Introduction
Epidermal growth factor-like domain (EGFL), an
evolutionarily conserved protein domain, is found in many
vertebrate proteins that are involved in several essential cellular
activities, such as blood coagulation, fibrinolysis, cell adhesion
and development (1). EGFL8, a
newly identified member of the EGFL family, was originally
identified as a paralog of EGFL7 by a BLAST search of the mouse
genome (2). EGFL7 has recently
emerged as a secreted angiogenic signaling molecule, which enhances
vasculogenesis and angiogenesis by promoting endothelial cell
adhesion, proliferation, chemoattraction, migration, sprouting and
invasion (3–7). Furthermore, EGFL7 stimulates
embryonic stem cell proliferation, but inhibits the proliferation
of adult neuronal stem cells, demonstrating its differential
mechanisms of action during cell proliferation between different
stem cell types (7,8).
However, little is known about the characterization
and the biological role of EGFL8. A structural analysis of the
EGFL8 protein predicted that it is a secretory protein (9). EGF-like repeats, located in the
extracellular domains of EGFL8 and Notch receptors, play a central
role in controlling the Notch signaling pathway (10). As recently demonstrated, EGFL8
expression was significantly decreased in patients with colorectal
and gastric cancer, suggesting that EGFL8 may have a distinct
expression pattern and mechanism of action in cancer progression
(11,12).
We have previously demonstated that the mouse EGFL8
gene plays a functional role in T-cell development in a
gain-of-function and loss-of-function study with an EGFL8 gene
overexpressing vector and EGFL8 siRNA (13). Based on these previous findings,
in the present study, we investigated the functional role of the
EGFL8 protein in mouse thymocytes and thymic epithelial cells
(TECs), which are pivotal for both T-cell development and T-cell
repertoire selection (14). To
identify the potential importance of the EGFL8 protein, in this
study, we designed and optimized a protocol to produce and purify
large amounts of mouse recombinant EGFL8 (rEGFL8) protein. Using
high-purity mouse rEGFL8, we demonstrate that EGFL8 inhibits the
survival and proliferation of thymocytes during T-cell development.
In addition, the mechanistic regulatory role of EGFL8 in mouse
thymocytes and TECs was determined.
Materials and methods
Cell line and cell culture
The mouse thymic cortical epithelial reticular cells
(1308.1) were kindly provided by Dr Barbara B. Knowles (The Jackson
Laboratory) (15). The cells were
cultured in Dulbecco’s modified Eagle’s medium (DMEM), containing
10% (v/v) fetal bovine serum (FBS), 100 U/ml penicillin and 100
μg/ml streptomycin (all from Gibco Life Technologies, Grand Island,
NY, USA) at 37°C in 5% CO2 incubator.
Cloning of mouse EGFL8 gene
The coding sequences of mouse EGFL8 total cDNA were
isolated and amplified by PCR and cloned into pcDNA3.1 (Life
Technologies, Carlsbad, CA, USA). They were amplified by PCR using
the following oligonucleotide primers: EGFL8 forward, 5′-TTT CAA
AGA GAG TTT GGG AGT G-3′ and reverse, 5′-CAC CAC GTG T′T CTG TGG
TA-3′ to create the Nco1 and Xho1 restriction sites
at the start and stop codon sites. The PCR product was cloned into
the pET28a vector, which carries a C-terminal
His·Tag/thrombin/T7·Tag configuration plus an optional C-terminal
His·Tag sequence.
Expression of mouse EGFL8 gene
The E. coli bacteria were cultured for 2 h,
stimulated by the addition of 0.2 mM IPTG, and then cultured for an
additional 4 h. The bacteria were harvested immediately by
centrifugation at 6,000 rpm for 8–10 min, and the pellet was either
frozen at −80°C until purification or directly resuspended in 60 ml
lysis buffer [50 mM Tris-HCl (pH 8.0), 100 mM NaCl and 5 mM EDTA].
Subsequently, 0.5% Triton X-100, 0.1 mM phenylmethylsulfonyl
fluoride (PMSF) and 1 mM dithiothreitol (DTT) were added to this
pellet. Subsequently, the pellet was sonicated and centrifuged at
12,000 rpm for 15 min. The supernatant was discarded and the pellet
was resuspended in lysis buffer. The following steps were repeated
as above except for the addition of 10 mM MgCl2, 0.01
mg/ml DNase and 0.1 mg/ml lysozyme to the Triton X-100, PMSF and
DTT mixture. The pellet mixture was incubated for 20 min at room
temperature, sonicated and centrifuged as described above. The
supernatant was discarded and the pellet was resuspended in 60 ml
lysis buffer. Subsequently, only PMSF and DTT were added, sonicated
and centrifuged as described above. The supernatant was discarded,
and the pellet was resuspended in 40 ml of 8 M urea [100 mM
Tris-HCl (pH 8.0), 50 mM glycine]. The pellet was then slowly
shaken for 1 h at room temperature to completely solubilize the
proteins. After a short centrifugation to remove non-solubilized
proteins, the protein concentration was determined by a NanoDrop
2000 spectrophotometer (Thermo Scientific, Wilmington, DE, USA).
The proteins (1–2 mg/ml) were dialyzed through a dialysis bag. Two
liters of dialysis buffers [20 mM Tris-HCl (pH 8.0), 150 mM NaCl
and 0.1 mM DTT] were used at 4°C for 12 to 15 h and the process was
repeated. The third dialysis buffer was used under the same
conditions except for the absence of DTT.
Ni-NTA column purification
Purification of His-tagged proteins for the
recombinant protein was then performed. The column was washed with
distilled water and then with 6X Ni-NTA washing buffer. The protein
mixture was added to the Ni-NTA column and slowly passed through
the column. The bound EGFL8 protein was eluted with 6X Ni-NTA
elution buffer. The eluted protein was subjected to sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) after
determining its concentration.
Mass spectrometry
The rEGFL8 protein was subjected to 10% SDS-PAGE,
stained with Coomassie blue, and the protein bands were excised
from the gel. The excised gel pieces were transferred to
microcentrifuge tubes containing 0.5 ml of distilled water. The
protein was then digested with trypsin. The single band was used
for matrix-assisted laser desorption/ionization time-of-flight mass
spectrometry (MALDI-TOF-MS/MS).
Experimental animals and treatment with
rEGFL8 in vivo
C57BL/6 mice (Dae Han Bio Link, Chungbuk, Korea)
were intravenously (i.v.) injected with rEGFL-8 (100 μg) and were
sacrificed 12 h, 1, 2 and 3 days after injection. Animal care and
all experimental procedures were conducted in accordance with the
‘Guide for Animal Experiments’ published by the Korean Academy of
Medical Sciences.
Proliferation and apoptosis assay
The mice were injected intraperitonealy (i.p.) with
BrdU (BD Biosciences Pharmingen, San Diego, CA, USA) or with PBS.
The thymus was isolated by dissection 2 h after BrdU injection. The
isolated cells were stained for CD4, CD8, CD25, CD44 and BrdU
according to the BrdU Flow kit manual (BD Biosciences Pharmingen).
For the apoptosis assay, the isolated cells were stained for CD4,
CD8, CD25, CD44 and Annexin V according to the Annexin V detection
kit protocol (BD Biosciences Pharmingen).
Flow cytometric analysis
The following fluorochrome-conjugated monoclonal
antibodies (mAbs) were purchased from BD Biosciences Pharmingen:
Pacific Blue-conjugated anti-CD4 (RM4-5), allophycocyanin
(APC)-Cy7-conjugated anti-CD8 (53-6.7), PE-labeled anti-CD25 (PC61)
and APC anti-CD44 (IM7). Flow cytometric analysis was performed
using a FACSCanto II flow cytometer (BD Biosciences Pharmingen),
and the acquired data were analyzed using the FlowJo software (Tree
Star, Ashland, OR, USA).
Electron microscopy
For electron microscopic investigation, all
specimens were processed according to a standard procedure. The
ultra-thin sections were examined under a JEOL-1200 EXII
transmission electron microscope.
Western blot analysis
The TECs were incubated with 100 ng/ml rEGFL8 for 12
h. The thymocytes were isolated from the mice injected with 100 μg
of rEGFL8 i.v., after 12 h, 1 and 2 days. Following treatment with
rEGFL8, the thymocytes and TECs were washed with cold PBS and the
total proteins were then extracted from the cultured cells using a
protein extraction solution (iNtRON Biotechnology, Seongnam, Korea)
supplemented with a protease inhibitor mixture (Sigma-Aldrich, St.
Louis, MO, USA). Protein concentrations were measured using the
Bradford protein assay kit (Bio-Rad, Hercules, CA, USA). Anti-Hes-1
(sc-166378), anti-Hey-1 (sc-28746) (both from Santa Cruz
Biotechnology, Santa Cruz, CA, USA) and anti-β-actin (Abcam,
Cambridge, UK) antibodies were used for immunoblot analysis. The
goat anti-mouse IgG-HRP (7076) and goat anti-rabbit IgG-HRP (7074)
(both from Cell Signaling Technology, Danvers, MA, USA) were used
as the secondary antibodies. Immunoreactivity was detected and
quantified using a LAS-3000 imaging system (Fujifilm, Tokyo,
Japan).
Statistical analysis
Data are expressed as the means ± SD. Statistical
analyses were performed using the Student’s t-test. A value of
P<0.05 was considered to indicate a statistically significant
difference.
Results
Expression and purification of mouse
rEGFL8 protein
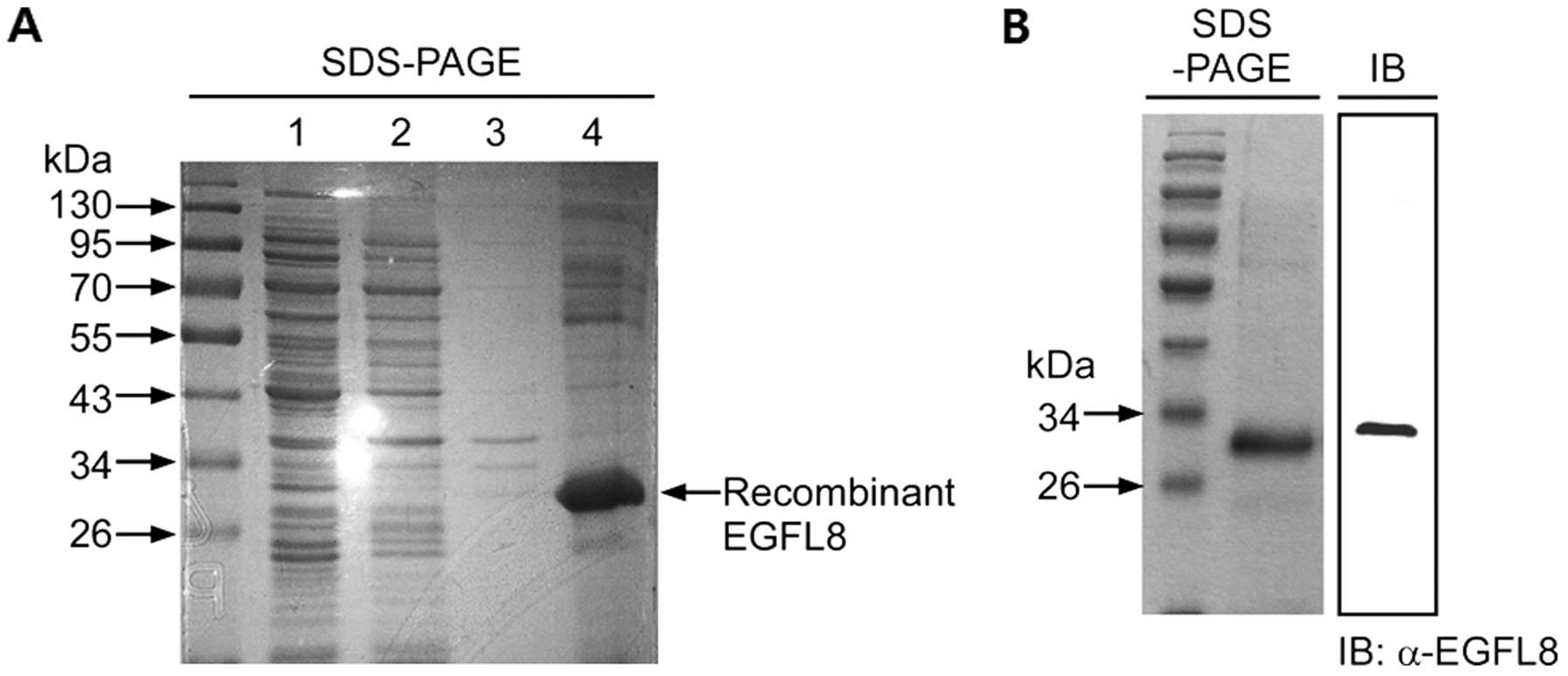
rEGFL8 was expressed in E. coli and purified.
The harvested rEGFL8 protein was stained with Coomassie blue in 10%
SDS-PAGE (Fig. 1A). We produced
high-purity proteins after the third lysis step, suggesting that
most of the secreted fusion proteins and most of the other
bacterial proteins were removed by the lysis procedure. The
dialysis and refolding steps were performed in the presence of
urea, mainly as EGFL8 is prone to precipitation at high
concentrations and is also prone to protein interactions with the
exchange groups on the columns. However, to recover the biological
activity of rEGFL8, urea needs to be removed and the protein should
be refolded properly. The dialysis protein was analyzed on SDS-PAGE
in comparison with cell lysates and supernatants to confirm the
successful recovery of the recombinant protein after solubilization
with 8 M urea. Fig. 1A
illustrates an exact size of the desired protein for this study
with no contaminated product from the E. coli cells. This
result also verified that most or all of the inclusion bodies of
rEGFL8 protein were solubilized in 8 M urea. The Ni-NTA-purified
protein was loaded onto 10% SDS-PAGE for total protein analysis
(Fig. 1B). The total Ni-NTA
column-purified protein showed a single protein band with a
specific size, which confirmed the successful rEGFL8 protein
purification through the Ni-NTA column. The Ni-NTA-purified protein
was also analyzed by western blot analysis for further confirmation
(Fig. 1B). The western blot
analysis result also showed a single and specific band of rEGFL8
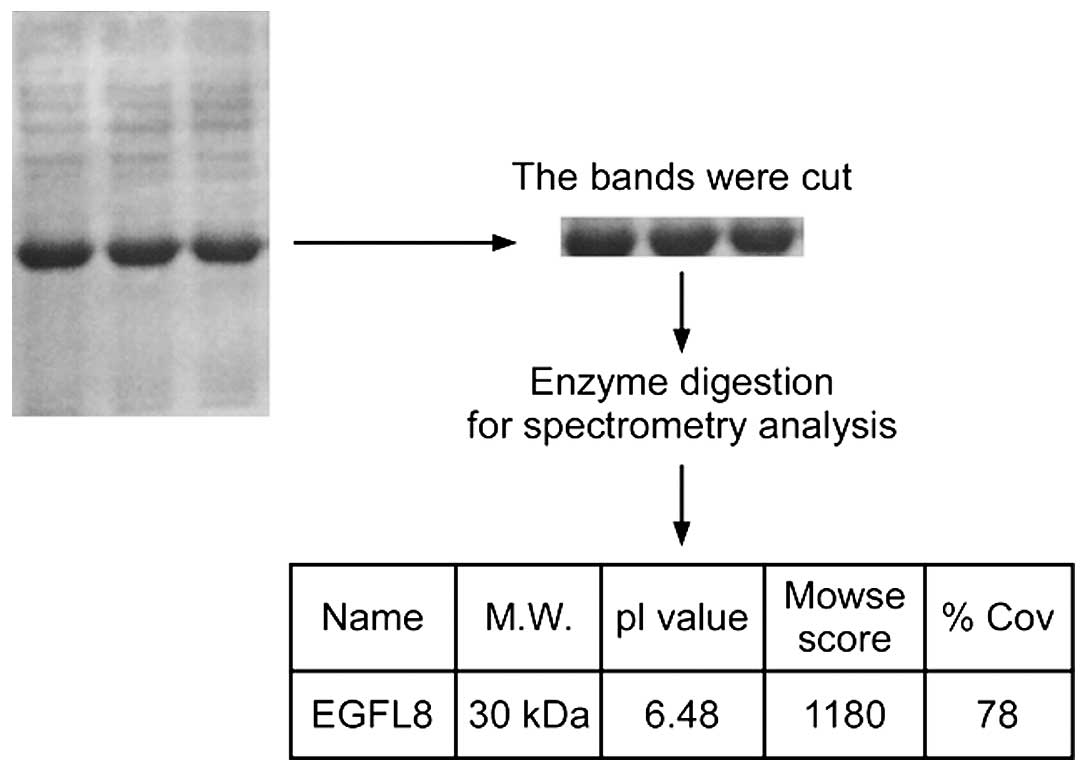
protein. For further characterization, the rEGFL8 protein was run
on 10% SDS-PAGE and the band was cut and trypsinized before loading
onto the spectrometer (Fig. 2).
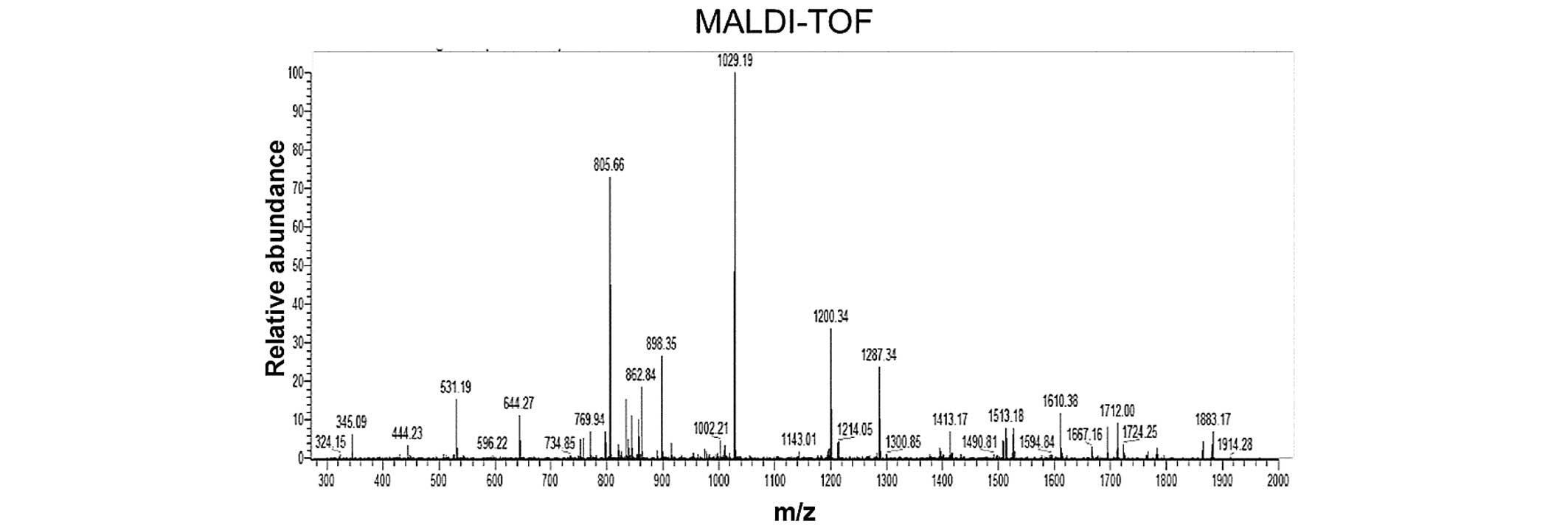
All liquid chromatography (LC)-MS/MS data were submitted to a
Mascot search; the peaks from the purified rEGFL8 protein sample
are shown in Fig. 3, with the
masses indicated on the top of each peak. The identified molecular
weight of purified rEGFL8 measured by MS was 30.09 kDa, which was
almost identical to the theoretical value.
Negative effects of rEGFL8 on the weight
of the thymus and the number of thymocytes
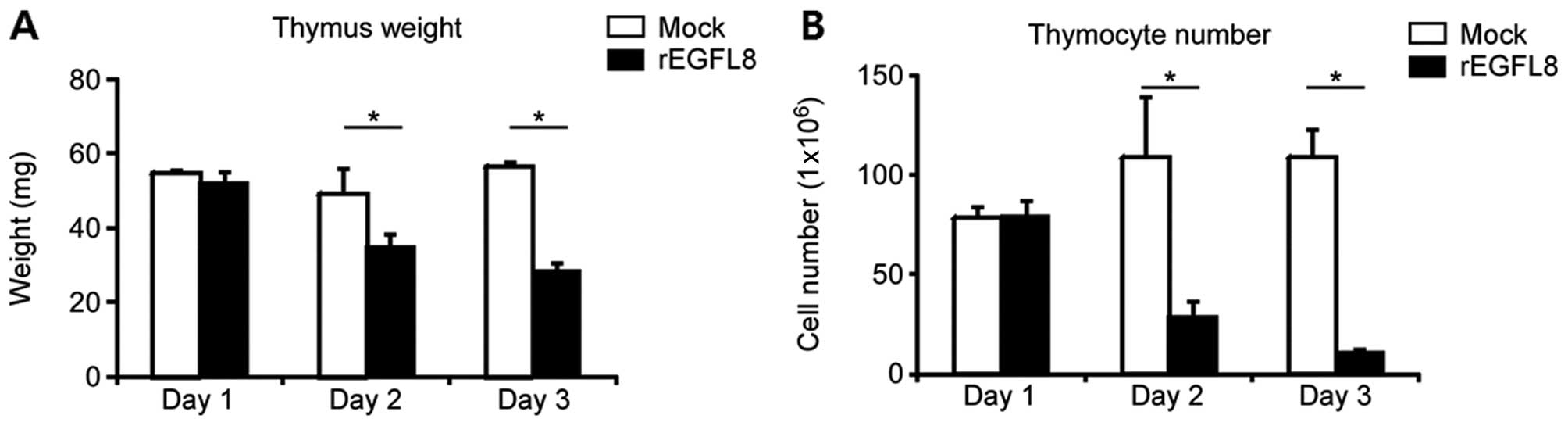
To investigate the biological activity of rEGFL8
protein in mouse thymocytes, 100 μg of rEGFL8 protein were injected
i.v. into the mice, and the weight of the thymus and the total
number of thymocytes were determined (Fig. 4). The weight of the thymus and the
total number of thymocytes were significantly decreased after the
rEGFL8 injection. The weight of the thymus and the number of
thymocytes diminished from 1 day after rEGFL8 injection, and these
effects reached a peak value at 3 days. The observation that the
number of thymocytes markedly decreased after the administration of
rEGFL8 raised the question of whether the subsets of thymocytes
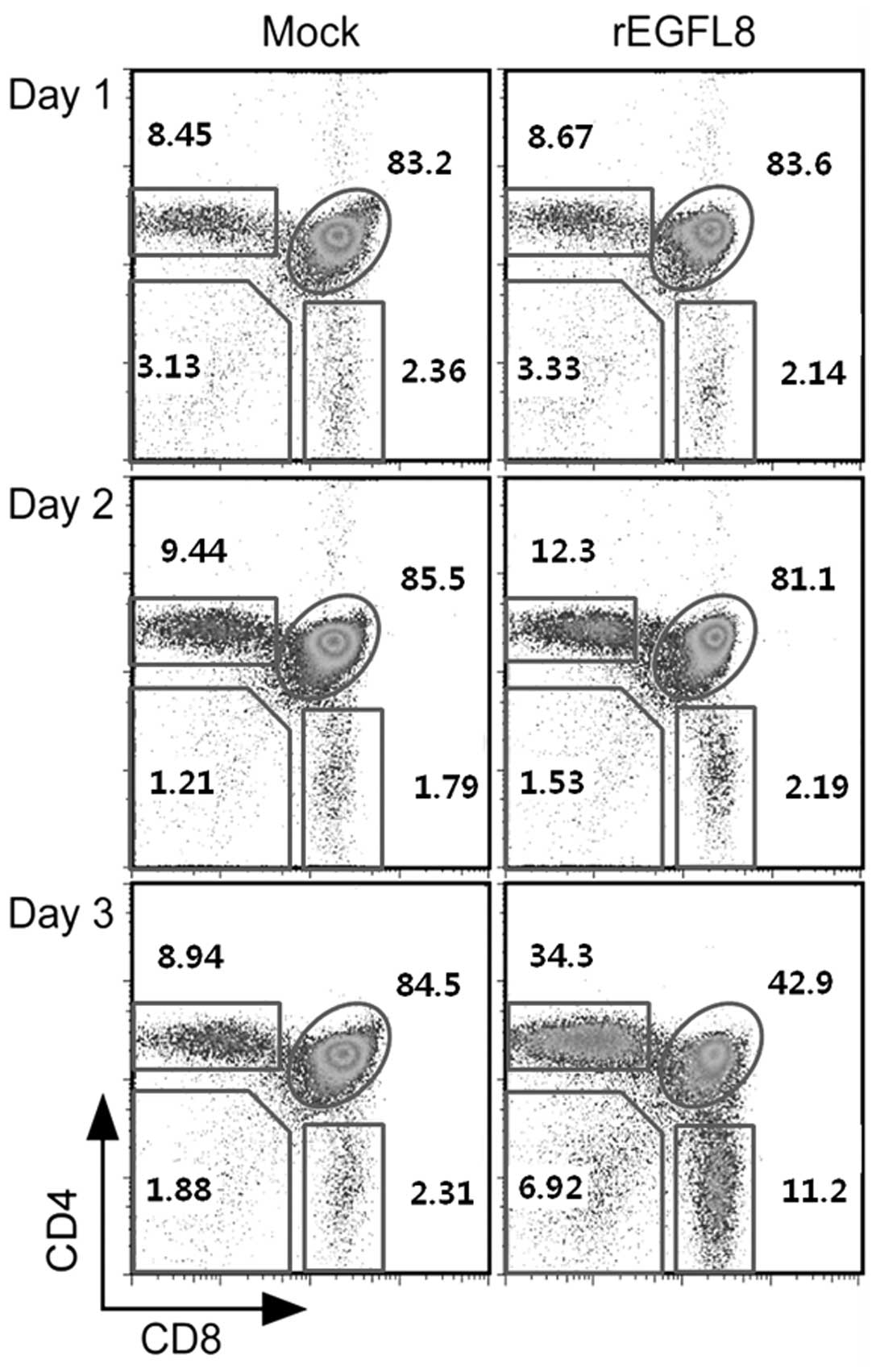
were differentially affected by treatment with rEGFL8. To address
this issue, mouse thymocytes were freshly isolated 1, 2 and 3 days
after rEGFL8 injection, and were stained with anti-CD4 and anti-CD8
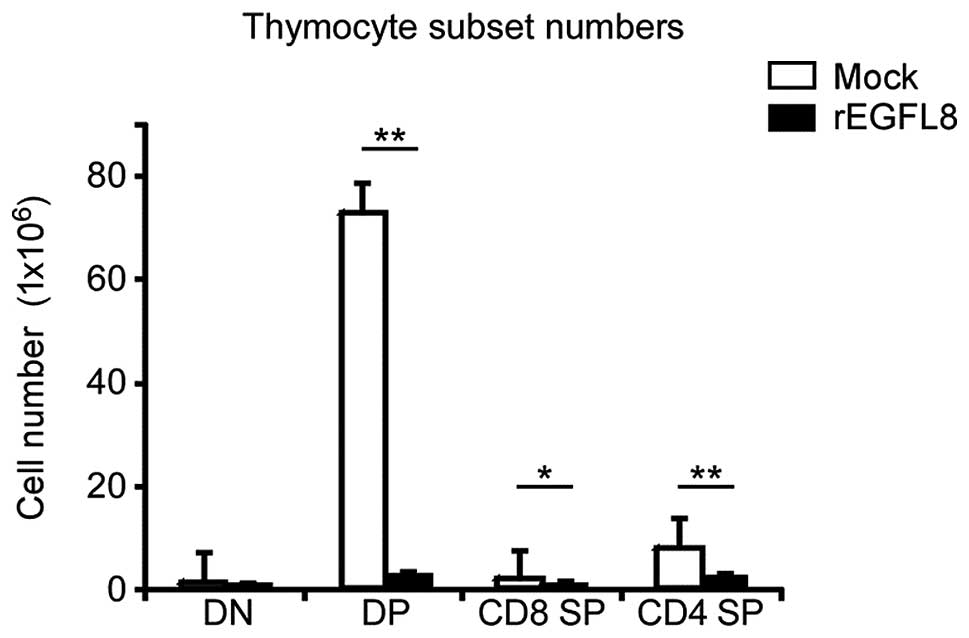
mAbs (Fig. 5). Of note, there was
a drastic decrease in both the percentage and number of
CD4+CD8+ double-positive (DP) thymocytes 3
days after rEGFL8 injection (Figs.
5 and 6). Moreover, the
CD4+ single-positive (SP) and CD8+ SP
thymocyte subsets were also significantly reduced in number
compared with the wild-type subset, although to a lesser degree
than the CD4+CD8+ DP thymocytes on day 3
(Fig. 6).
Inhibitory effect of rEGFL8 on the
survival and proliferation of thymocytes
The reduction in the number of thymocytes follwing
treatment with rEGFL8 also raised the question of whether these
changes occur due to the inhibition of cell proliferation or the
promotion of apoptosis of thymocytes by rEGFL8. To shed light on
this matter, the effect of rEGFL8 on cell proliferation was first
investigated 12 h, 1 and 3 days after rEGFL8 injection. Freshly
isolated thymocytes from the thymus 2 h after BrdU injection were
stained for anti-CD4, anti-CD8, anti-CD25, anti-CD44 and anti-BrdU,
and subsequently flow cytometric analysis was performed (Fig. 7). Remarkably, the BrdU+
thymocyte numbers were robustly reduced in the DN, DP,
CD4+ SP and CD8+ SP thymocyte subsets 12 h
and 1 day after rEGFL8 injection, indicating that rEGFL8 profoundly
inhibited the cell proliferation of all 4 major thymocyte subsets.
The number of BrdU+ thymocytes returned to a level
similar to that of the normal control mice at 3 days post-treatment
with rEGFL8 (Fig. 7).
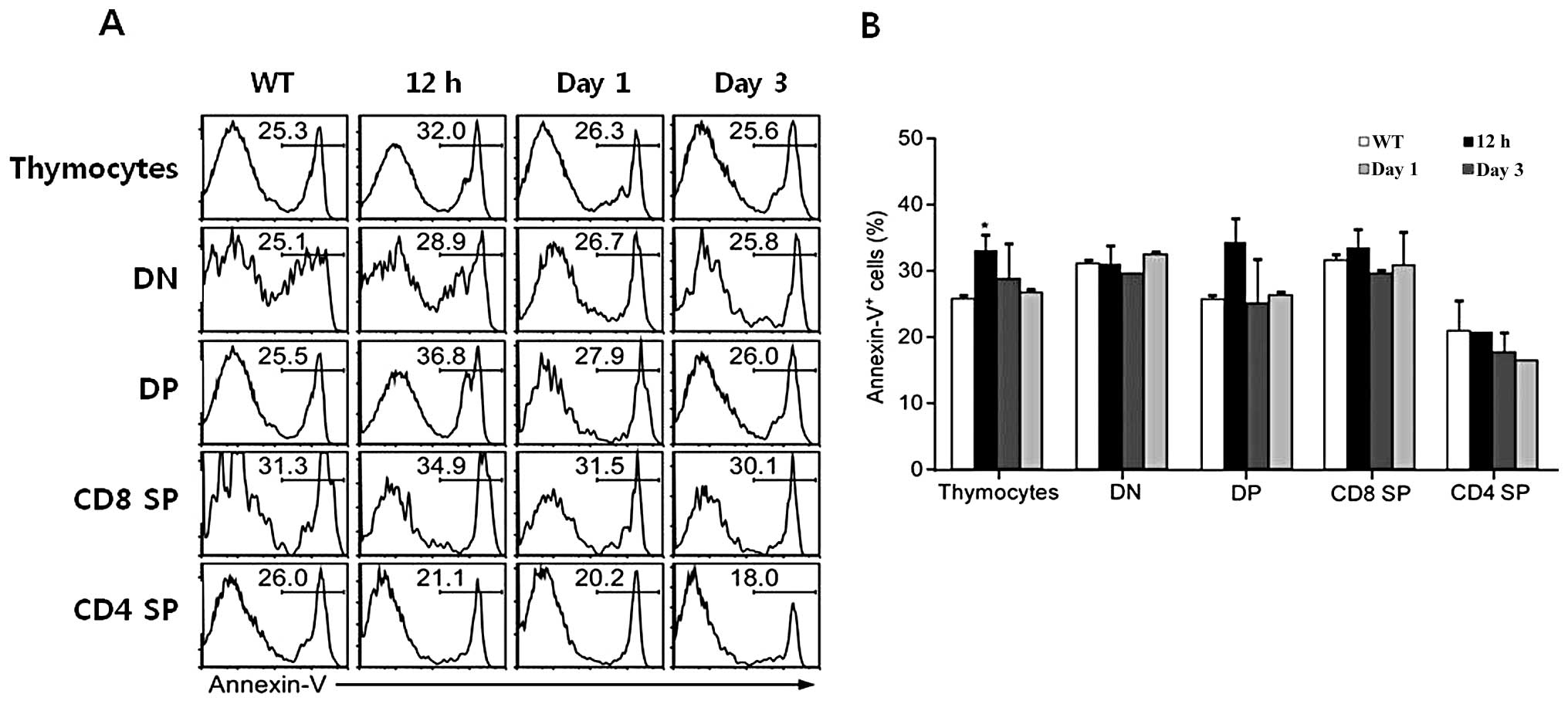
Subsequently, to determine whether rEGFL8 induces apoptosis during
thymocyte development, thymocytes were stained with Annexin V
antibody, as well as anti-CD4 and anti-CD8 mAbs after rEGFL8
injection for 12 h, 1 and 3 days. A relatively low but significant
level of apoptosis was induced in total thymocytes 12 h after
rEGFL8 injection (Fig. 8). The
number of Annexin V+ cells reached peak values 12 h
following treatment with rEGFL8 (Fig.
8). These results clearly indicate that EGFL8 is not a potent
inducer of apoptosis in mouse thymocytes.
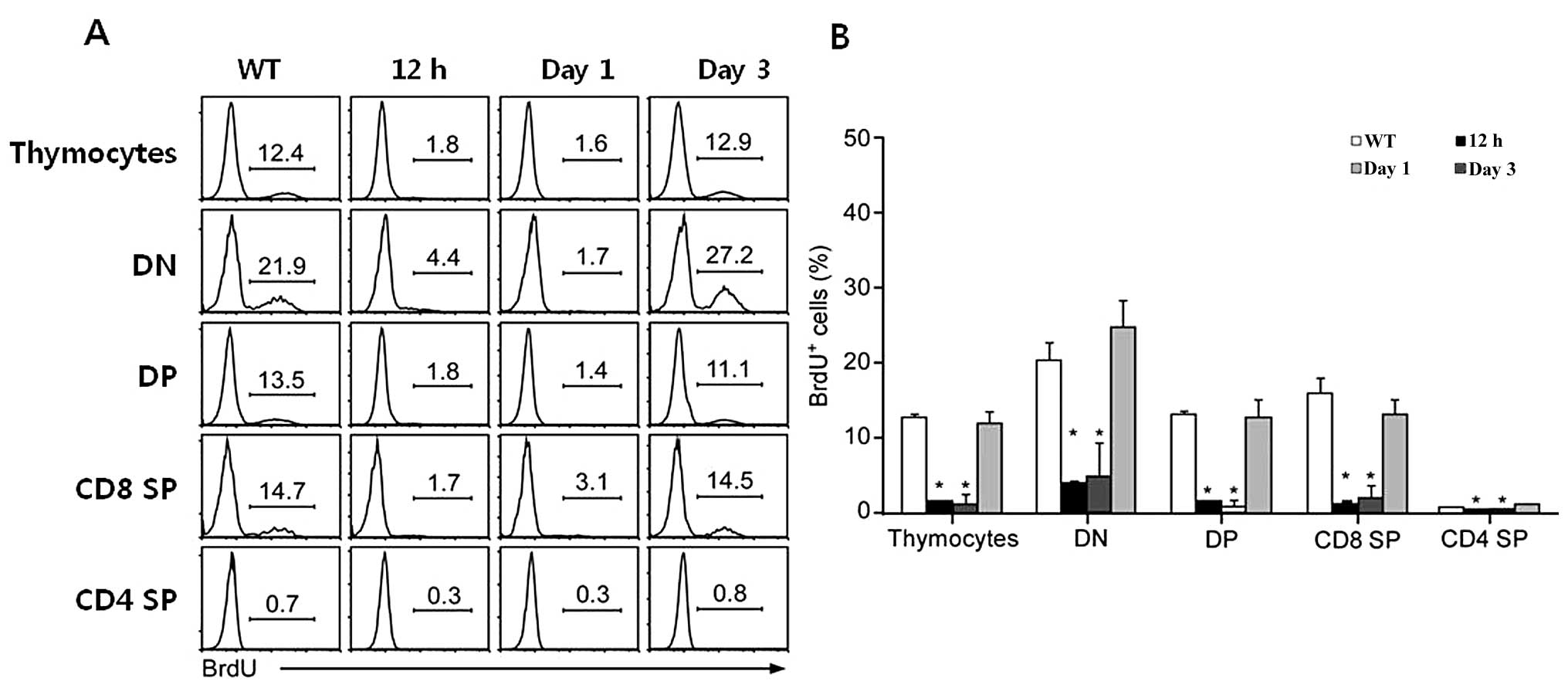 | Figure 7(A) The effect of epidermal growth
factor-like domain 8 (EGFL8) on cell proliferation was assessed by
a BrdU incorporation assay and subsequent FACS analysis. Mice were
injected intraperitonealy (i.p.) with BrdU 12 h, 1 and 3 days
following treatment with recombinant EGFL8 protein (rEGFL8) and
sacrificed 2 h later. Thymocytes were stained with anti-CD4,
anti-CD8, anti-CD25, anti-CD44 and anti-BrdU mAbs. The histogram
shows BrdU+ thymocytes gated on the 4 major subsets of
thymocytes (DN, DP, CD8 SP and CD4 SP) of rEGFL8-treated mice at
the indicated time points. (B) The bar graph is a summary of the
BrdU+ thymocyte frequency. All major thymocyte subsets,
particularly at 12 h and 1 day after EGFL8 injection, exhibited a
decrease in the percentage of BrdU+ cells compared with
the control mice. Groups of mice were analyzed at each time point
and data are presented as the means ± SD. *P<0.05.
DN, double-negative; DP, double-positive; SP, single-positive. |
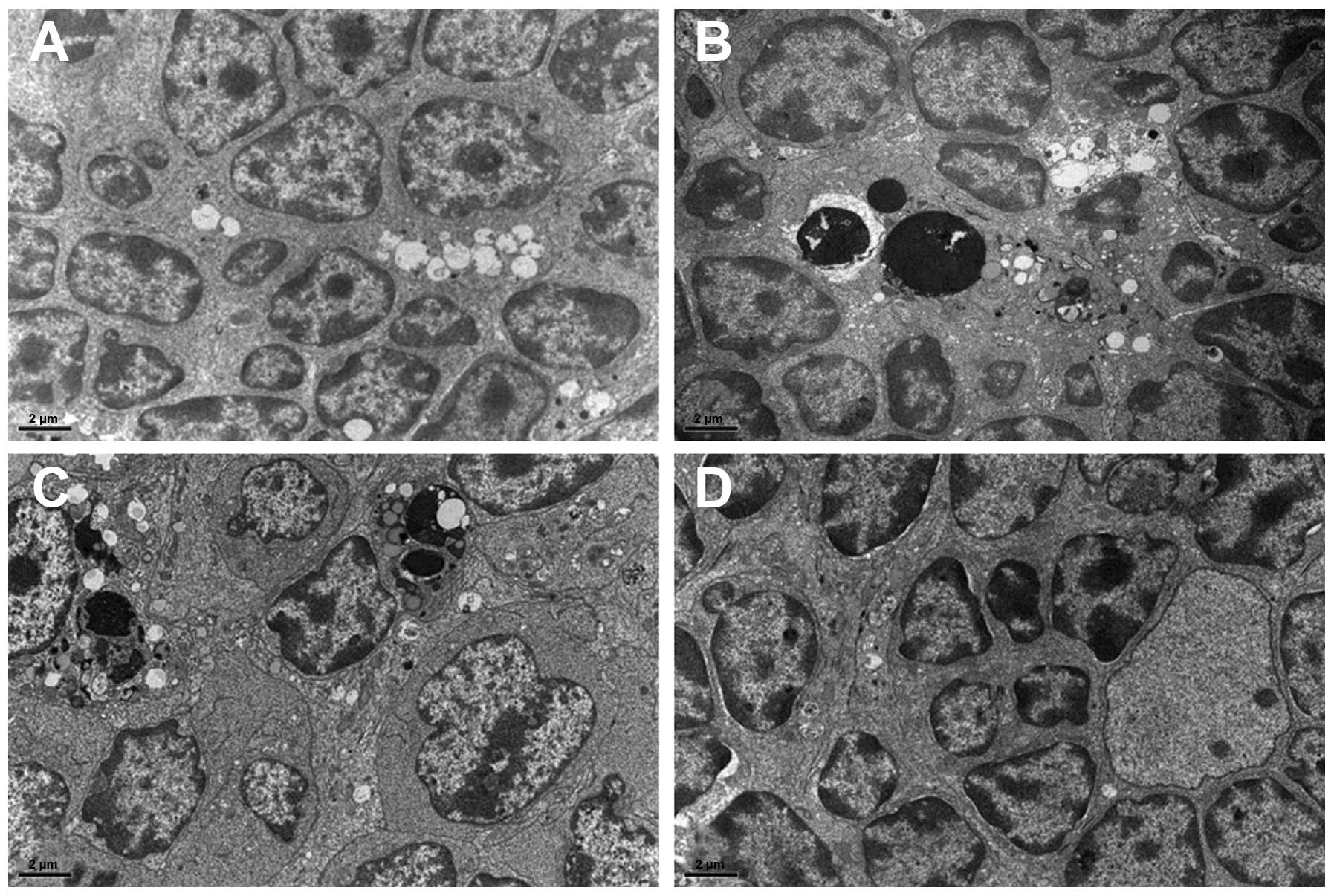
To determine whether EGFL8 causes morphological
changes in the mouse thymus at the ultrastructural level,
transmission electron microscopy was performed after the injection
of rEGFL8. The thymus from the control mice showed normal
ultrastructural features (Fig.
9A). However, electron micrographs of the thymus 12 h after the
administration of rEGFL8 revealed apoptotic thymus cells, although
their proportion was relatively small (Fig. 9B). Apoptotic cells (Fig. 9B and C) had typical morphological
changes; the chromatins were condensed and aggregated into large
dark, compact masses. Most of the apoptotic thymic cells appeared
to be thymocytes, whereas the apoptosis of TECs and other thymic
stromal cell types was rarely observed. In the thymus 1 day after
the administration of rEGFL8, apoptotic thymocytes were observed to
a much lesser extent than in at 12 h (Fig. 9C). Within 3 days after the rEGFL8
injection, apoptotic cells were rarely visible and the thymus
exhibited an almost normal ultrastructural appearance (Fig. 9D).
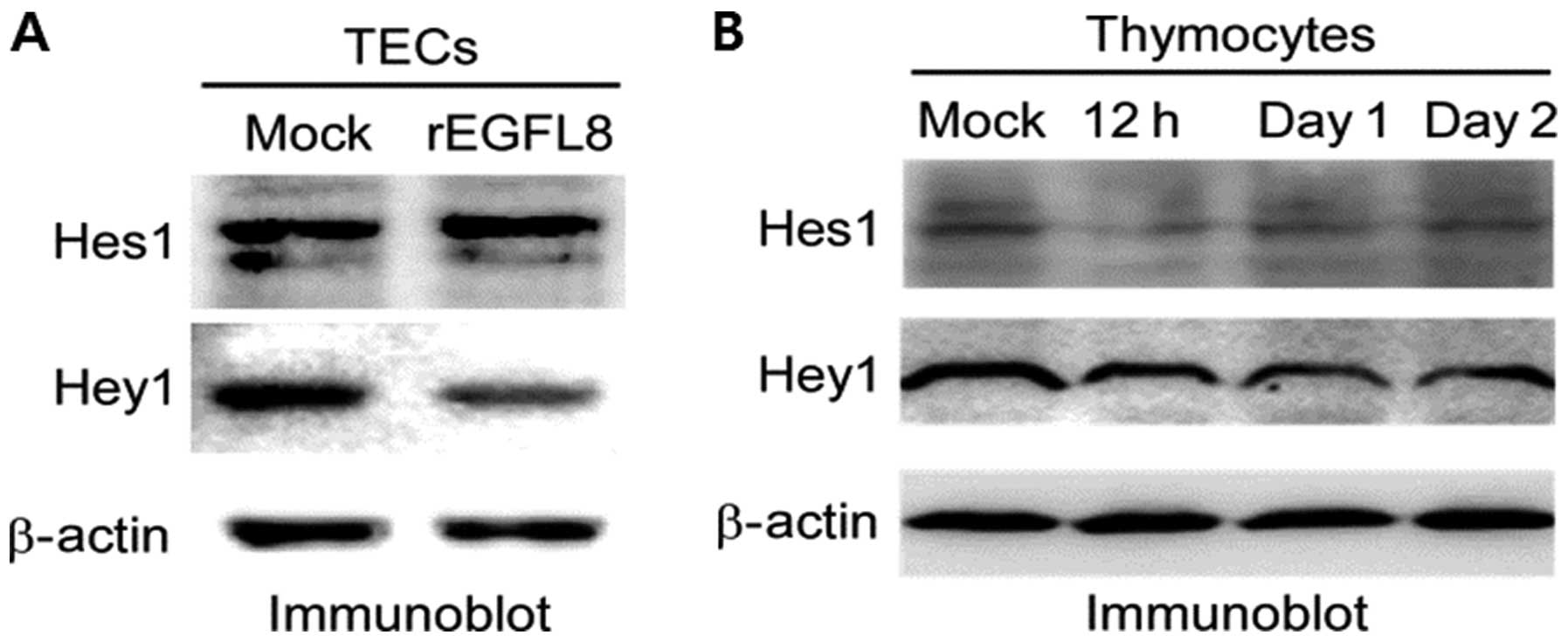
Inhibitory effects of rEGFL8 on the
expression of Hes1 and Hey1
To determine the effect of rEGFL8 on the Notch
signaling pathway in mouse TECs, TECs were treated with rEGFL8
protein and the expression of Hes1 and Hey1 was then assessed by
western blot analysis. Remarkably, the expression of Hes1 and Hey1
was downregulated in the TECs (Fig.
10A). To determine the effect of EGFL8 on the Notch signaling
pathway in mouse thymocytes, rEGFL8 protein was injected into the
mice, and the expression of Hes1 and Hey1 was assessed by western
blot analysis. The expression of Hes1 and Hey1 was significantly
downregulated in the mouse thymocytes (Fig. 10B). The inhibitory effect of
rEGFL8 on Hes1 expression was more pronounced 12 h after rEGFL8
injection. The decrease in Hey1 expression was most evident 1 day
following treatment with EGFL8.
Discussion
In the present study, to our knowledge, we present
for the first time an optimized protocol for the production of
high-purity mouse rEGFL8 protein; we also demonstrate the
biological activity of the EGFL8 protein in mouse thymocytes. The
biological activity of rEGFL8 in mouse thymocytes and TECs was
investigated as the modulation of the EGFL8 gene in TECs
provided evidence of its negative regulatory role on the activity
of TECs and the development of mouse thymocytes in our previous
study (13). The in vivo
experiment with rEGFL8 demonstrated its inhibitory effect on the
development of mouse thymocytes in a time-dependent manner in the
present study. rEGFL8 also induced a decrease in the weight of the
thymus, as well as in the number of mouse thymocytes primarily by
the suppression of thymocyte proliferation as assessed by BrdU cell
proliferation assay and, to a lesser extent, by the induction of
apoptosis in thymocytes as revealed by flow cytometric analysis and
electron microscopy. In a previous study, a differential equation
model of thymocyte dynamics was constructed, which showed that cell
proliferation, differentiation and cell death in the thymus may
account for both the total number of thymic cells and the fraction
of various types of immature and mature thymocytes (16). According to this model, a decrease
in the proliferation rate or an increase in the rate of apoptosis
may be some of the parameters that account for the reduction of
thymic size and thymocyte number that occurs due to thymic
involution.
As regards the reduction in the number of
thymocytes, the suppressive effect of rEGFL8 on thymocytes had no
subset specificity, although the administration of rEGFL8 induced a
profound decrease in the number of DP thymocytes. The reason why
there was a marked reduction in the number of DP thymocytes
compared with the other subsets may be related with their
proportion and the absolute number in the control mice.
As regards the molecular mechanisms underlying the
inhibitory effects of EGFL8 on the proliferation of mouse
thymocytes and its promotion of apoptosis in mouse thymocytes, the
involvement of EGFL8 in the Notch signaling pathway contributes, at
least in part, to this negative regulatory role of rEGFL8, since it
inhibited the expression of the Notch downstream effectors, Hes1
and Hey1, in the mouse thymocytes and TECs. Notch signaling is
directly involved in the regulation of thymic T-cell development
(17). Notch signaling has also
been found to play a central role in reconstitution after
transplantation, immunomodulation, or the development and
maturation of thymocytes (18).
Taken together, the data from the present study suggest that EGFL8
acts as a negative regulatory factor in the critical steps of mouse
T-cell development, such as thymocyte proliferation and survival,
through the inhibition of Notch signaling in mouse thymocytes and
TECs.
In conclusion, the pET-28a-EGFL8 vector was
expressed in E. coli (DE3) and a relatively large amount of
mouse rEGFL8 was successfully produced and purified in this study.
Treatment with rEGFL8 reduced the weight of the thymus and the
number of thymocytes, suppressed thymocyte proliferation, induced
thymocyte apoptosis and inhibited Notch signaling (downregulation
of Hes1 and Hey1 expression) in mouse thymocytes and TECs.
Therefore, the data from the present study suggest
that EGFL8 acts as a negative regulatory factor in the critical
steps of mouse T-cell development. Further studies are required to
fully elucidate the functional role of EGFL8 in diverse
physiological and pathological processes.
Acknowledgements
This study was supported by the National Research
Foundation of Korea (NRF) grant funded by the Korean government
(MEST) (no.≈2010-0014194).
References
|
1
|
Takahama Y: Journey through the thymus:
stromal guides for T-cell development and selection. Nat Rev
Immunol. 6:127–135. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fitch MJ, Campagnolo L, Kuhnert F and
Stuhlmann H: Egfl7, a novel epidermal growth factor-domain gene
expressed in endothelial cells. Dev Dyn. 230:316–324. 2004.
View Article : Google Scholar
|
|
3
|
Nichol D and Stuhlmann H: EGFL7: a unique
angiogenic signaling factor in vascular development and disease.
Blood. 119:1345–1352. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Campagnolo L, Leahy A, Chitnis S,
Koschnick S, Fitch MJ, Fallon JT, Loskutoff D, Taubman MB and
Stuhlmann H: EGFL7 is a chemoattractant for endothelial cells and
is up-regulated in angiogenesis and arterial injury. Am J Pathol.
167:275–284. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nichol D, Shawber C, Fitch MJ, Bambino K,
Sharma A, Kitajewski J and Stuhlmann H: Impaired angiogenesis and
altered Notch signaling in mice overexpressing endothelial Egfl7.
Blood. 116:6133–6143. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Parker LH, Schmidt M, Jin SW, Gray AM,
Beis D, Pham T, Frantz G, Palmieri S, Hillan K, Stainier DY, De
Sauvage FJ and Ye W: The endothelial-cell-derived secreted factor
Egfl7 regulates vascular tube formation. Nature. 428:754–758. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schmidt MH, Bicker F, Nikolic I, Meister
J, Babuke T, Picuric S, Muller-Esterl W, Plate KH and Dikic I:
Epidermal growth factor-like domain 7 (EGFL7) modulates Notch
signalling and affects neural stem cell renewal. Nat Cell Biol.
11:873–880. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Durrans A and Stuhlmann H: A role for
Egfl7 during endothelial organization in the embryoid body model
system. J Angiogenes Res. 2:42010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chim SM, Qin A, Tickner J, Pavlos N, Davey
T, Wang H, Guo Y, Zheng MH and Xu J: EGFL6 promotes endothelial
cell migration and angiogenesis through the activation of
extracellular signal-regulated kinase. J Biol Chem.
286:22035–22046. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kojika S and Griffin JD: Notch receptors
and hematopoiesis. Exp Hematol. 29:1041–1052. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu F, Shirahata A, Sakuraba K, Kitamura Y,
Goto T, Saito M, Ishibashi K, Kigawa G, Nemoto H, Sanada Y and Hibi
K: Down-regulation of EGFL8: a novel biomarker for advanced gastric
cancer. Anticancer Res. 31:3377–3380. 2011.PubMed/NCBI
|
|
12
|
Wu F, Shirahata A, Sakuraba K, Kitamura Y,
Goto T, Saito M, Ishibashi K, Kigawa G, Nemoto H, Sanada Y and Hibi
K: Down-regulation of EGFL8: a novel prognostic biomarker for
patients with colorectal cancer. Anticancer Res. 31:2249–2254.
2011.PubMed/NCBI
|
|
13
|
Choi HJ, Yoon TD, Muhammad I, Jeong MH,
Lee J, Baek SY, Kim BS and Yoon S: Regulatory role of mouse
epidermal growth factor-like protein 8 in thymic epithelial cells.
Biochem Biophys Res Commun. 425:250–255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Romano R, Palamaro L, Fusco A, Iannace L,
Maio S, Vigliano I, Giardino G and Pignata C: From murine to human
nude/SCID: the thymus, T-cell development and the missing link.
Clin Dev Immunol. 2012:4671012012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Faas SJ, Rothstein JL, Kreider BL, Rovera
G and Knowles BB: Phenotypically diverse mouse thymic stromal cell
lines which induce proliferation and differentiation of
hematopoietic cells. Eur J Immunol. 23:1201–1214. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mehr R, Globerson A and Perelson AS:
Modeling positive and negative selection and differentiation
processes in the thymus. J Theor Biol. 175:103–126. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ersvaer E, Hatfield KJ, Reikvam H and
Bruserud O: Future perspectives: therapeutic targeting of notch
signalling may become a strategy in patients receiving stem cell
transplantation for hematologic malignancies. Bone Marrow Res.
2011:5707962011. View Article : Google Scholar
|
|
18
|
Pui JC, Allman D, Xu L, DeRocco S, Karnell
FG, Bakkour S, Lee JY, Kadesch T, Hardy RR, Aster JC and Pear WS:
Notch1 expression in early lymphopoiesis influences B versus T
lineage determination. Immunity. 11:299–308. 1999. View Article : Google Scholar : PubMed/NCBI
|