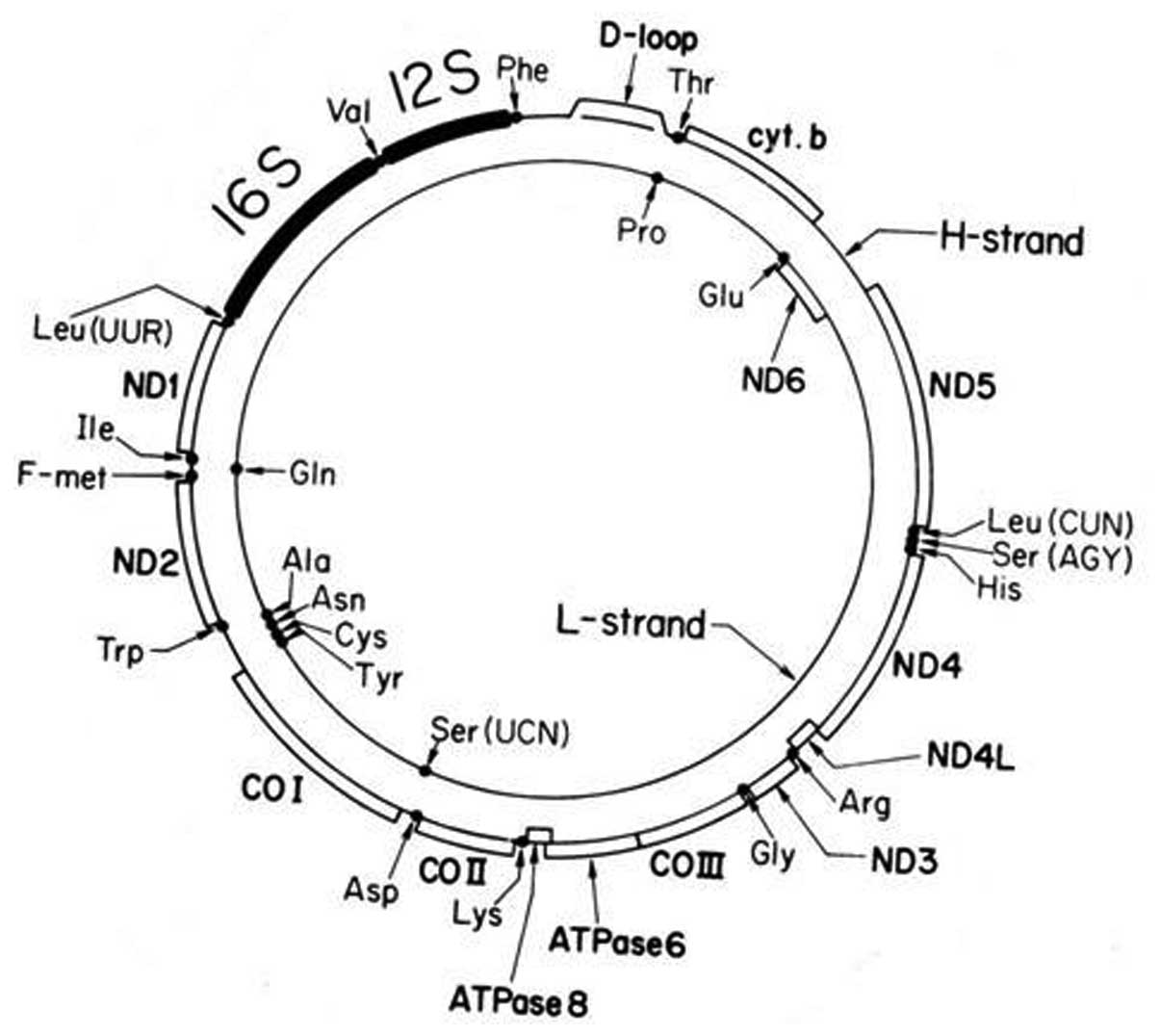
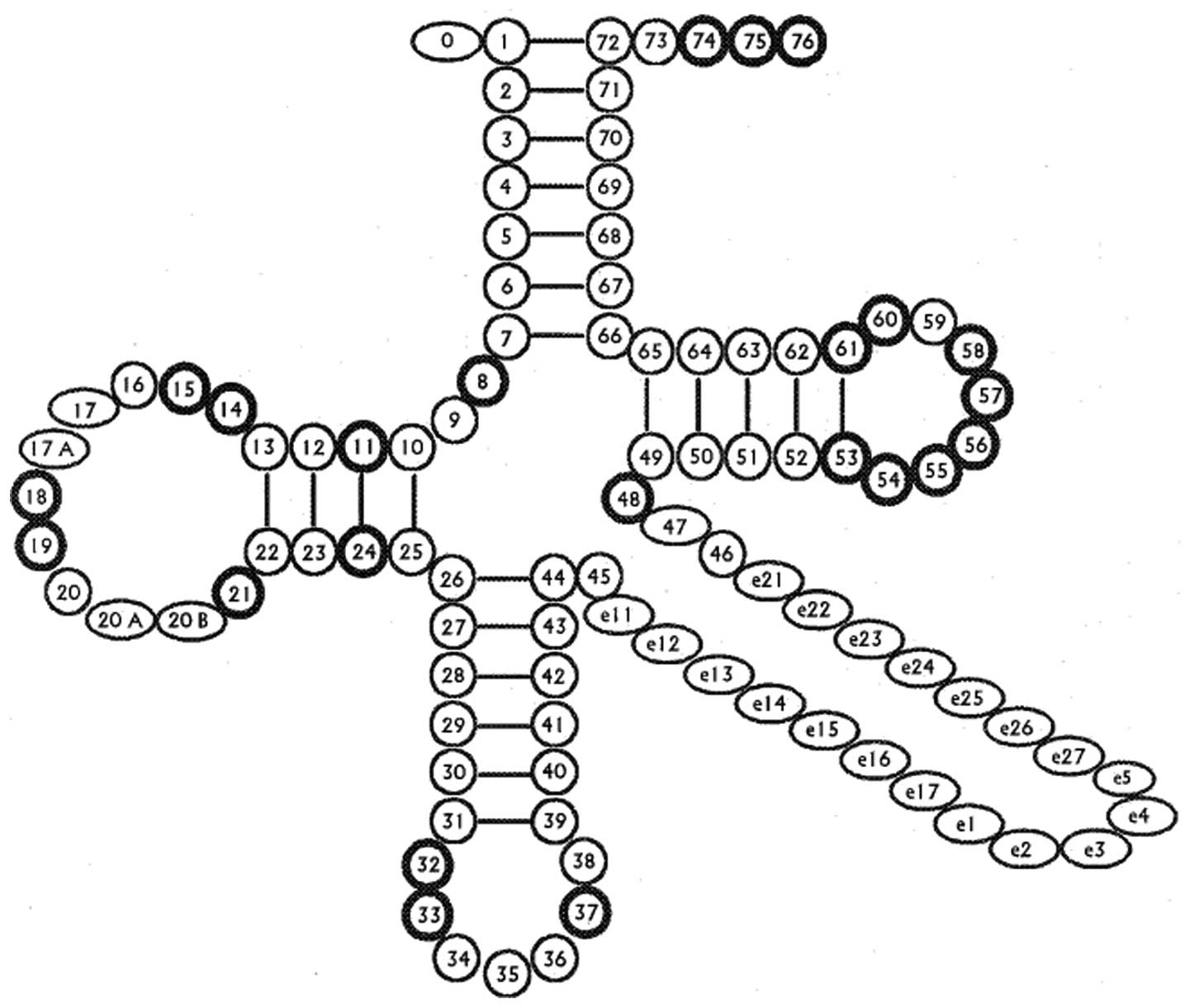
|
1
|
El Shamieh S, Herbeth B, Azimi-Nezhad M,
et al: Human formyl peptide receptor 1 C32T SNP interacts with age
and is associated with blood pressure levels. Clin Chim Acta.
413:34–38. 2012.PubMed/NCBI
|
|
2
|
Kearney PM, Whelton M, Reynolds K, et al:
Global burden of hypertension: analysis of worldwide data. Lancet.
365:217–223. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Messerli FH, Williams B and Ritz E:
Essential hypertension. Lancet. 370:591–560. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kunes J and Zicha J: Developmental windows
and environment as important factors in the expression of genetic
information: a cardiovascular physiologist’s view. Clin Sci.
111:295–305. 2006.PubMed/NCBI
|
|
5
|
Timberlake DS, O’Connor DT and Parmer RJ:
Molecular genetics of essential hypertension: recent results and
emerging strategies. Curr Opin Nephrol Hypertens. 10:71–79. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fan JB, Chen X, Halushka MK, et al:
Parallel genotyping of human SNPs using generic high-density
oligonucleotide tag arrays. Genome Res. 10:853–860. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Oliphant A, Barker DL, Stuelpnagel JR and
Chee MS: BeadArray technology: enabling an accurate, cost-effective
approach to high-throughput genotyping. Biotechniques. 56(Suppl
56–58): 60–61. 2002.PubMed/NCBI
|
|
8
|
Levy D, Ehret GB, Rice K, et al:
Genome-wide association study of blood pressure and hypertension.
Nat Genet. 41:677–687. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tobin MD, Tomaszewski M, Braund PS, et al:
Common variants in genes underlying monogenic hypertension and
hypotension and blood pressure in the general population.
Hypertension. 51:1658–1664. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Padmanabhan S, Melander O, Johnson T, et
al: Genome-wide association study of blood pressure extremes
identifies variant near UMOD associated with hypertension. PLoS
Genet. 6:e10011772010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schon EA: Mitochondria. Encyclopedia of
Human Biology. Dulbecco R: 5. 2nd edition. Academic Press; London:
pp. 713–724. 1997
|
|
12
|
Wallace DC: Mitochondrial DNA mutations in
disease and aging. Environ Mol Mutagen. 51:440–450. 2010.PubMed/NCBI
|
|
13
|
Garcia-Rodriguez LJ: Appendix 1. Basic
properties of mitochondria. Methods Cell Biol. 80:809–812. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
DiMauro S and Schon EA: Mitochondrial DNA
mutations in human disease. Am J Med Genet. 106:18–26. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cadenas E and Davies KJ: Mitochondrial
free radical generation, oxidative stress, and aging. Free Radic
Biol Med. 29:222–230. 2000.PubMed/NCBI
|
|
16
|
Schon EA, DiMauro S and Hirano M: Human
mitochondrial DNA: roles of inherited and somatic mutations. Nat
Rev Genet. 13:878–890. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
McBride HM, Neuspiel M and Wasiak S:
Mitochondria: more than just a powerhouse. Curr Biol. 16:R551–R560.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Choksi KB, Boylston WH, Rabek JP, et al:
Oxidatively damaged proteins of heart mitochondrial electron
transport complexes. Biochim Biophys Acta. 1688:95–101. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Murphy MP: Induction of mitochondrial ROS
production by electrophilic lipids: a new pathway of redox
signaling? Am J Physiol Heart Circ Physiol. 290:H1754–H1755. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Abou-Sleiman PM, Muqit MM and Wood NW:
Expanding insights of mitochondrial dysfunction in Parkinson’s
disease. Nat Rev Neurosci. 7:207–219. 2006.
|
|
21
|
Li P, Nijhawan D and Wang X: Mitochondrial
activation of apoptosis. Cell. 116(Suppl 2): S57–S59. 2004.
View Article : Google Scholar
|
|
22
|
Wu L and Juurlink BH: Increased
methylglyoxal and oxidative stress in hypertensive rat vascular
smooth muscle cells. Hypertension. 39:809–814. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lerman LO, Nath KA, Rodriguez-Porcel M, et
al: Increased oxidative stress in experimental renovascular
hypertension. Hypertension. 37:541–546. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Trolliet MR, Rudd MA and Loscalzo J:
Oxidative stress and renal dysfunction in salt-sensitive
hypertension. Kidney Blood Press Res. 24:116–123. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Romero JC and Reckelhoff JF:
State-of-the-Art lecture. Role of angiotensin and oxidative stress
in essential hypertension. Hypertension. 34:943–949. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hamilton CA, Berg G, McIntyre M, et al:
Effects of nitric oxide and superoxide on relaxation in human
artery and vein. Atherosclerosis. 133:77–86. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Paravicini TM and Touyz RM: Redox
signaling in hypertension. Cardiovasc Res. 71:247–258. 2006.
View Article : Google Scholar
|
|
28
|
Prezant TR, Agapian JV, Bohlman MC, et al:
Mitochondrial ribosomal RNA mutation associated with both
antibiotic-induced and non-syndromic deafness. Nat Genet.
4:289–294. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Estivill X, Govea N, Barceló E, et al:
Familial progressive sensorineural deafness is mainly due to the
mtDNA A1555G mutation and is enhanced by treatment of
aminoglycosides. Am J Hum Genet. 62:27–35. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Guan MX: Molecular pathogenetic mechanism
of maternally inherited deafness. Ann NY Acad Sci. 1011:259–271.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Van Camp G and Smith RJ: Maternally
inherited hearing impairment. Clin Genet. 57:409–414. 2000.
|
|
32
|
Chen H, Zheng J, Xue L, et al: The 12S
rRNA A1555G mutation in the mitochondrial haplogroup D5a is
responsible for maternally inherited hypertension and hearing loss
in two Chinese pedigrees. Eur J Hum Genet. 20:607–612. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hamasaki K and Rando RR: Specific binding
of aminoglycosides to a human rRNA construct based on a DNA
polymorphism which causes aminoglycoside-induced deafness.
Biochemistry. 36:12323–12328. 1997. View Article : Google Scholar
|
|
34
|
Cotney J, McKay SE and Shadel GS:
Elucidation of separate, but collaborative functions of the rRNA
methyltransferase-related human mitochondrial transcription factors
B1 and B2 in mitochondrial biogenesis reveals new insight into
maternally inherited deafness. Hum Mol Genet. 18:2670–2682. 2009.
View Article : Google Scholar
|
|
35
|
Hobbie SN, Bruell CM, Akshay S, et al:
Mitochondrial deafness alleles confer misreading of the genetic
code. Proc Natl Acad Sci USA. 105:3244–3249. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Guan MX, Fischel-Ghodsian N and Attardi G:
Nuclear background determines biochemical phenotype in the
deafness-associated mitochondrial 12S rRNA mutation. Hum Mol Genet.
10:573–580. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bernal-Mizrachi C, Gates AC, Weng S, et
al: Vascular respiratory uncoupling increases blood pressure and
atherosclerosis. Nature. 435:502–506. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li X, Fischel-Ghodsian N, Schwartz F, et
al: Biochemical characterization of the mitochondrial
tRNASer(UCN) T7511C mutation associated with
nonsyndromic deafness. Nucleic Acids Res. 32:867–877. 2004.
View Article : Google Scholar
|
|
39
|
Li R, Ishikawa K, Deng JH, et al:
Maternally inherited nonsyndromic hearing loss is associated with
the T7511C mutation in the mitochondrial tRNASerUCN gene
in a Japanese family. Biochem Biophys Res Commun. 328:32–37. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu Y, Li Z, Yang L, et al: The
mitochondrial ND1 T3308C mutation in a Chinese family with the
secondary hypertension. Biochem Biophys Res Commun. 368:18–22.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Anderson S, Bankier AT, Barrell BG, et al:
Sequence and organization of the human mitochondrial genome.
Nature. 290:457–465. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Guan MX, Enriquez JA, Fischel-Ghodsian N,
et al: The deafness-associated mitochondrial DNA mutation at
position 7445, which affects tRNASer(UCN) precursor
processing, has long-range effects on NADH dehydrogenase subunit
ND6 gene expression. Mol Cell Biol. 18:5868–5879. 1998.PubMed/NCBI
|
|
43
|
Teng L, Zheng J, Leng J and Ding Y:
Clinical and molecular characterization of a Han Chinese family
with high penetrance of essential hypertension. Mitochondrial DNA.
23:461–465. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Liu Z, Song Y, Gu S, et al: Mitochondrial
ND5 12338T>C variant is associated with maternally inherited
hypertrophic cardiomyopathy in a Chinese pedigree. Gene.
506:339–343. 2012.
|
|
45
|
Florentz C, Sohm B, Tryoen-Tóth P, et al:
Human mitochondrial tRNAs in health and disease. Cell Mol Life Sci.
60:1356–1375. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yu-Wai-Man P, Griffiths PG, Hudson G and
Chinnery PF: Inherited mitochondrial optic neuropathies. J Med
Genet. 46:145–158. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Guo H, Zhuang XY, Zhang AM, et al:
Presence of mutation m.14484T>C in a Chinese family with
maternally inherited essential hypertension but no expression of
LHON. Biochim Biophys Acta. 1822:1535–1543. 2012.PubMed/NCBI
|
|
48
|
Andreu AL, Hanna MG, Reichmann H, et al:
Exercise intolerance due to mutations in the cytochrome b gene of
mitochondrial DNA. N Engl J Med. 341:1037–1044. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Andreu AL, Bruno C, Dunne TC, et al: A
nonsense mutation (G15059A) in the cytochrome b gene in a patient
with exercise intolerance and myoglobinuria. Ann Neurol.
45:127–130. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Nikitin AG, Lavrikova EY and Chistiakov
DA: The heteroplasmic 15059G>A mutation in the mitochondrial
cytochrome b gene and essential hypertension in type 2 diabetes.
Diabetes Metab Syndr. 6:150–156. 2012.PubMed/NCBI
|
|
51
|
Chang DD and Clayton DA: Priming of human
mitochondrial DNA replication occurs at the light-strand promoter.
Proc Natl Acad Sci USA. 82:351–355. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kang D, Miyako K, Kai Y, et al: In vivo
determination of replication origins of human mitochondrial DNA by
ligation-mediated polymerase chain reaction. J Biol Chem.
272:15275–15279. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Bi R, Zhang AM, Zhang W, et al: The
acquisition of an inheritable 50-bp deletion in the human mtDNA
control region does not affect the mtDNA copy number in peripheral
blood cells. Hum Mutat. 31:538–543. 2010.PubMed/NCBI
|
|
54
|
Elango S, Govindaraj P, Vishwanadha VP, et
al: Analysis of mitochondrial genome revealed a rare 50 bp deletion
and substitutions in a family with hypertension. Mitochondrion.
11:878–885. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Pham XH, Farge G, Shi Y, et al: Conserved
sequence box II directs transcription termination and primer
formation in mitochondria. J Biol Chem. 281:24647–24652. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Li Z, Liu Y, Yang L, et al: Maternally
inherited hypertension is associated with the mitochondrial
tRNA(Ile) A4295G mutation in a Chinese family. Biochem Biophys Res
Commun. 367:906–911. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Merante F, Myint T, Tein I, et al: An
additional mitochondrial tRNA(Ile) point mutation (A-to-G at
nucleotide 4295) causing hypertrophic cardiomyopathy. Hum Mutat.
8:216–222. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Suzuki T, Nagao A and Suzuki T: Human
mitochondrial tRNAs: biogenesis, function, structural aspects, and
diseases. Annu Rev Genet. 45:299–329. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Levinger L, Giegé R and Florentz C:
Pathology-related substitutions in human mitochondrial tRNA(Ile)
reduce precursor 3′ end processing efficiency in vitro. Nucleic
Acids Res. 31:1904–1912. 2003.PubMed/NCBI
|
|
60
|
Gutiérrez Cortés N, Pertuiset C, Dumon E,
et al: Novel mitochondrial DNA mutations responsible for maternally
inherited nonsyndromic hearing loss. Hum Mutat. 33:681–689.
2012.PubMed/NCBI
|
|
61
|
Wang S, Li R, Fettermann A, et al:
Maternally inherited essential hypertension is associated with the
novel 4263A>G mutation in the mitochondrial tRNAIle
gene in a large Han Chinese family. Circ Res. 108:862–870. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Zhu HY, Wang SW, Liu L, et al: Genetic
variants in mitochondrial tRNA genes are associated with essential
hypertension in a Chinese Han population. Clin Chim Acta.
410:64–69. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Wilson FH, Hariri A, Farhi A, et al: A
cluster of metabolic defects caused by mutation in a mitochondrial
tRNA. Science. 306:1190–1194. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Sprinzl M, Steegborn C, Hübel F and
Steinberg S: Compilation of tRNA sequences and sequences of tRNA
genes. Nucleic Acids Res. 24:68–72. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Ashraf SS, Sochacka E, Cain R, et al:
Single atom modification (O→S) of tRNA confers ribosome binding.
RNA. 5:188–194. 1999.
|
|
66
|
Jaksch M, Kleinle S, Scharfe C, et al:
Frequency of mitochondrial transfer RNA mutations and deletions in
225 patients presenting with respiratory chain deficiencies. J Med
Genet. 38:665–673. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Qu J, Li R, Zhou X, et al: The novel
A4435G mutation in the mitochondrial tRNAMet may
modulate the phenotypic expression of the LHON-associated ND4
G11778A mutation. Invest Ophthalmol Vis Sci. 47:475–483.
2006.PubMed/NCBI
|
|
68
|
Guo LJ, Oshida Y, Fuku N, et al:
Mitochondrial genome polymorphisms associated with type-2 diabetes
or obesity. Mitochondrion. 5:15–33. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Kong QP, Bandelt HJ, Sun C, et al:
Updating the East Asian mtDNA phylogeny: a prerequisite for the
identification of pathogenic mutations. Hum Mol Genet.
15:2076–2086. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Liu Y, Li R, Li Z, et al: Mitochondrial
transfer RNAMet 4435A>G mutation is associated with maternally
inherited hypertension in a Chinese pedigree. Hypertension.
53:1083–1090. 2009.PubMed/NCBI
|
|
71
|
Lu Z, Chen H, Meng Y, et al: The
tRNAMet 4435A>G mutation in the mitochondrial
haplogroup G2a1 is responsible for maternally inherited
hypertension in a Chinese pedigree. Eur J Hum Genet. 19:1181–1186.
2011.
|
|
72
|
Postnov YV, Orlov SN, Budnikov YY, et al:
Mitochondrial energy conversion disturbance with decrease in ATP
production as a source of systemic arterial hypertension.
Pathophysiology. 14:195–204. 2007.
|
|
73
|
Zhu HY, Wang SW, Liu L, et al: A
mitochondrial mutation A4401G is involved in the pathogenesis of
left ventricular hypertrophy in Chinese hypertensives. Eur J Hum
Genet. 17:172–178. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Li R, Liu Y, Li Z, et al: Failures in
mitochondrial tRNAMet and tRNAGln metabolism
caused by the novel 4401A>G mutation are involved in essential
hypertension in a Han Chinese Family. Hypertension. 54:329–337.
2009.PubMed/NCBI
|
|
75
|
Ojala D, Montoya J and Attardi G: tRNA
punctuation model of RNA processing in human mitochondria. Nature.
290:470–474. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Qiu Q, Li R, Jiang P, et al: Mitochondrial
tRNA mutations are associated with maternally inherited
hypertension in two Han Chinese pedigrees. Hum Mutat. 33:1285–1293.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Levinger L, Mörl M and Florentz C:
Mitochondrial tRNA 3′ end metabolism and human disease. Nucleic
Acids Res. 32:5430–5441. 2004.
|
|
78
|
Kelley SO, Steinberg SV and Schimmel P:
Functional defects of pathogenic human mitochondrial tRNAs related
to structural fragility. Nat Struct Biol. 7:862–865. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Kelley SO, Steinberg SV and Schimmel P:
Fragile T-stem in disease-associated human mitochondrial tRNA
sensitizes structure to local and distant mutations. J Biol Chem.
276:10607–10611. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Arredondo JJ, Gallardo ME, García-Pavía P,
et al: Mitochondrial tRNA valine as a recurrent target for
mutations involved in mitochondrial cardiomyopathies.
Mitochondrion. 12:357–362. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Vilarinho L, Santorelli FM, Rosas MJ, et
al: The mitochondrial A3243G mutation presenting as severe
cardiomyopathy. J Med Genet. 34:607–609. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Zeviani M, Gellera C, Antozzi C, et al:
Maternally inherited myopathy and cardiomyopathy: association with
mutation in mitochondrial DNA tRNA(Leu)(UUR). Lancet. 338:143–147.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Sweeney MG, Brockington M, Weston MJ, et
al: Mitochondrial DNA transfer RNA mutation Leu(UUR)A→G 3260: a
second family with myopathy and cardiomyopathy. Q J Med.
86:435–438. 1993.
|
|
84
|
Nishino I, Komatsu M, Kodama S, et al: The
3260 mutation in mitochondrial DNA can cause mitochondrial
myopathy, encephalopathy, lactic acidosis, and strokelike episodes
(MELAS). Muscle Nerve. 19:1603–1604. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Goldstein JD, Shanske S, Bruno C and
Perszyk AA: Maternally inherited mitochondrial cardiomyopathy
associated with a C-to-T transition at nucleotide 3303 of
mitochondrial DNA in the tRNA(Leu(UUR)) gene. Pediatr Dev Pathol.
2:78–85. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Silvestri G, Santorelli FM, Shanske S, et
al: A new mtDNA mutation in the tRNA(Leu(UUR)) gene associated with
maternally inherited cardiomyopathy. Hum Mutat. 3:37–43. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Palecek T, Tesarova M, Kuchynka P, et al:
Hypertrophic cardiomyopathy due to the mitochondrial DNA mutation
m.3303C>T diagnosed in an adult male. Int Heart J. 53:383–387.
2012.PubMed/NCBI
|
|
88
|
Taniike M, Fukushima H, Yanagihara I, et
al: Mitochondrial tRNA(Ile) mutation in fatal cardiomyopathy.
Biochem Biophys Res Commun. 186:47–53. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Giordano C, Perli E, Orlandi M, et al:
Cardiomyopathies due to homoplasmic mitochondrial tRNA mutations:
morphologic and molecular features. Hum Pathol. 44:1262–1270. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Taylor RW, Giordano C, Davidson MM, et al:
A homoplasmic mitochondrial transfer ribonucleic acid mutation as a
cause of maternally inherited hypertrophic cardiomyopathy. J Am
Coll Cardiol. 41:1786–1796. 2003. View Article : Google Scholar
|
|
91
|
Arbustini E, Diegoli M, Fasani R, et al:
Mitochondrial DNA mutations and mitochondrial abnormalities in
dilated cardiomyopathy. Am J Pathol. 153:1501–1510. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Terasaki F, Tanaka M, Kawamura K, et al: A
case of cardiomyopathy showing progression from the hypertrophic to
the dilated form: association of Mt8348A→G mutation in the
mitochondrial tRNA(Lys) gene with severe ultrastructural
alterations of mitochondria in cardiomyocytes. Jpn Circ J.
65:691–694. 2001.PubMed/NCBI
|
|
93
|
Merante F, Tein I, Benson L and Robinson
BH: Maternally inherited hypertrophic cardiomyopathy due to a novel
T-to-C transition at nucleotide 9997 in the mitochondrial
tRNA(glycine) gene. Am J Hum Genet. 55:437–446. 1994.PubMed/NCBI
|
|
94
|
Shin WS, Tanaka M, Suzuki J, et al: A
novel homoplasmic mutation in mtDNA with a single evolutionary
origin as a risk factor for cardiomyopathy. Am J Hum Genet.
67:1617–1620. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
95
|
Van Hove JL, Freehauf C, Miyamoto S, et
al: Infantile cardiomyopathy caused by the T14709C mutation in the
mitochondrial tRNA glutamic acid gene. Eur J Pediatr. 167:771–776.
2008.PubMed/NCBI
|
|
96
|
Ruppert V, Nolte D, Aschenbrenner T, et
al: Novel point mutations in the mitochondrial DNA detected in
patients with dilated cardiomyopathy by screening the whole
mitochondrial genome. Biochem Biophys Res Commun. 318:535–543.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Dikalov S: Cross talk between mitochondria
and NADPH oxidases. Free Radic Biol Med. 51:1289–1301. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Leone TC and Kelly DP: Transcriptional
control of cardiac fuel metabolism and mitochondrial function. Cold
Spring Harb Symp Quant Biol. 76:175–182. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Cottrill KA and Chan SY: Metabolic
dysfunction in pulmonary hypertension: the expanding relevance of
the Warburg effect. Eur J Clin Invest. 43:855–865. 2013. View Article : Google Scholar : PubMed/NCBI
|