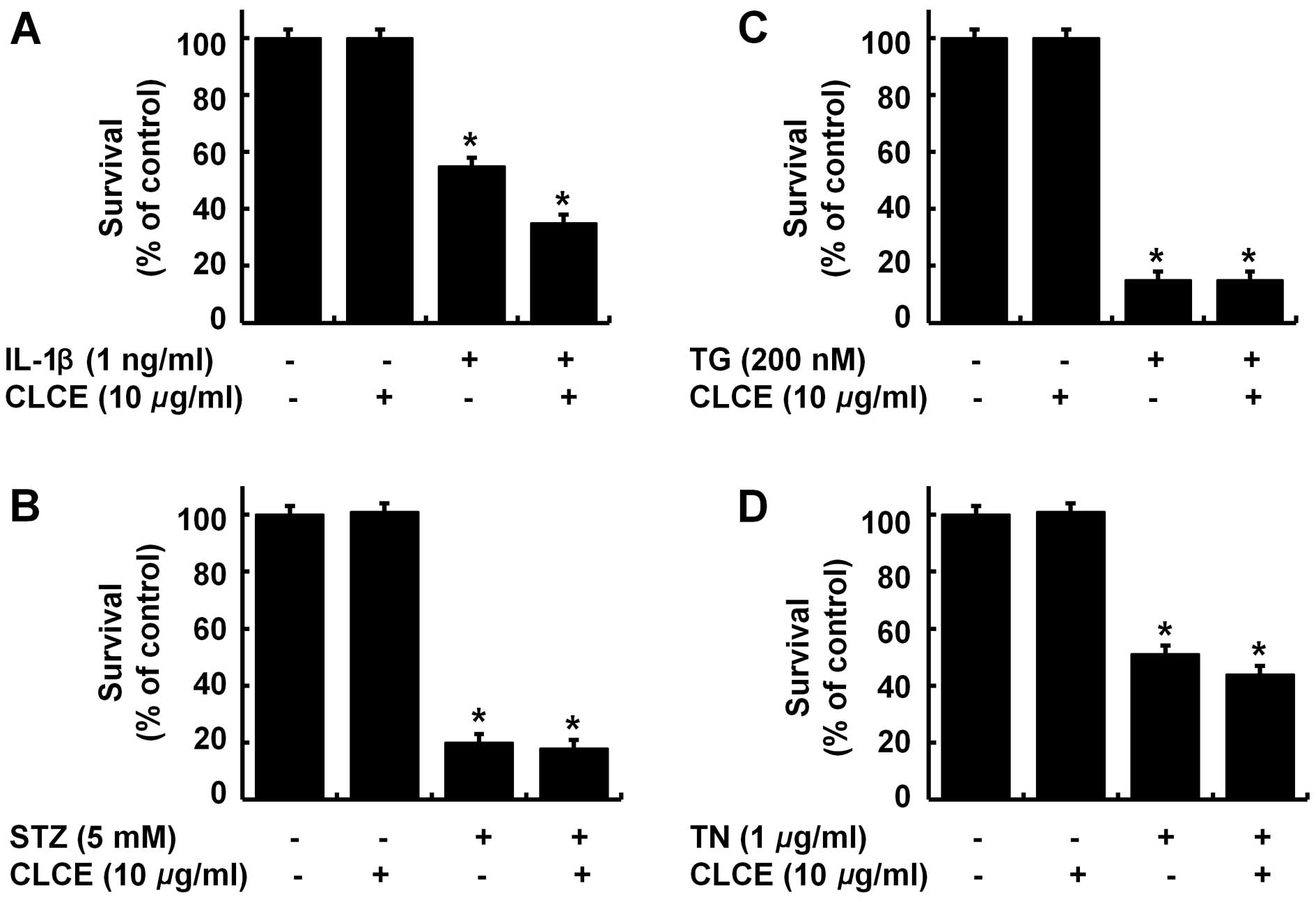
CLCE does not protect INS-1 cells against
the cytotoxicity induced by IL-1β, streptozotocin, thapsigargin or
tunicamycin
To ascertain specificity, we next investigated the
effect of CLCE on the cytotoxicity to INS-1 cells triggered by
other insults, such as interleukin-1β (IL-1β, an inflammatory
cytokine), streptozotocin (STZ, a diabetogenic durg), thapsigargin
(TG, a calcium mobilizing agent and an ER stress inducer), and
tunicamycin (TN, a protein N-glycosylation inhibitor and an ER
stress inducer). Treatment with IL-1β, STZ, TG or TN strongly
reduced the survival of INS-1 cells (Fig. 5A–D). CLCE treatment, however, did
not block the IL-1β-, STZ-, TG- or TN-induced cytotoxic effect in
INS-1 cells; rather CLCE treatment appeared to slightly enhance the
IL-1β- or TN-induced INS-1 cytotoxicity.
Discussion
Evidence suggests that excessive and/or long-term
glucocorticoid therapy reduces β-cell mass in association with
inhibition of β-cell survival and/or induction of β-cell apoptosis,
which may lead to steroid diabetes. In the present study, we
evaluated the effect of a crude extract from C. lacerata on
the survival and apoptosis of INS-1 insulin-secreting cells in
response to Dex exposure. Our data showed that CLCE has a
protective effect on Dex-induced INS-1 cytotoxicity through the
modulation of the PI3K/PKB pathway.
Through initial experiments, we demonstrated that
CLCE largely blocks the Dex-induced decrease in the survival of
INS-1 cells (Fig. 1A). Cells
undergoing apoptosis have distinct biochemical and morphological
characteristics, including nuclear DNA fragmentation (18,19). In our experimental conditions, Dex
treatment induced nuclear DNA fragmentation in the INS-1 cells
(Fig. 1B), suggesting the
Dex-induced apoptosis of INS-1 cells. However, the Dex-induced
apoptosis of INS-1 cells was strongly suppressed by CLCE,
indicating that CLCE inhibits the Dex-induced apoptotic death of
INS-1 cells.
Survival and/or apoptosis of cells, including INS-1,
is largely influenced by the expression and/or activities of many
intracellular signaling proteins, including PI3K, PKB (also known
as Akt) and ERK-1/2. For instance, numerous studies have
highlighted the role of PI3K, which catalyzes the production of
phosphatidylinositol-3,4,5-trisphosphate, in cell survival pathways
(20). The positive relationship
between activation of PKB, a known major downstream target of PI3K,
and cell survival has also been reported (21). There is further evidence that the
activation of PKB signaling is critical for the regulation of
β-cell mass and function in response to growth factors, incretins,
and nutrients (glucose, amino acids) (22-24), and mediates anti-apoptotic effects
induced by glucose, glucagon-like peptide-1, insulin-like growth
factor-1, and insulin in β-cells (23-27), as well as protects β-cells against
fatty acid-induced apoptosis (28). A role of ERK-1/2 in the
quercetin-mediated protection of INS-1 cells from oxidative damage
triggered by hydrogen peroxide (H2O2) has
been previously shown (29). In
numerous studies, glucocorticoids are shown to stimulate expression
of MKP-1, which results in inhibition of the phosphorylation of
numerous substrate proteins, including ERK-1/2 and p38 MAPK, which
impair proliferation in human and mouse osteoblast cell lines
(30-32). In line with this, a previous study
found that Dex induced apoptosis of INS-1 cells by inhibiting
phosphorylation of PKB and ERK-1/2 (11). Supporting these previous findings,
we herein showed the ability of Dex to inhibit phosphorylation of
PKB and ERK-1/2 but to induce expression of MKP-1 (Fig. 2). An important finding in this
study is that CLCE blocked Dex-induced PKB dephosphorylation but
did not interfere with the Dex-induced ERK-1/2 dephosphorylation
and MKP-1 expression in INS-1 cells (Fig. 3), indicating that CLCE has the
specific ability to restore (enhance) PKB phosphorylation in
Dex-treated INS-1 cells. Of further importance, the present study
showed that the protective effect of CLCE on Dex-induced INS-1
cytotoxicity was largely attributed to CLCE’s restorative activity
of PI3K/PKB, as deduced from the strong attenuation of the
protective effect of CLCE by LY294002, a PI3K/PKB inhibitor
(Fig. 4). The mechanism by which
CLCE restores PKB phosphorylation in Dex-treated INS-1 cells
remains uncertain. However, considering no effect of CLCE on the
Dex-induced MKP-1 expression in INS-1 cells (Fig. 3), it is unlikely that the
restorative effect of CLCE on PKB phosphorylation in the
Dex-treated INS-1 cells was the MKP-1-dependent. Recent studies
have shown that inhibition of PKB phosphorylation is linked to the
action of protein phosphatase-2A (PP-2A) (33) or the dephosphorylation of PIP3
molecules by the lipid phosphatase PTEN (34). Moreover, there are several lines
of evidence that Dex treatment increases the activity of PP-2B,
also called calcineurin (35),
and treatment with the calcineurin inhibitor, FK506 or
deltamethrin, attenuates Dex-induced apoptosis in INS-1 cells
(36). It will be important to
ascertain, in future experiments, whether CLCE modulates the
expression (activity) of PP-2A, PP-2B and/or PTEN in Dex-treated
INS-1 cells and whether treatment with pharmacological inhibitor or
siRNA of PP-2A, PP-2B and/or PTEN alters the restorative effect of
CLCE on PKB phosphorylation and/or the protective effect of CLCE on
the apoptosis of INS-1 cells induced by Dex.
Induction of apoptosis is also closely linked to
activation of caspases, a group of essential proteases required for
the execution of cell death by apoptotic stimuli (37). In resting cells, caspases are
synthesized as zymogens (inactive precursors), but upon exposure to
apoptotic stimuli, they become processed via partial proteolytic
cleavage and are activated in cells. Active caspase cleaves many
cellular proteins, including PARP, protein kinase C-δ, and other
vital proteins, leading to induction/execution of apoptosis
(38,39). The Dex-mediated apoptotic cell
death through the activation of the mitochondrial apoptotic pathway
was previously revealed (5,6,40).
A recent study also demonstrated that in INS-1 cells Dex induced
apoptosis through the activation of caspase-3, downregulation of
Bcl-2, dephosphorylation of BAD and mitochondrial depolarization
(36), which further points to
involvement of the mitochondrial-mediated activation of the caspase
pathway in Dex-induced apoptosis in INS-1 cells. However, the
present study, based on the fact that Dex treatment did not trigger
activation of the caspase pathway in INS-1 cells (Fig. 2), excludes the possibility of
involvement of the caspase pathway in the Dex-induced apoptosis of
INS-1 cells. Differential effects of the caspase pathway on the
Dex-induced apoptosis of INS-1 cells in the previous and present
studies may be due to the different experimental conditions used
(the treatment time of Dex: 48 vs. 2-8 h). Pancreatic β-cells have
a well-developed ER and express high amounts of chaperones and
protein disulfide isomerases to meet the high demand for
translation of many proteins. There is strong evidence that
excessive or severe ER stress in β-cells is linked to the
development of diabetes (41) and
also diabetes is a disease of ER stress (42). In general, cells undergoing ER
stress display many cellular alterations, including upregulation of
molecular chaperones (GRP78) (43), reduction of p90ATF-6 (44), and phosphorylation of eIF-2α
(45). Thus, assuming that Dex
treatment for 2 or 8 h does not change the expression and/or
activities of ER stress markers, GRP78, ATF-6, PERK and eIF-2α, in
INS-1 cells herein (Fig. 2), it
is unlikely that Dex induces ER stress in INS-1 cells and that ER
stress plays a role in the Dex-induced INS-1 cytotoxicity.
Evidence suggests that mushrooms have
anti-diabetogenic properties (46,47). It has also been suggested that
polysaccha-rides are the best known and most potent
mushroom-derived substances with antidiabetes and antitumor
activities (48,49). However, a recent study concerning
ternatin, a highly methylated cyclic heptapeptide isolated from the
mushroom Coriolus versicolor, was found to inhibit
hyperglycemia (50) suggesting
the anti-diabetogenic activity by mushroom peptides. In the present
study, we know through phenol-sulfuric acid and bicinchoninic acid
methods that the major components in CLCE are exopolysaccharides
(EPS) and proteins, and the amount of EPS and proteins is ~50:50%
(data not shown). It is therefore conceivable that the protective
effect of CLCE herein is closely associated with the components of
EPS and proteins (or peptides) in CLCE. Not only further
fractionation of each component (EPS, proteins, and/or EPS-protein
complex) in CLCE but also further biochemical characterization of
the action mechanism by which each component mediates the
protective effect of CLCE on Dex-induced INS-1 cytotoxicity warrant
future study.
In addition to glucocorticoids, there are other
diabetogenic factors that lead to type 1 and/or 2 diabetes. For
instance, the inflammatory cytokines, including IL-1β, are
well-known inducers of type 1 diabetes, and high levels of IL-1β
are detected in newly diagnosed type 1 diabetic patients (51,52). Numerous studies have also shown
that STZ, which is an antibiotic originally isolated from
Streptomyces achromo-genes and an N-acetyl-glucosamine
analog, is cytotoxic to pancreatic β-cells after being transported
through glucose transporter 2 (53) and is used to generate type 1
diabetes model animals (54).
Recently, the stimulation of islet cell and/ or β-cell apoptosis by
classic ER stress inducers, such as TG, a pharmacological inhibitor
of SERCA pumps that depletes ER calcium levels, or TN, an inhibitor
of protein N-glycosylation, has been reported (55-57). In the present study, treatment of
INS-1 cells with IL-1β, STZ, TG or TN strongly reduced the survival
of INS-1 cells (Fig. 5A–D),
suggesting that they all have a cytotoxic effect on INS-1 cells.
However, we demonstrated that CLCE did not protect INS-1 cells from
IL-1β-, STZ-, TG- or TN-induced cytotoxicity. These results
strongly suggest that CLCE may have a unique ability to protect
INS-1 cells against the cytotoxicity induced by Dex.
In conclusion, we demonstrated for the first time
that CLCE has a protective effect on Dex-induced INS-1
cytotoxicity, and the effect is mediated through the modulation of
the PI3K/PKB pathway. The findings presented herein may shed light
on the possibility of applying CLCE, as a single and/or
combinatorial regimen with other anti-diabetes therapies, for the
prevention and/or treatment of steroid diabetes in which reduction
of β-cell survival and induction of β-cell apoptosis play
pathogenic roles.
Acknowledgements
This study was supported by iPET
(Korea Institute of Planning and Evaluation for Technology in Food,
Agriculture, Forestry and Fisheries) (111123-2).
References
|
1.
|
Hoogwerf B and Danese RD: Drug selection
and the management of corticosteroid-related diabetes mellitus.
Rheum Dis Clin North Am. 25:489–505. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Schacke H, Docke WD and Asadullah K:
Mechanisms involved in the side effects of glucocorticoids.
Pharmacol Ther. 96:23–43. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Lambillotte C, Gilon P and Henquin JC:
Direct glucocorticoid inhibition of insulin secretion: an in vitro
study of dexamethasone effects in mouse islets. J Clin Invest.
99:414–423. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Jeong IK, Oh SH, Kim BJ, et al: The
effects of dexamethasone on insulin release and biosynthesis are
dependent on the dose and duration of treatment. Diabetes Res Clin
Pract. 51:163–171. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Hegardt C, Andersson G and Oredsson SM:
Spermine prevents cytochrome c release in glucocorticoid-induced
apoptosis in mouse thymocytes. Cell Biol Int. 27:115–121. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Tonomura N, McLaughlin K, Grimm L, et al:
Glucocorticoid-induced apoptosis of thymocytes: requirement of
proteasome-dependent mitochondrial activity. J Immunol.
170:2469–2478. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Renner K, Amberger A, Konwalinka G, et al:
Changes of mitochondrial respiration, mitochondrial content and
cell size after induction of apoptosis in leukemia cells. Biochim
Biophys Acta. 1642:115–123. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Roma LP, Bosqueiro JR, Cunha DA, et al:
Protection of insulin-producing cells against toxicity of
dexamethasone by catalase overexpression. Free Radic Biol Med.
47:1386–1393. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Amsterdam A, Tajima K and Sasson R:
Cell-specific regulation of apoptosis by glucocorticoids:
implication to their anti-inflammatory action. Biochem Pharmacol.
64:843–850. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Kovalovsky D, Refojo D, Holsboer F, et al:
Molecular mechanisms and Th1/Th2 pathways in corticosteroid
regulation of cytokine production. J Neuroimmunol. 109:23–29. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Avram D, Ranta F, Hennige AM, et al: IGF-1
protects against dexamethasone-induced cell death in insulin
secreting INS-1 cells independent of AKT/PKB phosphorylation. Cell
Physiol Biochem. 21:455–462. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Abraham SM, Lawrence T, Kleiman A, et al:
Antiinflammatory effects of dexamethasone are partly dependent on
induction of dual specificity phosphatase 1. J Exp Med.
203:1883–1889. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Wu W, Pew T, Zou M, et al: Glucocorticoid
receptor-induced MAPK phosphatase-1 (MPK-1) expression inhibits
paclitaxel-associated MAPK activation and contributes to breast
cancer cell survival. J Biol Chem. 280:4117–4124. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Miller AL, Webb MS, Copik AJ, et al: p38
mitogen-activated protein kinase (MAPK) is a key mediator in
glucocorticoid-induced apoptosis of lymphoid cells: correlation
between p38 MAPK activation and site-specific phosphorylation of
the human glucocorticoid receptor at serine 211. Mol Endocrinol.
19:1569–1583. 2005. View Article : Google Scholar
|
|
15.
|
Nicoletti-Carvalho JE, Lellis-Santos C,
Yamanaka TS, et al: MKP-1 mediates glucocorticoid-induced ERK1/2
dephosphorylation and reduction in pancreatic beta-cell
proliferation in islets from early lactating mothers. Am J Physiol
Endocrinol Metab. 299:1006–1015. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Paterson RR: Ganoderma - a therapeutic
fungal biofactory. Phytochemistry. 67:1985–2001. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Lee JW, Gwak KS, Park JY, et al:
Biological pretreatment of softwood Pinus densiflora by
three white rot fungi. J Microbiol. 45:485–491. 2007.
|
|
18.
|
Wyllie AH, Kerr JF and Currie AR: Cell
death: the significance of apoptosis. Int Rev Cytol. 68:251–306.
1980. View Article : Google Scholar
|
|
19.
|
Allen RT, Hunter WJ III and Agrawal DK:
Morphological and biochemical characterization and analysis of
apoptosis. J Pharmacol Toxicol Methods. 37:215–228. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Cantley LC: The phosphoinositide 3-kinase
pathway. Science. 296:1655–1657. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Song G, Ouyang G and Bao S: The activation
of Akt/PKB signaling pathway and cell survival. J Cell Mol Med.
9:59–71. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Elghazi L, Balcazar N and Bernal-Mizrachi
E: Emerging role of protein kinase B/Akt signaling in pancreatic
beta-cell mass and function. Int J Biochem Cell Biol. 38:157–163.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Brubaker PL and Drucker DJ: Glucagon-like
peptides regulate cell proliferation and apoptosis in the pancreas,
gut, and central nervous system. Endocrinology. 145:2653–2659.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Dickson LM and Rhodes CJ: Pancreatic
beta-cell growth and survival in the onset of type 2 diabetes: a
role for protein kinase B in the Akt? Am J Physiol Endocrinol
Metab. 287:192–198. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Dickson LM, Lingohr MK, McCuaig J, et al:
Differential activation of protein kinase B and p70(S6)K by glucose
and insulin-like growth factor 1 in pancreatic beta-cells (INS-1).
J Biol Chem. 276:21110–21120. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Srinivasan S, Bernal-Mizrachi E, Ohsugi M,
et al: Glucose promotes pancreatic islet beta-cell survival through
a PI 3-kinase/ Akt-signaling pathway. Am J Physiol Endocrinol
Metab. 283:784–793. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Wang Q, Li L, Xu E, et al: Glucagon-like
peptide-1 regulates proliferation and apoptosis via activation of
protein kinase B in pancreatic INS-1 beta cells. Diabetologia.
47:478–487. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Wrede CE, Dickson LM, Lingohr MK, et al:
Protein kinase B/ Akt prevents fatty acid-induced apoptosis in
pancreatic beta-cells (INS-1). J Biol Chem. 277:49676–49684. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Youl E, Bardy G, Maqous R, et al:
Quercetin potentiates insulin secretion and protects INS-1
pancreatic beta-cells against oxidative damage via the ERK1/2
pathway. Br J Pharmacol. 161:799–814. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Kassel O, Sancono A, Krätzschmar J, et al:
Glucocorticoids inhibit MAP kinase via increased expression and
decreased degradation of MKP-1. EMBO J. 20:7108–7116. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Lasa M, Abraham SM, Boucheron C, et al:
Dexamethasone causes sustained expression of mitogen-activated
protein kinase (MAPK) phosphatase 1 and phosphatase-mediated
inhibition of MAPK p38. Mol Cell Biol. 22:7802–7811. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Engelbrecht Y, de Wet H, Horsch K, et al:
Glucocorticoids induce rapid up-regulation of mitogen-activated
protein kinase phosphatase-1 and dephosphorylation of extracellular
signal-regulated kinase and impair proliferation in human and mouse
osteoblast cell lines. Endocrinology. 144:412–422. 2003. View Article : Google Scholar
|
|
33.
|
Gao T, Furnari F, Newton AC, et al: PHLPP:
A phosphatase that directly dephosphorylates Akt, promotes
apoptosis, and suppresses tumor growth. Mol Cell. 18:13–24. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Woodgett JR: Recent advances in the
protein kinase B signaling pathway. Curr Opin Cell Biol.
17:150–157. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Tumlin JA, Lea JP, Swanson CE, et al:
Aldosterone and dexamethasone stimulate calcineurin activity
through a transcription-independent mechanism involving steroid
receptor-associated heat shock proteins. J Clin Invest.
99:1217–1223. 1997. View Article : Google Scholar
|
|
36.
|
Ranta F, Avram D, Berchtold S, et al:
Dexamethasone induces cell death in insulin-secreting cells, an
effect reversed by exendin-4. Diabetes. 55:1380–1390. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Fearnhead HO, Dinsdale D, Cohen GM, et al:
An interleukin-1 beta-converting enzyme-like protease is a common
mediator of apoptosis in thymocytes. FEBS Lett. 375:283–288. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Emoto Y, Manome Y, Meinhardt G, et al:
Proteolytic activation of protein kinase C delta by an ICE-like
protease in apoptotic cells. EMBO J. 14:6148–6156. 1995.PubMed/NCBI
|
|
39.
|
Lazebnik YA, Kaufmann SH, Desnoyers S, et
al: Cleavage of poly(ADP-ribose) polymerase by a proteinase with
properties like ICE. Nature. 371:346–347. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Almawi WY, Melemedjian OK and Jaoude MM:
On the link between Bcl-2 family proteins and
glucocorticoid-induced apoptosis. J Leukoc Biol. 76:7–14. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Fonseca SG, Burcin M, Gromada J, et al:
Endoplasmic reticulum stress in beta-cells and development of
diabetes. Curr Opin Pharmacol. 9:763–770. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Thomas SE, Dalton LE, Daly ML, et al:
Diabetes as a disease of endoplasmic reticulum stress. Diabetes
Metab Res Rev. 26:611–621. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Lee AS: The glucose-regulated proteins:
stress induction and clinical applications. Trends Biochem Sci.
26:504–510. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Haze K, Yoshida H, Yanagi T, et al:
Mammalian transcription factor ATF6 is synthesized as a
transmembrane protein and activated by proteolysis in response to
endoplasmic reticulum stress. Mol Biol Cell. 10:3787–3799. 1999.
View Article : Google Scholar
|
|
45.
|
De Haro C, Méndez R and Santoyo J: The
eIF-2alpha kinases and the control of protein synthesis. FASEB J.
10:1378–1387. 1996.PubMed/NCBI
|
|
46.
|
Sun JE, Ao ZH, Lu ZM, et al:
Antihyperglycemic and antilipidperoxidative effects of dry mater of
culture broth of Inonotus obliquus in submerged culture on
normal and alloxan-diabetes mice. J Ethnopharmacol. 118:7–13. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
47.
|
Jeong SC, Jeong YT, Yang BK, et al: White
button mushroom (Agaricus bisporus) lowers blood glucose and
cholesterol levels in diabetic and hypercholesterolemic rats. Nutr
Res. 30:49–56. 2010.
|
|
48.
|
Mizuno T: Development of antitumor
polysaccharides from mushroom fungi. Food Food Ingred J Jpn.
167:69–85. 1996.
|
|
49.
|
Wasser SP and Weis AL: Medicinal
properties of substances occurring in higher basidiomycetes
mushrooms: current perspectives. Int J Med Mushrooms. 1:31–62.
1999. View Article : Google Scholar
|
|
50.
|
Kobayashi M, Kawashima H, Takemori K, et
al: Ternatin, a cyclic peptide isolated from mushroom, and its
derivative suppress hyperglycemia and hepatic fatty acid synthesis
in spontaneously diabetic KK-A(y) mice. Biochem Biophys Res Commun.
427:299–304. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51.
|
Netea MG, Hancu N, Blok WL, et al:
Interleukin 1 beta, tumour necrosis factor-alpha and interleukin 1
receptor antagonist in newly diagnosed insulin-dependent diabetes
mellitus: comparison to long-standing diabetes and healthy
individuals. Cytokine. 9:284–287. 1997. View Article : Google Scholar
|
|
52.
|
Kaizer EC, Glaser CL, Chaussabel D, et al:
Gene expression in peripheral blood mononuclear cells from children
with diabetes. J Clin Endocrinol Metab. 92:3705–3711. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
53.
|
Schnedl WJ, Ferber S, Johnson JH, et al:
STZ transport and cytotoxicity. Specific enhancement in
GLUT2-expressing cells. Diabetes. 43:1326–1333. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
54.
|
Deeds MC, Anderson JM, Armstrong AS, et
al: Single dose streptozotocin-induced diabetes: considerations for
study design in islet transplantation models. Lab Anim. 45:131–140.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
55.
|
Duprez J and Jonas JC: Role of activating
transcription factor 3 in low glucose- and thapsigargin-induced
apoptosis in cultured mouse islets. Biochem Biophys Res Commun.
415:294–299. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
56.
|
Rosengren V, Johansson H, Lehtiö J, et al:
Thapsigargin down-regulates protein levels of GRP78/BiP in INS-1E
cells. J Cell Biochem. 113:1635–1644. 2012.PubMed/NCBI
|
|
57.
|
Peng L, Men X, Zhang W, et al:
Dynamin-related protein 1 is implicated in endoplasmic reticulum
stress-induced pancreatic β-cell apoptosis. Int J Mol Med.
28:161–169. 2011.PubMed/NCBI
|