Introduction
Mast cells are broadly distributed throughout
mammalian tissue and play various functions as regulators of
allergic inflammation, such as asthma, atopic dermatitis and
sinusitis (1). Immediate-type
hypersensitivity (anaphylaxis) is mediated by the release of
histamine in response to the antigen crosslinking of immunoglobulin
E (IgE) bound to mast cells. Stimulated mast cells rapidly secrete
pre-formed and de novo synthesized allergic mediators, such
as histamine, cytokines and arachidonic derivatives (2). One of the allergic mediators,
histamine, plays a major role in normal physiology and in
pathophysiology, and regulates a variety of vital functions in the
allergic inflammatory response (3,4).
The activation of mast cells leads to the
phosphorylation of tyrosine kinase and the mobilization of internal
calcium (5). These are followed
by the activation of protein kinase C, mitogen-activated protein
kinases (MAPKs) and nuclear factor (NF)-κB, as well as an increase
in the expression of inflammatory cytokines (1). Activated mast cells release
histamine and other inflammatory mediators, such as eicosanoids,
proteoglycans and several pro-inflammatory cytokines, such as tumor
necrosis factor (TNF)-α, interleukin (IL)-1β, IL-4, IL-6 and IL-13
(2,6). Although these inflammatory cytokines
have beneficial effects on the host defense process, they cause
pathological conditions when overexpressed. Therefore, the
inhibition of the release of these inflammatory cytokines from mast
cells is crucial to reducing allergic inflammatory symptoms.
Vigna angularis (azuki bean) is one of the
largest crops in Asia and has long been used in alternative and
complementary medicine in Korea, China and Japan. It has been
prescribed for infection, edema and inflammation of the kidneys and
bladder (7). Vigna
angularis has been reported to exert tumor-suppressive,
anti-diabetic, antioxidant and anti-inflammatory effects (7–9).
In addition, Azuki bean seed coats, which are rich in polyphenols,
have recently been reported to attenuate vascular oxidative stress
in spontaneously hypertensive rats (10). However, the anti-allergic and
anti-inflammatory effects of Vigna angularis have not yet
been fully elucidated.
In the present study, we investigated the effects of
extracts of Vigna angularis (EVA) on mast cell-mediated
allergic inflammation using in vitro and in vivo
models. The release of histamine and intracellular calcium levels
were examined to clarify the mechanisms by which EVA inhibits the
release of histamine from mast cells. The effects of EVA on
pro-inflammatory cytokines and the role of NF-κB and MAPKs in these
effects were investigated using human mast cells (HMC-1). In
addition, to confirm the anti-allergic and anti-inflammatory
effects of EVA in an in vivo system, systemic and local
anaphylaxis mouse models were employed.
Materials and methods
Preparation of EVA
Vigna angularis was purchased from a herbal
medicine store in Jeongeup, Korea. The authenticity of the plants
was confirmed by Professor Y.H. Kim, at the College of Pharmacy of
Chungnam National University, Daejeon, Korea. The Vigna
angularis material (10 kg) was dried, pulverized to a fine
powder and extracted twice with 95% EtOH at 70°C for 4 h. The EtOH
extract (120 l) of Vigna angularis was then passed through a
0.45-μm filter and vaporized in a rotary evaporator, yielding 100 g
of residue. For the treatment of the cells, the stock solution (100
mg/ml) of EVA was dissolved in 100% DMSO and further diluted with
Iscove’s medium before use.
Reagents and cell culture
Compound 48/80, anti-dinitrophenyl (DNP) IgE,
DNP-human serum albumin (HSA), phorbol 12-myristate 13-acetate
(PMA) and calcium ionophore A23187 (PMACI) were purchased from
Sigma (St. Louis, MO, USA). The human mast cell line (HMC-1) was
grown in Iscove’s medium (Life Technologies, Grand Island, NY, USA)
supplemented with 10% fetal bovine serum (FBS) at 37°C in 5%
CO2. Passage 4–8 HMC-1 cells were used throughout the
study.
Determination of histamine levels
The histamine levels in the HMC-1 cells and serum
were measured using the o-phthaldialdehyde
spectrofluorometric procedure as previously described (11). The HMC-1 cells (1×106
cells/ml) were pre-incubated with EVA for 30 min, and then
incubated for 30 min with PMA (20 nM) and calcium ionophore A23187
(1 μM). The cells were separated from the released histamine by
centrifugation at 400 × g for 5 min at 4°C. The blood from the mice
was centrifuged at 400 × g for 10 min and the serum was withdrawn
to measure the histamine content.
Determination of intracellular calcium
levels
The intracellular calcium levels were measured with
the use of the fluorescence indicator, Fluo-3/AM (Molecular Probes,
Eugene, OR, USA). The HMC-1 cells were pre-incubated with Fluo-3/AM
for 30 min at 37°C. After washing the dye from the cell surface,
the cells were treated with EVA for 10 min prior to the addition of
PMACI. The fluorochrome was excited at 488 nm, and the emission was
filtered with 515 nm using a flow cytometer (BD Biosciences
Pharmingen, San Diego, CA, USA).
RNA extraction and mRNA detection
Total cellular RNA was isolated from the cells
(1×106/well in a 24-well plate) following stimulation
with PMA (20 nM) and A23187 (1 μM) with or without EVA for 4 h
using TRI reagent (Molecular Research Center, Cincinnati, OH, USA)
according to the manufacturer’s instructions. The first-strand
complementary DNA (cDNA) was synthesized using Superscript II
reverse-transcriptase (Invitrogen, Carlsbad, CA, USA). A
reverse-transcriptase polymerase chain reaction (RT-PCR) was used
to analyze the mRNA expression of TNF-α, IL-6 and β-actin (internal
control). The conditions for the reverse transcription and PCR
steps were similar to those described previously (12). The amplified products were
separated by electrophoresis on 2% agarose gels containing ethidium
bromide, documented using a Kodak DC 290 digital camera and
digitized using UN-SCAN-IT software (Silk Scientific, Inc., Orem,
UT, USA). The band intensity was normalized to that of β-actin in
the same sample.
Enzyme-linked immunosorbent assay
(ELISA)
The secretion of TNF-α and IL-6 was measured by
enzyme-linked immunosorbent assay (ELISA) according to a previously
described method (13) with
certain modifications. The HMC-1 cells were cultured in medium and
resuspended in Tyrode buffer A. The cells were sensitized with
PMACI for 8 h in the absence or presence of EVA. ELISA was
performed by coating 96-well plates with 6.25 ng/well of monoclonal
antibody with specificity for TNF-α or IL-6.
Western blot analysis
The HMC-1 cells were washed 3 times with PBS and
resuspended in lysis buffer. The samples were electrophoresed using
12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis as
previously described (14) and
then transferred onto a nitrocellulose membrane. The p38 MAPK, ERK
and JNK activation was determined using anti-phopspho-p38, -ERK and
-JNK antibodies (Cell Signaling Technology, Inc., Beverly, MA,
USA). The nuclear and cytosolic p65 NF-κB and IκBα were assayed
using anti-NF-κB (p65) and anti-IκBα antibodies, respectively
(Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA).
Immunodetection was carried out using SuperSignal West Pico
Chemiluminescent Substrate (Thermo Fisher Scientific Inc, Waltham,
MA, USA).
Animals
The original stock of male imprinting control region
(ICR) mice (6 weeks of age) was purchased from Dae Han Bio Link.
Co., Ltd. (Chungbuk, Korea). The animals were housed 5 per cage in
a laminar airflow room maintained under a temperature of 22±2°C and
a relative humidity of 55±5°C throughout the study. The care and
treatment of the mice were in accordance with the guidelines
established by the Public Health Service Policy on the Humane Care
and Use of Laboratory Animals and were approved the Animal Care and
Use Committee at Kyungpook National University.
Systemic anaphylaxis
The mice were administered an intraperitoneal
injection of 8 mg/kg body weight (BW) of the mast cell
degranulator, compound 48/80. EVA was dissolved in saline and
orally administered at various doses (10, 50 and 250 mg/kg BW) 2 h
prior to the injection of compound 48/80 (n=10/group). Mortality
was monitored for 1 h after the induction of anaphylactic shock.
After the mortality test, blood was obtained from the heart of each
mouse to measure the serum histamine content.
Passive cutaneous anaphylaxis (PCA)
An IgE-dependent cutaneous reaction was carried out
as previously described (12).
The PCA reaction was generated by sensitizing the skin with an
intradermal injection of anti-DNP IgE followed 48 h later with an
injection of DNP-HSA into the mouse tail vein. The anti-DNP IgE
antibody and DNP-HSA were diluted in PBS. The mice were injected
intradermally with 0.5 μg of anti-DNP IgE. EVA was orally
administered at doses of 10, 50 and 250 mg/kg BW 2 h prior to the
injection of anti-DNP IgE. After 48 h, each mouse (n=10/group)
received an injection of 1 μg of DNP-HSA containing 4% Evans blue
(1:4) via the tail vein. Thirty minutes after the challenge, the
mice were sacrificed and the dorsal skin (diameter, 1 cm) was
removed for measurement of the pigmented area. The amount of dye
was then determined colorimetrically following extraction with 1 ml
of 1 M potassium hydroxide (KOH) and 9 ml of a mixture of acetone
and phosphoric acid (5:13). The absorbance intensity of the
extraction was measured at 620 nm on a spectrophotometer (Shimadzu
UV-1201; Shimadzu Corp., Kyoto Japan).
Statistical analysis
Statistical analyses were performed using SAS
statistical software (SAS Institute, Cary, NC, USA). The effects of
treatment were analyzed using analysis of variance, followed by
Duncan’s multiple range tests. A P-value <0.05 was considered to
indicate a statistically significant difference.
Results
Effect of EVA on the release of histamine
and intracellular calcium levels
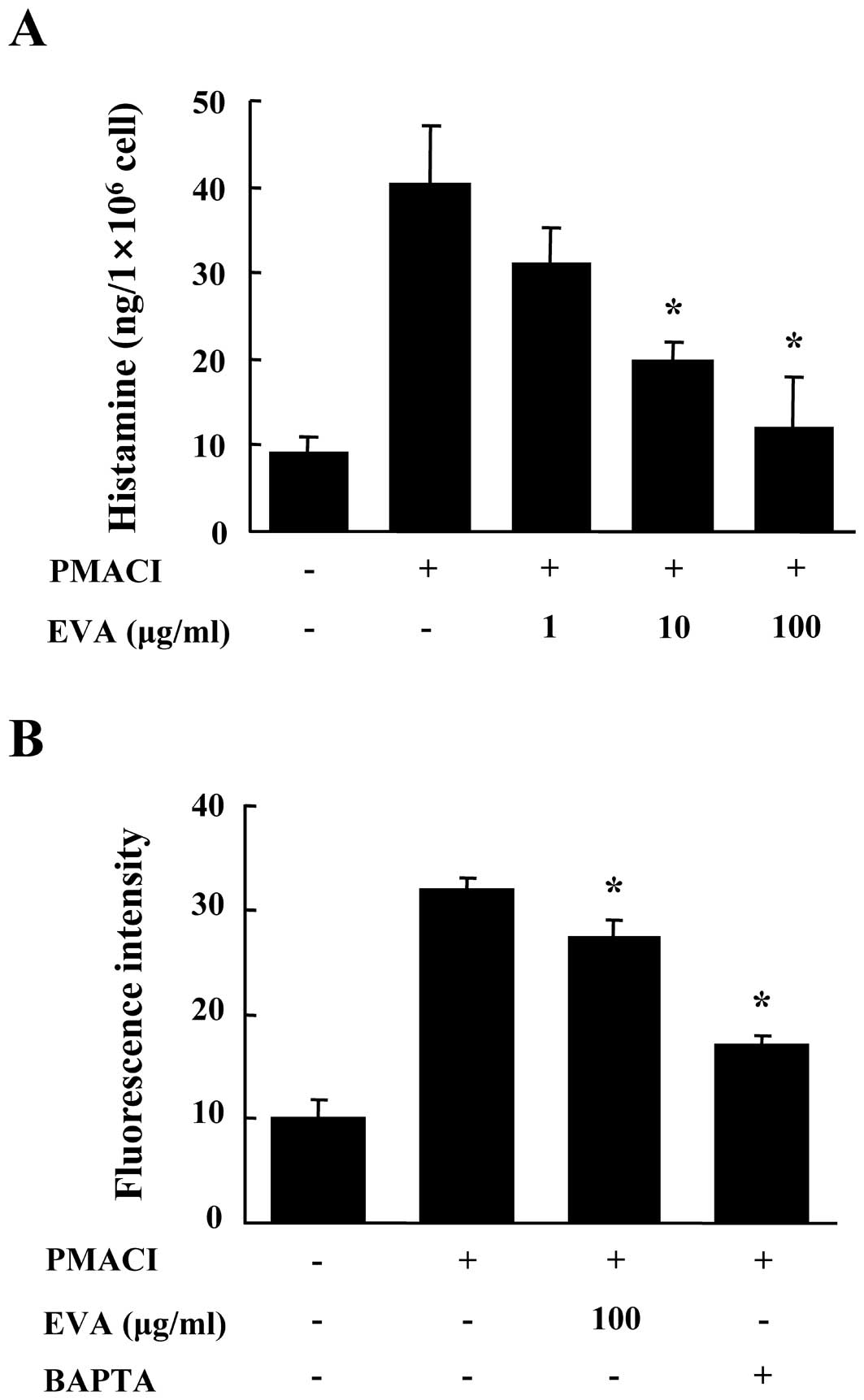
First, we evaluated the effects of EVA on the
release of histamine from PMACI-stimulated HMC-1 cells. The HMC-1
cells released high levels of histamine following stimulation with
PMACI (15). Pre-treatment with
EVA for 30 min reduced the release of histamine in a dose-dependent
manner (Fig. 1A). To eludicate
the mechanisms responsible for the reduction of histamine following
treatment with EVA, we measured the levels of intracellular
calcium. Calcium movements across the membranes of mast cells are
critical to the release of histamine (16). When the HMC-1 cells were
stimulated with PMACI, the intracellular calcium levels
significantly increased. The pre-incubation of HMC-1 cells with EVA
(100 μg/ml) decreased the intracellular calcium levels (Fig. 1B). BAPTA-AM (Molecular Probes) was
used as a positive control. The concentration and duration of EVA
treatment used in these experiments had no significant effect on
the viability of HMC-1 cells (data not shown).
Effects of EVA on the expression and
secretion of pro-inflammatory cytokines
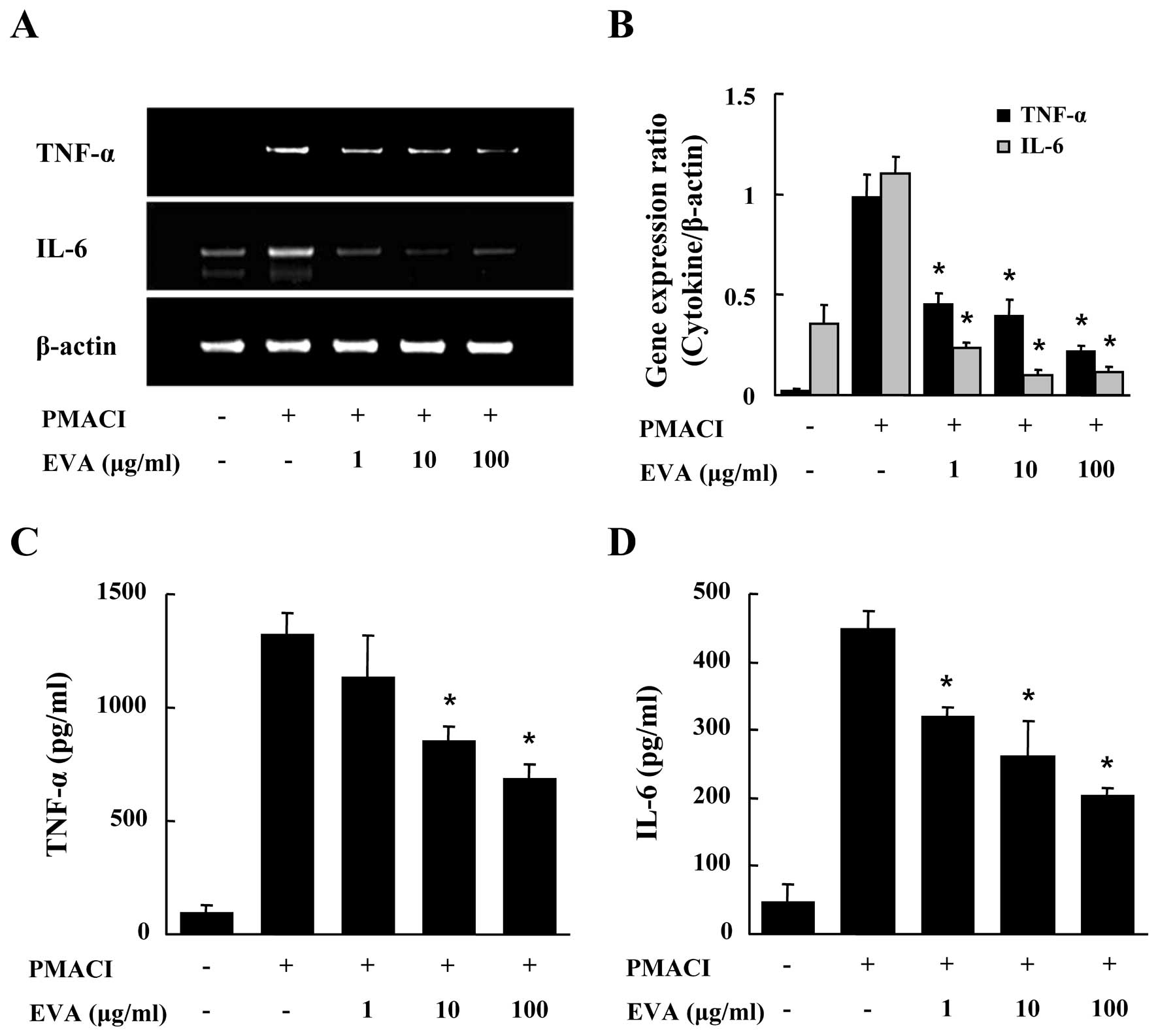
We evaluated the effects of EVA on the gene
expression and secretion of pro-inflammatory cytokines, such as
TNF-α and IL-6 in the PMACI-stimulated HMC-1 cells. After the HMC-1
cells were pre-incubated with EVA for 30 min, they were then
stimulated with PMACI for 4 h. As shown in Fig. 2A and B, EVA dose-dependently
inhibited the PMACI-induced gene expression of TNF-α and IL-6. To
confirm the effects of EVA on the gene expression of
pro-inflammatory cytokines, culture supernatants were collected and
the levels of TNF-α and IL-6 were measured by ELISA. EVA inhibited
the secretion of TNF-α and IL-6 in the PMACI-stimulated HMC-1 cells
(Fig. 2C and D).
Effect of EVA on the activation of NF-κB
and MAPKs
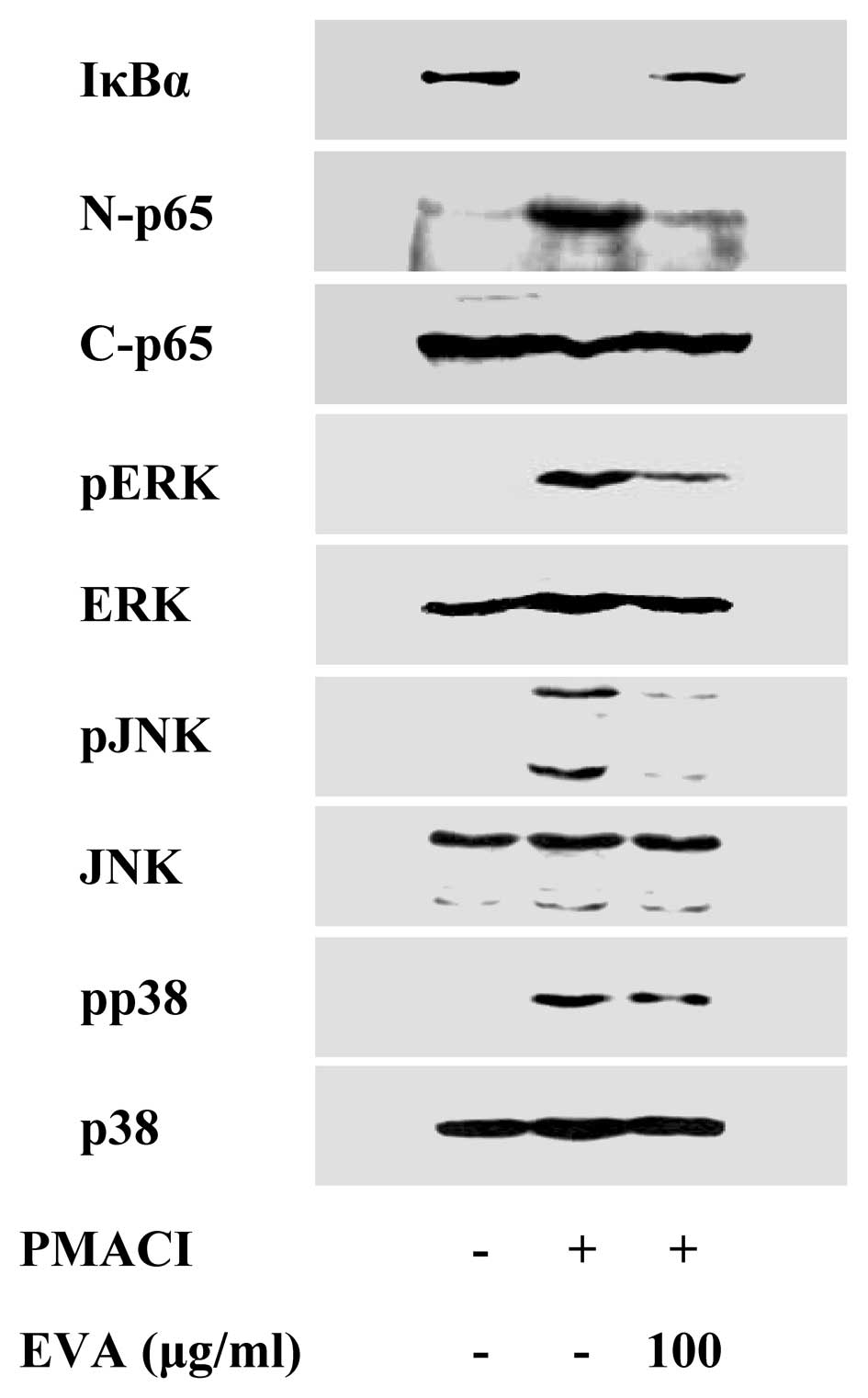
To elucidate the mechanisms responsible for the
reduction of cytokine levels, we examined the effects of EVA on the
activation of the transcription factors, NF-κB and MAPKs. NF-κB is
an important transcriptional regulator of inflammatory cytokines
and plays a crucial role in immune and inflammatory responses
(17). Stimulation of the HMC-1
cells with PMACI induced the degradation of IκBα and the nuclear
translocation of p65 NF-κB after 2 h of incubation. EVA inhibited
the PMACI-induced degradation of IκBα and the nuclear translocation
of p65 NF-κB (Fig. 3). MAPK
pathways also play a crucial role in the regulation of
pro-inflammatory molecules (17).
Previously, we demonstrated that PMACI activates all 3 types of
MAPKs, such as p38, JNK and ERK within 15–30 min in HMC-1 cells
(13). EVA markedly attenuated
the PMACI-induced activation of all 3 types of MAPKs (Fig. 3). These data indicate that EVA
decreases the expression of inflammatory cytokines by blocking the
activation of NF-κB and MAPKs.
Effect of EVA on systemic and local
anaphylaxis
To determine the effects of EVA on allergic
reaction, an in vivo mouse model of systemic anaphylaxis was
used. Compound 48/80 (8 mg/kg, BW) was used as a model of induction
for a systemic fatal allergic reaction. After the intraperitoneal
injection of compound 48/80, the mice were monitored for 1 h, after
which the mortality rate was determined. The injection of compound
48/80 into the mice induced fatal shock in 100% of the animals.
When the animals were pre-treated with EVA (oral administration) at
doses 10, 50 and 250 mg/kg (BW) for 2 h, the mortality rate was
dose-dependently reduced (Table
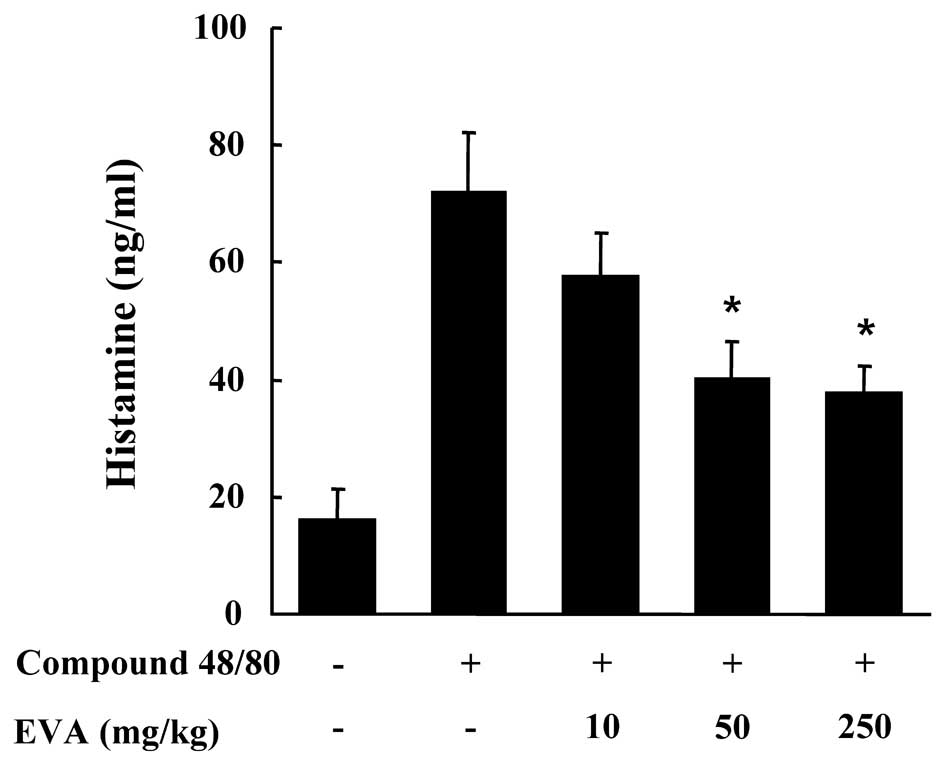
I). The effect of EVA on the compound 48/80-induced release of
histamine in serum was also investigated. The injection of compound
48/80 induced a marked increase in the release of histamine in
serum which was significantly inhibited by treatment with EVA at
doses of 50 and 250 mg/kg BW (Fig.
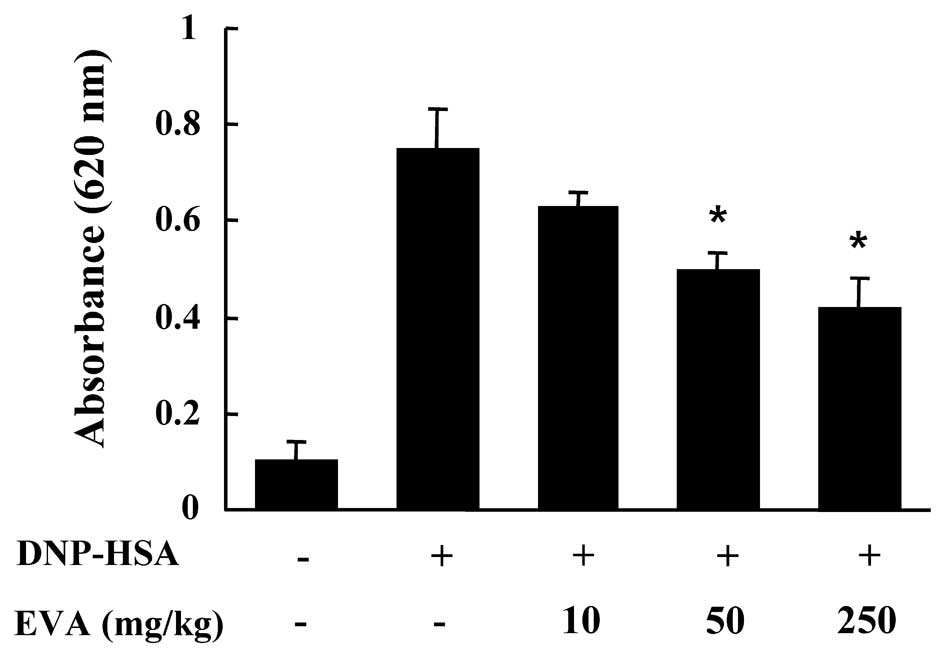
4). Another way to test the anaphylactic reaction is to induce
PCA. A local extravasation was induced by a local injection of IgE
followed by antigenic challenge. EVA was orally administered at 10,
50 and 250 mg/kg (BW) 2 h prior to challenge with the antigen. EVA
dose-dependently inhibited PCA (Fig.
5).
 | Table IDose-dependent effect of EVA on
compound 48/80-induced systemic anaphylaxis. |
Table I
Dose-dependent effect of EVA on
compound 48/80-induced systemic anaphylaxis.
| EVA treatment (mg/kg
BW) | Compound 48/80 (8
mg/kg BW) | Mortality (%) |
|---|
| None (saline) | + | 100 |
| 10 | + | 80 |
| 50 | + | 30 |
| 250 | + | 0 |
| 250 | − | 0 |
Discussion
Anaphylaxis is a life-threatening syndrome induced
by the sudden systemic release of inflammatory mediators, such as
histamine and various cytokines from mast cells (18). In this study, using in
vitro and in vivo models, we demonstrate that EVA
reduces mast cell-derived allergic inflammatory responses.
Histamine was originally considered as a mediator of
acute inflammatory and immediate hypersensitivity responses.
Previously, it was reported that histamine affects chronic
inflammation and regulates several essential events of immune
response, such as immune cell maturation, polarization and
lymphocyte responsiveness (19).
Studies have established that the stimulation of mast cells with
compound 48/80 or IgE initiates the activation of signal
transduction pathways, which lead to the release of histamine. It
has been demonstrated that compound 48/80 and other polybasic
compounds are able to directly activate G proteins (20). Compound 48/80 increases the
permeability of the lipid bilayer membrane by inducing a
perturbation in the membrane. These data indicate that the increase
in membrane permeability may be an essential trigger for the
release of the mediator from mast cells. In this sense,
anti-allergic agents having a membrane-stabilizing action may be
desirable (21). EVA may
stabilize the lipid bilayer membrane, thus preventing the compound
48/80-induced membrane perturbation.
Intracellular calcium is critical to the
degranulation of mast cells. Calcium movements across the membranes
of mast cells represent a major target for effective anti-allergic
drugs, as these are essential events linking stimulation to
secretion (22,23). The mode of action of EVA is
possibly associated with the prevention of the release of histamine
from mast cells due to the reduction in intracellular calcium
levels. Our results showing an attenuation of intracellular calcium
levels in mast cells following treatment with EVA are consistent
with those from other reports. According to these observations, we
strongly speculate that decreased intracellular calcium levels may
be involved in the inhibitory effects of EVA on the release of
histamine.
The HMC-1 cell line is useful for studying cytokine
activation pathways (24). The
various types of cytokines produced by HMC-1 cells with PMACI
stimulation supports the well-recognized role of mast cells in
immediate-type hypersensitivity. TNF-α and IL-6 play a major role
in triggering and sustaining the allergic inflammatory response in
mast cells. Mast cells are one of the major sources of TNF-α in the
human dermis (25). TNF-α
promotes inflammation, granuloma formation and tissue fibrosis and
is considered to be an initiator of cytokine-related inflammatory
states by stimulating cytokine production (26). TNF-α is involved in the survival
of eosinophils, thereby contributing to chronic inflammation
(27). IL-6 is also produced from
mast cells and its local accumulation is associated with PCA
(28). These reports indicate
that the decrease in the levels of TNF-α and IL-6 in mast cells is
one of the key indicators of reduced allergic inflammatory
symptoms. In our study, EVA decreased the elevated gene expression
of TNF-α and IL-6 in mast cells. These data suggest that EVA exerts
anti-inflammatory effects by inhibiting the production of
inflammatory cytokines.
To evaluate the mechanisms behind the inhibitory
effects of EVA on TNF-α and IL-6, we examined the effects of EVA on
NF-κB. NF-κB regulates the expression of multiple inflammatory and
immune genes and plays a critical role in chronic inflammatory
diseases. The role of NF-κB activation and regulation of cytokine
production in allergic inflammatory processes have been
characterized (29). The
activation of NF-κB requires the phosphorylation and proteolytic
degradation of the inhibitory protein, IκBα, an endogenous
inhibitor that binds to NF-κB in the cytoplasm (30). In our study, EVA decreased the
degradation of IκBα and the nuclear translocation of p65 NF-κB.
These results indicate that the inhibitory effects of EVA on
inflammatory cytokines are due to the regulation of the NF-κB
pathway.
The MAPK cascade is one of the important signaling
pathways in immune responses (31). The MAPK signaling cascade
regulates important cellular processes, including gene expression,
cell proliferation, cell survival and death, as well as cell
mobility (32). The expression of
TNF-α and IL-6 is regulated by MAPKs (24). The precise signaling pathways
among the 3 types of MAPKs, i.e., ERK, JNK and p38 remain unclear.
However, the induction of inflammatory cytokine production requires
the phosphorylation of all 3 types of MAPKs. In this study, the
PMACI-induced phosphorylation of all 3 types of MAPKs was reduced
by EVA. These data suggest that EVA exerts an inhibitory effect on
all 3 types of MAPKs and downstream cytokine expression.
To confirm the effects of EVA in animal models, we
evaluated the inhibitory effects of EVA on compound 48/80-induced
systemic anaphylaxis and histamine release. These results indicate
that mast cell-mediated immediate-type allergic reactions are
inhibited by EVA. In addition, EVA administered to mice protects
them against IgE-mediated PCA; the mouse model of PCA is one of the
most important in vivo models of anaphylaxis in local
allergic reactions. These finding suggest that EVA may be useful in
the treatment of allergic diseases, particularly skin reactions. As
we used whole extracts of Vigna angularis and not a purified
single compound, the biological effects of the individual active
components are not clear at this time. Nevertheless, EVA showed
remarkable anti-allergic and anti-inflammatory effects. Therefore,
we partitioned the Vigna angularis extracts based on
anti-allergic and anti-inflammatory effects. Through the
fractionation, we obtained 2 single compounds, oleanolic acid and
oleanolic acid acetate. The effort to confirm the actual role of
these compounds on mast cell-mediated allergic inflammation is
ongoing in our laboratory.
In conclusion, the present study demonstrates that
EVA significantly reduces mast cell-mediated allergic inflammation
in in vitro and in vivo models. We suggest that EVA
reduces the release of histamine through the modulation of
intracellular calcium. EVA inhibits the expression of inflammatory
cytokines by suppressing the activation of NF-κB and MAPKs. We
provide evidence that EVA may be used in the prevention or
treatment of mast cell-mediated allergic inflammatory diseases.
Acknowledgements
This study was supported by a KRIBB Research
Initiative Program, Korea Healthcare technology R&D Project,
Ministry for Health, Welfare and Family Affairs (A111375),
Kyungpook National University Research Fund 2012, and NRF funded by
the Ministry of Science, ICT & Future Planning
(2012M3A9B6055416).
References
|
1
|
Galli SJ, Kalesnikoff J, Grimbaldeston MA,
Piliponsky AM, Williams CM and Tsai M: Mast cells as ‘tunable’
effector and immunoregulatory cells: recent advances. Annu Rev
Immunol. 23:749–786. 2005.
|
|
2
|
Amin K: The role of mast cells in allergic
inflammation. Respir Med. 106:9–14. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Galli SJ, Nakae S and Tsai M: Mast cells
in the development of adaptive immune responses. Nat Immunol.
6:135–142. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Galli SJ, Tsai M and Piliponsky AM: The
development of allergic inflammation. Nature. 454:445–454. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu LC: Immunoglobulin E receptor signaling
and asthma. J Biol Chem. 286:32891–32897. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sismanopoulos N, Delivanis DA,
Alysandratos KD, et al: Mast cells in allergic and inflammatory
diseases. Curr Pharm Des. 18:2261–2277. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Itoh T, Umekawa H and Furuichi Y:
Potential ability of hot water adzuki (Vigna angularis)
extracts to inhibit the adhesion, invasion, and metastasis of
murine B16 melanoma cells. Biosci Biotechnol Biochem. 69:448–454.
2005.PubMed/NCBI
|
|
8
|
Itoh T, Kobayashi M, Horio F and Furuichi
Y: Hypoglycemic effect of hot-water extract of adzuki (Vigna
angularis) in spontaneously diabetic KK-A(y) mice. Nutrition.
25:134–141. 2009. View Article : Google Scholar
|
|
9
|
Itoh T and Furuichi Y: Hot-water extracts
from adzuki beans (Vigna angularis) stimulate not only
melanogenesis in cultured mouse B16 melanoma cells but also
pigmentation of hair color in C3H mice. Biosci Biotechnol Biochem.
69:873–882. 2005.PubMed/NCBI
|
|
10
|
Mukai Y and Sato S: Polyphenol-containing
azuki bean (Vigna angularis) seed coats attenuate vascular
oxidative stress and inflammation in spontaneously hypertensive
rats. J Nutr Biochem. 22:16–21. 2011.
|
|
11
|
Kim SH, Lee S, Kim IK, et al: Suppression
of mast cell-mediated allergic reaction by Amomum
xanthiodes. Food Chem Toxicol. 45:2138–2144. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bae Y, Lee S and Kim SH: Chrysin
suppresses mast cell-mediated allergic inflammation: involvement of
calcium, caspase-1 and nuclear factor-kappaB. Toxicol Appl
Pharmacol. 254:56–64. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim HH, Choi PH, Yoo JS, et al: Ripe fruit
of Rubus coreanus inhibits mast cell-mediated allergic
inflammation. Int J Mol Med. 29:303–310. 2012.
|
|
14
|
Lee S, Suk K, Kim IK, et al: Signaling
pathways of bisphenol A-induced apoptosis in hippocampal neuronal
cells: role of calcium-induced reactive oxygen species,
mitogen-activated protein kinases, and nuclear factor-kappaB. J
Neurosci Res. 86:2932–2942. 2008. View Article : Google Scholar
|
|
15
|
Singh TS, Lee S, Kim HH, Choi JK and Kim
SH: Perfluorooctanoic acid induces mast cell-mediated allergic
inflammation by the release of histamine and inflammatory
mediators. Toxicol Lett. 210:64–70. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Eisenhut M and Wallace H: Ion channels in
inflammation. Pflugers Arch. 461:401–421. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Newton K and Dixit VM: Signaling in innate
immunity and inflammation. Cold Spring Harb Perspect Biol.
4:a0060492012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Galli SJ and Tsai M: IgE and mast cells in
allergic disease. Nat Med. 18:693–704. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jutel M, Blaser K and Akdis CA: Histamine
in allergic inflammation and immune modulation. Int Arch Allergy
Immunol. 137:82–92. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Palomaki VA and Laitinen JT: The basic
secretagogue compound 48/80 activates G proteins indirectly via
stimulation of phospholipase D-lysophosphatidic acid receptor axis
and 5-HT1A receptors in rat brain sections. Br J Pharmacol.
147:596–606. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim SY, Kim SH, Shin HY, et al: Effects of
Prunella vulgaris on mast cell-mediated allergic reaction
and inflammatory cytokine production. Exp Biol Med (Maywood).
232:921–926. 2007.
|
|
22
|
Ma HT and Beaven MA: Regulators of Ca(2+)
signaling in mast cells: potential targets for treatment of mast
cell-related diseases? Adv Exp Med Biol. 716:62–90. 2011.
|
|
23
|
Manikandan J, Kothandaraman N, Hande MP
and Pushparaj PN: Deciphering the structure and function of
FcɛRI/mast cell axis in the regulation of allergy and anaphylaxis:
a functional genomics paradigm. Cell Mol Life Sci. 69:1917–1929.
2012.
|
|
24
|
Kim SH, Jun CD, Suk K, et al: Gallic acid
inhibits histamine release and pro-inflammatory cytokine production
in mast cells. Toxicol Sci. 91:123–131. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sarchio SN, Kok LF, O’Sullivan C, Halliday
GM and Byrne SN: Dermal mast cells affect the development of
sunlight-induced skin tumours. Exp Dermatol. 21:241–248. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vandenabeele P, Declercq W, Van Herreweghe
F and Vanden Berghe T: The role of the kinases RIP1 and RIP3 in
TNF-induced necrosis. Sci Signal. 3:re42010.PubMed/NCBI
|
|
27
|
Walczak H: TNF and ubiquitin at the
crossroads of gene activation, cell death, inflammation, and
cancer. Immunol Rev. 244:9–28. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mican JA, Arora N, Burd PR and Metcalfe
DD: Passive cutaneous anaphylaxis in mouse skin is associated with
local accumulation of interleukin-6 mRNA and immunoreactive IL-6
protein. J Allergy Clin Immunol. 90:815–824. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Barnes PJ: Pathophysiology of allergic
inflammation. Immunol Rev. 242:31–50. 2011. View Article : Google Scholar
|
|
30
|
Nakagomi D, Suzuki K and Nakajima H:
Critical roles of IkappaB kinase subunits in mast cell
degranulation. Int Arch Allergy Immunol. 158(Suppl 1): 92–95. 2012.
View Article : Google Scholar
|
|
31
|
Wang X and Liu Y: Regulation of innate
immune response by MAP kinase phosphatase-1. Cell Signal.
19:1372–1382. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wancket LM, Frazier WJ and Liu Y:
Mitogen-activated protein kinase phosphatase (MKP)-1 in immunology,
physiology, and disease. Life Sci. 90:237–248. 2012. View Article : Google Scholar : PubMed/NCBI
|