Introduction
Hypertension is a factor responsible for structural
and functional alterations in myocardial tissue leading to
hypertrophy of the left ventricle (LV), which is defined as an
increase in the LV mass in a compensatory response to elevated
blood pressure to minimize wall stress (1). Eventually, congestive heart failure
(CHF) develops after sustained load increase. LV hypertrophy is a
strong independent predictor of cardiovascular events and all-cause
mortality. Patients with LV hypertrophy are at an increased risk of
stroke, coronary heart disease, CHF and sudden cardiac death, while
the regression of LV hyper-trophy is associated with the reduced
risk of these clinical events (2,3).
Therefore, identifying the molecular mechanisms behind the
mechanical stress from elevated blood pressure in LV hypertrophy is
key to understanding and treating hyper-tensive heart disease. The
Src kinase plays a critical role in the development of cardiac
hypertrophy in response to mechanical stress (4).
The Src kinase, a non-receptor protein tyrosine
kinase, participates in multiple signaling pathways involved in
cardiac hypertrophy, such as integrin-mediated and G
protein-coupled receptor signal transduction (5–10).
Src kinase activity can be regulated by altering the
phosphorylation of specific tyrosine residues. There are 3 main
phosphorylation sites, tyrosines 529, 418 and 215, that are
involved in regulating Src activity. Phosphorylation at residues
418 and 215 increases Src kinase activity, while phosphorylation at
tyrosine 529 downregulates Src kinase activity in growth factor
signaling (11,12). Src autophosphorylates, and Src
kinase activity also transiently increases when cultured rat
cardiac myocytes are subjected to mechanical stress in vitro
(5). The Src kinase is also
activated during cardiac hypertrophy following acute pressure
overload in vivo (9). Our
previous study demonstrated that the Src kinase relocated from the
cytoplasm to the cell membrane and aggregated in the intercalated
discs during decompensated remodeling of cardiac myocytes in LV
hypertrophy resulting from hypertension (13). However, whether the Src kinase is
involved in signal transduction in the nuclei of cardiac myocytes
in the LV during hypertrophy resulting from hypertension remains
unknown.
Materials and methods
Animals
Two-, 6-, 12- and 18-month-old, lean, male
spontaneously hypertensive heart failure (SHHF) rats and
age-matched, male Wistar-Kyoto (WKY) rats, as the normotensive
controls, were acquired from Da-Shuo Scientific Co. (Chengdu,
China). Six rats from each age group were used for extracting
nuclear protein (NP) homogenates of the LV tissues for western blot
analysis and for cutting frozen sections of the LV tissues for
immunofluorescent staining. Six rats from each age group were used
for isolating cardiac myocytes for immunofluorescent staining. Six
of the 6-month-old SHHF and WKY rats (6 rats from each) were used
for immunoprecipitation. All procedures were performed in
accordance with the Guide for the Care and Use of Laboratory
Animals (US Department of Health and Human Services, NIH
publication no. 85-23) and approved by the Sun Yat-sen University
Animal Care and Use Committee.
Antibodies
Rabbit polyclonal antibodies against the N-terminus
of Src(N-16) for western blot analyses, mouse monoclonal antibody
against the N-terminus of Src(H-12) for immunofluorescent labeling
and immunoprecipitation, and mouse monoclonal antibody against
Src-associated in mitosis 68 kDa (Sam68) were obtained from Santa
Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Anti-Src
phosphorylated at tyrosine 529 (Src[pY529]) and anti-Src
phosphorylated at tyrosine 418 (Src[pY418]) phosphospecific
antibodies for western blot analysis and immunolabeling were
obtained from Abcam (Cambridge, UK) and BioSource International,
Inc. (Camarillo, CA, USA), respectively. Anti-Src phosphorylated at
tyrosine 215 (Src[pY215]) phosphospecific antibody was obtained
from Upstate Biotechnology, Inc. (Lake Placid, NY, USA).
Anti-fibrillarin monoclonal antibody was obtained from
Cytoskeleton, Inc. (Denver, CO, USA). Alexa Fluor 488 or 568
conjugated goat anti-rabbit IgG or goat anti-mouse IgG antibodies
for immunolabeling were obtained from Molecular Probes (Eugene, OR,
USA).
Myocyte isolation, frozen sections of
intact myocardium, immunolabeling and confocal microscopy
Cardiac myocytes were enzymatically isolated as
previously described (14).
Briefly, the hearts were removed and trimmed. The aorta was
cannulated for retrograde perfusion with oxygenated calcium-free
Joklik medium (Sigma, St. Louis, MO, USA) at 37°C followed by
incubation with the same medium containing 0.1% collagenase. After
collagenase perfusion, the heart became softened and was removed
from the perfusion apparatus. Tissue was minced in calcium-free
Joklik medium and the isolated cells were filtered through a nylon
mesh (250 μm) into 8% paraformaldehyde solution, fixed at a
final concentration of 4% for 10 min and subsequently suspended in
phosphate-buffered saline (PBS) for fluorescent labeling.
The LV tissue was placed in embedding medium and
frozen. Sections (6-μm-thick) were cut using a cryostat and
mounted onto poly-l-lysine-coated slides for immunofluorescent
labeling. Frozen tissue sections were thawed to room temperature,
fixed in cold acetone for 10 min and washed with PBS as previously
described (15). For the isolated
cardiac myocytes, cardiac myocyte suspensions were aliquoted onto
positively charged slides, permeated with 0.5% Triton X-100 for 30
min at room temperature and washed in PBS (16). Immunofluorescent labeling was
performed on both the frozen tissue sections and the isolated
cardiac myocyte sedimentary slides according to the following
procedure: After washing with PBS, the slides were blocked with 1%
bovine serum albumin to reduce non-specific binding and a primary
antibody (anti-c-Src antibody or anti-Src[pY529], anti-Src[pY418]
and anti-Src[pY215] phosphopecific antibodies, or anti-fibrillarin
antibody or anti-Sam68 antibody) was added for overnight incubation
at 4°C. The primary antibody was removed and the slides were washed
3 times with PBS. A secondary fluorochrome-conjugated antibody
(Alexa Fluor 488 conjugated goat anti-rabbit IgG antibody, Alexa
Fluor 488 conjugated goat anti-mouse IgG antibody, Alexa Fluor 568
conjugated goat anti-rabbit IgG antibody or Alexa Fluor 568
conjugated goat anti-mouse IgG antibody, based on the primary
antibody used in labeling) was added followed by incubation for 1 h
at room temperature. Unbound secondary antibody was then washed
away with PBS. The nuclei were counterstained with propidium iodide
(PI). The same procedure was repeated with the second set of
antibodies, except that the nuclei were not counterstained with PI
for double labeling. The slides were mounted in 60% glycerol in PBS
and sealed with nail polish for observation using an Olympus
FluoView Confocal Laser Scanning Microscope System. The negative
controls were incubated with the omission or substitution of
primary antibodies with rabbit serum under the same conditions.
Cardiac myocytes labeled without primary antibody or with rabbit
serum only showed weak diffuse background fluorescence.
NP extraction, gel electrophoresis and
western blot analysis
The extraction of NP was performed as previously
described (17). NPs were
separated by one-dimensional SDS-polyacrylamide gel electrophoresis
(SDS-PAGE) (Laemmli) based on their molecular weight. NP content
was determined for each sample using the Bradford method. Fifty
micrograms of protein from each LV sample were mixed with Laemmli
SDS sample buffer (2X stock Laemmli SDS sample buffer, 5%
2-mercaptoethenol and 0.005% bromophenol blue), and the samples
were then boiled and electrophoresed on 10% gels. The proteins were
then transferred onto nitrocellulose membranes. The membranes were
blocked with 3% BSA and 5% dry milk in PBS for 1 h and probed with
primary antibody. After thorough washing, the bound antibodies were
visualized with horseradish peroxidase-conjugated anti-rabbit IgG
antibody using the enhanced chemiluminescence technique (Amersham
Biosciences, Piscataway NJ, USA). The protein bands were scanned
and the densities were quantified using NIH image software. The
mean optical density of each band was regarded as the density unit
for the relative protein content.
Immunoprecipitation
The LV was homogenized with a PT1200C Polytron
homogenizer in ice-cold lysis buffer (50 mM Tris•HCl, 1% NP-40, 150
mM NaCl, 1 mM EDTA, 1 mM PMSF, 1 μg/ml aprotinin, 1
μg/ml leupeptin, 1 μg/ml pepstatin, 1 mM
Na3VO4 and 1 mM NaF, pH 7.4). The homogenates
were centrifuged at 14,000 x g for 15 min at 4°C. The supernatant
was quantified using the Bradford method. A total of 1,000
μg protein was placed into a new centrifuge tube and
adjusted to the same volume of 0.5 ml with ice-cold lysis buffer.
Following incubation with 30 μl of 50% protein A agarose
beads (Santa Cruz Biotechnology, Inc.) for 1 h at 4°C in a tube
rotator, the beads were removed by centrifugation. Primary antibody
was added to the supernatant and incubated overnight with rotation
at 4°C. The protein complex was pulled down by the addition of 30
μl of 50% protein A agarose beads. Non-specific binding was
removed by washing 3 times with ice-cold lysis buffer. The washed
protein complex was boiled with 30 μl of SDS sample buffer
and separated by SDS-PAGE for western blot analysis. For the
negative controls, irrelevant antibody or rabbit serum was used
instead of specific antibodies.
Statistical analysis
Data are expressed as the means ± SE. Two-sample,
independent-group t-tests were performed to compare physiological
data and the optical densities from the western blot analyses
between the SHHF and WKY rats. A value of P<0.05 was considered
to indicate a statistically significant difference.
Results
Comparison of physiological parameters
between SHHF and WKY rats
The heart weight (HW), the ratio of the heart weight
to the body weight (HW/BW) and the LV weight (LVW) significantly
increased in the 2-, 6-, 12- and 18-month-old SHHF rats as compared
with the age-matched WKY rats (Table
I). These results confirm that heart hyper-trophy, particularly
LV hypertrophy, exists in 2-, 6-, 12- and 18-month-old SHHF
rats.
 | Table I.Comparison of the physiological
data. |
Table I.
Comparison of the physiological
data.
| Group | HW, g | HW/BW, g | LVW, g |
|---|
| 2 M, SHHF | 0.85±0.03a | 0.40±0.01a | 0.51±0.02a |
| 2 M, WKY | 0.62±0.01 | 0.33±0.01 | 0.31±0.01 |
| 6 M, SHHF | 1.18±0.05a | 0.40±0.01a | 0.70±0.03a |
| 6 M, WKY | 0.99±0.02 | 0.30±0.00 | 0.52±0.01 |
| 12 M, SHHF | 1.35±0.02a | 0.43±0.00a | 0.78±0.02a |
| 12 M, WKY | 1.20±0.03 | 0.26±0.00 | 0.64±0.01 |
| 18 M, SHHF | 1.59±0.03a | 0.42±0.01a | 1.01±0.03a |
| 18 M, WKY | 1.40±0.04 | 0.25±0.00 | 0.79±0.02 |
Nuclear expression of c-Src, Src[pY529],
Src[pY418] and Src[pY215] in the LV of SHHF rats
We used rabbit antibodies against the N-terminus of
cellular Src (c-Src) to detect its expression in the NP extracts
from the LV of SHHF and WKY rats. Western blot analysis revealed
the expected band at 60 kDa, which represents c-Src. There were no
significant differences observed in c-Src expression in the NP
between the 2-, 6-, 12- and 18-month-old SHHF rats and their
age-matched WKY controls (104.5±11.1 vs. 104.4±10.7, 105.2±9.5 vs.
104.5±9.2, 113.9±10.9 vs. 104.1±12.7 and 126.9±10.3 vs. 111.7±10.7,
respectively; N=6, P>0.05) (Fig.
1A).
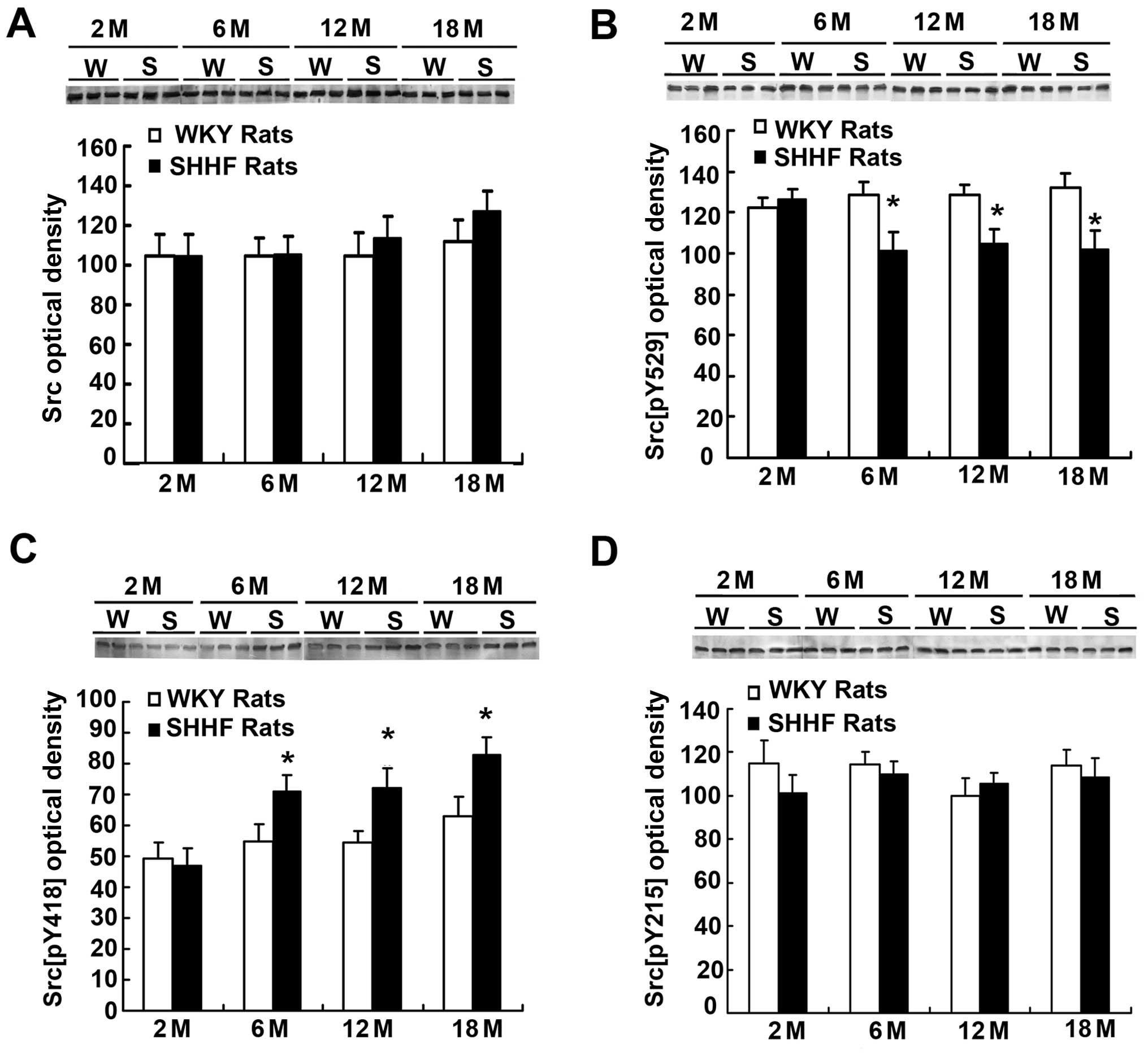 | Figure 1.(A) c-Src, (B) Src[pY529], (C)
Src[pY418] and (D) Src[pY215] expression in the nuclear protein
(NP) fraction of the left ventricle of 2- (2 M), 6- (6 M), 12- (12
M), 18- (18 M) month-old spontaneously hypertensive heart failure
(SHHF) (S) and [Wistar-Kyoto (WKY)] (W) rats (N=6). (A) Western
blot (top panel) showing a similar expression pattern of c-Src
between the SHHF and WKY rats; histogram (bottom panel) also
demonstrates that there is no significant difference in c-Src
content between the SHHF and WKY rats by densitometry analysis
(N=6, P>0.05). (B) Western blot (top panel) and histrogram
(bottom) showing a similar level of Src[pY529] between the
2-month-old SHHF and WKY rats by densitometry analysis; the level
of Src[pY529] in the 6-, 12- and 18-month-old SHHF rats is
significantly lower than that in the age-matched WKY rats (N=6,
P<0.05). (C) Western blot (top panel) and histogram (bottom
panel) also demonstrate a similar level of Src[pY418] between the
2-month-old SHHF and WKY rats by densitometry analysis; the level
of Src[pY418] in the 6-, 12- and 18-month-old SHHF is significantly
higher than that of the age-matched WKY rats (N=6, P<0.05). (D)
Western blot (top panel) and histogram (bottom panel) show a
similar level of Src[pY215] between the 2-, 6-, 12- and
18-month-old SHHF and age-matched WKY rats by densitometry analysis
(N=6, P>0.05). *P<0.05 compared with the
age-matched WKY control rats. N, number of animals. |
The activation of Src kinases is closely associated
with the phosphorylation and dephosphorylation of certain amino
sites. To determine whether Src phosphorylation levels were altered
in the NP extracts from the LV of the SHHF rats, we investigated 3
tyrosine phosphorylation sites: Src[pY529], Src[pY418] and
Src[pY215]. Western blot analysis revealed that the Src[pY529]
levels in the NP extracts from the LV did not significantly differ
between the 2-month-old SHHF rats and the age-matched WKY control
rats (126.5±5.0 vs. 122.2±5.0; N=6, P>0.05) (Fig. 1B). However, Src[pY529] levels
decreased in the NP extracts from the LV tissues from the 6-, 12-
and 18-month-old SHHF rats compared to the age-matched WKY rats
(101.6±8.7 vs. 128.8±6.2, 104.6±7.1 vs. 128.5±5.0 and 102.0±8.9 vs.
132.1±7.1, respectively; N=6, P<0.05) (Fig. 1B). Src phosphorylation levels at
the tyrosine 529 site were downregulated in the nuclei of the
cardiac myocytes of the hypertrophic LV of the 6- to 18-month-old
SHHF rats.
Src[pY418] expression in the NP extracts from the LV
myocytes of the 2-month-old SHHF rats did not differ significantly
from that of the 2-month-old WKY rats (47.0±5.6 vs. 49.3±5.0; N=6,
P>0.05) (Fig. 1C). However,
Src[pY418] expression significantly increased in the NP extracts
from the LV myocytes from the 6-, 12- and 18-month-old SHHF rats
compared to the age-matched WKY rats (71.2±5.1 vs. 54.8±5.6,
72.3±6.3 vs. 54.3±3.9 and 82.9±5.8 vs. 63.1±6.2, respectively; N=6,
P<0.05) (Fig. 1C). Src
phosphorylation levels at the tyrosine 418 site increased in the
nuclei of cardiac myocytes from the hypertrophic LV of the 6- to
18-month-old SHHF rats.
Finally, Src[pY215] levels were not significantly
altered in the NP extracts from the LV tissues from the 2-, 6-,
12-and 18-month-old SHHF rats and the age-matched WKY control rats
(101.6±7.8 vs. 114.9±10.8, 110.3±5.4 vs. 114.4±6.0, 105.6±4.9 vs.
100.1±8.0 and 108.6±8.9 vs. 114.0±7.4, respectively; N=6,
P>0.05) (Fig. 1D).
Subnuclear localization of c-Src in the
nuclei of cardiac myocytes
The subcellular localization of the Src kinase is
important for the regulation of specific cellular processes, such
as mitogenesis, cytoskeletal organization and membrane trafficking
(18). To further investigate the
subnuclear distribution and localization of c-Src in the nuclei of
cardiac myocytes, we labeled the cardiac myocytes from the LV of
the 2-, 6-, 12- and 18-month-old SHHF rats and the age-matched WKY
rats with anti-c-Src antibody, which we detected using
immunofluorescence. The result was that fluorescent brightness was
uniformly distributed in the nuclei of the cardiac myocytes from
all the rats (data not shown). When the slides were scanned using
higher magnification and lower photomultiplier tube voltage and
gain settings on the confocal microscope to decrease fluorescent
background brightness, we clearly observed bright fluorescent dots
of c-Src in the nuclei of the cardiac myocytes. Subsequent double
labeling of the cardiac myocyte sections with anti-c-Src antibody
and anti-Sam68 or anti-fibrillarin antibody revealed that c-Src was
co-localized mainly with Sam68 (Fig.
2A–C). A smaller fraction of c-Src co-localized with
fibrillarin (Fig. 2D–F) in the
nuclei of the cardiac myocytes.
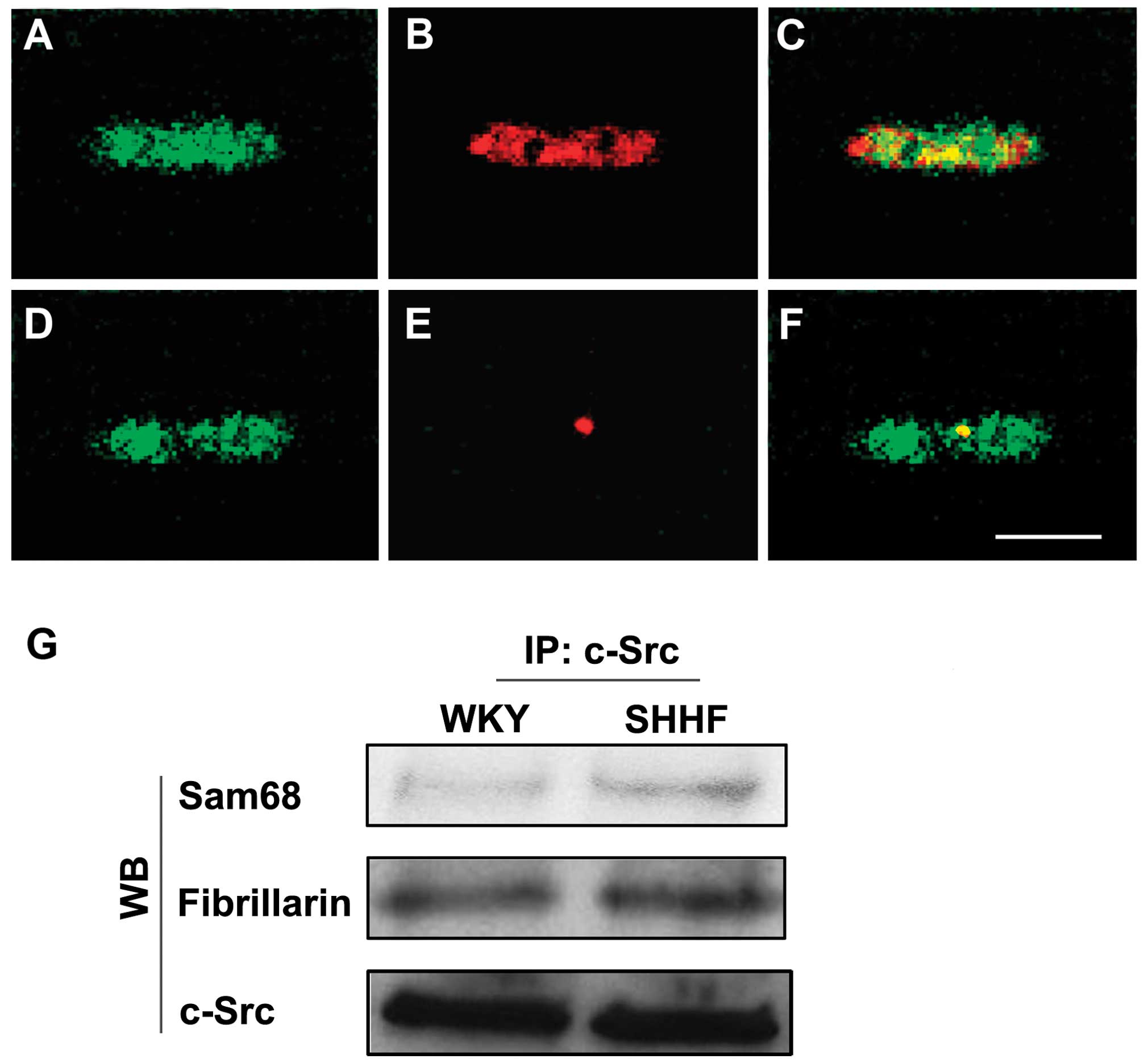
Immunoprecipitation also showed that c-Src
co-localized with Sam68 and fibrillarin in the nuclei of the
cardiac myocytes and that the amount of c-Src associated with Sam68
and fibrillarin increased in the cardiac myocytes of the
hypertrophic LV in the 6-month-old SHHF rats compared to the
age-matched WKY controls (Fig.
2G).
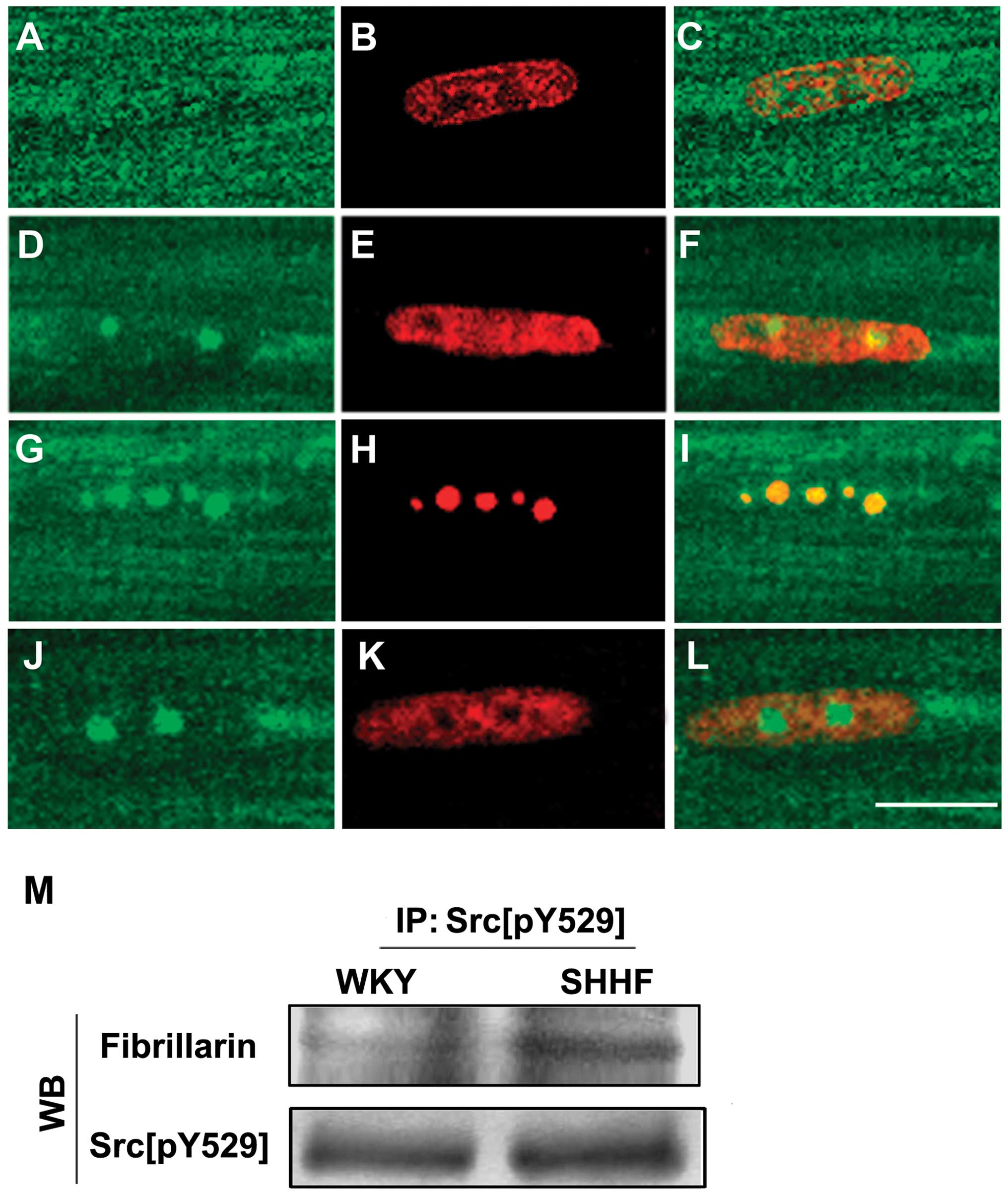
Subnuclear localization of Src[pY529] in
the nuclei of cardiac myocytes
To investigate the distribution and localization of
Src[pY529] in the nuclei of cardiac myocytes of the hypertrophic LV
of the SHHF rats, we performed immunofluorescent labeling of
Src[pY529] on the cardiac myocytes that were isolated from the LV
of the 2-, 6-, 12- and 18-month-old SHHF rats and the age-matched
WKY controls. The results revealed that a similar pattern of
Src[pY529] fluorescence was distributed in the nuclei of the
cardiac myocytes from the 6, 12 and 18-month-old SHHF rats;
Src[pY529] staining revealed 1–4 bright fluorescent dots on a
diffusely weak fluorescent background in the nuclei of the cardiac
myocytes (Fig. 3A–F). It was
further demonstrated, by means of the double staining of Src[pY529]
with antibodies against fibrillarin and Sam68, that the bright
green dot-like fluorescence of Src[pY529] overlaid with the red
dot-like fluorescence of fibrillarin; thus, Src[pY529] co-localized
with fibrillarin in the nucleoli, but not with Sam68 (Fig. 3G–L). In addition, it was also
shown, in the immunoprecipitation with anti-Src[pY529] antibody
followed by western blot analysis with anti-fibrillarin antibody,
that Src[pY529] co-localized with fibrillarin; this was more
evident in the cardiac myocytes of the hypertrophic LV from the
6-month-old SHHF rats compared to the age-matched WKY controls
(Fig. 3M).
Subnuclear localization of Src[pY418] in
the nuclei of cardiac myocytes
To investigate the distribution and localization of
Src[pY418] in the nuclei of the cardiac myocytes of the
hypertrophic LV from the SHHF rats, we labeled intact frozen
sections of LV tissues from the 2-, 6-, 12- and 18-month-old SHHF
rats and the age-matched WKY controls with anti-Src[pY418]
antibody. Confocal microscopy revealed a similar pattern of
macular-like fluorescent distribution of Src[pY418] in the nuclei
of the cardiac myocytes from the 6- to 18-month-old SHHF rats
(Fig. 4A–F). Double labeling
demonstrated that Src[pY418] fluorescence was co-localized mainly
with Sam68 but not with fibrillarin in the nuclei of the cardiac
myocytes (Fig. 4G–L).
Immunoprecipitation with anti-Sam68 antibody followed by western
blot analysis with anti-Src[pY418] antibody confirmed that
Src[pY418] co-localized with Sam68. The same technique also
revealed that the expression of Sam68-bound Src[pY418] increased in
the cardiac myocytes of the hypertrophic LV from the 6-month-old
SHHF rats compared to the age-matched WKY controls (Fig. 4M).
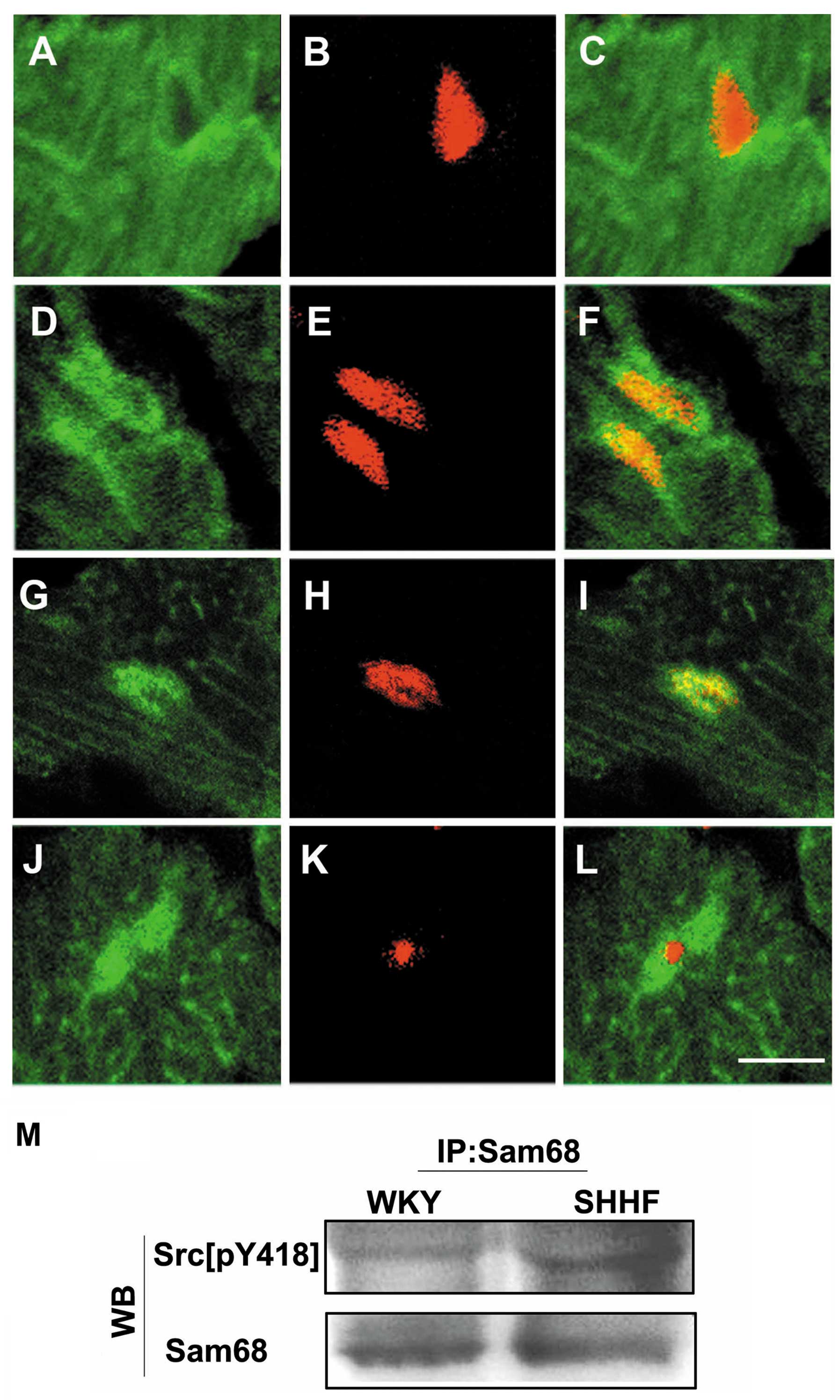 | Figure 4.(A–F) Immunofluorescent labeling of
Src[pY418] (A and D, green) and the myocardial nuclei [(B and E)
red, counterstained with propidium iodide] in frozen sections of
the LV tissues from (A–C) 6-month-old Wistar-Kyoto (WKY) rats and
(D–F) spontaneously hypertensive heart failure (SHHF) rats. (A and
C) Only a weak green Src[pY418] fluorescence is present in the
myocardial nuclei of WKY rats, (D and F) but stronger green
Src[pY418] fluorescence accumulated in the myocardial nuclei of the
SHHF rats. (G–L) Double labeling of Src[pY418] (G and J, green)
with Sam68 (H, red) or fibrillarin (K, red) shows that green
fluorescent Src[pY418] co-localized with the red fluorescence of
Sam68, which produced bright yellow fluorescence in the merged
image (I). There was no overlap with the red fluorescent
fibrillarin in the merged image (L) of the nuclei of the cardiac
myocytes in frozen sections of the LV tissues from the 6-month-old
SHHF rats. Scale bar, 10 μm. (M) Immunoprecipitation (IP)
with anti-Sam68 antibody followed by western blot analysis (WB)
with anti-Src[pY418] antibody. Levels of Src[pY418] bound to Sam68
increased in the cardiac myocytes of the left ventricle from the
6-month-old SHHF rats compared to the age-matched WKY controls,
whereas a similar levels of Sam68 was observed down, as shown by WB
on the same membrane after stripping (N=6, P<0.05). |
We also immunolabeled Src[pY215] on frozen sections
of the LV from the 2-, 6-, 12- and 18-month-old SHHF rats and the
age-matched WKY controls. No difference was observed between the
Src[pY215] signals in the myocardial nuclei of the LV of the WKY
and SHHF rats (data not shown).
Discussion
Hypertension-induced increases in sustained pressure
load cause increased left ventricular wall stress and thereby
stimulate myocardial hypertrophy (3). Wall stress stimulates sarcomeres to
grow in a parallel pattern by increasing protein synthesis, which
increases myocyte width in response to increased afterload
(19). Cardiac hypertrophy,
therefore, is a compensatory response to increased wall stress.
However, with the progression of cardiac hypertrophy, the LV can no
longer compensate for the sustained increased afterload and
eccentric hypertrophy of the LV occurs, which eventually results in
CHF (2,20).
In the SHHF rats, myocardial sarcomeres begin
increasing in width at as early as 2 months and reach a maximum
cross-sectional area at approximately 4–6 months (21). The pathological changes during
this period include concentric left ventricular hypertrophy and the
relative thickening of the LV wall. At 6 months, sarcomeres begin
to increase in length, which results in progressive lengthening and
maladaptive remodeling, leading to CHF at 18–24 months (21,22). The molecular mechanisms
controlling ‘a change-over switch’ in the hypertrophic pattern from
cross-sectional growth to myocyte lengthening are not entirely
clear. In the present study, we found that the late decompensatory
eccentric hypertrophy of the LV occurred at 6–18 months. At the
same time, the expression of Src[pY529] decreased and that of
Src[pY418] increased in the nuclei of the myocytes in the
hypertrophic LV from the SHHF rats. These results suggest that the
dephosphorylation of Src tyrosine 529 and the phosphorylation of
tyrosine 418 are involved in the regulation of cardiac remodeling
in the development and progression of the decompensated
hypertensive ventricular hypertrophy by the intranuclear signaling
transduction pathway in cardiac myocytes.
From the N- to C-terminus, c-Src contains an SH4
domain, a unique domain, an SH3 domain that directs specific
association with proline-rich motifs, an SH2 domain that provides
interaction with phosphotyrosine motifs, an SH2-kinase linker, an
SH1 domain that is responsible for the enzymatic activity and
contains tyrosine 418 and a C-terminal regulatory segment that
contains tyrosine 529 (23).
Under basal conditions in vivo, the majority of Src is
phosphorylated at tyrosine 529; phosphorylated tyrosine 529 binds
intra-molecularly with the c-Src SH2 domain while the SH3 domain
binds to proline-rich sequences located within the SH2-kinase
linker. These 2 intramolecular associations stabilize a restrained
form of the enzyme (23).
Dephosphorylation of the negative regulatory site, tyrosine 529, is
a mechanism by which Src may be activated in response to
extracellular stimuli. Candidate phosphotyrosine 529 phosphatases
include cytoplasmic protein-tyrosine phosphatase 1B (PTP1B) and
transmembrane enzymes, such as CD45, PTPα, PTPɛ and PTPλ (12). An alternative mechanism of
activating Src is the displacement of the negative regulatory
element from the SH2 domain and/or disruption of interaction by
presenting higher-affinity SH2 or SH3 domain-binding ligands
(24). In addition, Src with
phosphorylated tyrosine 529 cannot undergo autophosphorylation at
other sites, such as tyrosine 418; thus, the tyrosine 529 residue
must first be dephosphorylated to allow Src autophosphorylation at
other sites and activation (11).
In the present study, we found that the expression of Src
phosphorylated at tyrosine 529 decreased and that of Src
phosphorylated at tyrosine 418 increased; Src[pY529] aggregated
inhe nucleoli where it co-localized with a nucleolar protein,
fibrillarin, in the nuclei of the cardiac myocytes of the
hypertrophic LV from the SHHF rats. Src[pY529] aggregating in the
nucleoli and binding fibrillarin may lead to the dissociation of
pY529 from SH2, thereby disrupting the pY529-mediated
auto-inhibition, as fibrillarin competitively occupies the SH2
domain. Enzymes such as PTPα may be involved in dephosphorylating
pY529 from SH2. When pY529 dissociates from SH2, tyro-sine 418,
which is located in the SH1 domain, will be exposed, allowing it to
undergo autophosphorylation, resulting in the increased expression
of Src[pY418] and the activation of the tyrosine 418-phosphorylated
Src kinase.
The nucleolus is the most active and dynamic nuclear
domain that plays a prominent role in the organization of various
components of the nucleus; it is not only essential for ribosomal
production, but also for the control of cell survival and
proliferation (25,26). Fibrillarin is an evolutionarily
conserved, obligatory protein component of eukaryotic cell nucleoli
involved in the early processing and modification of pre-rRNA and
ribosomal and nucleolar assembly; it is also essential for early
embryonic development (25,27). Moreover, fibrillarin depletion
results in the reduction of cellular growth and abnormal nuclear
morphology, suggesting that fibrillarin plays a critical role in
cellular growth and the maintenance of nuclear shape (25). It is well known that cardiac
myocytes are terminally differentiated cells with limited or no
potential to re-enter the cell cycle (no-dividing cells), and
therefore, the post-natal growth of cardiac myocytes occurs through
hypertrophy of existing cells. The aggregation of Src[pY529] to the
nucleoli and its co-localization with fibrillarin during
hypertensive cardiac hypertrophy suggests that the Src kinase is
involved in the hypertrophic growth of myocardial cells and plays a
role in the endonucleolar signal transduction in myocardial
hypertrophy that is induced by hypertension.
Sam68 is the prototypical member of the signal
transducer and activator of RNA (STAR) family of RNA-binding
proteins that link signaling pathways to RNA processing (28). Sam68 was first identified as the
major phosphorylation substrate of the Src kinase during mitosis.
It can not only interact with the SH2/SH3 domain-containing adaptor
proteins and signaling enzymes, such as the Src family kinases,
phosphatidylinositol 3-kinase (PI3K) and growth factor
receptor-bound protein 2 (Grb2), but is also involved in several
steps of mRNA processing, including transcription, alternative
splicing and nuclear export (29,30). Therefore, Sam68 functions as a
multifunctional adaptor by linking signaling pathways to the
transcriptional and post-transcriptional regulation of gene
expression (28,31). The co-localization of Src and
Src[pY418] with Sam68, as observed in this study, suggests that the
Src kinase regulates signal transduction in myocardial hyper-trophy
by directly controlling hypertrophic gene transcription in the
nucleus. An earlier study demonstrated that SrcF527, a
constitutively active oncogenic mutant of c-Src, stimulated
hypertrophic expression in genes, such as atrial natriuretic factor
(ANF), skeletal muscle (SkM)-α-actin and β-myosin heavy chain
(β-MHC) (32). Thus, the Src
kinase is a potential candidate for hypertrophic gene transcription
and RNA processing in the nucleus during cardiac hypertrophy in
chronic hypertension.
In conclusion, the data from the present study
suggest that the Src kinase is involved in regulating cardiac
myocyte remodeling in eccentric hypertrophy of the LV by
participating in an intranuclear signaling transduction pathway,
constituted by the dephosphorylation of Src tyrosine kinase 529,
the phosphorylation of tyrosine 418, and intranuclear
redistribution in the nuclei of myocardial cells undergoing
hypertensive cardiac hypertrophy.
Acknowledgements
This study was supported by grants
from the National Nature Science Foundation of China (no. 30871046)
and the Nature Science Foundation of Guangdong Province, China (no.
8151008901000162).
References
|
1.
|
Drazner MH: The progression of
hypertensive heart disease. Circulation. 123:327–334. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Diamond JA and Phillips RA: Hypertensive
heart disease. Hypertens Res. 28:191–202. 2005. View Article : Google Scholar
|
|
3.
|
Krauser DG and Devereux RB: Ventricular
hypertrophy and hypertension: prognostic elements and implications
for management. Herz. 31:305–316. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Marin TM, Clemente CF, Santos AM, et al:
Shp2 negatively regulates growth in cardiomyocytes by controlling
focal adhesion kinase/Src and mTOR pathways. Circ Res. 103:813–824.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Torsoni AS, Constancio SS, Nadruz W Jr,
Hanks SK and Franchini KG: Focal adhesion kinase is activated and
mediates the early hypertrophic response to stretch in cardiac
myocytes. Circ Res. 93:140–147. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Kuppuswamy D, Kerr C, Narishige T, Kasi
VS, Menick DR and Cooper G IV: Association of
tyrosine-phosphorylated c-Src with the cytoskeleton of
hypertrophying myocardium. J Biol Chem. 272:4500–4508. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Kovacic B, Ilic D, Damsky CH and Gardner
DG: c-Src activation plays a role in endothelin-dependent
hypertrophy of the cardiac myocyte. J Biol Chem. 273:35185–35193.
1998.PubMed/NCBI
|
|
8.
|
Zou Y, Komuro I, Yamazaki T, et al: Both
Gs and Gi proteins are critically involved in isoproterenol-induced
cardiomyocyte hypertrophy. J Biol Chem. 274:9760–9770. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Heidkamp MC, Bayer AL, Scully BT, Eble DM
and Samarel AM: Activation of focal adhesion kinase by protein
kinase C epsilon in neonatal rat ventricular myocytes. Am J Physiol
Heart Circ Physiol. 285:H1684–H1696. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Aikawa R, Nagai T, Kudoh S, et al:
Integrins play a critical role in mechanical stress-induced p38
MAPK activation. Hypertension. 39:233–238. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Roskoski R Jr: Src protein-tyrosine kinase
structure and regulation. Biochem Biophys Res Commun.
324:1155–1164. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Roskoski R Jr: Src kinase regulation by
phosphorylation and dephosphorylation. Biochem Biophys Res Commun.
331:1–14. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Li LH, Wang XH, Xie YY, et al: Membrane
translocation of Src kinase in cardiac myocytes of hypertrophic
left ventricle of hypertensive rats. Chinese Heart Journal.
23:295–299. 2011.(In Chinese).
|
|
14.
|
Li ZY, Yi XP, Zhong L, et al: Expression
of focal adhesion kinase in cardiac myocytes of hypertrophic
ventricle. Zhonghua Bing Li Xue Za Zhi. 36:677–680. 2007.(In
Chinese).
|
|
15.
|
Zhong L, Yi XP, Li ZY and Faqian L:
Phosphorylation and nuclear translocation of serine 722 and serine
910 of focal adhesion kinase in hypertrophic cardiac myocytes of
left ventricle of spontaneously hypertensive rats. Zhonghua Bing Li
Xue Za Zhi. 37:328–332. 2008.(In Chinese).
|
|
16.
|
Yi XP, Gerdes AM and Li F: Myocyte
redistribution of GRK2 and GRK5 in hypertensive,
heart-failure-prone rats. Hypertension. 39:1058–1063. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Blough E, Dineen B and Esser K: Extraction
of nuclear proteins from striated muscle tissue. Biotechniques.
26:202–204. 206:1999
|
|
18.
|
Vielreicher M, Harms G, Butt E, Walter U
and Obergfell A: Dynamic interaction between Src and C-terminal Src
kinase in integrin alphaIIbbeta3-mediated signaling to the
cytoskeleton. J Biol Chem. 282:33623–33631. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Papadopoulos DP and Papademetriou V:
Hypertrophic and hypertensive hypertrophic cardiomyopathy - a true
association? Angiology. 61:92–99. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Li F, Wang X, Yi XP and Gerdes AM:
Structural basis of ventricular remodeling: role of the myocyte.
Curr Heart Fail Rep. 1:5–8. 2004. View Article : Google Scholar
|
|
21.
|
Heyen JR, Blasi ER, Nikula K, et al:
Structural, functional, and molecular characterization of the SHHF
model of heart failure. Am J Physiol Heart Circ Physiol.
283:H1775–H1784. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Johnsen DD, Kacimi R, Anderson BE, Thomas
TA, Said S and Gerdes AM: Protein kinase C isozymes in hypertension
and hypertrophy: insight from SHHF rat hearts. Mol Cell Biochem.
270:63–69. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Ingley E: Src family kinases: regulation
of their activities, levels and identification of new pathways.
Biochim Biophys Acta. 1784:56–65. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Schaller MD, Hildebrand JD and Parsons JT:
Complex formation with focal adhesion kinase: A mechanism to
regulate activity and subcellular localization of Src kinases. Mol
Biol Cell. 10:3489–3505. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Amin MA, Matsunaga S, Ma N, et al:
Fibrillarin, a nucleolar protein, is required for normal nuclear
morphology and cellular growth in HeLa cells. Biochem Biophys Res
Commun. 360:320–326. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Yanagida M, Hayano T, Yamauchi Y, et al:
Human fibrillarin forms a sub-complex with splicing factor
2-associated p32, protein arginine methyltransferases, and tubulins
alpha 3 and beta 1 that is independent of its association with
preribosomal ribonucleoprotein complexes. J Biol Chem.
279:1607–1614. 2004. View Article : Google Scholar
|
|
27.
|
Barygina VV, Veiko VP and Zatsepina OV:
Analysis of nucleolar protein fibrillarin mobility and functional
state in living HeLa cells. Biochemistry (Mosc). 75:979–988. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Rajan P, Gaughan L, Dalgliesh C, et al:
Regulation of gene expression by the RNA-binding protein Sam68 in
cancer. Biochem Soc Trans. 36:505–507. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Bielli P, Busa R, Paronetto MP and Sette
C: The RNA-binding protein Sam68 is a multifunctional player in
human cancer. Endocr Relat Cancer. 18:R91–R102. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Najib S, Martin-Romero C, Gonzalez-Yanes C
and Sanchez-Margalet V: Role of Sam68 as an adaptor protein in
signal transduction. Cell Mol Life Sci. 62:36–43. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Sette C: Post-translational regulation of
star proteins and effects on their biological functions. Adv Exp
Med Biol. 693:54–66. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Fuller SJ, Gillespie-Brown J and Sugden
PH: Oncogenic src, raf, and ras stimulate a hypertrophic pattern of
gene expression and increase cell size in neonatal rat ventricular
myocytes. J Biol Chem. 273:18146–18152. 1998. View Article : Google Scholar : PubMed/NCBI
|