Introduction
Attractin (Atrn), a dipeptidyl peptidase
IV/CD26-like enzyme rapidly expressed and released on activated T
cells, has attracted much attention in recent years (1). Scientists used positional cloning to
identify the candidate gene, mahogany (Mgca). The predicted protein
encoded by Mgca is a 1,428-amino acid, single-transmembrane-domain
protein expressed in many tissues, including pigment cells and the
hypothalamus (2–4). The extracellular domain of the Mgca
protein is the orthologue of human attractin, a circulating
molecule produced by activated T cells that has been implicated in
immune-cell interactions. Atrn mRNA was found to be widely
distributed throughout the central nervous system by in situ
hybridization (ISH). In the hypothalamus, Atrn mRNA is observed in
paraventricular and supraoptic nuclei, suggesting a potential role
in the regulation of posterior pituitary gland function (5). Investigations have focused on the
functional activity of attractin in every allelic line, in addition
to its physiological properties, subcellular location, unifying
mechanism and possible therapeutic interventions. Research has
shown that attractin is widely distributed in most organisms and is
involved in a number of physiological and pathological functions,
including immune system regulation, body weight control,
myelinization and tumor susceptibility. Particularly, attractin
plays a role in the switch of pigment synthesis of hair color and
in degenerative diseases of the central nervous system. Regarding
pigmentation, it has been reported that mice with a mutation at the
Atrn locus are darkly pigmented as the agouti-induced yellow band
on the hair is suppressed and the mutation also suppresses the
yellow fur caused by the overexpression of agouti in mutant animals
(6).
Paz et al (7) found that the age-dependent
progressive neurodegeneration, such as neuronal cell death,
hypomyelination and vacuolation, is closely correlated with
loss-of-function of attractin. This indicates that Atrn may have a
potential therapeutic effect on neurodegenerative diseases. It is
known that the function of the male reproductive system is brought
into play by the hypothalamus-pituitary-gonadal axis. However,
there are few reports of attractin expression in the male
reproductive system. To determine whether Atrn is expressed in the
reproductive system of the male rat and to determine its
localization, immunohistochemistry (IHC), indirect
immunofluorescence (IIF), ISH and western blotting were conducted
on testicular and epididymal tissue from male Sprague Dawley (SD)
rats of different ages.
Materials and methods
Tissue collection
SD male rats used in all of the experiments were
purchased from the animal center of Huazhong University of Science
and Technology, so as to exclude any effects of immunologic
interference. The animals were maintained at the animal facilities
of Tongji Medical College, Huazhong University of Science and
Technology. The experiments were conducted in accordance with the
guidelines approved by the China Association of Laboratory Animal
Care.
Testes and epididymides were obtained from 5 groups
of SD rats: newborn (8 h after birth), prepubertal (5 days),
pubertal (20 days), postpubertal (50 days) and mature (70 days).
Rats of the same age were born on the same day, and each group
included 8 animals.
Tissue preparation and staining
Preparation of paraffin tissue
sections
Following castration, testes and epididymides from
all animals of each age group (n=8) were transported in cold Hanks’
Balanced Salt Solution to the laboratory. Sections (~1
cm3) of the testicular parenchyma were removed and
placed in 4% paraformaldehyde at 4°C for 24 h. Tissues were then
transferred to 0.1 M phosphate-buffered saline (PBS) solution at
4°C for 24 h, dehydrated with 70, 80, 95 and 95% of ethanol
successively at room temperature for 15 min for each treatment and
then treated with 100% ethanol at room temperature for 10 min. The
sections were cleaned twice with xylene at room temperature for 20
min and were embedded in paraffin. Tissue sections (4 μm) of each
testis and epididymis were cut, mounted on poly-L-lysine-coated
slides (Sigma Chemical Co., St. Louis, MO, USA), dried and stored
at 4°C.
Preparation of frozen tissue
sections
Rats were deeply anesthetized with sodium
pentobarbital (100 mg/kg i.p.). The chest of the rat was opened by
midline incision, and then perfused transcardially with 300 ml of
0.9% NaCl containing 2% sodium nitrite and 600 ml of 4%
paraformaldehyde successively at 4°C. The testes and epididymides
were removed and immersed in a post-perfusion fixative of 4% fresh
paraformaldehyde solution for 4 h at 4°C, and then in 25% sucrose
distilled water solution at 4°C for 4–6 days until they sank to the
bottom of the sucrose solution. Fresh sucrose solution was replaced
daily. Testes and epididymides were carefully embedded at optimal
cutting temperature (OCT) and cut into 20-μm sections and stored in
cryoprotectant.
Direct immunofluorescence
Frozen tissue sections were washed 3 times with PBS
for 5 min each time, and then incubated in normal rabbit serum (New
Step Reagent Co., Fujian, China) for 10 min at 37°C and washed 3
times with PBS for 5 min each time. Afterwards, tissues were
incubated in the primary antibody (rabbit anti-mouse attractin
antibody) (provided by Dr Shiliang Shen, Department of Genetics and
HHMI Stanford University School of Medicine) diluted (1:400) in
Tris-buffered saline (TBS) containing 10% rabbit serum for 2 h at
37°C and then in goat anti-rabbit IgG labeled with fluorescein
isothiocyanate (FITC) for 10 min at 37°C in a dark humidified
chamber. Slides were rinsed 3 times in PBS and then 3 times in
distilled water. Each rinse lasted for 5 min. The tissue sections
were mounted in glycerin buffer onto slides and covered by
coverslips. Images of the tissues were captured immediately under
fluorescence microscopy. Controls for the specificity of the
antisera consisted of incubating tissue in antisera that had been
pre-absorbed with an antigen which blocked all staining.
Immunohistochemistry
Paraffin tissue sections were dewaxed in xylene,
rehydrated and washed in distilled water, and then immersed in 0.01
M citrate buffer (pH 6.0), and finally heated at 98°C for 15 min in
a microwave oven. After the tissue sections were cooled down to
room temperature, the slides were washed 3 times with distilled
water and 3 times with PBS successively. The slides were blocked
for endogenous peroxidase by incubation with 0.3%
H2O2 in PBS for 10 min at 37°C, and then
blocked with 10% goat serum (New Step Reagent Co., Fujian, China)
for 20 min at 37°C to prevent nonspecific binding of the
antibodies. Subsequently, the slides were incubated at 4°C
overnight with the primary antibody diluted (1:200) in TBS
containing 10% goat serum. After washing in PBS, the sections were
incubated for 20 min at 37°C with biotin-conjugated goat
anti-rabbit IgG (New Step Reagent Co., Fujian, China). The sections
were then rinsed in PBS and incubated for 20 min with
enyzme-conjugated horseradish peroxidase (HRP)-streptavidin (New
Step Reagent Co., Fujian, China), then rinsed in PBS. The attractin
antibody-peroxidase complex was stained with a solution containing
nickel sulfate (0.250 g), 3,3′-diaminobenzidine (0.002 g), and
H2O2 (8.3 ml of 3%) in 10 ml of 0.175 M
sodium acetate buffer for 2–3 min. Some sections were
counterstained with Mayer’s hematoxylin (New Step Reagent Co.,
Fujian, China), dehydrated step-wise with an ethanol series,
cleaned and coverslipped. Controls for the specificity of the
antisera consisted of incubating tissue in antisera that had been
preabsorbed with a stain-blocking antigen.
Confocal laser scanning
microscopy
Paraffin tissue sections were dewaxed in xylene,
rehydrated and washed in PBS. Tissues were then incubated in normal
rabbit serum for 10 min at 37°C, washed, and incubated again in the
primary antibody which was diluted with TBS containing 10% rabbit
serum at 1:400 for 2 h at 37°C. Goat anti-rabbit IgG was labeled
with FITC for 30 min at 37°C in a darkened humidified chamber.
Slides were rinsed 3 times with PBS buffer, and then three times
with distilled water for 5 min each rinsing. Sections were
coverslipped in 1,4-diasabicyclo [2.2.2]octane (Dabco). The tissue
sections were examined and photographed under confocal laser
scanning microscopy immediately. Controls for the specificity of
the antisera consisted of incubating tissue in antisera that had
been preabsorbed with stain-blocking antigen.
In situ hybridization
Paraffin tissue sections were dewaxed in xylene, and
rehydrated and washed in PBS. Endogenous peroxidase was blocked by
incubating the sections in 0.3% H2O2 in PBS
for 5 min at 37°C to reduce the non-specific background staining.
The sections were subsequently rinsed in distilled water, treated
with pepsin diluted in 3% fresh citric acid, and fixed in 4%
paraformaldehyde for 10 min at room temperature. Prehybridization
was performed for ~2 h at 40°C in prehybridization buffer [50%
deionized formamide, 0.3 M NaCl, 20 mM Tris-HCl (pH 8.0), 5 mM
ethylenediaminetetraacetic acid (EDTA), 10% dextran sulfate, and 1X
Denhardt’s solution, and 10 mM NaH2PO4].
Hybridization was performed for ~18 h at 40°C in hybridization
buffer containind digoxigenin (DIG)-labeled oligonucleotide
riboprobes. The in situ hybridization kit was synthesized by
Wuhan Boshide Biological Co. Ltd. The 5′-labeled attractin
digoxigenin oligonucleotide probe was synthesized using multi-phase
oligonucleotide probes and a highly sensitive labeling technique.
The mRNA sequences of attractin target genes for the rat were,
5′-CCGCTTCAGACTAACTGGAT CTTCTGGATTTGTAA-3′, 5′-ATATGTCTCCATTCACA
AATAGTTTGCTGCAGTGG-3′ and 5′-TATAAAGACTGT
TCCTAAGCCCATTGCCCTGGAGC-3′.
After hybridization, the coverslips were removed in
5X SSC (standard saline citrate). Sections were washed in 2X SSC at
37°C for 10 min, in 0.5X SSC at 37°C for 15 min, and then in 0.2X
SSC for 15 min successively. The hybridized DIG-labeled probes were
detected with anti-DIG monoclonal antibodies. Sections were
incubated with biotin-conjugated mouse anti-DIG (Boehringer,
Mannheim) for 1 h at 37°C, and then with 10% horse serum in TBS
[0.1 M Tris (pH 7.6) and 0.15 M NaCl] for 30 min at 37°C. After
washing, the sections were incubated in the
streptavidin-biotin-peroxidase complex (SABC) (Biological Co.,
Ltd., Wuhan Boshide, China) and then in enyzme-conjugated
HRP-streptavidin at 37°C for 20 min for each incubation. To
visualize the complex, sections were covered with 0.5 mg/ml of DAB
(Dako Corp., Carpintera, CA, USA) in 0.05 M Tris-HCl (pH 7.6)
containing 0.01% H2O2. Counterstaining and
other steps were performed as described above. Controls for the
specificity of the oligonucleotide riboprobe consisted of
incubating the tissue in oligonucleotide riboprobe hybridization
buffer that had been preabsorbed with prehybridization buffer which
blocked all staining.
Western blotting
Fresh testis tissues were isolated and put in 3
volumes of extract buffer. The sample was centrifuged at 10,000 rpm
for 10 min at 4°C and the supernatant was maintained at −70°C.
Then, 50 μl of the sample was added to an equal volume of 2X SDS
gel-loading buffer. The sample in the loading buffer was heated at
100°C for 3–5 min and then loaded for electrophoresis. The applied
voltage was 8 V/cm (6 × 8 = 48 V). When the dye (bromophenol blue)
front entered the separating gel, the voltage was increased to 15
V/cm (6 × 15 = 90 V). The power was turned off when the bromophenol
blue reached the bottom of the separation gel. The gel was prepared
for transfer in transfer buffer: 0.65 mA/cm2 (~100 V)
for 1.5–2 h, or 30 V overnight, on ice, and the filter was blocked
with blocking buffer for 1–2 h at room temperature (0. ml blocking
solution/cm2 filter), with gentle agitation on a
platform shaker. After blocking solution was discarded, the filter
was immediately incubated with the attractin antibody. The attactin
antibody (0.005 ml) (1:2,000) was added to the blocking solution
and incubated at 4°C for 2 h or overnight with gentle agitation on
a platform shaker. The blocking solution was discarded, and the
filter was washed 3 times (10 min each time) with 250 ml of PBS.
The filter was incubated in 150 mM NaCl, 50 mM Tris-HCl (pH 7.5)
(phosphate-free, azide-free blocking solution) 3 times for 10 min
each time. The filter was then immediately incubated with the
secondary antibody. Ten milliliters of phosphate-free, azide-free
solution [150 mM NaCl, 50 mM Tris-HCl, 5% nonfat dry milk (pH 7.5)]
was added. Secondary antibody solution (0.005 ml) (1:2,000) was
added, and incubated for 1–2 h at room temperature with gentle
agitation. The secondary antibody solution was discarded, and the
filter was then washed with 150 mM NaCl, 50 mM Tris-HCl (pH 7.5)
(phosphate-free, azide-free solution) 3 times for 10 min each time.
Five milliliters of the substrate 5-brono-4-chloro-3-indolyl
phosphate/nitro blue tetrazolium (BCIP/NBT) solution (Sigma) was
added. Blue color on the filter (~20 min) was observed. BCIP/NBT
solution was discarded when the bands were clear (~20 min). The
enzymatic reaction was halted immediately by adding water. The
filter was finally covered with a plastic membrane and the filter
was stored.
Results
In the rat, maturation of the testis starts early
after birth. Before puberty, the lumen of the seminiferous tubule
has not yet formed. The diameter of this tubule is small and has no
basement membrane. It contains gonocytes and undifferentiated cells
that will later be transformed into Sertoli cells. Comparatively,
in the interstitial tissue surrounding the seminiferous tubules,
there are only undifferentiated interstitial cells which later give
rise to Leydig cells.
Immunofluorescence and confocal laser
scanning microscopy
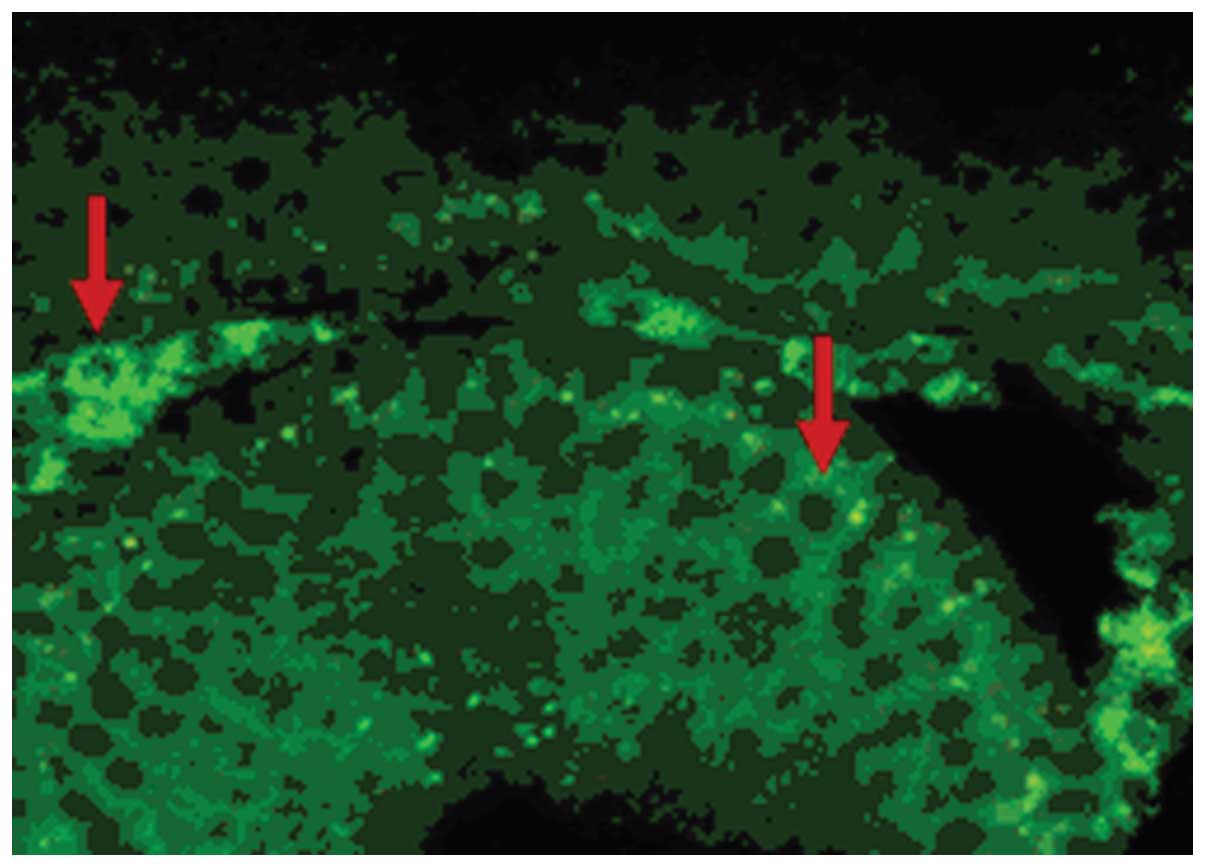
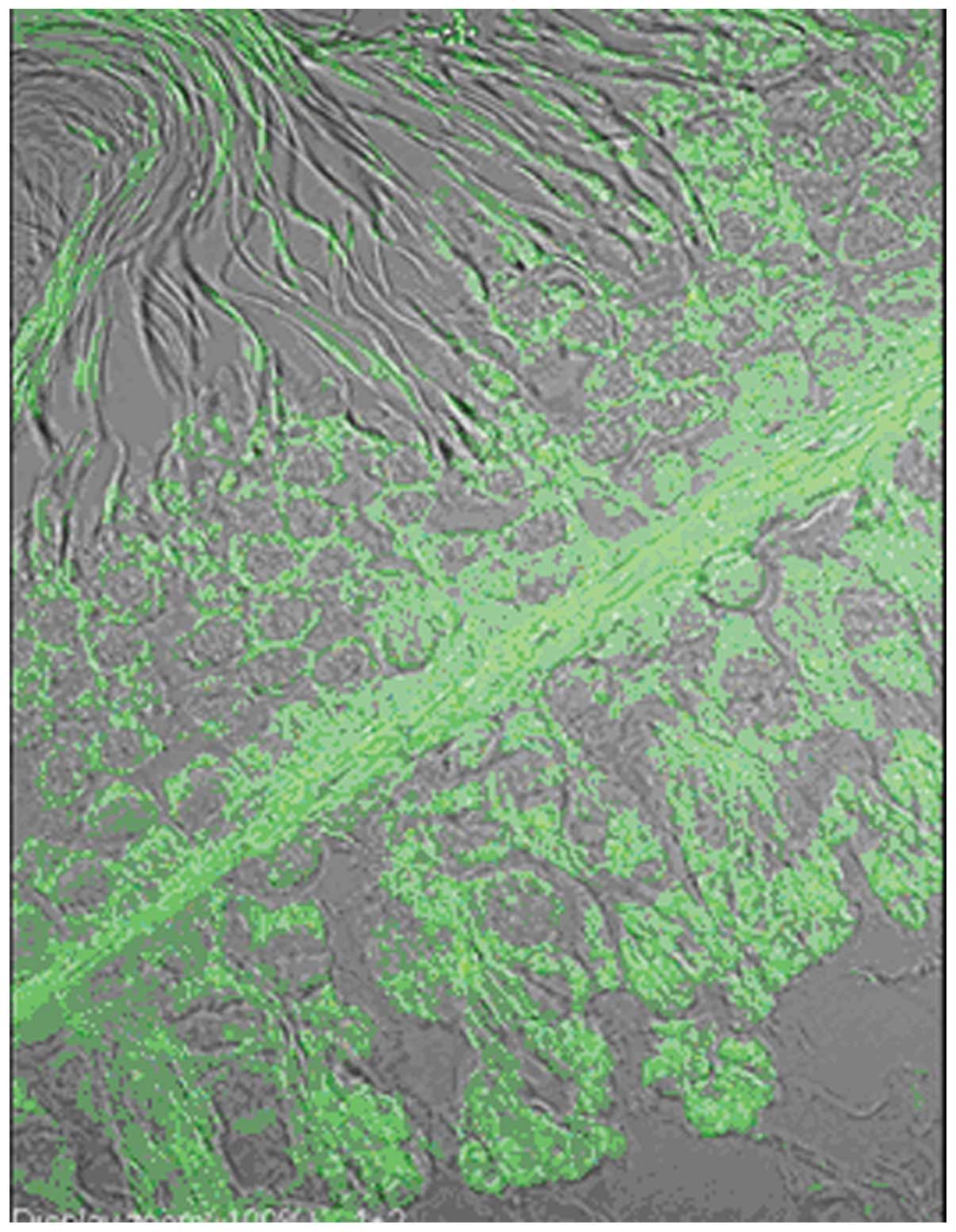
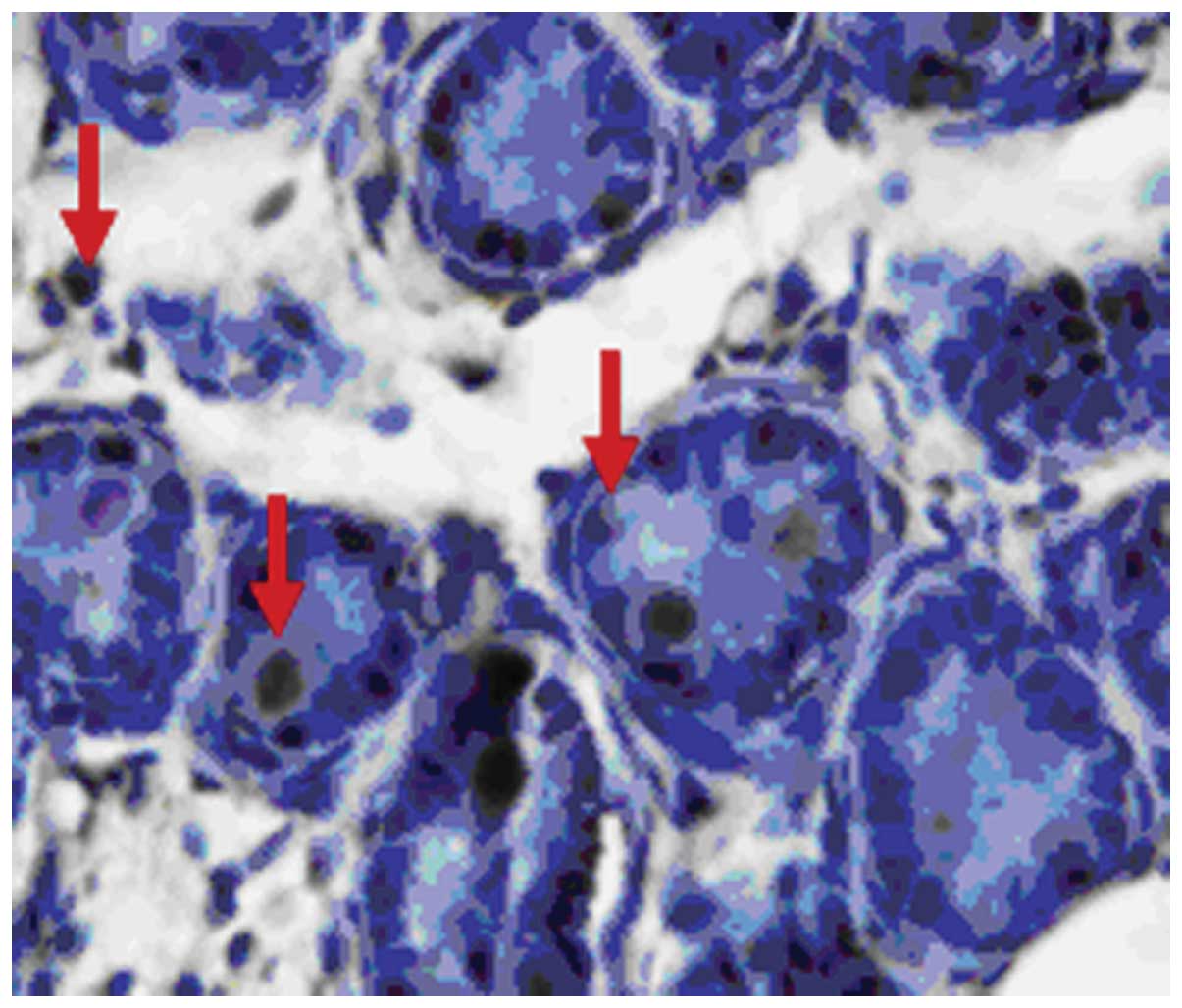
In the testis of the male mature rat, there was
distinct immunopositive staining of attractin on the cell membrane
and in the cytoplasm of Leydig cells, primitive spermatogonia,
primary spermatocytes, spermatids, Sertoli cells, and peritubular
myoid cells. In the epididymis, there was no definitive
immunopositive staining within the efferent ductules or the
epididymal ducts (Figs.
1–4).
Immunohistochemistry
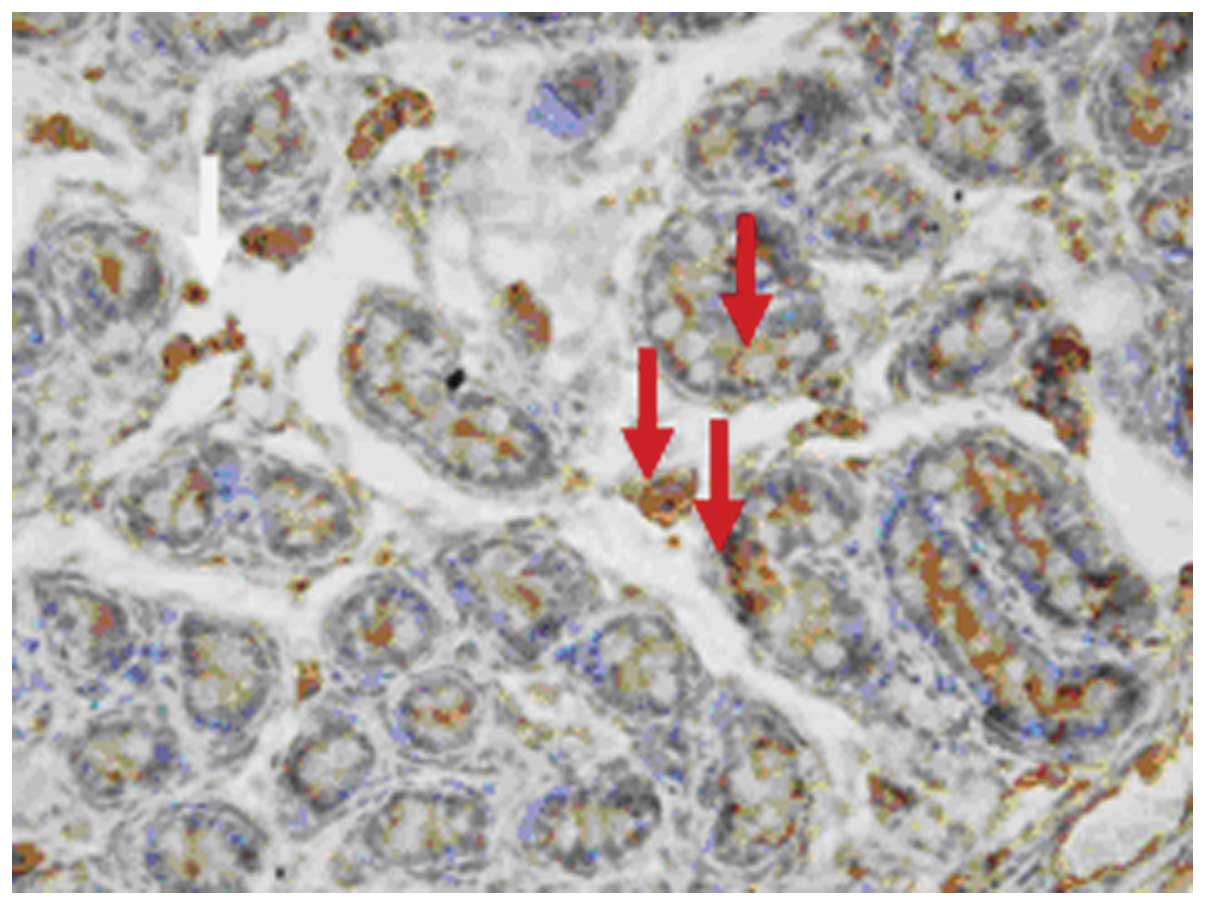
The attractin protein was positively stained with
DAB in undifferentiated interstitial cells, in the newborn (1 day)
gonocytes, in prepubertal (5 days) spermatogonia, in pubertal (20
days) primary spermatocytes, in postpubertal (50 days) spermatids
as well as in Sertoli cells, peritubular constrictive cells and
Leydig cells. The expression of Atrn in interstitial tissue was
stronger than that in the seminiferous tubule. Attractin protein
was also positively stained in Leydig and germ cells of the mature
rat, exhibiting a strong membrane and cytoplasmic presence. No
immunopositive staining was noted in spermatozoa or in the
epididymides (Figs. 5–10).
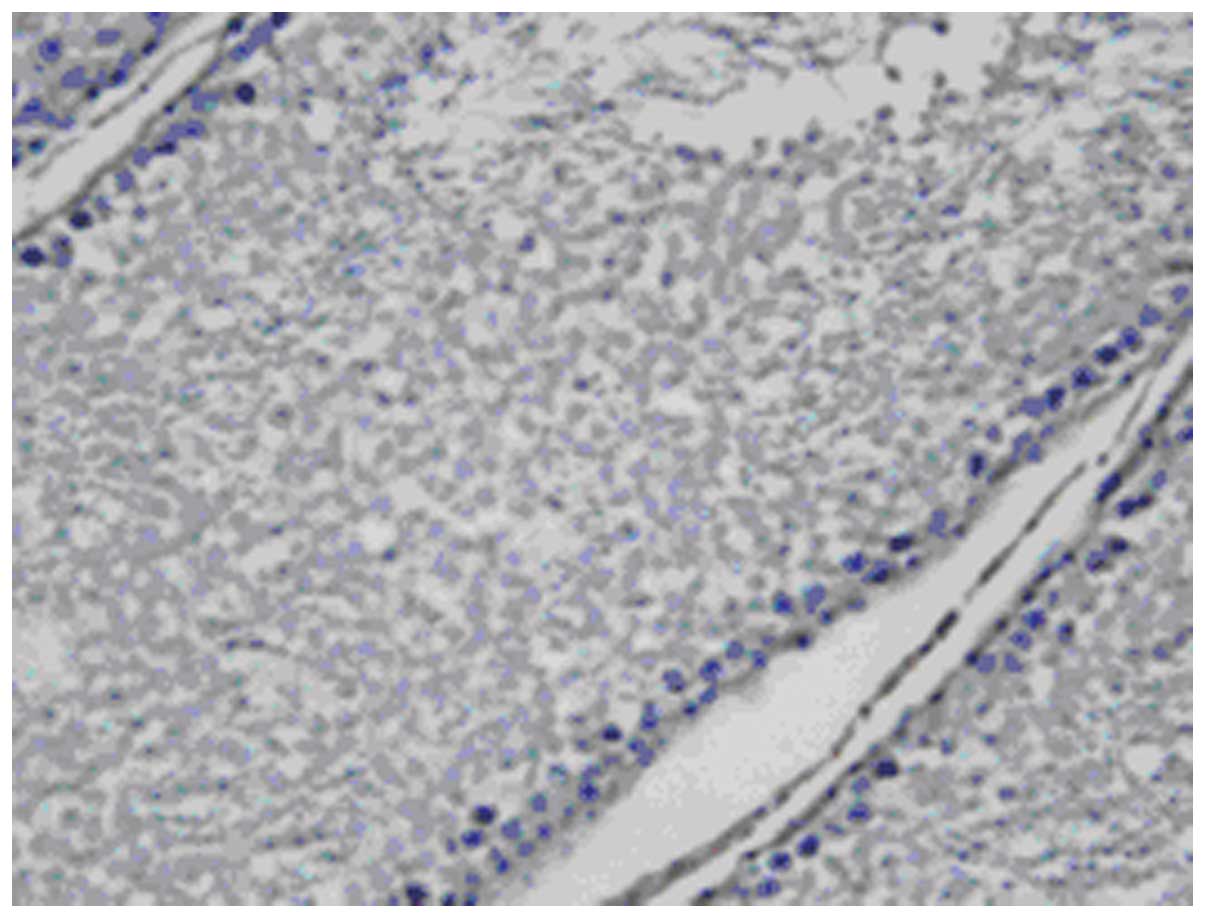
In situ hybridization
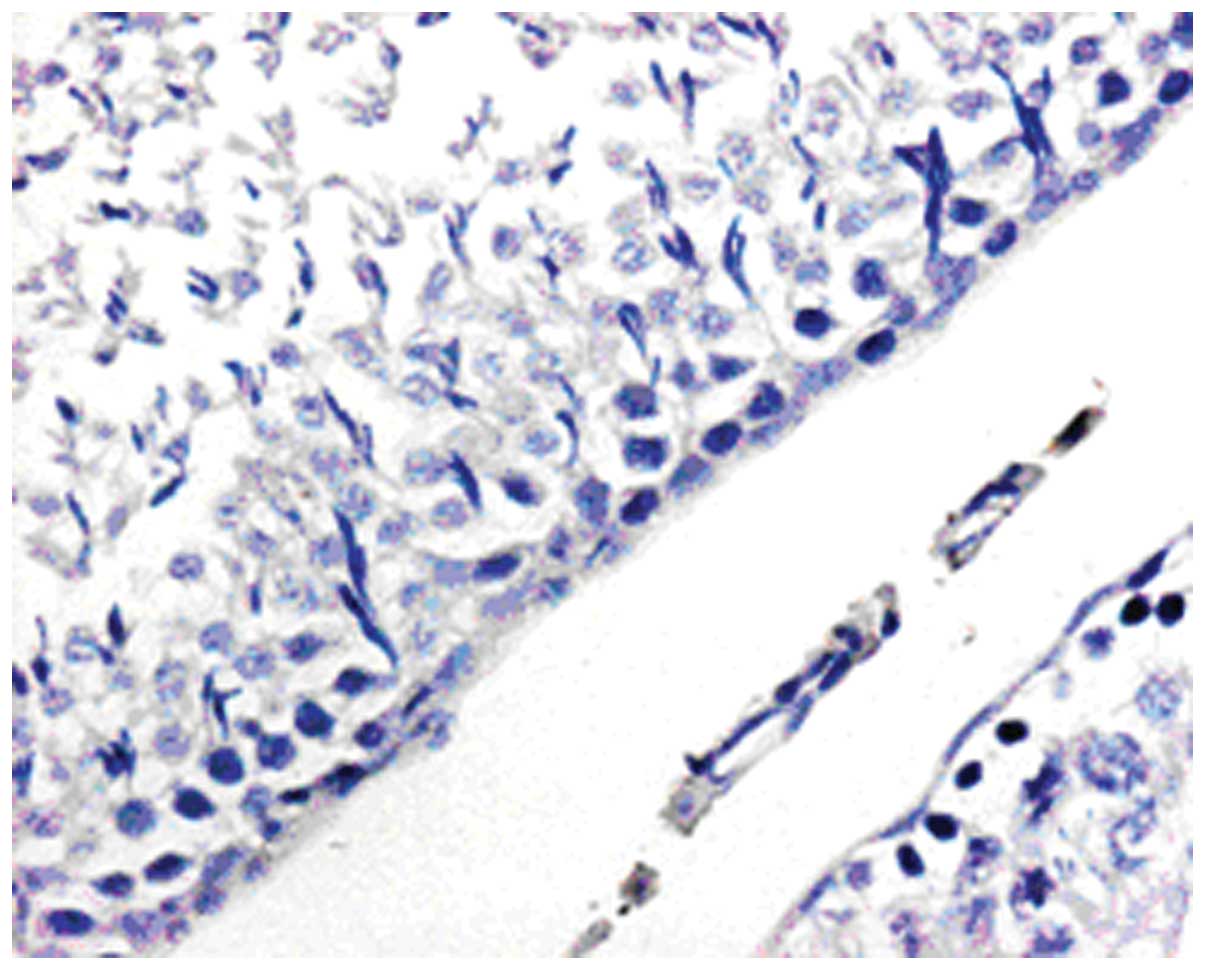
Expression of attractin mRNA by ISH was consistent
with the results of attractin protein staining within Leydig cells,
primitive spermatogonia, primary spermatocyte, spermatid, Sertoli
cells and peritubular myoid cells. Attractin mRNA was distributed
in the nucleus and the cytoplasm of these cells. No immunopositive
staining was noted in spermatozoa or in the epididymides (Figs. 11–16).
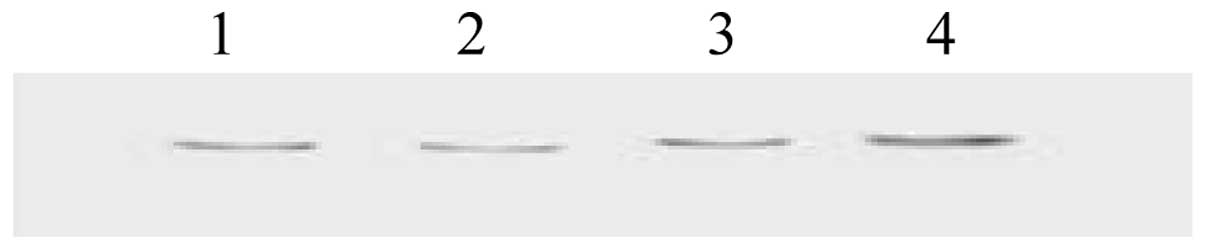
Western blot analysis
Color development was according to the protein
Marker Mix molecular weight between 215 and 120K. The Atrn protein
band was noted (Fig. 17). Lanes
1–3 show the 200-kDa Atrn protein band, respectively; lane 4 shows
the 215-kDa protein marker.
Discussion
The mouse mahogany gene mutation was first
recognized as a modifier of the Agouti phenotype in the late 1950s.
It was only in the last few years however, that a clear image of
mahogany gene structure and function emerged. In 1999, Gunn et
al (2) and Nagle et al
(3) discovered that it encodes a
1428-residue single transmembrane-spanning protein with a large
extracellular domain that contains two epidermal growth factor
(EGF) repeats, a complement C1r/C1s, Uegf, Bmp1 (CUB) domain and
two laminin-type EGF repeats by positional cloning. Concurrently,
Duke-Cohan et al (8,9)
cloned human attractin, a human serum glycoprotein secreted from
activated T cells. The extracellular domain of the mouse protein
was found to be 93% identical to attractin. Due to this homology
between the mouse mahogany protein and human attractin, the mouse
gene was renamed the attractin gene. Further analysis demonstrated
that humans produce two major isoforms of the Atrn protein: a
secreted isoform that is truncated just short of the transmembrane
domain, and a membrane-spanning isoform that is homologous to mouse
Atrn. The secreted isoform is present in humans and rats but not
mice, and is expressed in nearly every tissue. The
membrane-spanning isoform has a wide (10) but not a ubiquitous distribution
and is found at high levels in melanocytes and other components of
the skin, as well as in the brain, the heart, the kidney, the
liver, and the lung, but excluding the uterus, muscles or the
spleen.
In recent years, extensive research has been
conducted in regards to attractin in its involvement in
melanogenetic shifts of coat color, in diseases of the central
nervous system and in the development of obesity-regulating drugs,
with the development of molecular biology techniques (11). Recently, involvement of attractin
in the aspects of mammalian egg-sperm interactions has been
suggested (10). However, there
are no studies concerning the role of attractin in the male
reproductive system.
This study provides evidence based on IIF, IHC,
confocal laser microscopy techniques and ISH that Atrn protein and
mRNA may be localized within both interstitial tissues and the
seminiferous tubules in an age-dependent manner. In spermatozoa and
epididymides, including the efferent ductules and the epididymal
ducts, no definitive immunopositive staining was noted. This
suggests that rat testis has the ability to synthesize attractin
protein throughout sexual development, and Atrn has likely little
importance on maturation and deposition of spermatozoa. In the
testis of the male rat, there was distinct immunopositive staining
as detected by IHC on many cell membranes and also in the
cytoplasm. This is the result of antigen dispersement, since Atrn
is a transmembrane protein.
IIF, IHC, confocal laser microscopy data suggest
that the expression of Atrn protein in Leydig cells gradually
becomes stronger when compared with germ cells with the increasing
age of the rat. This phenomenon may be due to the fact that
seminiferous tubules contain only spermatocytes and Sertoli cells
during the prepubertal stages of the male rat. From puberty, by the
action of pituitary gonadotropin, Leydig cells gradually mature and
testosterone secretion starts to increase. Upon stimulation of
testosterone, the transcription of the Atrn gene is accelerated and
protein secretion is increased. There are two isoforms of rat Atrn
protein: the secreted isoform and the membrane-spanning isoform.
The secreted isoform of Atrn protein may act on Leydig cells in an
autocrine and paracrine manner by mechanisms which promote the
expression of Atrn protein and secretion of testosterone.
However, contrary to the above mentioned results
from IIF, IHC, confocal laser microscopy, ISH data indicate that
the expression of Atrn mRNA within germ cells was stronger than
that in Leydig cells, and increased with the age of the rats. This
may be due to the interaction of Atrn protein and testosterone
which results in increased secretion of testosterone, accelerated
transcription of Atrn gene in germ cells, and facilitation of the
development and maturation of germ cells. In addition, androgens
are the most important hormones in the regulation of
spermatogenesis, and testicular Sertoli cells and peritubular myoid
cells are the target cells of androgens. The expression of Atrn
protein and mRNA in Sertoli cells and peritubular myoid cells
gradually increased along with the maturation of the rat.
Spermatogenesis is the result of programmed differentiation of germ
cells with the help and regulation of Sertoli cells. It is logical
and foreseeable that the quantity of mRNA in a cell would be
representative of the protein expression. But in fact, this is not
entirely true. There are three levels of regulation from DNA and
RNA to protein: the transcription level, translation level and
post-translation level. From the mRNA point of view, it is only
affected by the regulation of the transcription level and cannot
represent the level of protein expression as the end result. Recent
experimentation has confirmed that there is a poor correlation
between the level of mRNA and the amount of protein in tissues,
particularly proteins with low expression levels or contents
(12). More importantly, based on
the abundance of mRNA, it is almost impossible to estimate the
complex modification afer protein translation, the subcellular
localization or migration of the protein, and protein-protein
interactions.
In the present study, Atrn protein in the testis of
the mature male rat using dot blotting and western blot techniques
was analyzed and the results showed that the protein content of
Atrn was lower at this age.
In prepubertal stages, Leydig cells in the
interstitial tissue of the testis have no ability to synthesize or
secrete androgens. However, ISH analysis showed that attractin mRNA
was positively stained in the gonocytes of newborn (1 day) and
prepubertal (5 days) rats, which implies that Atrn plays a key role
in gonocyte development and maturation (and its shift to
spermatogonia independently from the effects of androgens secreted
by mature Leydig cells). Prior to puberty, spermatogonia can still
continuously differentiate and proliferate in this manner.
We conclude that the expression of Atrn protein and
mRNA is related to the functional status of the cell. For example,
seminiferous tubules have no lumens in the newborn (1 day) rat and
are mainly composed of gonocytes and Sertoli cells. Strong mRNA
staining was present mainly in gonocytes at this age. Spermatogonia
emerged in the seminiferous tubules of the prepubertal (5 days)
rat, and the expression of Atrn in spermatogonia became the
strongest when compare to the other cells. This phenomenon was the
same in the spermatocytes that emerged in the seminiferous tubules
of the pubertal (20 days) rat and in spermatides that emerged in
the seminiferous tubules of the postpubertal (50 days) rat.
Models by Huckins and Oakberg (13) and Huckins (14) of spermatogonial stem cells
indicate that type A spermatogonia can be characterized into a
reserve type of spermatogonial stem cell (AS), renewing
spermatogonial stem cell (Apr-Aal-Aal), and differentiating
spermatogonial stem cell (A1–A4, type B). This experiment did not
sort type A spermatogonial stem cells into detailed types, but
results showed that from puberty, the expression of Atrn was
stronger in type A spermatogonia than type B, which suggests that
Atrn may play a distinct role in the differentiation and
proliferation of spermatogonia. Once type A spermatogonia had
differentiated into type B spermatogonia, cell mitosis stopped, and
the expression of Atrn was reduced or even disappeared. The
expression of Atrn protein and mRNA was found to be strongly
expressed in pachytene spermatocytes, which was demonstrated by the
experimental phenomenon of significantly increased brown particles
when compared to other cells. This suggests that, upon the action
of pituitary gonadotropin, spermatogenic cells continuously
proliferated and differentiated. Atrn may promote the meiosis of
spermatocytes which leads to the differentiation of spermatocytes
into spermatids and ultimately into spermatozoa.
The present study confirmed that Atrn protein and
mRNA is widely distributed in the testes of rats at different ages
of maturation and suggests that Atrn may take part in the
physiological function and differentiation of germ cells. Thus,
investigation of the function of Atrn in the male reproductive
system in future preclinical and clinical studies is warranted.
References
|
1
|
Wrenger S, Faust J, Friedrich D, et al:
Attractin, a dipeptidyl peptidase IV/CD26-like enzyme, is expressed
on human peripheral blood monocytes and potentially influences
monocyte function. J Leukoc Biol. 80:621–629. 2006. View Article : Google Scholar
|
|
2
|
Gunn TM, Miller KA, He L, et al: The mouse
mahogany locus encodes a transmembrane form of human attractin.
Nature. 398:152–156. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nagle DL, McGrail SH, Vitale J, et al: The
mahogany protein is a receptor involved in suppression of obesity.
Nature. 398:148–152. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gunn TM and Barsh GS: Mahogany/attractin:
en route from phenotype to function. Trends Cardiovasc Med.
10:76–81. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lu XY, Gunn TM, Shieh Kr, Barsh GS, Akil H
and Watson SJ: Distribution of Mahogany/Attractin mRNA in the rat
central nervous system. FEBS Lett. 462:101–107. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Malik R, Mares V, Kleibl Z, Pohlreich P,
Vlasicová K and Sedo A: Expression of attractin and its
differential enzyme activity in glioma cells. Biochem Biophys Res
Commun. 284:289–294. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Paz J, Yao H, Lim HS, Lu XY and Zhang W:
The neuroprotective role of attractin in neurodegeneration.
Neurobiol Aging. 28:1446–1456. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Duke-Cohan JS, Kim JH and Azouz A:
Attractin: cautionary tales for therapeutic intervention in
molecules with pleiotropic functionality. J Environ Pathol Toxicol
Oncol. 23:1–11. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Duke-Cohan JS, Gu J, McLaughlin DF, Xu Y,
Freeman GJ and Schlossman SF: Attractin (DPPT-L), a member of the
CUB family of cell adhesion and guidance proteins, is secreted by
activated human T lymphocytes and modulates immune cell
interactions. Proc Natl Acad Sci USA. 95:11336–11341. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cummins SF, Nichols AE, Schein CH and
Nagle GT: Newly identified water-borne protein pheromones interact
with attractin to stimulate mate attraction in Aplysia. Peptides.
27:597–606. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kuramoto T, Kitada K, Inui T, et al:
Attractin/mahogany/zitter plays a critical role in myelination of
the central nervous system. Proc Natl Acad Sci USA. 98:559–564.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tang W, Gunn TM, Mclaughlin DF, Barsh GS,
Schlossman SF and Duke-Cohan JS: Secreted and membrane attractin
result from alternative splicing of the human ATRN gene. Proc Natl
Acad Sci USA. 97:6025–6030. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huckins C and Oakberg EF: Morphological
and quantitative analysis of spermatogonia in mouse testes using
whole mounted seminiferous tubules, I. The normal testes. Anat Rec.
192:519–528. 1978. View Article : Google Scholar
|
|
14
|
Huckins C: Spermatogonial intercellular
bridges in whole-mounted seminiferous tubules from normal and
irradiated rodent testes. Am J Anat. 153:97–121. 1978. View Article : Google Scholar : PubMed/NCBI
|