Introduction
Chrysanthemums are perennial flowering plants in the
Asteraceae family which is native to Asia and northeastern Europe.
Extracts of chrysanthemum plants have been shown to have a variety
of potential medicinal properties, such as anti-AIDS (1,2),
antimicrobial (3), antioxidant
(4,5) and antimycotic activities (6). Chrysanthemum zawadskii is one
of the species of the genus Chrysanthemum and has
traditionally been used in folk medicine, known as ‘Gujeolcho’ in
Korea for the treatment of various diseases. Chrysanthemum
zawadskii extracts (CZE) has been shown to have
anti-inflammatory and anti-oxidative stress activities in RAW 264.7
murine macrophage cells (7) and
one of the fractions derived from CZE exerts protective effects
against carbon tetrachloride (CCl4)-induced
hepatotoxicity in mice via the induction of detoxifying enzyme,
NAD(P)H: (quinone acceptor) oxidoreductase 1 (8).
Oxidative stress results from a persistent imbalance
between antioxidant defenses and the production of highly reactive
oxygen species (ROS) (9). Chronic
hyperglycemia leads to oxidative stress which is involved in the
progression of pancreatic β-cell deterioration, as well as in the
development of diabetic complications (10). Bone complications in diabetes
include early onset osteopenia and osteoporosis (11,12). These cause an increase in bone
fractures and a delay in the healing of fractures, affecting the
quality of life (13). In
vitro studies have indicated that oxidative stress inhibits
osteoblast differentiation (14)
and induces osteoblastic insults and apoptosis (15). One of the mechanisms of
diabetes-associated bone disease may be the direct effects on
osteoblasts and bone turnover. An imbalance between bone-forming
osteoblasts and bone-resorbing osteoclasts leads to the
pathogenesis and etiology of certain bone metabolic diseases,
including osteoporosis and osteopetrosis (16).
Sugars that contain aldehyde groups that are
oxidized to carboxylic acids are classified as reducing sugars, and
they produce ROS through autoxidation and protein glycosylation
(17–19). 2-Deoxy-D-ribose (dRib) is a strong
reducing sugar that is highly reactive with proteins (20,21). Since glucose is the least reactive
of the reducing sugars and requires long-term exposure to provoke
oxidative stress (19), we
selected dRib as a surrogate for glucose to induce oxidative damage
in MC3T3-E1 osteoblastic cells. We have previously demonstrated
that dRib promotes apoptosis by increasing oxidative stress in
HIT-T15 pancreatic β-cells (20–23) and MC3T3-E1 osteoblastic cells
(24–26).
Although it is known that the health beneficial and
pharmacological effects of CZE are due to its antioxidant
activities, the molecular mechanisms behind its biological effects
on bone metabolism are still unknown. Oxidative stress is involved
in the modulation of the expression of transcription factors and
cellular signaling, which may affect osteoblast function. In the
present study, we aimed to investigate the effects of CZE on
oxidative stress-induced damage and cellular dysfunction in
MC3T3-E1 osteoblastic cells.
Materials and methods
Plant materials and reagents
The aerial parts of Chrysanthemum zawadskii
were collected from Nowha-do, Wando-gun, Jeollanam-do Province,
Korea. The botanical origin of the sample was classified by one of
the authors (Y.P. Jang) and the voucher specimen (KHUP-289) was
deposited in the Kyung Hee Museum of Korean Traditional Herbal
Medicine, Seoul, Korea. Dried aerial parts of Chrysanthemum
zawadskii (1 kg) were refluxed with 80% ethanol (in water, v/v)
at room temperature several times. This extract was filtered and
evaporated in a rotary vacuum evaporator and then finally
lyophilized with a freezing dryer. The extract (total yield, 26.7%)
was dissolved in dimethyl sulfoxide (DMSO) and diluted to the
appropriate concentrations with culture medium [final DMSO
concentration was 0.05% (v/v)]. Linarin was obtained from ChromaDex
(Irvine, CA, USA). High performance liquid chromatography (HPLC)
grade acetonitrile was purchased from Fisher Scientific (Seoul,
Korea). Formic acid of analytical reagent grade was obtained from
Wako Pure Chemical Industries, Ltd. (Osaka, Japan).
HPLC and HPLC-electrospray
ionization-tandem mass spectrometry (HPLC-ESI-MS) analysis of
CZE
Standard solutions containing linarin were prepared
in the concentration range from 50 to 400 μg/ml. A total of
5 mg of 80% ethanolic CZE was dissolved in 1 ml of initial solvent
mixture of HPLC [15% acetonitrile (in water, v/v)]. All the
standard and sample solutions were filtered through a 0.45
μm syringe filter (Millipore, Bedford, MA, USA) before being
subjected to HPLC. HPLC analysis was performed on a Waters system
(Waters Corp., Milford, MA, USA) equipped with a photodiode array
detector (Waters 996) running with Empower software. A Hector C18
column (250×4.6 mm, 5 μm) (RStech Co., Ltd., Daejeon, Korea)
was selected for the chemical fingerprint analysis of the ethanolic
extract of Chrysanthemum zawadskii. The UV data of the
effluent from the column ranging from 200 to 400 nm were collected.
The detection wavelength was set to 330 nm. The flow rate was 1
ml/min. The mobile phase comprised 0.1% formic acid in acetonitrile
(solvent A) and 0.1% formic acid in water (solvent B). The gradient
program commenced with linear gradient from 15 to 23% of solvent A
for 15 min, followed by isocratic elution for 35 min, then linear
gradient to 30% for 2 min and isocratic elution for 10 min. The
injection volume of the standard and the samples was 10
μl.
In order to identify major peaks from CZE,
HPLC-ESI-MS was performed. The HPLC flow rate was approximately 0.2
ml/min using a commercial splitter. An AccuTOF®
single-reflectron time-of-flight mass spectrometer was equipped
with an ESI and operated using Mass Center system version 1.3.7b
software (both from Jeol USA Inc., Peabody, MA, USA). In the
positive ion mode, the atmospheric pressure interface potentials
were typically set to the following values: orifice 1, 80 V and
ring lens and orifice 2, 10 and 5 V, respectively. The ion guide
potential and detector voltage were set to 2,000 and 2,300 V,
respectively. ESI parameters were set as follows: needle electrode
= 2,000 V, nitrogen gas was used as a nebulizer, desolvating and
the flow rate was 1 and 3 l/min, desolvating chamber temperature,
250°C, orifice 1 temperature, 80°C. Mass scale calibration was
performed using the YOKUDELNA calibration kit (Jeol Ltd., Tokyo,
Japan) for accurate mass measurements and calculations of the
elemental composition. MS acquisition was set with a scan range of
m/z 100 to 2,000.
Cell culture
MC3T3-E1 murine osteoblastic cells were obtained
from the American Type Culture Collection (Rockville, MD, USA). The
cells were cultured in α-modified minimal essential medium (α-MEM;
Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine
serum (FBS; Sigma Chemical Co., St. Louis, MO, USA), 100
μU/ml penicillin, and 100 μg/ml streptomycin. The
cultures were maintained at 37°C in a humidified 5% CO2
atmosphere and subcultured by trypsinization with 0.05%
trypsin-0.02% EDTA in Ca2+- and Mg2+-free
Dulbecco’s phosphate-buffered saline (DPBS) until they reached
approximately 70% confluence. For assessment of cell viability,
apoptosis and ROS production, the cells were plated in 24-well
culture plates at a density of 2×104 cells/well. Two
days after culture, the cells were treated with CZE for 24 h in
α-MEM containing 0.5% FBS. The cells were also seeded in a 6-well
culture plate at a density of 1×105 cells/well and
treated with culture medium containing 10 mM β-glycerophosphate and
50 μg/ml ascorbic acid to initiate in vitro
mineralization as previously described (27). The cell culture medium was changed
every 2 days. After 6 days, the cells were cultured in medium
containing dRib and/or CZE for 2 days and the alkaline phosphatase
(ALP) activity, collagen content and gene expression were then
measured.
Assessment of cell viability
Cell viability was determined by measuring cell
metabolic activity using the Cell Counting kit-8 (CCK-8) (Dojindo
Co., Kumamoto, Japan) (28).
CCK-8 contains
2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium,
monosodium salt (WST-8), which produces a water-soluble formazan
dye upon reduction in the presence of an electron carrier. The
amount of yellow formazan dye generated by the activity of
dehydrogenases in the cells is directly proportional to the number
of living cells. MC3T3-E1 osteoblastic cells were plated in 24-well
cell culture plates at a density of 2×104 cells/well. At
the end of the culture period, 50 μl of the CCK-8 solution
were added to each well of the culture plate, which contained 500
μl of medium. After a 4-h incubation, the absorbance was
measured using a Zenyth 3100 multimode detector (Anthos Labtec
Instruments GmbH, Wals/Salzburg, Austria) at 450 nm using a 650 nm
filter as a reference. Cells incubated with culture medium alone
were used to determine 100% viability and were included as the
control in all the experiments to allow the estimation of the
percentage viability of the cell samples.
Apoptosis determination by ELISA
A cell death ELISA kit (Roche Molecular
Biochemicals, Mannheim, Germany), which quantitatively detects
cytosolic histone-associated DNA fragments, was used to measure
apoptosis according to the manufacturer’s instructions. Briefly,
cells were seeded at a density of 2×104 cells in 24-well
culture plates. The culture conditions used were the same as those
described for the cell viability assay. Following incubation, the
cells were lysed and the intact nuclei were pelleted by
centrifugation. An aliquot of supernatant was used as the antigen
source for sandwich ELISA using a primary anti-histone monoclonal
antibody that was bound to the streptavidin-coated wells of a
microtiter plate. Subsequently, the cells were treated with a
second anti-DNA monoclonal antibody coupled to peroxidase.
Nucleosome levels were quantified by determining the amount of
peroxidase retained in the immunocomplex. Peroxidase activity was
determined photometrically at 405 nm using
2,2′-azino-di(3-ethylbenzthiazolin-sulfonate) (ABTS) as the
substrate.
Measurement of ROS
The fluorescent probe,
chloromethyl-2,7-dichlorodihydrofluorescein diacetate (DCFDA;
Molecular Probes, Eugene, OR, USA), was used to measure
intracellular ROS levels as previously described (28). MC3T3-E1 osteoblastic cells were
cultured for 24 h in α-MEM containing 0.5% FBS, rinsed twice with
DPBS, and then treated with 10 μM of DCFDA for 1 h. The
cells were then rinsed, scraped and their fluorescence was measured
(excitation 485 nm, emission 515 nm) using a Zenyth 3100 multimode
detector.
ALP activity
At the time of cell harvesting, the medium was
removed and the cell monolayer was gently washed twice with PBS.
The cells were then lysed with 0.2% Triton X-100 and the lysate was
centrifuged at 14,000 × g for 5 min. The cleared supernatant was
used for the measurement of ALP activity and protein concentration.
ALP activity was determined using an ALP activity assay kit (Somang
Engineering Co., Seoul, Korea) and normalized using the number of
cells.
Collagen content
Cellular collagen content was measured using a
Sircol Collagen Assay kit (Biocolor Ltd., Newtownabbey, Northern
Ireland). This assay is a quantitative dye-binding method designed
for the analysis of collagen extracted from mammalian tissues and
cells during in vitro culture. The dye reagent binds
specifically to the [Gly-X-Y]n helical structure found
in mammalian collagen (types I–V). Measurements were normalized
using the number of cells.
RNA extraction
Total RNA was isolated from the cells using TRIzol
reagent (Invitrogen). Following isolation, RNA integrity was
assessed using an Agilent 2100 Bioanalyzer (Agilent Technologies,
Inc., Palo Alto, CA, USA). cDNA was synthesized using the
Transcriptor First Strand cDNA synthesis kit (Roche Diagnostics
GmbH, Mannheim, Germany) and stored at −70°C until further
processing. All procedures were carried out according to the
manufacturer’s instructions.
Real-time RT-PCR
Real-time PCR was performed to verify the
differential expression of selected genes using a Roche LightCycler
480 system (Roche Diagnostics GmbH) and the TaqMan method using the
Roche Universal Probe Library (UPL) kit. Relative gene expression
was determined by employing the comparative CT method. All
reactions were carried out in a total volume of 20 μl of
reaction mixture containing 10.0 μl of 2X UPL master mix,
1.0 μl of 5′ primer (10 pmol/μl), 1.0 μl of 3′
primer (10 pmol/ml), 0.2 μl of UPL probe, 1.0 μl of
cDNA and 6.8 μl of sterile water. The thermal cycling
conditions for PCR were an initial denaturation for 10 min at 95°C,
followed by 40 cycles of 94°C for 10 sec and 60°C for 30 sec. The
primers summarized in Table I
were designed using the Roche ProbeFinder assay tool. For RT-PCR
analysis, duplicate PCRs were carried out for each cDNA. Negative
controls (except templates) were included in the PCR reaction to
ensure specific amplification. LightCycler 480 software version 1.2
(Roche Diagnostics GmbH) was used for the analysis of the
quantitative PCR. The values obtained from each sample were
normalized to hypoxanthine guanine phosphoribosyltransferase (HPRT)
expression. The levels of each gene expression in all experimental
groups were compared to the expression levels of the control
group.
 | Table I.Primer sequences used in this
study. |
Table I.
Primer sequences used in this
study.
| Genes | Primer
sequences |
|---|
| AKT1 | 5′-TCG TGT GGC AGG
ATG TGT AT-3′ |
| 5′-ACC TGG TGT CAG
TCT CAG AGG-3′ |
| AKT2 | 5′-CGA CCC AAC ACC
TTT GTC A-3′ |
| 5′-GAT AGC CCG CAT
CCA CTC T-3′ |
| AKT3 | 5′-TGG ACC ACT GTT
ATA GAG AGA ACA TTT-3′ |
| 5′-TGG ATA GCT TCC
GTC CAC TC-3′ |
| ALP | 5′-GGC CAG CTA CAC
CAC AAC A-3′ |
| 5′-CTG AGC GTT GGT
GTT ATA TGT CTT-3′ |
| BMP2 | 5′-GGT CAC AGA TAA
GGC CAT TGC-3′ |
| 5′-GCT TCC GCT GTT
TGT GTT TG-3′ |
| BMP4 | 5′-GAG GAG TTT CCA
TCA CGA AGA-3′ |
| 5′-GCT CTG CCG AGG
AGA TCA-3′ |
| BMP7 | 5′-CGA TAC CAC CAT
CGG GAG TTC-3′ |
| 5′-AAG GTC TCG TTG
TCA AAT CGC-3′ |
| BSP | 5′-GAA AAT GGA GAC
GGC GAT AG-3′ |
| 5′-CAT TGT TTT CCT
CTT CGT TTG A-3′ |
| Collagen | 5′-AGA CAT GTT CAG
CTT TGT GGA C-3′ |
| 5′-GCA GCT GAC TTC
AGG GAT G-3′ |
| GP×1 | 5′-GGT TTC CCG TGC
AAT CAG T-3′ |
| 5′-TCG GAC GTA CTT
GAG GGA AT-3′ |
| GP×4 | 5′-TAA GAA CGG CTG
CGT GGT-3′ |
| 5′-GTA GGG GCA CAC
ACT TGT AGG-3′ |
| OPG | 5′-ATG AAC AAG TGG
CTG TGC TG-3′ |
| 5′-CAG TTT CTG GGT
CAT AAT GCA A-3′ |
| OPN | 5′-TGA GAT TGG CAG
TGA TTT GC-3′ |
| 5′-ATC TGG GTG CAG
GCT GTA AA-3′ |
| OC | 5′-CAC CAT GAG GAC
CCT CTC TC-3′ |
| 5′-TGG ACA TGA AGG
CTT TGT CA-3′ |
| PI3K | 5′-TTT GGG AGA CTG
AAT CTC TGG-3′ |
| 5′-GTG GCA TCC TTT
ACA ATC TCG-3′ |
| SOD1 | 5′-CCA TCA GTA TGG
GGA CAA TAC A-3′ |
| 5′-GGT CTC CAA CAT
GCC TCT CT-3′ |
| SOD2 | 5′-GAC CCA TTG CAA
GGA ACA A-3′ |
| 5′-GTA GTA AGC GTG
CTC CCA CAC-3′ |
| SOD3 | 5′-GGG GAG GCA ACT
CAG AGG-3′ |
| 5′-TGG CTG AGG TTC
TCT GCA C-3′ |
Statistical analysis
All results are expressed as the means ± SD.
Statistical analysis was performed using one-way ANOVA with a
subsequent Tukey’s multiple comparison test. A P-value <0.05 was
considered to indicate a statistically significant difference.
Statistical analysis was performed using SAS software (SAS Inc.,
Cary, NC, USA).
Results and Discussion
To our knowledge, this is the first study to
investigate the effects of CZE on dRib-induced oxidative damage
using a MC3T3-E1 osteoblastic culture model. One of the mechanisms
of diabetes-associated bone disease may be the direct effects on
osteoblastic cells. In vitro studies have shown that
hyperglycemia inhibits osteoblastic cell proliferation and
differentiation (29,30), indicating that extracellular high
glucose directly impairs osteoblastic function, resulting in bone
disease. In our previous studies, we demonstrated that dRib induced
cellular damage in pancreatic β-cells by increasing oxidative
stress and protein glycation (20–23). Recently, we also reported that
dRib induces cellular dysfunction and apoptosis in the MC3T3-E1
mouse osteoblastic cell line by increasing oxidative stress
(26).
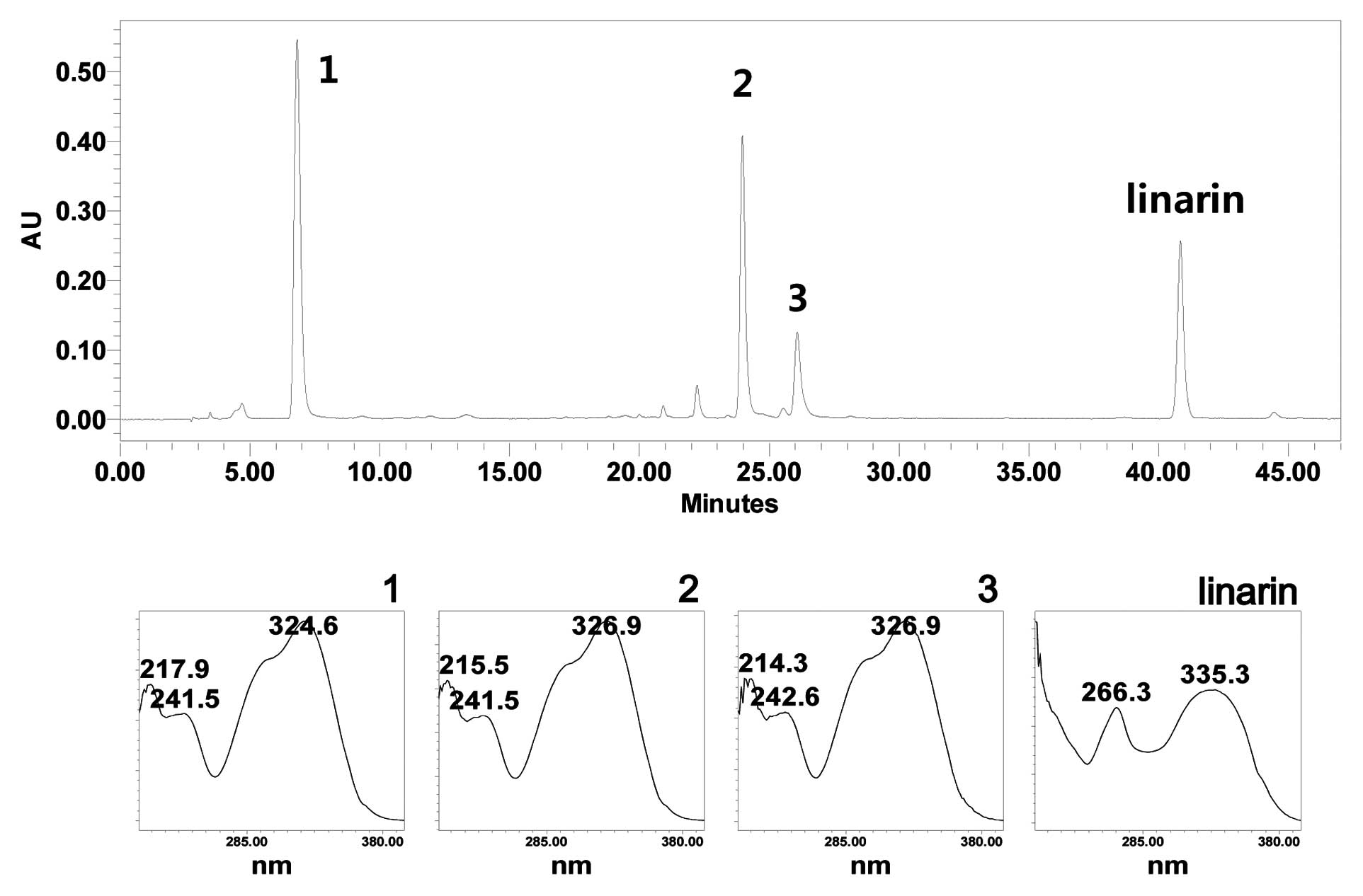
Due to the absence of a previous HPLC fingerprint
study on CZE, we aimed to establish a HPLC profiling method.
Gradient elution system was the best choice to obtain an entire
HPLC profile of the secondary metabolites from this plant. An
optimized HPLC chromatogram is shown in Fig. 1. Compared to the main active
compound from a previous report, linarin was identified from the
chromatogram (31). A calibration
curve was established using methanol stock solution containing
linarin diluted to the appropriate concentration. The co-efficient
value (r2) was 0.996, linearity in this range was
sufficient to provide a highly accurate value for the linarin
content in the samples. The relative standard deviation (RSD) was
<5.5%. Using the established calibration curves, the content of
linarin in the extract was quantified. The calculated content of
linarin in the extract was 4.05±0.27% (w/w) (Table II).
 | Table II.Regression data, precision and
quantification of linarin from the 80% ethanolic extract of
Chrysanthemum zawadskii. |
Table II.
Regression data, precision and
quantification of linarin from the 80% ethanolic extract of
Chrysanthemum zawadskii.
| Compound | Regression
equation | R2 | Linear range
(μg/ml) | RSD (%) (n=3) | Contents of linarin
in 80% ethanolic extract (μg/mg) |
|---|
| Linarin | y = 19,819 x +
31,363 | 0.996 | 50–200 | 1.2–5.5 | 40.57±2.72 |
The retention time, observed mass, mass difference
and proposed compounds of 3 peaks are listed in Table III. From the mass spectra, major
ion peaks 1–3 contributed to the protonated molecular ion of
m/z 355, 517 and 517, respectively. Comparing the reference
molecular weight and UV-Visible absorption spectrum, peak 1
corresponds to that of caffeoylquinic acid (32,33). Since caffeoylquinic acid has the
same molecular weight and UV-Visible absorption spectrum, it is
difficult to assign the exact identification of the peak only by
HPLC-(diode-array detection) DAD-MS. Considering the UV-Visible
absorption spectrum and molecular weight, unidentified peaks 2 and
3 are supposed to be isomers of dicaffeoylquinic acids (Table III). In order to elucidate the
exact identity of the peaks, further sets of experiments, including
semi-quantitative scale isolation and spectroscopical analysis are
required.
 | Table III.The observed and calculated mass
numbers of HPLC peaks of CZE. |
Table III.
The observed and calculated mass
numbers of HPLC peaks of CZE.
| Peak no. | Rt (min) | Theoretical mass [M
+ H]+ | Observed mass [M +
H]+ | Mass difference
(mmu) | Identification |
|---|
| 1 | 6.55 | 355.10291 | 355.09980 | −3.11 | Caffeoylquinic
acid |
| 2 | 23.76 | 517.13460 | 517.13413 | −0.47 | Dicaffeoylquinic
acid |
| 3 | 25.90 | 517.13460 | 517.13498 | 0.38 | Dicaffeoylquinic
acid |
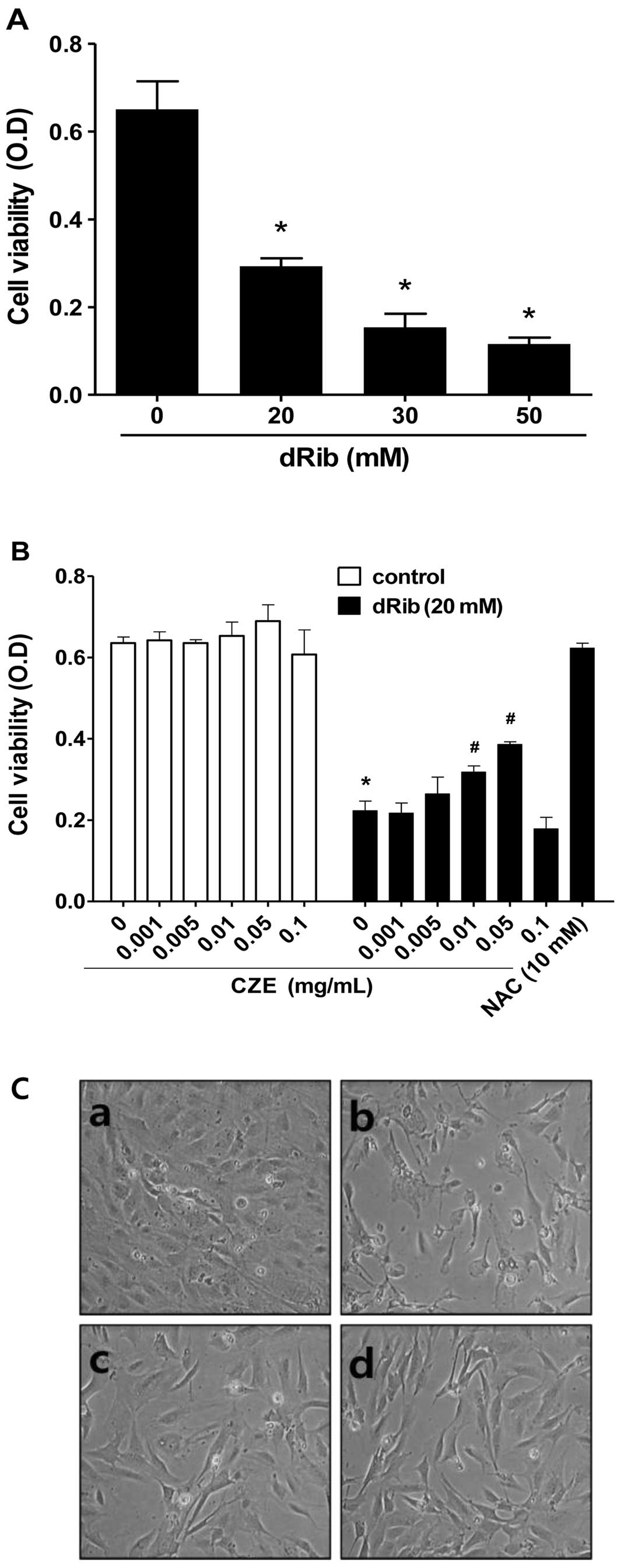
To evaluate the effect of dRib on MC3T3-E1
osteoblastic cell survival, cell viability was determined using the
CCK-8 assay. We observed a dose-dependent decrease in cell
viability in the cells exposed to various concentrations of dRib
for 24 h (Fig. 2A). Based on the
results of these viability assays, we used 20 mM dRib in subsequent
biochemical assays. At this concentration, approximately 50%
inhibition of cell viability occurred in 24 h under our
experimental culture conditions.
To evaluate the effect of CZE on MC3T3-E1
osteoblastic cell survival, the cells were incubated in α-MEM
containing 0.5% FBS with increasing concentrations of CZE
(0.001-0.1 mg/ml) for 24 h and then cell viability was determined.
CZE at these concentrations had no effect on cell viability
(Fig. 2B), while higher doses
(>0.1 mg/ml) were cytotoxic (data not shown).
To determine whether CZE has an effect on the
dRib-induced decrease in cell survival, cells were pre-incubated
with CZE for 30 min and then cultured with 20 mM dRib for 24 h.
CCK-8 assays revealed that CZE (0.01–0.05 mg/ml) partially reversed
the dRib-mediated reduction in cell viability in a dose-dependent
manner (Fig. 2B). Therefore, we
selected the highest non-toxic concentration of CZE (0.01 and 0.05
mg/ml) for all subsequent cell culture experiments. The
antioxidant, N-acetyl-L-cysteine (NAC), was used to
investigate the mechanism of dRib-induced cell damage.
Pre-treatment of the cells with 10 mM NAC almost completely
reversed the dRib-induced cytotoxicity. These findings suggest that
the dRib-induced cytotoxicity was most likely due to oxidative
stress-induced effects. In a recent study, we showed that the
antioxidants, NAC and α-lipoic acid (ALA), almost reversed the
dRib-mediated reduction in the viability of MC3T3-E1 osteoblastic
cells (26). Morphological
changes were compared between the dRib-treated and control cells
under an inverted microscope. The control cells were flat,
polygonal in shape and arranged in a monolayer. Following exposure
to dRib for 24 h, the cells were degenerated and had a
spindle-shaped appearance. CZE improved the morphological changes
of osteoblastic cells induced by dRib (Fig. 2C).
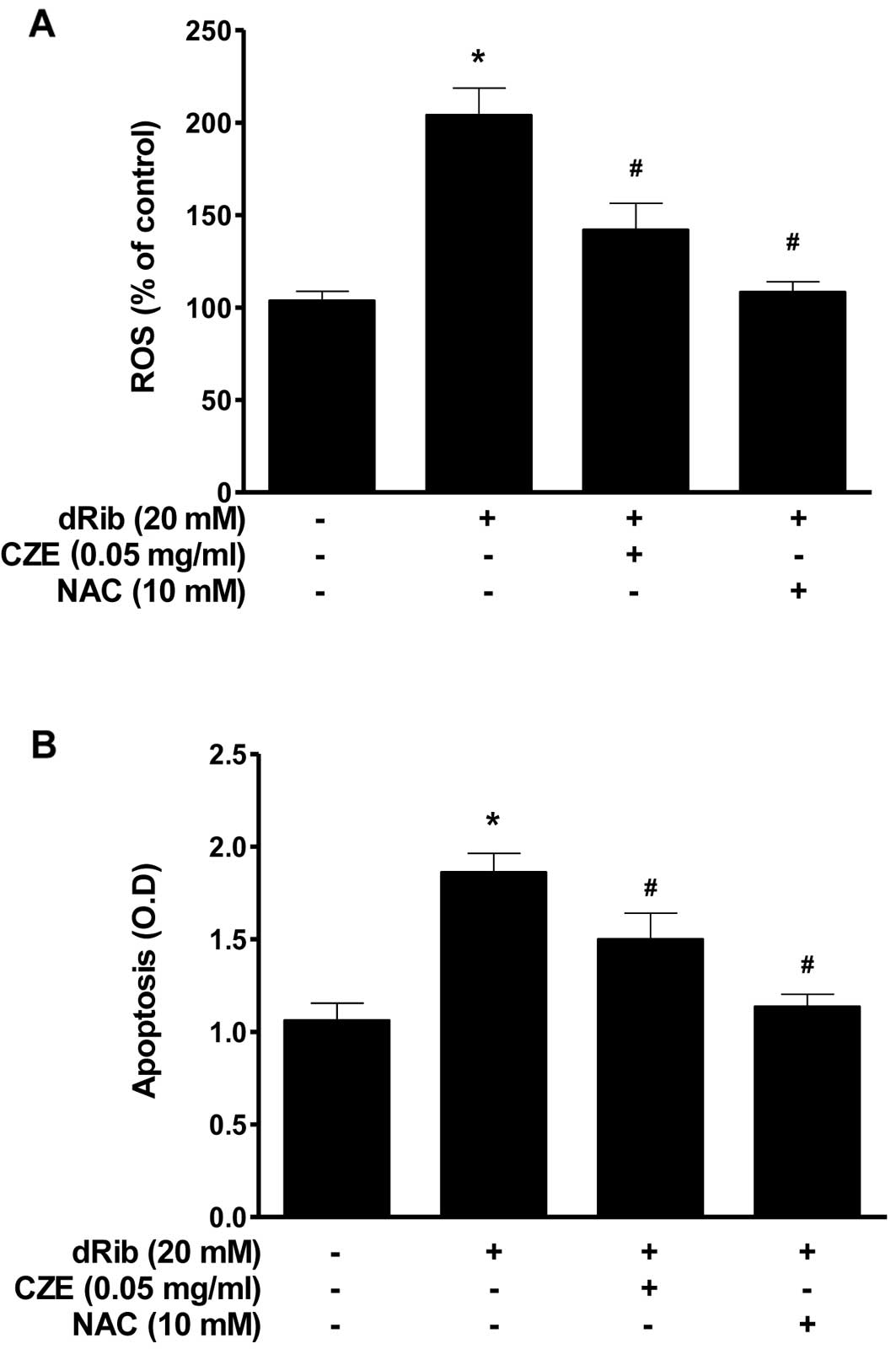
The oxidative stress caused by dRib in MC3T3-E1
osteoblastic cells was evaluated by measuring ROS generation and
apoptosis. Oxidative stress may initiate a mitochondrial
permeability transition event, which is an early mediator of
cellular apoptosis. When the cells were treated with 20 mM dRib,
ROS generation and apoptosis increased, while treatment with CZE
(0.05 mg/ml) in the presence of dRib attenuated all the
dRib-induced effects (Fig. 3). We
used the antioxidant, NAC, to investigate the effect of oxidative
stress on the cells. NAC prevented the dRib-induced cellular
effects. These data are consistent with those from previous our
studies, indicating that the antioxidants, NAC and ALA, protect
pancreatic β-cells and MC3T3-E1 osteoblastic cells against
oxidative stress, as shown by a reduction in ROS generation and
apoptosis (20– 22, 26, 28). These findings indicate that CZE
can function as an antioxidant and thereby protect MC3T3-E1
osteoblastic cells from dRib-induced oxidative cell damage.
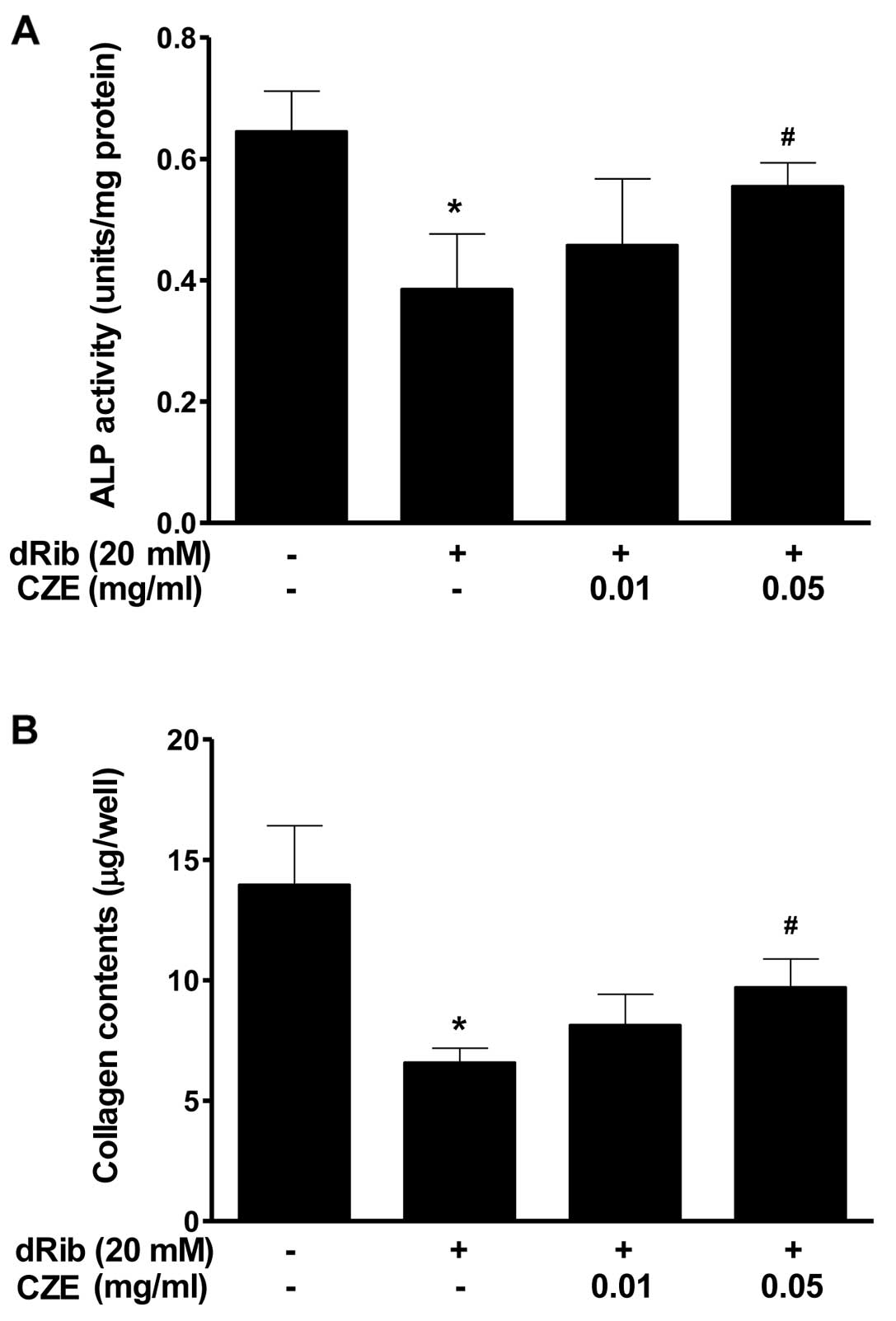
MC3T3-E1 cells are an osteoblastic precursor cell
line which was cloned from newborn mouse calvaria (34), and it is frequently used to study
osteoblast differentiation. Osteoblast differentiation is the
primary event of bone formation. Bone ALP is a glycoprotein
localized in the plasma membrane of osteoblastic cells which is one
of the osteoblastic phenotype markers (35). Alterations in its activity have
been observed in osteoporosis and other metabolic bone diseases.
High levels of ALP activity have been observed in both
pre-osteoblasts and osteoblasts in vivo and in
differentiating osteoblasts in vitro. Osteoblastic cells
produce type I collagen, which is the most abundant protein in the
bone matrix, serves an early marker of osteoblast differentiation,
and is the major organic component of mineralized bone matrix
(36). The present study
demonstrates that the reducing sugar, dRib, exerts a profound
inhibitory effect on osteoblastic cell differentiation; however,
when osteoblastic cells were treated with CZE in the presence of 20
mM dRib, significant increases in the major osteoblast-specific ALP
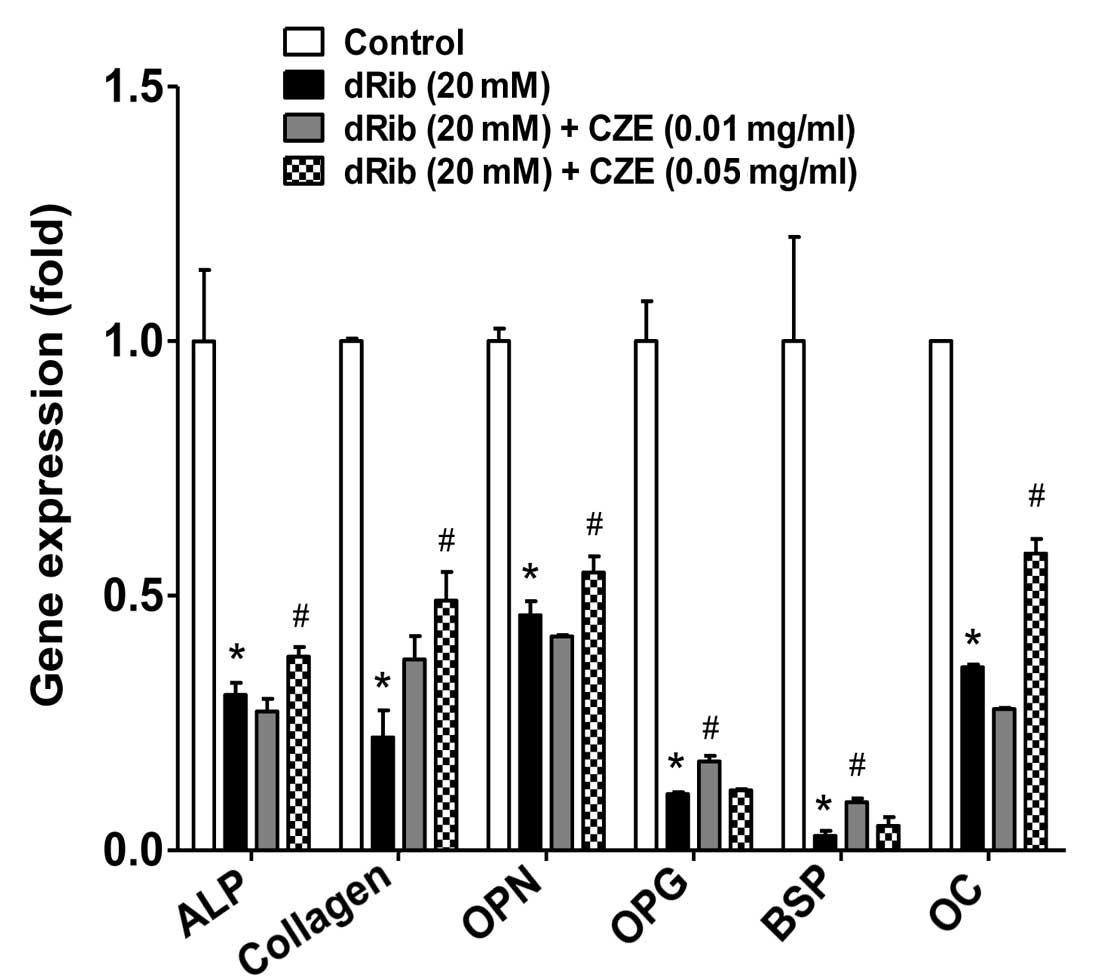
activity and collagen content were observed (Fig. 4).
In addition to measuring the levels of the
differentiation marker, ALP and collagen, we evaluated the
differentiated function of the osteoblastic cells at the
transcriptional level. We analyzed the gene expression of a number
of molecular markers of osteoblast differentiation. Osteopontin
(OPN) is a major acidic phosphorylated glycoprotein secreted by
osteoblasts and acts as a regulator of bone formation (37). Osteoprotegerin (OPG), produced by
osteoblasts is one of the regulators of bone metabolism and
inhibits bone resorption by regulating the unction of osteoclasts
(38). Bone sialoprotein (BSP) is
thought to function in the initial mineralization of bone and may
be crucial for osteoblast differentiation. The flavonoid,
kaempferol, has been shown to stimulate BSP gene transcription and
new bone formation (39). Thus,
these molecular markers are important and best known regulators of
osteoblast function. In this study, osteoblastic cells were treated
with dRib in the presence or absence of CZE. Six differentiation
makers [ALP, collagen, OPN, OPG, BSP and osteocalcin (OC)] were
down-regulated in response to exposure to dRib; however, treatment
with CZE partially inhibited the dRib-induced downregulation of
gene expression of the differentiation markers (Fig. 5).
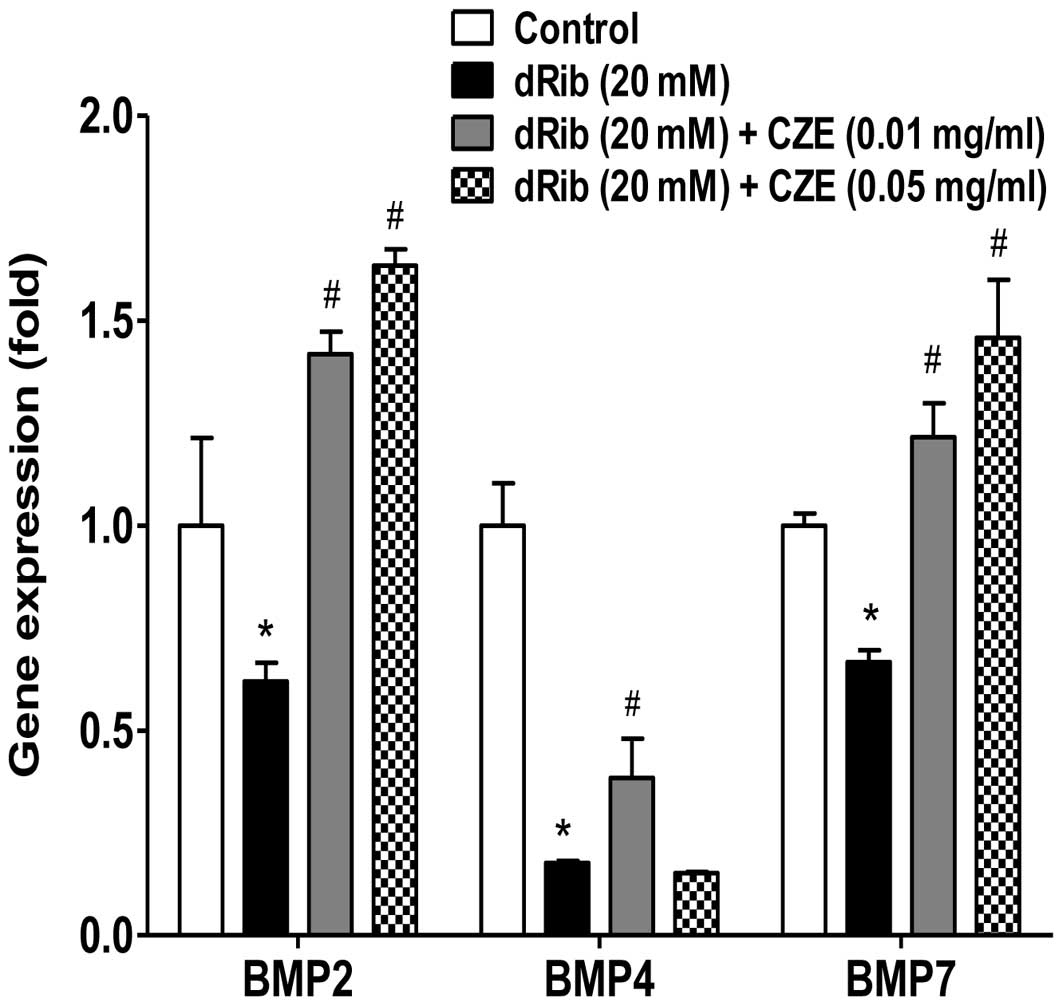
Bone morphogenetic proteins (BMPs) are known to be
the most potent regulators of osteoblast differentiation among many
local factors. MC3T3-E1 cells are highly BMP-responsive and can
complete the differentiation process in long term culture. BMPs
stimulate ALP activity, collagen synthesis, parathyroid hormone
(PTH) responsiveness and OC production in osteoblastic cells
(40–41), suggesting that BMPs stimulate the
differentiation of osteoblastic cells. The present study
demonstrates that the reducing sugar, dRib, exerts a profound
inhibitory effect on the gene expression of BMPs; however, when the
osteoblastic cells were treated with CZE in the presence of 20 mM
dRib, the expression of BMPs, including BMP2, BMP4 and BMP7 was
significantly increased (Fig. 6).
These results suggest that CZE exerts a positive effect on the
differentiation of osteoblastic cells through the stimulation of
BMP production.
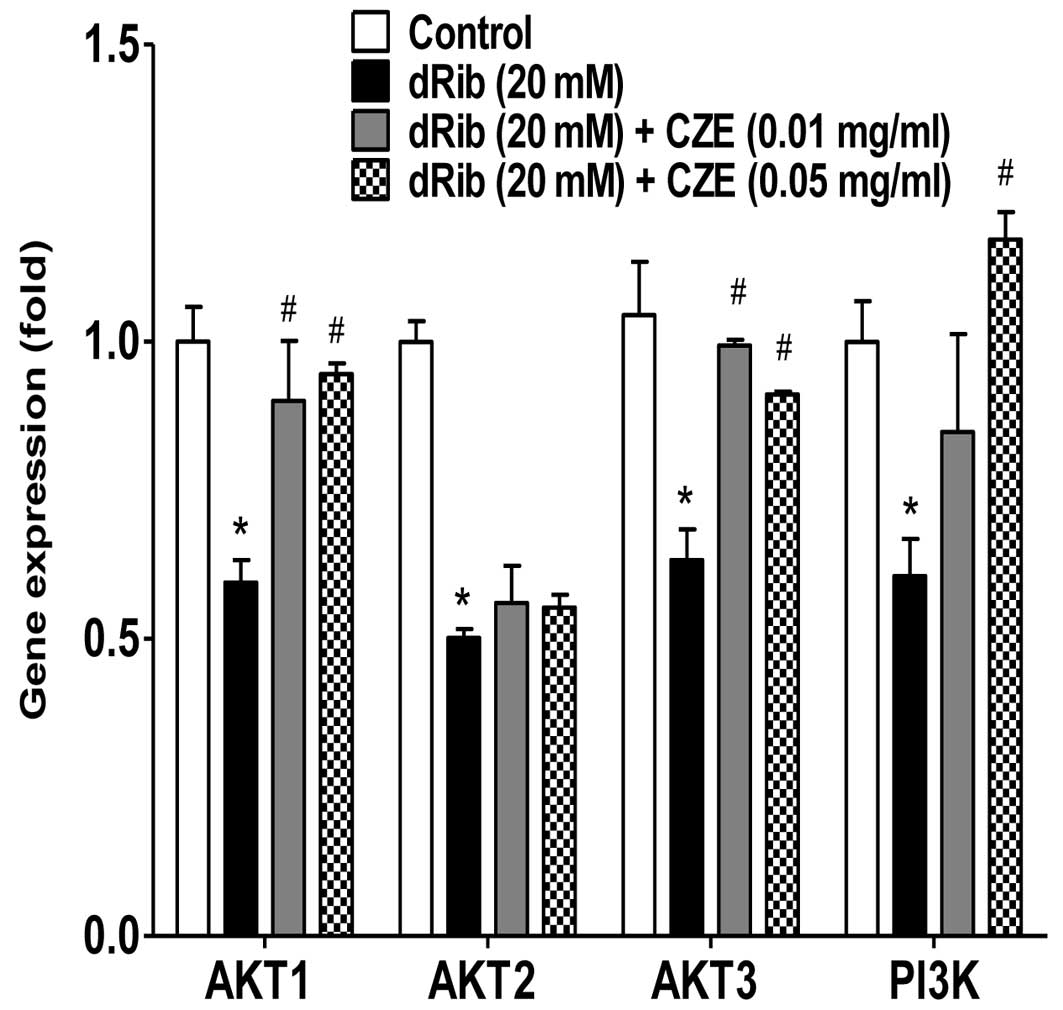
The phosphatidylinositol 3-kinase (PI3K)-protein
kinase B (AKT) signaling pathway is activated by many types of
cellular stimuli or toxic insults and regulates fundamental
cellular functions, such as transcription, translation,
proliferation, growth and survival (42). One important function of activated
PI3K in cells is the inhibition of apoptosis (43). AKT is a good candidate for
mediating these PI3K-dependent cell survival responses. AKT has
been implicated as an anti-apoptotic factor in several different
cell death paradigms, including the withdrawal of extracellular
signaling factors, oxidative and osmotic stress, irradiation and
the treatment of cells with chemotherapeutic drugs and ischemic
shock (44). The flavonoid,
honokiol, has been shown to exert a protective effect against
antimycin A (an inhibitor of mitochondrial electron
transport)-induced oxidative cell damage via the activation of PI3K
and/or AKT in MC3T3-E1 osteoblastic cells (45). In the present study, CZE also
induced the activation of PI3K, AKT1 and AKT3, which was inhibited
by dRib (Fig. 7). Since these
signaling molecules are involved in cellular survival pathways, CZE
may be cytoprotective for osteoblastic cells during oxidative
stress responses.
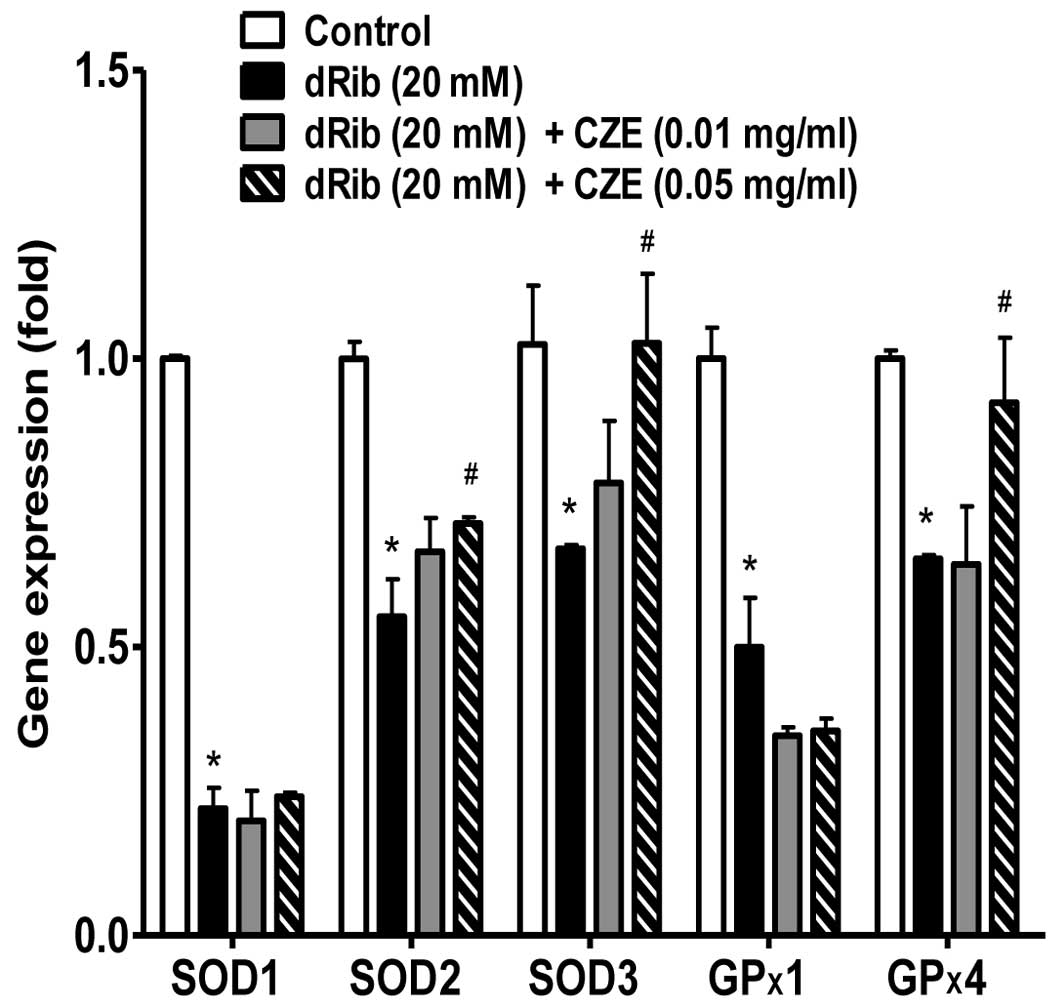
Excess ROS must be promptly eliminated from the
cells by a variety of antioxidant defense mechanisms. Cellular
antioxidant enzymes and other redox molecules serve to
counterbalance ROS generated in cells. Superoxide dismutase (SOD),
which catalyzes the dismutation of the superoxide anion into
hydrogen peroxide and molecular oxygen, is one of the most
important antioxidant enzymes (46). SOD enzymes are classified into 3
groups: CuZn-SOD (SOD1) is located in the cytoplasm, Mn-SOD (SOD2)
in the mitochondria and EC-SOD (SOD3) is extracellular. Glutathione
peroxidase (GPx) catalyzes the reduction of hydroperoxides,
including hydrogen peroxides, by reduced glutathione and functions
to protect the cell from oxidative damage. GP×1 is the most
abundant version, found in the cytoplasm of almost all mammalian
tissues, whose preferred substrate is hydrogen peroxide. GP×4 has a
high preference for lipid hydroperoxides. It has been reported that
various flavonoids increase the activity of antioxidant enzymes in
osteoblastic cells. Quercetin can diminish oxidative human
osteoblastic cell damage by scavenging the radicals and by
upregulating the expression of heme oxygenase-1 (HO-1) and SOD1
exposed to cigarette smoke medium (47). An extract of total flavonoids from
persimmon leaves has been shown to significantly decrease the level
of ROS and malondialdehyde (MDA), while increasing the activity of
catalase (CAT), SOD and GPx in MC3T3-E1 cells (48). Simvastatin has been shown to abate
oxidative stress by enhancing catalase, HO-1 and SOD activity and
suppressing NADPH oxidase activity in an aged and ovariectomized
rat model (49). Another study
demonstrated that intracellular redox imbalance caused by SOD1
deficiency plays a pivotal role in the development and progression
of bone fragility both in vivo and in vitro (51). In
this study, in addition to the biochemical aspects of oxidative
stress, the gene expression of antioxidative enzymes was
investigated. The reducing sugar, dRib, exerted a profound
inhibitory effect on the gene expression of SOD1, SOD2, SOD3, GP×1
and GP×4. However, when the osteoblastic were treated with CZE in
the presence of 20 mM dRib, a significant increase in the gene
expression levels of SOD2, SOD3 and GP×4, but not SOD1 and GP×1 was
observed (Fig. 8).
In conclusion, in the present study, we demonstrate
that CZE attenuates dRib-induced cell damage in MC3T3-E1
osteoblastic cells due to its antioxidant activity and postive
effect on differentiation, which may promote bone recovery in
diabetes-associated bone diseases.
Acknowledgements
This study was supported by
‘Industrialization Propulsion Unit for Chrysanthemum and Abalone’,
Wando-gun, Jeollanam-do, Republic of Korea.
References
|
1.
|
Collins RA, Ng TB, Fong WP, Wan CC and
Yeung HW: A comparison of human immunodeficiency virus type 1
inhibition by partially purified aqueous extracts of Chinese
medicinal herbs. Life Sci. 60:PL345–PL351. 1997. View Article : Google Scholar
|
|
2.
|
Hu CQ, Chen K, Shi Q, Kilkuskie RE, Cheng
YC and Lee KH: Anti-AIDS agents, 10.
Acacetin-7-O-beta-D-galactopyranoside, an anti-HIV principle from
Chrysanthemum morifolium and a structure-activity
correlation with some related flavonoids. J Nat Prod. 57:42–51.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Sassi AB, Harzallah-Skhiri F, Bourgougnon
N and Aouni M: Antimicrobial activities of four Tunisian
Chrysanthemum species. Indian J Med Res. 127:183–192.
2008.PubMed/NCBI
|
|
4.
|
Liu Q, Liu H, Yuan Z, Wei D and Ye Y:
Evaluation of antioxidant activity of chrysanthemum extracts and
tea beverages by gold nanoparticles-based assay. Colloids Surf B
Biointerfaces. 92:348–352. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
He J, Chen F, Chen S, Lv G, Deng Y, Fang
W, Liu Z, Guan Z and He C: Chrysanthemum leaf epidermal surface
morphology and antioxidant and defense enzyme activity in response
to aphid infestation. J Plant Physiol. 168:687–693. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Marongiu B, Piras A, Porcedda S, Tuveri E,
Laconi S, Deidda D and Maxia A: Chemical and biological comparisons
on super-critical extracts of Tanacetum cinerariifolium
(Trevir) Sch. Bip with three related species of chrysanthemums of
Sardinia (Italy). Nat Prod Res. 23:190–199. 2009.PubMed/NCBI
|
|
7.
|
Wu TY, Khor TO, Saw CL, Loh SC, Chen AI,
Lim SS, Park JH, Cai L and Kong AN:
Anti-inflammatory/anti-oxidative stress activities and differential
regulation of Nrf2-mediated genes by non-polar fractions of tea
Chrysanthemum zawadskii and licorice Glycyrrhiza
uralensis. AAPS J. 13:1–13. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Seo JY, Lim SS, Park J, Lim JS, Kim HJ,
Kang HJ, Yoon Park JH and Kim JS: Protection by Chrysanthemum
zawadskii extract from liver damage of mice caused by carbon
tetrachloride is maybe mediated by modulation of QR activity. Nutr
Res Pract. 4:93–98. 2010.
|
|
9.
|
Robertson RP, Harmon J, Tran PO, Tanaka Y
and Takahashi H: Glucose toxicity in beta-cells: type 2 diabetes,
good radicals gone bad, and the glutathione connection. Diabetes.
52:581–587. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Robertson RP: Chronic oxidative stress as
a central mechanism for glucose toxicity in pancreatic islet beta
cells in diabetes. J Biol Chem. 279:42351–42354. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
López-Ibarra PJ, Pastor MM,
Escobar-Jiménez F, Pardo MD, González AG, Luna JD, Requena ME and
Diosdado MA: Bone mineral density at time of clinical diagnosis of
adult-onset type 1 diabetes mellitus. Endocr Pract. 7:346–351.
2001.PubMed/NCBI
|
|
12.
|
Tuominen JT, Impivaara O, Puukka P and
Ronnemaa T: Bone mineral density in patients with type 1 and type 2
diabetes. Diabetes Care. 22:1196–1200. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Herskind AM, Christensen K,
Nørgaard-Andersen K and Andersen JF: Diabetes mellitus and healing
of closed fractures. Diabete Metab. 18:63–64. 1992.PubMed/NCBI
|
|
14.
|
Bai XC, Lu D, Bai J, Zheng H, Ke ZY, Li XM
and Luo SQ: Oxidative stress inhibits osteoblastic differentiation
of bone cells by ERK and NF-kappaB. Biochem Biophys Res Commun.
314:197–207. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Fatokun AA, Stone TW and Smith RA:
Hydrogen peroxide-induced oxidative stress in MC3T3-E1 cells: The
effects of glutamate and protection by purines. Bone. 39:542–551.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Seeman E: Reduced bone formation and
increased bone resorption: rational targets for the treatment of
osteoporosis. Osteoporos Int. 14(Suppl 3): S2–S8. 2003.PubMed/NCBI
|
|
17.
|
Thornalley P, Wolff S, Crabbe J and Stern
A: The autoxidation of glyceraldehyde and other simple
monosaccharides under physiological conditions catalysed by buffer
ions. Biochim Biophys Acta. 797:276–287. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Kaneto H, Fujii J, Myint T, Miyazawa N,
Islam KN, Kawasaki Y, Suzuki K, Nakamura M, Tatsumi H, Yamasaki Y
and Taniguchi N: Reducing sugars trigger oxidative modification and
apoptosis in pancreatic beta-cells by provoking oxidative stress
through the glycation reaction. Biochem J. 320:855–863.
1996.PubMed/NCBI
|
|
19.
|
Bunn HF and Higgins PJ: Reaction of
monosaccharides with proteins: possible evolutionary significance.
Science. 213:222–224. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Koh G, Suh KS, Chon S, Oh S, Woo JT, Kim
SW, Kim JW and Kim YS: Elevated cAMP level attenuates
2-deoxy-D-ribose-induced oxidative damage in pancreatic beta-cells.
Arch Biochem Biophys. 438:70–79. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Koh G, Lee DH and Woo JT: 2-Deoxy-D-ribose
induces cellular damage by increasing oxidative stress and protein
glycation in a pancreatic beta-cell line. Metabolism. 59:325–332.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Lee YJ, Suh KS, Choi MC, Chon S, Oh S, Woo
JT, Kim SW, Kim JW and Kim YS: Kaempferol protects HIT-T15
pancreatic beta cells from 2-deoxy-D-ribose-induced oxidative
damage. Phytother Res. 24:419–423. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Suh KS, Oh S, Woo JT, Kim SW, Kim JW, Kim
YS and Chon S: Apigenin attenuates 2-deoxy-D-ribose-induced
oxidative cell damage in HIT-T15 pancreatic β-cells. Biol Pharm
Bull. 35:121–216. 2012.PubMed/NCBI
|
|
24.
|
Choi EM and Kim YH: Hesperetin attenuates
the highly reducing sugar-triggered inhibition of osteoblast
differentiation. Cell Biol Toxicol. 24:225–231. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Lee KH and Choi EM: Myricetin, a naturally
occurring flavonoid, prevents 2-deoxy-D-ribose induced dysfunction
and oxidative damage in osteoblastic MC3T3-E1 cells. Eur J
Pharmacol. 591:1–6. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Suh KS, Choi EM, Kwon M, Chon S, Oh S, Woo
JT, Kim SW, Kim JW and Kim YS: Kaempferol attenuates
2-deoxy-D-ribose-induced oxidative cell damage in MC3T3-E1
osteoblastic cells. Biol Pharm Bull. 32:746–749. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Kanno S, Anuradha CD and Hirano S:
Localization of zinc after in vitro mineralization in osteoblastic
cells. Biol Trace Elem Res. 83:39–47. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Suh KS, Chon S, Oh S, Kim SW, Kim JW, Kim
YS and Woo JT: Prooxidative effects of green tea polyphenol
(-)-epigallocate-chin-3-gallate on the HIT-T15 pancreatic beta cell
line. Cell Biol Toxicol. 26:189–199. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Balint E, Szabo P, Marshall CF and Sprague
SM: Glucose-induced inhibition of in vitro bone mineralization.
Bone. 28:21–28. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Terada M, Inaba M, Yano Y, Hasuma T,
Nishizawa Y, Morii H and Otani S: Growth-inhibitory effect of a
high glucose concentration on osteoblast-like cells. Bone.
22:17–23. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Han SH, Sung KH, Yim DS, Lee SK, Lee CK,
Ha NJ and Kim KJ: The effect of linarin on LPS-induced cytokine
production and nitric oxide inhibition in murine macrophages cell
line RAW264.7. Arch Pharm Res. 25:170–177. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Clifford MN, Wu W, Kirkpatrick J and
Kuhnert N: Profiling the chlorogenic acids and other caffeic acid
derivatives of herbal chrysanthemum by LC-MSn. J Agric
Food Chem. 55:929–936. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Lin LZ and Harnly JM: Identification of
the phenolic components of chrysanthemum flower (Chrysanthemum
morifolium Ramat). Food Chem. 120:319–326. 2010. View Article : Google Scholar
|
|
34.
|
Sudo H, Kodama HA, Amagai Y, Yamamoto S
and Kasai S: In vitro differentiation and calcification in a new
clonal osteogenic cell line derived from newborn mouse calvaria. J
Cell Biol. 96:191–198. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Bellows CG, Aubin JE and Heersche JN:
Initiation and progression of mineralization of bone nodules formed
in vitro: the role of alkaline phosphatase and organic phosphate.
Bone Miner. 14:27–40. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Domon S, Shimokawa H, Yamaguchi S and Soma
K: Temporal and spatial mRNA expression of bone sialoprotein and
type I collagen during rodent tooth movement. Eur J Orthod.
23:339–348. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Chen Y, Bal BS and Gorski JP: Calcium and
collagen binding properties of osteopontin, bone sialoprotein, and
bone acidic glycoprotein-75 from bone. J Biol Chem.
267:24871–24878. 1992.PubMed/NCBI
|
|
38.
|
Khosla S: Minireview: the OPG/RANKL/RANK
system. Endocrinology. 142:5050–5055. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Yang L, Takai H, Utsunomiya T, Li X, Li Z,
Wang Z, Wang S, Sasaki Y, Yamamoto H and Ogata Y: Kaempferol
stimulates bone sialoprotein gene transcription and new bone
formation. J Cell Biochem. 110:1342–1355. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Takuwa Y, Ohse C, Wang EA, Wozney JM and
Yamashita K: Bone morphogenetic protein-2 stimulates alkaline
phosphatase activity and collagen synthesis in cultured
osteoblastic cells, MC3T3-E1. Biochem Biophys Res Commun.
174:96–101. 1991. View Article : Google Scholar
|
|
41.
|
Nakase T, Takaoka K, Masuhara K, Shimizu
K, Yoshikawa H and Ochi T: Interleukin-1β enhances and tumor
necrosis factor-α inhibits bone morphogenetic protein-2-induced
alkaline phosphatase activity in MC3T3-E1 osteoblastic
cells. Bone. 21:17–21. 1997.
|
|
42.
|
Vivanco I and Sawyers CL: The
phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev
Cancer. 2:489–501. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Yao R and Cooper GM: Requirement for
phosphatidylinositol-3 kinase in the prevention of apoptosis by
nerve growth factor. Science. 267:2003–2006. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Franke TF, Kaplan DR and Cantley LC: PI3K:
Downstream AKTion blocks apoptosis. Cell. 88:435–437. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
45.
|
Choi EM: Honokiol protects osteoblastic
MC3T3-E1 cells against antimycin A-induced cytotoxicity. Inflamm
Res. 60:1005–1012. 2011. View Article : Google Scholar
|
|
46.
|
Zelko IN, Mariani TJ and Folz RJ:
Superoxide dismutase multigene family: a comparison of the CuZn-SOD
(SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures,
evolution, and expression. Free Radic Biol Med. 33:337–349. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
47.
|
Braun KF, Ehnert S, Freude T, Egaña JT,
Schenck TL, Buchholz A, Schmitt A, Siebenlist S, Schyschka L,
Neumaier M, Stöckle U and Nussler AK: Quercetin protects primary
human osteoblasts exposed to cigarette smoke through activation of
the anti-oxidative enzymes HO-1 and SOD-1. ScientificWorldJournal.
11:2348–2357. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48.
|
Sun L, Zhang J, Lu X, Zhang L and Zhang Y:
Evaluation to the antioxidant activity of total flavonoids extract
from persimmon (Diospyros kaki L.) leaves. Food Chem
Toxicol. 49:2689–2696. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49.
|
Yin H, Shi ZG, Yu YS, Hu J, Wang R, Luan
ZP and Guo DH: Protection against osteoporosis by statins is linked
to a reduction of oxidative stress and restoration of nitric oxide
formation in aged and ovariectomized rats. Eur J Pharmacol.
674:200–206. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50.
|
Nojiri H, Saita Y, Morikawa D, Kobayashi
K, Tsuda C, Miyazaki T, Saito M, Marumo K, Yonezawa I, Kaneko K,
Shirasawa T and Shimizu T: Cytoplasmic superoxide causes bone
fragility owing to low-turnover osteoporosis and impaired collagen
cross-linking. J Bone Miner Res. 26:2682–2694. 2011. View Article : Google Scholar : PubMed/NCBI
|