Introduction
Lung cancer, predominantly non-small cell lung
cancer, is the leading cause of cancer-related mortality worldwide.
Patients with non-small cell lung cancer are mostly treated with
platinum-based chemotherapy, often in combination with radiation
therapy. However, the development of chemoresistance, either
intrinsic or acquired, is a major obstacle limiting successful
treatment (1). Previous studies
have indicated the cytological mechanisms of drug resistance in
cancer cells, such as increased detoxification of anticancer drugs
by the glutathione system, a defective apoptotic pathway, enhanced
DNA repair or increased tolerance to DNA damage, decreased uptake
of water-soluble drugs and enhanced drug efflux from cancer cells
mediated by ATP-binding cassette (ABC) transporters (1–4).
Studies have shown that epigenetic changes, including aberrant CpG
island methylation, histone modifications and abnormal expression
of microRNAs (miRNAs or miRs) may be responsible for the drug
resistance of cancer cells. Specifically, epigenetic changes as
opposed to genetic mutations may play a crucial role in the
acquired drug resistance of cancer cells (5–7).
Among these, miRNAs, as a class of small non-coding RNAs of 18–24
nucleotides, are post-transcriptional regulators that bind
complementary sequences of target mRNAs, usually resulting in
translational repression or target degradation and gene silencing
(8). By regulating gene
expression at the post-transcriptional level, miRNAs have been
linked to pathways associated with cell differentiation,
proliferation and survival, and their aberrant expression has been
shown to be involved in drug resistance in different types of
tumors (9), such as breast
cancer, colorectal carcinoma, gastric cancer and glioblastoma
multiforme (10–15).
Recently, there has been increasing interest in
understanding the role of miR-503 in cancer. miR-503 has been shown
to be downregulated in oral cancer cells and hepatocellular
carcinoma cells (16,17), whereas it is overexpressed in
parathyroid carcinoma, retinoblastoma and adrenocortical carcinoma
(18–20). The upregulation of miR-503 is
associated with a shorter overall survival rate among patients with
adrenocortical carcinoma (18). A
previous study demeonstrated that miR-503 is a novel regulator of
RBP CUG-binding protein 1 (CUGBP1) expression, and thus increases
the sensitivity of intestinal epithelial cells to apoptosis
(21). Moreover, miR-503 has been
shown to reduce the S phase cell population and to inhibit cell
growth by suppressing endogenous CCND1 at the protein and mRNA
level, suggesting that miR-503 is a putative tumor suppressor
(22). miR-503 has also been
shown to induce G1 phase arrest and to inhibit the migration and
invasion of HCCLM3 hepatocellular carcinoma cells in vitro
(16). The altered expression of
miR-503 affects the immunity response to radiotherapy and is a
negative regulator of CD40 in irradiated U937 cells (23). However, little is known about the
effects of miR-503 in the development of drug resistance in lung
cancer.
In this study, we demonstrate that miR-503 is
downregulated in the drug-resistant A549/CDDP human non-small cell
lung cancer cell line, compared with the parental A549 cell line.
Our results suggest that miR-503 plays a role in the development of
drug resistance in human non-small cell lung cancer cells, at least
in part by targeting the anti-apoptotic protein, Bcl-2.
Materials and methods
Cell culture
The A549 human lung cancer cell line and its
drug-resistant variant, A549/CDDP (both obtained from Biosis
Biotechnology Co., Ltd., Shanghai, China), were cultured in
RPMI-1640 medium supplemented with 10% fetal calf serum (Gibco-BRL,
Grand Island, NY, USA) in a humidified atmosphere containing 5%
CO2 at 37°C. To maintain the drug-resistant phenotype,
cisplatin (at a final concentration of 4 μg/ml) was added to the
culture medium for the A549/CDDP cells.
Real-time PCR analysis for miRNA
Total RNA from the A549 and A549/CDDP cells was
isolated using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and
the miRNA fraction was further purified using a mirVana™ miRNA
isolation kit (Ambion, Austin, TX, USA). The concentration and
purity of the RNA samples were determined spectroscopically.
Real-time reverse transcription PCR (qRT-PCR) was performed using
the ViiA 7 Real-Time PCR System with SYBR-Green Master Mix. As a
control, the small housekeeping U6 gene was amplified and
quantified. The EzOmics™ miRNA qPCR Detection Primer Set (cat no.
BK1010) and the EzOmics™ One-Step qPCR kit (cat no. BK2100), which
were purchased from Biomics Biotechnologies Co., Ltd. (Nantong,
China), were used for real-time PCR analysis for miR-503 and U6
snRNA, respectively (24). The
fold-change for miRNA from the A549/CDDP cells relative to the
control A549 cells was calculated using the 2−ΔΔCt
method (25), where ΔΔCt = ΔCt
A549/CDDP − ΔCt A549 and ΔCt = Ct miRNA − Ct U6 small nuclear RNA
(snRNA). PCR was performed in triplicate.
In vitro drug sensitivity assay
The A549/CDDP and A549 cells were plated in 6-well
plates (6×105 cells/well) and 100 nM of the miR-503
mimic or 100 nM miRNA mimic control were transfected into the
A549/CDDP cells, while 100 nM of the miR-503 inhibitor or 100 nM
miRNA inhibitor control were transfected into the A549 cells, using
Lipofectamine-2000 (Invitrogen, Grand Island, NY, USA) according to
the manufacturer’s instructions The miR-503 mimic, miRNA mimic
control, 2′-O-methyl (2′-O-Me) modified miR-503 inhibitor, and
miRNA inhibitor control were chemically synthesized by Shanghai
GenePharma Co., Ltd. (Shanghai, China).
Twenty-four hours after transfection, the cells were
seeded in 96-well plates (5×103 cells/well) for the
following experiment. After cellular adhesion, freshly prepared
anticancer drug (cisplatin; Qilu Pharmaceutical Co., Ltd., Jiinan,
China) was added at a final concentration of 0.01-, 0.1-, 1- and
10-fold higher than the human peak plasma concentration as
previously described (24). The
peak serum concentration was 2.0 μg/ml for cisplatin as previously
described (26,27). Forty-eight hours after the
addition of the drug, cell viability was assessed by the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT)
assay. The absorbance at 490 nm (A490) of each well was read on a
spectrophotometer. The concentration at which the drug produced 50%
inhibition of growth (IC50) was estimated by the relative survival
curve. Three independent experiments were performed in
duplicate.
Dual luciferase activity assay
The 3′-UTR of human Bcl-2 cDNA containing the
putative target site for miR-503 was chemically synthesized and
inserted into the XbaI site, immediately downstream of the
luciferase gene in the pGL3-control vector (Promega, Madison, WI,
USA) by Biomics Biotechnologies Co., Ltd. Twenty-four hours prior
to transfection, the cells were plated at 1.5×105
cells/well in 24-well plates. Two hundred nanograms of
pGL3-Bcl-2-3′-UTR plus 80 ng pRL-TK (Promega) were transfected in
combination with 60 pmol of the miR-503 mimic or miRNA mimic
control using Lipofectamine 2000 (Invitrogen) according to the
manufacturer’s instructions as previously described (24). Luciferase activity was measured 24
h after transfection using the Dual Luciferase Reporter Assay
System (Promega). Firefly luciferase activity was normalized to
Renilla luciferase activity for each transfected well. Three
independent experiments were performed in duplicate.
Western blot analysis
A549/CDDP cells were plated in 6-well plates
(6×105 cells/well). Seventy-two hours after the
transfection of the miR-503 mimic or miRNA mimic control, the cells
were harvested and homogenized with lysis buffer. Total protein was
separated by denaturing on 10% SDS-polyacrylamide gel
electrophoresis. Western blot analysis was performed as previously
described (14). The primary
antibodies against Bcl-2 and α-tubulin were purchased from Cell
Signaling Technology (Danvers, MA, USA) and Bioworld Technology
(Minneapolis, MN, USA), respectively. Protein levels were
normalized to α-tubulin. Fold-changes were determined.
Apoptosis assay
Cells were plated in 6-well plates (6×105
cells/well). Twenty-four hours after transfection, the A549/CDDP
cells were treated with cisplatin at a final concentration of 5 and
20 μg/ml. Forty-eight hours following treatment with cisplatin,
flow cytometry was used to detect the apoptosis of the transfected
A549/CDDP cells by determining the relative amount of Annexin
V-FITC-positive-PI-negative cells as previously described (14).
Statistical analysis
Each experiment was repeated at least three times.
Numerical data are presented as the means ± SD. The differences
between means were analyzed using the Student’s t-test. All
statistical analyses were performed using SPSS 11.0 software (SPSS
Inc., Chicago, IL, USA). A P-value <0.01 was considered to
indicate a statistically significant difference.
Results
miR-503 is downregulated in A549/CDDP
cells compared with A549 cells
To determine whether miR-503 is involved in the
development of drug resistance in non-small cell lung cancer cells,
the level of miR-503 was analyzed in the A549/CDDP cells compared
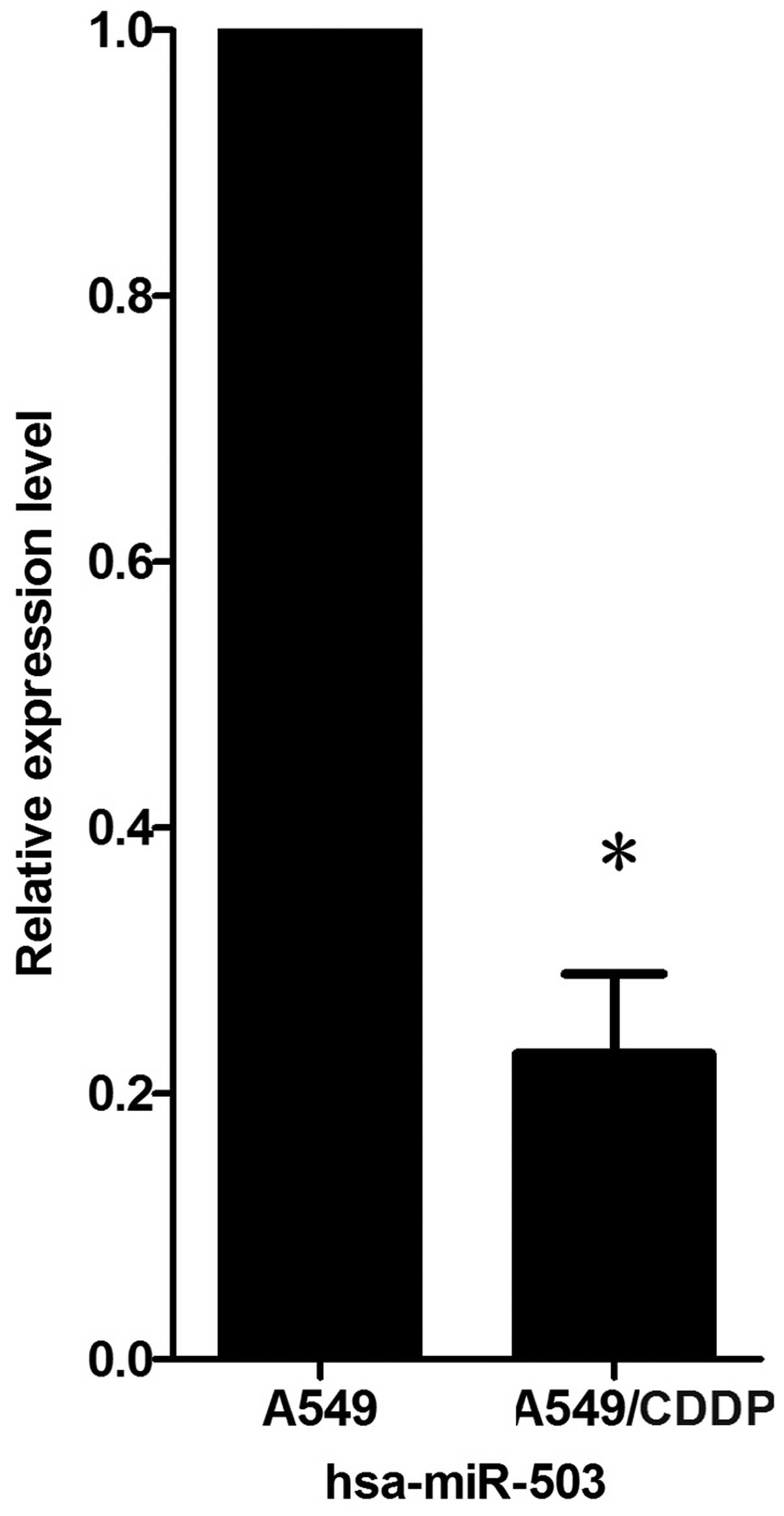
with their parent cell line, A549. Real-time PCR for miR-503
verified that miR-503 was significantly downregulated in the
A549/CDDP cells compared with the parental cells, A549, and the
decreased fold-change was 4.35±0.06 (Fig. 1).
miR-503 regulates the resistance of human
lung cancer cells to cisplatin
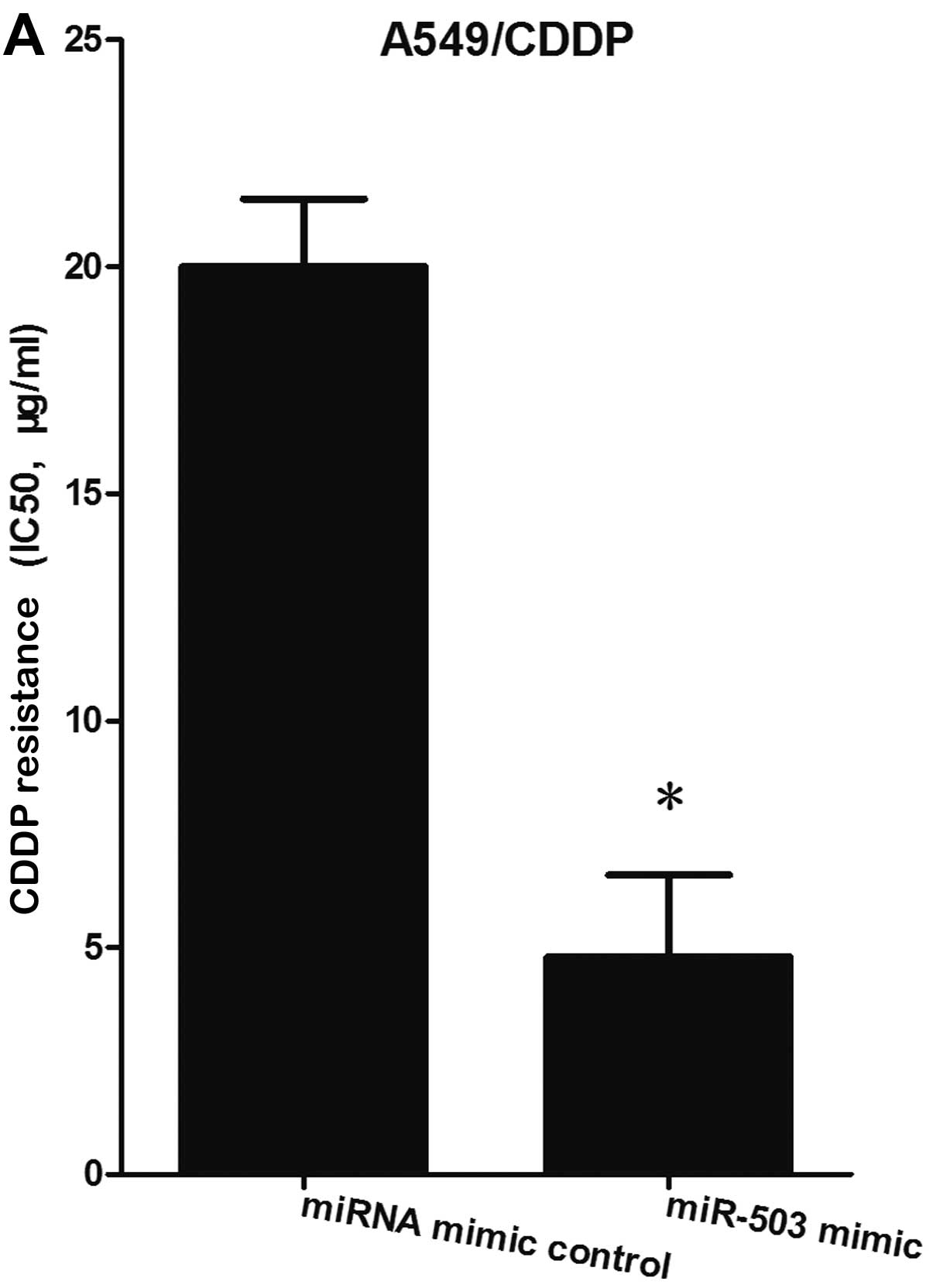
In the A549/CDDP cells, MTT assay revealed that the
cells transfected with the miR-503 mimic exhibited significantly
decreased resistance to cisplatin compared with the miRNA mimic
control-transfected cells (Fig.
2A); whereas in the A549 cells, the cells transfected with the
miR-503 inhibitor exhibited significantly enhanced resistance to
cisplatin compared with the miRNA inhibitor control-transfected
cells (Fig. 2B). These results
suggest that miR-503 regulates the resistance of human lung cancer
cells to cisplatin.
Anti-apoptotic Bcl-2 is the target gene
of miR-503
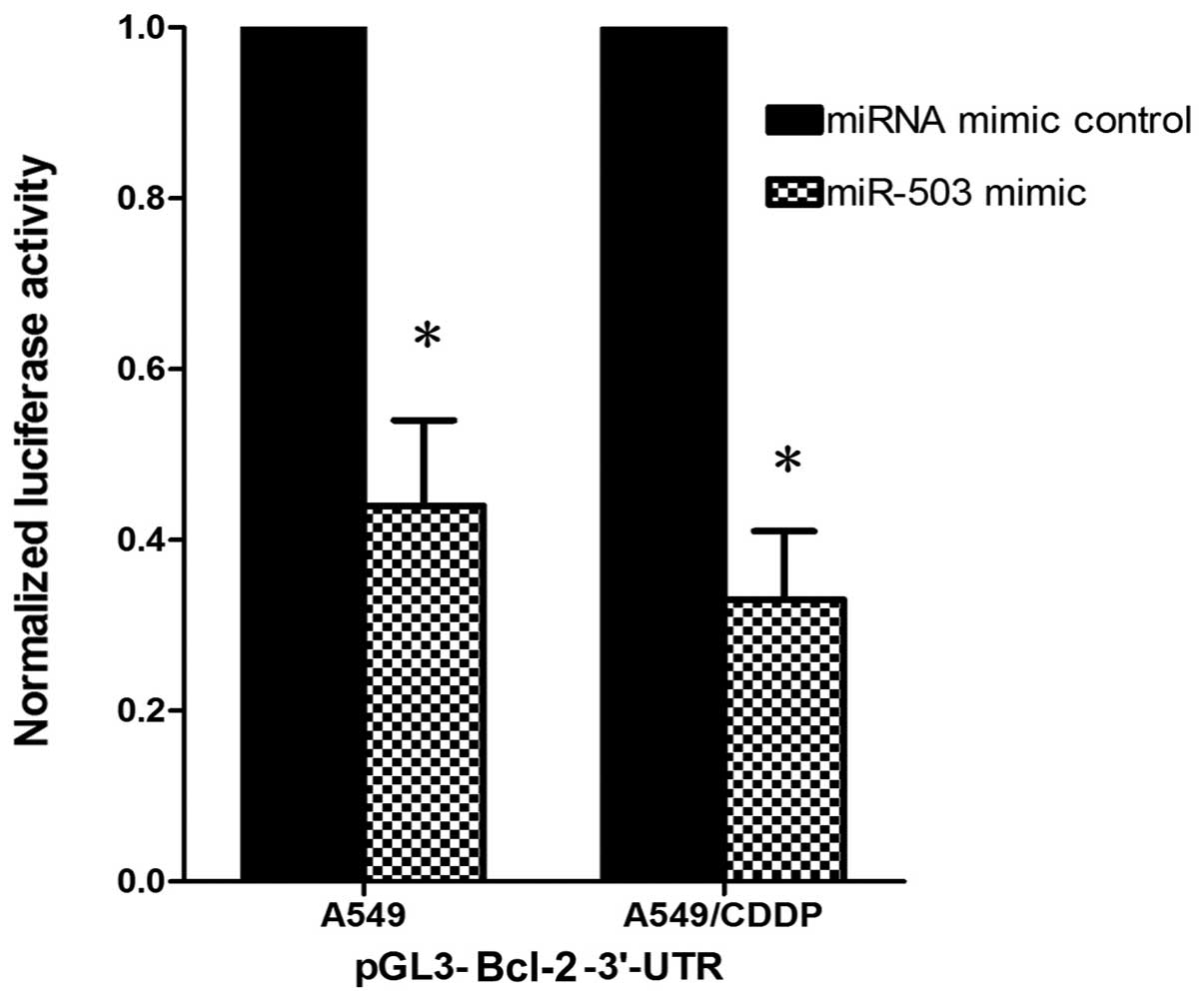
TargetScan Human 5.1 (http://www.targetscan.org) predicted that Bcl-2 is the
target gene of miR-503 conserved between different species. To
determine whether Bcl-2 is the target gene of miR-503, we
constructed a luciferase reporter vector with the putative Bcl-2
3′-UTR target site for miR-503 downstream of the luciferase gene
(pGL3-Bcl-2-3′-UTR). The luciferase reporter vector together with
the miR-503 mimic or the miRNA mimic control was transfected into
the A549 and A549/CDDP cells. In both cell lines, a significant
decrease in relative luciferase activity was observed when the
pGL3-Bcl-2-3′-UTR vector was co-transfected with the miR-503 mimic
but not with the miRNA mimic control, suggesting that Bcl-2 is the
target gene of miR-503 (Fig.
3).
miR-503 modulates resistance to cisplatin
by suppressing Bcl-2 protein expression
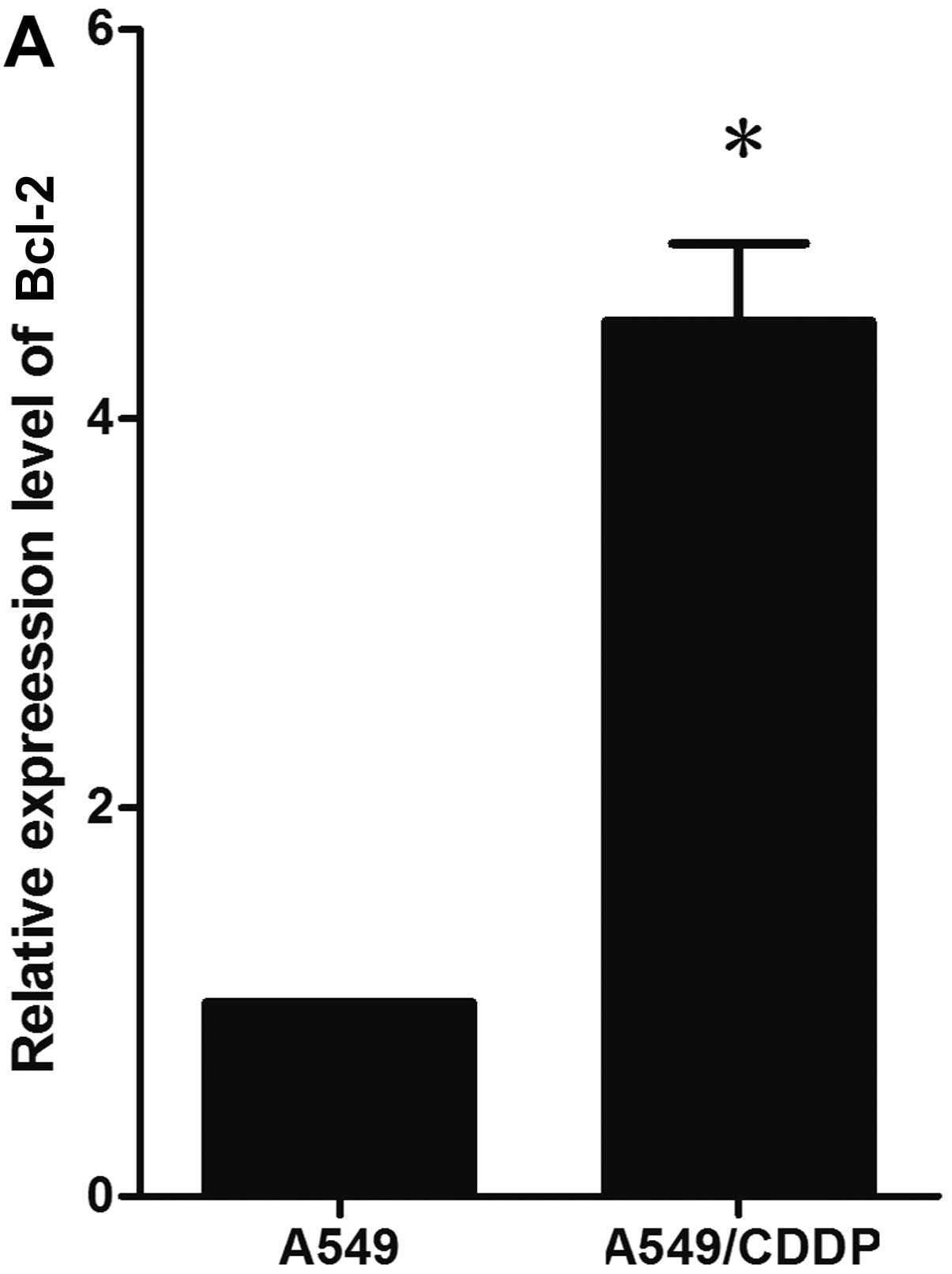
A noteworthy observation was that the decreased
expression of miR-503 in the A549/CDDP cells was concurrent with
the overexpression of Bcl-2 protein, compared with the parental
A549 cells in our study (Fig. 4A and
C). Since the anti-apoptotic Bcl-2 protein is the target of
miR-503, we hypothesized that miR-503 may modulate the drug
resistance of cancer cells by suppressing Bcl-2 protein expression.
To confirm our hypothesis, we transfected the miR-503 mimic and the
control miRNA mimic into the A549/CDDP cells and detected changes
in Bcl-2 expression levels. In the A549/CDDP cells, 72 h after
transfection, western blot analysis revealed a significantly
decreased Bcl-2 protein expression level in the miR-503
mimic-transfected cells compared with the miRNA mimic
control-transfected cells (Fig. 4B
and C). These results demonstrate that miR-503 modulates the
resistance of cancer cells to cisplatin, at least in part by
suppressing Bcl-2 protein expression.
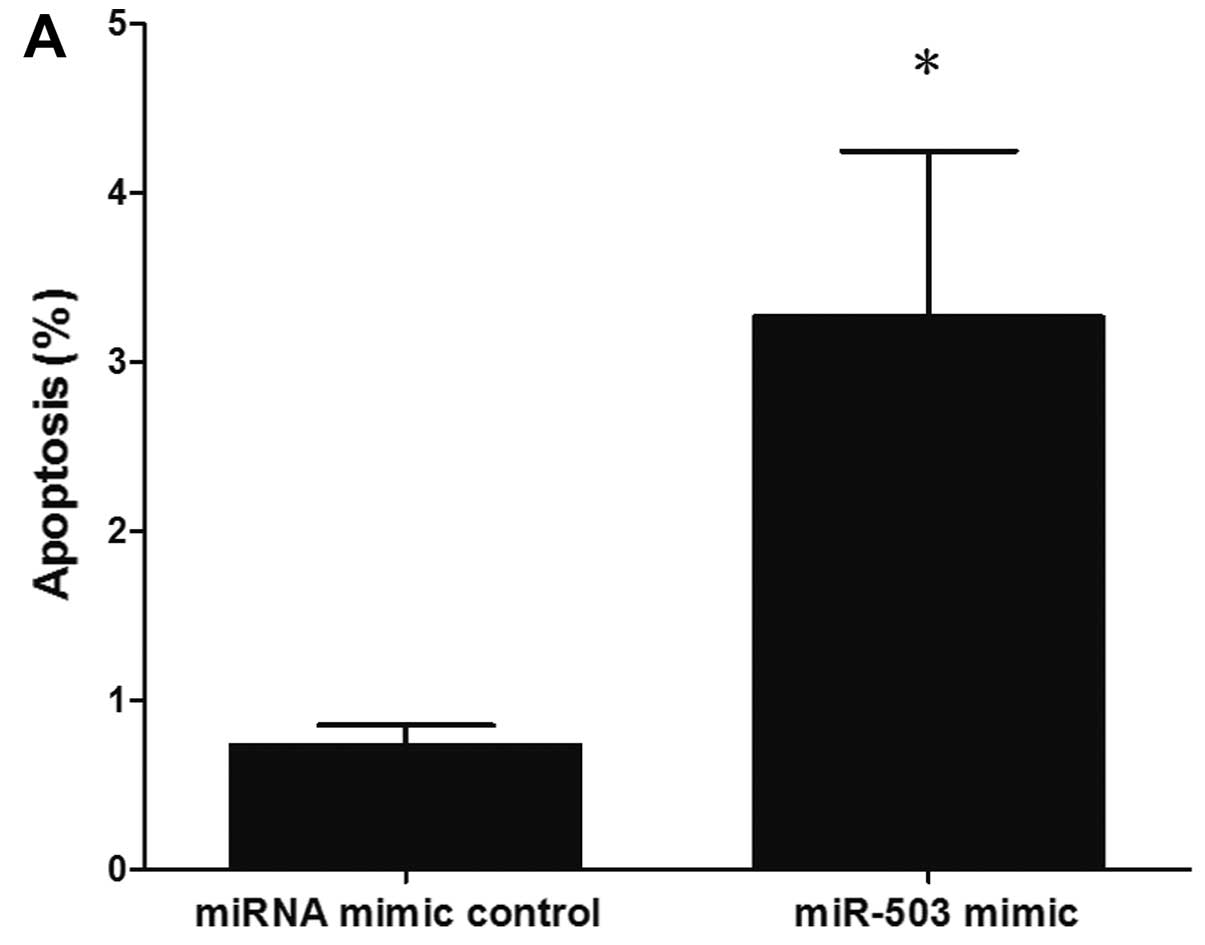
miR-503 sensitizes A549/CDDP cells to
CDDP-induced apoptosis
The development of drug resistance in various cancer
cells has been linked to a reduced susceptibility to drug-induced
apoptosis, which has been shown to be a consequence of the
overexpression of anti-apoptotic proteins, such as Bcl-2 and Bcl-xL
(10,28,29). Since miR-503 regulates the drug
resistance of cancer cells, at least in part by suppressing Bcl-2
protein expression, considering the well-characterized role of
Bcl-2 in apoptosis and drug resistance, we hypothesized that
miR-503 plays a role in the development of drug resistance, at
least in part through the modulation of apoptosis by targeting
Bcl-2. To confirm this hypothesis, we evaluated cisplatin-induced
apoptosis following the transfection of A549/CDDP cells with the
miR-503 mimic or the miRNA mimic control. In the A549/CDDP cells, a
marked increase in apoptosis, as assessed by flow cytometry, was
observed in the miR-503 mimic-transfected cells following treatment
with cisplatin, compared with the miRNA mimic control-transfected
cells (Fig. 5).
Discussion
Platinum-based drugs, cisplatin in particular, are
the major clinical treatment drugs for non-small cell lung cancer
at present. Despite tremendous efforts, cisplatin treatment often
results in the development of drug resistance, leading to
therapeutic failure. Therefore, cisplatin drug resistance has
become an important clinical issue which needs to be resolved. It
has been suggested that the mechanisms of cisplatin resistance
involve reduced intracellular cisplatin accumulation, increased
inactivation of cisplatin by thiol-containing molecules, enhanced
DNA damage repair and the inhibition of transmitted DNA damage
recognition to the apoptotic pathway (30). Among these, the acquired imbalance
of apoptotic pathways is thought to be one of the most important
mechanisms involved in drug resistance. Several apoptotic
inhibitors have been associated with platinum resistance, including
X-linked inhibitor of apoptosis (XIAP), Bcl-2 and Bcl-xL (31–34). Bcl-2 is an important survival
factor which suppresses apoptosis in a variety of cell systems and
regulates cell death by controlling the mitochondrial membrane
permeability. The resistance of cancer cells to cisplatin may be,
in some cases, caused by the overexpression of Bcl-2 (31). Consistent with this, in our study,
we found that the anti-apoptotic protein, Bcl-2, was upregulated in
the cisplatin-resistant A549/CDDP cells compared with the A549
cells.
Since defective apoptosis may contribute to
cisplatin drug resistance, miRNAs may modulate the drug sensitivity
of cancer cells, at least in part through this mechanism (14,24,35). Of note, miR-503 was downregulated
in the A549/CDDP cells and the elevated levels of miR-503 not only
downregulated the expression of Bcl-2 protein but also increased
the sensitivity of these cells to cisplatin. The data presented in
this study, provide evidence that miR-503 may be involved in the
development of the resistance of non-small cell lung cancer cells
to cisplatin by targeting Bcl-2.
miR-503 is located on chromosomal band Xq26.3, based
on the sequence AGCAGC starting at the second nucleotide from the
5′ end of the mature (~22 nt, single-stranded) miRNA, a motif which
is referred to as AGC X2. As shown in previous studies, miR-503 is
differentially expressed in different types of tumors, suggesting
that the expression of miR-503 is tissue- specificity (16–20). In the present study, we found that
miR-503 was downregulated in the cisplatin resistant non-small cell
lung cancer cells. A previous study demonstrated that epigenetic
alterations promote the dysregulation of miRNAs (36). Among these, the DNA methylation of
CpG islands has been observed in the promoter region of miRNAs with
tumor suppressor functions in human cancer, such as miRNAs miR-497,
miR-127 and miR-1 (37–39). According to the data from the UCSC
database (http://genome.ucsc.edu/cgi-bin/hgTracks?hgHubConnect.destUrl=..%2Fcgi-bin%2FhgTracks&clade=mammal&org=Human&db=hg19&position=miR-503&hgt.positionInput=miR-503&hgt.suggestTrack=knownGene&Submit=submit&hgsid=298724897),
the DNA methylation of CpG islands in the promoter region may lead
to the downregulation of miR-503 in drug-resistant cells. However,
further studies are required to elucidate the underlying
mechanisms.
In conclusion, the data presented in this study
provide evidence of the involvement of miR-503 in the development
of the resistance of human non-small cell lung cancer cells to
cisplatin. Hsa-miR-503 sensitizes human drug-resistant lung cancer
cells to cisplatin, at least in part by targeting Bcl-2 expression.
Our findings may contribute to the further understanding of the
regulation of drug resistance in cancer cells. Therapeutic
strategies targeting the miRNAs associated with drug resistance,
such as hsa-miR-503, may prove to be a promising approach for the
development of safe and effective clinical treatments. However, it
should be noted that our data were derived from cell lines which
have been removed from their in vivo context and cannot be
considered accurate surrogates for clinical tumors. Thus, further
clinical studies are warranted to assess the role of hsa-miR-503
in vivo.
Acknowledgements
The authors are grateful for the funding provided by
the National Natural Science Foundation of China (grant nos.
81171908 and 81201705) and the Research and Innovation Project for
College Graduates of Jiangsu Province (grant no. JX22013193).
References
|
1
|
Szakács G, Paterson JK, Ludwig JA, et al:
Targeting multidrug resistance in cancer. Nat Rev Drug Discov.
5:219–234. 2006.
|
|
2
|
Zhang K, Mack P and Wong KP:
Glutathione-related mechanisms in cellular resistance to anticancer
drugs. Int J Oncol. 12:871–882. 1998.PubMed/NCBI
|
|
3
|
Johnstone RW, Ruefli AA and Lowe SW:
Apoptosis: a link between cancer genetics and chemotherapy. Cell.
108:153–164. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rabik CA and Dolan ME: Molecular
mechanisms of resistance and toxicity associated with platinating
agents. Cancer Treat Rev. 33:9–23. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fojo T: Multiple paths to a drug
resistance phenotype: mutations, translocations, deletions and
amplification of coding genes or promoter regions, epigenetic
changes and microRNAs. Drug Resist Updat. 10:59–67. 2007.
View Article : Google Scholar
|
|
6
|
Glasspool RM, Teodoridis JM and Brown R:
Epigenetics as a mechanism driving polygenic clinical drug
resistance. Br J Cancer. 94:1087–1092. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sharma SV, Lee DY, Li B, et al: A
chromatin-mediated reversible drug-tolerant state in cancer cell
subpopulations. Cell. 141:69–80. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee RC, Feinbaum RL and Ambros V: The
C. elegans heterochronic gene lin-4 encodes small RNAs with
antisense complementarity to lin-14. Cell. 75:843–854. 1993.
|
|
9
|
Garzon R, Marcucci G and Croce CM:
Targeting microRNAs in cancer: rationale, strategies and
challenges. Nat Rev Drug Discov. 9:775–789. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen GQ, Zhao ZW, Zhou HY, et al:
Systematic analysis of microRNA involved in resistance of the MCF-7
human breast cancer cell to doxorubicin. Med Oncol. 27:406–415.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bourguignon LY, Spevak CC, Wong G, et al:
Hyaluronan-CD44 interaction with protein kinase C(epsilon) promotes
oncogenic signaling by the stem cell marker Nanog and the
production of microRNA-21, leading to down-regulation of the tumor
suppressor protein PDCD4, anti-apoptosis, and chemotherapy
resistance in breast tumor cells. J Biol Chem. 284:26533–26546.
2009.
|
|
12
|
Li Y, Li W, Yang Y, et al: MicroRNA-21
targets LRRFIP1 and contributes to VM-26 resistance in glioblastoma
multiforme. Brain Res. 1286:13–18. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu K, Liang X, Cui D, et al: miR-1915
inhibits Bcl-2 to modulate multidrug resistance by increasing
drug-sensitivity in human colorectal carcinoma cells. Mol Carcinog.
52:70–78. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xia L, Zhang D, Du R, et al: miR-15b and
miR-16 modulate multidrug resistance by targeting BCL2 in human
gastric cancer cells. Int J Cancer. 123:372–379. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen J, Tian W, Cai H, et al:
Down-regulation of microRNA-200c is associated with drug resistance
in human breast cancer. Med Oncol. 29:2527–2534. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou J and Wang W: Analysis of microRNA
expression profiling identifies microRNA-503 regulates metastatic
function in hepatocellular cancer cell. J Surg Oncol. 104:278–283.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lu YC, Chen YJ, Wang HM, et al: Oncogenic
function and early detection potential of miRNA-10b in oral cancer
as identified by microRNA profiling. Cancer Prev Res (Phila).
5:665–674. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Özata DM, Caramuta S, Velázquez-Fernández
D, et al: The role of microRNA deregulation in the pathogenesis of
adrenocortical carcinoma. Endocr Relat Cancer. 18:643–655.
2011.PubMed/NCBI
|
|
19
|
Corbetta S, Vaira V, Guarnieri V, et al:
Differential expression of microRNAs in human parathyroid
carcinomas compared with normal parathyroid tissue. Endocr Relat
Cancer. 17:135–146. 2010. View Article : Google Scholar
|
|
20
|
Zhao JJ, Yang J, Lin J, et al:
Identification of miRNAs associated with tumorigenesis of
retinoblastoma by miRNA microarray analysis. Childs Nerv Syst.
25:13–20. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cui YH, Xiao L, Rao JN, et al: miR-503
represses CUG-binding protein 1 translation by recruiting CUGBP1
mRNA to processing bodies. Mol Biol Cell. 23:151–162. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jiang Q, Feng MG and Mo YY: Systematic
validation of predicted microRNAs for cyclin D1. BMC Cancer.
9:1942009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cheng G, Sun S, Wang Z and Jin S:
Investigation of the interaction between the miR-503 and CD40 genes
in irradiated U937 cells. Radiat Oncol. 7:382012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhu W, Zhu D, Lu S, et al: miR-497
modulates multidrug resistance of human cancer cell lines by
targeting BCL2. Med Oncol. 29:384–391. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yamaue H, Tanimura H, Noguchi K, et al:
Chemosensitivity testing of fresh human gastric cancer with highly
purified tumor cells using the MTT assay. Br J Cancer. 66:794–799.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yamaue H, Tani M, Onishi H, et al:
Locoregional chemotherapy for patients with pancreatic cancer
intra-arterial adjuvant chemotherapy after pancreatectomy with
portal vein resection. Pancreas. 25:366–372. 2002. View Article : Google Scholar
|
|
28
|
Wang S, Yang D and Lippman ME: Targeting
Bcl-2 and Bcl-XL with nonpeptidic small-molecule antagonists. Semin
Oncol. 30(Suppl 16): S133–S142. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Reed JC: Drug insight: cancer therapy
strategies based on restoration of endogenous cell death
mechanisms. Nat Clin Prac Oncol. 3:388–398. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tanida S, Mizoshita T, Ozeki K, et al:
Mechanisms of cisplatin-induced apoptosis and of cisplatin
sensitivity: potential of BIN1 to act as a potent predictor of
cisplatin sensitivity in gastric cancer treatment. Int J Surg
Oncol. 2012:8628792012.PubMed/NCBI
|
|
31
|
Beale PJ, Rogers P, Boxall F, et al: BCL-2
family protein expression and platinum drug resistance in ovarian
carcinoma. Br J Cancer. 82:436–440. 2000.PubMed/NCBI
|
|
32
|
Yu L and Wang Z: Difference in expression
of Bcl-2 and Bcl-xl genes in cisplatin-sensitive and
cisplatin-resistant human in ovarian cancer cell lines. J Huazhong
Univ Sci Technolog Med Sci. 24:151–153. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dong Z and Wang J: Hypoxia selection of
death-resistant cells. A role for Bcl-X(L). J Biol Chem.
279:9215–9221. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Williams J, Lucas PC, Griffith KA, et al:
Expression of Bcl-xL in ovarian carcinoma is associated with
chemoresistance and recurrent disease. Gynecol Oncol. 96:287–295.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhu W, Shan X, Wang TS, et al: miR-181b
modulates multidrug resistance by targeting BCL2 in human cancer
cell lines. Int J Cancer. 127:2520–2529. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Baylin SB: DNA methylation and gene
silencing in cancer. Nat Clin Pract Oncol. 2(Suppl 1): S4–S11.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li D, Zhao Y, Liu C, et al: Analysis of
MiR-195 and MiR-497 expression, regulation and role in breast
cancer. Clin Cancer Res. 17:1722–1730. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Saito Y, Liang G, Egger G, et al: Specific
activation of microRNA-127 with downregulation of the
proto-oncogene BCL6 by chromatin-modifying drugs in human cancer
cells. Cancer Cell. 9:435–443. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Datta J, Kutay H, Nasser MW, et al:
Methylation mediated silencing of MicroRNA-1 gene and its role in
hepatocellular carcinogenesis. Cancer Res. 68:5049–5058. 2008.
View Article : Google Scholar : PubMed/NCBI
|